Abstract
Background/Objectives
This study aimed to analyse the role of the choroid in early age-related macular degeneration (AMD) by analysing choroidal vascularity index (CVI) in pure cohorts of patients with subretinal drusenoid deposits (SDD) or conventional drusen (CD).
Subjects/Methods
This was an observational cross-sectional study. Comprehensive ophthalmologic examination and multimodal imaging including fundus photography, autofluorescence, near infrared reflectance, and spectral domain optical coherence tomography (SDOCT) was performed. CVI processing was performed on a foveal horizontal SDOCT scan with binarization using Image J Image software and calculated as the ratio between luminal area (LA) and total area (TA).
Results
Sixty-nine eyes of 69 participants were included; 23 eyes with SDD alone, 22 eyes with CD alone, and 24 control eyes of healthy age-matched subjects. CVI was significantly reduced in the SDD and CD group compared to controls (p = 0.0001). Post-hoc analysis revealed a significant reduction of CVI in the SDD versus the control group (p = 0.0002), in the CD versus the control group (p = 0.001), and in the SDD versus the CD group (p = 0.006). Covariance analysis showed a significant difference of LA (p = 0.033) but no significant difference of TA (p = 0.106) between the three groups. Direct comparison between CD and SDD showed a significant reduction of LA and TA in the SDD group.
Conclusions
CVI may have prognostic implications in early AMD. SDD is a biomarker of AMD progression and the mechanism for this could be via reduction of the CVI.
Subject terms: Predictive markers, Macular degeneration
Introduction
A major cause of irreversible vision impairment in patients above 65 years of age in the western world is advanced age-related macular degeneration (AMD). The majority of studies on the epidemiology of AMD were performed in North America and Australia but the prevalence of disease is similar in Europe, including Italy [1–3]. A recent systematic review based on data from fourteen population-based studies included in the European Eye Epidemiology consortium, reported a prevalence of 13.2% and 3.0%, for early and late AMD, respectively, similar to persons of European ancestry living in other continents [4].
AMD is caused by a complex interplay of genetic and environmental mechanisms that still need to be clarified despite extensive research. One pathological pathway seems to relate to impairment of the choroid, the multifunctional, prevalently vascular tissue, that supplies oxygen and nutrients to the outer retina. Similar to systemic generalized vessel alterations in other regions of the body, the choroid becomes thinner with age and research has suggested that choroidal vascular loss is an early alteration in AMD [5–8].
The classic hallmark of AMD is drusen that are extracellular accumulations beneath the retinal pigment epithelium (RPE) [9]. A unique phenotypic feature and risk factor for AMD progression are subretinal drusenoid deposits (SDD) or reticular pseudodrusen that are characterized by accumulations above the RPE [10]. This phenotypic characteristic has a strong correlation with choroidal alterations, retinal angiomatous proliferation, and geographic atrophy [10, 11].
The advent of enhanced depth imaging in SDOCT and further technological advances in imaging have enabled a new era of research in evaluating the role of the choroid, in vivo, in systemic and ophthalmic disease. Many studies have evaluated choroidal thickness in AMD but results have been somewhat inconsistent [12–16]. One factor that may account for the variability of results in previous reports is that choroidal thickness can be affected by multiple factors which may not be standardized between studies. These factors include age, gender, systemic disease, axial length, and diurnal variations [17, 18]. Another factor may be the presence of SDD. Indeed, reports of choroidal thinning in AMD associated with an SDD phenotype has led to the concept that these deposits may have a marked influence on choroidal structure and vascularization, and subsequent progression to late-stage AMD [10, 11, 19].
A relatively novel quantitative parameter in choroidal analysis is the choroidal vascular index (CVI) where the vascular lumen area and stromal component of the choroid is quantified and the CVI is calculated as the ratio of the luminal area (LA) and total area (TA) [20]. CVI has been shown to not undergo variations with changes in mean choroidal thickness and aging [21, 22]. Thus, this parameter represents a more sensitive biomarker to detect choroidal alterations in AMD.
In the current literature some authors have recently assessed CVI in AMD associated with SDD. Some studies included eyes with mixed phenotypes [23, 24], and a few recent investigations evaluated pure cohorts of patients [25–28]. However, given the diverse inclusion criteria among studies, including heterogeneous stages of AMD, results in the literature are somewhat variable. Thus, the present report aims to further clarify the role of the choroid in AMD associated with SDD by analysing the CVI in pure cohorts of patients with either SDD or CD in early AMD.
Methods
This was an observational cross-sectional study including 69 eyes of 69 Caucasian participants of Italian origin (23 eyes with SDD, 22 eyes with CD, and 24 eyes of 24 healthy age-matched subjects) enrolled in an ongoing prospective study on retinal and choroidal vascular components in eyes with AMD and SDD. The examinations were performed at the Retina Centre of the Ophthalmology Unit of the Sapienza University of Rome, St. Andrea Hospital. The study had Institutional Review Board approval from the Sapienza University. Informed consent was obtained from all patients and subjects and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
Inclusion criteria for patients with early AMD were as follows: age ≥50 years; SDOCT and near infrared reflectance (NIR) evidence of CD (defined as the CD group) or SDD (defined as the SDD group); clear optical media to obtain good quality images. Exclusion criteria were the presence of neovascular membranes or signs of complete RPE/ outer retinal atrophy, history of any other chorioretinal pathology, diabetic or hypertensive retinopathy, previous retinal treatment including intravitreal anti-vascular endothelial growth factor injections, spherical equivalent above 4 Dioptres, glaucoma or intraocular pressure above 18 mmHg, SDOCT evidence of epiretinal membranes, and presence of neurological disease. Healthy age matched control subjects were defined as the control group. Only one eye of each subject was included in the study. If both eyes were eligible, we used a random number generator where odd numbers were for right eyes and even numbers were for left eyes; if one eye had exclusion criteria it was excluded.
All patients and subjects underwent comprehensive ophthalmologic examination including assessment of best-corrected visual acuity, slit lamp evaluation of the anterior segment, tonometry, and fundus examination. Multimodal imaging included photographic documentation of the posterior pole (Centervue), fundus autofluorescence and NIR imaging (Heidelberg HRT II), SDOCT (RTvue XR Avanti, Optovue, Inc, Fremont, CA). Two operators evaluated the presence of SDD or CD with NIR and SDOCT images. Patients were included in the SDD group with evidence of at least five deposits in a papillary disc area. Patients were included in the CD group with evidence of at least one CD > 125 micron or five drusen between 63 and 125 micron according to the criteria by Spaide et al. and Zweifel et al. [29, 30]. Mixed phenotypes were excluded in order to avoid bias and obtain differential data between the groups examined.
Imaging analysis
The SDOCT protocol was performed with the grid scan with 5 vertical and 5 horizontal lines and the horizontal grid scan centred on the fovea was selected for binarization of the 3 mm choroidal area beneath the fovea. Image binarization was made with Image J (https://imagej.net/Fiji/Downloads) according to the method described by Sonoda et al. [20] In brief, after setting the image scale, with the polygon tool of the software, a region of interest (ROI) with the upper and the lower limit was selected at the RPE and the sclerochoroidal junction, respectively (Fig. 1). Three major choroidal vessels >100 microns of the ROI were selected in order to adjust the contrast and the mean vascular brightness for real lumen area. Successively, images were binarized on the ROI with the Niblack’s autolocal threshold technique, in order to analyse mean and standard deviation of dark and light pixels in the ROI (Fig. 2). The software analysis system, enabled to identify areas with dark pixels, the totality of which gave the choroidal LA; while the totality of pixels in the ROI gave the TA. The ratio between LA and TA identified the CVI.
Fig. 1. Spectral domain optical coherence tomography (SDOCT) and selection of region of interest (ROI) to calculate choroidal vascularity index.
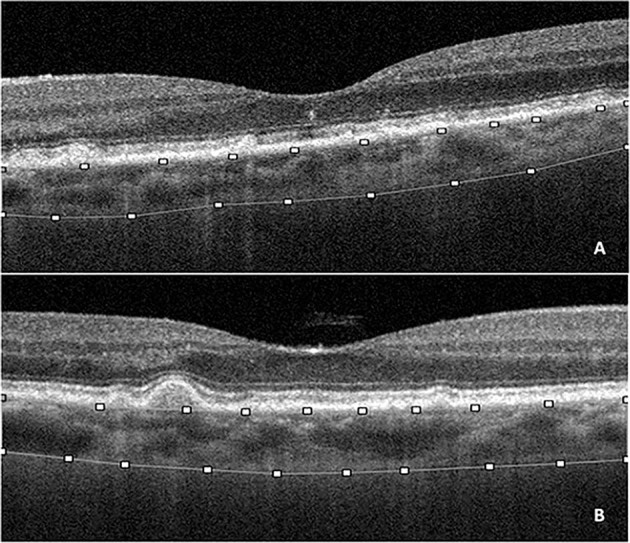
Horizontal SDOCT grid scan (3mm-area centred on the fovea) showing subretinal drusenoid deposits (A) and conventional drusen (B). The horizontal SDOCT grid scan was manually segmented to select the inner and outer borders of the choroid; the upper limit corresponding to the retinal pigment epithelium and the lower limit corresponding to the sclerochoroidal junction to select the ROI (superimposed lines).
Fig. 2. Spectral domain optical coherence tomography (SDOCT) and choroidal vascularity index (CVI) calculation.
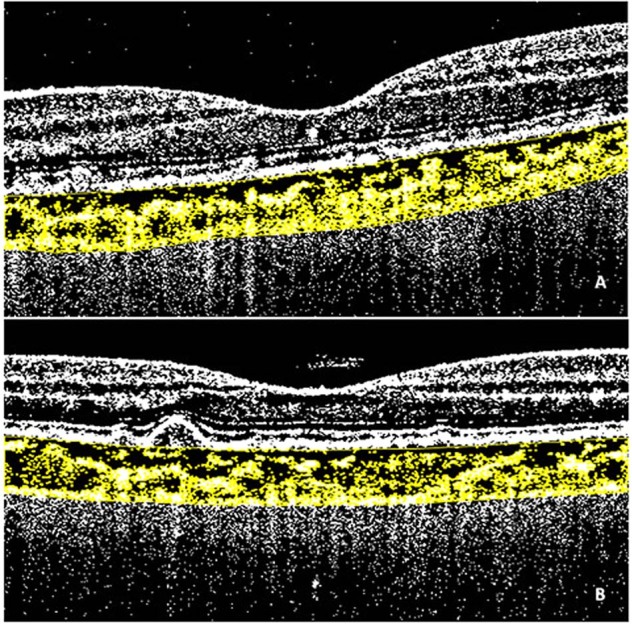
Horizontal SDOCT grid scan (3mm-area centred on the fovea) showing subretinal drusenoid deposits (A) and conventional drusen (B). Binarization of images performed with Image J software according to Niblack’s method. The region of interest corresponds to the yellow area, the totality of yellow dots corresponds to the stromal area while the black dots correspond to the luminal area. The ratio between the luminal area and the stromal area corresponds to the CVI.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (version 28.0.0.1, Chicago, IL, USA). Data were expressed as mean ± standard deviation or median and interquartile range for continuous variables, and the number of cases (and percentages) for categorical variables. All variables were tested for normality using the non-parametric Kolmogorov-Smirnov test. Demographic normally distributed continuous variables were compared by the analysis of variance (ANOVA); Tukey’s HSD post-hoc test was performed for multiple comparison between groups. Categorical variables were evaluated using the χ-square test or Fisher exact test when appropriate, using Bonferroni correction. Analysis of covariance was performed on CVI, adjusting for age, BCVA and spherical equivalent. A p value ≤ 0.05 was considered statistically significant.
Results
The sample consisted of three distinct cohorts; 23 patients in the SDD group, 22 patients in the CD group, and 24 healthy age-matched subjects in the control group; a total of 6 patients had been excluded due to low image quality. The mean age was 78.74 ± 8.99, 77.18 ± 8.27, and 76.13 ± 8.13 years for the SDD, CD, and control groups, respectively. All groups were homogeneous and did not show statistically significant differences (p = 0.572). No difference in gender, BCVA, and spherical equivalent between groups were found. Demographic data are shown in Table 1.
Table 1.
Demographic data of patients with subretinal drusenoid deposits (SDD), conventional drusen (CD), and control group.
| SDD (n = 23) | CD (n = 22) | P-valuea | Control group (n = 24) | P-valueb | |
|---|---|---|---|---|---|
| Age (Years) | 78.74 ± 8.99 | 77.18 ± 8.27 | 0.812c | 76.13 ± 8.13 | 0.572d |
| Sex (M/F) | 12/11 | 9/13 | 0.465e | 9/15 | 0.572e |
| BCVA (logMAR) | 0.90 ± 0,14 | 0.82 ± 0,19 | 0.291c | 0.90 ± 0.14 | 0.205d |
| Spherical Equivalent. (Dioptres) | 0.21 ± 1.01 | 0.23 ± 1.10 | 0.998c | 0.25 ± 1.40 | 0.995d |
| Phakic/Pseudophakic | 14/9 | 16/6 | 0.275e | 19/5 | 0.376e |
BCVA best-corrected visual acuity, SDD subretinal drusenoid deposits, CD conventional drusen.
aComparison of eyes with SDD versus eyes with CD.
bComparison of eyes with SDD, eyes with CD and control group eyes (control group).
cPost-hoc test with Tukey’s HSD.
dANOVA.
ePearson’s chi square test, Bonferroni correction.
Analysis of choroidal vascularity index
The analysis of CVI showed a significant reduction in the SDD and the CD group compared to controls (p = 0.0001). Post-hoc analysis revealed a significant reduction of CVI in the SDD versus the control group (p = 0.0002), in the CD versus the control group (p = 0.001), and in the SDD versus the CD group (p = 0.006). Covariance analysis of LA showed a significant difference between the three groups (p = 0.033), while no statistical differences were detected in the analysis of TA between the three groups (p = 0.106).
Post hoc analysis performed on the LA and TA showed no differences between the CD and SDD with respect to controls. Direct comparison between CD and SDD showed a significant reduction of LA and TA in the SDD group. All results are summarized in Table 2.
Table 2.
Choroidal vascularity index analysis in eyes with subretinal drusenoid deposits (SDD), conventional drusen (CD), and control group.
| SDD (n = 23) | CD (n = 22) | P-valuea | Control group (n = 24) | P-valueb | P-valuec | P-valued | |
|---|---|---|---|---|---|---|---|
| Luminal Area | 0.690 ± 0.20 | 0.907 ± 0.33 | 0.010e* | 0.861 ± 0.36 | 0.033f* | 0.114e | 0.273e |
| Total Area | 1.101 ± 0.33 | 1.379 ± 0.48 | 0.042e* | 1.260 ± 0.55 | 0.106f | 0.608e | 0.118e |
| CVI | 0.627 ± 0.03 | 0.655 ± 0.04 | 0.006e* | 0.687 ± 0.02 | 0.0001f* | 0.0002e* | 0.001e* |
SDD subretinal drusenoid deposits, CD conventional drusen, CVI choroidal vascularity index.
aComparison of eyes with SDD versus eyes with CD.
bComparison of eyes with SDD, eyes with CD, and control group eyes (control group).
cComparison of eyes with SDD and control group eyes (control group).
dComparison of eyes with CD and control group eyes (control group).
ePost hoc Tukey HSD, multiple comparison between groups.
fANCOVA, adjusted for age, BCVA and spherical equivalent.
*Statistically significant (p < 0.05).
Discussion
In the present study we found that in early AMD, the CVI in patients with pure phenotypes of SDD or CD was reduced with respect to controls, and CVI was significantly reduced in SDD eyes with respect to CD eyes.
Previous studies included mixed phenotypes and SDD were evaluated in association with soft drusen [23, 24]. Keenan et al. described groups of patients with increasing severity of AMD and found that AMD status was significantly associated with CVI. However, they found that eyes with large drusen >125 µm had a higher CVI index with respect to eyes with no or small drusen ≤125 µm. They hypothesized that there could be a first “compensatory” phase when drusen start forming and CVI may increase owing to inflammatory changes or engorgement of vasculature but, as disease progresses, in what they defined as the “second phase”, there is decreased CVI. Indeed, they reported that eyes with SDD (regardless of the presence of large drusen) had a lower CVI with respect to eyes with no or early AMD [24]. In contrast, Velaga et al. found a higher CVI in subjects with non-neovascular AMD associated with SDD compared with healthy control subjects and with non-neovascular AMD eyes without SDD [23]. A likely explanation for the discrepant results of Velaga et al. with respect to the literature is the study population and differences in patient recruitment criteria.
We are aware of three studies in the literature with similar designs to our investigation [25–27]. Corvi et al. performed a longitudinal, retrospective study on CVI in patients with drusen alone, SDD alone, and healthy controls. They found that the mean luminal, stromal, and total choroidal areas were reduced in the SDD group with respect to the drusen and control groups. While we did not find any difference between the TA between the three arms of our study, we found a significant difference in the LA of the three groups. Corvi et al. found a significantly lower CVI in the SDD group with respect to the drusen and control groups, and suggested that the vascular area is less represented in SDD. They also found reduced CVI in eyes with drusen with respect to controls although this did not reach statistical significance [25]. This is in agreement with our results where we found a significant reduction of CVI of the SDD versus the CD group, but we also found reduced CVI of the CD group versus controls. Querques et al. found choroidal atrophy and fibrosis [31] and Ueda-Arakawa et al. [32] found thinned choroidal vessels in eyes with SDD further corroborating the concept of pronounced choroidal impairment in eyes with SDD and supporting the results of a lower LA in SDD eyes reported by Corvi et al. and our study [25]. Zheng et al. found a relationship between choroidal thickness and CVI in nonexudative age-related macular degeneration independent of the presence of SDD [33]. The impairment of the choroidal circulation in AMD was reported by Berenberg et al. who showed an association between reduced choroidal blood flow and drusen load [34]. Based on histological studies on AMD eyes, primary damage is hypothesized to initiate at the choroidal level [35, 36]. Thus, it is reasonable to assume that CVI is reduced in both the CD and SDD groups with respect to controls with a more pronounced reduction in eyes with SDD.
Sacconi et al. recently performed a study where patients were grouped in eyes with intermediate AMD with drusen only, eyes with SDD only, eyes with geographic atrophy, and control eyes [26]. These authors found significant reduction of the CVI and LA in the SDD group with respect to controls, similar to our results and those of Corvi et al. [25] We are in agreement with their hypothesis that the choroid may undergo stromal replacement especially in the SDD group leading to a faster progression to advanced AMD. Interestingly, Sacconi et al. found that the CVI had a different distribution among their cohorts; the geographic atrophy group showed a lower CVI in comparison to the other groups. They concluded that the LA was progressively more impaired in increasing severity from healthy controls to eyes with drusen, to eyes with SDD, and finally to eyes with geographic atrophy [26]. We are in agreement with their hypothesis as we found a lower CVI in the CD group with respect to controls, and a lower CVI in the SDD group with respect to the CD group indicating that CVI can be correlated to worsening AMD stages linked to phenotype, indicating progression risk. Thus, CVI could have prognostic implications in AMD. Choroidal alterations in different AMD cohorts were reported by Borelli et al. who found choriocapillaris flow alterations in the areas underlying, and in proximity of drusen [37], this was shown to be more pronounced in eyes with SDD with respect to drusen alone [38].
Lains et al. in a large sample sized study used swept source OCT to evaluate 88 eyes with classic drusen alone in intermediate AMD, 10 eyes with SDD alone, and 84 eyes in a mixed group of CD and SDD deposits. These authors found that eyes with SDD showed a significantly reduced choroidal vessel volume as compared eyes without SDD [28]. Although, we are in agreement that SDD have a greater impact on choroidal vascularization, we cannot directly compare our results as we did not use swept source OCT and also because our cohorts were homogenous in size whereas the group of pure SDD eyes in the study by Lains et al. was minimally represented.
The strengths of our study include the prospective design and strict inclusion criteria in homogenous cohorts selected by detailed phenotypic characterization of eyes allowing for comparison of pure groups of eyes with either CD or SDD. The main limitation was the small sample size but considering our distribution of eyes with CD or SDD in distinct pure cohorts based on rigid exclusion criteria for mixed phenotypes, we obtained significant results that contribute to the current literature. A further limitation of our study was that we did not analyse pigmentary alterations of the fundus as such changes, together with the number and size of drusen, are also used for staging disease severity. The focus of our study was the comparison of patients with SDD or CD where a combination of NIR and SDOCT are the gold standard. Recent additions to some commercially available SDOCT devices are segmentation algorithms to discern the position of Bruch’s membrane and the RPE layer. This method enables quantification of areas of RPE elevation to determine drusen number, area and volume in order to track modifications in a longitudinal manner, although small drusen do not elevate the RPE layer sufficiently to be captured by automatic segmentation [39–41]. We did not use automated software for calculation of drusen area and load, although this could have been of value, as the instrument used in our study was not equipped with the appropriate software. Furthermore, to our knowledge, automated software has not been validated in SDD load calculation. We did not evaluate smoking in the patients examined and this could possibly be a limitation even though there is no unanimous consensus on the effect of smoking on choroidal thickness [42–44]. However, recently Wei et al. reported that although the choroidal thickness did not show change, the choroidal vascular index decreased in smokers in a dose-dependent manner [45].
Optical coherence tomography (OCTA) is a functional extension of structural SDOCT where motion contrast is detected with repeated B-scans in order to produce high contrast and well- defined images of retinal capillary plexuses and the choriocapillaris microvasculature [46, 47]. However, given that the choriocapillaris is thin, possible errors in segmentation can result in the segmented region missing the choriocapillaris. Furthermore, flow in the deeper layers of the choroid cannot be detected mainly because of scattering by the pigment in the RPE and projection artefacts on deeper choroidal layers due to the blood flow in the choriocapillaris. Advances in imaging technology using swept source OCT seem to offer better visualization of the deeper choroid as there is less sensitivity loss with depth, although larger vessels are not clearly visible unless there are atrophic phenomenon of the RPE and choriocapillaris [48, 49]. It must be considered, however, that although choroidal structure is predominantly vascular, it is also composed of a connective structure, nerves, melanocytes, and extracellular fluid. OCTA provides visualization of the vascular component whereas CVI enables to evaluate both the vascular lumen area and stromal component of the choroid.
In conclusion, we found that in early AMD the CVI in patients with pure phenotypes of SDD or CD was reduced with respect to controls, and CVI was significantly reduced in SDD with respect to CD eyes. Our results are comparable to the studies with recruitment criteria similar to our investigation that eyes with SDD have more pronounced choroidal vascular alterations. SDD is a biomarker of AMD progression and this work suggests that the mechanism for this is via reduction of the CVI. Further studies on larger patient populations are warranted to corroborate this hypothesis.
Summary table
What was known before
Subretinal drusenoid deposits are correlated to choroidal thinning and progression to advanced age-related macular degeneration
Choroidal vascularity index is a sensitive parameter to detect choroidal alterations in age-related macular degeneration as it does not undergo variations with changes in mean choroidal thickness and aging
Results on choroidal vascularity index in age-related macular degeneration and the presence of subretinal drusenoid deposits are contrasting mainly owing to the inclusion of mixed phenotypes rather than cohorts of patients with pure deposits.
What this study adds
In early age-related macular degeneration choroidal vascularity index is reduced with a significant reduction in eyes with subretinal drusenoid deposits alone with respect to eyes with conventional drusen alone
Subretinal drusenoid deposits are a biomarker of age-related macular degeneration progression and the mechanism for this could be via reduction of the choroidal vascularity index.
Author contributions
SA, MDP, and AJL made substantial contributions to the conception and design of the work; ES and MC contributed to the acquisition of data; ES, MC, MDP, and SA contributed to analysis of data; SA, MDP, and ES contributed to in interpretation of data; SA, MDP, ES, MC, and AJL drafted the work, SA, MDP, and AJL revised the work critically for important intellectual content; all the authors approved the final version of the manuscript to be published.
Data availability
Data is available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colijn JM, Buitendijk GHS, Prokofyeva E, Alves D, Cachulo ML, Khawaja AP, et al. Prevalence of age-related macular degeneration in europe. The past and the future. Ophthalmology. 2017;124:1753–63. doi: 10.1016/j.ophtha.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augood CA, Vingerling JR, De Jong TVM, Chakravarthy U, Seland J, Soubrane G, et al. Prevalence of age-related maculopathy in older Europeans, The European Eye Study (EUREYE) Arch Ophthalmol. 2006;124:529–35. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Piermarocchi S, Segato T, Scopa P, Masetto M, Ceca S, Cavarzeran F, et al. for the PAMDI Study Group. The prevalence of age-related macular degeneration in Italy (PAMDI) Study: Report 1. Ophthalmic Epidemiol. 2011;18:129–36. doi: 10.3109/09286586.2011.574334. [DOI] [PubMed] [Google Scholar]
- 4.Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet GlobHealth. 2014;2:e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich R, Harris A, Kheradiya NS, Winston DM, Ciulla TA, Wirostko B. Age-related macular degeneration and the aging eye. Clin Inter Aging. 2008;3:473–82. doi: 10.2147/CIA.S2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirco KR, Sohn EH, Stone EM, Tucker BA, Mullins RF. Structural and molecular changes in the aging choroid: Implications for age-related macular degeneration. Eye. 2017;31:10–25. doi: 10.1038/eye.2016.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdolrahimzadeh S, Parisi F, Scavella V, Recupero SM. Optical coherence tomography evidence on the correlation of choroidal thickness and age with vascularized retinal layers in normal eyes. Retina. 2016;36:2329–38. doi: 10.1097/IAE.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 9.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2011;30:1441–54. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivaprasad S, Bird A, Nitiahpapand R, Nicholson L, Hykin P, Chatrizalli I. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016;16:521–37. doi: 10.1016/j.survophthal.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Spaide RF, Ooto S, Curcio CA. Subretinal drusenodi deposits AKA pseudodrusen. Surv Ophthlmol. 2018;63:782–815. doi: 10.1016/j.survophthal.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Sigler EJ, Randolph JC. Comparison of macular choroidal thickness among patients older than age 65 with early atrophic age-related macular degeneration and normals. Investig Ophthalmol Vis Sci. 2013;54:6307–13. doi: 10.1167/iovs.13-12653. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Lee DH, Lee JY, Yoon YH. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Investig Ophthalmol Vis Sci. 2013;54:7812–8. doi: 10.1167/iovs.13-12284. [DOI] [PubMed] [Google Scholar]
- 14.Yiu G, Chiu SJ, Petrou PA, Stinnett S, Sarin N, Farsiu S, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159:617–26. doi: 10.1016/j.ajo.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Jonas JB, Forster TM, Steinmetz P, Schlichtenbrede FC, Harder BC. Choroidal thickness in age-related macular degeneration. Retina. 2014;34:1149–55. doi: 10.1097/IAE.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 16.Wood A, Binns A, Margrain T, Drexler W, Povazay B, Esmaeelpour M, et al. Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol. 2011;152:1030–8.e2. doi: 10.1016/j.ajo.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Tan KA, Gupta P, Agarwal A, Chhablani J, Cheng CY, Keane PA, et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol. 2016;61:566–81. doi: 10.1016/j.survophthal.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Ooto S, Hangai M, Yoshimura N. Effects of sex and age on the normal retinal and choroidal structures on optical coherence tomography. Curr Eye Res. 2015;40:213–25. doi: 10.3109/02713683.2014.952828. [DOI] [PubMed] [Google Scholar]
- 19.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117:1775–81. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159:1123–31.e1. doi: 10.1016/j.ajo.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Dai Y, Shi Y, Russell JF, Liu C, Noorikolouri J, et al. Age-related changes in choroidal thickness and the volume of vessels and stroma using swept-source OCT and fully automated algorithms. Ophthalmol Retin. 2020;4:204–15. doi: 10.1016/j.oret.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breher K, Terry L, Bower T, Wahl S. Choroidal Biomarkers: A repeatability and topographical comparison of choroidal thickness and choroidal vascularity index in healthy eyes. Transl Vis Sci Technol. 2020;9:8. doi: 10.1167/tvst.9.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velaga SB, Nittala MG, Vupparaboina KK, Jana S, Chhablani J, Haines J, et al. Choroidal vascularity index and choroidal thickness in eyes with reticular pseudodrusen. Retina. 2020;40:612–7. doi: 10.1097/IAE.0000000000002667. [DOI] [PubMed] [Google Scholar]
- 24.Keenan TG, Klein B, Agròn E, Chew EY, Cukras CA, Wong WT. choroidal thickness and vascularity vary with disease severity and subretinal drusenoid deposit presence in non advanced age-related macular degeneration. Retina. 2020;40:632–42. doi: 10.1097/IAE.0000000000002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corvi F, Souied EH, Capuano V, Costanzo E, Bennati L, Querques L, et al. Choroidal structure in eyes with drusen and reticular pseudodrusen determined by binarisation of optical coherence tomographic images. Br J Ophthalmol. 2017;101:348–52. doi: 10.1136/bjophthalmol-2016-308548. [DOI] [PubMed] [Google Scholar]
- 26.Sacconi R, Vella G, Battista M, Borrelli E, Balasubramanian S, Querques L, et al. choroidal vascularity index in different cohorts of dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10:26. doi: 10.1167/tvst.10.12.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viggiano P, Toto L, Ferro G, Evangelista F, Porreca A, Mastropasqua R. Choroidal structural changes in different intermediate AMD patterns. Eur J Ophthalmol. 2021. 10.1177/1120672121992009. [DOI] [PubMed]
- 28.Lains I, Wang J, Providencia J, Mach S, Gil P, Gil J, et al. Choroidal changes associated with subretinal drusenoid deposits in age-related macular degeneration using swept-source optical coherence tomography. Am J Ophthalmol. 2017;180:55–63. doi: 10.1016/j.ajo.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Zweifel SA, Spaide RF, Curcio CA, Malek G, Mamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–12. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Spaide RF, Yannuzzi L, Freund KB, Mullins R, Stone E. Eyes with subretinal drusenoid deposits and no drusen: Progression of macular findings. Retina. 2019;39:12–26. doi: 10.1097/IAE.0000000000002362. [DOI] [PubMed] [Google Scholar]
- 31.Querques G, Querques L, Forte R, Massamba N, Coscas F, Souied EH. Choroidal changes associated with reticular pseudodrusen. Investig Ophthalmol Vis Sci. 2012;53:1258–63. doi: 10.1167/iovs.11-8907. [DOI] [PubMed] [Google Scholar]
- 32.Ueda-Arakawa N, Ooto S, Ellabban AA. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol. 2014;157:994–1004. doi: 10.1016/j.ajo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Zheng F, Gregori G, Schaal KB, Legarreta AD, Miller AR, Roisman L, et al. Choroidal thickness and choroidal vessel density in nonexudative age-related macular degeneration using swept-source optical coherence tomography imaging. Investig Ophthalmol Vis Sci. 2016;57:6256–64. doi: 10.1167/iovs.16-20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berenberg TL, Metelitsina TI, Madow B, Dai Y, Ying GS, Dupont JC, et al. The association between drusen extent and foveolar choroidal blood flow in age-related macular degeneration. Retina. 2012;32:25–31. doi: 10.1097/IAE.0b013e3182150483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35:2562–73. doi: 10.1016/j.neurobiolaging.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Huisingh C, Messinger J, Dolz-Marco R, Ferrara D, Freund KB, et al. Histology of geographic atrophy secondary to age-related macular degeneration a multilayer approach. Retina. 2018;38:1937–53. doi: 10.1097/IAE.0000000000002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrelli E, Shi Y, Uji A, Balasubramanian S, Nassisi M, Sarraf D, et al. Topographic analysis of the choriocapillaris in intermediate age related macular degeneration. Am J Ophthalmol. 2018;196:34–43. doi: 10.1016/j.ajo.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2017;174:42–55. doi: 10.1016/j.ajo.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlanitz FG, Ahlers C, Sacu S, Schutze C, Rodriguez M, Schriefl S, et al. Performance of drusen detection by spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2010;51:6715–21. doi: 10.1167/iovs.10-5288. [DOI] [PubMed] [Google Scholar]
- 40.Nathoo NA, Or C, Young M, Chui L, Fallah N, Kirker AW, et al. Optical coherence tomography–based measurement of drusen load predicts development of advanced age-related macular degeneration. Am J Ophthalmol. 2014;158:757–61. doi: 10.1016/j.ajo.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Diniz B, Ribeiro R, Heussen FM, Maia M, Sadda S. Drusen measurements comparison by fundus photograph manual delineation versus optical coherence tomography retinal pigment epithelial segmentation automated analysis. Retina. 2014;34:55–62. doi: 10.1097/IAE.0b013e31829d0015. [DOI] [PubMed] [Google Scholar]
- 42.Yang TK, Huang XG, Yao JY. Effects of cigarette smoking on retinal and choroidal thickness: a systematic review and meta-analysis. J Ophthalmol. 2019;2019:8079127. [DOI] [PMC free article] [PubMed]
- 43.El-Shazly AAE, Farweez YAT, Elzankalony YA, Elewa LS, Farweez BAT. Effect of smoking on macular function and structure in active smokers versus passive smokers. Retina. 2018;38:1031–40. doi: 10.1097/IAE.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 44.Kantarci FA, Tatar MG, Colak HN, Uslu H, Yildirim A, Goker H, et al. A pilot study of choroidal thickness in long-term smokers. Retina. 2016;36:986–91. doi: 10.1097/IAE.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Kumar S, Ding J, Khandelwal N, Agarwal M, Agrawal R. Choroidal structural changes in smokers measured using choroidal vascularity index. Investig Ophthalmol Vis Sci. 2019;60:1316–20. doi: 10.1167/iovs.18-25764. [DOI] [PubMed] [Google Scholar]
- 46.Braaf B, Vermeer KA, Vienola KV, de Boer JF. Angiography of the retina and the choroid with phase-resolved OCT using interval-optimized backstitched B-scans. Opt Express. 2012;20:20516–34. doi: 10.1364/OE.20.020516. [DOI] [PubMed] [Google Scholar]
- 47.Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One. 2013;8:e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130–55. doi: 10.1016/j.preteyeres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Spaide RF, Fujimotob JG, Waheedc NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


