Abstract
Background
People with human immunodeficiency virus (HIV) with and without hepatitis C virus (HCV) coinfection had poor outcomes after liver transplant (LT). Integrase strand transfer inhibitors (INSTIs) and direct-acting antivirals (DAAs) have changed the treatment landscape for HIV and HCV, respectively, but their impact on LT outcomes remains unclear.
Methods
This retrospective analysis of adults with HIV monoinfection (n = 246) and HIV/HCV coinfection (n = 286) who received LT compared mortality in patients with HIV who received LT before versus after approval of INSTIs and in patients with HIV/HCV coinfection who received LT before versus after approval of DAAs. In secondary analysis, we compared the outcomes in the different eras with those of propensity score–matched control cohorts of LT recipients without HIV or HCV infection.
Results
LT recipients with HIV monoinfection did not experience a significant improvement in survival between the pre-INSTI and INSTI recipients with HIV (adjusted hazard ratio [aHR], 0.70 [95% confidence interval {CI}, .36–1.34]). However, recipients with HIV/HCV coinfection in the DAA era had a 47% reduction (aHR, 0.53 [95% CI, .31–9.2] in 1-year mortality compared with coinfected recipients in the pre-DAA era. Compared to recipients without HIV or HCV, HIV-monoinfected recipients had higher mortality during the pre-INSTI era, but survival was comparable between groups during the INSTI era. HIV/HCV-coinfected recipients also experienced comparable survival during the DAA era compared to recipients without HCV or HIV.
Conclusions
Post-LT survival for people with HIV monoinfection and HIV/HCV coinfection has improved with the introduction of INSTI and DAA therapy, suggesting that LT has become safer in these populations.
Keywords: INSTI, DAA, transplant, HIV, HCV
This era-based analysis examines the impact of DAA and INSTI therapy on liver transplant survival in patients with HIV monoinfection and HIV/HCV coinfection. Improved survival suggests that we should remove barriers to care in these previously marginalized populations.
The demand for liver donor organs continues to outpace supply, disproportionately affecting vulnerable patients, including those with human immunodeficiency virus (HIV) [1–3]. As a response to the shortage of donors and to increase opportunities for liver transplant (LT) among individuals with HIV, the United States (US) Congress passed the HIV Organ Policy Equity (HOPE) Act in 2013, which allowed for transplantation from donors with HIV to recipients with HIV. Historically, LT recipients with HIV, and particularly those coinfected with hepatitis C virus (HCV), were reported to have inferior post-LT outcomes including higher rates of mortality and graft rejection [4, 5], resulting in limited access to LT for patients with HIV.
In the past decade, 2 classes of medications have changed the treatment landscape for patients with HIV and HCV—integrase strand transfer inhibitors (INSTIs) and direct-acting antivirals (DAAs), respectively. INSTIs, which received US Food and Drug Administration (FDA) approval for the treatment of HIV as part of combination antiretroviral therapy (ART) in 2011, are potent antiretrovirals with minimal drug–drug interactions, thus limiting risk of interactions with posttransplant immunosuppressants [6–8]. Similarly, FDA approval for DAA therapy for HCV, in December 2013, has led to improved outcomes for patients with chronic liver disease from HCV including those necessitating LT, with sustained virologic response rates >95% and a favorable side effect profile when compared to interferon-based therapies [9]. The advantages of both classes of medications have led to their rapid adoption as standard of care for the treatment of HIV and HCV.
In an era-based analysis, we aimed to evaluate the impact of INSTI and DAA therapy on post-LT outcomes in people with HIV and people with HIV/HCV coinfection.
METHODS
We conducted a retrospective analysis using patient data from the United Network for Organ Sharing (UNOS) liver transplant database. This study was reviewed and deemed exempt from Baylor College of Medicine Institutional Review Board.
Study Cohorts
We identified adults (≥18 years old) who underwent LT in the US between 1 March 2002 (implementation date of Model for End-Stage Liver Disease [MELD] score for LT allocation) and 31 December 2020. Patient HIV and HCV serostatus was based on reporting in the UNOS system by each transplant center. Patients reported to be seropositive for both HIV and HCV were categorized as coinfected. For these analyses, all patients undergoing simultaneous organ transplant, having previous LT, and those transplanted for acute liver failure (status 1A) were excluded.
Based on FDA approval for INSTI (2012) and oral combination DAA therapy (2013), patients were divided into multiple cohorts; patients with HIV were divided into a pre-INSTI cohort if they received LT between 1 March 2002 and 31 December 2011 and an INSTI cohort if they underwent LT between 1 January 2012 and 31 December 2020. Similarly, patients with HIV/HCV were divided into pre-DAA (1 March 2002 through 31 December 2013) and DAA (1 January 2014 through 31 December 2020) era cohorts based on the dates of LT [10, 11].
Statistical Analyses
The primary outcome was post-LT patient survival. Clinical donor and recipient characteristics at LT were compared between patients in the INSTI and DAA eras. Absolute frequencies and relative proportions were calculated for categorical variables and were compared across cohorts using 2-sided χ2 tests. Median values and interquartile ranges (IQRs) were calculated for nonparametric continuous variables and were compared across cohorts with Wilcoxon rank-sum testing.
We followed patients from the time of waitlist enrollment to death or last follow-up, whichever came first. We used Kaplan-Meier survival curves to compare post-LT patient mortality among recipients with HIV in the pre-INSTI versus INSTI eras, and recipients with HIV/HCV coinfection in the pre-DAA and DAA eras. Cox hazard regression analysis was performed to assess the impact of the INSTI and DAA eras on outcomes of recipients with HIV monoinfection and HIV/HCV coinfection, respectively. A P value <.05 was used for statistical significance. All statistical analyses were performed using Stata software version 16.0.
Secondary Analysis
In a secondary analysis, we compared post-LT mortality of recipients with HIV monoinfection and HCV/HIV coinfection in the INSTI and DAA eras to mortality in a propensity score–matched cohort of LT recipients without HIV or HCV. Patients in the INSTI and DAA cohorts were propensity score matched based on year of LT, age, gender, and race/ethnicity. The patients in the INSTI cohort were additionally matched based on MELD score at transplant, whereas patients in the DAA cohort were matched based on HCV serology. To account for HCV donor utilization, we additionally matched for donor HCV serology in our recipients with HIV/HCV coinfection. With effective DAA therapy, some HCV recipients may have been cured prior to transplant but were reinfected after receiving LT from an HCV-seropositive donor. HCV-seropositive donors were not utilized in recipients with HIV monoinfection. Additional analysis was performed to compare cumulative 1-year mortality in the coinfection cohort compared with a propensity score–matched HCV monoinfection cohort in the pre-DAA, DAA, pre-INSTI, and INSTI eras due to a short follow-up time in the propensity score–matched cohort in the DAA era. Details regarding matching can be found in Supplementary Table 1.
RESULTS
From 1 March 2002 through 31 December 2020, 246 LT recipients had HIV monoinfection and 286 LT recipients had HIV/HCV coinfection at the time of LT. The number of LTs performed in recipients with HIV monoinfection and HIV/HCV coinfection increased annually from 2002 to 2020 (Supplementary Figure 1). In 2002, there were 3 recipients with HIV monoinfection and 4 recipients with HIV/HCV coinfection, whereas in 2020, there were 31 and 21, respectively (Supplementary Figure 1A and 1B). Differences in clinical characteristics and survival for HIV-monoinfected and HIV/HCV-coinfected LT recipients, stratified by INSTI and DAA era, respectively, are described further in Supplementary Figures 2 and 3.
Figure 1.
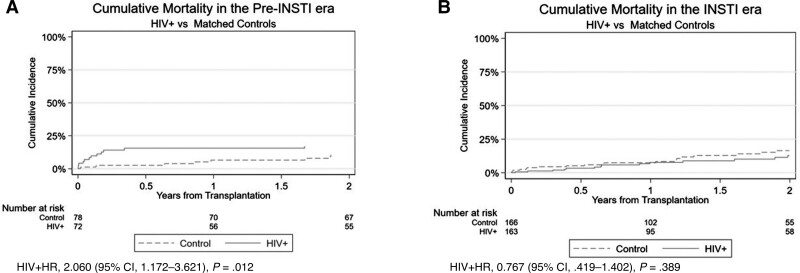
Cumulative mortality in the pre–integrase strand transfer inhibitor (INSTI) (A) and INSTI eras (B). Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; INSTI, integrase strand transfer inhibitor.
HIV Monoinfection
Clinical Characteristics
Of the recipients with HIV monoinfection (n = 246), 78 (31.7%) were transplanted in the pre-INSTI era, with a mean follow-up time of 6.4 years (standard deviation [SD], 4.9 years), and 168 (68.3%) in the INSTI era with a mean follow-up time of 1.8 years (SD, 1.7 years). Overall, the median age of HIV-monoinfected recipients was 53 years (IQR, 43–58 years); most were male (78.5%) and of White race (56.1%). Median MELD score at time of LT was 22.5 (IQR, 13–32), with a median wait time of 2.2 months (IQR, 0.2–7.5 months) to LT. Differences in clinical characteristics among HIV-monoinfected recipients between the pre-INSTI and INSTI eras are detailed in Table 1. Between eras, recipients were older in the INSTI era (pre-INSTI, 48 [IQR, 44–55] years vs INSTI, 54 [IQR, 47–60] years; P = .002). In addition, the proportion of recipients who were Hispanic (pre-INSTI, 6.4% vs INSTI, 21.4%; P = .003), with obesity (pre-INSTI, 16.7% vs INSTI, 29.2%; P = .036), and with nonalcoholic steatohepatitis (NASH) (pre-INSTI, 1.3% vs INSTI, 22.0%; P < .001) also increased. Conversely, the proportion with concomitant active hepatitis B virus infection (hepatitis B surface antigen positive) decreased between eras. A higher percentage of patients who were seronegative for cytomegalovirus (CMV) received grafts from CMV-seropositive donors in the pre-INSTI era compared with the INSTI era (7.7% vs 0%; P < .001). Among donor characteristics, cold ischemia time (pre-INSTI, 6.9 [IQR, 5–8.2] vs INSTI, 5.5 [IQR 4.5–7]; P < .001) decreased and the proportion of donors who died from drug overdose (pre-INSTI, 28.2% vs INSTI, 49.4%; P = .002) increased over time.
Table 1.
Comparison of Clinical Recipient and Donor Characteristics at Time of Transplant Among Liver Transplant Recipients With Human Immunodeficiency Virus Monoinfection
| Characteristic | LT Recipients With HIV Monoinfection | |||
|---|---|---|---|---|
| Overall (N = 246) |
Pre-INSTI (n = 78) |
INSTI (n = 168) |
P Value | |
| Age at transplant, y, median (IQR) | 53 (45–58) | 48 (44–55) | 54 (47–60) | .002 |
| Age, y | ||||
| ȃ<40 | 32 (13.1) | 10 (12.8) | 22 (13.1 | .952 |
| ȃ40–49 | 64 (26.0) | 31 (39.7) | 33 (19.6) | .001 |
| ȃ50–65 | 129 (52.4) | 34 (43.6) | 95 (56.6) | .058 |
| ȃ>65 | 21 (8.5) | 3 (3.9) | 18 (10.7) | .073 |
| Gender | ||||
| ȃFemale | 53 (21.5) | 17 (21.8) | 36 (21.4) | .948 |
| ȃMale | 193 (78.5) | 61 (78.2) | 132 (78.6) | .948 |
| Race/ethnicity | ||||
| ȃWhite | 138 (56.1) | 48 (61.5) | 90 (53.6) | .241 |
| ȃBlack/African American | 54 (22.0) | 22 (28.2) | 32 (19.1) | .106 |
| ȃHispanic/Latino | 41 (16.7) | 5 (6.4) | 36 (21.4) | .003 |
| ȃAsian | 10 (4.1) | 3 (3.9) | 7 (4.2) | .906 |
| Etiology of liver disease | ||||
| ȃAlcohol-related liver disease | 29 (11.8) | 2 (2.6) | 27 (16.1) | .002 |
| ȃNonalcoholic steatohepatitis | 38 (15.5) | 1 (1.3) | 37 (22.0) | <.001 |
| ȃHepatocellular carcinoma | 46 (18.7) | 19 (24.4) | 27 (16.1) | .121 |
| Diabetes mellitus | 52 (21.1) | 11 (14.1) | 41 (24.4) | .066 |
| Obesity (BMI ≥30 kg/m2) | 62 (25.2) | 13 (16.7) | 49 (29.2) | .036 |
| Dialysis | 23 (9.4) | 5 (6.4) | 18 (10.7) | .281 |
| Hepatic decompensation at transplantation | ||||
| ȃSevere hepatic encephalopathy | 38 (15.5) | 17 (21.8) | 21 (12.5) | .061 |
| ȃModerate ascites | 66 (26.8) | 16 (20.5) | 50 (29.8) | .128 |
| ȃPortal venous thrombosis | 39 (15.9) | 7 (9.0) | 32 (19.1) | .044 |
| ȃHistory of SBP | 15 (6.1) | 1 (1.3) | 14 (8.3) | .031 |
| Donor CMV IgG+/recipient CMV IgG– | 6 (2.4) | 6 (7.7) | 0 | <.001 |
| Donor EBV IgG+/recipient EBV IgG– | 18 (7.3) | 7 (9.0) | 11 (6.6) | .496 |
| Wait time to LT, mo, median (IQR) | 2.2 (0.2–7.5) | 2.8 (0.2–13.2) | 1.9 (0.2–6.0) | .125 |
| Laboratory MELD score at LT, median (IQR) | 22.5 (13–32) | 19.5 (12–32) | 24 (14–32) | .299 |
| Recipient HBsAg positive | 68 (27.6) | 32 (41.0) | 36 (21.4) | .001 |
| Donor characteristics | ||||
| ȃAge, y, median (IQR) | 41 (26–54) | 45 (31–56) | 39 (25–53) | .080 |
| ȃBlack/African American | 46 (18.7) | 15 (19.2) | 31 (18.5) | .884 |
| ȃMale | 143 (58.1) | 34 (43.6) | 109 (64.9) | .002 |
| ȃCold ischemia time, h, median (IQR) | 5.9 (4.6–7.6) | 6.9 (5–8.2) | 5.5 (4.5–7) | <.001 |
| ȃWarm ischemia time, min, median (IQR) | 13 (10–19) | 11 (10–13) | 21 (19–23) | .053 |
| ȃHCV antibody positive | 4 (1.6) | 0 | 4 (2.4) | .169 |
| ȃHIV positive | 14 (5.7) | 0 | 14 (8.3) | .009 |
| ȃHistory of drug use | 105 (42.7) | 22 (28.2) | 83 (49.4) | .002 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; LT, liver transplant; MELD, Model for End-Stage Liver Disease; SBP, spontaneous bacterial peritonitis.
Posttransplant Survival
In recipients with HIV monoinfection, overall 1- and 2-year survival rates were 86% (95% confidence interval [CI], 85%–88%) and 81% (95% CI, 80%–82%), respectively. Two-year survival rates were significantly higher in the INSTI era (87% [95% CI, 86%–88%] compared to the pre-INSTI era (83% [95% CI, 81%–84%]). After adjusting for recipient age, race/ethnicity, and gender, recipients in the INSTI era had a 30% higher survival rate than those in the pre-INSTI era; however, it did reach statistical significance (Supplementary Figure 2).
Recipients with HIV monoinfection were propensity score matched to recipients with chronic liver disease without HIV or HCV infection within the pre-INSTI and INSTI eras (Supplementary Table 1). When compared to recipients without HIV or HCV infection, the HIV-monoinfected recipients had higher risk for mortality in the pre-INSTI era (HIV monoinfected, 17% vs without HIV or HCV, 11%; unadjusted hazard ratio [HR], 2.060 [95% CI, 1.172–3.621]; P = .012; Figure 1A). However, in the INSTI era, there was no statistical difference in mortality between recipients with or without HIV, respectively (HIV monoinfected, 13% vs without HIV or HCV, 16%; unadjusted HR, 0.767 [95% CI, .419–1.402]; P = .389; Figure 1B).
HIV/HCV Coinfection
Clinical Characteristics
Among the recipients with HIV/HCV coinfection, 166 (58.0%) were transplanted in the pre-DAA era with a mean follow-up time of 5.6 years (SD, 5.1 years) and 120 (42.0%) in the DAA era with a mean follow-up time of 2.0 years (SD, 1.7 years). Overall, the median age of HIV/HCV-coinfected recipients was 56 years (IQR, 49–62 years); most were predominantly male (79.7%) and of White race (57.7%). Median MELD score at time of LT was 17 (IQR, 12–25), with a median wait time of 4.1 months (IQR, 0.6–11.8 months) to LT. Differences in clinical characteristics among HIV/HCV-coinfected recipients between the pre-DAA and DAA eras are detailed in Table 2. Similar to the HIV cohort, between eras, recipients were older in the DAA era (pre-DAA, 55 [IQR, 49–61] years vs DAA, 58 [IQR, 50–64] years; P < .001). A higher percentage of patients who were seronegative for CMV received grafts from CMV-seropositive donors in the pre-DAA era compared with the DAA era (7.8% vs 0.8%; P = .007). In the DAA era, LT recipients with HIV/HCV had a higher percentage with hepatocellular carcinoma (HCC) (43.3% vs 19.3%) and MELD score at LT (19 vs 16) compared to LT recipients with HIV/HCV coinfection in the pre-DAA era.
Table 2.
Comparison of Clinical Recipient and Donor Characteristics at Time of Transplant Among Liver Transplant Recipients With Human Immunodeficiency Virus/Hepatitis C Virus Coinfection
| Characteristic | HIV/HCV LT Recipients | |||
|---|---|---|---|---|
| Overall (N = 286) |
Pre-DAA (n = 166) |
DAA (n = 120) |
P Value | |
| Age at transplant, y, median (IQR) | 56 (49–62) | 55 (49–61) | 58 (50–64) | <.001 |
| Age, y | ||||
| ȃ<40 | 15 (5.2) | 12 (7.2) | 3 (2.5) | .077 |
| ȃ40–49 | 61 (21.3) | 46 (27.7) | 15 (12.5) | .002 |
| ȃ50–65 | 187 (65.4) | 106 (63.9) | 81 (67.5) | .523 |
| ȃ>65 | 23 (8.0) | 2 (1.2) | 21 (17.5) | <.001 |
| Gender | ||||
| ȃFemale | 58 (20.3) | 27 (16.3) | 31 (25.8) | .047 |
| ȃMale | 228 (79.7) | 139 (83.7) | 89 (74.2) | .047 |
| Ethnicity/race | ||||
| ȃWhite | 165 (57.7) | 104 (62.7) | 61 (50.8) | .046 |
| ȃBlack/African American | 62 (21.7) | 36 (21.7) | 26 (21.7) | .997 |
| ȃHispanic/Latino | 50 (17.5) | 24 (14.5) | 26 (21.7) | .113 |
| ȃAsian | 5 (1.8) | 2 (1.2) | 3 (2.5) | .410 |
| Etiology of liver disease | ||||
| ȃAlcohol-related liver disease | 4 (1.4) | 2 (1.2) | 2 (1.7) | .743 |
| ȃNonalcoholic steatohepatitis | 2 (0.7) | 0 | 2 (1.7) | .095 |
| ȃHepatocellular carcinoma | 84 (29.4) | 32 (19.3) | 52 (43.3) | <.001 |
| Diabetes | 49 (17.1) | 30 (18.1) | 19 (15.8) | .620 |
| Obesity (BMI >30 kg/m2) | 68 (23.8) | 33 (19.9) | 35 (29.2) | .069 |
| BMI <18.5 kg/m2 | 3 (1.1) | 2 (1.2) | 1 (0.8) | .761 |
| Hepatic decompensation at transplant | ||||
| ȃSevere hepatic encephalopathy | 23 (8.0) | 18 (10.8) | 5 (4.2) | .040 |
| ȃModerate ascites | 57 (19.9) | 40 (24.1) | 17 (14.2) | .038 |
| ȃDialysis | 9 (3.2) | 5 (3.0) | 4 (3.3) | .878 |
| ȃPortal venous thrombosis | 24 (8.4) | 9 (5.4) | 15 (12.5) | .033 |
| ȃHistory of SBP | 18 (6.3) | 14 (8.4) | 4 (3.3) | .080 |
| Laboratory MELD score at LT, median (IQR) | 17 (12–25) | 16 (11–22) | 19 (12–28) | .011 |
| Wait time to LT, mo, median (IQR) | 4.1 (0.6–11.8) | 3 (0.5–8.9) | 6.7 (1.0–13) | .029 |
| Recipient HBsAg positive | 18 (6.3) | 12 (7.2) | 6 (5.0) | .444 |
| Donor CMV IgG+/recipient CMV IgG– | 14 (4.9) | 13 (7.8) | 1 (0.8) | .007 |
| Donor EBV IgG+/recipient EBV IgG– | 14 (4.9) | 10 (6.0) | 4 (3.3) | .298 |
| Donor characteristics | ||||
| ȃAge, y, median (IQR) | 42 (27–54) | 43 (26–54) | 41 (28–54) | .268 |
| ȃBlack/African American | 60 (21.0) | 37 (22.3) | 23 (19.2) | .522 |
| ȃCold ischemia time, h, median (IQR) | 6.0 (4.8–7.8) | 6.0 (5.0–7.9) | 5.8 (4.7–7.7) | .268 |
| ȃHCV antibody positive | 42 (14.7) | 13 (7.8) | 29 (24.2) | <.001 |
| ȃHIV positive | 13 (4.6) | 3 (1.8) | 10 (8.3) | .009 |
| ȃHistory of drug use | 122 (42.7) | 55 (33.1) | 67 (55.8) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; DAA, direct-acting antiviral; EBV, Epstein-Barr virus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IQR, interquartile range; LT, liver transplant; MELD, Model for End-Stage Liver Disease; SBP, spontaneous bacterial peritonitis.
Post-LT Survival
In the cohort with HIV/HCV coinfection, unadjusted 1- and 2-year survival rates were 84% (IQR 82%–85%) and 78% (IQR 77%–79%), respectively. Two-year survival rates for the cohort with HIV/HCV coinfection were statistically significantly higher (P = .018) in the DAA era (83% [IQR 82%–84%]) than the pre-DAA era (75% [IQR 74%–76%]). Recipients with HIV/HCV coinfection in the pre-DAA era experienced higher 1-year mortality of 22% compared with 8% mortality in the post-DAA era (HR, 0.536 [95% CI, .311–.922]; P = .024; Supplementary Figure 3). Cox proportion regression analysis confirmed these findings for the HIV/HCV-coinfected recipients when adjusting for age, race/ethnicity, and gender (Supplementary Table 2).
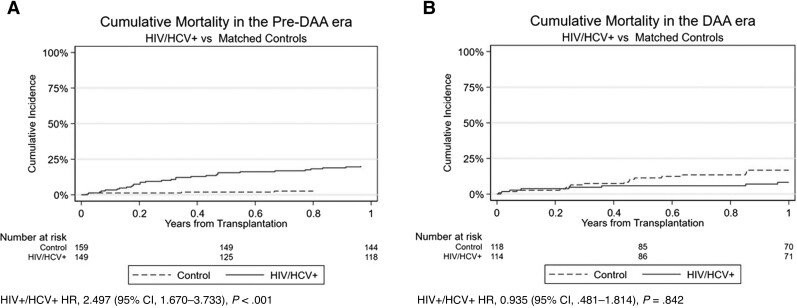
Secondary analysis was performed to compare the post-LT survival in HIV/HCV-coinfected patients with a propensity score–matched patient cohort without HIV or HCV infection. In similar fashion, the cohort with HIV/HCV coinfection had a higher 1-year mortality in the pre-DAA era compared with the matched control cohort (22% vs 3%; HR, 2.497 [95% CI, 1.670–3.733]; P < .001; Figure 2A), but no difference was seen in the DAA era (8% vs 17%; HR, 0.935 [95% CI, .481–1.814]; P = .842; Figure 2B).
Figure 2.
Cumulative mortality in the pre–direct-acting antiviral (DAA) (A) and DAA eras (B). Abbreviations: CI, confidence interval; DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio.
Post-LT Survival in Patients With HIV/HCV Compared to Propensity Score–Matched Patients With HCV Monoinfection
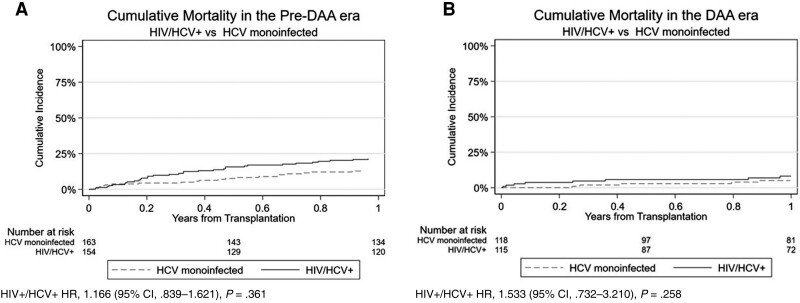
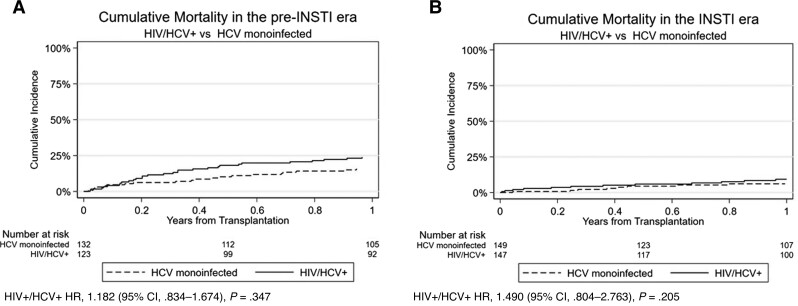
An additional propensity score–matched cohort of patients with HCV monoinfection was created to compare post-LT mortality with patients with HIV/HCV coinfection. The cumulative mortality was numerically lower in the cohort with HCV monoinfection in the pre-DAA (13%) and DAA (5%) eras compared with the cohort with HIV/HCV coinfection (Figure 3A and 3B), though this difference was not statistically significant. Likewise, the cohort with HCV monoinfection had lower mortality in the pre-INSTI (16%) and INSTI (6%) eras compared with the cohort with HIV/HCV coinfection (Figure 4A and 4B).
Figure 3.
Cumulative mortality in the pre–direct-acting antiviral (DAA) (A) and DAA eras (B). Abbreviations: CI, confidence interval; DAA, direct-acting antiviral; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio.
Figure 4.
Cumulative mortality in the pre–integrase strand transfer inhibitor (INSTI) (A) and INSTI eras (B). Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; INSTI, integrase strand transfer inhibitor.
DISCUSSION
Historically, HIV monoinfection and HIV/HCV coinfection have been associated with higher post-LT mortality in recipients compared to uninfected persons. Recent studies have shown that patient and graft survival have improved for patients with HIV monoinfection and HIV/HCV coinfection over time, and here we show that this is at least in part due to improvement of both HIV and HCV therapy [12, 13].
Early ART for HIV resulted in direct hepatotoxicity, as well as higher rates of metabolic syndrome, nonalcoholic fatty liver disease, and HCC [14]. Studies of people with HIV with end-stage liver disease revealed similar sequelae presenting at a younger age, and decreased survival after their first episode of decompensation, compared to uninfected peers. These findings were thought to result from drug toxicity of early ART as well as HIV-related inflammation and immune dysfunction [14]. Furthermore, several early ART medications had interactions with posttransplant immunosuppression [15]. These factors posed a challenge to treating patients with both decompensated cirrhosis and HIV, even after transplant.
INSTIs became an initial option for HIV treatment in the International Antiviral Society–USA panel's 2012 recommendations [10]. They quickly demonstrated their effectiveness in transplant recipients, especially due to their minimal side effects when combined with posttransplant immunosuppressive medications [16, 17]. Our data show that the gap in mortality between patients with HIV undergoing LT and matched controls decreased from the pre-INSTI to INSTI eras [16, 17]. In the pre-INSTI period, patients with HIV monoinfection had a 2-fold higher risk of death than matched controls. On the other hand, in the INSTI era, there was no statistically significant difference in mortality between the 2 cohorts. The findings suggest that INSTI and/or some other changes in HIV or transplant care have had a positive impact on the survival of patients with HIV undergoing LT.
Even after INSTI therapy was adopted, HCV treatment with interferon- and ribavirin-based regimens had numerous side effects and offered relatively low rates of sustained virologic response before the widespread adoption of DAA therapy [9]. We show that patients with HIV/HCV coinfection had lower 1-year mortality outcomes in the DAA era compared with the pre-DAA era. These findings persist when controlling for age, race/ethnicity, and gender. In addition, the gap in mortality between patients with HIV/HCV coinfection undergoing LT and matched controls also decreased from the pre-DAA to DAA era. In the pre-DAA period, patients with HIV/HCV coinfection had a nearly 2.5-fold higher risk of death than matched controls. In the DAA era there was no difference in mortality between the 2 cohorts. These findings are supportive of the positive impact seen of DAA on the care and clinical outcomes post-LT for all people with HCV infection.
When cumulative 1-year mortality in the HIV/HCV coinfection cohort was compared with a matched HCV monoinfection cohort, the difference in 1-year mortality between the 2 groups decreased from the pre-DAA and pre-INSTI eras (8%–9%) to the DAA and INSTI era (3%), demonstrating the possible impact of DAA and INSTI therapy over time.
Another metric that could reflect the impact of these medications is the number of transplants performed in each cohort. For instance, there was a 10-fold increase in number of transplants performed in the cohort of people with HIV between 2002 and 2020, compared with a 5-fold increase in the number of transplants performed in the cohort with HIV/HCV coinfection during the same period. This improvement is despite the fact that not all US transplant centers are performing LT in patients with HIV infection; the hope is that the improvement in outcomes encourages more centers to participate, improving access to care for the HIV population.
Furthermore, the demographics of disease in transplant recipients with HIV are changing, reflecting national patterns in the overall population [18]; we have observed an increase of NASH and alcoholic cirrhosis and of transplantation in the Hispanic population.
Limitations of this study include its retrospective nature and lack of granular data regarding donor and recipient viremic and treatment status for HIV and HCV prior to or after LT. In addition, candidates for transplantation with HIV and HIV/HCV are often carefully selected by each center, which cannot be replicated for the matched cohort, and may have a significant impact on posttransplant outcomes. The centers that offer transplantation for patients with HIV and HIV/HCV are likely higher-volume tertiary centers, which might have better overall transplant outcomes. We could not verify INSTI and DAA use in each individual, so the use of INSTIs and DAAs was hypothesized based on the respective era. Last, we only measured 1- or 2-year post-LT survival in this study to ensure that each group had adequate follow-up time. Data with longer follow-up are important to fully understand post-LT patient outcomes in the era of INSTIs and DAAs.
Although patients with HIV monoinfection or HIV/HCV coinfection have previously been reported to have poorer outcomes post-LT, the posttransplant mortality has greatly improved after the adoption of INSTI and DAA therapy, with an increased number of patients across the country receiving transplantation. It is important to ensure that people with HIV have fair access to liver transplants when indicated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the United Network for Organ Sharing (UNOS), a nonprofit organization that administrates the Organ Procurement and Transplantation Network (OPTN), for providing us with the registry from which our data were collected and analyzed.
Disclaimer. The interpretation and reporting of these data are the responsibility of the authors and should not be seen as an official policy of or interpretation by UNOS/OPTN or the US government.
Financial support. This material is based on work supported by the Cancer Prevention and Research Institute of Texas (CPRIT) (grant number RP200633). This work was also supported by the National Cancer Institute (grant numbers U01 CA230997 and R01 CA256977).
Supplementary Material
Contributor Information
Jake Sheraj Jacob, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, USA.
Anjiya Shaikh, Department of Internal Medicine, University of Connecticut, Mansfield, Connecticut, USA.
Karthik Goli, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, USA.
Nicole E Rich, Division of Digestive and Liver Diseases, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Jihane N Benhammou, Division of Gastroenterology and Hepatology, University of California, Los Angeles, California, USA.
Aijaz Ahmed, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, California, USA.
Donghee Kim, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, California, USA.
Abbas Rana, Hepatology Program, Division of Abdominal Transplantation, Baylor College of Medicine, Houston, Texas, USA.
John A Goss, Hepatology Program, Division of Abdominal Transplantation, Baylor College of Medicine, Houston, Texas, USA.
Susanna Naggie, Division of Infectious Diseases, Department of Medicine, Duke University School of Medicine, Durham, North Carolina, USA.
Tzu-Hao Lee, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, USA; Hepatology Program, Division of Abdominal Transplantation, Baylor College of Medicine, Houston, Texas, USA.
Fasiha Kanwal, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, USA.
George Cholankeril, Department of Internal Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, USA; Hepatology Program, Division of Abdominal Transplantation, Baylor College of Medicine, Houston, Texas, USA.
References
- 1. Gridelli B, Remuzzi G. Strategies for making more organs available for transplantation. N Engl J Med 2000; 343:404–10. [DOI] [PubMed] [Google Scholar]
- 2. Shingina A, Dewitt PE, Dodge JL, et al. Future trends in demand for liver transplant: birth cohort effects among patients with NASH and HCC. Transplantation 2019; 103:140–8. [DOI] [PubMed] [Google Scholar]
- 3. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open 2020; 3:e201997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper C, Kanters S, Klein M, et al. Liver transplant outcomes in HIV-infected patients: a systematic review and meta-analysis with synthetic cohort. AIDS 2011; 25:777–86. [DOI] [PubMed] [Google Scholar]
- 5. Miro JM, Montejo M, Castells L, et al. Outcome of HCV/HIV-coinfected liver transplant recipients: a prospective and multicenter cohort study. Am J Transplant 2012; 12:1866–76. [DOI] [PubMed] [Google Scholar]
- 6. Mayer S, Rayeed N, Novak RM, Li J, Palella FJ, Buchacz K. INSTI-based initial antiretroviral therapy in adults with HIV, the HIV outpatient study, 2007–2018. AIDS Res Hum Retroviruses 2021; 37:768–75. [DOI] [PubMed] [Google Scholar]
- 7. Werbel WA, Durand CM. Solid organ transplantation in HIV-infected recipients: history, progress, and frontiers. Curr HIV/AIDS Rep 2019; 16:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taramasso L, Cenderello G, Riccardi N, Tunesi S, di Biagio A. Role of raltegravir in patients co-infected with HIV and HCV in the era of direct antiviral agents. New Microbiol 2017; 40:227–33. [PubMed] [Google Scholar]
- 9. Baumert TF, Berg T, Lim JK, Nelson DR. Status of direct-acting antiviral therapy for hepatitis C virus infection and remaining challenges. Gastroenterology 2019; 156:431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA Panel. JAMA 2012; 308:387–402. [DOI] [PubMed] [Google Scholar]
- 11. Yee HS, Chang MF, Pocha C, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol 2012; 107:669–89. [DOI] [PubMed] [Google Scholar]
- 12. Campos-Varela I, Dodge JL, Berenguer M, et al. Temporal trends and outcomes in liver transplantation for recipients with HIV infection in Europe and United States. Transplantation 2020; 104:2078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotter TG, Wang J, Lieber SR, et al. “Raising HOPE”: improved outcomes for HIV/HCV-coinfected liver transplant recipients in the direct-acting antiviral era. Transplant Direct 2021; 7:e707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merchante N, Girón-González JA, González-Serrano M, et al. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS 2006; 20:49–57. [DOI] [PubMed] [Google Scholar]
- 15. Frassetto LA, Browne M, Cheng A, et al. Immunosuppressant pharmacokinetics and dosing modifications in HIV-1 infected liver and kidney transplant recipients. Am J Transplant 2007; 7:2816–20. [DOI] [PubMed] [Google Scholar]
- 16. Azar MM, Malinis MF, Moss J, Formica RN, Villanueva MS. Integrase strand transferase inhibitors: the preferred antiretroviral regimen in HIV-positive renal transplantation. Int J STD AIDS 2017; 28:447–58. [DOI] [PubMed] [Google Scholar]
- 17. Tricot L, Teicher E, Peytavin G, et al. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. Am J Transplant 2009; 9:1946–52. [DOI] [PubMed] [Google Scholar]
- 18. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.