Abstract
Background
Health care workers (HCW) have a higher exposure to SARS-CoV-2 virus than other professionals and to protect both HCW and patients, HCW have been prioritized for vaccination against SARS-CoV-2 in many countries. Estimating the COVID-19 vaccine effectiveness among HCW is important to provide recommendations to protect risk groups.
Methods
We estimated vaccine effectiveness against SARS-CoV-2 infections using Cox proportional hazard models among HCW with comparisons in the general population, from 1 August 2021 to 28 January 2022. Vaccine status is specified as a time-varying covariate and all models incorporated explicit time and were adjusted for age, sex, comorbidities, county of residence, country of birth, and living conditions. Data from the adult Norwegian population (aged 18–67 years) and HCW workplace data (as registered 1 January 2021) were collated from the National Preparedness Register for COVID-19 (Beredt C19).
Results
Vaccine effectiveness was higher for Delta than for the Omicron variant in HCW (71 % compared to 19 %) as well as in non-HCW (69 % compared to −32 %). For the Omicron variant a 3rd dose provides significantly better protection against infection than 2 doses in both HCW (33 %) and non-HCW (10 %). Further, HCW seem to have better vaccine effectiveness than non-HCW for the Omicron, but not for the Delta variant.
Conclusions
Vaccine effectiveness were comparable between HCW and non-HCW for the delta variant, but significantly higher in HCW than non-HCW for the omicron variant. Both HCW and non-HCW got increased protection from a third dose.
Keywords: Vaccine effectiveness, Health personnel, Delta, Omicron, Norway, SARS-CoV-2
1. Background
The coronavirus disease 2019 (COVID-19) pandemic challenged health care systems world-wide and has only partially been abated by non-pharmaceutical control measures. Therefore, vaccines would be essential to control the outbreak. Due to the rapid development, various COVID-19 vaccines have been approved for Emergency Use Listing/Authorization (EUL/EUA) by the European Medical Agency (EMA), including Comirnaty (Pfizer/BioNTech; BNT162b2), Spikevax (Moderna; mRNA-1273), and Vaxzevria (AstraZeneca; ChAdOx nCoV-19; AZD1222). In general, these vaccines have shown high efficacy in clinical trials and good effectiveness from observational studies [1], [2], [3], [4], [5], [6], [7]. However, all vaccines show waning over time and protection is variant dependent, with lower protection against infection with the Omicron than the Delta variant [8], [9], [10], [11], [12], [13].
In Norway, COVID-19 vaccination was rolled out in December 2020 and certain groups were prioritised, including residents of long-term care facilities, elderly (over 65 years), those with underlying medical conditions and health care workers (HCW). The majority of those vaccinated in Norway received Comirnaty, Spikevax or a combination of these. Vaxzevria was initially used for the prioritisation of HCW, but it was taken out of the Norwegian national vaccine programme on 11th of March 2021. Those who received one dose of Vaxzevria were offered a second dose with an mRNA vaccine (either Comirnaty or Spikevax). When booster doses were offered, these could be either of the mRNA vaccines regardless of the vaccine product used for the primary series. Several vaccine effectiveness studies using registry data have been done in Norway, showing good protection against severe disease among the general population as well as specific population groups [7], [11], [14], [15]. In line with other studies, the vaccine effectiveness differed between SARS-CoV variants, effectiveness estimates were higher against more severe outcomes and protection waned with time since last received dose. During the second half of 2021 and 2022, SARS-CoV-2 PCR testing was freely available for anyone, including for those with symptoms, contacts, risk groups as well as those wanting to test preventively (for example before going to an event). However, with the introduction of rapid tests and high number of cases during the spread of the Omicron variant, confirmation of rapid tests with PCR was gradually reduced after January 2022. Therefore, SARS-CoV-2 positive PCR tests cannot reliably be used to estimate vaccine effectiveness after this period.
HCW constitute an important occupational group during outbreaks of infectious diseases, such as COVID-19, since they may have an increased risk of exposure as well as an essential role in managing the outbreak [16], [17]. In addition, HCW are often in contact with individuals who have an increased risk of severe disease and thus transmission from HCW could cause a higher burden in the population than among HCWs only. In Norway, as in many other countries, extensive measures were put in place to limit both the risk of infection as well as transmission from and to HCW, including strict measures concerning the work environment as well as discouragement of working in more than one institution or sharing rides to and from work. In addition, HCW were prioritised for COVID-19 vaccination when immunization started and were offered a first dose in the first quarter of 2021. In general, HCW is a group that stand out in a few key ways: they are relatively healthy and young, they were prioritized for COVID-19 vaccination in many countries and were often more systematically tested or prioritized for testing throughout the pandemic. HCW are therefore often used for studies, including those to estimate vaccine effectiveness. However, due to the differences between HCW and the general population it is important to understand how to interpretate estimates from HCW when generalising these finding to the general population. Estimating and comparing the vaccine effectiveness against SARS-CoV-2 infection in this group compared to the general population could therefore shed light on potentially confounders and support interpretation of effectiveness estimates among HCW. In this study we estimated the vaccine effectiveness against Delta and Omicron SARS-CoV-2 infection among HCW and compared these with estimates for the general population in Norway to identify factors influencing found differences.
2. Methods
2.1. Study design and data sources
We conducted a retrospective population-based cohort study for the period 31 July 2021 to 30 January 2022 including all individuals aged 18 to 66 years old, who were registered as living in Norway with a valid national identity number. We obtained data from the Norwegian national preparedness registry for COVID-19 (Beredt-C19) and linked individual-level data from central health registries, national clinical registries, and other national administrative registries, using the national identification number. This dataset covers all residents in Norway and includes data on all SARS-CoV-2 PCR positive cases, and COVID-19 vaccinations. Individual-level data used for this study included age (in years), sex, county of residence (12 levels), dates of vaccination, underlying comorbidities (three levels), crowded living conditions (two levels), and sampling. In this dataset HCW (including their specific type of workplace and position) were identified by selecting individuals registered as a HCW on 1 January 2021 in the State Register of Employers and Employees. Further details on data sources and definitions are provided in the supplementary section 1. For estimates of the general population presented here, we excluded the individuals defined as HCW as described above. We extracted data from the registries on 6 September 2022.
To eliminate non-standard vaccination histories, we excluded individuals with more than three doses before the end of the study period and excluded individuals for which the interval between first and second dose was shorter than the recommended minimum intervals and censored those with a third dose registered before the recommended 120 days of the second dose. Based on the vaccine type given as the first dose, 19 days was the recommended minimum interval between first and second dose for Comirnaty, 22 days for Spikevax, and 21 days for Vaxzevria. We only included individuals who had received Comirnaty, Spikevax or Vaxzevria (including heterologous regiments), which were part of the Norwegian vaccination programme.
2.2. Definitions
SARS-CoV-2 infection: We defined SARS-CoV-2 infection as a positive SARS-CoV-2 PCR test reported to the Norwegian Surveillance System for Communicable Diseases (MSIS) registry. We use testing date as time of infection (positive PCR test) and included only the first SARS-CoV-2-infection per individual, to reduce biases related to natural immunization. Both symptomatic and asymptomatic reported cases have been included as it is not possible to distinguish between these in MSIS.
Variant waves: In Norway, SARS-CoV-2 Delta or Omicron variants were identified using Sanger partial S-gene sequencing, or PCR screening targeting specific single nucleotide polymorphisms, insertions or deletions; details for laboratory testing for variants previously described [18]. We identified the Delta and Omicron variant waves based on the date of the positive test considering periods where over 90 % of the screened samples in Norway were identified as Delta or Omicron variant (dominant variant). We defined the delta dominant wave between 31 July 2021 to 12 November 2021 and the Omicron dominant wave between 3 January to 30 January 2022. The Omicron wave was not extended after the end of January 2022 because of the gradual downscaling of the national testing strategy, and to ensure analysis when Omicron sublineage (BA.1) was predominant.
Vaccination status: Vaccine status was defined based on number of doses and date of vaccination recorded in the Norwegian Immunisation Registry (SYSVAK). We included only Comirnaty, Spikevax and Vaxzevria. To consider and quantify the possibility for vaccines to show reduced protection over time since becoming fully vaccinated (waning), vaccine status is treated as a fixed factor where individuals contribute time at risk depending on their individual vaccination schedule. These intervals are split by 16-week (=112 days) durations after the receipt of the 2nd dose. The following vaccine status groups were thus used:
-
•
Unvaccinated: unvaccinated up to seven days before the first dose.
-
•
1st dose: ≥ 21 days after first vaccine dose up to date of second vaccine dose.
-
•
2nd dose: > 7 days after the 2nd dose, until date of 3rd dose, divided in periods of 8 weeks.
-
•
3rd dose (booster): > 7 days after a vaccine dose given 120 days or more after completion of the primary vaccine regimen.
We included the time span from seven days before until 21 days after the first vaccine dose as a separate status not reported here, in order to take into account induction time as well as the fact that vaccinations are usually only administered to healthy individuals. Similarly, individuals were included as a separate status and not reported here for the first 7 days after receiving the second and third dose.
2.3. Data analysis
Per-week testing activity (regardless of test result) was calculated as the proportion of the population tested at least once per calendar week, in order to reduce the effect of certain individuals with extremely high testing activity.
We estimated the overall vaccine effectiveness against SARS-CoV-2 infection during the Delta and Omicron periods separately, using Cox proportional hazards models with vaccination status as a time-dependent covariate, and with explicit calendar time to account for changes in the baseline hazard over time. All analyses used the unvaccinated proportion as the control group. For vaccine effectiveness estimates, we stratified (using the strata functionality in the survival package) for available factors that could affect the likelihood of being vaccinated or infected with SARS-CoV-2. These factors were sex, country of birth (split into Norway, outside Norway, or unknown), county of residence, crowded living condition, and underlying comorbidities associated with increased risk of severe COVID-19. Including these factors as strata entails a less stringent assumption than treating them as fixed variables, which assumes that a given factor level has the same proportional hazard to the reference level over the whole period. Vaccine effectiveness is defined as 100*(1 – β), with β the proportional hazard associated with vaccine status. Separate regression models were run for each age group, as well as each workplace and occupation in the HCW population, using non-HCW as reference.
We included one model also stratified by age and estimated the total vaccine effectiveness including only being HCW or not, as well as its interaction terms, i.e., to which extent being HCW affects the vaccine effectiveness, compared to non-HCW. In addition, we conducted sensitivity analyses in order to identify potential factors that could explain the potential difference in the overall estimated vaccine effectiveness among HCW. For that purpose, we similarly built (as overall vaccine effectiveness) separate models for HCW and the general population after a) excluding all individuals that had received at least one dose of Vaxzevria according to SYSVAK, or b) excluding all individuals with relevant underlying comorbidities. We also investigated differences in age groups, as well as the occupational position and workplace among the HCW as recorded in the Norwegian State Register of Employers and Employees. The latter analyses for age, occupation and workplace are presented in the supplementary sections 4.2 and 4.5 respectively.
All analyses were performed in R version 4.0.2 with the R packages survival version 3.1–12.
2.4. Eth ics approval
Ethical approval was granted by Regional Committees for Medical and Health Research Ethics (REC) Southeast (reference number 122745).
2.5. Fun ding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was performed as part of routine work at the Norwegian Institute of Public Health.
3. Results
3.1. Demographics
In the Delta and Omicron wave assessed here, 3,399,379 individuals were included of which 415,657 (12 %) were categorized as HCW and 2,983,722 (88 %) as non-HCW. Females were overrepresented in the HCW group (82 %) whereas males were slightly overrepresented in the non-HCW group (56 %). Of the HCW, 6,276 (1.5 %) tested positive during the period with Delta variant and 24,442 (5.9 %) tested positive during the Omicron period, while 34,650 (1.2 %) of the non-HCW tested positive during the Delta period and 172,026 (5.8 %) tested positive during the Omicron period. Further details of the population and cohort included are presented in Table 1 .
Table 1.
Characteristics of study population and cases among health care workers (HCW) and non-HCW during Delta (31 July-12 November 2021) and Omicron (3–30 January 2022) wave in Norway.
|
Non-HCW |
HCW |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Population |
Delta cases |
Omicron cases |
Population |
Delta cases |
Omicron cases |
|||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||
| Population | 2,983,722 | 88* | 34,650 | 1.2* | 172,026 | 5.8* | 415,657 | 12* | 6,276 | 1.5* | 24,442 | 5.9* | ||
| Age | 18–27 | 528,647 | 18 | 8,776 | 25 | 42,922 | 25 | 90,595 | 22 | 1,553 | 25 | 7,040 | 29 | |
| 28–35 | 531,818 | 18 | 6,821 | 20 | 37,842 | 22 | 81,742 | 20 | 1,310 | 21 | 5,790 | 24 | ||
| 36–45 | 624,892 | 21 | 8,894 | 26 | 50,285 | 29 | 84,672 | 20 | 1,681 | 27 | 6,806 | 28 | ||
| 46–55 | 665,381 | 22 | 6,775 | 20 | 29,587 | 17 | 83,078 | 20 | 1,163 | 19 | 3,497 | 14 | ||
| 56–66 | 632,984 | 21 | 3,384 | 9.8 | 11,390 | 6.6 | 75,570 | 18 | 569 | 9.1 | 1,309 | 5.4 | ||
| Sex | Female | 1,328,932 | 45 | 15,834 | 46 | 82,111 | 48 | 334,772 | 81 | 4,828 | 77 | 19,305 | 79 | |
| Male | 1,654,790 | 56 | 18,816 | 54 | 89,915 | 52 | 80,885 | 20 | 1,448 | 23 | 5,137 | 21 | ||
| Underlying conditions | No known risk | 2,558,687 | 86 | 30,689 | 89 | 155,924 | 91 | 361,413 | 87 | 5,604 | 89 | 22,041 | 90 | |
| Medium risk | 381,052 | 13 | 3,611 | 10 | 14,903 | 8.7 | 49,717 | 12 | 631 | 10 | 2,246 | 9.2 | ||
| High risk | 43,983 | 1.5 | 350 | 1 | 1,199 | 0.7 | 4,527 | 1.1 | 41 | 0.7 | 155 | 0.6 | ||
| Country of birth | Norway | 2,215,070 | 74 | 19,935 | 58 | 118,627 | 69 | 327,610 | 79 | 4,025 | 64 | 17,445 | 71 | |
| Outside Norway | 684,341 | 23 | 14,364 | 42 | 51,909 | 30 | 77,390 | 19 | 2,175 | 35 | 6,805 | 28 | ||
| Unknown | 84,311 | 2.8 | 351 | 1 | 1,490 | 0.9 | 10,657 | 2.6 | 76 | 1.2 | 192 | 0.8 | ||
| Crowded living | Yes | 275,847 | 9.2 | 6,836 | 20 | 26,852 | 16 | 41,696 | 10 | 1,141 | 18 | 4,078 | 17 | |
| No | 2491,711 | 84 | 24,913 | 72 | 133,894 | 78 | 358,967 | 86 | 4,915 | 78 | 19,404 | 79 | ||
| Unknown | 216,164 | 7.2 | 2,901 | 8.4 | 11,280 | 6.6 | 14,994 | 3.6 | 220 | 3.5 | 960 | 3.9 | ||
Abbreviations: HCW: health care workers,
: These percentages have been calculated horizontally using the relevant population in the denominator. The rest of the percentages have been calculated vertically.
At the start of the Delta period, the vaccine coverage for 2 doses was 69 % for HCW and 24 % for non-HCW in the study population. Coverage for three doses was 0.04 % for HCW and 0.01 % for non-HCW. At the start of the Omicron period, the vaccine coverage for two doses was 92 % for HCW and 84 % for non-HCW in the study population. Coverage for three doses was 55 % for HCW and 21 % for non-HCW. At the end of the study period, 30 % of HCW had received at least one dose of Vaxzevria, compared to 12 % for non-HCW. The vaccine coverage over time is presented in supplement section 2.
In general, infection rates were higher in HCW than in non-HCW, reflected in a hazard ratio of 1.38 (95 % confidence interval [CI]: 1.29–1.44) for the Delta wave and 1.13 (95 % CI: 1.12–1.15) for the Omicron wave, with limited variation within the HCW job positions and workplaces (See Supplementary section 2, figure S1). The difference in risk ratio between the two waves can be associated to the higher average testing activity for HCW during the periods, as shown in Fig. 1 .
Fig. 1.
Relative difference in per-week testing activity among health care workers (HCW) compared to non-HCW during the Delta (31 July-12 November 2021) and Omicron (3–30 January 2022) waves. Dashed line is the average relative testing difference for the Delta and Omicron wave, respectively.
3.2. Vaccine effectiveness against SARS-CoV-2 infection
3.2.1. Delta wave
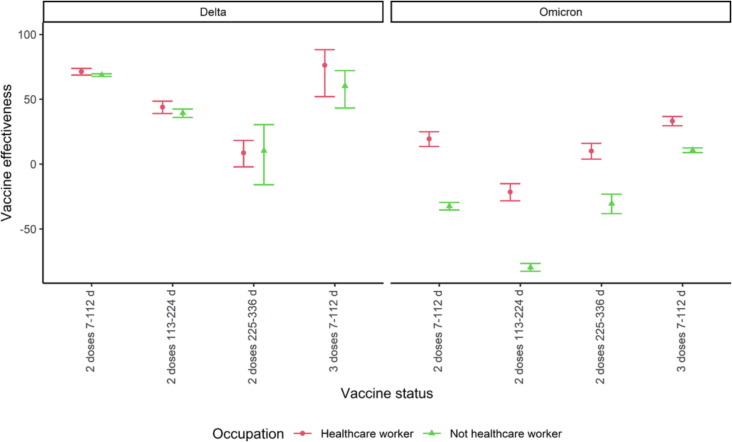
The first period (7–112 days) after two doses the vaccine effectiveness against Delta infection was 71 % (95 % CI: 69–74 %) for HCW and 69 % (95 % CI: 68–70 %) for non-HCW. The vaccine effectiveness decreased to 9 (95 % CI: −2–18 %) and 10 % (95 % CI: −16–31 %) after 225 days or more, respectively. After the third dose, the vaccine effectiveness was 76 % (95 % CI: 52–88 %) and 60 % (95 % CI: 43–72 %) among HCW and non-HCW, respectively. These analyses showed that there were no significant differences in the vaccine effectiveness between HCW and non-HCW (Fig. 2 , overlapping CI). In addition, we observed a decrease in vaccine effectiveness over time since last vaccination with two doses (Fig. 2, no overlapping CI).
Fig. 2.
Vaccine effectiveness among health care workers (HCW) and non-HCW during Delta (31 July-12 November 2021) and Omicron (3–30 January 2022) wave in Norway. The data behind the figure are available in the Supplementary section 4.1.
3.2.2. Omicron wave
The first period (7–112 days) after two doses the vaccine effectiveness against Omicron infection was 20 % (95 % CI: 14–25 %) for HCW and –32 % (95 % CI: −35- −30 %) for non-HCW. The vaccine effectiveness first decreased in the period 113–224 days after the second dose and increased after 225 days or more to 10 % (95 % CI: 4–16 %) and −31 % (95 % CI: −38- –23 %), respectively. The highest vaccine effectiveness was observed after receiving the third dose (7–112 days) and was 33 % (95 % CI: 30–37 %) and 11 (95 % CI: 9–13 %) respectively. These analyses showed that there were significant differences in the vaccine effectiveness against Omicron infection between HCW and non-HCW (Fig. 2, no overlapping CI). Here we did not observe the same waning pattern as we did in the Delta wave.
In the analysis where we estimated the difference in vaccine effectiveness for HCW as compared to non-HCW (interaction term) against infections, we found that HCW yielded significantly higher efficiencies (12–29 %) for a first dose and 2 doses from 7 to 224d post vaccination, and all investigated vaccine statuses for Omicron (29–40 %). Further details on these results are presented in the Supplementary section 4.1.
The estimated vaccine effectiveness patterns were similar in all age subgroups, as well as workplaces and occupations (Supplementary sections 4.2, 4.5). There were no differences in vaccine effectiveness between the age groups during the Delta period, apart from the age groups 18–27 and 28–35 demonstrating a slightly higher effectiveness for some vaccine statuses among both HCW and non-HCW. For Omicron the pattern was somewhat reversed, with older recipients tending to demonstrate the highest vaccine effectiveness for HCW, while both the youngest and oldest among non-HCW show higher effectiveness.
3.2.3. Excluding individuals with underlying conditions
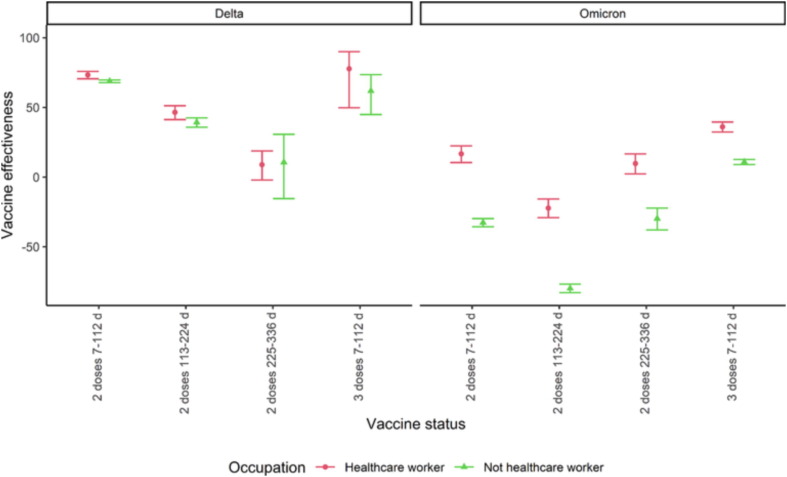
While HCW were in general offered vaccination earlier than non-HCW, within the non-HCW, vaccination was preferentially offered to individuals with underlying conditions. Furthermore, HCW may on average be of better health than non-HCW. This could, through the analysis, have led to differences in vaccine effectiveness between HCW and non-HCW. Repeating the analysis with the exclusion of all individuals with the relevant underlying conditions, we obtained similar results (Fig. 3 ).
Fig. 3.
Vaccine effectiveness among health care workers (HCW) and non-HCW against infections during the Delta (31 July-12 November 2021) and Omicron (3–30 January 2022) wave in Norway after excluding individuals with underlying conditions. The data behind the figure are available in the Supplementary section 4.3. The number of cases who received three doses during the delta wave were less than 5 and estimates are not provided.
3.2.4. Excluding individuals who received Vaxzevria
In contrast to Comernaty and Spikevax, the Vaxzevria vaccine is not based on mRNA delivery technology. As the proportion vaccinated with Vaxzevria was higher among HCW than non-HCW, the analysis was repeated with the exclusion of all individuals that received at least one dose of Vaxzevria, with similar results (Fig. 4 ).
Fig. 4.
Vaccine effectiveness among health care workers (HCW) and non-HCW against infections during the Delta (31 July-12 November 2021) and Omicron (3–30 January 2022) wave in Norway after excluding individuals with at least one dose of Vaxzevria. The data behind the figure are available in the Supplement section 4.4.
4. Discussion
This study shows a higher estimated vaccine effectiveness against infection with the SARS-CoV-2 Delta variant than the Omicron variant, both among HCW and the general population. During Delta wave, we found a declining effectiveness with time since receiving the last dose, which was observed less for Omicron. However, receiving a booster (3rd) dose improved effectiveness in both groups and was even significantly better than the first period after the 2nd dose in the Omicron period. We show that the estimated vaccine effectiveness during the Omicron wave was higher among HCW than the general population, but no difference was observed between the two groups during the Delta wave.
Our results are in line with previous studies, showing a higher estimated vaccine effectiveness against the Delta variant than the Omicron variant in the general population [8], [9], [10], [12], [13]. One previous study has investigated vaccine effectiveness in HCW in Wales, but this study covered predominantly the Alpha variant period and did not compare HCW with non-HCW [19]. To the best of our knowledge the comparison between HCW and non-HCW have not previously been shown. A proposed mechanism underlying the reduction in vaccine effectiveness from the Delta to Omicron variant period is a reduction in neutralizing activity which has been observed in serum specimens in several studies [20]. Overall vaccine effectiveness is higher against more severe COVID-19 outcomes, such as hospitalizations and death, than against infection [7], [9], [13]. As COVID-19 hospitalizations and mortality among HCW in the Norwegian population was very rare, we lacked statistical power for determining vaccine effectiveness against severe outcomes. The reduced effectiveness with time since last dose is similar to those reported by other countries as well as other studies from Norway [7], [8], [9], [10], [13]. However, we observed different patterns of waning of vaccine effectiveness between the Delta and Omicron variants. While the vaccine effectiveness against the Delta variant waned with time since 2nd dose, the vaccine effectiveness against the Omicron variant recovered after showing a decline. One possible interpretation of this phenomenon is different patterns of behaviour in terms of exposure in the vaccinated and unvaccinated cohorts resulting in underestimation of vaccine effectiveness, as has been previously indicated by a Danish population study [9], [21].
In this study, the confirmed COVID-19 infections rates among HCW were higher than non-HCW, but this risk was reduced during the Omicron period. During Omicron period, there was a higher spread in the general population, which might have reduced the difference between HCW and non-HCW.
Differences in vaccine effectiveness between HCW and the remaining population might be related to the differences in testing behaviour between HCW and non-HCW. In general, HCW have been subject to a higher degree of routine testing, which would give a more accurate incidence estimate both among vaccinated and unvaccinated. The unvaccinated non-HCW might have a higher threshold for getting tested for COVID-19, which might have been more evident during Omicron, which has been shown to cause milder disease than Delta [22]. We showed that the differences in effectiveness could not be explained by HCW receiving Vaxzevria more often, nor being heathier than the general population. In addition, we found no differences between age, type of health care worker or workplace. Non-pharmaceutical measures, both in the general population and those targeted to HCW, and behavioural elements might therefore play an important role in the differences found.
This study was performed using data for the entire Norwegian population, enabling the linkage of several nationwide registries, which is unique in an international context and limits a range of potential sources of selection bias. By investigating vaccine effectiveness for different virus variants, different age groups, occupational groups, and subgroups, we have provided findings at a detailed level. Identification of the most susceptible occupational subgroups among HCW adds important knowledge for health policy makers in order to design tailormade and more effective vaccination programs. However, data used for these estimates are not specifically collected for this purpose and will therefore have its limitations. It is possible that the rate of testing in vaccinated compared with unvaccinated individuals constitutes a bias which could underestimate positive COVID-19 test among unvaccinated and thus underestimate overall vaccine effectiveness. This bias could in turn be affected by the pathogenicity of the SARS-CoV-2 variant, as a proportional increase in asymptomatic infections among the unvaccinated may remain undetected. Additionally, a proportion of the population might have had a previous (undocumented) SARS-CoV-2 infection, which could underestimate the true vaccine effectiveness. This bias could be larger among non-HCW as HCW were subject to routine testing and therefore (asymptomatic) infection was more likely identified in this group. In addition, the number of unvaccinated individuals (both among HCW and non-HCW) in Norway is relatively low, which limits our statistical power.
In conclusion, we showed that the estimated vaccine effectiveness was similar between HCW and non-HCW during the period of Delta predominance, but effectiveness was significantly higher among HCW in the omicron period. The most likely explanation for this difference is related to prevention measures in the community and health care setting, as well as routine testing among HCW (https://www.helsedirektoratet.no/veiledere/koronavirus/kommunale-helse-og-omsorgstjenester/sykehjem/smitteverntiltak-i-helse-og-omsorgstjenesten?tidligere-versjoner). In line with previous reports, we found that effectiveness waned with time since last dose for both groups but was restored after receiving a booster.
Authors’ contributions
LV, PE, and HM were involved in the conceptualisation of the study. LV drafted the study protocol with feedback from PE, HM and PL. LV coordinated the study in collaboration with PL and PE. PL, LV, JS, had access to the data and the rest of the authors had the opportunity to request access to the data for this study. PL conducted the statistical analysis in consultation with JS, LV, PE, HM and MT. All co-authors contributed to the interpretation of the results. MT and PL drafted the manuscript with support from LV and HM. All co-authors contributed to the revision of the manuscript and approved the final version for submission. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We wish to thank all those who have helped to collect and report data to the national emergency preparedness registry at the Norwegian Institute of Public Health (NIPH) throughout the pandemic. We are grateful to all health professionals that contributed to vaccinating the Norwegian population and those performing millions of laboratory tests for COVID-19. Thanks also to the staff at the regional laboratories and the Virology and Bacteriology departments at NIPH involved in the analyses of samples, national variant identification and whole genome analysis of SARS-CoV-2 viruses. We would also like to acknowledge our colleagues at the NIPH who have contributed to the data cleaning from different registries throughout the pandemic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was performed as part of routine work at the Norwegian Institute of Public Health.
Data sharing statement
The dataset analyzed in the study contains individual-level linked data from various central health registries, national clinical registries, and other national administrative registries in Norway. The researchers had access to the data through the national emergency preparedness registry for COVID-19 (Beredt C19), housed at the Norwegian Institute of Public Health (NIPH). In Beredt C19, only fully anonymised data (i.e. data that are neither directly nor potentially indirectly identifiable) are permitted to be shared publicly. Legal restrictions therefore prevent the researchers from publicly sharing the dataset used in the NH study that would enable others to replicate the study findings. However, external researchers are freely able to request access to linked data from the same registries from outside the structure of Beredt C19, as per normal procedure for conducting health research on registry data in Norway. Further information on Beredt C19, including contact information for the Beredt C19 project manager, and information on access to data from each individual data source, is available at https://www.fhi.no/en/id/infectious-diseases/coronavirus/emergency-preparedness-register-for-covid-19/.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.05.022.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan Y.J., Chan K.H., Hung I.F. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of different vaccines at phase 3. Vaccines (Basel) 2021;9(9) doi: 10.3390/vaccines9090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higdon M.M., Wahl B., Jones C.B., Rosen J.G., Truelove S.A., Baidya A., et al. A systematic review of COVID-19 vaccine efficacy and effectiveness against SARS-CoV-2 infection and disease. medRxiv. 2021 2021.09.17.21263549. [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scobie H.M., Johnson A.G., Suthar A.B., Severson R., Alden N.B., Balter S., et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1284–1290. doi: 10.15585/mmwr.mm7037e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 caccine through 6 months. N Engl J Med. 2021 doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starrfelt J., Danielsen A.S., Buanes E.A., Juvet L.K., Lyngstad T.M., Rø G., et al. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July-November 2021. BMC Med. 2022;20(1):278. doi: 10.1186/s12916-022-02480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28(5):1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gram M.A., Emborg H.D., Schelde A.B., Friis N.U., Nielsen K.F., Moustsen-Helms I.R., et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022;19(9):e1003992. doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchan S.A., Chung H., Brown K.A., Austin P.C., Fell D.B., Gubbay J.B., et al. Estimated effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5(9):e2232760. doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veneti L., Berild J.D., Watle S.V., Starrfelt J., Greve-Isdahl M., Langlete P., et al. Vaccine effectiveness with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine against reported SARS-CoV-2 Delta and Omicron infection among adolescents, Norway, August 2021 to January 2022. medRxiv. 2022 doi: 10.1016/j.ijid.2023.03.004. 2022.03.24.22272854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starrfelt J., Danielsen A.S., Kacelnik O., Wang Børseth A., Seppälä E., Meijerink H. High vaccine effectiveness against coronavirus disease 2019 (COVID-19) and severe disease among residents and staff of long-term care facilities in Norway, November 2020-June 2021. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e10. doi: 10.1017/ash.2021.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seppala E., Veneti L., Starrfelt J., Danielsen A.S., Bragstad K., Hungnes O., et al. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill. 2021;26(35) doi: 10.2807/1560-7917.ES.2021.26.35.2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey B.B., Villabona-Arenas C.J., Campbell F., Keeley A.J., Parker M.D., Shah D.R., et al. Characterising within-hospitalSARS-CoV-2 transmission events using epidemiological and viral genomic data across two pandemic waves. Nat Commun. 2022;13(1):671. doi: 10.1038/s41467-022-28291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnusson K., Nygård K., Methi F., Vold L., Telle K. Occupational risk of COVID-19 in the first versus second epidemic wave in Norway, 2020. Euro Surveill. 2021;26(40) doi: 10.2807/1560-7917.ES.2021.26.40.2001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health NIoP. Påvisning og overvåkning av SARS-CoV 2-virusvarianter 2022 [updated 11.11.2022].
- 19.Bedston S., Akbari A., Jarvis C.I., Lowthian E., Torabi F., North L., et al. COVID-19 vaccine uptake, effectiveness, and waning in 82,959 health care workers: A national prospective cohort study in Wales. Vaccine. 2022;40(8):1180–1189. doi: 10.1016/j.vaccine.2021.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruell H., Vanshylla K., Tober-Lau P., Hillus D., Schommers P., Lehmann C., et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022;28(3):477–480. doi: 10.1038/s41591-021-01676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen C.H., Schelde A.B., Moustsen-Helm I.R., Emborg H.-D., Krause T.G., Mølbak K., et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv. 2021 2021.12.20.21267966. [Google Scholar]
- 22.Sigal A., Milo R., Jassat W. Estimating disease severity of Omicron and Delta SARS-CoV-2 infections. Nat Rev Immunol. 2022;22(5):267–269. doi: 10.1038/s41577-022-00720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.