Abstract
Increased vigilance in settings of potential threats or in states of vulnerability related to pain is important for survival. Pain disrupts sleep and conversely, sleep disruption enhances pain, but the underlying mechanisms remain unknown. Chronic pain engages brain stress circuits and increases secretion of dynorphin, an endogenous ligand of the kappa opioid receptor (KOR). We therefore hypothesized that hypothalamic dynorphin/KOR signalling may be a previously unknown mechanism that is recruited in pathological conditions requiring increased vigilance.
We investigated the role of KOR in wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep in freely moving naïve mice and in mice with neuropathic pain induced by partial sciatic nerve ligation using EEG/EMG recordings. Systemic continuous administration of U69,593, a KOR agonist, over 5 days through an osmotic minipump decreased the amount of NREM and REM sleep and increased sleep fragmentation in naïve mice throughout the light-dark sleep cycle. We used KORcre mice to selectively express a Gi-coupled designer receptor activated by designer drugs (Gi-DREADD) in KORcre neurons of the hypothalamic paraventricular nucleus, a key node of the hypothalamic-pituitary-adrenal stress response. Sustained activation of Gi-DREADD with clozapine-N-oxide delivered in drinking water over 4 days, disrupted sleep in these mice in a similar way as systemic U69,593. Mice with chronic neuropathic pain also showed disrupted NREM and total sleep that was normalized by systemic administration of two structurally different KOR antagonists, norbinaltorphimine and NMRA-140, currently in phase II clinical development, or by CRISPR/Cas9 editing of paraventricular nucleus KOR, consistent with endogenous KOR activation disrupting sleep in chronic pain. Unexpectedly, REM sleep was diminished by either systemic KOR antagonist or by CRISPR/Cas9 editing of paraventricular nucleus KOR in sham-operated mice.
Our findings reveal previously unknown physiological and pathophysiological roles of dynorphin/KOR in eliciting arousal. Physiologically, dynorphin/KOR signalling affects transitions between sleep stages that promote REM sleep. Furthermore, while KOR antagonists do not promote somnolence in the absence of pain, they normalized disrupted sleep in chronic pain, revealing a pathophysiological role of KOR signalling that is selectively recruited to promote vigilance, increasing chances of survival. Notably, while this mechanism is likely beneficial in the short-term, disruption of the homeostatic need for sleep over longer periods may become maladaptive resulting in sustained pain chronicity. A novel approach for treatment of chronic pain may thus result from normalization of chronic pain-related sleep disruption by KOR antagonism.
Keywords: sleep disruption, chronic pain, vigilance, kappa opioid receptor, hypothalamus
Ito et al. show that hypothalamic kappa opioid receptor (KOR) signalling promotes arousal and sleep stage transitions, and is selectively recruited to promote vigilance in states such as chronic pain. KOR antagonists normalize disrupted sleep in mice with chronic pain, without promoting somnolence in control mice.
Introduction
The regulation of sleep is complex and involves many interacting neural systems that affect the sleep-wake cycle.1 Neurons in the preoptic area of the anterior hypothalamus promote sleep while orexin and histamine containing neurons in the lateral and posterior hypothalamus, respectively, promote wakefulness and are main components of the arousal system. These systems follow circadian rhythms and additionally respond to physiological demands such as those associated with energy and fluid homeostasis. Additionally, however, disturbance of the sleep-wake cycle may occur in settings of threat to the organism that require increased vigilance to promote survival.2 The neural circuits that are specifically engaged to promote wakefulness in conditions of vulnerability including injuries, stress or threats remain unknown.
A bidirectional relationship between sleep and pain is well established clinically. Approximately 50–80% of patients with chronic pain complain of poor sleep.3–6 The relative hazard ratio of sleep disturbances in patients with chronic pain is two to five times higher than in healthy individuals7–11 and the prevalence of severe sleep disturbances increases with pain severity.12,13 On the other hand, people fulfilling the criteria for insomnia disorders have high comorbidity with chronic pain and show higher sensitivity to nociceptive stimulation than those without insomnia.14 Chronic exposure of healthy volunteers to restricted sleep was shown to increase sensitivity, decrease habituation, and increase temporal summation to noxious stimuli.15 Even acute sleep-deprivation of only 1 day was reported to amplify pain reactivity16,17 and this finding has also been demonstrated preclinically.18 Sleep disturbances are also a potential cause of mental and physical health problems beyond pain. Sleep disturbances increase the risk for mental disorders such as depression, anxiety and substance misuse,19–21 and could be a significant factor in risk of obesity, diabetes, hypertension and critical vascular diseases including stroke and myocardial infarction.22–25 Quality of life is reduced by many factors that promote sleep disturbances including pain.26
Dynorphin, an endogenous ligand at the kappa opioid receptor (KOR), has been repeatedly demonstrated to play a key role in stress responses.27,28 Antagonism of KOR signalling blocks stress-related aversive behaviours23,28,29 and both preclinical and clinical studies have suggested that KOR antagonists may be useful in blocking negative affective states associated with depression, anxiety and drug seeking behaviour. Recent preclinical studies have suggested that KOR activation promotes pain aversiveness and that KOR antagonists may be useful in targeting affective qualities of chronic pain.30–33 Additionally, KOR signalling promotes the loss of descending control of nociception/diffuse noxious inhibitory controls (DCN/DNIC)34 observed in rodent models of chronic pain likely through the activation of stress-related circuits in the limbic system.35,36
The disruption of sleep by chronic pain may reflect the need for increased vigilance in states of vulnerability. We therefore hypothesized that dynorphin/KOR signalling in stress-related circuits may be a fundamental mechanism that promotes adaptive behaviours to increase survival. Our data reveal previously unknown roles of hypothalamic dynorphin/KOR signalling in increasing arousal and wakefulness in both physiological and pathological conditions.
Materials and methods
Study design
We recorded sleep/wake data continuously for one or more 24-h light/dark cycles (starting at 07:00 am) in freely moving male mice that had been implanted with head mounts for EEG and EMG recording electrodes and evaluated the amount of total, non-rapid eye movement (NREM) and REM sleep and the average duration of wake, NREM and REM episodes. We investigated the effects of chronic pain on sleep in a model of chronic neuropathic pain induced by partial sciatic nerve ligation. The role of KOR signalling on sleep and pain was studied using: (i) sustained systemic administration of KOR agonists or KOR antagonists by subcutaneously implanted osmotic minipumps; (ii) cell specific manipulation of KOR expressing neurons in the paraventricular nucleus (PVN) of KORCre mice37 to mimic effects of KOR agonists in a specific brain region; or (iii) CRISPR/Cas9 editing of KOR expression in the PVN. We measured pain behaviours using evoked sensory thresholds elicited by probing the hind paws with von Frey filaments or with learning behaviours that capture pain-related motivation to seek relief, i.e. conditioned place preference (CPP).38 Expression of DREADDs or CRISPR/Cas9-mediated deletions were verified post hoc.
Animals
The study was conducted in accordance with the NIH guidelines for use of laboratory animals and approval from the Institutional Animal Care and Use Committee at the University of Arizona. Male C57BL/6J mice (8 weeks) or KORCre mice (8–10 weeks) were used for all experiments. KORCre mice were generated as previously described37 and were backcrossed to C57BL/6 background for at least eight generations. Mice were maintained under conditions with 12-h light/dark cycle. Light onset and offset times were 07:00 and 19:00, respectively. Food and water were available ad libitum. Every effort was made to minimize numbers and suffering of animals used in the experiments. Mice were randomly assigned to the treatment groups and the experiments were conducted in a blinded fashion.
Partial sciatic nerve ligation surgery
We produced a partial sciatic nerve ligation (PSNL) model as described previously.39 Mice were anaesthetized with 2–5% isoflurane. The right sciatic nerve was ligated by a tight ligature with 8–0 silk suture around approximately one-half of the diameter. In sham-operated mice, the nerve was only exposed without ligation. The muscle was sutured with a 5-0 braided suture and the skin was closed with an Auto-clip.
Pain measurements
Thermal pain responses were assessed using tail flick test (see Supplementary material for details). Mechanical allodynia was assessed at different time points before and after PSNL/sham surgery using von Frey filaments (Supplementary material).40 CPP with a 5-day conditioning protocol involving: acclimation, baseline chamber preference, two conditioning days using saline in one chamber and gabapentin (30 mg/kg, i.p.) in the opposite chamber, and a CPP test day, was used to assess ongoing pain (Supplementary material).
EEG/EMG recording and sleep analysis
After acclimatization, mice were implanted with EEG and EMG electrodes for polysomnographic recordings (Pinnacle Technology),41 as detailed in the Supplementary material. EEG/EMG recording was performed in individual recording chambers after at least 5 days recovery. The collected EEG/EMG data were analysed by Sleepsign software (Kissei Comtec, Japan) with every 5-s epoch automatically classified into either wakefulness, REM or NREM sleep, according to standard criteria41 (Supplementary material and Supplementary Fig. 1).
Drug administrations
U69,593 (Tocris) was dissolved in hydrochloric acid and adjusted to pH 7.4 with sodium hydroxide. Mice received continuous infusion of U69,593 (30 mg/kg/day) or vehicle by an implanted micro-osmotic pump (Model 2991, Alzet Cupertino) under the skin (Supplementary material). This dose of sustained U69,593 delivery was determined by evaluating anti-nociceptive effects in the spinally mediated tail-flick test as in our previous report with a single bolus administration.42 Continuous delivery of U69,593 (30 mg/kg/day) produced persistent antinociception from Days 1 to 6 after osmotic-pump implantation (Supplementary Fig. 2). Norbinaltorphimine (nor-BNI) was purchased from Tocris and dissolved in 0.9% saline to 10 mg/kg just before injection. Mice received a single intraperitoneal injection of nor-BNI or vehicle (saline) 1 day before EEG/EMG recording at 10 days after PSNL/sham surgery. NMRA-140 (formerly BTRX-335140, or CYM-53093) was synthesized as described previously42 and was dissolved in DMSO, Tween and saline at a ratio of 1:1:8. Mice were implanted subcutaneously with osmotic minipumps at 8 days after PSNL/sham surgery and received vehicle or NMRA-140 at 10 mg/kg/day. EEG/EMG recording started after a 1-day recovery. Gabapentin (Spectrum Chemical MFG) was dissolved in water and was administered i.p. at 30 mg/kg.
Design and in vivo transfection of CRISPR/Cas9 targeting KOR
To delete the KOR we targeted the second exon of the oprk1 common to all KOR splice variants (ENSMUSG00000025905, gRNA: gTCCCCCATTCAGATCTTCCG, on-score 65.7, off-score 78.8). The indicated gRNA sequence was inserted into the Esp3I restriction site of the pL-CRISPR.EFS.tRFP lentiplasmid (Cat# 57819, Addgene) as described before.43–45 Plasmids were verified by Sanger sequencing (Eurofins). KOR CRISPR plasmid (500 nl) was injected into the PVN (bregma: 0.8 mm posterior, 1.2 mm lateral, 5.3 mm or 4.9 mm ventral, at an angle of 10°). Decreased KOR expression was verified using western blotting with the anti-KOR primary antibody (Cat# sc-9112, Thermo Fisher Scientific) (Supplementary material).
Adeno-associated virus vector microinjection
AAV8-hSyn-DIO-hM4D(Gi)-mCherry (150 nl; Addgene, viral prep #44362-AAV8) encoding hM4Di-mCherry was injected into the same PVN location as described for KOR CRISPR plasmid. Expression of hM4D(Gi)-mCherry in the targeted brain region was verified using fluorescent microscopy on an Olympus BX51 microscope (Olympus) (Supplementary material).
Clozapine-N-oxide administration
After baseline EEG/EMG recordings, the water bottle was filled with water or 0.1 mg/ml clozapine-N-oxide (CNO). KORCre and KORWT mice weighed 20–25 g and consumed ∼5 ml/day for an approximate self-administered dose of 0.8–1.0 mg/kg/h CNO for 6 days. EEG/EMG was recorded at test Days 4 and 5 and the tail flick test was performed at test Days 2 and 6.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). The statistical significance of differences between the groups was evaluated with one-way or two-way repeated measures ANOVA followed by the Bonferroni multiple comparisons test as appropriate. P < 0.05 was considered significant. All statistical analyses were performed with Prism 9 (GraphPad Software, California, USA) and are reported in Supplementary Table 1.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Results
Sustained systemic administration of KOR agonist disrupts sleep in naïve mice
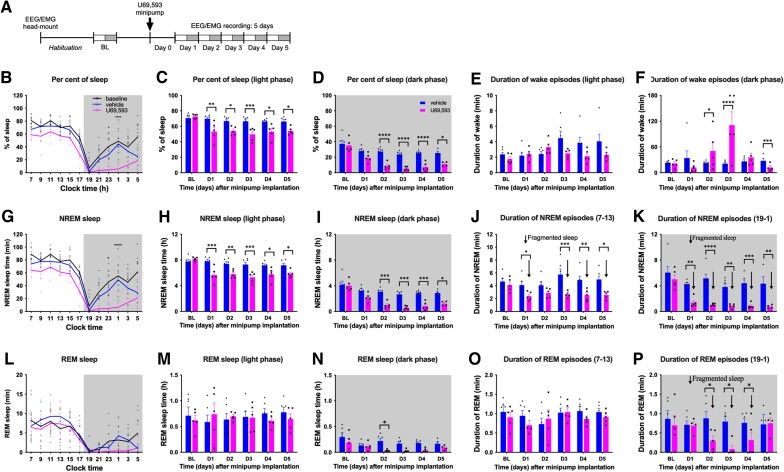
EEG/EMG was recorded in male C57Bl6 mice continuously to monitor wakefulness, NREM and REM sleep across multiple days. Mice are nocturnal and typically sleep during the light phase and are active during the dark phase. In addition, mice show repeated short episodes of wakefulness during the light phase and sleep during the dark phase. We recorded sleep for 5 days beginning 1 day after s.c. implantation of an osmotic minipump delivering the KOR agonist U69,593 or saline (Fig. 1A). NREM and REM sleep were analysed during the light and dark phases in 2, 6 or 12 h bins as (i) percent of sleep time (for total sleep); and (ii) amount of sleep time. Sleep fragmentation was assessed by calculating the average duration of individual NREM or REM sleep episodes for each 6-h period defining early (07:00–13:00) and late (13:00–19:00) light phase and early (19:00–01:00) and late (01:00–07:00) dark phase. Sleepiness (i.e. ‘daytime napping’ in the dark phase) was assessed by calculating the average duration of individual wake episodes for each 6-h period. Figure 1B shows an example of 24-h sleep cycles during baseline and at Day 4 after saline or U69,593 infusion. The per cent of sleep in the 12-h light and dark phases are plotted for all 5 days of infusion in Fig. 1C and D. The average duration of wake episodes for the 6-h early light and early dark period is shown in Fig. 1E and F. The amount of sleep and average duration of individual NREM and REM sleep episodes are shown in Fig. 1G–K and Fig. 1L–P, respectively.
Figure 1.
Sustained U69,593 administration disrupts sleep. (A) Experimental timeline. Baselines were collected after EEG/EMG head mount implantation and habituation in recording chambers. Mice then received osmotic minipumps delivering U69,593 or vehicle. Continuous sleep recording during the light (white rectangle) and dark (grey-shaded rectangle) started at 7:00 am 1 day after the minipump implant and continued non-stop for 5 days. (B) Sleep time at baseline and at Day 4 is expressed in per cent of total time in 2-h bins over a 24 h cycle. Dark phase from 7:00 pm to 7:00 am is shaded in grey. (C and D) Per cent of sleep time in the 12-h light (C) and dark (D) phases for all recorded days (BL, D1–5). (E and F) Average duration of wake episodes during the light (E) and dark (F) phases for all recorded days. (G) Amount of NREM sleep time (in min) at baseline and at Day 4 in 2-h bins. (H and I) Total amount of NREM sleep time (in hours) during the light (H) and dark (I) phases for baseline and Days 1–5. (J and K) Average duration of NREM episodes during the light (J) and dark (K) phases. Fragmented sleep (i.e. reduced duration of NREM episodes) is indicated by down arrows. (L) Amount of REM sleep time at baseline and at Day 4 in 2-h bins. (M and N) Total amount of REM sleep time (in hours) during the light (M) and dark (N) phases. (O and P) Average duration of REM episodes during the light (O) and dark (P) phases. Fragmented sleep is indicated by down arrows. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 6 for vehicle; n = 5 for U69,593; baseline data are combined from both vehicle and U69,593 groups. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated measures ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
Under pretreatment baseline conditions, mice showed a typical nocturnal cycle with 70.6 ± 2.3% and 37.3 ± 4.4% sleep during the 12-h light (white graph area) and dark (shaded graph area) phases, respectively (Fig. 1C and D and Supplementary Table 1). Mice showed short wake episodes during the light phase (mean duration of 2.1 ± 0.2 min) and long continuous wake episodes especially in the first half of the dark phase (mean duration of 22.5 ± 2.0 min). They spent on average 7.8 ± 0.3 h in NREM and 0.71 ± 0.17 h in REM sleep during the 12-h light phase and 4.2 ± 0.6 h in NREM and 0.30 ± 0.09 h in REM sleep during the dark phase. The duration of individual NREM episodes was similar between the light and dark phases (4.4 ± 0.3 min and 5.6 ± 0.6 min, respectively). Likewise, the duration of REM episodes was similar in the light and dark phases (1.0 ± 0.1 min and 0.8 ± 0.2 min, respectively). REM sleep episodes were always preceded by NREM sleep.
Compared to baseline measures, U69,593, but not vehicle, reduced the percent of sleep (Fig. 1B–D) and amount of NREM sleep (Fig. 1G–I) during both the light and dark phases (Supplementary Table 1). U69,593 reduced per cent of sleep consistently throughout the 5-day recording period. U69,593 did not significantly reduce amount of REM sleep during the light phase (Fig. 1L and M). However, U69,593 decreased amount of REM sleep during the dark phase likely reflecting the little, if any, preceding NREM sleep in this active phase (Fig. 1L and N). The average duration of wake episodes in the vehicle group was similar to baseline (Fig. 1E). In contrast, U69,593 significantly prolonged the duration of wake episodes in the dark phase when compared to the vehicle group (50.6 ± 20.2 min versus 22.3 ± 2.4 min at test Day 2 and 110.8 ± 31.1 min versus 21.1 ± 2.4 min at test Day 3; Fig. 1F) demonstrating less daytime sleep. Mice treated with U69,593 were unable to stay awake at test Day 5 with short duration of wake episodes (12.2 ± 4.2 min) likely reflecting homeostatic rebound sleep due to accumulated sleep debt (Fig. 1F). During all periods through the 5-day time course, U69,593 produced shorter episodes of NREM sleep compared to baseline or vehicle-treatment revealing sleep fragmentation (Fig. 1J and K). Additionally, U69,593 produced fragmentation of REM sleep in the dark phase also likely reflecting loss of NREM sleep (Fig. 1O and P). U69,593 decreased the power density of slow δ waves suggesting a decrease in depth of NREM sleep (Supplementary Fig. 2B and C).
Chemogenetic activation of hypothalamic KOR signalling disrupts sleep in naïve mice
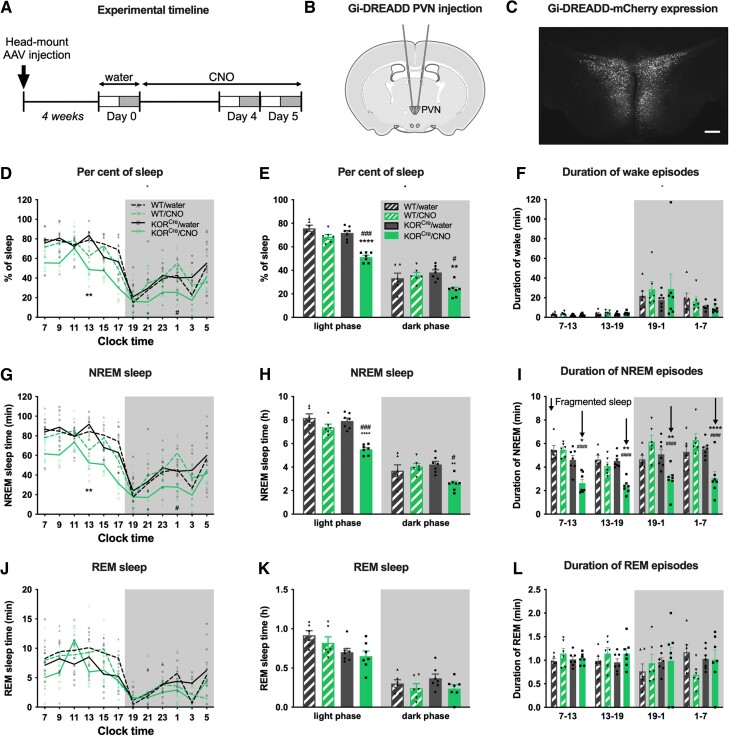
Both the KOR and the PVN are strongly associated with stress responses. As U69,593 disrupted sleep, we evaluated whether this effect is mediated by KOR signalling (i.e. inhibitory Gi-coupling) in the PVN. Prior to EEG/EMG head mount implantation, we injected AAV8-hSyn-DIO-hM4D(Gi)-mCherry bilaterally into the PVN of KORCre mice (Fig. 2A and B). Sleep recordings began after 4 weeks to allow expression of hM4D(Gi)-mCherry, a Gi coupled designer receptor activated by designer drugs (Gi-DREADD), which is activated by CNO. Expression in the PVN was verified post hoc by mCherry fluorescence (Fig. 2C and Supplementary Fig. 3A). We first collected baseline EEG/EMG recordings while mice had water available for drinking (Day 0). Mice then received CNO (∼20 mg/kg/day, p.o.) in their drinking water for the 5-day experimental period. There were no significant differences in CNO/water consumption between KORCre and KORWT mice (Supplementary Fig. 3B and C) and this treatment had no anti-nociceptive effect in the tail flick test (Supplementary Fig. 3D). EEG/EMG was recorded on Days 4 and 5 after starting CNO and compared to sleep measures in the same animals during water consumption (Fig. 2A). Reduced sleep time was observed on both days in KORCre but not KORWT mice (Fig. 2D and E, Supplementary Fig. 3E and Supplementary Table 1). The average duration of wake episodes during either the light or dark phases was not significantly influenced (Fig. 2F). Gi-DREADD activation of PVN KORCre neurons reduced the amount of NREM sleep (Fig. 2G and H and Supplementary Fig. 3F) and the mean duration of NREM sleep episodes in both the light and dark phases (Fig. 2I and Supplementary Fig. 3G), reflecting sleep fragmentation. Notably, the effect of Gi-DREADD activation on NREM sleep in the dark phase was not as strong as that observed with systemic U69,593 (Fig. 1I and K). Consequently, CNO did not affect the amount of REM sleep nor the mean duration of REM episodes on Day 4 (Fig. 2J–L) although a small decrease in REM sleep was observed on Day 5 (Supplementary Fig. 3H). Also, unlike U69,593 infusion, CNO did not change sleep depth (δ wave power) in KORCre and KORWT when compared to water (Supplementary Fig. 3I–L).
Figure 2.
Chemogenetic activation of KOR in the PVN disrupts sleep. (A) Experimental timeline: KORCre mice received bilateral AAV8-hSyn-DIO-hM4D(Gi)-mCherry into the PVN and (B) EEG/EMG head mount implantation. Sleep was recorded 4 weeks later (Day 0) when all mice had drinking water available. Water was replaced with a CNO and water solution and sleep was recorded again on Days 4 and 5 after starting CNO self-administration. (C) Verification of hM4D(Gi)-mCherry expression by fluorescent microscopy. Scale bar, 100 μm. (D) Percent of sleep time in 2-h bins is plotted in WT and KORCre mice at Day 0 (water) and Day 4 (CNO). (E) Per cent of sleep time in the light and dark phases. (F) Average duration of wake episodes during the 6-h early and late light and early and late dark phases. (G) Amount of NREM sleep time in 2-h bins over the 24 h day cycle. (H) Amount of NREM sleep time during the light and dark phases. (I) Average duration of NREM episodes during the 6-h phases; fragmented sleep is indicated by down arrows. (J) Amount of REM sleep time in 2-h bins. (K) Amount of REM sleep time during the light and dark phases. (L) Average duration of REM episodes during the early/late light and dark phases. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 6 for wild-type (WT); n = 7 for KORCre. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated mearues ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
Chronic pain disrupts sleep
We next investigated the impact of chronic pain on sleep using the PSNL model. Sleep was recorded at baseline and at 11 days after sham or PSNL surgery (Fig. 3A) when sham-operated mice fully recovered from surgery-related allodynia while mice with PSNL demonstrate tactile allodynia (Fig. 3B) that lasts for many weeks.39 Ongoing pain was demonstrated by CPP to systemic administration of gabapentin (30 mg/kg, i.p.), an analgesic used clinically for treatment of neuropathic pain, in PSNL but not sham-operated animals (Fig. 3C). While sleep did not change significantly in sham-operated mice compared to baseline, in mice with PSNL we observed significantly reduced per cent of sleep including reduction in the amount of both NREM and REM sleep during the light phase (Fig. 3D–I and Supplementary Table 1). More frequent wake episodes were observed during the light phase along with shorter average duration of NREM episodes, suggesting fragmented sleep in PSNL mice (Fig. 3J and K). Additionally, more frequent sleep episodes and significantly reduced average duration of wake episodes was observed during the dark (i.e. active) phase in animals with PSNL (Fig. 3L and M), indicative of increased ‘daytime’ sleepiness (i.e. ‘napping’). We did not observe a change in sleep depth (i.e. power density of delta wave) following PSNL surgery (Supplementary Fig. 4A and B). Therefore, chronic pain and KOR activation exert overlapping but not identical effects on sleep.
Figure 3.
Chronic neuropathic pain disrupts sleep. (A) Experimental timeline. Top: The timeline for sleep measurements that were performed before PSNL/sham surgery and again on Day 11 after the surgery. All mice received saline (s.c.) on Day 10 as part of the study presented in Fig. 4. Bottom timeline shows the times when pain behaviour was measured in a different cohort of mice: von Frey (VF) testing was done before PSNL/sham surgery and on Days 7, 11 and 15. A 5-day CPP protocol started on Day 11 after the surgery with final testing on Day 15. (B) Tactile allodynia was measured as a reduction in withdrawal thresholds of the injured hind paw. (C) Ongoing pain was assessed by CPP to gabapentin. (D–F) Per cent of sleep (D), and amount of NREM (E) and REM sleep (F) in 2-h bins is shown at baseline and on Day 11 after PSNL or sham injury. (G–I) The same data are presented for the light and dark phases. (J) Examples of EMG integral and EEG δ-power traces during the light phase at baseline and at Day 11 after PSNL demonstrating more frequent wake episodes (arrows) and shorter duration of NREM and REM episodes after PSNL. (K) Average duration of NREM episodes during the 6-h early/late light and dark phases; fragmented sleep is indicated by down arrows. (L) Examples of EMG integral during the dark phase at baseline and after PSNL show more frequent and shorter wake episodes (i.e. ‘napping’) in mice after PSNL. (M) Average duration of wake episodes during the 6-h early/late light and early/late dark phases. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 11 for both groups in the pain behavioural study and n = 8 for both groups in the sleep study. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated measures ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
Nor-BNI normalizes pain-induced sleep disruption without producing somnolence in sham-operated mice
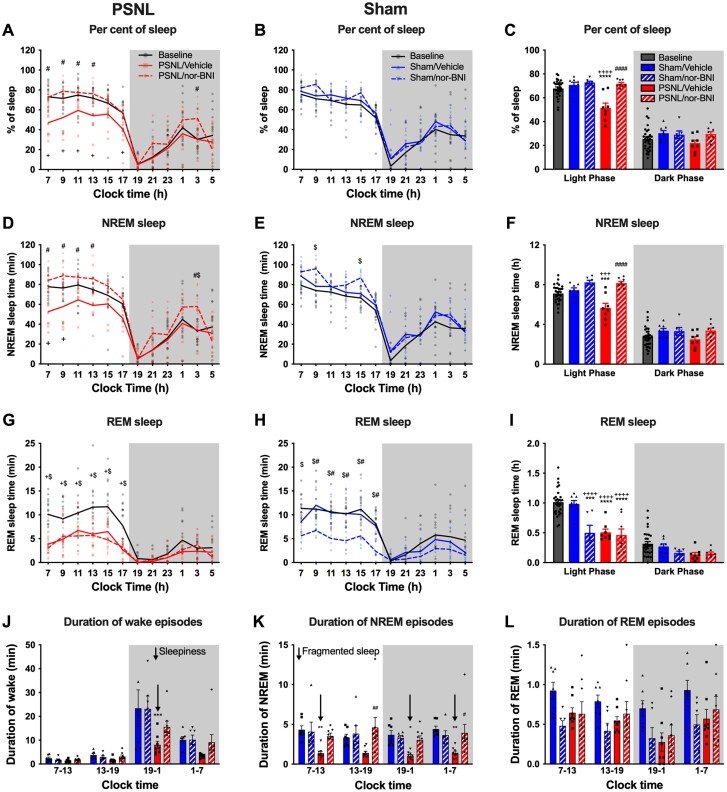
To determine the role of endogenous KOR signalling in chronic pain-induced sleep disruption, we evaluated the sleep-wake cycle of sham and PSNL mice after systemic administration of a long-lasting KOR antagonist, nor-BNI (10 mg/kg, i.p), or vehicle. We confirmed that PSNL reduced the paw withdrawal threshold, and this was not affected by systemic administration of nor-BNI (Supplementary Fig. 4C). The per cent of sleep in the PSNL/vehicle group (46.1 ± 2.6%) was significantly lower than at baseline (69.2 ± 1.1%; P < 0.0001) or in the sham/vehicle group (69.9 ± 3.1%; P < 0.0001) during the light phase (Fig. 4A–C and Supplementary Table 1). Nor-BNI normalized the per cent of sleep (71.4 ± 1.3%) of PSNL group to the same level as baseline and sham (Fig. 4A–C). This effect was due to normalization of the amount of NREM sleep (Fig. 4D–F) since nor-BNI had no effect on the amount of REM sleep in PSNL animals (Fig. 4G–I).
Figure 4.
KOR antagonist nor-BNI restores sleep in mice with PSNL. The sleep study was performed according to the timeline shown in Fig. 3A. Nor-BNI or vehicle was administered on Day 10 after PSNL/sham surgery, i.e. 1 day before EEG/EMG recording. (A and B) Per cent of sleep time in 2-h bins is shown at baseline and 11 days after PSNL (A) or sham (B) injury in mice treated with nor-BNI or vehicle. (C) Summary of per cent of total sleep data in light and dark phases for all groups. (D and E) NREM sleep time in 2-h bins for mice with PSNL (D) or sham (E) injury. (F) NREM sleep in the light and dark phases for all groups. (G and H) REM sleep time in 2-h bins for mice with PSNL (G) or sham (H) injury. (I) REM sleep in the light and dark phases for all groups. (J) Average duration of wake episodes during the 6-h early/late light and early/late dark phases. ‘Daytime sleepiness’ is indicated by a down arrow. (K) Average duration of NREM episodes during the 6-h early/late light and dark phases; fragmented sleep is indicated by down arrows. (L) Average duration of REM sleep episodes during the 6-h early/late light and dark phases. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 6 for sham/nor-BNI; n = 8 for sham/vehicle and both PSNL groups. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated measure ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
Importantly, systemic nor-BNI did not alter per cent of sleep time in sham-operated animals (Fig. 4B and C) suggesting that dynorphin/KOR does not promote wakefulness in the absence of ongoing pain. Additionally, in sham animals, there was no significant difference in the amount of NREM sleep between nor-BNI and vehicle groups (Fig. 4E and F). Unexpectedly, however, nor-BNI significantly reduced the amount of REM sleep in sham-operated mice (Fig. 4H and I) suggesting a role of dynorphin/KOR in REM sleep. We compared the average duration of awake, NREM and REM sleep episodes across treatment groups. Although nor-BNI tended to reverse the decreased wake duration observed in PSNL/vehicle mice in the dark phase (i.e. ‘daytime’ sleepiness), this was not statistically significant (Fig. 4J). However, the duration of NREM episodes was normalized in PSNL/nor-BNI mice compared to PSNL/vehicle mice demonstrating improvement of sleep fragmentation (Fig. 4K). Nor-BNI had no significant effect on the duration of REM episodes (Fig. 4L) and the depth of sleep (i.e. delta power) was unchanged by nor-BNI administration in either PSNL or sham-operated animals (Supplementary Fig. 4D–G).
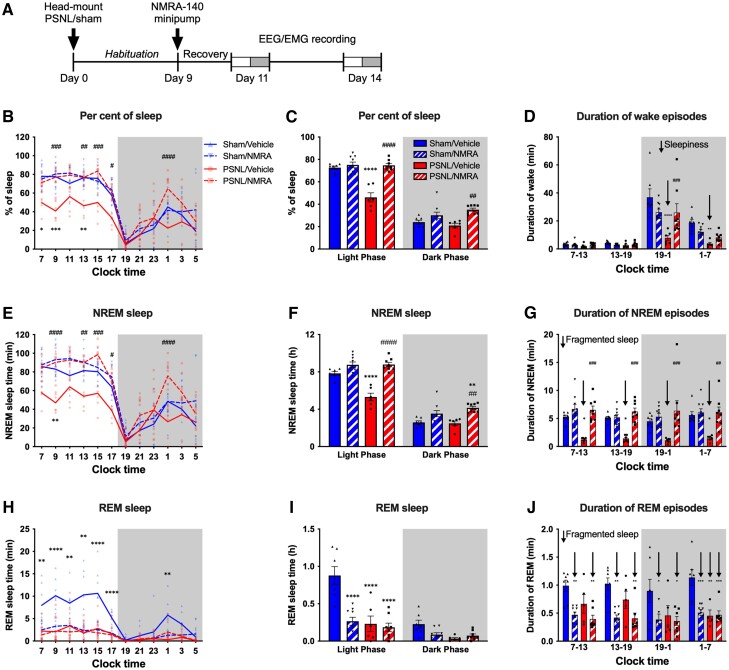
Continuous systemic administration of NMRA-140 normalizes sleep disruption in chronic pain
We also evaluated the effect of a selective KOR antagonist NMRA-140 currently in phase 2 clinical trials, on PSNL-induced sleep disruption. NMRA-140 (10 mg/kg/day) was delivered continuously by osmotic minipump implanted 9 days after sham or PSNL surgery and sleep was recorded at Days 11 and 14 (Fig. 5A). The effects of NMRA-140 were similar to those found with nor-BNI with normalization of pain disrupted sleep but no somnolence in sham-operated animals. We observed normalization of PSNL-induced reduction of per cent of sleep on both days (Fig. 5B and C, Supplementary Fig. 5A and B and Supplementary Table 1) and improvement of the decreased duration of wake episodes in the first half of dark phase, i.e. ‘daytime’ sleepiness (Fig. 5D and Supplementary Fig. 5C). NMRA-140 also normalized the amount of NREM sleep (Fig. 5E and F and Supplementary Fig. 5D and E) and PSNL-induced fragmented NREM sleep in both the light and dark phases (Fig. 5G and Supplementary Fig. 5F). Also like nor-BNI, NMRA-140 did not normalize PSNL-induced disruption of REM sleep and additionally decreased the amount of REM sleep and the duration of REM episodes in sham-operated animals (Fig. 5H–J and Supplementary Fig. 5G–I). The depth of sleep was not altered by NMRA-140 (Supplementary Fig. 5J–M).
Figure 5.
KOR antagonist NMRA-140 restores sleep in mice with PSNL. (A) Experimental timeline: mice were given PSNL or sham surgery and were instrumented with EEG/EMG head mounts. After habituation, on Day 9, mice were implanted with osmotic minipumps delivering NMRA-140 or vehicle and sleep was assessed on Days 11 and 14 after PSNL/sham surgery. Day 11 recordings are shown in this figure, for Day 14 data see Supplementary Figure 5. (B) Per cent of sleep time in 2-h bins is shown for NMRA-140 or vehicle treated mice at 11 days after PSNL or sham surgery. (C) Summary of total sleep data in light and dark phases. (D) Average duration of wake episodes during the 6-h early/late light and early/late dark phases. ‘Daytime sleepiness’ is indicated by down arrows. (E and F) NREM sleep time in 2-h bins (E) and during light and dark phases (F). (G) Average duration of NREM episodes during the 6-h early/late light and dark phases; fragmented sleep is indicated by down arrows. (H and I) REM sleep time in 2-h bins (H) and in the light and dark phases (I). (J) Average duration of REM sleep episodes during the 6-h early/late light and dark phases. Fragmented sleep is indicated by down arrows. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 6 for sham/vehicle; n = 8 for sham/NMRA; n = 7 for PSNL/vehicle and n = 9 for PSNL/NMRA groups. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated measures ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
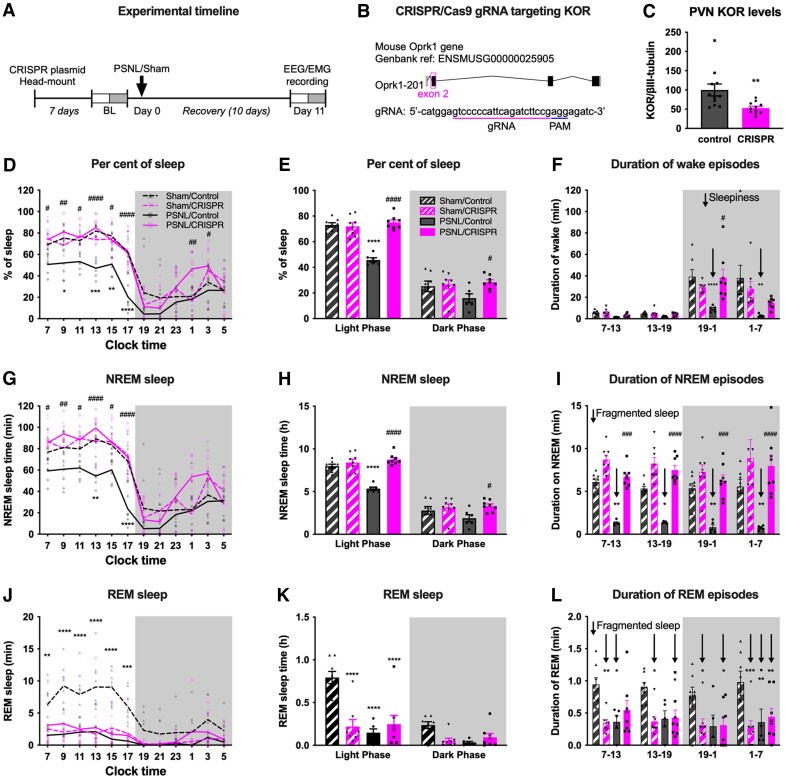
CRISPR/Cas9 editing of PVN KOR normalizes chronic pain-induced sleep disruption
To determine if the sleep disruption associated with chronic pain was due to hypothalamic KOR signalling, we microinjected a CRISPR/Cas9 plasmid with a guide RNA (gRNA) targeting KOR (i.e. KOR CRISPR) bilaterally into the PVN (Fig. 6A and B) and using western blot analysis demonstrated decreased PVN KOR expression (Fig. 6C). PVN KOR CRISPR had no effect on tactile withdrawal responses in either sham or PSNL mice (Supplementary Fig. 6A). While PSNL mice injected with a control CRISPR/Cas9 plasmid (i.e. control CRISPR) showed decreased per cent of sleep, this was not observed in PSNL mice injected with KOR CRISPR (Fig. 6D and E and Supplementary Table 1), suggesting prevention of chronic pain-induced sleep disruption. PVN KOR CRISPR but not control CRISPR also prevented PSNL-induced reduction in the duration of wake episodes during the dark phase (i.e. ‘daytime’ sleepiness) (Fig. 6F). Compared to control CRISPR treated PSNL mice, KOR CRISPR treated PSNL mice showed normalization of NREM sleep (Fig. 6G and H) and increased duration of NREM sleep episodes revealing decreased sleep fragmentation (Fig. 6I). Interestingly, similar to treatment with KOR antagonists, PVN KOR CRISPR did not normalize REM sleep in PSNL animals, and this treatment also disrupted REM sleep in sham-operated animals (Fig. 6J–L). PVN KOR CRISPR had no effect on sleep depth (i.e. delta power) (Supplementary Fig. 6B and C).
Figure 6.
Deletion of KOR in the PVN with CRISPR normalizes sleep in mice with PSNL. (A) Experimental timeline: mice received KOR CRISPR or control plasmid bilaterally in the PVN as well as an EEG/EMG implant. Seven days later, baseline sleep recordings were collected, mice then underwent PSNL, or sham surgery and sleep was assessed on Day 11 after the surgery. (B) Design of gRNA to target Oprk1 gene. (C) KOR levels in the PVN were assessed at the end of the experiment by western blotting. KOR CRISPR reduced KOR expression by 47 ± 15% compared to control plasmid. (D) Per cent of sleep time in 2-h bins is shown for at 11 days after PSNL or sham surgery. (E) Total sleep time in light and dark phases. (F) Average duration of wake episodes during the 6-h early/late light and early/late dark phases. ‘Daytime sleepiness’ is indicated by down arrows. (G and H) NREM sleep time in 2-h bins (G) and during light and dark phases (H). (I) Average duration of NREM episodes during the 6-h early/late light and dark phases; fragmented sleep is indicated by down arrows. (J and K) REM sleep time in 2-h bins (J) and in the light and dark phases (K). (L) Average duration of REM sleep episodes during the 6-h early/late light and dark phases. Fragmented sleep is indicated by down arrows. Data values for individual mice are shown as small symbols; lines represent the group means; bars represent the means ± SEM; n = 8 for PSNL/CRISPR and both sham groups; n = 6 for PSNL/control. *P < 0.05; **P < 0.01; ***P < 0.0001; ****P < 0.00001; two-way repeated measures ANOVA with Bonferroni post hoc test. Detailed statistics in Supplementary Table 1.
Discussion
Our study reveals that dynorphin/KOR signalling is a previously unknown mechanism that promotes arousal and wakefulness. We found that in naïve mice activation of brain KOR signalling with exogenous systemic agonists or by engagement of KORCre cells in the PVN promotes wakefulness. Additionally, inhibition of KOR signalling or deletion of hypothalamic KOR (i) disrupts REM sleep without promoting somnolence in physiological conditions; and (ii) normalizes sleep in animals with chronic pain. Collectively, these observations suggest that KOR in brain hypothalamic circuits is a critical mediator of arousal that likely serves both physiological functions and additionally represents a mechanism that can be recruited in situations of vulnerability that require increased vigilance. Finally, given the known homeostatic need for sleep and the increase in pain that follows sleep disruption, continued dynorphin/KOR signalling may become maladaptive in chronic states resulting in a cycle that reinforces and sustains chronic pain.
Patients with insomnia complain of difficulty initiating sleep (sleep-onset insomnia), difficulty maintaining sleep (sleep fragmentation), poor quality of sleep, circadian rhythm disorder (abnormal sleep timing) and/or daytime impairment related to sleep difficulties such as sleepiness, fatigue, reduced motivation and headache.46,47 Dynorphin, the endogenous opioid ligand at the KOR, was found at higher levels in the rat hypothalamus when measured at night than in the day suggesting possible circadian expression.48 Additionally, the secretion of dynorphin is facilitated by stress.49 These observations suggested that dynorphin/KOR signalling might play a role in promoting arousal. Consistent with this, we found that sustained administration of a selective KOR agonist, U69,593, increased alertness during both light and dark phases, largely mimicking signs of clinically observed insomnia (see Table 1 for summary). Uninjured animals treated with U69,593 showed a reduction of NREM sleep with minimal direct effects on REM sleep. Unlike humans, mice normally have short wake episodes lasting only for a few seconds during their sleep. Treatment with U69,593 increased arousal as well as the frequency of these brief wake episodes and decreased the duration of NREM episodes revealing increased sleep fragmentation. U69,593 treatment did not produce ‘daytime’ sleepiness during the dark phase (i.e. no rebound sleep) suggesting that continued exogenous KOR activation sustains wakefulness. The duration and number of REM episodes in the dark phase were also decreased following U69,593. As the occurrence of REM sleep requires preceding NREM sleep, the observed decreased amount of REM sleep in the dark phase likely reflects the extreme decrease in preceding NREM sleep. Furthermore, U69,593 decreased the power of slow (i.e. low frequency) δ waves characteristic of deep NREM sleep, suggesting a role of KOR signalling in reducing sleep depth and consistent with increased overall arousal. While KOR agonists are uncommon in clinical practice, nalfurafine is marketed in Japan for the treatment of pruritis but is not used for pain control. Consistent with our finding that KOR activation disrupts sleep in mice, one of the most frequently reported clinical side-effects of nalfurafine is insomnia.50,51
Table 1.
Summary of main effects of KOR signalling on sleep in uninjured and PSNL mice
| Treatment | Total sleepa (%) | NREMb (amount) | REMc (amount) | Fragmented sleepd | Daytime sleepinesse | Sleep depthf | |
|---|---|---|---|---|---|---|---|
| Naïve | KOR agonist (U69,593) | Reduced | Reduced | Reduced (dark) | Yes | No effect | Reduced |
| PVN KORCre Gi-DREADD |
Reduced | Reduced | No effect | Yes | No effect | No effect | |
| PSNL | PSNL | Reduced (light) | Reduced (light) | Reduced (light) | Yes | Yes (dark) | No effect |
| KOR antagonists | Normalized | Normalized | No effect | Normalized | Normalized | No effect | |
| PVN KOR CRISPR | Normalized | Normalized | No effect | Normalized | Normalized | No effect | |
| Sham | KOR antagonists | No effect | No effect | Reduced (light) | No effect | No effect | No effect |
| PVN KOR CRISPR | No effect | No effect | Reduced (light) | No effect | No effect | No effect |
Measures indicating disrupted sleep are highlighted in bold; normalization of sleep disruption observed in PSNL is indicated by italics. Light/dark indicates whether the effect is limited to a specific sleep phase.
Per cent of sleep time.
Amount of NREM sleep time.
Amount of REM sleep time.
Sleep fragmentation was assessed as significant decrease in the average duration of individual NREM or REM sleep episodes.
Sleepiness (i.e. ‘daytime napping’) was assessed as significant decrease in the average duration of individual wake episodes in the dark phase.
Reduced depth of sleep signifies a shift in the power of delta waves to higher wavelengths.
Stress engages the HPA (hypothalamus-pituitary-adrenal) axis in part through the release of dynorphin.27–29 Both dynorphin and KOR are localized in the PVN suggesting that chronic dynorphin/KOR activity may result in dysfunction of the HPA axis.27,52,53 We therefore hypothesized that increased endogenous KOR signalling in this hypothalamic nucleus may promote sleep disturbances in conditions requiring increased vigilance such as chronic pain. The profile of sleep disturbance following Gi-DREADD activation of PVN KORCre neurons was similar, but not identical, to that seen with systemic administration of a KOR agonist (Table 1). Chemogenetic PVN KOR activation reduced NREM sleep and increased sleep fragmentation without significantly affecting REM sleep. Notably, while the effects of Gi-DREADD activation of PVN KORCre neurons were similar to systemic U69,593, the magnitude of changes was smaller suggesting that KOR mechanisms beyond the PVN likely play a role in arousal. The precise mechanisms by which PVN KOR activation may disrupt sleep remain to be identified. However, it is possible that hypothalamic KOR signalling could disrupt sleep through disinhibition of arousal-related neurons including those that express orexin and/or histamine. Neurons expressing orexin, a neuropeptide that promotes arousal,54 arise in the lateral hypothalamus and project to many brain regions including the PVN.55 Dynorphin is often co-expressed in orexinergic neurons56 and has been reported to disinhibit these neurons.57 Furthermore, dynorphin disinhibits arousal-promoting histaminergic neurons in the tuberomammillary nucleus.58 Notably, following Gi-DREADD activation of PVN KORCre neurons we did not observe a change in power density of δ waves as was seen with systemic U69,593 administration suggesting that KOR signalling in PVN is not sufficient to impact the depth of sleep. It should be noted that other factors beyond stress and dynorphin/KOR signalling undoubtedly contribute to sleep disturbances associated with chronic pain. Previous reports suggest that the activity of ascending serotonin or noradrenaline neurons from the brainstem reticular activating system may be altered in experimental neuropathic pain resulting in facilitation of arousal.41,59
Patients with chronic pain show a high degree of comorbidity with stress-related disorders including anxiety and depression, conditions that are also associated with insomnia. In our preclinical model of chronic neuropathic pain induced by PSNL, mice showed long-lasting allodynia and ongoing pain. Consistent with clinical observation, mice with PSNL exhibited sleep disturbances including reduced sleep and increased sleep fragmentation that largely mimicked those observed in uninjured mice treated with systemic U69,593 or by activation of Gi-DREADD signalling in PVN KORCre neurons (Table 1).
Supporting the conclusion that chronic pain-induced sleep disruption is mediated, in part, by increased KOR signalling, treatment with systemic nor-BNI, a long-acting KOR antagonist, or with sustained infusion of NMRA-140, a relatively short-acting and selective KOR antagonist currently in phase 2 clinical trials, normalized disrupted sleep in PSNL mice (Table 1) to the same levels as observed at pre-injury baselines and in sham-operated mice. In PSNL animals, both KOR antagonists increased the duration of NREM sleep episodes in the light phase resulting in decreased sleep fragmentation. KOR antagonists also increased the duration of wake episodes in the dark phase suggesting improvement of daytime sleepiness. Critically, unlike blockade of orexin or histaminergic signalling, KOR antagonists did not produce somnolence in sham-operated mice suggesting that endogenous dynorphin/KOR signalling does not play a role in maintaining wakefulness under physiological conditions.
Surprisingly, however, we found that KOR antagonists (i) decreased REM sleep in sham-operated animals; and (ii) did not improve REM sleep in the PSNL model. Decreased REM sleep following KOR antagonist administration suggests that endogenous dynorphin/KOR signalling promotes REM sleep in normal physiological conditions. The failure of KOR antagonists to improve REM sleep in conditions of chronic pain may indicate that dynorphin/KOR signalling likely does not contribute to disruption of REM sleep by pain. The relationship between REM sleep and pain, however, is not well understood and requires further study.60,61
To determine if the sleep disruption associated with chronic pain was due to hypothalamic KOR signalling, we microinjected a CRISPR/Cas9 plasmid targeting KOR to decrease KOR expression in the PVN. Unlike PSNL mice injected with control CRISPR, PSNL mice with PVN KOR CRISPR did not show decreased amount of sleep and had a normal amount of NREM sleep. Editing of PVN KOR in PSNL mice also increased the duration of NREM episodes, revealing an improvement in PSNL-induced sleep fragmentation, and increased duration of wake episodes during the dark phase suggesting normalization of chronic pain-induced day-time sleepiness. In agreement with outcomes from KOR antagonist treatment, PVN KOR CRISPR did not normalize REM sleep in PSNL animals and additionally disrupted REM sleep in sham-operated animals.
Translational implications
Pain is a warning alarm that promotes protective behaviours to avoid tissue damage. For this reason, pain interrupts deep sleep and increases vigilance. In conditions requiring alertness, sleep is shallow allowing responses to external stimuli and behaviours associated with increased survival to be prioritized over sleep. In our studies, we observed shallower sleep in KOR agonist treated mice, but importantly, KOR antagonism improved sleep without altering the power density of δ (slow-wave, NREM) and θ (REM) waves in animals with chronic pain. KOR antagonists can therefore improve pain-disrupted sleep without impairment of protective behavioural responses.
Human studies suggest that fragmented sleep has a stronger influence on pain thresholds than decreased amount of sleep.16 While increased wakefulness and arousal may be an adaptive response to situations of organismal threat, there is a homeostatic need for sleep. Continued KOR activation as a consequence of chronic pain can then lead to a cycle of sleep disruption that may ultimately become maladaptive and reinforce and sustain the chronic pain state. We have previously reported that nor-BNI reduces aversiveness of ongoing pain.30 In the present study, we showed that nor-BNI, NMRA-140 and knockdown of KOR in PVN could improve pain-related sleep disruption. As pain and sleep have a bidirectional relationship, the improvement in sleep that we observed with blockade of KOR signalling could be due to relief of pain or, alternatively, improvement of pain could be the result of improved sleep. Future studies will be required to determine whether the improvement in sleep is a direct effect of prevention of KOR signalling or an indirect effect via the improvement of pain. Nevertheless, these findings support the conclusion that targeting the KOR may break the detrimental vicious spiral of pain and sleep.
We also note that there are many overlapping clinical conditions including anxiety and depression that are associated with sleep disruption. Such conditions are often co-morbid with chronic pain and could also be amenable to treatment with KOR antagonists. KOR antagonists including NMRA-140 and aticaprant (i.e. CERC-501) have not shown addictive properties and are currently in clinical development for stress-related indications including anxiety and depression providing an opportunity to rapidly explore the potential of this mechanism in sleep disruption associated with chronic pain. We note that KOR antagonists, or genetic deletion of PVN KOR disrupted REM sleep suggesting a potential side effect of KOR antagonist therapy. While the clinical impact of disruption of REM sleep with KOR antagonists remains unknown, such risks appear tolerable. Many drugs in clinical use disrupt REM sleep including duloxetine, an SNRI used both for the treatment of depression as well as for chronic pain.62 In spite of the link between REM sleep and memory consolidation, antidepressants including SNRIs have been reported to have either no effects or to even modestly improve memory.63 These observations suggest that the benefits of KOR antagonism in the dual modulation of chronic pain aversiveness and pain-related sleep disruption may outweigh potential negative effects arising from disruption of REM sleep.
It should also be emphasized that KOR antagonists did not produce direct hypnotic effects in animals without pain suggesting that upregulation of dynorphin is a key factor in the maladaptive consequences of chronic pain to induce insomnia. This observation provides an important distinction between KOR antagonists and orexin antagonists that produce sleep in the absence of chronic pain potentially limiting their daytime use. Finally, we note that the blockade of KOR signalling may be useful in normalization of sleep disruption associated with stress-related disorders beyond chronic pain including generalized anxiety, depression and others.
Supplementary Material
Acknowledgements
We thank Dr Sarah Ross for providing the KORCre mouse line.
Contributor Information
Hisakatsu Ito, Department of Pharmacology, University of Arizona, Tucson, USA; Department of Anesthesiology, University of Toyama, Toyama, Japan.
Edita Navratilova, Department of Pharmacology, University of Arizona, Tucson, USA; Department of Collaborative Research, Mayo Clinic, Scottsdale, USA.
Barbora Vagnerova, Department of Pharmacology, University of Arizona, Tucson, USA.
Moe Watanabe, Department of Pharmacology, University of Arizona, Tucson, USA.
Carol Kopruszinski, Department of Pharmacology, University of Arizona, Tucson, USA.
Luiz H Moreira de Souza, Department of Pharmacology, University of Arizona, Tucson, USA.
Xu Yue, Department of Pharmacology, University of Arizona, Tucson, USA.
Daigo Ikegami, Department of Pharmacology, University of Arizona, Tucson, USA.
Aubin Moutal, Department of Pharmacology, University of Arizona, Tucson, USA.
Amol Patwardhan, Department of Pharmacology, University of Arizona, Tucson, USA.
Rajesh Khanna, Department of Pharmacology, University of Arizona, Tucson, USA.
Mitsuaki Yamazaki, Department of Anesthesiology, University of Toyama, Toyama, Japan.
Miguel Guerrero, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, USA.
Hugh Rosen, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, USA.
Ed Roberts, Department of Molecular Medicine, The Scripps Research Institute, La Jolla, USA.
Volker Neugebauer, Department of Pharmacology and Neuroscience and Garrison Institute on Aging, Texas Tech University Health Sciences Center, Lubbock, USA.
David W Dodick, Department of Neurology, Mayo Clinic, Phoenix, USA.
Frank Porreca, Department of Pharmacology, University of Arizona, Tucson, USA; Department of Collaborative Research, Mayo Clinic, Scottsdale, USA.
Funding
This work was supported by grants from the National Institutes of Health: P01DA041307 (F.P., E.N.), R01 NS106902 (F.P., E.N., V.N.), R01 NS109255 (E.N., V.N., F.P.).
Competing interests
F.P. has served as a consultant or received research funding from Voyager, Nektar, Amgen, Acadia, Blackthorn, Teva, Eli Lilly, Hoba, Allergan, Abbvie, Ipsen, PeptideLogic and Proximagen and is a founder of Catalina Pharma. R.K. is a stakeholder in Regulonix Holding Inc. and Eleutheria LLC. A.P. is a founder of Catalina Pharma. D.W.D. reports the following conflicts within the past 12 months: Consulting: AEON, Amgen, Clexio, Cerecin, Cooltech, Ctrl M, Allergan, Alder, Biohaven, GSK, Linpharma, Lundbeck, Promius, Eli Lilly, eNeura, Novartis, Impel, Satsuma, Theranica, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Praxis, Revance, Equinox. Honoraria: Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, MJH Lifesciences, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Ctrl M (options), Aural analytics (options), ExSano (options), Palion (options), Healint (Options), Theranica (Options), Second Opinion/Mobile Health (Options), Epien (Options/Board), Nocira (options), Matterhorn (Shares/Board), Ontologics (Shares/Board), King-Devick Technologies (Options/Board), Precon Health (Options/Board). Patent 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. M.G. reports financial interests in NMRA-140. E.R. and H.R. are paid consultants and hold shares in Blackthorn. Other authors declare no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. [DOI] [PubMed] [Google Scholar]
- 2. Ricketts EJ, Price RB, Siegle GJ, et al. . Vigilant attention to threat, sleep patterns, and anxiety in peripubertal youth. J Child Psychol Psychiatry. 2018;59:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emery PC, Wilson KG, Kowal J. Major depressive disorder and sleep disturbance in patients with chronic pain. Pain Res Manag. 2014;19:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCracken LM, Williams JL, Tang NK. Psychological flexibility may reduce insomnia in persons with chronic pain: a preliminary retrospective study. Pain Med. 2011;12:904–912. [DOI] [PubMed] [Google Scholar]
- 6. Tang NK, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. 2007;16:85–95. [DOI] [PubMed] [Google Scholar]
- 7. Karaman S, Karaman T, Dogru S, et al. . Prevalence of sleep disturbance in chronic pain. Eur Rev Med Pharmacol Sci. 2014;18:2475–2481. [PubMed] [Google Scholar]
- 8. Louie GH, Tektonidou MG, Caban-Martinez AJ, Ward MM. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Data from the 2007 National Health Interview Survey. Arthritis Care Res (Hoboken). 2011;63:247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–159. [DOI] [PubMed] [Google Scholar]
- 10. Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–116. [DOI] [PubMed] [Google Scholar]
- 11. Zhang J, Lam SP, Li SX, et al. . Long-term outcomes and predictors of chronic insomnia: a prospective study in Hong Kong Chinese adults. Sleep Med. 2012;13:455–462. [DOI] [PubMed] [Google Scholar]
- 12. Mazzotti DR, Guindalini C, Sosa AL, Ferri CP, Tufik S. Prevalence and correlates for sleep complaints in older adults in low and middle income countries: a 10/66 Dementia Research Group study. Sleep Med. 2012;13:697–702. [DOI] [PubMed] [Google Scholar]
- 13. Stubbs B, Vancampfort D, Thompson T, et al. . Pain and severe sleep disturbance in the general population: Primary data and meta-analysis from 240,820 people across 45 low- and middle-income countries. Gen Hosp Psychiatry. 2018;53:52–58. [DOI] [PubMed] [Google Scholar]
- 14. Sivertsen B, Lallukka T, Petrie KJ, Steingrimsdottir OA, Stubhaug A, Nielsen CS. Sleep and pain sensitivity in adults. Pain. 2015;156:1433–1439. [DOI] [PubMed] [Google Scholar]
- 15. Simpson NS, Scott-Sutherland J, Gautam S, Sethna N, Haack M. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: A brain characterization in humans. J Neurosci. 2019;39:2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eichhorn N, Treede RD, Schuh-Hofer S. The Role of sex in sleep deprivation related changes of nociception and conditioned pain modulation. Neuroscience. 2018;387:191–200. [DOI] [PubMed] [Google Scholar]
- 18. Alexandre C, Latremoliere A, Ferreira A, et al. . Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat Med. 2017;23:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blank M, Zhang J, Lamers F, Taylor AD, Hickie IB, Merikangas KR. Health correlates of insomnia symptoms and comorbid mental disorders in a nationally representative sample of US adolescents. Sleep. 2015;38:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung KH, Li CY, Kuo SY, Sithole T, Liu WW, Chung MH. Risk of psychiatric disorders in patients with chronic insomnia and sedative-hypnotic prescription: a nationwide population-based follow-up study. J Clin Sleep Med. 2015;11:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paunio T, Korhonen T, Hublin C, et al. . Poor sleep predicts symptoms of depression and disability retirement due to depression. J Affect Disord. 2015;172:381–389. [DOI] [PubMed] [Google Scholar]
- 22. Mims KN, Kirsch D. Sleep and stroke. Sleep Med Clin. 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 23. Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. [DOI] [PubMed] [Google Scholar]
- 24. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. . Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21:57–64. [DOI] [PubMed] [Google Scholar]
- 26. Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–349. [DOI] [PubMed] [Google Scholar]
- 27. Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knoll AT, Carlezon WA Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Navratilova E, Ji G, Phelps C, et al. . Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain. 2019;160:824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie JY, De Felice M, Kopruszinski CM, et al. . Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia. 2017;37:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Massaly N, Copits BA, Wilson-Poe AR, et al. . Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron. 2019;102:564–573.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu SS, Pickens S, Burma NE, et al. . Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci. 2019;39:4162–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bannister K, Kucharczyk MW, Graven-Nielsen T, Porreca F. Introducing descending control of nociception: a measure of diffuse noxious inhibitory controls in conscious animals. Pain. 2021;162:1957–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nation KM, De Felice M, Hernandez PI, et al. . Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain. 2018;159:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K. Kappa Opioid Signaling in the Right Central Amygdala Causes Hindpaw Specific Loss of Diffuse Noxious Inhibitory Controls (DNIC) in Experimental Neuropathic Pain. Pain. 2019;160:1614–1621. [DOI] [PubMed] [Google Scholar]
- 37. Cai X, Huang H, Kuzirian MS, et al. . Generation of a KOR-Cre knockin mouse strain to study cells involved in kappa opioid signaling. Genesis. 2016;54:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King T, Vera-Portocarrero L, Gutierrez T, et al. . Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. [DOI] [PubMed] [Google Scholar]
- 40. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 41. Ito H, Yanase M, Yamashita A, et al. . Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol Brain. 2013;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guerrero M, Urbano M, Kim EK, et al. . Design and synthesis of a novel and selective kappa opioid receptor (KOR) antagonist (BTRX-335140). J Med Chem. 2019;62:1761–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moutal A, Cai S, Luo S, Voisin R, Khanna R. CRMP2 is necessary for Neurofibromatosis type 1 related pain. Channels (Austin). 2018;12:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moutal A, Sun L, Yang X, et al. . CRMP2-Neurofibromin interface drives NF1-related pain. Neuroscience. 2018;381:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moutal A, Yang X, Li W, et al. . CRISPR/Cas9 editing of Nf1 gene identifies CRMP2 as a therapeutic target in neurofibromatosis type 1-related pain that is reversed by (S)-Lacosamide. Pain. 2017;158:2301–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buysse DJ. Insomnia. JAMA. 2013;309:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morin CM, Drake CL, Harvey AG, et al. . Insomnia disorder. Nat Rev Dis Primers. 2015;1:15026. [DOI] [PubMed] [Google Scholar]
- 48. Przewlocki R, Lason W, Konecka AM, Gramsch C, Herz A, Reid LD. The opioid peptide dynorphin, circadian rhythms, and starvation. Science. 1983;219:71–73. [DOI] [PubMed] [Google Scholar]
- 49. Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. [DOI] [PubMed] [Google Scholar]
- 50. Kumagai H, Ebata T, Takamori K, et al. . Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol. 2012;36:175–183. [DOI] [PubMed] [Google Scholar]
- 51. Kumada H, Miyakawa H, Muramatsu T, et al. . Efficacy of nalfurafine hydrochloride in patients with chronic liver disease with refractory pruritus: A randomized, double-blind trial. Hepatol Res. 2017;47:972–982. [DOI] [PubMed] [Google Scholar]
- 52. Szeto HH. Dynorphin and the hypothalamo-pituitary-adrenal axis during fetal development. Life Sci. 2003;73:749–758. [DOI] [PubMed] [Google Scholar]
- 53. Liyanarachchi K, Ross R, Debono M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract Res Clin Endocrinol Metab. 2017;31:459–473. [DOI] [PubMed] [Google Scholar]
- 54. Saito YC, Maejima T, Nishitani M, et al. . Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J Neurosci. 2018;38:6366–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 2002;104:97–103. [DOI] [PubMed] [Google Scholar]
- 56. Chowdhury S, Hung CJ, Izawa S, et al. . Dissociating orexin-dependent and -independent functions of orexin neurons using novel Orexin-Flp knock-in mice. Elife. 2019;8:e44927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferrari LL, Park D, Zhu L, Palmer MR, Broadhurst RY, Arrigoni E. Regulation of lateral hypothalamic orexin activity by local GABAergic neurons. J Neurosci. 2018;38:1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Eriksson KS, Sergeeva OA, Selbach O, Haas HL. Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci. 2004;19:1278–1284. [DOI] [PubMed] [Google Scholar]
- 59. Koh K, Hamada A, Hamada Y, et al. . Possible involvement of activated locus coeruleus-noradrenergic neurons in pain-related sleep disorders. Neurosci Lett. 2015;589:200–206. [DOI] [PubMed] [Google Scholar]
- 60. Stroemel-Scheder C, Karmann AJ, Ziegler E, et al. . Sleep, experimental pain and clinical pain in patients with chronic musculoskeletal pain and healthy controls. J Pain Res. 2019;12:3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. [DOI] [PubMed] [Google Scholar]
- 62. Kluge M, Schussler P, Steiger A. Duloxetine increases stage 3 sleep and suppresses rapid eye movement (REM) sleep in patients with major depression. Eur Neuropsychopharmacol. 2007;17:527–531. [DOI] [PubMed] [Google Scholar]
- 63. Prado CE, Watt S, Crowe SF. A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and non-depressed samples. Neuropsychol Rev. 2018;28:32–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.