Abstract
Background
Antibiotics are prescribed to most pediatric intensive care unit (PICU) patients, but data describing indications and appropriateness of antibiotic orders in this population are lacking.
Methods
We performed a multicenter point prevalence study that included children admitted to 10 geographically diverse PICUs over 4 study days in 2019. Antibiotic orders were reviewed for indication, and appropriateness was assessed using a standardized rubric.
Results
Of 1462 patients admitted to participating PICUs, 843 (58%) had at least 1 antibiotic order. A total of 1277 antibiotic orders were reviewed. Common indications were empiric therapy for suspected bacterial infections without sepsis or septic shock (260 orders, 21%), nonoperative prophylaxis (164 orders, 13%), empiric therapy for sepsis or septic shock (155 orders, 12%), community-acquired pneumonia (CAP; 118 orders, 9%), and post-operative prophylaxis (94 orders, 8%). Appropriateness was assessed for 985 orders for which an evidence-based rubric for appropriateness could be created. Of these, 331 (34%) were classified as inappropriate. Indications with the most orders classified as inappropriate were empiric therapy for suspected bacterial infection without sepsis or septic shock (78 orders, 24%), sepsis or septic shock (55 orders, 17%), CAP (51 orders, 15%), ventilator-associated infections (47 orders, 14%), and post-operative prophylaxis (44 orders, 14%). The proportion of antibiotics classified as inappropriate varied across institutions (range, 19%–43%).
Conclusions
Most PICU patients receive antibiotics. Based on our study, we estimate that one-third of antibiotic orders are inappropriate. Improved antibiotic stewardship and research focused on strategies to optimize antibiotic use in critically ill children are needed.
Keywords: pediatric intensive care unit, antimicrobial stewardship, sepsis, antibiotic
In a 10-center point prevalence study, we found that one-third of antibiotics prescribed to critically ill children are inappropriate. Empiric therapy for suspected bacterial infections, pneumonia, and post-operative prophylaxis account for the majority of inappropriate antibiotic orders in this population.
Children admitted to the pediatric intensive care unit (PICU) are a highly complex and vulnerable patient population who frequently experience serious bacterial infections, either as the primary reason for PICU admission or as a complication of hospitalization for critical illness [1–4]. Further, nonspecific signs of systemic inflammation are common among critically ill children and often prompt initiation of broad-spectrum antimicrobials, an approach driven in part by the deleterious impacts of delayed antimicrobial therapy in patients with bacterial septic shock [5–7]. Collectively, these factors contribute to high utilization of broad-spectrum antimicrobials in the PICU, with prior studies demonstrating that 56%–80% of children hospitalized in the PICU receive 1 or more antibiotics during their stay [8–12].
While undoubtedly beneficial for patients with bacterial infections, antibiotics also carry a risk of adverse drug events, acute kidney injury, or Clostridioides difficile infection and contribute to the burgeoning issue of antimicrobial resistance [13–17]. These potential harms are amplified in the PICU, as critically ill children are at increased risk for infections due to antimicrobial resistant organisms, may be prone to developing serious adverse events from coexisting organ system failures (eg, preexisting acute kidney injury exacerbated by vancomycin administration), and are often on numerous medications, heightening the potential risk of drug interactions (eg, interactions between ciprofloxacin and other QTc prolonging medications) [18, 19]. Optimized antibiotic stewardship approaches that integrate the need for urgent antimicrobials in a population at high risk for septic shock, the challenge of differentiating true infections from noninfectious mimics, and the high risk of preventable harm from unnecessary antibiotic exposure are therefore critical.
There is significant hospital-level variation in antibiotic use across PICUs [20]. Single-center studies have reported estimates of inappropriate antibiotic use in the PICU ranging from 0% to 61% [11, 12, 21]. A large multicenter cohort study of hospitalized children in the United States estimated that approximately 34% of antibiotics prescribed to children hospitalized in the PICU were suboptimal [22]. Our aim in this multicenter study was to build on this prior work by comprehensively characterizing antibiotic indications and systematically assessing the appropriateness of antibiotics prescribed to critically ill children to define targets for improved antimicrobial stewardship in the PICU.
METHODS
Study Design and Setting
This cross-sectional, point prevalence study was conducted in the PICUs of 10 tertiary care, geographically diverse children's hospitals in the United States. All participating centers have antimicrobial stewardship programs. The participating PICUs ranged in size from 18 to 84 beds. Data were collected on 4 weekdays during specified 2-week periods between January 2019 and June 2019. Institutional review board approval was obtained at each institution.
Study Population
All PICU patients aged 0–20 years with 1 or more nontopical antibiotics ordered at 8:00 Am on each study day were included. Within this overall study population, patients were further classified as having received broad-spectrum antibiotics if they received at least 1 of the following: third- or fourth-generation cephalosporin, fluoroquinolone, carbapenem, piperacillin-tazobactam, ceftazidime-avibactam, vancomycin, linezolid, or daptomycin. Patients with only antifungal or antiviral medication orders were excluded.
Data Collection
Data were collected by attending or fellow physicians in pediatric critical care and/or infectious diseases using a standardized data collection form through manual review of clinical documentation available in the electronic health record. Data were entered into a Research Electronic Data Capture database hosted at Children's Hospital of Philadelphia. Approximately 100 rules for checking data quality were integrated, and potential discrepancies were resolved with site principal investigators. Patient-level variables included clinical and demographic data. Because each patient may have had active orders for more than 1 antibiotic, antibiotic indication and appropriateness were assessed for each individual order.
Defining Antibiotic Indication
Antibiotic indication was determined based on clinical documentation (including PICU progress notes, consultant notes, and orders) and reflected the use intended by the ordering clinician. Indications were grouped into 6 categories: prophylaxis, defined as an antibiotic prescribed to prevent an infection in a patient without a suspected or definite infection; empiric therapy, defined as an antibiotic prescribed for a suspected infection for which a diagnosis of a definite infection has not yet been made by the clinician; treatment of a clinician-diagnosed infection; a noninfectious condition; cystic fibrosis or bronchiectasis exacerbation; or febrile neutropenia (eg, while awaiting neutrophil count recovery). Antibiotics prescribed for prophylaxis were subcategorized into those prescribed for post-operative prophylaxis and non–-post-operative prophylaxis. Antibiotics prescribed for empiric therapy were subcategorized into those ordered for suspected bacterial infections without sepsis-associated organ dysfunction or shock and those ordered for suspected bacterial infection with sepsis-associated organ dysfunction or septic shock, as defined by published criteria [23]. All clinician-diagnosed infections were reported by source. Nonsystemic antibiotics, including inhaled, intraventricular, and intravesical antibiotics, were considered separately as one group.
Defining Antibiotic Appropriateness
Antibiotic appropriateness was assessed using a standardized indication-specific rubric based on national or international guidelines, published literature, and expert consensus (Supplementary Table 1). Investigators were also permitted to enter “other” reasons for inappropriate antibiotic prescribing as well as to “disagree” with the established rubric. These responses were independently evaluated by 2 investigators (K. C. and J. S. G.) and reconciled with the site principal investigator. All orders for post-operative prophylaxis, empiric therapy, and clinician-diagnosed infections were assessed for appropriateness. Antibiotic orders for all other indications were not assessed for appropriateness, as decisions for using these antibiotics are generally not made by intensivists and/or guidelines informing optimal use are not available (Supplementary Table 2). Inappropriate antibiotic orders for clinician-diagnosed infections were further subclassified as “definitely” or “likely” inappropriate; likely inappropriate orders are those where there is greater subjectivity in assessing appropriateness (Supplementary Table 1).
Data Analyses
We analyzed antibiotic choices and indication using standard descriptive statistics, using frequencies and proportions. In the primary analysis, all definite or likely inappropriate antibiotic use was classified as inappropriate. As a secondary analysis, we report only the frequency and proportions of antibiotics classified as definitely inappropriate.
RESULTS
Patient Demographic and Clinical Characteristics
Among the 1462 patients admitted to the 10 PICUs over the 4 study days, 843 patients (58%) were prescribed at least 1 antibiotic and 494 (34%) received at least 1 broad-spectrum antibiotic. A total of 311 (37%) received 2 or more antibiotics. Most patients who received antibiotics (670 of 843, 79%) had 1 or more comorbid medical conditions; chronic respiratory failure and static encephalopathy/severe developmental delay were most common, each occurring in 20% of patients. Almost two-thirds (63%) of patients received mechanical ventilation within 7 days of the study day, and 22% received vasoactive infusions (Table 1).
Table 1.
Clinical and Demographic Characteristics of Pediatric Intensive Care Unit Patients Who Received Antibiotics
| Characteristic | Any Antibiotic, n (%) (Total N = 843) | Broad-Spectrum Antibiotic,a n (%) (Total N = 494) |
|---|---|---|
| Age, median (interquartile range), months | 47 (13–149) | 72 (13–162) |
| Male sex | 448 (53) | 250 (51) |
| Race | ||
| ȃWhite | 490 (58) | 302 (61) |
| ȃBlack | 152 (18) | 82 (17) |
| ȃAsian | 46 (5) | 29 (6) |
| ȃAmerican Indian/Alaskan Native | 6 (1) | 2 (<1) |
| ȃNative Hawaiian/Pacific Islander | 3 (<1) | 3 (<1) |
| ȃOther/Mixed Race | 61 (7) | 30 (6) |
| ȃNot reported | 85 (10) | 46 (9) |
| Ethnicity | ||
| ȃHispanic or Latino | 142 (17) | 86 (17) |
| ȃNot Hispanic or Latino | 605 (72) | 354 (72) |
| ȃNot reported | 95 (11) | 53 (11) |
| Institution | ||
| ȃ1 | 173 (21) | 110 (22) |
| ȃ2 | 144 (17) | 73 (15) |
| ȃ3 | 113 (13) | 71 (14) |
| ȃ4 | 73 (9) | 44 (9) |
| ȃ5 | 69 (8) | 35 (7) |
| ȃ6 | 69 (8) | 45 (9) |
| ȃ7 | 67 (8) | 37 (7) |
| ȃ8 | 63 (7) | 35 (7) |
| ȃ9 | 39 (5) | 25 (5) |
| ȃ10 | 34 (4) | 21 (4) |
| Study day | ||
| ȃ1 | 211 (25) | 127 (26) |
| ȃ2 | 197 (23) | 127 (26) |
| ȃ3 | 226 (27) | 128 (26) |
| ȃ4 | 209 (25) | 112 (23) |
| Comorbid medical conditions | ||
| ȃPreviously healthy | 173 (21) | 108 (22) |
| ȃImmunocompromisedb | 199 (24) | 116 (23) |
| ȃRespiratory | ||
| ȃȃChronic respiratory failurec | 172 (20) | 82 (17) |
| ȃȃChronic respiratory insufficiencyd | 74 (9) | 39 (8) |
| ȃCardiac | ||
| ȃȃPulmonary hypertension | 77 (9) | 39 (8) |
| ȃȃCongenital heart disease | 105 (12) | 51 (10) |
| ȃNeurologic | ||
| ȃȃStatic encephalopathy/severe developmental delaye | 168 (20) | 100 (20) |
| ȃȃSeizure disorder | 140 (17) | 81 (16) |
| ȃȃNeuromuscular disease | 58 (7) | 33 (7) |
| ȃȃOther neurologic condition | 31 (4) | 20 (4) |
| ȃRenal | ||
| ȃȃChronic dialysis dependence or chronic kidney disease | 29 (3) | 15 (3) |
| ȃȃNeurogenic bladder or abnormal genitourinary tract anatomy | 46 (5) | 30 (6) |
| ȃPrematurity (<32 weeks’ gestation) | 80 (9) | 45 (9) |
| ȃGenetic or metabolic syndrome | 76 (9) | 45 (9) |
| ȃEndocrine disease | 13 (2) | 11 (2) |
| ȃHematologic disease | 19 (2) | 9 (2) |
| ȃHepatic disease | 7 (1) | 5 (1) |
| ȃNonhepatic gastrointestinal disease | 29 (3) | 22 (4) |
| ȃOther comorbidityf | 12 (2) | 2 (<1) |
| Invasive medical devices (within 7 days) | ||
| ȃNone | 143 (17) | 76 (15) |
| ȃCentral venous catheter | 461 (55) | 310 (63) |
| ȃArterial line | 231 (27) | 163 (33) |
| ȃEndotracheal tube | 328 (39) | 215 (44) |
| ȃTracheostomy | 209 (25) | 107 (22) |
| ȃFoley catheter | 211 (25) | 142 (29) |
| ȃExternalized ventricular drain | 42 (5) | 29 (6) |
| Intensive care unit therapies (within 7 days) | ||
| ȃNone | 139 (16) | 77 (16) |
| ȃInvasive mechanical ventilation | 532 (63) | 318 (64) |
| ȃNoninvasive mechanical ventilation | 174 (21) | 96 (19) |
| ȃVasoactive infusion | 185 (22) | 139 (28) |
| ȃExtracorporeal membrane oxygenation | 17 (2) | 14 (3) |
| ȃInhaled nitric oxide | 31 (4) | 27 (6) |
| Penicillin or cephalosporin allergy | 68 (8) | 41 (8) |
| Severe penicillin or cephalosporin allergy g | 4 (<1) | 3 (<1) |
Includes third- or fourth-generation cephalosporin, fluoroquinolone, carbapenem, piperacillin-tazobactam, ceftazidime-avibactam, vancomycin, linezolid, or daptomycin.
Includes patients who have undergone a bone marrow transplant, solid organ transplant, are being actively treated for a malignancy, or are receiving systemic immunosuppression with tacrolimus, sirolimus, oral or subcutaneous methotrexate ≥5 mg, prednisone 2 mg/kg/day or ≥ 20 mg daily for >2 weeks, cyclophosphamide, rituximab, mycophenolate mofetil, azathioprine, anakinra, infliximab, etanercept, adalimumab, or any other monoclonal antibody or any tumor necrosis factor-alpha inhibitor when taken for nonmalignant, nontransplant condition.
Includes patients with tracheostomies requiring long-term mechanical ventilation for all or part of the day prior to admission.
Includes patients requiring noninvasive mechanical ventilation (continuous positive airway pressure or bilevel positive airway pressure) for all or part of the day prior to admission.
Impaired consciousness severe enough to result in patients being nonverbal or nonambulatory.
Lymphatic disorder/lymphangectasia (6), cardiomyopathy (4), other/not specified (2).
Includes anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis, or drug reaction with eosinophilia and systemic symptoms.
Antibiotic Choice
Of the 1277 antibiotic orders, 897 (70%) were administered intravenously, 360 (28%) enterally, and 20 (2%) by the intraventricular, intravesicular, or inhaled routes combined. The most common antibiotic orders were intravenous vancomycin (152, 12%), ceftriaxone (148, 12%), cefepime (146, 12%), trimethoprim-sulfamethoxazole (134, 11%), and metronidazole (65, 5%). Across the 10 institutions, at least 3 of these 5 overall top antibiotics appeared in each institution's top 5 antibiotics, though there was substantial variability in the other top antibiotics (Supplementary Table 3A).
Antibiotic Indications
Treatment for clinician-diagnosed infections accounted for most antibiotic orders (476, 38%), followed by empiric therapy for suspected bacterial infections (415, 33%), nonoperative prophylaxis (164, 13%), and post-operative prophylaxis (94 orders, 7%). Less common indications for antibiotic orders included noninfectious indications, including antiinflammatory effect and gastrointestinal motility (76, 6%), febrile neutropenia (18, 1%), and cystic fibrosis exacerbation (5, 0.4%). We were unable to determine indication for 9 orders (<1%; Table 2).
Table 2.
Indications for Antibiotic Orders in Pediatric Intensive Care Unit Patients
| Indication Category | Subcategory | n (%) Total N = 1277 |
|---|---|---|
| Prophylaxis: antibiotic ordered to prevent an infection when no suspected or documented infection for which this antibiotic ordered was present Total antibiotic orders for prophylaxis = 258/1277 (20%) |
Post-operative | 94 (7) |
| Non–post-operativea | 164 (13) | |
| Empiric: antibiotic ordered for a suspected infection in which a diagnosis of a definite infection had not yet been made by the clinician Total antibiotic orders for empiric therapy = 415/1277 (32%) |
Suspected bacterial infection (no sepsis or septic shock) | 260 (20) |
| ȃSpecific source suspected | 188 | |
| ȃNo specific source suspected | 72 | |
| Sepsis-associated organ dysfunction or septic shock | 155 (12) | |
| ȃSpecific source suspected | 104 | |
| ȃNo specific source suspected | 51 | |
| Clinician-diagnosed infection Total orders for treatment of a clinician-diagnosed infection = 476/1277 (37%) |
Bacteremia (central line–associated bloodstream infection or noncatheter-associated) | 47 (4) |
| Meningitis or ventriculitis | 28 (2) | |
| Community-acquired pneumonia | 123 (10) | |
| Hospital-acquired pneumonia | 34 (3) | |
| Ventilator-associated infection (includes pneumonia or tracheitis) | 86 (7) | |
| Intraabdominal infection | 33 (3) | |
| Urinary tract infection (catheter-associated urinary tract infection or noncatheter-associated) | 39 (3) | |
| Skin or soft tissue infection | 23 (20) | |
| Culture-negative sepsis | 5 (<1) | |
| Other | 58 (12) | |
| ȃClostridioides difficile | 18 | |
| ȃHead, eye, ear, nose, or throat infection | 25 | |
| ȃHome medication | 7 | |
| ȃOther respiratory | 4 | |
| ȃDonor-derived infection | 2 | |
| ȃEndovascular infection | 2 | |
| Noninfectious condition Total orders for noninfectious condition = 76/1277 (6%) |
Antiinflammatory | 29 (2) |
| Gastrointestinal motility | 33 (3) | |
| Small bowel bacterial overgrowth | 10 (<1) | |
| Other | 4 (<1) | |
| Cystic fibrosis or bronchiectasis exacerbation Total orders for cystic fibrosis or bronchiectasis exacerbation = 5/1277 (<1%) |
No subcategories | 5 (<1) |
| Febrile neutropenia (secondary prophylaxis while awaiting count recovery) Total orders for febrile neutropenia = 18/1277 (1%) |
No subcategories | 18 (1) |
| Nonsystemic route Total orders administered by a nonsystemic route = 20/1277 (2%) |
Inhaled | 16 (1) |
| Intraventricular | 2 (<1) | |
| Intravesical | 2 (<1) | |
| Intraperitoneal | 0 (0) | |
| Cannot determine Total orders for which an indication could not be determined = 9/1277 (<1%) |
No subcategories | 9 (<1) |
Nonoperative prophylaxis includes prophylaxis for opportunistic infections in immunocompromised hosts, urinary tract infection prophylaxis, endocarditis prophylaxis, and extracorporeal membrane oxygenation prophylaxis.
Within these broad indications, antibiotic orders for empiric therapy for suspected bacterial infections without sepsis-associated organ dysfunction or shock (260, 21%), prophylaxis for nonoperative indications (164, 13%), empiric therapy for suspected bacterial infection with sepsis-associated organ dysfunction or septic shock (155, 12%), community-acquired pneumonia (CAP; 118, 9%), and post-operative prophylaxis (94, 8%) were most common. Among antibiotic orders for empiric therapy for suspected bacterial infection, a specific source of infection was suspected in 70% (Table 2). Across the 10 institutions, at least 3 of the 5 overall top indications appeared in each institution's top 5 indications, though there was substantial variability regarding the other top indications (Supplementary Table 3B).
Antibiotic Appropriateness
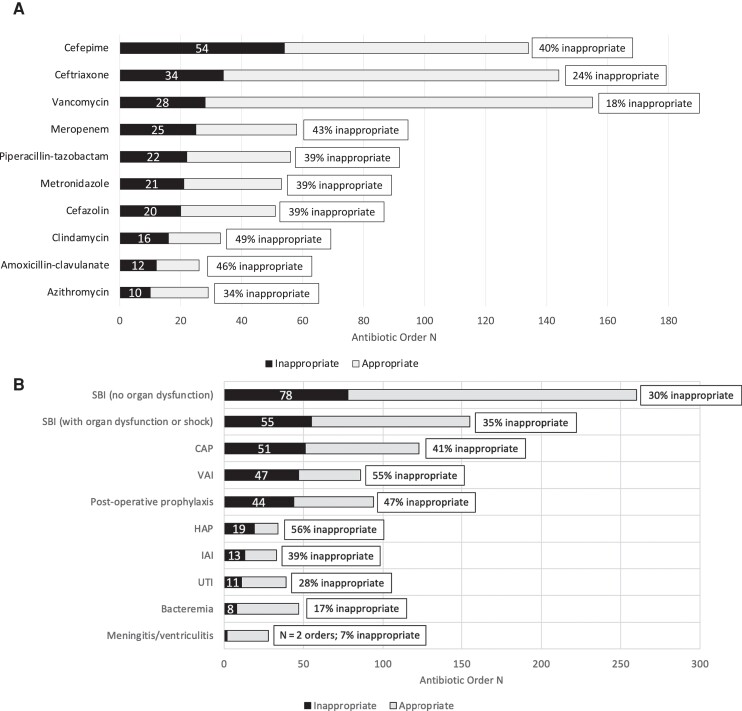
Of the 843 patients who received antibiotics during the study, 283 (34%) received 1 or more antibiotics classified as inappropriate. Of the 1277 individual antibiotic orders, 985 (77%) were assessed for appropriateness using our standardized rubric; the majority of the remaining 292 orders were antibiotics administered for nonoperative prophylaxis or noninfectious indications (Supplementary Table 2). A total of 331 (34%) antibiotic orders assessed were classified as inappropriate. The antibiotics accounting for the greatest proportion of all inappropriate orders were cefepime (54, 16%), ceftriaxone (34, 11%), vancomycin (28, 8%), meropenem (25, 8%), and piperacillin-tazobactam (22, 7%; Figure 1A). The indications accounting for the greatest proportion of all inappropriate antibiotic orders were inappropriate empiric therapy for suspected bacterial infections without organ dysfunction (78 of 331, 24%), empiric therapy for suspected bacterial infection with sepsis-associated organ dysfunction or septic shock (55 of 331, 17%), CAP (51 of 331, 15%), ventilator-associated infections (VAI) including ventilator-associated pneumonia (VAP) and ventilator-associated tracheitis (47 of 331, 14%), and post-operative prophylaxis (44 of 331, 13%; Figure 1B).
Figure 1.
A, Top inappropriate antibiotic orders by drug. The total bar is the number of antibiotic orders that were reviewed for appropriateness, sorted by antibiotic. The black stacked bar indicates antibiotic orders classified as inappropriate. The gray portion of the stacked bar indicates the antibiotic orders classified as appropriate. The numbers on each bar indicate the total number of antibiotic orders. B, Top inappropriate antibiotic order indications. The total bar is the number of antibiotics that were reviewed for appropriateness, sorted by indication. The black stacked bar indicates antibiotic orders classified as inappropriate. The gray portion of the stacked bar indicates the antibiotic orders classified as appropriate. The numbers on each bar indicate the total number of antibiotic orders. Abbreviations: CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; IAI, intraabdominal infection; SBI, suspected bacterial infection; UTI, urinary tract infection; VAI, ventilator-associated infection.
Common reasons for classifying antibiotic orders as inappropriate include prolonged empiric therapy (78, 24%), antibiotics ordered for a clinician-diagnosed infection where evidence of a bacterial infection was lacking (70, 21%), overly broad-spectrum therapy for a clinician-diagnosed infection (58, 18%), prolonged post-operative prophylaxis (41, 12%), and excessive duration of therapy for a clinician-diagnosed infection (36, 11%; Tables 3 and 4).
Table 3.
Inappropriate Antibiotic Orders and Reasons for Inappropriate Classification by Indication Category
| Category | n (%), Total Inappropriate Orders = 331 |
|---|---|
| Prophylaxisa | 44 (13) |
| ȃPost-operative prophylaxis >24 hours (clean/clean contaminated procedure) | 41 (12) |
| ȃPost-operative antibiotic choice not aligned with national guidelines (allergy or multidrug-resistant organism excluded) | 3 (1) |
| Empiric therapya | 133 (40) |
| ȃEmpiric therapy given ≥4 days without a clinician-diagnosed infection defined, unless pending surgical procedure or microbiologic or other diagnostic test | 78 (24) |
| ȃAntipseudomonal beta-lactam ordered for a community-onset infection,b absent an invasive device or residence in a long-term care facility | 25 (8) |
| ȃAntibiotic choice not compliant with local guidelines (absent an allergy, multidrug-resistant organism history, or treatment failure) | 23 (7) |
| ȃAntibiotic choice noncompliant with Surviving Sepsis Campaign guidelines and administered for sepsis | 6 (2) |
| ȃAntibiotic orders for community-acquired pneumonia with no radiographic infiltrate and no need for new or escalated respiratory support | 6 (2) |
| ȃAntibiotic orders for urinary tract infection with no clinical symptoms, pyuria, or nitrites | 2 (<1) |
| Clinician-diagnosed infectiona,c | 154 (47) |
| ȃLack of supporting clinical evidence of bacterial infection | 70 (21) |
| ȃToo broad | 58 (18) |
| ȃInappropriate duration | 36 (11) |
| ȃInappropriate route | 27 (8) |
| ȃOverlapping coverage | 9 (3) |
| ȃToo narrow | 3 (1) |
Antibiotic orders may be classified as inappropriate for multiple reasons. Therefore, the column totals and percentages will exceed 331 (100%) inappropriate orders.
Community-onset infection was defined as an infection that was present upon admission or occurred within 48 hours of hospitalization. Patients with invasive devices or those who reside in long-term care facilities were excluded from this definition.
See Supplementary Table 1 for detailed criteria for assessing appropriateness of antibiotic orders for clinician-diagnosed infection.
Table 4.
Reasons for Inappropriate Antibiotic Classification Among Patients Being Treated for Clinician-Diagnosed Infections
| Indication | Total Orders for Indication, N | Inappropriate Orders, n (%) | Too Narrow, n (%) | Too Broada, n (%) | Overlapping Coverage, n (%) | Inappropriate Duration, n (%) | No Infection, n (%) | Suboptimal Route, n (%) |
|---|---|---|---|---|---|---|---|---|
| Bacteremia | 47 | 8 (17) | 0 | 6 (13) | 2 (4) | 4 (9) | 2 (4) | 0 |
| Meningitis/ventriculitis | 28 | 2 (7) | 0 | 2 (7) | 0 | 0 | 0 | 0 |
| Community-acquired pneumonia | 123 | 51 (41) | 0 | 18 (15) | 2 (2) | 2 (2) | 32 (26) | 10 (8) |
| Hospital-acquired pneumonia | 34 | 19 (56) | 1 (3) | 6 (18) | 0 | 7 (21) | 7 (21) | 6 (18) |
| Ventilator-associated infection | 86 | 47 (55) | 2 (2) | 16 (19) | 1 (1) | 14 (16) | 24 (28) | 8 (9) |
| Intraabdominal infection | 33 | 13 (39) | 0 | 3 (9) | 4 (12) | 8 (24) | 1 (3) | 0 |
| Urinary tract infection | 39 | 11 (28) | 0 | 7 (18) | 0 | 1 (3) | 2 (5) | 2 (5) |
| Skin or soft tissue infection | 23 | 1 (4) | 0 | 0 | 0 | 0 | 0 | 1 (4) |
| Culture-negative sepsis | 5 | 2 (40) | 0 | 0 | 0 | 0 | 2 (40) | 0 |
| Other | 58 | NA | NA | NA | NA | NA | NA | NA |
| Totalb | 476 | 154 (32) | 3 (<1) | 58 (12) | 9 (2) | 36 (8) | 70 (15) | 27 (6) |
Of the N orders classified as inappropriate due to therapy that is too broad, 2 orders for each community-acquired pneumonia, hospital-acquired pneumonia, and ventilator-associated infection were classified as inappropriate anti–methicillin-resistant Staphylococcus aureus therapy.
Antibiotic orders may be classified as inappropriate for multiple reasons. Therefore, the row totals and percentages classified as inappropriate for each reason will exceed 154 inappropriate orders.
In a secondary analysis limited to antibiotic orders classified as definitely inappropriate (265 of 331 total inappropriate orders, 80%), hospital-acquired pneumonia (HAP), VAI, and CAP remained the clinician-diagnosed infections accounting for the greatest number of inappropriate orders. The most common reasons antibiotic orders were classified as inappropriate were therapy that was too broad, duration of therapy that was too long, and suboptimal route of administration (Supplementary Table 4).
Institutional Variation
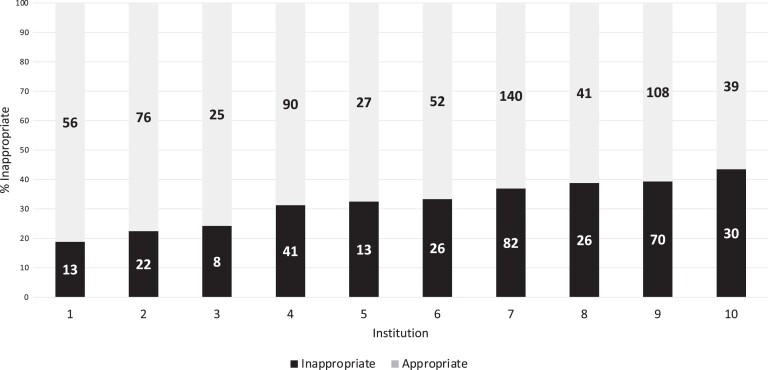
The proportion of patients who received any antibiotics varied across institutions (range, 47%–62%), as did the proportion of patients receiving broad-spectrum antibiotics (range, 28%–39%). There was variability in the proportion of antibiotic orders classified as inappropriate, ranging from 19% to 43% (Figure 2).
Figure 2.
Variation in inappropriate antibiotic orders by institution. The black portion of the stacked bar indicates the proportion of antibiotic orders classified as inappropriate. The gray portion of the stacked bar indicates the proportion of antibiotic orders classified as appropriate. The numbers on each bar indicate the total number of antibiotic orders.
DISCUSSION
In this multicenter study, we determined that 58% of children hospitalized in tertiary care US PICUs received antibiotics, with empiric therapy for suspected bacterial infections and treatment of clinician-diagnosed respiratory infections being the most common indications for antibiotic use. More than one-third of patients received 1 or more antibiotics classified as inappropriate, and 34% of all antibiotic orders assessed for appropriateness were classified as inappropriate. Prolonged empiric antibiotic therapy for suspected bacterial infection with or without shock, antibiotics prescribed absent clinical evidence of a bacterial infection (particularly antibiotics ordered for respiratory infections), unnecessarily broad antibiotic therapy, antibiotics ordered for excessive durations, and prolonged post-operative prophylaxis were common reasons antibiotic orders were classified as inappropriate. Collectively, these findings identify targets for improved antibiotic stewardship in critically ill children and identify future research needs in this area.
To date, studies evaluating antibiotic appropriateness in the PICU consist primarily of single-center reports and studies conducted outside of the US, and these reports estimate that 18%–61% of patients receive inappropriate antibiotics [11, 12, 21, 24, 25]. In the largest US study performed to date that evaluated antibiotic use in hospitalized children, 34% of nonneonatal ICU patients received at least 1 inappropriate antibiotic [22]. While granularity of antibiotic indications and the reasons for inappropriate use were not provided specifically for the PICU patients in this report, the consistency of the overall estimate across these 2 large studies supports the robustness of this estimate in quantifying the magnitude of inappropriate antibiotic use in US PICUs.
Our study builds on this prior work and introduces several novel findings. First, empiric therapy for suspected bacterial infections, with or without sepsis-associated organ dysfunction or septic shock, was the most common indication for antibiotic orders in the PICU. Efforts to improve antibiotic prescribing in the PICU must therefore include efforts to optimize empiric therapy, particularly given that many stewardship programs use prospective audit with feedback, where reviews may not occur until 48–72 hours after antibiotics are initiated [26]. Such efforts should target both the urgency of empiric antibiotic administration and the empiric antibiotic choice. National guidelines, regulatory mandates, and quality improvement collaboratives have appropriately emphasized the need for emergent broad-spectrum antibiotics administered within 1 hour for patients with septic shock given associations between prolonged time to antibiotics and deleterious clinical outcomes [7, 27]. However, for less severely ill patients, this association is weak or absent, such that overemphasis on emergent broad-spectrum antibiotic administration for all patients risks overtreatment of patients in whom a focal source of infection or a noninfectious condition could have been identified prior to antibiotic administration [5, 6, 28]. In our cohort, almost two-thirds of patients who received empiric antibiotics did not have septic shock or sepsis-associated organ dysfunction, highlighting that this strategy may be feasible in many cases. Further, overuse of empiric anti–methicillin-resistant Staphylococcus aureus and anti-pseudomonal antibiotics in patients unlikely to be infected with these resistant organisms (eg, patients with community-onset sepsis) is supported by both the current study and published literature [29–31]. While improved clinical prediction rules and rapid molecular diagnostic tests hold promise for tailoring empiric antibiotic choices to patient risk of multidrug-resistant organisms, quality improvement initiatives that target reductions in empiric vancomycin administration in the PICU based on readily available clinical information have been shown to be both safe and effective, highlighting this current potential opportunity for improved stewardship [30, 31].
Second, inappropriately prolonged durations of empiric antibiotic therapy accounted for almost one-quarter of inappropriate antibiotic use, consistent with published literature and demonstrating that once initiated, antibiotics are often not deescalated or discontinued in a timely fashion [32, 33]. This underscores the importance of an appropriate infectious evaluation at the time of antibiotic initiation to inform subsequent decision making, as well as the need for reassessment after 48–72 hours of antibiotic therapy [34]. However, there are significant knowledge gaps regarding current deescalation strategies that warrant further study in the critical care setting, including the optimal approach for implementing a prospective audit with feedback, the impact of clinician-driven antibiotic time-outs, and the role for rapid molecular diagnostic testing and biomarkers.
Third, almost 50% of antibiotics ordered for post-operative prophylaxis were classified as inappropriate, the majority of which were classified as inappropriate due to being administered for >24 hours after surgery. This estimate of inappropriate antibiotic use is conservative, as the Surgical Infection Society recommends against any antibiotic administration after skin closure [35]. Given these guideline recommendations and published data quantifying the additive risk of antibiotic adverse events with each additional day of unnecessary antibiotics following surgery, reducing duration of post-operative antibiotics should be a shared priority of intensivists, surgeons, and antimicrobial stewardship programs [36].
Finally, respiratory infections represented common indications for antibiotics, but evidence supporting a diagnosis of bacterial infection was lacking in up to 28%, while overly broad and excessively long treatment durations occurred in nearly 20% of HAP and VAP cases. Undoubtedly, the lack of a “gold standard” for diagnosing bacterial pneumonia hampers both individual clinical decision making and stewardship efforts in this area, highlighting the need for improved strategies for diagnosing these common conditions in the PICU. Nevertheless, stewardship interventions that are focused on optimizing the duration of antibiotics for VAP and HAP, with 7 days of therapy recommended by the 2016 Infectious Diseases Society of America /American Thoracic Society guidelines and supported by the results of randomized trials, represent an actionable target based on our results [37–39].
Despite these strengths, our work is subject to several limitations. First, assessment of antibiotic appropriateness is inherently subjective, particularly given that this assessment was performed retrospectively and by different individuals at each site. We sought to mitigate this by using a standardized rubric for assessing appropriateness, rather than allowing individuals to classify antibiotic orders as appropriate or inappropriate based on their own judgment. Assessing the appropriateness of antibiotic use for CAP, HAP, and VAI is particularly vulnerable to this limitation given the lack of a diagnostic gold standard for these infections. Second, while our assessment tool was based on available evidence-based guidelines, guidelines were not available for all scenarios encountered in the PICU and were often not specific to children. We were therefore permissive in classifying scenarios where evidence was weak or lacking as “appropriate” antibiotic use in the rubric, such that we may have underestimated inappropriate antibiotic use for some conditions. An example of this is empiric vancomycin use, which was classified as appropriate in all cases if administered for fewer than 4 calendar days. Third, our assessment spanned 4 days in 10 tertiary care PICUs, the majority of which were medical–surgical ICUs without cardiac surgical patients. Generalizability of our findings to smaller centers, where case mix may differ, is therefore unknown. However, our findings are strengthened given the geographic diversity of the PICUs, diversity in bed size, and given that 2 of the 10 PICUs did care for cardiac surgical patients. Finally, this study was performed in 2019, prior to the coronavirus disease 2019 pandemic. While this is a potential limitation, these prepandemic data and stewardship targets are arguably more reflective of the current case mix than 2020 or 2021 data given the changes in PICU case mix and decreases in PICU admissions during the pandemic [40].
Our study, which is the largest to date focused on antibiotic use in the PICU setting, demonstrated that more than one-third of critically ill children receive inappropriate antimicrobials. Tailoring empiric antibiotic choices, deescalating antibiotics when no bacterial infection has been identified, limiting the duration of post-operative antibiotics, and ensuring antibiotic durations are aligned with national guidelines represent actionable stewardship targets in this population. Future research that is focused on improving diagnostic modalities to differentiate bacterial infections from viral infections or noninfectious mimics, developing clinical prediction rules to more appropriately risk-stratify patients with regard to infections with antibiotic-resistant bacteria, and evaluating optimal implementation of evidence-based practices in the PICU setting are key opportunities for future research.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kathleen Chiotos, Division of Critical Care Medicine, Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Jennifer Blumenthal, Division of Infectious Diseases, Department of Pediatrics, Boston Children's Hospital, Boston, Massachusetts, USA; Division of Critical Care Medicine, Department of Anesthesiology, Critical Care, and Pain Medicine, Boston Children's Hospital, Boston, Massachusetts, USA.
Juri Boguniewicz, Section of Infectious Diseases and Epidemiology, Department of Pediatrics, University of Colorado School of Medicine, Aurora, Colorado, USA.
Debra L Palazzi, Infectious Diseases Division, Department of Pediatrics, Baylor College of Medicine, Houston, Texas, USA.
Erika L Stalets, Division of Critical Care Medicine, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Jessica H Rubens, Division of Infectious Diseases, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Pranita D Tamma, Division of Infectious Diseases, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Stephanie S Cabler, Division of Infectious Diseases, Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, USA.
Jason Newland, Division of Infectious Diseases, Department of Pediatrics, Washington University in St. Louis, St. Louis, Missouri, USA.
Hillary Crandall, Division of Pediatric Critical Care, Department of Pediatrics, University of Utah, Primary Children's Hospital, Salt Lake City, Utah, USA.
Emily Berkman, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Seattle Children's Hospital, University of Washington School of Medicine, Seattle, Washington, USA.
Robert P Kavanagh, Division of Pediatric Critical Care Medicine, Department of Pediatrics, Penn State University College of Medicine, Hershey, Pennsylvania, USA.
Hannah R Stinson, Division of Critical Care Medicine, Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Jeffrey S Gerber, Division of Infectious Diseases, Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA; Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Notes
Acknowledgments. The authors thank Giyoung Lee, MPH; Kellie Liston, MSc; Debbie Spear, RN; and Toni Yunger, BS, for their assistance with study coordination and data management. The authors also thank Sara Liechti, PharmD, for her assistance with data collection. This research was supported by the Centers for Disease Control and Prevention Cooperative Agreement FOA#CK16-004–Epicenters for the Prevention of Healthcare Associated Infections.
Financial support. This work was supported by the Agency for Healthcare Research and Quality (K12-HS026393 to K. C.) and the National Institutes of Health (T32AI052071 to J. H. R.).
References
- 1. Jung M, Park H, Kang D, et al. Age-specific distribution of diagnosis and outcomes of children admitted to ICUs: a population-based cohort study. Pediatr Crit Care Med 2019; 20:e301–10. [DOI] [PubMed] [Google Scholar]
- 2. Ibiebele I, Algert CS, Bowen JR, Roberts CL. Pediatric admissions that include intensive care: a population-based study. BMC Health Serv Res 2018; 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the Sepsis Prevalence, Outcomes, and Therapies study. Am J Respir Crit Care Med 2015; 191:1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Pediatrics 1999; 103:e39. [DOI] [PubMed] [Google Scholar]
- 5. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 2020; 21:e52–106. [DOI] [PubMed] [Google Scholar]
- 6. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA 2018; 320:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 8. Gerber JS, Newland JG, Coffin SE, et al. Variability in antibiotic use at children's hospitals. Pediatrics 2010; 126:1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J 2005; 24:766–73. [DOI] [PubMed] [Google Scholar]
- 10. Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC Project Group . The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother 2016; 71:1106–17. [DOI] [PubMed] [Google Scholar]
- 11. Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med 2000; 26:959–66. [DOI] [PubMed] [Google Scholar]
- 12. Blinova E, Lau E, Bitnun A, et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr Crit Care Med 2013; 14:e280–8. [DOI] [PubMed] [Google Scholar]
- 13. Same RG, Hsu AJ, Cosgrove SE, et al. Antibiotic-associated adverse events in hospitalized children. J Pediatr Infect Dis Soc 2021; 10:622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lovegrove MC, Geller AI, Fleming-Dutra KE, Shehab N, Sapiano MRP, Budnitz Ds. US emergency department visits for adverse drug events from antibiotics in children 2011–2015. J Pediatr Infect Dis Soc 2019; 8:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downes KJ, Cowden C, Laskin BL, et al. Association of acute kidney injury with concomitant vancomycin and piperacillin/tazobactam treatment among hospitalized children. JAMA Pediatr 2017; 171:e173219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:990–1001. [DOI] [PubMed] [Google Scholar]
- 17. Sanden L, Paul M, Leibovici Let al. Quantifying the associations between antibiotic exposure and resistance—a step towards personalised antibiograms. Eur J Clin Microbiol Infect Dis 2016; 35:1989–96. [DOI] [PubMed] [Google Scholar]
- 18. Chiotos K, Tamma PD, Flett KB, et al. Multicenter study of the risk factors for colonization or infection with carbapenem-resistant Enterobacteriaceae in children. Antimicrob Agents Chemother 2017; 61:e01440–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiner-Lastinger LM, Abner S, Benin AL, et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: summary of data reported to the National Healthcare Safety Network 2015–2017. Infect Control Hosp Epidemiol 2020; 41:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brogan TV, Thurm C, Hersh AL, et al. Variability in antibiotic use across PICUs. Pediatr Crit Care Med 2018; 19:519–27. [DOI] [PubMed] [Google Scholar]
- 21. Ergul AB, Gokcek I, Celik T, Altuner Torun Y. Assessment of inappropriate antibiotic use in pediatric patients: point-prevalence study. Turk Pediatri Arsivi 2018; 53:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tribble AC, Lee BR, Flett KB, et al. Appropriateness of antibiotic prescribing in United States children's hospitals: a national point prevalence survey. Clin Infect Dis 2020; 71:e226. [DOI] [PubMed] [Google Scholar]
- 23. Goldstein B, Giroir B, Randolph A. International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 24. Abbas Q, Ul Haq A, Kumar R, Ali SA, Hussain K, Shakoor S. Evaluation of antibiotic use in pediatric intensive care unit of a developing country. Indian J Crit Care Med 2016; 20:291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazzaz YM, AlTurki H, Aleisa L, Alahmadi B, Alfattoh N, Alattas N. Evaluating antimicrobial appropriateness in a tertiary care pediatric ICU in Saudi Arabia: a retrospective cohort study. Antimicrob Resist Infect Control 2020; 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newland JG, Gerber JS, Weissman SJ, et al. Prevalence and characteristics of antimicrobial stewardship programs at freestanding children's hospitals in the United States. Infect Control Hosp Epidemiol 2014; 35:265–71. [DOI] [PubMed] [Google Scholar]
- 27. Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014; 42:2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alam N, Oskam E, Stassen PM, et al. Prehospital antibiotics in the ambulance for sepsis: a multicentre, open label, randomised trial. Lancet Respir Med 2018; 6:40–50. [DOI] [PubMed] [Google Scholar]
- 29. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open 2020; 3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanata MM, Diaz A, Hecht SM, et al. Empiric vancomycin reduction in a pediatric intensive care unit. Pediatrics 2021; 148:e2020009142. [DOI] [PubMed] [Google Scholar]
- 31. Chiotos K, Fitzgerald JC, Hayes M, et al. Improving vancomycin stewardship in critically ill children. Pediatrics 2022; 149:e2021052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis 2016; 16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32–40. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . Core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 25 May 2022. [DOI] [PMC free article] [PubMed]
- 35. Ban KA, Minei JP, Laronga C, et al. Executive summary of the American College of Surgeons/Surgical Infection Society surgical site infection guidelines–2016 update. Surg Infect 2017; 18:379–82. [DOI] [PubMed] [Google Scholar]
- 36. Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dimopoulos G, Poulakou G, Pneumatikos IA, Armaganidis A, Kollef MH, Matthaiou DK. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 2013; 144:1759–67. [DOI] [PubMed] [Google Scholar]
- 38. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61– 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 2003; 290:2588–98. [DOI] [PubMed] [Google Scholar]
- 40. Kanthimathinathan HK, Buckley H, Davis PJ, et al. In the eye of the storm: impact of COVID-19 pandemic on admission patterns to paediatric intensive care units in the UK and Eire. Crit Care Lond Engl 2021; 25:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.