Abstract
Using interleukin-10 (IL-10)-deficient (IL-10−/−) mice, previous studies revealed a pathological immune response after infection with Trypanosoma cruzi that is associated with CD4+ T cells and overproduction of proinflammatory cytokines. In this study we further investigate the pathology and potential mediators for the mortality in infected animals. T. cruzi-infected IL-10−/− mice showed reduced parasitemia accompanied by increased systemic release of gamma interferon (IFN-γ), IL-12, and reactive nitrogen intermediates and overproduction of tumor necrosis factor alpha (TNF-α). Despite this early resistance, IL-10−/− mice died within the third week of infection, whereas all control mice survived acute infection. The clinical manifestation with weight loss, hypothermia, hypoglycemia, hyperkalemia, and increased liver-derived enzymes in the blood together with hepatic necrosis and intravascular coagulation in moribund mice indicated a toxic shock-like syndrome, possibly mediated by the systemic TNF-α overproduction. Indeed, high production of systemic TNF-α significantly correlated with mortality, and moribund mice died with critically high TNF-α concentrations in the blood. Consequent treatment with anti-TNF-α antiserum attenuated pathological changes in T. cruzi-infected IL-10−/− mice and significantly prolonged survival; the mice died during the fourth week postinfection, again with a striking correlation between regaining high systemic TNF-α concentrations and the time of death. Since elevated serum IL-12 and IFN-γ concentrations were not affected by the administration of antiserum, these studies suggest that TNF-α is the direct mediator of this toxic shock syndrome. In conclusion, induction of endogenous IL-10 during experimentally induced Chagas' disease seems to be crucial for counterregulating an overshooting proinflammatory cytokine response resulting in TNF-α-mediated toxic shock.
Interleukin-10 (IL-10) is an anti-inflammatory cytokine that has immunosuppressive functions through downregulation of gamma interferon (IFN-γ) production (12) and macrophage activation in vitro, e.g., cytokine production (16), generation of nitric oxide (NO) (5), and surface expression of major histocompatibility complex class II (14) and costimulatory molecules (15), also influencing NK cell activation and T-cell effector functions.
Efficient stimulation of these cells during innate and adaptive immunity is critical in the control and elimination of pathogens in many infectious-disease models. In order to mount effective microbicidal effector functions, macrophages have to be stimulated by IFN-γ produced by IL-12-activated NK cells and T cells. Therefore, neutralization of endogenous IL-10 in vivo increases resistance to infection with certain pathogens, as demonstrated for Candida albicans (37), Mycobacterium avium (13), Listeria monocytogenes (44), and Trypanosoma cruzi (35) infections. After the infection of IL-10-deficient (IL-10−/−) mice with L. monocytogenes, the absence of endogenous IL-10 increases the inflammatory cytokine responses (e.g., IL-12, IFN-γ, IL-1α, and tumor necrosis factor alpha [TNF-α]) of macrophages and NK cells in innate immunity and positively influences Th1-cell responses in acquired immunity, thus leading to reduced susceptibility (11). In contrast to this IL-10-dependent susceptibility, endogenous IL-10 is protective in lipopolysaccharide (LPS)- or staphylococcal enterotoxin B-induced endotoxic shock in mice (17, 20). Consistently, IL-10−/− mice are extremely susceptible to LPS-induced mortality (3).
In agreement with the dual role of IL-10, infection of IL-10−/− mice with the protozoan parasite T. cruzi resulted in a reduced parasitemia but caused increased mortality (1, 22). Elevated systemic levels of IL-12, IFN-γ, and TNF-α have been found, and neutralization of IL-12 as well as depletion of CD4+ T cells delayed mortality. This illustrates that under certain conditions IL-10 prevents the development of a pathological immune response, which seems to be associated with CD4+ T cells and overproduction of IFN-γ.
To gain more insights into this protective role of IL-10, we analyzed the immunopathological consequences of the absence of endogenous IL-10 during experimentally induced Chagas' disease. Infection of IL-10−/− mice with T. cruzi caused a systemic proinflammatory host response with a TNF-α-mediated pathology which is similar to toxic shock and responsible for the observed mortality in the absence of IL-10.
MATERIALS AND METHODS
Mice.
Breeding pairs of IL-10−/− mice (129sv × C57BL/6) (23) were kindly provided by W. Müller (Cologne, Germany). After embryo transfer into our specific-pathogen-free (SPF) animal facility (Max-Planck-Institute for Immunobiology, Freiburg, Germany), the mice were backcrossed to C57BL/6 mice for eight generations. Six- to 10-week-old homozygous IL-10−/− mice and their heterozygous (IL-10+/−) littermates were used for experiments. The mice were kept in filter cap cages during the infection studies.
T. cruzi infection.
The reticulotropic T. cruzi strain MHOM/CH/00/Tulahuen C2 (34) was routinely maintained in mice, and trypomastigotes for infection studies were isolated as recently described (21). For recording of parasitemia and preparation of plasma, blood was collected in heparinized tubes every 3 to 4 days. The resulting parasitemia was determined by hemacytometer counting of phosphate-buffered saline–1% glucose-diluted tail vein blood, and mortality was monitored daily. An infection dose of 500 blood trypomastigotes was used to induce a detectable inflammatory-cytokine response in wild-type mice. For determining parasitemia and mortality, a dose below the 50% lethal dose of 75 parasites was generally used.
Histopathological analyses.
Between days 14 and 17, infected moribund IL-10−/− mice and their heterozygous littermates were killed by cervical dislocation. Tissue specimens of liver and lung were collected and fixed in paraformaldehyde (4% in phosphate-buffered saline) for further processing. The paraffin-embedded tissues were cut in 4-μm-thick sections, stained by hematoxylin and eosin, and subjected to microscope analysis.
Measurement of body weights and body temperatures of mice after infection with T. cruzi.
The body weights of mice infected with T. cruzi were scored daily with a laboratory scale which was supplied by Mettler (Giessen, Germany). Rectal body temperature was measured daily with a temperature measuring unit (Testo 110) obtained from Fisher Scientific (Ulm, Germany).
Determination of cytokines, NO, liver-derived enzymes, glucose, and potassium in plasma of T. cruzi-infected mice.
Cytokine levels in plasma were analyzed in threefold serial dilutions using a two-site sandwich enzyme-linked immunosorbent assay employing purified and biotinylated antibodies for IFN-γ, TNF-α, and IL-12 obtained from Pharmingen (San Diego, Calif.). After incubation with alkaline phosphatase coupled to streptavidin supplied by Southern Biotechnology (Birmingham, Ala.) and developed with p-nitrophenyl phosphate purchased from Sigma, the absorbance was read on a microplate reader (MR 600) from Dynatech Scientific Inc. (Cambridge, Mass.). Using a test wavelength of 405 nm and a reference wavelength of 495 nm, samples were compared to appropriate standards in threefold serial dilutions. Recombinant murine cytokines were purchased from Pharmingen. The detection limits of cytokines were as follows: IFN-γ, 0.1 ng/ml; TNF-α, 0.01 ng/ml; and IL-12, 0.02 ng/ml.
For quantification of NO in individual plasma samples, the Griess reaction was adapted as described previously (36). Briefly, 50-μl volumes of serial dilutions of plasma samples and sodium nitrate (1 μM to 1 mM) as standards were made in normal mouse plasma in 96-well microtiter plates, which were purchased from Nunc. Twenty microliters of freshly prepared reaction solution (1.8 μg of NADPH/ml and 2.5 μU of nitrate reductase/ml) purchased from Boehringer (Mannheim, Germany) was added, and the plates were incubated at room temperature for 20 min, after which 50 μl of freshly prepared Griess reagent [1% sulfanilamide and 0.1% N-(1-naphthyl) ethylene diamine; Sigma] and 80 μl of 10% trichloroacetic acid were added. After centrifugation, the optical densities of the supernatants were read at 550 nm with a reference wavelength of 630 nm. The detection limit of NO was 1.5 μM.
Levels of the liver-derived enzymes glutamine oxaloacetic transaminase (GOT) and glutamine pyruvate transaminase (GPT), glucose, or potassium in the plasma of T. cruzi-infected mice were determined in the laboratories of the University Hospital, Freiburg, Germany, using standard methods (2, 6, 38).
In vivo neutralization of TNF-α.
Neutralizing sheep anti-murine TNF-α antiserum and sheep control serum was kindly provided by Chris Galanos (Max-Planck-Institute for Immunobiology). In the D-galactosamine–endotoxin model, a single injection of 0.7 μl of this antiserum still protects mice from a 10-fold lethal dose of LPS (C. Galanos, personal communication). Ten microliters of the antiserum (corresponding to a 14-fold anti-TNF-α concentration) was injected intravenously into a tail vein 1 day prior to infection and twice a week during infection.
Statistical analysis.
The nonparametric Mann-Whitney and Wilcoxon U test that was used in the endotoxin model were used to evaluate significant differences between experimental groups. For determining correlation, a linear regression analysis was performed, and the respective r values were calculated.
RESULTS
Infection of IL-10−/− mice with T. cruzi is lethal despite reduced parasitemia and increased inflammatory response.
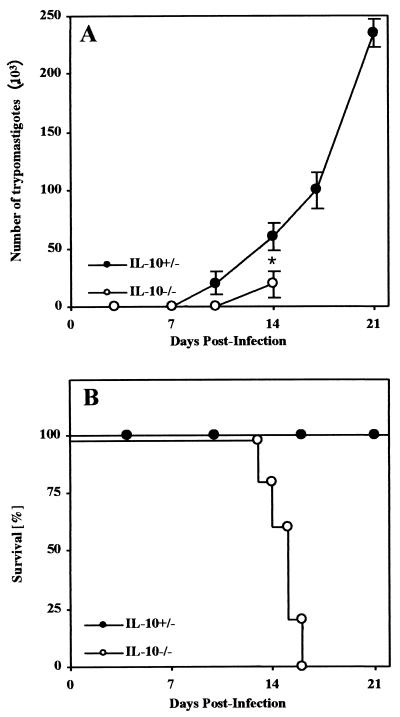
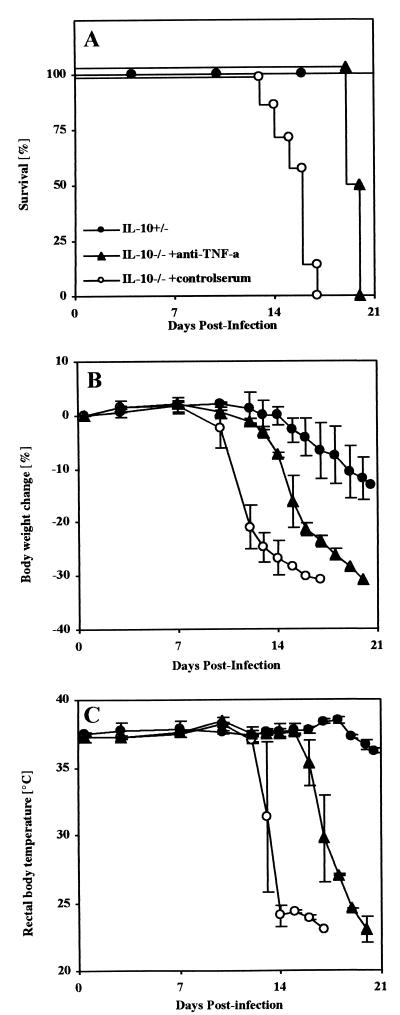
Mice deficient in IL-10 and their heterozygous littermates were infected intraperitoneally (i.p.) with a normally sublethal dose of 50 blood trypomastigotes of the Tulahuen strain of T. cruzi. IL-10−/− mice showed significantly reduced parasitemia compared to control mice (Fig. 1A). Nevertheless, all T. cruzi-infected IL-10−/− mice succumbed between day 14 and day 17 postinfection, whereas all control mice survived the acute infection (Fig. 1B).
FIG. 1.
IL-10−/− mice succumbed to infection with a regular sublethal dose of T. cruzi. Groups of five mice were infected i.p. with 50 blood trypomastigotes of the Tulahuen strain, and parasitemia was recorded twice a week. Shown are the kinetics of parasitemia (A) and survival (B). The means and standard deviations of one representative of three independent experiments are shown. ∗, significantly different from values of heterozygous littermates (P < 0.05; Mann-Whitney and U Wilcoxon test).
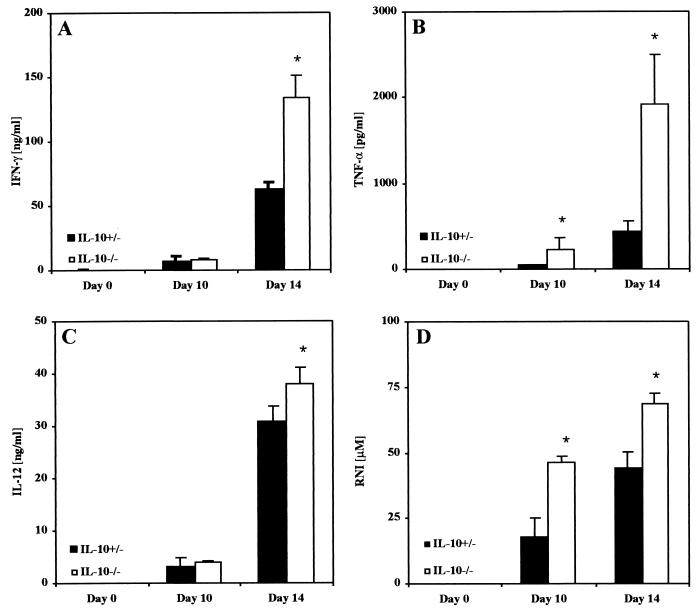
After infection with 500 blood trypomastigotes, T. cruzi induces a systemic inflammatory response with relatively large amounts of IFN-γ, TNF-α, IL-12, and reactive nitrogen intermediates (RNI) in the blood of control mice (Fig. 2). Compared to control mice, we observed significantly larger amounts of TNF-α and RNI in the blood of infected IL-10−/− mice as early as day 10 after infection (Fig. 2B and D). At day 14, shortly before the mice succumbed to the infection, the concentrations of IFN-γ, TNF-α, IL-12, and RNI were all increased (Fig. 2). The systemic overproduction of TNF-α (up to 2,500 pg/ml) is potentially able to induce a toxic shock-like syndrome in IL-10−/− mice.
FIG. 2.
In IL-10−/− mice, production of generalized proinflammatory cytokine and NO is exacerbated after infection with T. cruzi. Groups of five mice were infected with 500 trypomastigotes of the Tulahuen strain. IL-10−/− mice (open bars) or heterozygous littermates (solid bars) were bled prior to infection and at day 10 and day 14 postinfection. The concentrations of IFN-γ (A), TNF-γ (B), IL-12 (C), and RNI (D) in the plasma were determined as described in Materials and Methods. The results are presented as the mean of individually analyzed mice, including the standard deviation. One representative of three independent experiments is shown. ∗, significantly different from values of heterozygous littermates (P < 0.05; Mann-Whitney and Wilcoxon U test).
Pathophysiology of T. cruzi-infected IL-10−/− mice is similar to toxic shock syndrome.
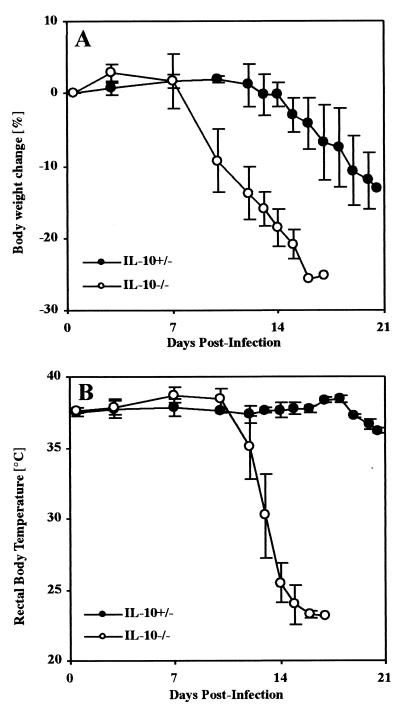
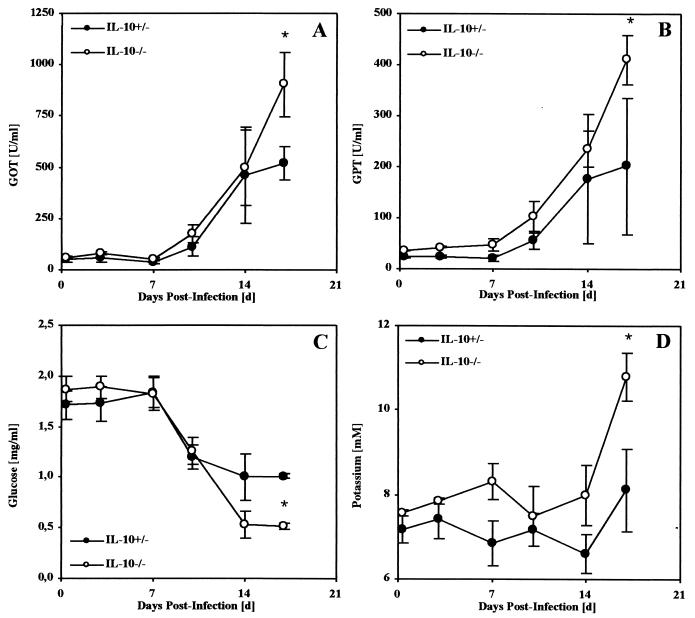
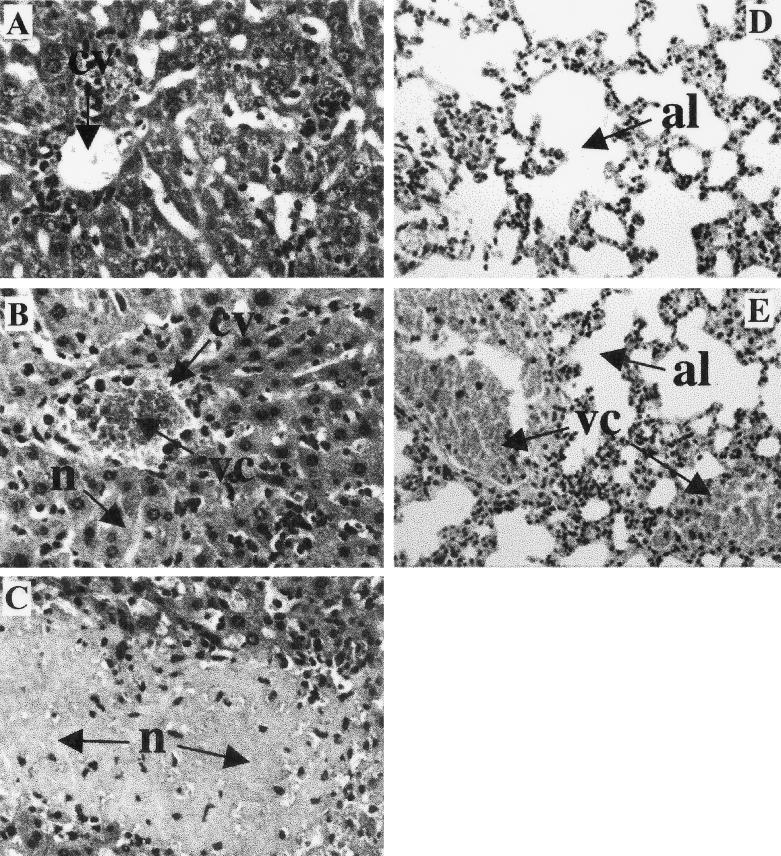
In TNF-α-mediated toxic shock, microvascular changes like vasodilatation and vascular coagulation can lead to multiple organ failure, especially of the lungs, liver, and kidneys (31, 39). In murine models of toxic shock, body weight loss, hypothermia, increased levels of liver-derived enzymes in serum, hypoglycemia, and hyperkalemia characterize this state (24, 25, 40). To further investigate a causative role of TNF-α for the observed mortality, these pathological parameters were determined in mice after infection with 50 blood trypomastigotes of T. cruzi. Infected control mice showed a maximal body weight loss of 13% (Fig. 3A) and a constant body temperature of 37°C (Fig. 3B). In contrast, infection of IL-10−/− mice with T. cruzi resulted in a body weight loss of 25% (Fig. 3A) and a loss of body temperature to less than 25°C (hypothermia) (Fig. 3B), and the mice succumbed during the third week of infection. Moreover, rising serum levels of GOT and GPT (Fig. 4A and B), hypoglycemia (Fig. 4C), and profound hyperkalemia (Fig. 4D) were observed in moribund mice (Fig. 4), symptomatic of hepatic and renal failure. Moribund IL-10−/− mice and the infected but vital control mice were sacrificed and analyzed for histopathology. Both groups showed several foci of inflammation within the heart (data not shown) and liver (Fig. 5) containing T. cruzi amastigotes. Lower numbers of amastigotes were present in infected organs of IL-10−/− mice, consistent with the observed decreased parasitemia. Nevertheless, inflammation was more prominent in the livers of IL-10−/− mice (Fig. 5B and C) and, in contrast to that in T. cruzi-infected control mice (Fig. 5A), was accompanied by vascular coagulation (Fig. 5B) as well as focal and centroacinar hepatocyte necrosis (Fig. 5C). Whereas the lungs of control mice revealed no pathological changes after infection with T. cruzi (Fig. 5D), the lungs of IL-10−/− mice showed hemorrhagic effusion and intra-alveolar coagulation (Fig. 5E). Obviously, the relatively low parasite load could not explain the severe pathology in T. cruzi-infected IL-10−/− mice. Rather, this clinical manifestation indicated a toxic shock-like syndrome (24, 25, 40), which could be mediated by the systemic overproduction of TNF-α
FIG. 3.
T. cruzi-infected IL-10−/− mice develop a profound body weight loss and hypothermia. Groups of five mice were infected with 50 trypomastigotes of the Tulahuen strain, and their body weights and rectal body temperatures were measured in IL-10−/− mice (open symbols) or heterozygous-littermate controls (solid symbols). The results are presented as the mean of individually analyzed mice, including the standard deviation. The means and standard deviations of one representative of two independent experiments are shown.
FIG. 4.
Blood of T. cruzi-infected IL-10−/− mice contains enhanced levels of liver-derived enzymes, with hypoglycemia and hyperkalemia. Groups of five mice were infected with 500 trypomastigotes of the Tulahuen strain, and their plasma was analyzed twice a week until the IL-10−/− mice died. The kinetics GOT (A), GPT (B), glucose (C), and potassium (D) were determined in IL-10−/− mice (open symbols) or heterozygous-littermate controls (solid symbols). The results are presented as the mean of individually analyzed mice, including the standard deviation. ∗, significantly different from values for heterozygous littermates (P < 0.05; Mann-Whitney and Wilcoxon U test).
FIG. 5.
Livers and lungs of T. cruzi-infected IL-10−/− mice show increased pathology. The mice were infected i.p. with 50 trypomastigotes of the Tulahuen strain, and the moribund IL-10−/− mice and the corresponding vital heterozygous controls were killed. Histological samples were stained with hematoxylin and eosin. In the livers of the infected control mice, only small granulomas without pathological changes of the central vein (cv) were observed (A), whereas the livers of infected IL-10−/− mice showed striking inflammation with vascular coagulation (vc) of central veins (B) and focal and centroacinar necrosis (n) (C). The lungs of the infected control mice showed no pathological change of alveoli (al) (D), whereas in IL-10−/− mice this organ revealed severe coagulation (vc) (E), resembling the pathology also observed in TNF-α-mediated toxic shock. (Magnification, 1:500)
Neutralization of TNF-α attenuates hypothermia and body weight loss and prolongs survival of infected IL-10−/− mice.
To examine whether TNF-α is directly involved in the toxic shock-like syndrome, IL-10−/− mice were infected with 50 blood trypomastigotes of T. cruzi and treated with anti-TNF-α antiserum, and the course of infection was followed. In contrast to T. cruzi-infected IL-10−/− mice treated with a control serum, which died between days 15 and 17 postinfection, administration of anti-TNF-α antiserum prolonged the survival time of IL-10−/− mice (Fig. 6A). TNF-α neutralization also had an effect on the clinical manifestation of the observed toxic shock-like syndrome, causing a significant delay of body weight loss (Fig. 6B) and attenuation of the observed hypothermia (Fig. 6C) in IL-10−/− mice. Nevertheless, continuous treatment could delay but not prevent mortality in IL-10−/− mice after infection with T. cruzi, and the mice eventually died between days 20 and 21 postinfection. Similar results were found in a neutralization experiment using a monoclonal rat anti-TNF-α antibody (MP6-XT3; Pharmingen) in which anti-TNF-α-treated T. cruzi-infected IL-10−/− mice died 6 days later than control animals, at days 20 and 21 postinfection.
FIG. 6.
Neutralization of TNF-α increases survival time and delays body weight loss and hypothermia in T. cruzi-infected IL-10−/− mice. Groups of two to seven mice were infected i.p. with 50 trypomastigotes of the Tulahuen strain of T. cruzi. One day prior to infection and twice a week after infection, the IL-10−/− mice were treated with either antiserum (▴) or control serum (○). Infected IL-10 heterozygous littermates (●) were included as a positive control for survival. Shown are the survival time (A), the kinetics of the body weight change (B), and the rectal body temperature (C). The results in panels B and C are presented as the mean of individually scored mice (twice a week), including the standard deviation. One representative of two independent experiments is shown.
Systemic TNF-α levels but not IFN-γ or IL-12 levels correlate with mortality.
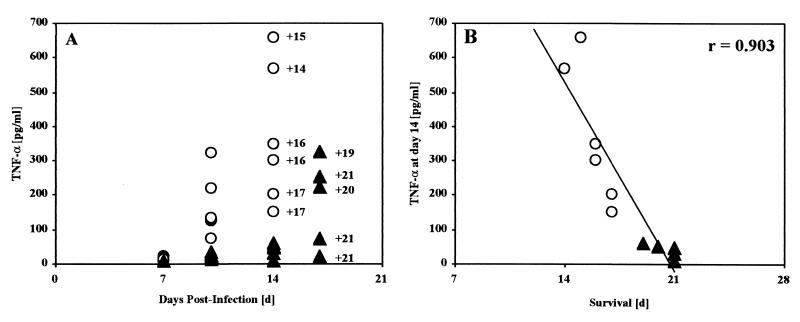
The inability of anti-TNF-α treatment to prevent death may be due to an incomplete neutralization of TNF-α or to other factors, such as IFN-γ or IL-12, contributing to the observed toxic shock-like syndrome. To investigate and distinguish between these two possibilities, neutralization studies were repeated and blood cytokine levels were determined during the infection and neutralization studies shown in Table 1. At day 14 postinfection, infected IL-10−/− mice showed only slightly increased IFN-γ and IL-12 blood levels in comparison to heterozygote control animals. Conversely, TNF-α levels were strikingly increased (37-fold) in the mice that succumbed, with an average survival time of 15.8 days (Table 1). Also importantly, there was a significant correlation between high systemic TNF-α concentration at day 14 postinfection and the time of death shortly thereafter (Fig. 7). Anti-TNF-α-treated T. cruzi-infected IL-10−/− mice showed IFN-γ or IL-12 concentrations similar to those of mice treated with control serum, but as expected from the neutralization activity of anti-TNF-α, strikingly reduced TNF-α blood levels were present at day 14 postinfection (Table 1). However, anti-TNF-α treatment became ineffective during the following week, and the mice regained high TNF-α levels, as shown on day 17 postinfection (Fig. 7A). Again, a striking correlation between high systemic TNF-α levels and the time of death was observed in anti-TNF-α-treated IL-10−/− mice (Fig. 7B). In summary, these results conclusively demonstrate that TNF-α is the direct mediator of the observed toxic shock-like syndrome in T. cruzi-infected IL-10−/− mice.
TABLE 1.
T. cruzi-induced toxicity is associated with enhanced systemic production of TNF-α
| IL-10 | Treatmenta | IL-12b (pg/ml) | IFN-γb (pg/ml) | TNF-αb (pg/ml) | Survival time (days)a |
|---|---|---|---|---|---|
| +/− | Control serum | 234 ± 210 | 494 ± 44 | 10 ± 0 | >30 |
| −/− | Control serum | 400 ± 50 | 835 ± 11 | 370 ± 200 | 15.8 ± 1.1 |
| −/− | TNF-α antiserum | 509 ± 110 | 1,060 ± 23 | 40 ± 20d | 20.5 ± 0.7d |
Five to six mice per group were infected i.p. with 50 blood trypomastigotes and treated twice a week with control serum or anti-TNF-α antiserum as described in Materials and Methods.
At day 14 postinfection, plasma cytokine concentrations of individual mice were determined. The mean values and standard deviations are shown.
Mortality of T. cruzi-infected mice was monitored daily. The mean values and standard deviations are shown.
Significant difference in mortality between TNF-α antiserum-treated IL-10−/− mice and control-serum-treated IL-10−/− mice (P < 0.05; Mann-Whitney and Wilcoxon U test).
FIG. 7.
Striking correlation between high systemic TNF-α concentrations and time of mortality. (A) Kinetics of blood TNF-α concentrations and day of mortality from the study shown in Table 1. Control-serum-treated T. cruzi-infected IL-10−/− mice (○) showed strikingly increased TNF-α concentrations during the second week postinfection. The mice developed critically high TNF-α concentrations at day 14 postinfection and died shortly thereafter (the numbers with pluses indicate the days of death). TNF-α neutralization by anti-TNF-α antiserum treatment of infected IL-10−/− mice (▴) was effective until day 14, but high TNF-α concentrations were present at day 17 and the mice died shortly thereafter. (B) Linear regression analysis revealed a striking correlation (r = 0.903) between high blood TNF-α concentration and time of death in control and antiserum-treated IL-10−/− mice.
DISCUSSION
As expected from previous studies (1, 22), infection of IL-10−/− mice with T. cruzi was fatal. Whereas wild-type mice survived the acute phase of infection, all mutant mice died during the third week postinfection, despite reduced parasitemia and increased proinflammatory cytokine response with increased systemic IFN-γ, IL-12, and TNF-α levels. In the present study, we have shown the pathological consequences of the absence of endogenous IL-10 after infection with T. cruzi and defined the direct mediator of this pathology. The observation of weight loss, hypothermia, hypoglycemia, and hyperkalemia accompanied by intravascular coagulation and hemorrhagic effusion in the liver and lungs of infected IL-10−/− mice resembles the clinical manifestation of a toxic shock-like syndrome (2, 24, 25). TNF-α is known as a direct mediator of cachexia (TNF-α-mediated toxic shock) (40) and has also been shown to be deleterious in some severe systemic inflammatory responses (4, 40, 41). Indeed, 14 days after infection with T. cruzi, several IL-10−/− mice reached critically high concentrations of systemic TNF-α (up to 650 pg/ml), and these mice succumbed within 24 h. Those mice which had increased but still tolerable TNF-α concentrations at day 14 postinfection succumbed soon after, and all of the mice showed highly significant correlations between these systemic TNF-α concentrations and their times of death (Fig. 7). Furthermore, TNF-α neutralization not only attenuated disease progression but also prolonged the survival of T. cruzi-infected IL-10−/− mice. In vivo anti-TNF-α treatment apparently lost its activity during the third week postinfection, and the mice regained high systemic TNF-α concentrations. Again, there was a significant correlation between the high systemic TNF-α concentration and time of death (Fig. 7). Together with the observed clinical manifestation of a toxic shock syndrome, these results strongly suggest that TNF-α is the direct mediator of mortality due to toxic shock in T. cruzi-infected IL-10−/− mice. This is in line with a recent study demonstrating that the endogenous balance of soluble TNF-α receptors and TNF-α modulates cachexia and mortality in mice acutely infected with T. cruzi (42). Although we used concentrations of anti-TNF-α antiserum that have been shown to completely protect mice from LPS-induced toxic shock syndrome (see Materials and Methods), it is perhaps not surprising in the context of our studies, which were comparatively long term, that such treatment had limited success. During the 3 weeks of infection, the host will undoubtedly produce allotypic antibodies which will negate the neutralizing activity of the sheep- or rat-derived antibodies used. Although we have demonstrated in these studies the paramount role of TNF-α in toxic shock-mediated death, it is likely that other factors involved in activating cells to produce TNF-α or involved in pathology also contribute to the disease outcome. Indeed, previous studies have shown that neutralization of endogenous IL-12 but not IFN-γ is also able to delay mortality in T. cruzi-infected IL-10−/− mice (22). Moreover, on a cellular level CD4+ T cells seem to play a role in early mortality in IL-10−/− mice, since SCID IL-10−/− mice (1, 22) or CD4+- but not CD8+-T-cell-depleted IL-10−/− mice (22) showed a delay in time of death. Hunter et al. also found significantly reduced serum IFN-γ levels in CD4+-T-cell-depleted mice but no reduction in the high systemic IL-12 production observed in IL-10−/− mice. They speculated from these results that a combination of IL-12 and CD4+ T cells may be required for the development of the pathological immune response observed in T. cruzi-infected IL-10−/− mice. A direct toxic effect of IL-12 as the cause for mortality cannot be ruled out, but it is rather unlikely, since neither CD4+-T-cell depletion (22) nor TNF-α neutralization (Table 1) had any significant effect on systemic IL-12 concentrations in IL-10−/− mice. Our own unpublished data indicate that both T cells and macrophages substantially contribute to the overall increased production of TNF-α. Since IL-12 is able to promote T helper cell differentiation and is also able to sensitize the host to the lethal effects of TNF-α (9), it is possible that IL-12 neutralization as well as CD4+-T-cell depletion contribute to reduced TNF-α concentration; this would explain the observed delay in time of death. Unfortunately, TNF-α levels were not measured in the IL-12 neutralization or CD4+-T-cell depletion study, leaving this hypothesis unresolved. However, IL-12-deficient mice are highly susceptible to T. cruzi infection and show a reduced TNF-α response (unpublished data), indicating that the presence of IL-12 is important for protection. Increased systemic IFN-γ levels, previously observed (22) and confirmed in this study, seemed not to be involved in the pathological effects. First, anti-IFN-γ treatment did not delay mortality (22), and second, TNF-α neutralization had no marked influence on systemic IFN-γ production (Table 1). Moreover, we recently showed that IFN-γ is necessary for macrophage NO production mediated by the inducible nitric oxide synthase which is a crucial killing effector molecule in resistance to T. cruzi (21).
Together, these results strongly suggest that during infection with T. cruzi there is a critical requirement for IL-10 to suppress systemic overproduction of TNF-α-producing cells to prevent toxic shock.
In the absence of endogenous IL-10, an enhanced susceptibility was also observed after infection with Toxoplasma gondii (19), Plasmodium chabaudi chabaudi (27, 28), or Schistosoma mansoni (45), which was also accompanied by an overproduction of proinflammatory cytokines. In contrast, IL-10−/− mice were more resistant after infection with L. monocytogenes (11), showing reduced mortality, whereas all control mice succumbed within 7 days (unpublished results). A similar resistance has been shown for IL-10−/− mice during infection with C. albicans (43) or Mycobacterium bovis BCG (33). How can we explain the contrary outcome of infection in the absence of endogenous IL-10 that seems to depend on particular properties of the infectious agent? One possible explanation could be that pathogens like T. cruzi or P. chabaudi show a pronounced parasitemia during the acute course of infection and are able to spread into every part of the host's body. This may lead to a more systemic immune response which, if not regulated by IL-10, results in a toxic overproduction of proinflammatory cytokines, like TNF-α. An equally likely determinant for the regulatory role of IL-10 during infection could be whether or not the respective pathogens themselves produce molecules that directly induce a proinflammatory cytokine response. For example, D-galactosamine-sensitized mice are susceptible to a lethal inflammatory-cytokine shock after injection of antigens derived from T. gondii tachyzoites (30). This may explain why in the absence of endogenous IL-10 even an infection with a nonpathogenic parasite strain is sufficient to induce a lethal inflammatory response (19). Pathogen-derived superantigens lead to hyperactivation of the immune system and superantigen-mediated toxic shock (18, 29, 32). It has been shown, for instance, that the polyclonal immune response after infection with T. cruzi is associated with skewed expansion of Vβ chain-expressing T cells, which may be induced by superantigenic activities associated with this parasite (8, 10, 26). Therefore, it is likely that antigens from T. cruzi directly promote the production of TNF-α by lymphocytes and macrophages. Evidence for this hypothesis arose from a recent study showing that glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from T. cruzi are indeed able to induce the production of TNF-α by macrophages (7). However, further studies are necessary to identify the mechanisms for inducing TNF-α production and the regulatory effect of IL-10 on its target cells in experimentally induced Chagas' disease. In summary, a tight counterregulation by induction of immunosuppressive endogenous IL-10 seems to be essential to prevent an otherwise lethal systemic toxic cytokine response during infection with particular pathogens like T. cruzi, whereas induction of IL-10 during infection with other pathogenic agents, like L. monocytogenes, may be rather disadvantageous for the host.
ACKNOWLEDGMENTS
We thank M. Held, F. Grünemayer, U. Stauffer, and K.-H. Widmann for excellent technical assistance; I. Neumann for performing analysis of plasma samples; J. Juritz, and G. H. Aguilar for help with statistical analysis; C. Galanos for providing antiserum; and L.-G. Bekker, J. Wood, A. Dorfmüller, G. Alber, and J. Alexander for critically reviewing the manuscript. We are also grateful to W. Müller for breeding pairs of IL-10−/− mice.
This work was supported by the Franco/South African Science and Technology Agreement (grant no. 2043232). C.H. is supported by a UCT Postdoctoral Fellowship grant. F.B. is the holder of a Wellcome Trust Senior Research Fellowship in Medical Science in South Africa.
REFERENCES
- 1.Abrahamsohn I A, Coffman R L. Trypanosoma cruzi—IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired-immunity to infection. Exp Parasitol. 1996;84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 2.Bauss F, Droge W, Mannel D N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987;55:1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg D J, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Investig. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutler B, Grau G E. Tumor necrosis factor in the pathogenesis of infectious diseases. Crit Care Med. 1993;21:423–435. [PubMed] [Google Scholar]
- 5.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandzaeg P, Ovstebo P, Kierulf P. Bacteremia and compartmentalization of LPS in meningococcal disease. In: Levin J, Alving C R, Munford R, Redl H, editors. Lipopolysaccharides from genes to therapy. New York, N.Y: Wiley; 1995. pp. 219–233. [PubMed] [Google Scholar]
- 7.Camargo M M, Almeida I C, Pereira M E, Ferguson M A, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 8.Cardoni R L, Antunez M I, Orn A, Gronvik K O. T-cell receptor v-beta repertoire in the thymus and spleen of mice infected with Trypanosoma cruzi. Cell Immunol. 1996;169:238–245. doi: 10.1006/cimm.1996.0114. [DOI] [PubMed] [Google Scholar]
- 9.Cauwels A, Fiers W, Brouckaert P. Murine IL-12 is involved in Calmette-Guerin bacillus-induced sensitization and is by itself sufficient to sensitize mice to the lethal effects of human TNF1. J Immunol. 1996;156:4686–4690. [PubMed] [Google Scholar]
- 10.Cordeiro da Silva A, Lima E C, Vicentelli M H, Minoprio P. V beta 6-bearing T cells are involved in resistance to Trypanosoma cruzi infection in XID mice. Int Immunol. 1996;8:1213–1219. doi: 10.1093/intimm/8.8.1213. [DOI] [PubMed] [Google Scholar]
- 11.Dai W J, Köhler G, Brombacher F. Both innate and acquired immunity to Listeria monocytogenes infection are increased in IL-10-deficient mice. J Immunol. 1997;158:2259–2267. [PubMed] [Google Scholar]
- 12.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 14.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 16.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 17.Florquin S, Amraoui Z, Abramowicz D, Goldman M. Systemic release and protective role of IL-10 in staphylococcal enterotoxin B-induced shock in mice. J Immunol. 1994;153:2618–2623. [PubMed] [Google Scholar]
- 18.Gaus H, Miethke T, Wagner H, Heeg K. Superantigen-induced anergy of V beta 8+ CD4+ T cells induces functional but non-proliferative T cells in vivo. Immunology. 1994;83:333–340. [PMC free article] [PubMed] [Google Scholar]
- 19.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 20.Gerard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hölscher C, Köhler G, Müller U, Mossmann H, Schaub G A, Brombacher F. Defective nitric-oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma-interferon receptor or inducible nitric-oxide synthase. Infect Immun. 1998;66:1208–1215. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter C A, Ellis-Neyes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 23.Kühn R, Lohler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 24.Leist M, Gantner F, Bohlinger I, Germann P G, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 25.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann P G, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 26.Leite-de-Moraes M C, Coutinho A, Hontebeyrie-Joskowicz M, Minoprio P, Eisen H, Bandeira A. Skewed V beta TCR repertoire of CD8+ T cells in murine Trypanosoma cruzi infection. Int Immunol. 1994;6:387–392. doi: 10.1093/intimm/6.3.387. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linke A, Kühn R, Müller W, Honarvar N, Li C, Langhorne J. Plasmodium chabaudi chabaudi—differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol. 1996;84:253–263. doi: 10.1006/expr.1996.0111. [DOI] [PubMed] [Google Scholar]
- 29.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall A J, Denkers E Y. Toxoplasma gondii triggers granulocyte-dependent cytokine-mediated lethal shock in D-galactosamine-sensitized mice. Infect Immun. 1998;66:1325–1333. doi: 10.1128/iai.66.4.1325-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayeux P R. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997;51:415–435. doi: 10.1080/00984109708984034. [DOI] [PubMed] [Google Scholar]
- 32.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray P J, Young R A. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzi T, Taliaffero W H. Connective tissue reactions in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi. J Infect Dis. 1955;96:199–215. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- 35.Reed S G, Brownell C E, Russo D M, Silva J S, Grabstein K H, Morrissey P J. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135–3140. [PubMed] [Google Scholar]
- 36.Rockett K A, Awburn M M, Rockett E J, Clark I A. Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am J Trop Med Hyg. 1994;50:735–742. doi: 10.4269/ajtmh.1994.50.735. [DOI] [PubMed] [Google Scholar]
- 37.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 38.Ruetten H, Thiemermann C, Vane J R. Effects of the endothelin receptor antagonist, SB 209670, on circulatory failure and organ injury in endotoxic shock in the anaesthetized rat. Br J Pharmacol. 1996;118:198–204. doi: 10.1111/j.1476-5381.1996.tb15386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijs L G, Groeneveld A B, Hack C E. Multiple organ failure in septic shock. Curr Top Microbiol Immunol. 1996;216:209–237. doi: 10.1007/978-3-642-80186-0_10. [DOI] [PubMed] [Google Scholar]
- 40.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J D, Zentella A, Albert J D, Shires G T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 41.Tracey K J, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993;21:415–422. [PubMed] [Google Scholar]
- 42.Truyens C, Torrico F, Lucas R, de Baetselier P, Buurman W A, Carlier Y. The endogenous balance of soluble tumor necrosis factor receptors and tumor necrosis factor modulates cachexia and mortality in mice acutely infected with Trypanosoma cruzi. Infect Immun. 1999;67:5579–5586. doi: 10.1128/iai.67.11.5579-5586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Torres A, Jones-Carson J, Wagner R D, Warner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67:670–674. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner R D, Maroushek N M, Brown J F, Czuprynski C J. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infect Immun. 1994;62:2345–2353. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wynn T A, Cheever A W, Williams M E, Hieny S, Caspar P, Kühn R, Müller W, Sher A. IL-10 regulates liver pathology in acute murine schistosomiasis-mansoni but is not required for immune down-modulation of chronic disease. J Immunol. 1998;160:4473–4480. [PubMed] [Google Scholar]