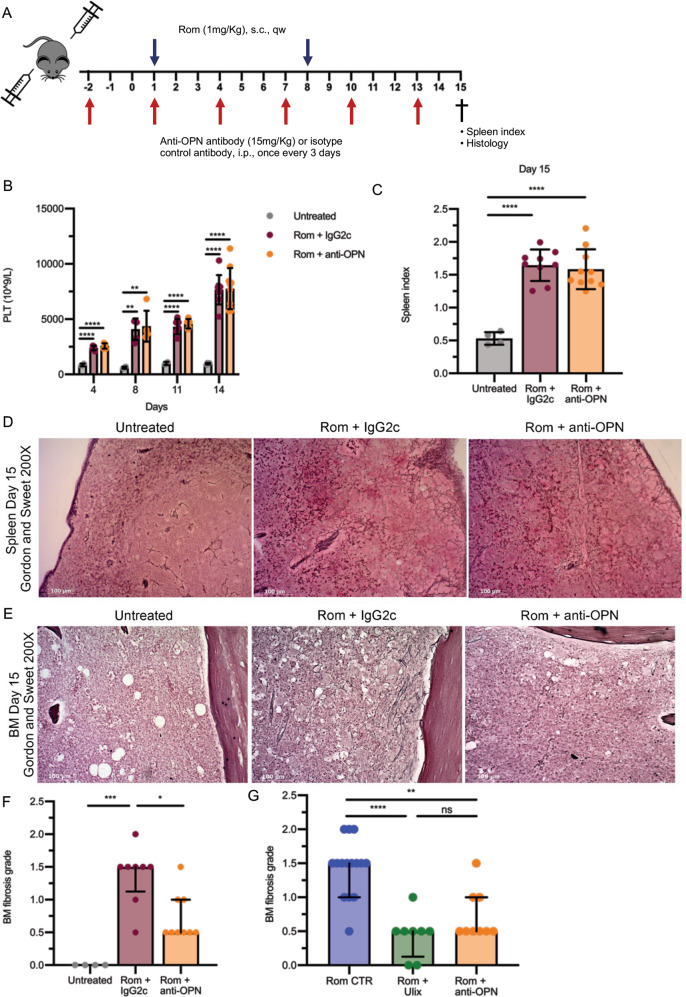
Fig. 7. Treatment with anti-OPN neutralizing antibody recapitulates the antifibrotic effect of Ulixertinib.
A Schematic outline of the experimental design. Myelofibrosis was induced by sub-cutaneous injection of Romiplostim (1 mg/kg, once weekly). Mice were given anti-OPN antibody 15 mg/kg (Rom + anti-OPN, in orange) or an isotype control 15 mg/kg (Rom + IgG2c, in brown) through intraperitoneal injection once every 3 days starting 3 days before the first Romiplostim injection. Mice were sacrificed after 15 days of Rom treatment for the evaluation of spleen index and bone marrow and spleen fibrosis. B Platelet count was assessed at days 4, 8, 11, and 14 (n = 4–10/group). C Spleen index was calculated at sacrifice (day 15) (n = 4–10/group). D Spleen fibrosis as shown by Gordon and Sweet’s reticulin staining in Untreated and treated mice. Magnification 200×. E Representative images of Gordon and Sweet’s reticulin staining of bone marrow (BM) sections from mice belonging to Untreated, Rom + IgG2c and Rom + anti-OPN groups. Magnification 200×. F Blinded fibrosis grade quantification (n = 4–10/group) was performed by a specialized pathologist. G Comparison of BM fibrosis grade reduction after ERK1/2 inhibition through Ulixertinib and anti-OPN treatment, a comprehensive analysis of data from Fig. 6B and Fig. 7F was performed (n = 8–15/group). In B and C histograms represent mean values while bars indicate the standard deviation; comparisons were performed by means of one-way ANOVA. In F and G histograms represent median values while bars indicate the interquartile range; comparisons were performed by means of Kruskal-Wallis’ test. *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001; ****: P ≤ 0.0001 Abbreviations: n number of samples; s.c. sub-cutaneous; qw once weekly; i.p. intraperitoneal; OPN osteopontin; Rom romiplostim; PLT platelets; Ulix Ulixertinib; BM bone marrow.