Abstract
We describe here the participation of a Trichomonas vaginalis 30-kDa proteinase (CP30) with affinity to the HeLa cell surface in attachment of this parasite to host epithelial cells. The CP30 band is a cysteine proteinase because its activity was inhibited by E-64, a thiol proteinase inhibitor. In two-dimensional substrate gel electrophoresis of total extracts of the trichomonad isolate CNCD 147, three spots with proteolytic activity were detected in the 30-kDa region, in the pI range from 4.5 to 5.5. Two of the spots (pI 4.5 and 5.0) bound to the surfaces of fixed HeLa cells corresponding to the CP30 band. The immunoglobulin G fraction of the rabbit anti-CP30 antiserum that recognized a 30-kDa band by Western blotting and immunoprecipitated CP30 specifically inhibited trichomonal cytoadherence to HeLa cell monolayers in a concentration-dependent manner and reacted with CP30 at the parasite surface. CP30 degraded proteins found on the female urogenital tract, including fibronectin, collagen IV, and hemoglobin. Interestingly, CP30 digested fibronectin and collagen IV only at pH levels between 4.5 and 5.0. Moreover, trichomonosis patients whose diagnosis was confirmed by in vitro culture possessed antibody to CP30 in both sera and vaginal washes, and CP30 activity was found in vaginal washes. Our results suggest that surface CP30 is a cysteine proteinase necessary for trichomonal adherence to human epithelial cells.
Trichomonas vaginalis is a flagellate protozoan which infects the urogenital tract of humans. It is responsible for trichomonosis, one of the most prevalent sexually transmitted diseases. Cytoadherence, one of the early steps in trichomonosis, is essential for cervicovaginal epithelium colonization. Previous studies on the specificity of the adherence of T. vaginalis to vaginal epithelial cells (VECs) have demonstrated that adherence is time, temperature, and pH dependent (1) and that it is a multifactorial process in which microtubules, microfilaments (16, 17), four adhesins (7), and cysteine proteinases (5) participate.
This parasite has many proteinases, most if not all of which are cysteine proteinases (CPs) (10, 11, 18). At least 23 different CPs were identified by two-dimensional (2-D) substrate gel electrophoresis (18). Some of them are involved in cytotoxicity (4, 6), hemolysis (12), immune response evasion (19), and cytoadherence (5, 6).
Previously we identified a 30-kDa T. vaginalis proteinase (CP30) that binds to host cell surfaces. Its proteolytic activity was inhibited by leupeptin, a cysteine-serine proteinase inhibitor that also reduced trichomonal attachment to HeLa cell monolayers by up to 80%. Furthermore, T. vaginalis isolates with low levels of cytoadherence had little or none of the 30-kDa-proteinase activity (6). These data suggested a relationship between the CP30 proteolytic activity and cytoadherence.
The main goal of this study was to demonstrate the role of CP30 in trichomonal cytoadherence and to characterize it as a virulence factor. Here we show that CP30 may be active under the environmental conditions found in the vagina, where it may degrade some extracellular matrix (ECM) proteins, i.e., fibronectin and collagen IV, as well as hemoglobin. This proteinase is immunogenic and is secreted into the vagina during infection. We also determined that the surface localization of CP30 is consistent with its role in cytoadherence, the first event in an infection.
MATERIALS AND METHODS
Growth and radiolabeling of trichomonads.
The T. vaginalis isolate CNCD 147 was used in this study (4, 21). Trichomonads were cultured in Diamond's Trypticase-yeast extract-maltose (TYM) medium (13) and supplemented with 10% heat-inactivated horse serum (JRS, Lenexa, Kans.) for 24 h at 37°C. Only late-logarithmic-phase organisms were used for assays. For cytoadherence assays, trichomonads were radiolabeled for 18 h at 37°C with 7 μCi of [3H]methyl-thymidine per ml (7.85-Ci/mmol specific activity [Amersham Life Science, Little Chalfont, England]).
Pretreatment of parasites.
Before lysis, parasites were treated with different proteinase inhibitors to determine their effect on the CP30 proteolytic activity by substrate gel electrophoresis as before (4). Briefly, 2 × 107 parasites suspended in phosphate-buffered saline (PBS) were treated for 20 min at 4°C with l-3-carboxy-2,3-trans-epoxypropionyl-leucylamido(4-guanidino)butane (E-64), p-tosyl-l-lysine chloromethyl ketone (TLCK), and leupeptin for cysteine proteinases; for serine proteinases, phenylmethylsulfonyl fluoride (PMSF); and for metalloproteinases, EDTA and EGTA (all purchased from Sigma Chemical Co., St. Louis, Mo.). Pretreated parasites were washed with PBS, pH 7.0, and suspended in PBS, pH 8.0, for the ligand assay. As a negative control, parasites treated identically but in the absence of the proteinase inhibitors were used.
Ligand proteinase assay.
The ligand assay for proteinases was performed as previously described (6). Briefly, a clarified detergent extract from 2 × 107 parasites interacted with 1 × 106 fixed HeLa cells for 18 h at 4°C. Eluted proteins from HeLa cell surfaces were separated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) copolymerized with different substrates (0.2% gelatin [Bio-Rad Laboratories, Richmond, Calif.], 500 μg of laminin-1, 800 μg of collagen IV, 500 μg of fibronectin, or 0.2% hemoglobin [all purchased from Sigma]) (4). Then gels were renatured with 2.5% Triton X-100 (Sigma) for 1 h at room temperature, activated with 100 mM sodium acetate buffer, pH 4.5, with 0.1% β-mercaptoethanol for 24 h at 37°C, and stained with 0.25% Coomassie blue R-250 (Sigma). Clear bands were indicative of proteolytic activity. Proteinase activity was also analyzed by 2-D substrate gel electrophoresis (18). Both one-dimensional (1-D) and 2-D electrophoresis analyses of total proteins and proteins bound to the HeLa cell surfaces were performed at least three times, obtaining identical patterns.
Generation of antiserum.
CP30 bound to HeLa cell surfaces was purified from preparative SDS-polyacrylamide gelatin gels. Gel fragments containing approximately 0.2 mg of proteinase were homogenized with Freund's complete adjuvant (Gibco Laboratories, Grand Island, N.Y.) and used to immunize rabbits. The animals were given booster injections three times with 0.1 mg of proteinase in Freund's incomplete adjuvant (Gibco) at 3- to 4-week intervals (15). This antiserum was used in Western blot analysis, cytoadherence inhibition, immunoprecipitation, and indirect immunofluorescence assays. Preimmune normal rabbit serum (NRS) was obtained before the immunization schedule began and was used as a negative control in all the experiments with antibodies.
Cytoadherence inhibition assays.
Cytoadherence inhibition assays were performed with confluent HeLa cell monolayers on 96-well microtiter plates, as described before (7). Two million [3H]thymidine-labeled parasites suspended in PBS, pH 7.0, were treated for 20 min at 4°C with different concentrations (100, 166, and 500 μg/ml) of anti-CP30 immunoglobulin G (IgG) fraction. Parasites treated identically with the same concentrations of preimmune rabbit serum (NRS) IgGs were used as negative controls. Then, antibody-treated parasites were washed with PBS and suspended in Dulbecco's minimal essential medium (DMEM)-TYM interaction media (2:1 [vol/vol]) without serum, added to confluent HeLa cell monolayers (4 × 104 cells per well) at a ratio of 5:1 (parasites to host cells), and incubated for 30 min at 37°C under a 5% CO2 atmosphere. The radioactivity (in counts per minute) associated with the HeLa cells was used to determine the extent of trichomonal cytoadherence. Each sampling was done in triplicate, and each experiment was performed at least three times with similar results.
Indirect immunofluorescence.
For confocal microscopy, parasites were fixed with 2% formaldehyde–0.5% glutaraldehyde for 1 h at 25°C, washed with PBS, and blocked with 0.2 M glycine, and half of the fixed parasites were permeabilized with cold acetone for 3 min (15). Then trichomonads were incubated for 20 min at 4°C, with anti-CP30 serum or NRS used as a negative control, at a 1:50 dilution. Washed parasites were incubated with a secondary antibody—a fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulins (Pierce, Rockford, Ill.)—at a 1:50 dilution for 20 min at 4°C, washed, and mounted with FluoroGuard solution (Bio-Rad).
Immunoprecipitation and Western blot assays.
Immunoprecipitations were conducted by a modification of a standard procedure, recently used (4), on 28 human sera and 43 vaginal washes (VWs). These samples were obtained from females attending the Centro Nacional de Clínicas de Displasias (CNCD) del Hospital General de México (HGM). The 28 sera were from women with diagnoses of trichomonosis (21 of 28) and other sexually transmitted diseases (STDs), including human papillomavirus infections (2 of 28) and Gardnerella infections (2 of 28), and from healthy people (3 of 28). The 43 VWs were from women with diagnoses of trichomonosis (20 of 43) and other STDs (14 of 43) and from healthy people (9 of 43). Western blot assay of trichloroacetic acid-precipitated proteins was carried out by standard procedures (4, 7), using antitrichomonad and anti-CP30 rabbit serum IgGs.
Biological samples, serum, and VWs.
Patients attending the CNCD at the HGM were diagnosed as being infected with T. vaginalis by positive culture and by their clinical presentation. The same patients and healthy women controls were also examined for the presence of other STD agents. Samples of blood (5 ml) and VWs (2 to 3 ml) were obtained as described before (2, 3). The samples were processed by a previously published procedure on the removal of vaginal epithelial cells from the VWs, before analysis for soluble proteinases (2).
RESULTS
The 30-kDa protein, which binds to HeLa cell surfaces, is a CP (CP30).
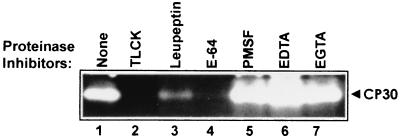
To determine the type of proteinase of the 30-kDa-activity band, we tested the effects of different proteinase inhibitors (PIs), including E-64, PMSF, EDTA, and EGTA, on the proteinase activity, by substrate-gel electrophoresis on gelatin gels. Leupeptin and TLCK were used as controls (6). After interaction of the total parasite extract with fixed HeLa cells (ligand assay), we detected that the proteolytic activity of the 30-kDa band was abolished by E-64 and TLCK, reduced by leupeptin, and unaffected by PMSF, EDTA, or EGTA (Fig. 1). These data indicated that the 30-kDa proteinase with affinity to HeLa cell surfaces is a CP (CP30).
FIG. 1.
CP30, a 30-kDa proteinase with affinity to HeLa cell surfaces. The effect of proteinase inhibitors on CP30 activity was analyzed. Before lysis, 2 × 107 parasites were treated for 20 min at 37°C with different proteinase inhibitors: 1 mM TLCK (lane 2); 0.2 mM leupeptin (lane 3); 180 μM E-64 (lane 4); 1 mM PMSF (lane 5); 0.2 mM EDTA (lane 6); and 0.2 mM EGTA (lane 7). Lane 1, parasites without inhibitors but with the same treatment. Then parasites were washed with PBS, lysed, and incubated with 106 fixed HeLa cells for 24 h at 4°C for a ligand assay. Proteinase activity was determined by substrate gel electrophoresis by SDS–10% PAGE with 0.2% gelatin, as described in Materials and Methods.
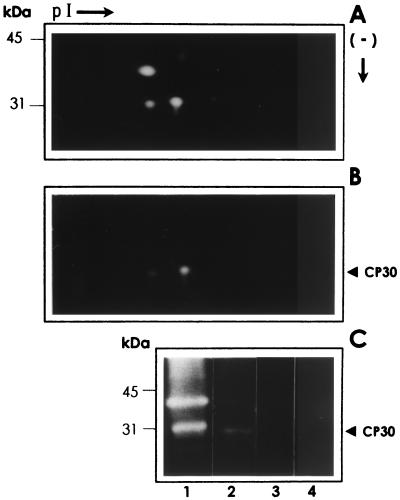
In T. vaginalis, numerous CPs are detected by 1-D and 2-D substrate-gelatin SDS-PAGE, including several spots in the 30-kDa region (11, 18). By 2-D SDS-PAGE of the T. vaginalis isolate CNCD 147, used in this study, we found three spots in the 30-kDa region, with proteolytic activity ranging between 4.5 and 5.5 pI (Fig. 2A). Then we performed a ligand assay using fixed HeLa cells to investigate which proteinase spots from the 30-kDa region bind to the host cell surface. On gelatin gels, we detected that two of the three spots with proteolytic activity from the 30-kDa region bound to the HeLa cell surfaces (Fig. 2B). As in 1-D gels, we found a single 30-kDa band with proteolytic activity that bound to the HeLa cell surface, and we assumed that the two spots appearing in the 2-D gels, which bound to HeLa cells and had proteolytic activity, correspond to the CP30 in 1-D SDS-PAGE.
FIG. 2.
2-D substrate SDS-PAGE analyses of proteinases with affinity to HeLa cell surfaces. The proteinase patterns corresponding to the trichomonad lysates (A) and with affinity to HeLa cell surfaces obtained after a ligand assay (B) (described in Materials and Methods) were analyzed by 2-D substrate gelatin gel electrophoresis. (C) 1-D substrate gelatin gels (experimental controls) of the trichomonad lysate (2 × 105 parasites) (lane 1); proteinases obtained after a ligand assay performed with the lysate from 2 × 107 parasites that interacted with 1 × 106 HeLa cell surfaces (lane 2); 1 × 106 fixed HeLa cells used in the ligand assays that had not been exposed to parasite lysates (lane 3); and 20 μl of fresh culture medium (TYM) with horse serum (lane 4). Positions of the molecular size markers are on the left. Only the region from 50 to 25 kDa is shown. pI →, direction of isoelectrofocusing using ampholines 3/10 and 5/8 (Bio-Rad); (−) ↓, direction of the SDS-denaturing gel electrophoresis by size.
Figure 2C shows that the CP30 activity band was present only on the parasite extracts, and it was absent in the fixed HeLa cells used in the ligand assays that had not been in contact with parasite extracts. The fresh culture media used to grow the trichomonad parasites did not have any proteolytic activity. These results suggest that CP30 is indeed a parasite proteinase.
Gel pieces containing the CP30 band were used to immunize rabbits to obtain antibodies that were utilized for cytoadherence inhibition assays and immunolocalization experiments.
CP30 participates in cytoadherence and is on the plasma membrane.
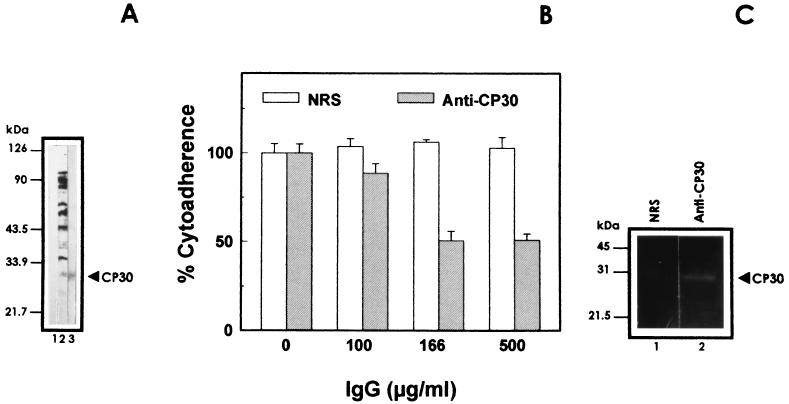
To study the role of the CP30 in cytoadherence, we used the anti-CP30 IgGs to inhibit trichomonal cytoadherence levels. Anti-CP30 antibodies that specifically recognized the CP30 by Western blotting (Fig. 3A) among the entire spectrum of T. vaginalis proteins inhibited T. vaginalis adherence to HeLa cell monolayers in a concentration-dependent manner, with maximum inhibition of approximately 50%, using a concentration of 166 μg of antibody per ml. Remarkably, raising the antibody concentrations further (threefold) did not affect cytoadherence levels; IgGs from control serum did not reduce parasite cytoadherence (Fig. 3B). The antibody concentrations that gave maximal inhibition of cytoadherence did not cause agglutination of parasites. Next, the effect of the anti-CP30 antibody (500 μg/ml) on trichomonal cytotoxicity over HeLa cell monolayers was also tested (7). At the maximum anti-CP30 IgG concentration used, this antibody was unable to protect HeLa cell monolayers from T. vaginalis destruction. These data show that CP30 is involved in parasite attachment to the host cells.
FIG. 3.
CP30 participation in T. vaginalis cytoadherence. (A) Western blotting experiments performed with nitrocellulose membrane containing trichloroacetic acid-precipitated total parasite proteins. Lanes: 1, IgG from NRS; 2, antitrichomonad IgGs; 3, test anti-CP30 IgGs. (B) The IgG fraction of the anti-CP30 antiserum was used for cytoadherence inhibition experiments. [3H]Thymidine-labeled parasites (2 × 106) were incubated for 20 min at 4°C with different concentrations (100, 166, and 500 μg/ml) of anti-CP30 or preimmune rabbit serum (NRS) before interaction with HeLa cell monolayers. Each point is the mean of the percentage of cytoadherence of two experiments with triplicate samples, and error bars represent the standard deviations. (C) Immunoprecipitation experiments were performed as described in Materials and Methods, with IgGs from NRS (lane 1) or anti-CP30 rabbit serum (lane 2) at a 1:50 dilution. Immunoprecipitated proteins were analyzed on gelatin substrate gels. (A and C) Positions of the molecular size markers are on the left.
Immunoprecipitation assays were performed on the proteins with affinity to HeLa cell surfaces obtained from ligand assays, using the antibodies from the anti-CP30 IgG fraction. The specific IgGs immunoprecipitated CP30, whereas normal serum IgGs (NRS) did not react (Fig. 3C), showing the monospecificity of the IgG fraction used in the cytoadherence inhibition experiments.
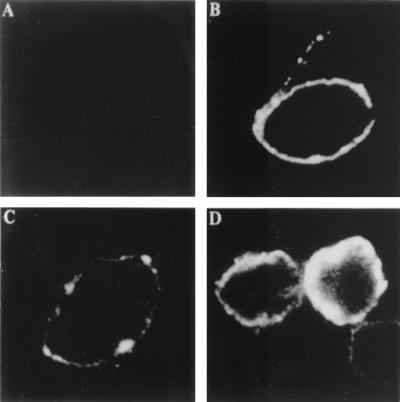
By indirect immunofluorescence assays on live and fixed parasites using the specific rabbit antibody to CP30, we initially determined that CP30 was located on the plasma membrane with a typical patchy distribution (Fig. 4C), but only in about 50% of the parasite population. In permeabilized trichomonads, fluorescence was detected both on the membrane and in the cytoplasm, where 100% of parasites were positive (Fig. 4D). As controls, we used preimmune rabbit serum, which gave no reaction (Fig. 4A), and an antitrichomonad rabbit serum, which showed a ring pattern (Fig. 4B). These results showed that CP30 is a surface proteinase as previously suggested (5, 6).
FIG. 4.
Localization of CP30 on the surface of T. vaginalis isolate CNCD 147. Fixed (A through D) and permeabilized (D) parasites were incubated with the antibody raised against CP30 (C and D) and with NRS (A) and antitrichomonad (B) rabbit serum used as negative and positive controls, respectively. All the antibodies were used at a 1:50 dilution. The samples were analyzed by confocal microscopy (Bio-Rad 1024 laser, krypton-argon; λ-564 nm) in the green channel at a ×40 magnification.
CP30 in vitro secretion.
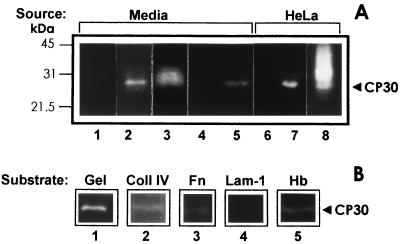
Next we investigated whether secretion of CP30 was increased by contact with the host cell. Ligand assays were performed with trichomonad culture supernatant obtained from the same amounts of parasites grown in suspension or in coculture over a fixed HeLa cell monolayer, in serum-containing TYM medium for 18 h, and in interaction media (DMEM-TYM) obtained after an adherence assay, and results were analyzed on gelatin gels. After a ligand assay, CP30 was found in the trichomonad culture media together with other secreted proteinases. As a negative control, the same number of fixed HeLa cells was equally treated, but they were not in contact with parasite lysates. Interestingly, parasites grown for 18 h in contact with HeLa cells seem to release higher levels of CP30, and 30 min of contact was enough to stimulate its secretion (Fig. 5A). Moreover, this proteinase was also deposited on the surfaces of the fixed HeLa cells used in the coculture. These results suggested that parasites seem to release more CP30 into the media when they interact with HeLa cells and that they deposited it on the surface of the epithelial cells that were in contact with the parasite.
FIG. 5.
CP30 is secreted (A) and is selective for different human protein substrates (B). (A) In vitro secretion of CP30 from parasites grown in suspension was compared to trichomonads grown in coculture over a HeLa cell monolayer (2 × 106). The same amounts of parasites (10 × 106) were inoculated in 30 ml of culture media for both conditions and incubated at 37°C for 18 h to produce 40 × 106 parasites. After parasites were pelleted by centrifugation at 900 × g, 1-ml aliquots from different sample supernatants (Media) were processed for a ligand assay (see Materials and Methods). Lanes: 1, samples from control fresh media; 2, spent culture media from parasites grown in suspension; 3, spent culture media from parasites grown in coculture with fixed HeLa cell monolayers; 4 and 5, clarified supernatant (1 ml) from parasites after 30 min in suspension (control [lane 4]) or in interaction with HeLa cell monolayers (lane 5) (adherence assay) which also were processed for a ligand assay; 6 through 8, material eluted from the same amount (106) of fixed HeLa cells was loaded from a mock control without contact with parasite lysates (lane 6) or in contact with extracts from parasites grown in suspension (lane 7) or from monolayers obtained from coculture with T. vaginalis (lane 8). All samples were analyzed by substrate gelatin gel electrophoresis. The positions of the molecular size markers are on the left. (B) The CP30 proteolytic activity was tested by SDS-PAGE using different human proteins as substrates. Lanes: 1, 0.2% gelatin (Gel); 2, 800 μg of collagen IV (Coll IV); 3, 500 μg of fibronectin (Fn); 4, 500 μg of laminin-1 (Lam-1); 5, 0.2% hemoglobin (Hb). All substrate gels were activated under the same experimental conditions, 24 h at 37°C and pH 5.0.
Collagen IV, fibronectin, and hemoglobin are substrates of CP30.
Degradation of collagen IV, fibronectin, laminin-1, and hemoglobin—proteins found in the vagina—by CP30 was analyzed on polyacrylamide gels. CP30 digested collagen IV, fibronectin, and human hemoglobin but not laminin-1. Gelatin was used as a control substrate (Fig. 5B).
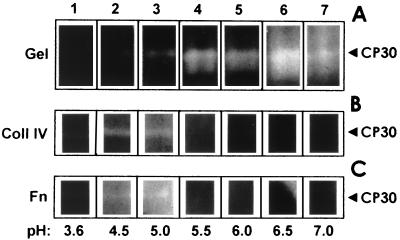
In addition, CP30 activity was evaluated at different pHs on gelatin, collagen IV, and fibronectin as substrates (Fig. 6). CP30 digested gelatin from pH 4.5 to 7.0 (Fig. 6A). In contrast, CP30 was active on collagen IV and fibronectin only at pH 4.5 and 5.0; beyond this pH, no CP30 activity was detected (Fig. 6B and C). Moreover, the CP30 proteolytic activity on gelatin gels at a constant pH of 4.5 was stable up to 50°C (not shown). These results indicated that the in vitro optimal conditions for CP30 activity are consistent with the environmental conditions found in the urogenital tract of women. For example, the vaginal pH ranges in healthy women from 4.0 to 5.0 and in women with ongoing trichomonosis from 4.4 to 7.0 (2). Thus, CP30 could degrade some ECM proteins in the first step of infection, when the vaginal microenvironment is acidic.
FIG. 6.
CP30 degrades different substrates at specific pHs. The CP30 proteolytic activity was analyzed on 0.2% gelatin (Gel); 800 μg of collagen IV (Coll IV); and 500 μg of fibronectin (Fn). The proteolytic activation was performed at 37°C at different pHs, as follows: lane 1, 3.6; lane 2, 4.5; lane 3, 5.0; lane 4, 5.5; lane 5, 6.0; lane 6, 6.5; and lane 7, 7.0. pH 4.5 was used as a control (lane 2).
The CP30 proteinase is immunogenic and secreted into the vaginas of patients with trichomonosis.
The immunogenicity (Fig. 7A and B) and secretion (Fig. 7C) of CP30 during trichomonal infection was investigated in 28 human sera and 43 human VWs, by immunoprecipitation assays and substrate gelatin gels. The presence of anti-CP30 antibodies in serum and VWs was determined by using the trichomonad proteins obtained from cultured parasites that bound to HeLa cell surfaces after a ligand assay as antigen.
FIG. 7.
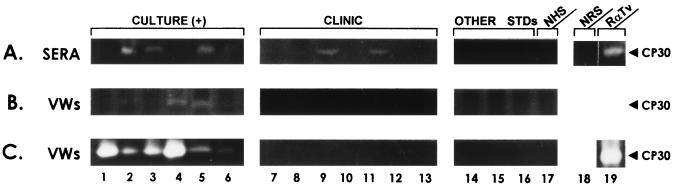
Anti-CP30 antibodies present in human sera (A) and VWs (B) and in vivo secretion of CP30 (C). Antibodies to CP30 were detected by immunoprecipitation assays, using as antigen HeLa cell-bound proteinases including the CP30 obtained from cultured parasites after a ligand assay, and the activity was then detected in gelatin gels as in Fig. 1. The lane numbers in parts A, B, and C correspond to the same patients. Culture-positive samples (Culture [+]) from lanes 1 through 6 are representative patterns of immunoprecipited proteinases and proteins with antibodies to trichomonad molecules present in human sera (A) or VWs (B) from patients with clinical trichomonosis confirmed by in vitro culture. Clinical samples (Clinic) from lanes 7 through 13 show representative patterns of immunoprecipitation assays with human sera (A) or VWs (B) from patients with clinical trichomonosis with negative in vitro culture. Lanes 14 through 16, immunoprecipitation patterns of human sera (A) or VWs (B) from patients with other STDs, such as human papillomavirus infection (lanes 14 and 15) and Gardnerella infection (lane 16). Lane 17 (NHS) corresponds to representative patterns of negative control serum (A) or VWs (B and C) obtained from healthy people. (A) Other internal controls for immunoprecipitation assays were used: lane 18, NRS (negative); lane 19, rabbit serum against total T. vaginalis protein (positive). The white bands indicate immunoprecipitation of CP30 activity, and the black ones indicate protein without proteinase activity from T. vaginalis, sera, or both. (C) Detection of proteolytic activity in the region of the CP30 band in VWs directly analyzed on substrate gelatin gels. Lanes 1 through 17 correspond to those in panel B. Lane 19 corresponds to the CP30 proteolytic activity used as a control, obtained from proteins bound to the surface and eluted from fixed HeLa cells after a ligand assay.
Of 28 sera used, 21 were from patients with clinically diagnosed trichomonosis, but only 7 of them were confirmed by in vitro culture. Four sera from patients with other STDs and three from healthy people were used as controls. All sera from patients with culture-positive trichomonosis (7 of 7) and two (2 of 14) with clinical diagnosis of trichomonosis, but who were negative by in vitro culture, contained anti-CP30 antibodies. Sera from patients with other STDs or healthy people (normal human serum [NHS]) did not immunoprecipitate CP30 (Fig. 7A). These data show that CP30 is immunogenic during trichomonal infection. Our results are consistent with the presence of antibodies to some trichomonad proteinases in sera from trichomonosis patients (3). In addition, we show that antibodies to CP30 were also present in some culture-negative patients, due to presumable low-level parasite infection.
The local immune response against CP30 was also checked on VWs by immunoprecipitation assays and gelatin gels (Fig. 7B). Twenty out of forty-three VWs used in these experiments were from patients with clinically diagnosed trichomonosis, but only seven were culture positive. We also used 14 VWs with other STDs and 9 from healthy people. Only three of the seven culture-positive VWs had anti-CP30 antibodies. In contrast, none of the VWs from in vitro-culture-negative patients, patients with other STDs, or healthy people had anti-CP30 antibodies (Fig. 7B). These results show the presence of anti-CP30 antibodies only in VWs from patients with trichomonosis confirmed by in vitro culture, but not all of them had anti-CP30 antibodies. The absence of anti-CP30 antibodies in some VWs from culture-positive patients could be due to the presence of an immunoglobulin-degrading proteinase (19).
The presence of CP30 proteolytic activity on the VWs used in this study was analyzed directly on gelatin gels (Fig. 7C). All of the culture-positive VWs exhibited CP30 activity, whereas none of the VWs from patients with clinical trichomonosis or other STDs or from healthy people was positive in this assay. These data show that CP30 is secreted in vivo into the vagina during infection. However, to assess that the procedure for clarifying VWs from VECs did not destroy T. vaginalis parasites, we performed a mock experiment as previously reported (2) with VWs from normal women that were contaminated with in vitro grown trichomonads. These VWs had no detectable soluble proteinases after being processed identically. However, we could not rule out that some parasites were lysed during the infection, as part of the host defense mechanisms.
DISCUSSION
In this study, we demonstrated the involvement of a 30-kDa CP (CP30) in trichomonal adherence and characterized it. It was determined earlier that a 30-kDa proteinase bound to HeLa and VECs is related to cytoadherence. Inhibition studies suggested a role for this protein in the parasite virulence (6). Treatment of live parasites with leupeptin or TLCK inhibitors decreased trichomonal cytoadherence and the 30-kDa-proteinase activity (5, 6). Since these two inhibitors inhibit both cysteine and serine proteinases and most T. vaginalis proteolytic activities are due to cysteine proteinases (10, 11, 18), it was important to use a specific cysteine proteinase inhibitor such as E-64 on the 30-kDa activity to confirm the thiol nature of this proteinase, which we have named CP30.
The 2-D substrate gelatin gels showing that two spots with proteolytic activity and affinity to HeLa cell surfaces formed the CP30 band suggest that these spots might represent isoforms of CP30 with specific pIs, which may share the cell-binding domain, since both of them bind to HeLa cell surfaces. Cloning the genes for this proteinase will help to determine whether these spots are products of a gene family.
Remarkably, antibody to CP30 inhibited cytoadherence in a concentration-dependent mode up to 50% of the saturation point. Similar results were previously obtained with antibodies against adhesins, which showed a maximum of 50% cytoadherence inhibition either individually or as a pool (7). These data confirmed that T. vaginalis recognition of and binding to host cells are multifactorial events. A cysteine proteinase activity is needed (5), and several other factors participate (7, 8, 20, 21). Due to this complexity, it might not be possible to totally abolish cytoadherence by using only one blocking agent at a time. Alternatively, we could not rule out immunoglobulin degradation of the anti-CP30 antibody by CP30 or by other parasite proteinases (19) during a cytoadherence assay.
Immunofluorescence experiments demonstrated the surface localization of CP30. Interestingly, half of the parasite population from the isolate analyzed in this study possessed CP30 on their surface, although all showed CP30 reactivity on their cytoplasm. The heterogeneity of CP30 surface expression suggests that CP30 may be another T. vaginalis surface protein that undergoes phenotypic variation (7) that could depend on the growth or cell cycle phases, environmental regulation, or isolate heterogeneity. Alternatively, it might also suggest different stages of proteinase maturation (pre-pro-CP30, pro-CP30, or CP30) or the parasite physiological stage. Both possibilities could influence or affect the translocation of the mature CP30 to the parasite plasma membrane. Studies in progress are directed toward understanding this variability.
CP30 degraded gelatin over a broad range of pHs at body temperature. Interestingly, the pH range at which the enzyme was active was narrowed when fibronectin or collagen IV was used as the substrate. These data suggest that the pH might be an environmental signal regulating the activity of this virulence factor, as has been observed in Candida albicans (9), another urogenital pathogen. In addition, our results indicate how critical it is to use the right protein as the substrate to study proteinases that could play a role in the host-parasite relationship during infection. Alternatively, it is also possible that the amino acids recognized by CP30 in these substrates are not accessible to the proteinase at a pH range between 5.5 and 7.0 due to conformational changes in the substrate at these pH values. The fact that CP30 was active against gelatin at higher pH levels, where it was inactive against fibronectin or collagen, could suggest that CP30 is active over a wide pH range. Interestingly, these pH ranges are detected in the infected vaginas of women with ongoing trichomonosis (2).
Another cysteine proteinase with a molecular weight similar to that of CP30 has been recently analyzed by Fiori et al. (14). Although the two proteinases have similar sizes, 30 kDa, we have evidence that suggests that they are different enzymes. (i) The CP30 binds to the surface of HeLa and VECs (6). (ii) It is secreted into the vaginal environment, as well as into the culture media during in vitro growth. (iii) Its secretion is increased by contact with the HeLa cell monolayers, and it is able to bind to HeLa cell surfaces. It should be noted that HeLa cell monolayers are only a working model. This does not rule out the possibility that VECs may yield different results. (iv) CP30 also degrades proteins found in the urogenital environment, i.e., hemoglobin, fibronectin, and collagen IV but not laminin-1. (v) An antibody raised against CP30 was able to inhibit cytoadherence but not cytotoxicity. However, at this point we cannot be sure that CP30 is not also involved in host cell damage, since cytotoxicity is a multifactorial process. By contrast, the proteinase described by Fiori et al. (14) (i) is not secreted, (ii) degrades a cytoskeleton protein, spectrin, from the red blood cells, and (iii) could be involved in cellular damage (14). We have recently identified a 65-kDa CP (CP65) with affinity to HeLa cell surfaces and characterized it as one of the molecules involved in trichomonal cytotoxicity. It also degrades fibronectin and collagen IV and is immunogenic in trichomonosis patients (4, 6), which is also different from the proteinase described by Fiori et al. (14).
In conclusion, we show that CP30 from T. vaginalis is a cysteine proteinase located on the plasma membrane that seems to be secreted during trichomonal infection. Our data suggest a role for this proteinase in trichomonal attachment to cervical and VECs (5, 6). In addition, CP30 may degrade hemoglobin present during menstruation and, at acidic pHs, collagen IV and fibronectin—ECM proteins present in the vaginal environment and basal lamina of the transitional epithelium between vagina and cervix. CP30 stimulates both a local and a systemic humoral immunological response in patients with active trichomonosis, although the role of the anti-CP30 antibody response in host protection remains unclear.
ACKNOWLEDGMENTS
This work was supported by grants 0579P-N and 25572-N from CONACyT México (to R.A.). M.R.M.-L. and C.B.-G. were scholarship recipients from CONACyT. L.V.F.-F. was supported by a scholarship from IPN.
We thank Esther Orozco and José Antonio Mendoza for their critical review of the manuscript. The excellent technical assistance of Alberto García, Alfredo Padilla-Barberi, and the personnel from the photography facility is greatly appreciated. We thank Beatriz Urrutia for her excellent secretarial assistance. We are also thankful to Leobardo Mendoza for his help with the confocal microscopy at the CICATA-IPN microscopy facility and the personnel at the Electron Microscopy Facility at our institution for allowing us to use the epifluorescence microscope for the in vivo observations. We thank José Cruz-Talonia (CNCD of the HGM) and Gloria López-Jimenez for their help in the collection of the T. vaginalis isolates, human sera, and vaginal washes used in this study.
REFERENCES
- 1.Alderete J F, Garza G E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete J F, Newton E, Dennis C, Neale K A. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin Med. 1991;67:469–474. doi: 10.1136/sti.67.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete J F, Newton E, Dennis C, Neale K A. Antibody in sera of patient infected with Trichomonas vaginalis is to trichomonad proteinases. Genitourin Med. 1991;67:331–334. doi: 10.1136/sti.67.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Sánchez M E, Avila-González L, Becerril-García C, Fattel-Facenda L V, Ortega-López J, Arroyo R. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb Pathol. 2000;28:193–202. doi: 10.1006/mpat.1999.0336. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo R, Alderete J F. Trichomonas vaginalis surface proteinase activity is necessary for parasite adherence to epithelial cells. Infect Immun. 1989;57:2992–2997. doi: 10.1128/iai.57.10.2991-2997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arroyo R, Alderete J F. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch Med Res. 1995;26:279–285. [PubMed] [Google Scholar]
- 7.Arroyo R, Engbring J, Alderete J F. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo R, González-Robles A, Martínez Palomo A, Alderete J F. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernardis F, de Mühlschlegel F A, Cassone A, Fonzi W A. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–3325. doi: 10.1128/iai.66.7.3317-3325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozner P, Demes P. Proteinases of Trichomonas vaginalis and Trichomonas mobilensis are not exclusively of cysteine type. Parasitology. 1990;102:113–120. doi: 10.1017/s0031182000060418. [DOI] [PubMed] [Google Scholar]
- 11.Coombs G H, North M J. An analysis of the proteinases of Trichomonas vaginalis by acrylamide gel electrophoresis. Parasitology. 1983;86:1–6. doi: 10.1017/s0031182000057103. [DOI] [PubMed] [Google Scholar]
- 12.Dailey D C, Chang T, Alderete J F. Characterisation of a hemolysin of proteinases of the parasitic protozoan Trichomonas vaginalis. Parasitology. 1990;101:171–177. doi: 10.1017/s0031182000063204. [DOI] [PubMed] [Google Scholar]
- 13.Diamond L S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- 14.Fiori P L, Rappelli P, Addis M F, Mannu F, Cappuccinelli P. Contact-dependent disruption of the host cell membrane skeleton induced by Trichomonas vaginalis. Infect Immun. 1997;65:5142–5148. doi: 10.1128/iai.65.12.5142-5148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 53–137. [Google Scholar]
- 16.Juliano C, Monaco G, Bandiera P, Tedde G, Cappuccinelli P. Action of anticytoskeletal compounds on in vitro cytopathic effect, phagocytosis, and adhesiveness of Trichomonas vaginalis. Genitourin Med. 1987;63:256–263. doi: 10.1136/sti.63.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger J N, Ravdin J I, Rein M F. Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun. 1985;50:778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neale K A, Alderete J F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun. 1990;58:157–162. doi: 10.1128/iai.58.1.157-162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provenzano D, Alderete J F. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect Immun. 1995;63:3388–3395. doi: 10.1128/iai.63.9.3388-3395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva-Filho F C, de Souza W, Lopes J D. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci USA. 1988;85:8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva-Filho F C, Ortega-López J, Arroyo R. YIGSR is the preferential laminin-1 residing adhesion sequence for Trichomonas vaginalis. Exp Parasitol. 1998;88:240–242. doi: 10.1006/expr.1998.4227. [DOI] [PubMed] [Google Scholar]