Abstract
Background
The diagnosis of infective endocarditis (IE) can be difficult, particularly if blood cultures fail to yield a pathogen. This study evaluates the potential utility of microbial cell-free DNA (mcfDNA) as a tool to identify the microbial etiology of IE.
Methods
Blood samples from patients with suspected IE were serially collected. mcfDNA was extracted from plasma and underwent next-generation sequencing. Reads were aligned against a library containing DNA sequences belonging to >1400 different pathogens. mcfDNA from organisms present above a statistical threshold were reported and quantified in molecules per milliliter (MPM). Additional mcfDNA was collected on each subject every 2–3 days for a total of 7 collections or until discharge.
Results
Of 30 enrolled patients with suspected IE, 23 had definite IE, 2 had possible IE, and IE was rejected in 5 patients by modified Duke Criteria. Only the 23 patients with definite IE were included for analysis. Both mcfDNA and blood cultures achieved a sensitivity of 87%. The median duration of positivity from antibiotic treatment initiation was estimated to be approximately 38.1 days for mcfDNA versus 3.7 days for blood culture (proportional odds, 2.952; P = .02771), using a semiparametric survival analysis. mcfDNA (log10) levels significantly declined (−0.3 MPM log10 units, 95% credible interval −0.45 to −0.14) after surgical source control was performed (pre- vs postprocedure, posterior probability >0.99).
Conclusion
mcfDNA accurately identifies the microbial etiology of IE. Sequential mcfDNA levels may ultimately help to individualize therapy by estimating a patient’s burden of infection and response to treatment.
Keywords: Infective endocarditis, microbial cell free DNA, bacteremia
Microbial cell-free DNA accurately identified the microbial etiology of infective endocarditis, was detectable for significantly longer than conventional blood cultures, and rapidly declined with surgical source control.
Infective endocarditis (IE) is a rare but life-threatening infection, with 1-year mortality rates reaching up to 30% [1]. Although the diagnosis of IE typically requires isolating a causative pathogen from the bloodstream, failure to identify an organism occurs in 15%–25% of IE cases [2–4]. The most frequent cause of culture negative IE is receipt of antibiotics before collecting blood cultures, which can transiently sterilize the blood but not eradicate the underlying infection [5, 6]. In these instances, treatment is empiric, requiring multiple long-term antibiotics with significant toxicity [7]. A number of alternative diagnostics in culture-negative IE have been explored, including enriched culture media, serological tests, and broad-range polymerase chain reaction (PCR) [2]. However, despite their additive diagnostic value, each test has limitations, including slow turnaround times, serological cross reactivity, false-positive results, or the requirement for surgically excised valve tissue [3]. Sensitive, rapid diagnostics that maintain a high level of accuracy despite initiation of antibiotics are needed for the optimal management of patients with IE.
The Karius Test developed by Karius, Inc. (Redwood City, CA, USA) detects microbial cell-free DNA (mcfDNA) by next-generation sequencing from plasma. We recently found that mcfDNA accurately identifies the causative pathogen in bacterial bloodstream infections for a significantly longer duration than conventional blood cultures [8]. Thus, mcfDNA represents a potentially valuable diagnostic for IE in general, and culture-negative IE in particular. In the present study, we prospectively evaluate the accuracy of mcfDNA in diagnosing the microbial etiology of definite IE.
METHODS
Study Cohort
This was a prospective observational cohort study of inpatients at Duke University Hospital from July 2016 to January 2018. Subjects were eligible if they were at least 18 years of age, were admitted to Duke University Hospital, and had suspected IE. Only patients meeting criteria for definite IE based on the modified Duke Criteria were included for analysis [9]. Subjects were ineligible for the study if they failed to understand instructions or comply with study-related procedures. This study was approved by the Duke institutional review board. Patients (or legally authorized representative) provided written informed consent. If a patient died before notification of blood culture results, the subjects were enrolled using an institutional review board-approved Notification of Decedent Research.
Clinical Data Abstraction
Clinical data were collected from the electronic medical record of each participant using a standardized case report form. Microbiological cause of IE was determined by an adjudication panel of 3 board-certified physicians (E. E., J. F., and V. G. F.). E. E. and J. F. independently reviewed patient clinical, histopathologic, and culture data and determined the microbiological cause of IE. Cases in which there was a disagreement were to be adjudicated by V. G. F. The kappa statistic for interrater reliability was 1.0.
Laboratory Studies
Blood cultures were drawn per physician discretion. Bacterial isolates were identified by the Duke Clinical Microbiology Laboratory using standard techniques. The BD Bactec instrument and BD Bactec routine aerobic and anaerobic Plus bottles are used by the Duke Clinical Microbiology Laboratory. Blood culture “set positivity” was defined as at least 1 bottle in a blood culture set testing positive for microbial growth.
mcfDNA Sequencing
Blood samples from each subject were collected in a K2-EDTA tube at any timepoint during their hospitalization after study enrollment. Additional blood samples for mcfDNA testing were collected on each subject periodically throughout the duration of the hospitalization. Methods and materials for mcfDNA sequencing have previously been described [10]. Briefly, mcfDNA is detected in plasma of patients in Karius’ Clinical Laboratory Improvement Amendments certified/College of American Pathologists–accredited laboratory (Redwood City, CA, USA). After mcfDNA is extracted and next-generation sequencing performed, human reads are removed, and remaining sequences are aligned to a curated database of more than 20 000 organisms, only approximately 1400 of which are reported if detected. mcfDNA from organisms present above a statistical threshold are reported and quantified in molecules per microliter (MPM).
The pathogen with the most abundant mcfDNA was compared with the pathogen identified by conventional blood culture. Longitudinal analysis was performed comparing duration of positivity of the mcfDNA to the duration of positivity of conventional blood culture. Day 0 was defined as the day targeted/effective antimicrobials were initiated, and duration of positivity was calculated using day 0 of initiation of targeted antimicrobials.
Statistical Analysis
The distributions of continuous measures are presented as means and standard deviations and categorical variables are evaluated using counts and percentages. Sensitivity of mcfDNA and blood cultures for diagnosing IE was calculated using the adjudicated microbiological cause of IE as the reference standard. Comparison of sensitivity of blood culture versus mcfDNA was calculated using the Fisher exact test. Comparison of duration of positivity for blood culture (any bottle positive or all bottles positive) versus mcfDNA was performed using semiparametric and Bayesian survival methods implemented in the icenReg R package (version 2.0.15). The icenReg package accommodates left, right, and interval censored data, definitions, and rules for the duration of positivity intervals are described in detail in the Supplemental Methods.
A Bayesian mixed-effects model was used to estimate the effect of surgical debridement on MPMs (brms R package, version 2.16.2). The model used default Bayesian priors and adjusted for duration of antibiotics, prior MPM abundance, a pre/current/postprocedure indicator variable, and a group-level effect for study participant. Additional details are provided in Supplemental Methods.
RESULTS
Clinical Characteristics
Thirty patients with suspected IE were enrolled in the study. Based on the modified Duke criteria, IE was “rejected” in 5 patients, “possible” in 2 patients, and “definite” in 23 patients (Figure 1, Supplemental Table 1). One patient with possible IE (KA01979) had 1 major criterion (positive blood culture) and 2 minor criteria (predisposing heart condition/prosthetic aortic valve and fever). Blood cultures were positive for Enterococcus faecalis, and serial mcfDNA testing revealed E faecalis. The other patient with possible IE met 3 minor criteria (history of injection drug use, fever >38.0°C, and a single blood culture that yielded Streptococcus mitis) but negative mcfDNA testing. Echocardiographic findings are detailed in Supplementary Table 2.
Figure 1.
Study cohort. Thirty patients were enrolled in the study. Of these, 23 had definite endocarditis, 2 had possible endocarditis, and 5 were rejected as having endocarditis as determined by the modified Duke criteria.
mcfDNA performance was evaluated among only the 23 patients with definite IE. Clinical and microbial characteristics are described in Table 1. Six (26.1%) of the patients endorsed prior injection drug use. Fifteen (65.2%) had native valve IE and 5 (21.7%) had prosthetic valve IE. Seven (30.4%) patients had an infected pacemaker or cardioverter defibrillator lead. Sixteen (69.6%) of the patients exhibited evidence of septic emboli during their presentation. Twenty (87.0%) of the patients required cardiac surgery.
Table 1.
Clinical and Microbiological Characteristics
| Definite Endocarditis Cohort N = 23 |
|
|---|---|
| Demographics | |
| Age, median y (Q1, Q3) | 58.0 [37.0–65.5] |
| Female | 5 (21.7%) |
| Race | |
| Black | 1 (4.3%) |
| White | 21 (91.3%) |
| Hispanic | 1 (4.3%) |
| Comorbidities | |
| Dialysis dependent | 1 (4.3%) |
| Diabetes mellitus | 6 (26.1%) |
| Injection drug use | 6 (26.1%) |
| Corticosteroid use (30 day) | 5 (21.7%) |
| APACHE II, median (Q1, Q3) | 12.0 [9.50–20.0] |
| Acute Physiology Score, median (Q1, Q3) | 10.0 [5.00–18.0] |
| True microbial etiology of endocarditis | |
| Staphylococcus aureus b | 14 (60.7%) |
| Streptococcus agalactiae | 1 (4.3%) |
| Enterococcus faecalis | 2 (8.7%) |
| Staphylococcus epidermidis | 2 (8.7%) |
| Candida albicans | 2 (8.7%) |
| Pantoea ananatis | 1 (4.3%) |
| Echocardiogram findings | |
| Transthoracic echocardiogram performed | 23 (100.0%) |
| Findings suggestive of IE present | 15 (65.2%) |
| Transesophageal echocardiogram performed | 20 (86.9%) |
| Findings suggestive of IE present | 16 (80.0%) |
| Type of endocarditis | |
| Native valve | 15 (65.2%) |
| Prosthetic valve | 5 (21.7%) |
| ICD/PM lead infectiona | 7 (30.4%) |
| Valve type | |
| Aortic | 7 (30.4%) |
| Mitral | 4 (17.4%) |
| Tricuspid | 7 (30.4%) |
| Aortic and mitral | 2 (8.7%) |
| ICD/PM lead only | 3 (13.0%) |
| Complications | |
| Septic emboli | 14 (60.9%) |
| Stroke | 4 (17.4%) |
| Vertebral osteomyelitis | 3 (13.0%) |
| Septic shock | 5 (21.7%) |
| Acute respiratory distress | 6 (26.1%) |
| Acute renal failure | 14 (60.9%) |
| Outcomes at 90 days | |
| Cured | 17 (73.9%) |
| Recurrent bacteremia | 2 (8.7%) |
| Death | 3 (13.0%) |
| Lost to follow-up | 1 (4.0%) |
Abbreviations: ICD/PM, implantable cardioverter defibrillator/pacemaker; IE, infective endocarditis; Q, quartile.
Four of the patients with pacemaker or defibrillator lead infection also had native valve involvement.
Includes 8 cases of methicillin-resistant S aureus and 6 cases of methicillin-susceptible S aureus.
Sensitivity of mcfDNA for Determining Microbiological Cause of IE
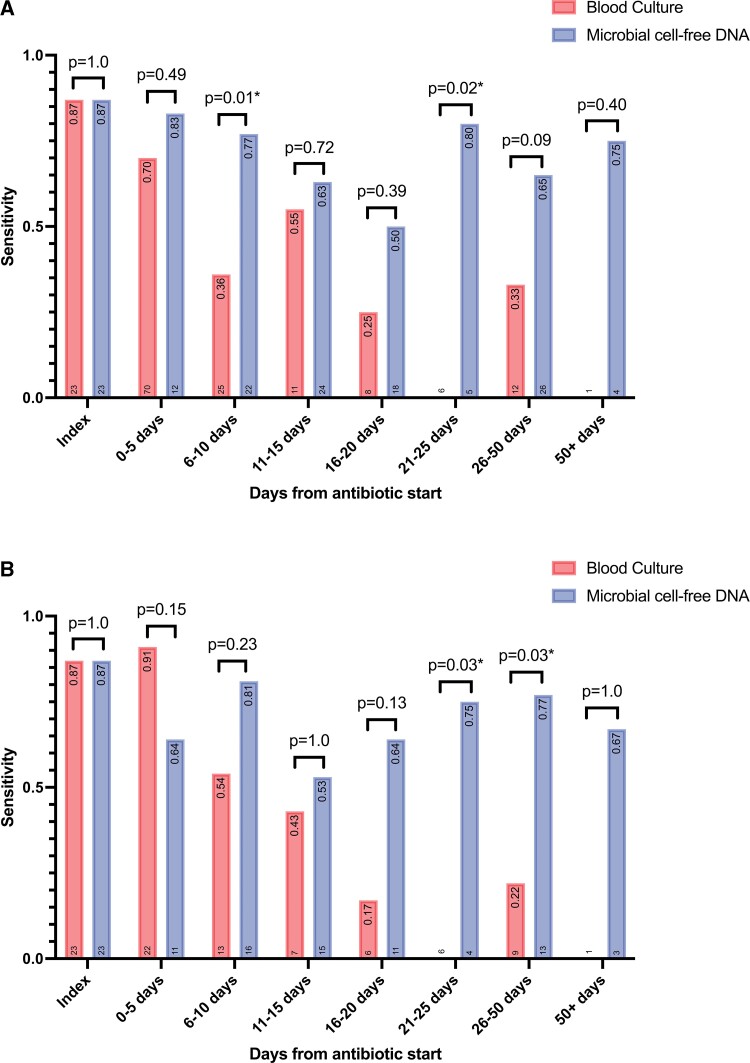
The mean time between mcfDNA collection and antibiotic initiation was significantly longer than mean time between blood culture collection and antibiotic initiation (mcfDNA 11.1 days; standard deviation [SD], 11.7 days vs blood culture −0.09 days [SD, 0.5 days]; P < 0.01) (Table 2). Despite this difference, both mcfDNA and blood cultures were 87% (20/23) sensitive in diagnosing the microbial etiology of IE.
Table 2.
Comparison of mcfDNA vs Blood Culture for the Detection of the Microbiologic Agent of Definite Infective Endocarditisa
| mcfDNA | ||||
|---|---|---|---|---|
| True Positive | False Negative | False Positive | ||
| Blood culture | True Positive | 19 | 1 | 0 |
| False Negative | 1 | 0 | 1 | |
| False Positive | 0 | 1 | 0 | |
Blood culture sensitivity: 20/23 = 87%. mcfDNA sensitivity: 20/23 = 87%.
Abbreviation: mcfDNA, microbial cell-free DNA.
True microbiologic agent of infective endocarditis for each case was determined by an adjudication panel of 3 independent board-certified physicians using clinical, microbiological, and histopathologic data.
Mean time from antibiotic start to index test draw was −0.09 days (±0.5 days) for blood culture and 11.1 days (±11.7 days) for mcfDNA.
To determine the effect of antibiotic duration on mcfDNA performance at different time intervals (ie, 5-day intervals), the sensitivity was calculated for a single patient’s index test and subsequent tests (Figure 2). Results of index and follow-up blood cultures are presented in Supplemental Table 3.
Figure 2.
Comparison of sensitivity of blood culture to mcfDNA for the detection of the microbiologic etiology of definite infectious endocarditis. (A) Results per test and (B) results per patient. The sensitivity value for each bar is listed at the top and the number of patients is listed at the bottom of each graph. Day 0 is set as the day appropriate antibiotics were started. Statistical difference between blood culture and mcfDNA was calculated using Fisher exact test; the associated P values are displayed above the corresponding bars. *Statistically significant (P < 0.05). mcfDNA, microbial cell-free DNA.
Duration of mcfDNA Detection
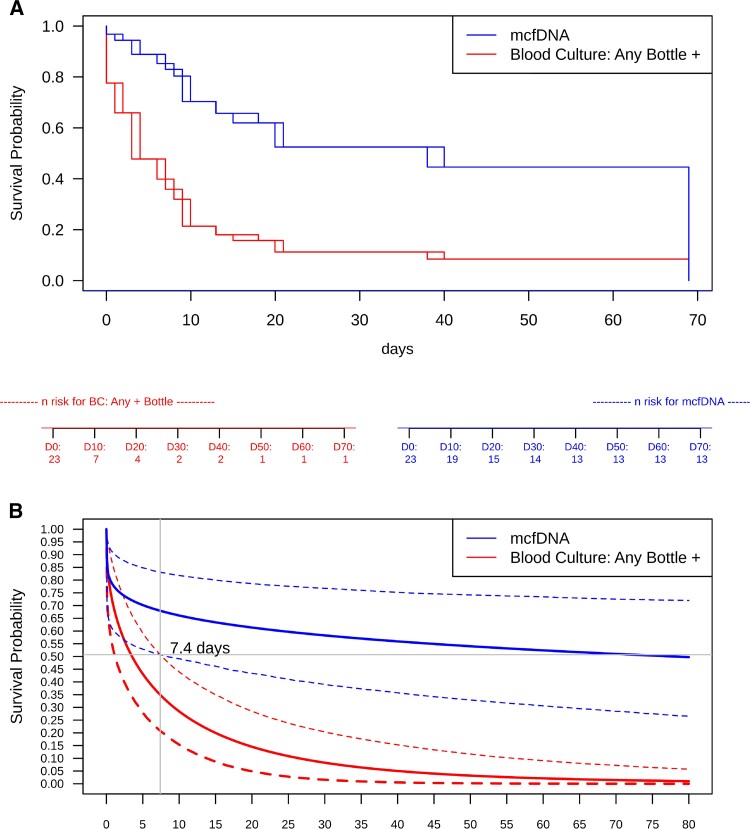
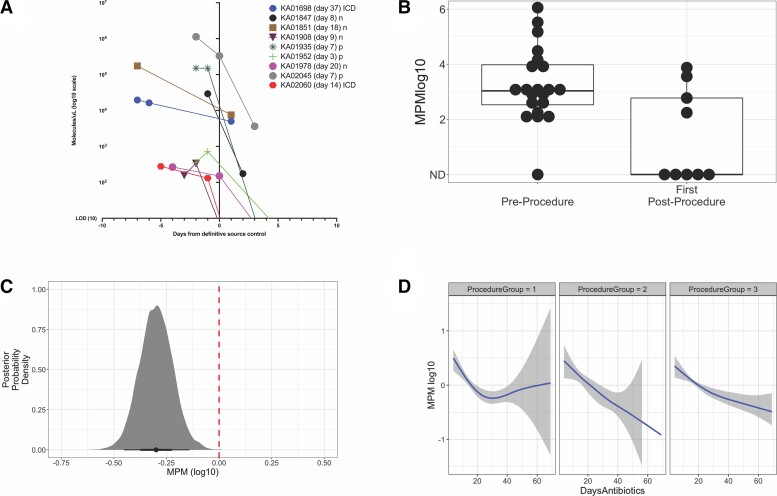
Survival analysis with interval censoring was used to estimate the period in days between the start of antibiotics and the last positive result for both mcfDNA and blood culture. mcfDNA results were compared with blood cultures set positivity. Semiparametric and Bayesian results are presented as a Kaplan-Meier and Bayesian survival curves in Figure 3A and B for mcfDNA versus blood culture set positivity. The point of separation between the detection of the causative pathogen in blood cultures versus mcfDNA began at 7.4 days by a Bayesian survival model (P < 0.025, blood culture survival probability 95% credible interval 0.654 [0.493–0.792]; mcfDNA survival probability 95% credible interval 0.316 [0.168–0.493] at 7.4 days). Median duration of positivity from antibiotic treatment initiation was estimated to be approximately 38.1 days for mcfDNA and 3.7 days for any bottle (ie, blood culture set) being positive (proportional odds, 2.952; P = 0.02771) using a semiparametric interval censored survival analysis (Figure 3A, Supplemental Table 4, and Supplemental Figure 2). Median duration of positivity from antibiotic treatment initiation was also calculated using the Bayesian interval censored survival model yielding an inflated estimate of 81.2 days for mcfDNA positivity compared with 3.5 days for any blood culture-bottle that was positive (P = .0126).
Figure 3.
Duration of last positivity (days) after antibiotic treatment initiation for mcfDNA and blood culture for any positive bottle (ie, blood culture set). (A) Semiparametric survival model estimation and (B) Bayesian survival model estimation. Divergence of the Bayesian survival credible intervals for blood culture (any bottle +) and mcfDNA occurred at 7.4 days (P value <0.025), demonstrated by lack of overlap between the credible intervals (outer bounds) on survival probability for mcfDNA and blood culture for any positive bottle. The number at risk below the figure indicates how many tests remain positive at that time point. Survival curves represent duration of last positivity for the causative pathogen of IE: false-positive and false-negative mcfDNA and blood cultures were treated as completely left-censored. IE, infective endocarditis; mcfDNA, microbial cell-free DNA.
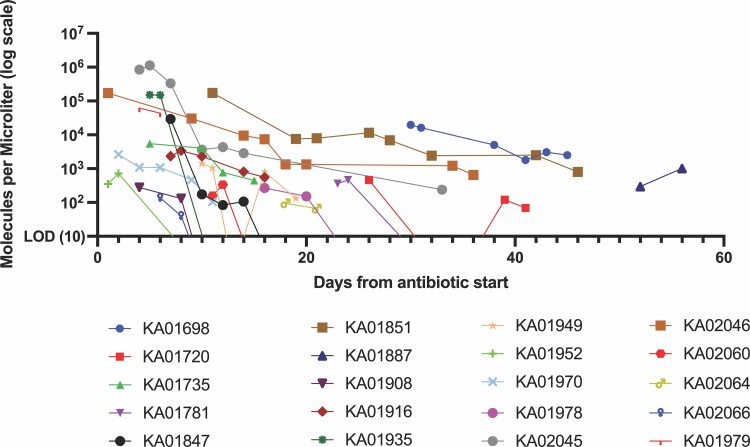
mcfDNA decay curves for each patient are shown in Figure 4 and aggregate curve is shown in Supplemental Figure 1.
Figure 4.
Change in molecules per microliter after antibiotic start (n = 20). The molecules per microliter of the pathogen detected is plotted on the y-axis against the time from antibiotic start in each patient (day 0) on the x-axis for 19 patients with definite infective endocarditis with positive serial mcfDNA testing. Three patients with definitive endocarditis with mcfDNA that did not identify the IE organism and 1 patient with a single positive mcfDNA timepoint were excluded from this graph. One patient (KA01979) with possible infective endocarditis with positive serial mcfDNA testing was included for comparison. LOD, limit of detection of assay; mcfDNA, microbial cell-free DNA.
Effect of Source Control on mcfDNA Dynamics
Seventeen (73.9%) of the 23 patients underwent a cardiothoracic surgical procedure for source control during their hospitalization. Nine (52.9%) of the 17 patients had mcfDNA collected before and after a surgical source control procedure. We fit a Bayesian hierarchical model to estimate the effect of cardiovascular debridement on mcfDNA levels. After adjusting for the duration of antibiotic treatment, prior MPM abundance, a pre/current/postprocedure indicator variable, and a group-level indicator for each patient, the regression model estimated surgical debridement was associated with a reduction of 0.30 log10 MPM units (95% CI, −0.45 to −0.14; posterior probability, >0.99; evidence ratio, 2249) at the first postprocedure timepoint (Figure 5).
Figure 5.
mcfDNA decay after cardiovascular surgical debridement. (A) mcfDNA (log10) timepoints pre- and postsource control by deidentified subject identification. Decay curves of subjects with mcfDNA sampling before and after cardiovascular surgical debridement where day 0 is set as the day of surgery; 2 presurgical time points (if performed) and 1 postsurgical time points are shown. The mcfDNA sampling on the day of surgery for 2 subjects (KA01978 and KA02045) were presurgical. (B) Boxplot of pre- and postcardiovascular debridement MPM levels used for the Bayesian hierarchical regression model. (C) Association of cardiovascular debridement with change in MPM levels (−0.30 log10 MPM units [95% CI, −0.45 to −0.14]; posterior probability > .99) at the first postprocedure timepoints estimated using a Bayesian hierarchical regression model; the red dashed line set at 0 represents the baseline mcfDNA abundance before cardiovascular debridement. (D) Conditional smoothed estimate of the decay function of days of antibiotics (4-knot spline) for MPM levels before (ProcedureGroup1), at first measurement postcardiac debridement procedure (ProcedureGroup2), and at subsequent postcardiac debridement (ProcedureGroup3) mcfDNA measurements. CI, confidence interval; mcfDNA, microbial cell-free DNA; MPM, molecules per milliliter.
DISCUSSION
This study evaluates whether an assay that detects microbial DNA directly from blood can identify the causative pathogen in patients with definite IE relative to physician-adjudicated microbiological cause of IE. We found that mcfDNA accurately identifies the microbial etiology of IE and that mcfDNA continues to accurately identify the causative pathogen days, and in some cases, even weeks after blood cultures have become negative. Our findings provide incremental evidence for mcfDNA as a possible diagnostic in patients with culture-negative IE because of prior treatment with antibiotics, although future directed studies are needed to fully evaluate its use in culture-negative IE.
Conventional blood cultures are negative in up to 40% of patients with IE [4]. The most common cause of these episodes of culture negative IE is prior antibiotic therapy [3], in which the causative bacteria have been temporarily sterilized from the bloodstream but not eradicated from the cardiac infection. In such cases of diagnostic uncertainty, guideline-recommended empiric antibiotic treatment is complex and toxic [7, 11]. Reliably identifying the causative pathogen in these cases of previously treated culture-negative IE could transform the management and outcome of this condition. The mean time to mcfDNA collection in our study was ∼11 days after starting antibiotics, when 80% of blood cultures were already negative. This is a key result. It highlights that mcfDNA is robust in the face of prior antibiotic treatment and it underscores the potential value of mcfDNA detection in the diagnosis of culture-negative IE. Additional studies dedicated to the diagnostic efficacy of mcfDNA in culture-negative IE are warranted.
To date, no studies have systematically evaluated the performance of mcfDNA detection in the diagnosis of IE. A steady stream of case reports have now described the use of mcfDNA detection for the microbiologic cause of IE or sepsis caused by a variety of fastidious organisms including Coxiella burnetii [12, 13], Bartonella species [14–19], Pasteurella multocida [20], Capnocytophaga canimorsus [20, 21], Tropheryma whipplei [22], Listeria monocytogenes [23], Mycobacterium chimera [24–26], and Bacille Calmette-Guerin/Mycobacterium bovis [27]. In our recently published study of 140 patients with bloodstream infection, mcfDNA correctly identified a pathogen in 88.5% of patients, including 9 patients with IE [8]. In the present study, mcfDNA correctly diagnosed the microbiological etiology of IE in almost 90% of the cases. Collectively, these reports support mcfDNA as a valid and accurate microbial diagnostic tool in IE.
The two cases of possible IE in this study and their correlative culture and mcfDNA data lend insight into the future potential use of mcfDNA in a modified set of Duke diagnostic criteria. One patient with possible IE met 1 major criterion and 2 minor criteria. mcfDNA tests (index and serial) demonstrated the same organism E faecalis on culture at a very high MPM: 61 259 MPM on index testing, which is in the range of other patients with definitive IE. If clinically validated, high index mcfDNA results such as in this patient may ultimately be used to further adjudicate a case from possible to definite IE within the Duke criteria. The second patient with possible IE met 3 minor criteria but had negative mcfDNA testing. In real time, it is often difficult to determine whether culture results in this context represent true infection or a contaminant and the uncertainty can lead to unnecessary long-term treatment with associated toxicities or undertreatment of a serious infection risking significant morbidity and mortality. In this clinical scenario, a negative result from the mcfDNA test may eventually be used to further adjudicate a possible IE case to “rejected,” thereby eliminating the need for additional antibiotics or diagnostic testing. Future studies will evaluate the performance of mcfDNA alone and in combination with other emerging diagnostic modalities (eg, pathogen-specific PCR testing, high-resolution imaging, positron emission tomography) to better refine the category of possible IE.
There are currently no biomarkers with which to evaluate the burden of infection and response to treatment in patients with IE. We found that the mcfDNA signal in IE patients rapidly declines with surgical source control, consistent with prior reports [15–17]. If validated in larger IE studies, this finding could have direct therapeutic implications. Current standard of care for the treatment of IE mandates prolonged courses of intravenous antibiotic therapy, a practice that is based on limited data and expert opinion [7]. Ultimately, serial monitoring of mcfDNA may help to individualize decisions on durations of antibiotic therapy by allowing clinicians to estimate when a patient’s infection has been adequately treated.
There were 3 cases in which mcfDNA failed to correctly identify the causative pathogen. In the first case (KA01741), valve cultures grew Candida albicans but blood cultures grew Staphylococcus lugdunensis and mcfDNA detected no pathogens. Valve histopathology did not show any evidence of infection or vegetation, 16sRNA PCR of the valve did not detect bacteria, and 23S RNA sequencing for yeast/fungal pathogens was not performed. Despite the conflicting clinical, pathologic, and culture data, owing to the valve culture and risks associated in not diagnosing fungal endocarditis, this patient was adjudicated to have C albicans endocarditis. In the second case (KA01784), mcfDNA identified Enterobacter in a case of Staphylococcus aureus IE in which blood cultures were also negative. In this instance, the mcfDNA was collected 62 days after antibiotic initiation and 4 days after aortic valve replacement. S aureus was adjudicated to be the microbial etiology of the IE owing to recent S aureus bacteremia and pacemaker lead vegetation 2 months before the current presentation. In the third case (KA01693), the mcfDNA collected 15 days after initiation of antibiotics did not identify a pathogen in another case of S aureus IE. In all 3 cases, the mcfDNA from causative organism was present in the raw data but below the established threshold for a positive interpretation.
There was 1 case (KA1698) in which mcfDNA identified 2 clinically relevant pathogens with MPMs over the statistical threshold: S aureus and Candida tropicalis. The microbial cause of IE was adjudicated to be S aureus, and blood cultures did not grow C tropicalis. Notably, 8 days before mcfDNA collection, the patient experienced a massive gastrointestinal bleed requiring rectal artery embolization, which may have led to C tropicalis translocation.
Our study has limitations. First, the sample size is small, limiting the power of the study. Second, biobanking and storage of samples can potentially introduce contamination. Third, the study is limited by the nonsystematic use of both blood cultures and mcfDNA test, as ideally blood cultures and mcfDNA would have been performed on the same days and with the same time intervals. Fourth, S aureus represented 57% of IE cases in this study, and other common causes of IE (eg, viridans group Streptococci, and non-S aureus Staphylococci) were underrepresented in our cohort. Fifth, the total duration of mcfDNA positivity based on the initiation of targeted antibiotics may have been overestimated because of the protracted courses of antibiotics in subjects with complicated, back-to-back episodes of IE. Similarly, the Bayesian model may have overestimated the duration of detection because of the limited number of patients with a final negative mcfDNA test. Thus, it is possible that we may not have captured a more rapid mcfDNA decay toward the conclusion of IE treatment because we did not collect postdischarge mcfDNA specimens. The semiparametric interval censored survival model offers a more conservative estimate of the duration of mcfDNA positivity (∼40 days), which is consistent with the expectation that current IE standard treatment regimens of 6 weeks most often eradicate the source of mcfDNA. The clinical consequence of remnant mcfDNA upon completion of medically treated IE remains to be determined.
Despite these limitations, we found that mcfDNA is highly accurate in diagnosing the microbial etiology of IE, and thus represents a promising diagnostic in blood culture–negative IE. mcfDNA may be a useful marker of burden of infection to follow over the course of an individual’s therapy, and future studies should evaluate the role of mcfDNA in therapeutic decision making.
Notes
Acknowledgments. V. G. F., A. A. A., F. R., and D. H. conceptualized the study. E. M. E., N. D., F. R., J. F., B. S. K., P. S., L. B., D. H., S. D., C. H., L. W., A. A. A., and C. D. contributed to the data collection and validation. E. R. S. and N. D. conducted the statistical analysis and created the figures. E. M. E. and N. D. wrote the original draft with input from all authors. All authors critically reviewed and approved the final version of the manuscript.
Financial support. This work was supported by funding from Karius Inc. V. G. F. was supported by 1R01AI165671 from the National Institutes of Health.
Supplementary Material
Contributor Information
Emily M Eichenberger, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Nicholas Degner, Karius, Inc., Redwood City, California, USA.
Erick R Scott, Karius, Inc., Redwood City, California, USA.
Felicia Ruffin, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
John Franzone, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Batu Sharma-Kuinkel, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Pratik Shah, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
David Hong, Karius, Inc., Redwood City, California, USA.
Sudeb C Dalai, Karius, Inc., Redwood City, California, USA.
Lily Blair, Karius, Inc., Redwood City, California, USA.
Desiree Hollemon, Karius, Inc., Redwood City, California, USA.
Eliza Chang, Karius, Inc., Redwood City, California, USA.
Carine Ho, Karius, Inc., Redwood City, California, USA.
Lisa Wanda, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Christiaan R de Vries, Karius, Inc., Redwood City, California, USA.
Vance G Fowler, Jr, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Asim A Ahmed, Karius, Inc., Redwood City, California, USA.
References
- 1. Cahill TJ, Baddour LM, Habib G, et al. . Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69:325–44. [DOI] [PubMed] [Google Scholar]
- 2. Fournier PE, Thuny F, Richet H, et al. . Comprehensive diagnostic strategy for blood culture-negative endocarditis: a prospective study of 819 new cases. Clin Infect Dis 2010; 51:131–40. [DOI] [PubMed] [Google Scholar]
- 3. Subedi S, Jennings Z, Chen SC. Laboratory approach to the diagnosis of culture-negative infective endocarditis. Heart Lung Circ 2017; 26:763–71. [DOI] [PubMed] [Google Scholar]
- 4. Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory diagnosis of infective endocarditis. J Clin Microbiol 2017; 55:2599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA 2018; 320:72–83. [DOI] [PubMed] [Google Scholar]
- 6. Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005; 84:162–73. [DOI] [PubMed] [Google Scholar]
- 7. Baddour LM, Wilson WR, Bayer AS, et al. . Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 8. Eichenberger EM, de Vries CR, Ruffin F, et al. . Microbial cell-free DNA identifies etiology of bloodstream infections, persists longer than conventional blood cultures, and its duration of detection is associated with metastatic infection in patients with Staphylococcus aureus and gram-negative bacteremia. Clin Infect Dis 2022; 74:2020–7. doi: 10.1093/cid/ciab742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li JS, Sexton DJ, Mick N, et al. . Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 10. Blauwkamp TA, Thair S, Rosen MJ, et al. . Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019; 4:663–74. [DOI] [PubMed] [Google Scholar]
- 11. Habib G, Lancellotti P, Antunes MJ, et al. . 2015. ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075–128. [DOI] [PubMed] [Google Scholar]
- 12. Kondo M, Dalai SC, Venkatasubrahmanyam S, et al. . Diagnosis and genotyping of Coxiella burnetii endocarditis in a patient with prosthetic pulmonary valve replacement using next-generation sequencing of plasma microbial cell-free DNA. Open Forum Infect Dis 2019; 6:ofz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Degner N, Castillo-Galvan R, Alexander J, et al. . 1026. Following the hoof prints: detecting Coxiella and Brucella infections with a plasma-based microbial cell-free DNA next-generation sequencing test. Open Forum Infect Dis 2021; 8(Supplement_1):S604. [Google Scholar]
- 14. Guo S, Pottanat ND, Herrmann JL, Schamberger MS . Bartonella endocarditis and diffuse crescentic proliferative glomerulonephritis with a full-house pattern of immune complex deposition. BMC Nephrol 2022; 23(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downey RD, Russo SM, Hauger SB, et al. . Identification of an emergent pathogen, Bartonella vinsonii, using next-generation sequencing in a patient with culture-negative endocarditis. J Pediatric Infect Dis Soc 2021; 10:213–6. [DOI] [PubMed] [Google Scholar]
- 16. Solanky D, Ahmed AA, Fierer J, et al. Utility of plasma microbial cell-free DNA decay kinetics after aortic valve replacement for Bartonella endocarditis: case report. Frontiers in Tropical Diseases 2022; 3:842100. [Google Scholar]
- 17. Hauger S, Fernandez M, Murphey D, et al. . Detection of Bartonella species in culture-negative endocarditis using the Karius Test, a plasma next-generation sequencing test for pathogen detection. San Francisco, CA: ASM, 2019. [Google Scholar]
- 18. Degner N, Smollin M, Arun A, et al. . Non-invasive detection of invasive Bartonella infections using a plasma-based microbial cell-free DNA next-generation sequencing test. American Society for Microbiology, 2021. [Google Scholar]
- 19. Patel R, Koran K, Call M, Schnee A. A case of Bartonella henselae native valve endocarditis presenting with crescentic glomerulonephritis. IDCases 2022; 27:e01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Degner N, Castillo-Galvan R, Alexander J, et al. . Chasing the long tail of infectious diseases: detecting Capnocytophaga canimorsus and Pasteurella multocida infections with a plasma-based microbial cell-free DNA next generation sequencing test. Open Forum Infect Dis 2021; 8(Supplement_1):S605–8. [Google Scholar]
- 21. Abril MK, Barnett AS, Wegermann K, et al. . Diagnosis of Capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. Open Forum Infect Dis 2016; 3:ofw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Vries CR, Macintyre A, Buggy B. 710. Non-invasive diagnosis of Whipple endocarditis using next-generation sequencing for microbial cell-free DNA in plasma. Open Forum Infectious Diseases 2020; 7(Suppl_1):S407-S. [Google Scholar]
- 23. Paras ML, Khurshid S, Foldyna B, et al . Case 13-2022: a 56-year-old man with myalgias, fever, and bradycardia. N Engl J Med 2022; 386(17):1647–57. [DOI] [PubMed] [Google Scholar]
- 24. Nomura J, Rieg G, Bluestone G, et al. . Rapid detection of invasive Mycobacterium chimaera disease via a novel plasma-based next-generation sequencing test. BMC Infect Dis 2019; 19:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Provenzano N, Boris J, Nelluri B, et al . An unusual case of prosthetic valve endocarditis. Cureus 2022; 14(6):e25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tai DGB, Eberly A, Abu Saleh O . 2180. Decreasing the time to diagnosis: nextgeneration sequencing in blood to detect disseminated nontuberculous mycobacteria. ECCMID 2021. [Google Scholar]
- 27. Vudatha V, Ranson M, Blair L, Ahmed AA. Rapid detection of bacille Calmette-Guérin-associated mycotic aortic aneurysm using novel cell-free DNA assay. J Vasc Surg Cases Innov Tech 2019; 5:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.