Abstract
Late-life depression occurring in older adults is common, recurrent, and malignant. It is characterized by affective symptoms, but also cognitive decline, medical comorbidity, and physical disability. This behavioral and cognitive presentation results from altered function of discrete functional brain networks and circuits. A wide range of factors across the lifespan contributes to fragility and vulnerability of those networks to dysfunction. In many cases, these factors occur earlier in life and contribute to adolescent or earlier adulthood depressive episodes, where the onset was related to adverse childhood events, maladaptive personality traits, reproductive events, or other factors. Other individuals exhibit a later-life onset characterized by medical comorbidity, pro-inflammatory processes, cerebrovascular disease, or developing neurodegenerative processes. These later-life processes may not only lead to vulnerability to the affective symptoms, but also contribute to the comorbid cognitive and physical symptoms. Importantly, repeated depressive episodes themselves may accelerate the aging process by shifting allostatic processes to dysfunctional states and increasing allostatic load through the hypothalamic–pituitary–adrenal axis and inflammatory processes. Over time, this may accelerate the path of biological aging, leading to greater brain atrophy, cognitive decline, and the development of physical decline and frailty. It is unclear whether successful treatment of depression and avoidance of recurrent episodes would shift biological aging processes back towards a more normative trajectory. However, current antidepressant treatments exhibit good efficacy for older adults, including pharmacotherapy, neuromodulation, and psychotherapy, with recent work in these areas providing new guidance on optimal treatment approaches. Moreover, there is a host of nonpharmacological treatment approaches being examined that take advantage of resiliency factors and decrease vulnerability to depression. Thus, while late-life depression is a recurrent yet highly heterogeneous disorder, better phenotypic characterization provides opportunities to better utilize a range of nonspecific and targeted interventions that can promote recovery, resilience, and maintenance of remission.
Subject terms: Depression, Pathogenesis, Prognostic markers
Introduction
Late-life depression (LLD) is major depressive disorder (MDD) occurring in adults age 60 years or older [1]. It is common, with ~5% of community-dwelling elders meeting DSM5 diagnostic criteria [2] and 10–16% of older adults exhibiting clinically significant depressive symptoms that may not meet full criteria [2, 3]. LLD is a malignant illness that increases disability [4], contributes to poorer medical outcomes [5], and is associated with increased suicide risk and mortality [6, 7].
LLD is further characterized by poor or impaired cognitive performance. Reduced executive functioning is common, affecting verbal fluency, response inhibition, set-shifting, working memory, and problem-solving [8]. Individuals with LLD also exhibit poor performance in other cognitive domains, including episodic memory, visuospatial skills, and processing speed [9–11]. Slower processing speed is particularly important, partly mediating impaired performance in other cognitive domains [11–13]. Although cognitive performance improves with successful treatment, deficits typically persist, and older depressed adults have an increased risk of dementia [14].
Such adverse outcomes may be related to LLD’s recurrent nature [15]. LLD is often a recurrent or chronic illness [16], although continuing antidepressant medication during remission reduces recurrence risk [17, 18]. However, even with maintenance treatment, ~35–40% of depressed elders experience recurrence in 2 years, with over 50% experiencing recurrence over four years [16, 18, 19].
Recurrence is particularly relevant for individuals with an initial onset of depression in early- or midlife. These individuals often experience multiple prior depressive episodes and are now in the geriatric age range. Individuals with early-onset LLD, typically defined as occurring before age 50–60 years, exhibit greater residual depression severity over time, more frequent suicidal thoughts, and a greater risk of recurrence following remission [16, 20]. Early-onset depression is characterized by stronger familial history and genetic loading, greater anxiety and reactions to stressful life events, maladaptive personality traits, and hormonal fluctuations associated with early-life reproductive events [21–26]. Individuals with late-onset depression are more often widowed, present with more apathy and somatic symptoms, poorer cognitive performance, greater cognitive decline and medical morbidity, and more severe atrophic and vascular changes on neuroimaging [26–32]. Although useful for clinical characterization, this age of onset dichotomization obfuscates potentially important differences in causal factors that influence the onset or recurrence of depression across the lifespan. Moreover, it does not address a parallel hypothesis that depression itself is toxic, with recurrent episodes increasing the allostatic load or “wear and tear” on the body, contributing to accelerated brain aging and vulnerability to poor longitudinal clinical, cognitive, and medical outcomes [15].
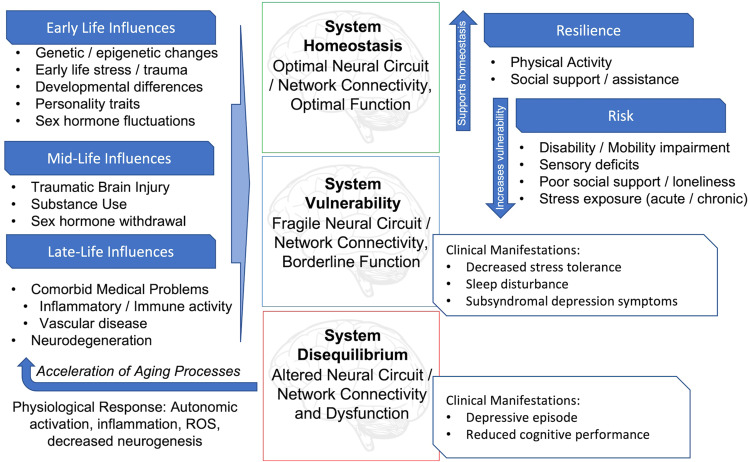
Based on past work [15], we present a model (Fig. 1) where disruption of functional brain network homeostasis contributes first to subclinical depressive symptoms and decreased stress tolerance. If unchecked, this progresses to discrete depressive episodes and reduced cognitive performance. Various biological and environmental factors across the lifespan increase the vulnerability of key networks to disequilibrium, with potentially modifiable behavioral and social factors contributing either to vulnerability or resilience. In turn, depressive episodes alter physiological systems that accelerate aging processes and contribute to adverse longer-term outcomes.
Fig. 1. Lifespan model of late-life depression.
Symptoms of depression are the behavioral manifestation of increasingly disrupted brain networks. Multiple influences contribute to network fragility and dysfunction across the lifespan, with some being linked to clear developmental periods. These may be additive and cumulative over time, although other risk and resiliency factors may be modifiable and targeted by specific treatments. Unchecked, repeated depressive episodes and their associated physiological responses may have deleterious effects contributing to accelerated aging.
Building on this model, we first present a network-based model of depression. We then focus on etiological influences across the lifespan that increase depression risk, primarily focusing on those salient to later life. Next, we consider depression as a contributor to accelerated aging. Finally, we review treatment for LLD and how interventions may support resilience to future episodes.
Neural networks implicated in depression
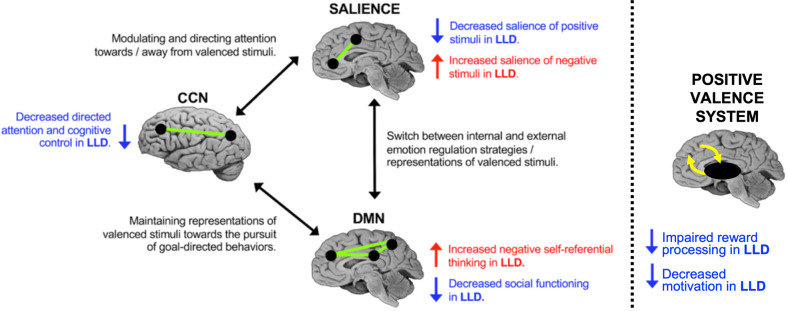
Altered neural network function is thought to result in the behavioral manifestations of depression [33]. The triple network model (Fig. 2) [33, 34] posits that depression is related to the aberrant function of the default mode network (DMN), cognitive control network (CCN), and anterior salience network (ASN). Positive valence system circuits involved in reward function are additional contributors [35, 36], although this has received less attention in LLD [37]. These networks likely influence depressive behavior across the lifespan, although the etiological factors contributing to network dysfunction change with aging. Although heterogeneity in LLD prevents sweeping generalizations [38], there is support for this network-centric model [34, 39].
Fig. 2. Network model of late-life depression.
The model details findings within each intrinsic functional network and between functional networks, specifically the default mode network (DMN), the cognitive control network (CCN), and the anterior salience network (ASN). Disruption in network connectivity influences the cognitive processes, giving risk to the behavioral manifestations of depression. Impaired function of the positive valence system involving the mesolimbic system likely also contributes to depressive behavior [37], although how this system interacts with interacts with intrinsic functional networks in LLD is not entirely clear. The model and figure [34] used with permission. Reprinted from Gunning et al. [34], Copyright 2021, with permission from Elsevier.
The DMN is implicated in self-referential processes [40] including rumination [41], making it a target of investigation in depression [33, 42]. However, there is little consensus on how the DMN is altered in depression [43]. Early studies reported hyperconnectivity within the DMN relative to healthy controls [42], though recent meta-analyses reported no difference in DMN connectivity [44] or even hypoconnectivity between DMN regions [45]. DMN functional connectivity appears to be altered in LLD [46, 47] and such differences may persist into remission [46].
The CCN is primarily involved in top-down executive functions [48]. Substantial evidence suggests CCN integrity differentiates healthy controls from depressed individuals [42] and influences treatment response [49]. The CCN may be especially relevant in LLD characterized by executive dysfunction [50, 51], where aberrant functional connectivity of the CCN (particularly the dorsolateral prefrontal cortex [DLPFC] hub), is associated with executive deficits [52].
The ASN is implicated in switching and control of attentional processes [53], and ASN dysfunction in depression biases individuals towards negative stimuli and processing [54, 55]. Unlike the DMN, the ASN has proven relatively reliable in distinguishing between healthy controls and depressed individuals [42, 44, 56]. Both structural and functional connectivity of the ASN are reduced in LLD [57].
Alterations in the positive valence system are additive. Conceptually, much work focuses on the dopaminergic mesolimbic pathway, projecting from the ventral tegmental area to the ventral striatum, nucleus accumbens, and medial temporal structures [37]. Dysfunction in this system influences a range of reward functions including valuation, decision-making, effort, and learning [37, 58]. Behaviorally, this contributes to anhedonia, motivational disturbances, and willingness to expend effort [37].
Neural networks and aging
The connectivity and function of these networks change with age. Cross-sectional and longitudinal studies demonstrate that frontal regions comprising the associative networks described above (plus the dorsal attention network) exhibit reduced intra-network connectivity and increased inter-network connectivity with aging [59–69]. These changes may reflect a decline in network efficiency and/or serve as a compensatory mechanism that maintains normal brain function in context of gray matter loss or white matter degradation [70, 71]. Supporting this latter hypothesis are studies showing increased activation in frontal regions in older adults compared to younger adults that are associated with better cognitive performance with aging [72]. Increased prefrontal activation is further associated with reduced white matter integrity, again supporting compensation [73, 74]. These age-related network changes have been replicated using both structural and functional network measures, further associating aging with lower strength and density of structural connections and decoupling of structural and functional connectivity, particularly in network hubs [63]. This may represent a rerouting of information flow in the brain intended to circumnavigate degraded white matter pathways or avoid regions that have suffered neuronal loss. The ability of the brain to successfully “rewire” during aging may be crucial for maintaining cognitive performance and reflect cognitive reserve [70]. Moreover, age-related changes may contribute to network fragility, increasing risk for LLD. While there is some evidence that network organization properties may differ according to age of onset [57], clear differences in neural network configuration between early- and late-onset LLD have not been identified.
Factors contributing to depression vulnerability
Early- and midlife risk factors
Older depressed adults carry the same vulnerabilities that increased risk for depression earlier in life. As the list of potential contributors to MDD risk is beyond this review, we focus on mechanisms of relevance to LLD.
Genetic factors that influence MDD vulnerability likely persist with aging. Genome-wide association studies (GWAS) identified several hundred potential genetic risk variants, including genes involved in synaptic structure and neurotransmission [75]. In order to influence depression risk, such genetic factors would need to directly or indirectly alter brain network function or stability [76]. However, concerns persist about translating these findings to the individual level, both due to the contributions of small-effect polymorphisms that may be missed on GWAS, and due to diagnostic heterogeneity within MDD [77]. What risk genes contribute to depression vulnerability may change across the lifespan, particularly if they affect brain aging. For example, some work supports that vascular risk genes may be germane in LLD [78].
Adverse childhood experiences (ACEs), such as abuse, parental loss, and bullying, are associated with a host of health disorders, including depression [79]. They are also associated with differences on neuroimaging and cognitive testing [80]. ACEs may contribute to depression vulnerability through hypothalamic–pituitary–adrenal (HPA) axis responses leading to increased activity of corticotropic releasing factor neurons [81]. ACEs can further result in epigenetic changes [24] that may increase depression vulnerability by influencing glucocorticoid signaling, serotonergic function, and neurotrophic factors [24]. The relationship between ACEs and depression vulnerability persists into later life [82, 83], where the relationship between ACEs and depression may be mediated by inflammation [82]. Stressful events occurring in adulthood or later life also increase the risk for new depressive episodes and depression persistence [84, 85].
Personality traits are similarly associated with depression. Traits that influence how individuals interact with and respond to their environments originate from variability in functional brain networks [22]. They arise from a complex interplay between brain development, genetic predisposition, and early environmental exposures. Neuroticism, a predisposition to experience psychological distress and negative mood states, is well-studied and shares some conceptual overlap with LLD [86]. Higher levels of neuroticism in LLD are associated with poorer antidepressant response and greater risk of cognitive decline [86–88].
Reproductive events including puberty, menstrual cycling, pregnancy, and menopause are associated with both new-onset and recurrent depression [23]. These events contribute to a higher risk of depression for women than men [89], particularly during reproductive years [90]. These relationships are due to fluctuations of ovarian hormones that influence neurotransmitter function, neuroendocrine processes, and inflammation [91]. Menopause is particularly relevant to aging, with decreased estrogen production potentially reducing its neuroprotective effects [92]. A longer exposure to endogenous estrogens, operationalized as an older age at menopause, is associated with a lower risk of subsequent depression [93], while earlier menopause, including surgically-induced menopause, is associated with cognitive decline and dementia [94].
Other exposures influence MDD, including comorbid mental health disorders, substance misuse, and traumatic brain injury [95]. Medical comorbidity also contributes, including a bidirectional relationship between depression and obesity [96] that may be mediated through immune system activation and inflammation [96]. Obesity increases the risk for other morbidities associated with LLD, including pain syndromes, vascular risk factors, and disability [97, 98].
Later-life risk factors
Despite LLD being associated with a range of medical comorbidities, few may directly contribute to depression pathogenesis. Age-related morbidities that are a focus of mechanistic models include inflammation, vascular disease, and neurodegeneration (Table 1).
Table 1.
Support for etiological hypotheses of late-life depression.
| Inflammation | Vascular | Neurodegeneration | |
|---|---|---|---|
| Clinical |
• Neurovegetative symptoms (lethargy, reduced appetite) • Greater medical morbidity • Increased levels of pro-inflammatory cytokines; Decreased levels of anti-inflammatory cytokines • Associated with treatment resistance and poor antidepressant efficacy |
• Dysphoria, anhedonia, apathy, psychomotor retardation, functional disability • Higher rates of vascular risk factors • Increased disability and mortality |
• Apathy, subjective memory loss • AD pathology or development of dementia associated with poor antidepressant efficacy |
| Cognitive | • Executive dysfunction, slowed processing and motor speed, reduced memory | • Executive dysfunction, reduced processing speed and visuospatial skills, retrieval-based memory deficits |
• Depression co-occurring with dementia worsens cognitive performance • Amnestic cognitive profile (often, but not always) |
| Imaging |
• Peripheral inflammatory markers linked with: • altered fronto-subcortical activation • gray and white matter volume loss • Higher markers of central inflammation found in anterior cingulate and temporal cortices |
• WMH volumes increase over time • Higher WMH volumes worsen cognitive outcomes • Persistent depressive symptoms exhibit greater change in WMH volume over time |
• LLD with greater beta-amyloid deposition have: • greater temporal lobe volume loss • lower functional connectivity in fronto-subcortical regions • functional DMN alterations |
| Negative findings | • When excluding comorbid medical conditions, studies do not show relationship between depression and inflammatory cytokines | • Inconsistent findings between LLD, vascular burden, and antidepressant response. |
• Some report less beta-amyloid deposition in LLD compared to controls • APOE ε4 does not clearly influence development of LLD |
AD Alzheimer’s disease, APOE apolipoprotein E, CSF cerebrospinal fluid, DMN default mode network, LLD late-life depression, WMH white matter hyperintensities.
Table inspired by and adapted from Alexopoulos [164].
Inflammation
The inflammation hypothesis proposes that immune dysregulation influences vulnerability to and the development of LLD [99]. Neurovegetative depressive symptoms are akin to immune responses to infectious diseases including lethargy, cognitive slowing, and reduced appetite [100, 101]. In younger adults, elevated pro-inflammatory cytokines levels in response to psychological stress are associated with depressive symptoms [101] and induction of peripheral inflammation results in fatigue and worsening of mood [102, 103]. Depressed patients across the adult lifespan can exhibit elevated levels of pro-inflammatory cytokines including c-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor (TNF) alpha and lower anti-inflammatory cytokine levels [104, 105]. Higher pro-inflammatory cytokine levels are associated with depression severity, suicide risk, and poor treatment response in adult and geriatric samples [105–107]. Pro-inflammatory cytokines are associated with worse function in executive processes, memory, and processing and motor speed [108].
Aging is itself associated with chronic, low-grade inflammation, dubbed “inflammaging” [109]. This process has multiple contributors, including immune system aging, mitochondrial changes, and gut microbiota [110, 111]. These changes may be secondary to common pro-inflammatory medical conditions that increase depression risk, including diabetes, cardiovascular disease, autoimmune disorders, rheumatoid arthritis, cirrhosis, and kidney disease [112–115]. These comorbidities may explain the observed relationship between depression and inflammation, as evidenced by a study that did not associate LLD with higher levels of central or peripheral inflammatory cytokines. However, this study employed rigorous entry criteria for medical comorbidities that excluded almost 95% of potentially eligible participants [116]. This raises issues about its generalizability and highlights the extent of comorbidity between LLD and medical illnesses.
Although most work examines peripheral inflammatory markers, it remains relevant to brain function. The CNS was long considered to be immunoprotected due to the blood-brain barrier. However, immune responses via peripheral immune cell secretion of pro-inflammatory cytokines can convey the inflammatory response to brain microglia via humoral and neural pathways [117, 118]. Microglia can thus become activated by peripheral cytokines inducing a neuroinflammatory response [119]. Both aging and psychological stress further prime microglia toward an activated state, tilting the CNS toward a pro-inflammatory state [120, 121]. In the aged brain, activated microglia exhibit an exaggerated response to pro-inflammatory cytokines, inducing oxidative stress and delayed clearance of neurotoxic molecules, resulting in disrupted neuronal function, impaired neurogenesis, and neural degeneration [119, 120]. Central inflammation further affects multiple neurotransmitter systems, contributing to reduced serotonin synthesis via induction of indoleamine 2,3-dioxygenase [100], glutamate system dysregulation [122–124], and altered dopamine synthesis, binding, and reuptake [37, 125–128].
While less examined in LLD, chronic inflammation induces an altered cellular environment in the brain parenchyma capable of modulating neural circuits and influencing depressive behavior [15]. Pro-inflammatory cytokines including CRP and IL-6 are associated with global gray matter and white matter loss [129]. A meta-analysis including participants across the lifespan associated peripheral inflammatory markers with altered activation of the prefrontal cortex, insula, striatum, amygdala, hippocampus, and various subcortical regions [130]. These regions overlap with the DMN, ASN, limbic, and corticostriatal networks. Studies of individuals with depression and suicide attempts report increased microglial activation in the anterior cingulate cortex (ACC), a key hub of both the ASN and CCN, in individuals with depression and suicide attempts [131, 132]. Experimental induction of acute inflammation similarly alters glutamate metabolism in the ACC and basal ganglia [124].
Treatment implications of pro-inflammatory processes are unclear. Work in midlife suggests that low-grade inflammation may decrease antidepressant efficacy [133–136]. However, successful antidepressant treatment can decrease pro-inflammatory cytokine levels [137, 138] Conversely, antidepressant augmentation with anti-inflammatory agents may reduce depressive symptom severity and improve treatment outcomes [139, 140]. If existing trials are supported, such interventions may most benefit individuals with higher inflammatory cytokine levels, such has been seen with infliximab, a TNF-alpha antagonist [141, 142]. Inflammation could identify a distinct phenotype [37, 143] who would benefit from anti-inflammatory treatments.
Vascular disease
Cerebrovascular system changes are common with normal aging [144]. Cerebral small vessel disease (CSVD) describes leakage of the blood-brain barrier and dysfunction of cerebral autoregulation, neurovascular coupling, and capillary blood flow [145]. This causes cerebral perfusion deficits and hypoxia, triggering inflammation and neuronal death. Vascular risk factors including hypertension, atherosclerosis, and diabetes contribute to CSVD and result in the thickening of the penetrating small arteries, fibrosis of the vessel wall, and depletion of vascular smooth muscle cells.
The “vascular depression hypothesis” [146, 147] posits that CSVD may predispose, precipitate, or perpetuate LLD. This process likely begins in adulthood, as midlife cerebrovascular burden predicts increased depression severity over time [148]. The neuroradiological manifestation of “vascular depression” includes white matter hyperintensities (WMHs) on T2-weighted MRI, subcortical lacunes, and microbleeds [147, 149]. Mechanistically, WMHs may contribute to a disconnection syndrome where damage to communicating cortical-subcortical pathways involved in mood regulation and cognitive processes increases LLD vulnerability [78, 150, 151]. Further supporting a mechanistic role, meta-analyses have shown that late-onset depression show significantly greater WMH burden in late-onset LLD than in early-onset LLD [152, 153]. The extent of ischemic injury extends beyond visible WMHs, as vascular risk factors compromise microstructural integrity in normal-appearing white matter [154, 155]. Location of WMHs and microstructural changes may be critical, as LLD is associated with damage to the cingulum bundle, uncinate fasciculus, and superior longitudinal fasciculus [156–159].
These processes influence longer-term negative outcomes. Even without depression, vascular changes are associated with cognitive deficits (including executive dysfunction and retrieval-based memory deficits) and altered emotion processing [160]. Cross-sectionally, depressive symptoms with greater WMH volumes worsen cognitive outcomes in the early stages of CSVD [161]. Greater WMH volume in LLD contributes to greater longitudinal decline in executive functions and increased risk for dementia [162]. Individuals with persistent depressive symptoms similarly exhibit greater increases in WMH volume over time [163]. Greater vascular burden may be associated with poorer response to pharmacological and nonpharmacological treatments [158, 164–166], although this relationship for medication response is somewhat weak [49, 78]. The higher vascular burden is further associated with greater disability [167], gait and other motor deficits [168], and frailty [169]. Depression can also worsen cardiovascular and cerebrovascular disease outcomes [170, 171], suggesting a bidirectional relationship.
Neurodegeneration
Aging is the strongest risk factor for dementia [172], a collective term for cognitive impairment negatively affecting independent functional activities. Alzheimer’s disease (AD), the most common dementia, is characterized by abnormal accumulation of beta-amyloid plaques and tau tangles in the brain. Early AD typically affects memory centers, including the entorhinal cortex and hippocampus. With disease progression, neuropathology spreads to frontal and parietal regions and affects language, executive abilities, and social behaviors.
Depression and dementia exhibit a bidirectional relationship. Depression in mid-to-late life increases risk for AD and all-cause dementia [14, 173, 174]. Depression can also be a precursor to or symptom of dementia, with prevalence rates ranging from 17 to 56% across all stages of AD [175]. Depression co-occurring with dementia worsens cognitive performance beyond what would be expected based on neuropathology alone [176]. Dementia risk may be highest in individuals exhibiting persistent or worsening depressive symptoms over time [177].
Such observations led to work searching for common genetic factors. While the apolipoprotein E (APOE) ε4 allele significantly increases risk for AD, it does not clearly influence the development of LLD [178, 179]. A genome-wide association study found that depression had a causal role in AD through Mendelian randomization, but there was no evidence for a causal role of AD on depression [180]. That study identified 53 brain transcripts and proteins regulated by the depression GWAS signals that also were associated with rates of cognitive decline over time [180].
The “amyloid hypothesis of LLD” [181] is supported by observations of increased beta-amyloid deposition in older adults with a depression history [182] and in LLD patients exhibiting a cognitive profile suggestive of amnestic Mild Cognitive Impairment [183]. Individuals with LLD exhibiting greater beta-amyloid deposition show greater volume loss in the temporal lobe, lower functional connectivity in fronto-subcortical regions, and greater functional alterations in the DMN [184, 185]. These findings are not universal, and the Alzheimer’s Disease Neuroimaging Initiative depression group reported less beta-amyloid deposition in LLD compared to a control group [186]. While beta-amyloid deposition was associated with worse memory performance in that study, the association between amyloid and cognitive performance did not differ between diagnostic groups. More recent work has focused on tau pathology as others report that individuals with elevated tau, but not amyloid, are twice as likely to be depressed [187].
Poorer cognitive performance, comorbid dementia, and AD pathology are associated with poorer prognosis for response to antidepressant medications. Both poorer cognitive performance, particularly executive dysfunction or slowed processing speed, and dementia are associated with poorer responses to antidepressant medications [12, 165, 188, 189]. Similarly, higher levels of beta-amyloid deposition, particularly in the temporal lobe, are associated with poor response or treatment resistance to antidepressant medications, even in cognitively intact elders [190, 191]. Alternative treatment approaches are not entirely clear, although some individuals with cognitive impairment may benefit from nonpharmacological interventions such as computerized cognitive training [192].
Depression is not unique to a single neuropathological process [176]. Beyond AD, it occurs in context of alpha-synuclein, a constituent of Lewy bodies and the pathological hallmark of synucleinopathies, including Parkinson’s disease (PD), dementia with Lewy bodies, and multiple system atrophy. Depression is a common non-motor symptom of PD [193] and depressed individuals exhibit a 2.2-fold increase in risk of subsequent parkinsonism [194]. There is a positive association between alpha-synuclein messenger RNA expression levels and depression severity [195], while levels of CSF alpha-synuclein may mediate associations between LLD, markers of synaptic dysfunction, and memory ability [196].
Depression and accelerated aging
Both vascular [170, 171] and neurodegenerative processes [14, 173, 174] occur more frequently or more severely in LLD. These may represent bidirectional relationships, where depressive episodes may both contribute to and result from accelerated aging. Biological aging is an inevitable process at molecular, cellular, and organ levels reducing a system’s reparative or regenerative potential [197]. “Accelerated aging” is when biological aging occurs more rapidly than expected, resulting in biological characteristics that are more severe than would be expected based on chronological age [198]. In the brain, this may include ventricular enlargement, cerebrovascular injury, or gray matter atrophy. For this review, we distinguish accelerated aging from “pathological brain aging”, characterized by neurodegenerative processes involving amyloid, tau, or other abnormal protein deposition.
Accelerated aging is observed in multiple neuropsychiatric disorders and quantified using a range of markers including telomere length, oxidative stress markers, epigenetic markers, physiological functioning, and neuroimaging [199–201]. In adult MDD, accelerated aging is observed on molecular and cellular markers, including reduced telomere length, epigenetic aging, and metabolomic aging [201, 202]. Accelerated aging in MDD is further observed on both structural and functional neuroimaging, where depressed individuals appear on average 1–2 years older than nondepressed cohorts [203, 204]. In the largest of these studies, the difference between calculated brain age and chronological age was independently replicated [205] and not associated with age of depression onset, recurrence, or remission [204].
An accelerated aging hypothesis of LLD implies a bidirectional process [206]. Depressed older adults exhibit an advanced biological age on a multibiomarker index of metabolic and inflammatory measures [207, 208] and on structural MRI, where they appear approximately 4 years older than nondepressed individuals [198]. This accelerated brain aging is further associated with disability and cognitive performance [198], with depression severity moderating the relationship between brain age and some cognitive measures. As the difference between calculated brain age and chronological age differs between midlife adult depressed samples and LLD [198, 203, 204, 209], depression may be associated with an altered trajectory of biological aging [210].
Accelerated aging also influences physical function and contributes to physical frailty. Frailty is characterized by deficits in strength and mobility, decreased physical activity, and reduced energy capacity that results from dysregulation in metabolic, musculoskeletal, and stress-response systems [211, 212]. Frailty is common, bidirectionally associated with LLD, and associated with increased mortality and poor antidepressant treatment responses [213–215]. Frailty may be an outcome of depression, as depression is associated with worsening trajectories in functional status, including reduced walking speed and hand strength [216].
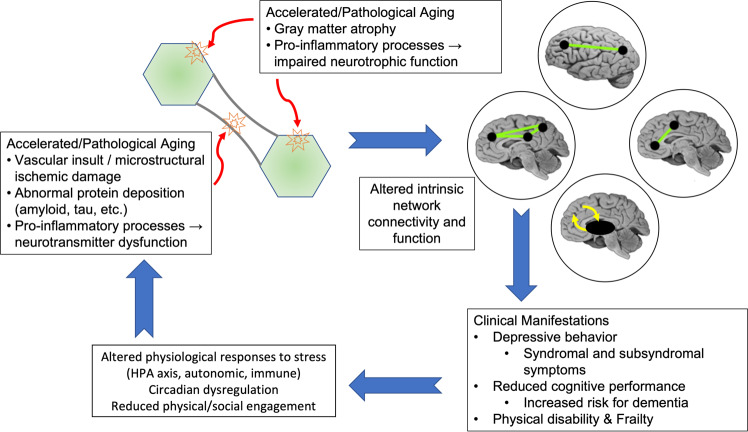
The model of a bidirectional relationship between depression and accelerated and pathological aging (Fig. 3) may start with age-related changes increasing vulnerability to depression. Individuals experiencing accelerated biological aging, operationalized as advanced medical morbidity, cerebrovascular pathology, pro-inflammatory processes, or pathological aging, such as increasing amyloid or tau burden, are at higher risk for LLD. Such etiological factors can disrupt the structure, function, and homeostasis of key intrinsic functional networks implicated in depression [78, 164]. These etiological factors may all occur to varying extents in the same individual, each challenging functional network homeostasis. As the underlying systems become more dysregulated or as pathologies progress, and networks experience greater homeostatic imbalance and impairment in function, the clinical presentation of the depressive syndrome emerges (Fig. 1) [15]. It is possible that the predominant underlying pathology and what brain networks are most affected influence the clinical presentation and phenotype [37, 217].
Fig. 3. Accelerated aging hypothesis of late-life depression.
Aging processes such as inflammation, vascular disease, or pathological neurodegeneration impair neurotrophic function and contribute to both gray matter atrophy and impairment of white matter microstructure. These changes in turn alter function of key intrinsic networks, leading to the clinical manifestations of late-life depression. In turn, repeated depressive episodes result in altered or sustained physiological responses increasing allostatic load. These effects may then further accelerate biological aging processes, shifting an individual further away from normative aging.
Depressive episodes may also accelerate biological aging (Fig. 2). One potential mechanism involves the altered neural and physiological responses to stress observed in depression. Such altered responses are observed across a range of systems, including altered function of brain regions involved in emotion processing, autonomic reactivity, HPA axis function, and dysregulation of the immune system or circadian processes [78, 99, 218–224]. Normally, these processes are meant to facilitate allostasis, the body’s ability to respond to environmental challenges and maintain normal functioning. However, with repeated stressors and depressive episodes, these responses contribute to increased allostatic load, the wear-and-tear resulting from stress and the dysregulation of processes meant to maintain homeostasis [15, 225]. Such canonical stress-response systems interact and over time contribute to accelerated aging, leading to regional brain atrophy, development of cerebrovascular pathology, and reduction of neurotrophic function [226–228]. This hypothesis is supported by longitudinal studies associating persistent or recurrent depression with greater increases in WMH volume and greater hippocampal volume decline [163, 229]. Other mechanisms explaining this relationship are possible, including shared genetic vulnerabilities [180].
Such bidirectional relationships have long-term implications. Accelerated or pathological aging also contribute to impaired cognitive performance [198, 230], increased risk for dementia [14], and risk of physical disability, sensory function loss, and frailty [230]. Accelerated brain aging may be associated with higher risk for depression relapse after achieving remission [15]. Even if the initial antidepressant treatment were successful, progressive aging may further challenge functional networks and result in a return of depressive symptoms [15].
Updates on established somatic treatments
Currently, treatment decisions for patients with LLD are guided more by clinical history and patient preference than potential biological causal factors. Robust blinded clinical trials data for LLD are scant and clinical guidelines tend to derive from expert opinion or are extrapolated from data in younger populations [231].
Antidepressant medications
Antidepressants are more effective than placebo in the treatment of LLD, although the response rate is lower for older than younger adults [232–234]. However, while antidepressants alter DMN and CCN connectivity [235], age of initial onset does not influence antidepressant medication response rates, although early-onset patients may respond more slowly [236, 237]. Antidepressants remain beneficial, with a number needed to treat (NNT) for an antidepressant response being 6.7 (95% CI, 4.8–10) [234, 238]. As in younger adults, augmentation strategies in LLD are more efficacious than strategies involving a switch to a different antidepressant [239]. Methylphenidate augmentation of an SSRI is superior to monotherapy with either agent alone [240]. Augmentation with lithium, bupropion, or aripiprazole in patients who did not respond to monotherapy can be well-tolerated and improve depressive symptoms [239, 241, 242]. Despite clear benefits of augmentation, the likelihood of achieving remission decreases with increasing number of failed antidepressant trials within the current episode [243].
Few studies examine outcomes of the N-methyl-d-aspartate (NMDA) receptor channel inhibitors ketamine and esketamine in LLD. Both a small, randomized trial of subcutaneously administered ketamine and larger open-label study of intravenous ketamine improved depression severity in older adults with treatment-resistant depression [244, 245]. Intravenous ketamine resulted in a remission rate of 12.8% for older individuals, which is comparable to remission rates seen in patients progressing to later stages of the STAR*D study [245, 246]. A randomized trial of esketamine in treatment-resistant LLD resulted in a comparable remission rate of 17.3%, with a NNT of 10, although that study did not detect a statistically significant difference in their primary endpoint [247]. Secondary analyses suggested that participants with an earlier life onset of depression or who were less than 75 years of age exhibited greater improvement [247]. Both ketamine and esketamine were well-tolerated, with common side effects including dizziness, dissociative symptoms, fatigue, and transiently elevated blood pressure [245, 247].
Electroconvulsive therapy (ECT)
ECT continues to be used for severe and treatment-resistant LLD. In LLD, ECT exhibits remission rates between 70 and 90%, although rates in community samples may be lower [248]. Individuals with late-onset depression tend to respond better to ECT than individuals with early-life depression onset [249], which may be related to illness chronicity or recurrence in the early-onset group. However, strong remission rates should be balanced by high relapse rates after the initial ECT course, with 40–50% of patients relapsing within 6 months [250]. Cognitive side effects in older adults tend to be limited and transient [251].
Recent work has refined ECT to improve tolerability while preserving efficacy. This includes administering ECT using right unilateral electrode placement with ultrabrief pulse width stimuli, an approach with fewer cognitive side effects [252]. When combined with venlafaxine in LLD, this results in remission rates of 61% and response rates of 70% [253]. Unilateral brief pulse ECT combined with venlafaxine only modestly affects cognitive performance, specifically letter fluency and cognitive flexibility [254]. This study also included a 24-week continuation phase, where participants were randomized to either medication only (venlafaxine plus lithium) or venlafaxine plus continuation ECT, administered weekly for the first month with additional sessions as needed. Continuation ECT resulted in lower levels of depression severity at study endpoint than medication only [255] and better quality of life [256].
Transcranial magnetic stimulation (TMS)
Repetitive TMS (rTMS) uses a pulsed magnetic field to induce a local electrical field on the brain’s surface, stimulating cortical pathways. rTMS treatment of depression typically targets the DLPFC, with the best-studied techniques including unilateral high-frequency left-sided (HFL), unilateral low-frequency right-sided (LFR), or sequential bilateral treatment of LFR followed by HFL [257]. Parallel work supports that targeting the DLPFC modulates functional connectivity within and between the DMN and CCN, with clinical benefit deriving from modulation of subgenual cingulate cortex connectivity [258]. While many randomized trials support rTMS efficacy, few have been conducted in LLD [259, 260]. However, rTMS is well-tolerated in older adults [261], and LLD trials generally support the efficacy of HFL rTMS, with bilateral treatment being more efficacious in treatment-resistant patients [257].
Recent work has modified rTMS to improve outcomes and reduce burden. A sham-controlled trial in LLD that examined bilateral deep rTMS reported efficacy and good tolerability when administered over four weeks [262]. This deep TMS approach addressed concerns that age-associated atrophy may contribute to poor treatment responses by increasing the distance between the scalp and cortex [263], however it requires longer administration sessions. More recent work in LLD compared rTMS to theta-burst stimulation (TBS), a bilateral approach that reduces session administration from 47 min for rTMS to 4 min for TBS. This randomized trial established non-inferiority of TBS, with comparable reductions in depression severity between groups [264].
Resilience factors: opportunities for intervention
Although these treatments are effective, benefit depends on continued treatment. If pharmacotherapy or neuromodulation stops, the risk of recurrence can be high [18, 250, 253]. This risk may be reduced through interventions that target vulnerability factors to depression and strengthen resiliency (Table 2).
Table 2.
Resilience factors influencing depressive episode risk in later life.
| Domain | Factors | Resilience correlates | Depression vulnerability correlates |
|---|---|---|---|
| Trait-like factors | Temperament | Positive emotionality | Greater harm avoidance |
| Personality | Extroversion, conscientiousness | Neuroticism | |
| Psychological factors | Beliefs | Self-esteem, self-efficacy, mastery, sense of purpose | Internalized self-blame or stigma |
| Coping | Active or accommodative coping | Avoidance or passive coping | |
| Social factors | Social support | Social engagement | Social withdrawal, loneliness |
| Altruism | Formal volunteering | Social role absences | |
| Cognitive factors | Cognitive reserve | Maintained cognitive performance | Poorer executive function and processing speed |
| Physical factors | Physical activity | Exercise, regularly active | Sedentary lifestyle |
| Sensory function | Sustained or corrected vision and hearing | Impaired sensory function | |
| Healthy diet | Good nutrition | Poor nutrition, substance abuse | |
| Healthy sleep | Regular sleep patterns | Disrupted, irregular sleep |
Correlates of factors that may contribute to resilience from depression, or vulnerability to depression. Some factors are modifiable (psychological factors, social factors, and lifestyle factors) while others are not (trait-like factors). Some depression correlates may both increase the risk of depression and also be an outcome of depression. Table inspired by and adapted from Laird et al. [267] and Andreescu et al. [15].
Resilience is broadly defined as the capacity to maintain or regain psychological well-being despite challenges, or the adaptive maintenance of homeostasis despite adversity [265–267]. Resilience is a multidimensional, dynamic process influenced by both internal factors and external resources. Resilience factors may decrease the risk of a depressive episode, reduce severity or duration of that episode, or increase likelihood of recovery [267, 268]. If depression contributes to accelerated biological aging, then bolstering such resilience factors and reducing the frequency or duration of depressive episodes could hypothetically shift the biological aging process towards a more normal trajectory. We discuss resilience factors and their corresponding vulnerability factors (Table 2) in context of treatments.
Psychological factors: role for brief psychotherapy
While factors such as temperament and personality may be challenging to target with brief therapy, progress can improve negative beliefs and coping strategies. Individuals’ beliefs about themselves and their environment influence how they cope with stressors or challenges. Depression risk is associated with lower self-esteem, anxiety sensitivity, and an external locus of control (i.e., a feeling that one cannot influence outcomes in one’s life) [269]. The converse of these beliefs contribute to resilience, as does a sense of purpose and grit, defined as perseverance in achieving goals despite setbacks [267, 270–272]. Greater self-efficacy enhances individuals’ ability to flexibly apply coping strategies, including active coping strategies that directly address the stressor, or accommodative strategies involving adaptation to the stressor. Such a flexible approach improves mental health outcomes and reduces depression [273, 274].
Evidence for psychotherapy
Psychological factors addressing vulnerability or promoting resilience may be particularly amenable to psychotherapy. Evidence-based treatments in LLD include cognitive-behavioral, problem-solving, interpersonal, and life-review therapies [275, 276]. Such therapies influence functional network connectivity, such as cognitive-behavioral therapy increasing connectivity between the amygdala and CCN [277]. Meta-analyses in LLD support that psychotherapy is quite effective, with a NNT of 3 [278]. More recently developed, “Engage” therapy targets neurobiologically-informed processes in LLD using streamlined behavioral techniques that can be effectively applied in the community [279, 280].
Social factors: opportunities for engagement
Aging adults tend to maintain close social partners but have fewer peripheral social contacts [281]. In contrast, larger objective social network size and greater perceived social support protects against LLD and predicts a better response to depression interventions [282–284]. Such benefits may occur through mechanisms including emotional support, tangible assistance (instrumental support), or opportunities for pleasurable activities [285]. Recent work has focused on loneliness, or perceived social isolation that is distinct from having fewer objective social contacts. Loneliness is bidirectionally associated with a host of negative outcomes, including depression, poor physical health, cognitive and functional decline, and mortality [286–288]. Loneliness may be a neuropsychiatric manifestation of preclinical AD, as it is associated with an elevated dementia risk and higher levels of amyloid and tau pathology [289–291].
Evidence for targeting social connectedness
Few intervention studies directly target social factors in depression [292]. Group therapy benefits LLD [293] but does not typically focus on social connectedness. Recent novel work has examined remote, layperson-delivered interventions intended to improve social connectedness and reduce loneliness in younger and older adults. Although not directly targeting individuals with a depression diagnosis, they reduced depressive and anxiety symptoms [294–297]. Modifying existing psychotherapies to target social disconnection may also reduce suicide risk [294].
Cognitive factors: role for cognitive training
As previously discussed, LLD is associated with cognitive changes in executive functioning, processing speed, and episodic memory [8–11]. However, not all individuals with LLD have cognitive difficulties. There appear to be separate cognitive phenotypes within LLD: “High Normal”, “Reduced Normal” (with a relative weakness in episodic recall), and “Low Executive Functions” [298]. The “High Normal” phenotype maintained cognitive performance despite similar levels of depression severity as the other phenotypes. They also had higher levels of education and less vascular risk factors, suggesting that cognitive reserve and vascular health may contribute to cognitive resilience in LLD [299]. Identifying cognitive difficulties unique to the depressed individual allows for the prescription of personalized cognitive training interventions.
Evidence for benefits of cognitive training
For computerized cognitive training to work, it must have an adequate duration and be appropriately intense or difficult [300]. Evidence for the potential benefit of neurobiologically-informed computerized cognitive training in LLD comes from the approaches by Morimoto and others that are optimized to treat LLD with executive dysfunction [301]. They demonstrate that by targeting the underlying deficient neural circuitry and associated cognitive deficits in LLD, cognitive functions and depressive symptoms improve, benefits transfer to non-trained domains such as memory, and there are positive changes to underlying neural structural-functional connections [192, 300, 302]. Targeted cognitive training appears to modulate network functional connectivity in the DMN and CCN [303, 304].
Interventions targeting memory deficits (such as the Mayo Clinic’s Healthy Action to Benefit Independence and Thinking (HABIT) program) [305] benefit Mild Cognitive Impairment both with in-person and virtual platforms. While this intervention has not been conducted in LLD, the approach could translate to other populations with primary memory issues. Similarly, processing speed training shows benefit for up to 10-years in nondepressed older adults [306] and may be particularly favorable for LLD characterized by predominant cognitive and motor slowing. Augmenting cognitive training with neuromodulation approaches or other nonpharmacological treatments may provide additional benefit but need study in LLD [307].
Physical disability: need for sustainable movement-based interventions
Motor deficits are common with aging, including slowing, coordination deficits, and balance difficulties [308, 309] and they contribute to falls, disability, and mortality [310–314]. Depressed older adults are at increased risk for these motor problems [310, 314, 315]. This may be due to common contributors or comorbidities, such as vascular disease or other brain pathology [316, 317]. However, by increasing sedentary behavior and isolation, depression may also hasten muscle atrophy, deconditioning, and frailty [37, 213–215].
Evidence for movement-based interventions
Structured physical exercise benefits depression symptoms across the adult lifespan [318], including moderately benefitting older adults [319, 320]. It has positive benefits on hippocampal volume [321], may modulate connectivity between key DMN and CCN regions [322, 323], and also augments the response to antidepressant medications [324]. Physical activity improves global cognitive function in unimpaired elders and benefits cognitive domains sensitive to aging, including attention, executive function, and memory [325, 326]. Similarly, physical exercise, particularly aerobic activity, may reduce the risk of dementia and benefit older adults with existing cognitive impairment or dementia [327, 328]. Interestingly, recent work suggests that mind-body therapies that combine movement-based approaches with mindfulness, such as yoga or tai chi, may be superior to conventional exercise for mood and cognitive outcomes [267, 329]. However, questions remain about optimal practices needed to obtain such benefit, including frequency, intensity, duration, and type of exercises [303]. Strategies to facilitate initiation and maintenance of exercise in community-based elders are sorely needed, particularly for individuals with chronic pain or disabilities that limit physical function.
Sensory impairment: can improving sensory function help?
Sensory impairment, including vision and hearing loss, is also common in later life. Uncorrected hearing loss and vision loss are associated with greater depressive symptom severity and increased risk of developing LLD [217, 330–335], particularly for dual sensory loss affecting both vision and hearing [336]. Several hypotheses could explain these relationships, including how sensory loss limits social activities, leading to isolation and worsening psychological health [332, 337]. This may not account for the entire relationship, as sensory loss is further associated with physical decline [338], cognitive decline, and risk of dementia [335, 339].
Evidence for interventions improving sensory function
Optimizing sensory function benefits depressive symptoms and reduces depression risk. For impaired vision, improving residual vision and self-management programs can reduce depressive symptoms [340, 341]. Integration of psychotherapy techniques, such as behavioral activation can prevent depression in high-risk patients with macular degeneration [342]. A similar benefit is seen in preliminary clinical trials demonstrating that hearing aids may benefit depressive symptoms, quality of life, and cognitive performance in older adults [343–345].
Conclusions
We repeatedly describe bidirectional relationships between LLD and lifespan factors such as aging, inflammation, vascular disease, and more. These reciprocal relationships in essence describe positive feedback loops. In the absence of counterbalancing forces, such feedback loops can spiral out of control. Reframed in the allostatic framework, maintaining stability requires adaptive regulation and resiliency.
This dynamic nature of allostatic processes contributes to LLD heterogeneity. Vulnerability to developing depressive episodes results from accumulated factors, many of which have an initial onset earlier in life. Other vulnerability factors are unique to later life and may contribute to a new diagnosis of depression or a relapse of symptoms in previously remitted individuals. We propose that these vulnerability factors (Fig. 1) have negative effects on functional brain networks that predispose networks towards a state of fragility and instability.
Etiological heterogeneity creates challenges for understanding both LLD’s neurobiology and variability in treatment responses. It also creates opportunities to use this heterogeneity to probe specific mechanisms and guide focused, personalized treatment approaches. Such examples include examining dopaminergic system influences on LLD in patients with psychomotor slowing, testing anti-inflammatory medications in patients with elevated inflammation, or using targeted cognitive training to treat patients with LLD and executive dysfunction. While no single treatment will improve symptoms in all patients with LLD, combining established treatments such as pharmacotherapy, psychotherapy, and neuromodulation alongside personalized interventions that bolster resilience or address comorbid disability may improve outcomes for otherwise treatment-resistant patients.
Acknowledgements
This work was supported by National of Institute of Health grants R01 MH121619, R01 MH121620, R01 MH121384, and R01 MH123662. SMS is additionally supported by NIH L30MH127619 and the McKnight Clinical Translational Research Scholarship in Cognitive Aging and Age-Related Memory Loss, funded by the McKnight Brain Research Foundation through the American Brain Foundation and the American Academy of Neurology. ARG is supported by NIH T32MH019986.
Author contributions
All authors (SMS, ARG, DH, and WDT) contributed to manuscript conceptualization and writing, as well as critical review of the manuscript for important intellectual content. WDT additionally contributed to early versions of the scientific model and supervision.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sarah M. Szymkowicz, Andrew R. Gerlach.
References
- 1.Taylor WD. Clinical practice. Depression in the elderly. N Engl J Med. 2014;371:1228–36. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- 2.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–65. doi: 10.1093/gerona/58.3.M249. [DOI] [PubMed] [Google Scholar]
- 3.Lyness JM, Caine ED, King DA, Cox C, Yoediono Z. Psychiatric disorders in older primary care patients. J Gen Intern Med. 1999;14:249–54. doi: 10.1046/j.1525-1497.1999.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steffens DC, Hays JC, Krishnan KR. Disability in geriatric depression. Am J Geriatr Psychiatry. 1999;7:34–40. doi: 10.1097/00019442-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161:1849–56. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 6.Conwell Y, Thompson C. Suicidal behavior in elders. Psychiatr Clin North Am. 2008;31:333–56. doi: 10.1016/j.psc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penninx BW, Geerlings SW, Deeg DJ, van Eijk JT, van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56:889–95. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 8.Manning KJ, Steffens DC. State of the science of neural systems in late-life depression: impact on clinical presentation and treatment outcome. J Am Geriatr Soc. 2018;66:S17–S23. doi: 10.1111/jgs.15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, et al. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med. 2012;42:1195–202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- 10.Boone KB, Lesser IM, Miller BL, Wohl M, Berman N, Lee ABP, et al. Cognitive functioning in older depressed outpatients: relationship of presence and severity of depression to neuropsychological test scores. Neuropsychology. 1995;9:390–8. doi: 10.1037/0894-4105.9.3.390. [DOI] [Google Scholar]
- 11.Sheline YI, Barch DM, Garcia K, Gersing K, Piper C, Welsh-Bohmer KA, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 13.Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30:679–91. doi: 10.1017/S0033291799001968. [DOI] [PubMed] [Google Scholar]
- 14.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreescu C, Ajilore O, Aizenstein HJ, Albert K, Butters MA, Landman BA, et al. Disruption of neural homeostasis as a model of relapse and recurrence in late-life depression. Am J Geriatr Psychiatry. 2019;27:1316–30. doi: 10.1016/j.jagp.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, McQuoid DR, Potter GG, Steffens DC, Albert K, Riddle M, et al. Predictors of recurrence in remitted late-life depression. Depress Anxiety. 2018;35:658–67. doi: 10.1002/da.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds CF, Frank E, Perel JM, Imber SD, Cornes C, Miller MD, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. J Am Med Assoc. 1999;281:39–45. doi: 10.1001/jama.281.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds CF, 3rd, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–8. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–90. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 20.Sachs-Ericsson N, Corsentino E, Moxley J, Hames JL, Rushing NC, Sawyer K, et al. A longitudinal study of differences in late- and early-onset geriatric depression: depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging Ment Health. 2013;17:1–11. doi: 10.1080/13607863.2012.717253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH, et al. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect Disord. 1999;55:149–57. doi: 10.1016/S0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 22.Markett S, Montag C, Reuter M. Network neuroscience and personality. Personal Neurosci. 2018;1:e14. doi: 10.1017/pen.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newhouse P, Albert K. Estrogen, stress, and depression: a neurocognitive model. JAMA Psychiatry. 2015;72:727–9. doi: 10.1001/jamapsychiatry.2015.0487. [DOI] [PubMed] [Google Scholar]
- 24.Park C, Rosenblat JD, Brietzke E, Pan Z, Lee Y, Cao B, et al. Stress, epigenetics and depression: a systematic review. Neurosci Biobehav Rev. 2019;102:139–52. doi: 10.1016/j.neubiorev.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Janssen J, Beekman AT, Comijs HC, Deeg DJ, Heeren TJ. Late-life depression: the differences between early- and late-onset illness in a community-based sample. Int J Geriatr Psychiatry. 2006;21:86–93. doi: 10.1002/gps.1428. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin RC, Tomenson B. Depression in later life: a comparison of symptoms and risk factors in early and late onset cases. Br J Psychiatry. 1995;167:649–52. doi: 10.1192/bjp.167.5.649. [DOI] [PubMed] [Google Scholar]
- 27.Lavretsky H, Lesser IM, Wohl M, Miller BL. Relationship of age, age at onset, and sex to depression in older adults. Am J Geriatr Psychiatry. 1998;6:248–56. doi: 10.1097/00019442-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Dillon C, Allegri RF, Serrano CM, Iturry M, Salgado P, Glaser FB, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517–26. doi: 10.2147/NDT.S7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, Gorman JM. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691–8. doi: 10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- 30.Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–74. doi: 10.1212/WNL.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 31.Riddle M, Potter GG, McQuoid DR, Steffens DC, Beyer JL, Taylor WD. Longitudinal cognitive outcomes of clinical phenotypes of late-life depression. Am J Geriatr Psychiatry. 2017;25:1123–34. doi: 10.1016/j.jagp.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ly M, Karim HT, Becker JT, Lopez OL, Anderson SJ, Aizenstein HJ, et al. Late-life depression and increased risk of dementia: a longitudinal cohort study. Transl Psychiatry. 2021;11:147. doi: 10.1038/s41398-021-01269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Gunning FM, Oberlin LE, Schier M, Victoria LW. Brain-based mechanisms of late-life depression: implications for novel interventions. Semin Cell Dev Biol. 2021;116:169–79. doi: 10.1016/j.semcdb.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. Am J Psychiatry. 2018;175:1111–20. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord. 2013;151:531–9. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 37.Taylor WD, Zald DH, Felger JC, Christman S, Claassen DO, Horga G, et al. Influences of dopaminergic system dysfunction on late-life depression. Mol Psychiatry. 2022;27:180–91. doi: 10.1038/s41380-021-01265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saberi A, Mohammadi E, Zarei M, Eickhoff SB, Tahmasian M. Structural and functional neuroimaging of late-life depression: a coordinate-based meta-analysis. Brain Imaging Behav. 2022;16:518–31. doi: 10.1007/s11682-021-00494-9. [DOI] [PubMed] [Google Scholar]
- 39.Manning K, Wang L, Steffens D. Recent advances in the use of imaging in psychiatry: functional magnetic resonance imaging of large-scale brain networks in late-life depression. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.17399.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhou HX, Chen X, Shen YQ, Li L, Chen NX, Zhu ZC, et al. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206:116287. doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–11. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scalabrini A, Vai B, Poletti S, Damiani S, Mucci C, Colombo C, et al. All roads lead to the default-mode network-global source of DMN abnormalities in major depressive disorder. Neuropsychopharmacology. 2020;45:2058–69. doi: 10.1038/s41386-020-0785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Javaheripour N, Li M, Chand T, Krug A, Kircher T, Dannlowski U, et al. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl Psychiatry. 2021;11:511. doi: 10.1038/s41398-021-01619-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tozzi L, Zhang X, Chesnut M, Holt-Gosselin B, Ramirez CA, Williams LM. Reduced functional connectivity of default mode network subsystems in depression: meta-analytic evidence and relationship with trait rumination. Neuroimage Clin. 2021;30:102570. doi: 10.1016/j.nicl.2021.102570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatr Res. 2011;194:39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandelman JA, Albert K, Boyd BD, Park JW, Riddle M, Woodward ND, et al. Intrinsic functional network connectivity is associated with clinical symptoms and cognition in late-life depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:160–70. doi: 10.1016/j.bpsc.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–68. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerlach AR, Karim HT, Pecina M, Ajilore O, Taylor WD, Butters MA, et al. MRI predictors of pharmacotherapy response in major depressive disorder. Neuroimage Clin. 2022;36:103157. doi: 10.1016/j.nicl.2022.103157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dotson VM, McClintock SM, Verhaeghen P, Kim JU, Draheim AA, Syzmkowicz SM, et al. Depression and cognitive control across the lifespan: a systematic review and meta-analysis. Neuropsychol Rev. 2020;30:461–76. doi: 10.1007/s11065-020-09436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–26. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Kim Y-K. Crosstalk between depression and dementia with resting-state fMRI studies and its relationship with cognitive functioning. Biomedicines. 2021;9:82. doi: 10.3390/biomedicines9010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11:85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91:561–71. doi: 10.1016/j.biopsych.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yun JY, Kim YK. Graph theory approach for the structural-functional brain connectome of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110401. doi: 10.1016/j.pnpbp.2021.110401. [DOI] [PubMed] [Google Scholar]
- 58.Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. 2016;65:21–35. doi: 10.1016/j.neubiorev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jockwitz C, Caspers S. Resting-state networks in the course of aging—differential insights from studies across the lifespan vs. amongst the old. Pflügers Arch - Eur J Physiol. 2021;473:793–803. doi: 10.1007/s00424-021-02520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coelho A, Fernandes HM, Magalhães R, Moreira PS, Marques P, Soares JM, et al. Reorganization of brain structural networks in aging: a longitudinal study. J Neurosci Res. 2021;99:1354–76. doi: 10.1002/jnr.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng KK, Lo JC, Lim JKW, Chee MWL, Zhou J. Reduced functional segregation between the default mode network and the executive control network in healthy older adults: a longitudinal study. NeuroImage. 2016;133:321–30. doi: 10.1016/j.neuroimage.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage. 2014;102:345–57. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 64.Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci USA. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 2014;25:1987–99. doi: 10.1093/cercor/bhu012. [DOI] [PubMed] [Google Scholar]
- 66.Grady C, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016;41:159–72. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 67.Hughes C, Faskowitz J, Cassidy BS, Sporns O, Krendl AC. Aging relates to a disproportionately weaker functional architecture of brain networks during rest and task states. NeuroImage. 2020;209:116521. doi: 10.1016/j.neuroimage.2020.116521. [DOI] [PubMed] [Google Scholar]
- 68.Setton R, Mwilambwe-Tshilobo L, Girn M, Lockrow AW, Baracchini G, Hughes C, et al. Age differences in the functional architecture of the human brain. Cereb Cortex. 2022;33:114–34. doi: 10.1093/cercor/bhac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zonneveld HI, Pruim RH, Bos D, Vrooman HA, Muetzel RL, Hofman A, et al. Patterns of functional connectivity in an aging population: the Rotterdam Study. Neuroimage. 2019;189:432–44. doi: 10.1016/j.neuroimage.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 70.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morcom AM, Johnson W. Neural reorganization and compensation in aging. J Cogn Neurosci. 2015;27:1275–85. doi: 10.1162/jocn_a_00783. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Z, Johnson NF, Kim C, Gold BT. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex. 2013;25:138–46. doi: 10.1093/cercor/bht212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Z, Johnson NF, Kim C, Gold BT. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex. 2015;25:138–46. doi: 10.1093/cercor/bht212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buch AM, Liston C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology. 2021;46:156–75. doi: 10.1038/s41386-020-00789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen TD, Harder A, Xiong Y, Kowalec K, Hagg S, Cai N, et al. Genetic heterogeneity and subtypes of major depression. Mol Psychiatry. 2022;27:1667–75. doi: 10.1038/s41380-021-01413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–74. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. 2019;4:e517–e28. doi: 10.1016/S2468-2667(19)30145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychol Med. 2017;47:171–81. doi: 10.1017/S0033291716002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Ann Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 82.Iob E, Lacey R, Steptoe A. Adverse childhood experiences and depressive symptoms in later life: longitudinal mediation effects of inflammation. Brain Behav Immun. 2020;90:97–107. doi: 10.1016/j.bbi.2020.07.045. [DOI] [PubMed] [Google Scholar]
- 83.Cheong EV, Sinnott C, Dahly D, Kearney PM. Adverse childhood experiences (ACEs) and later-life depression: perceived social support as a potential protective factor. BMJ Open. 2017;7:e013228. doi: 10.1136/bmjopen-2016-013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zannas AS, McQuoid DR, Steffens DC, Chrousos GP, Taylor WD. Stressful life events, perceived stress, and 12-month course of geriatric depression: direct effects and moderation by the 5-HTTLPR and COMT Val158Met polymorphisms. Stress. 2012;15:425–34. doi: 10.3109/10253890.2011.634263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 86.Hayward RD, Taylor WD, Smoski MJ, Steffens DC, Payne ME. Association of five-factor model personality domains and facets with presence, onset, and treatment outcomes of major depression in older adults. Am J Geriatr Psychiatry. 2013;21:88–96. doi: 10.1016/j.jagp.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manning KJ, Chan G, Steffens DC. Neuroticism traits selectively impact long term illness course and cognitive decline in late-life depression. Am J Geriatr Psychiatry. 2017;25:220–9. doi: 10.1016/j.jagp.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steffens DC, McQuoid DR, Smoski MJ, Potter GG. Clinical outcomes of older depressed patients with and without comorbid neuroticism. Int Psychogeriatr. 2013;25:1985–90. doi: 10.1017/S1041610213001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75:336–46. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci. 2008;33:331–43. [PMC free article] [PubMed] [Google Scholar]
- 91.Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165–80. doi: 10.1016/j.yfrne.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 93.Georgakis MK, Thomopoulos TP, Diamantaras AA, Kalogirou EI, Skalkidou A, Daskalopoulou SS, et al. Association of age at menopause and duration of reproductive period with depression after menopause: a systematic review and meta-analysis. JAMA Psychiatry. 2016;73:139–49. doi: 10.1001/jamapsychiatry.2015.2653. [DOI] [PubMed] [Google Scholar]
- 94.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 95.Stein MB, Jain S, Giacino JT, Levin H, Dikmen S, Nelson LD, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry. 2019;76:249–58. doi: 10.1001/jamapsychiatry.2018.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24:18–33. doi: 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 97.Taylor WD, McQuoid DR, Krishnan KR. Medical comorbidity in late-life depression. Int J Geriatr Psychiatry. 2004;19:935–43. doi: 10.1002/gps.1186. [DOI] [PubMed] [Google Scholar]
- 98.Griffin SC, Young JR, Naylor JC, Allen KD, Beckham JC, Calhoun PS. Reciprocal effects between depressive symptoms and pain in veterans over 50 years of age or older. Pain Med. 2022;23:295–304. doi: 10.1093/pm/pnab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26:1109–18. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–36. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 103.Grigoleit JS, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE. 2011;6:e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 105.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. [DOI] [PubMed]
- 106.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–92. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 107.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 108.Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J Neurosci Nurs. 2012;44:206–17. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 110.Franceschi C, Zaikin A, Gordleeva S, Ivanchenko M, Bonifazi F, Storci G, et al. Inflammaging 2018: an update and a model. Semin Immunol. 2018;40:1–5. doi: 10.1016/j.smim.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 111.Meszaros A, Molnar K, Nogradi B, Hernadi Z, Nyul-Toth A, Wilhelm I, et al. Neurovascular inflammaging in health and disease. Cells. 2020;9:1614. doi: 10.3390/cells9071614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nair D, Cukor D, Taylor WD, Cavanaugh KL. Applying a biopsychosocial framework to achieve durable behavior change in kidney disease. Semin Nephrol. 2021;41:487–504. doi: 10.1016/j.semnephrol.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rutledge T, Linke SE, Krantz DS, Johnson BD, Bittner V, Eastwood JA, et al. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Psychosom Med. 2009;71:958–64. doi: 10.1097/PSY.0b013e3181bd6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drosselmeyer J, Jacob L, Rathmann W, Rapp MA, Kostev K. Depression risk in patients with late-onset rheumatoid arthritis in Germany. Qual Life Res. 2017;26:437–43. doi: 10.1007/s11136-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 115.Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70:812–20. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- 116.Luning Prak ET, Brooks T, Makhoul W, Beer JC, Zhao L, Girelli T, et al. No increase in inflammation in late-life major depression screened to exclude physical illness. Transl Psychiatry. 2022;12:118. doi: 10.1038/s41398-022-01883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–35. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 119.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–9. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: a liability factor in the etiology of psychiatric disorders. Neurobiol Stress. 2016;4:62–70. doi: 10.1016/j.ynstr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, et al. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: preliminary findings. Brain Behav Immun. 2015;46:17–22. doi: 10.1016/j.bbi.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, et al. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39:1777–85. doi: 10.1038/npp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–82. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 126.Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, et al. Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology. 2013;38:2179–87. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seaman KL, Smith CT, Juarez EJ, Dang LC, Castrellon JJ, Burgess LL, et al. Differential regional decline in dopamine receptor availability across adulthood: linear and nonlinear effects of age. Hum Brain Mapp. 2019;40:3125–38. doi: 10.1002/hbm.24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karrer TM, Josef AK, Mata R, Morris ED, Samanez-Larkin GR. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging. 2017;57:36–46. doi: 10.1016/j.neurobiolaging.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu Y, Vorburger R, Scarmeas N, Luchsinger JA, Manly JJ, Schupf N, et al. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav Immun. 2017;65:150–60. doi: 10.1016/j.bbi.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun. 2019;81:24–40. doi: 10.1016/j.bbi.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 132.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun. 2014;42:50–9. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 133.Vogelzangs N, Comijs HC, Oude Voshaar RC, Stek ML, Penninx BW. Late-life depression symptom profiles are differentially associated with immunometabolic functioning. Brain Behav Immun. 2014;41:109–15. doi: 10.1016/j.bbi.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 134.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171:1278–86. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 135.Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214:11–9. doi: 10.1192/bjp.2018.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–43. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 137.Kohler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: An umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207. doi: 10.1016/j.jpsychires.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 138.Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:339–50. doi: 10.1038/s41380-019-0474-5. [DOI] [PubMed] [Google Scholar]
- 139.Kohler-Forsberg O, C NL, Hjorthoj C, Nordentoft M, Mors O, Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404–19. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 140.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 141.Bavaresco DV, Uggioni MLR, Ferraz SD, Marques RMM, Simon CS, Dagostin VS, et al. Efficacy of infliximab in treatment-resistant depression: A systematic review and meta-analysis. Pharm Biochem Behav. 2020;188:172838. doi: 10.1016/j.pbb.2019.172838. [DOI] [PubMed] [Google Scholar]
- 142.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, et al. Melancholic and atypical major depression—connection between cytokines, psychopathology and treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:1–6. doi: 10.1016/j.pnpbp.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Zimmerman B, Rypma B, Gratton G, Fabiani M. Age-related changes in cerebrovascular health and their effects on neural function and cognition: a comprehensive review. Psychophysiology. 2021;58:e13796. doi: 10.1111/psyp.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mustapha M, Nassir C, Aminuddin N, Safri AA, Ghazali MM. Cerebral small vessel disease (CSVD)—lessons from the animal models. Front Physiol. 2019;10:1317. doi: 10.3389/fphys.2019.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 147.Krishnan KRR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 148.Blochl M, Schaare HL, Kunzmann U, Nestler S. The age-dependent association between vascular risk factors and depressed mood. J Gerontol B Psychol Sci Soc Sci. 2022;77:284–94. doi: 10.1093/geronb/gbab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krishnan KRR, Tayor WD, McQuoid DR, MacFall JR, Payne ME, Provenzale JM, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;55:390–7. doi: 10.1016/j.biopsych.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 150.Fang Y, Qin T, Liu W, Ran L, Yang Y, Huang H, et al. Cerebral small-vessel disease and risk of incidence of depression: a meta-analysis of longitudinal cohort studies. J Am Heart Assoc. 2020;9:e016512. doi: 10.1161/JAHA.120.016512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smagula SF, Aizenstein HJ. Brain structural connectivity in late-life major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:271–7. doi: 10.1016/j.bpsc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–24. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 153.Salo KI, Scharfen J, Wilden ID, Schubotz RI, Holling H. Confining the concept of vascular depression to late-onset depression: a meta-analysis of MRI-defined hyperintensity burden in major depressive disorder and bipolar disorder. Front Psychol. 2019;10:1241. doi: 10.3389/fpsyg.2019.01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meinert S, Nowack N, Grotegerd D, Repple J, Winter NR, Abheiden I, et al. Association of brain white matter microstructure with cognitive performance in major depressive disorder and healthy controls: a diffusion-tensor imaging study. Mol Psychiatry. 2022;27:1103–10. doi: 10.1038/s41380-021-01330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taylor WD, Payne ME, Krishnan KRR, Wagner HR, Provenzale JM, Steffens DC, et al. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry. 2001;50:179–83. doi: 10.1016/S0006-3223(01)01160-X. [DOI] [PubMed] [Google Scholar]
- 156.Taylor WD, Zhao Z, Ashley-Koch A, Payne ME, Steffens DC, Krishnan RR, et al. Fiber tract-specific white matter lesion severity Findings in late-life depression and by AGTR1 A1166C genotype. Hum Brain Mapp. 2013;34:295–303. doi: 10.1002/hbm.21445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–32. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taylor WD, Kudra K, Zhao Z, Steffens DC, MacFall JR. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: a pilot study. J Affect Disord. 2014;162:8–11. doi: 10.1016/j.jad.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wen MC, Steffens DC, Chen MK, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. Int J Geriatr Psychiatry. 2014;29:1173–84. doi: 10.1002/gps.4129. [DOI] [PubMed] [Google Scholar]
- 160.Zanon Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin Neurol. 2021;34:246–57. doi: 10.1097/WCO.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arola A, Levanen T, Laakso HM, Pitkanen J, Koikkalainen J, Lotjonen J, et al. Neuropsychiatric symptoms are associated with exacerbated cognitive impairment in covert cerebral small vessel disease. J Int Neuropsychol Soc. 2022; 1–8. 10.1017/S1355617722000480. [DOI] [PubMed]
- 162.Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF, 3rd, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–57. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–6. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 164.Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9:188. doi: 10.1038/s41398-019-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boutzoukas EM, O’Shea A, Kraft JN, Hardcastle C, Evangelista ND, Hausman HK, et al. Higher white matter hyperintensity load adversely affects pre-post proximal cognitive training performance in healthy older adults. Geroscience. 2022;44:1441–55. doi: 10.1007/s11357-022-00538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cui K, Song R, Xu H, Shang Y, Qi X, Buchman AS, et al. Association of cardiovascular risk burden with risk and progression of disability: mediating role of cardiovascular disease and cognitive decline. J Am Heart Assoc. 2020;9:e017346. doi: 10.1161/JAHA.120.017346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hou Y, Li Y, Yang S, Qin W, Yang L, Hu W. Gait impairment and upper extremity disturbance are associated with total magnetic resonance imaging cerebral small vessel disease burden. Front Aging Neurosci. 2021;13:640844. doi: 10.3389/fnagi.2021.640844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem. 2019;65:80–6. doi: 10.1373/clinchem.2018.287318. [DOI] [PubMed] [Google Scholar]
- 170.Harshfield EL, Pennells L, Schwartz JE, Willeit P, Kaptoge S, Bell S, et al. Association between depressive symptoms and incident cardiovascular diseases. J Am Med Assoc. 2020;324:2396–405. doi: 10.1001/jama.2020.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zahodne LB, Gilsanz P, Glymour MM, Gibbons LE, Brewster P, Hamilton J, et al. Comparing variability, severity, and persistence of depressive symptoms as predictors of future stroke risk. Am J Geriatr Psychiatry. 2017;25:120–8. doi: 10.1016/j.jagp.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/WNL.51.3.728. [DOI] [PubMed] [Google Scholar]
- 173.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75:27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69:493–8. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leung DKY, Chan WC, Spector A, Wong GHY. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36:1330–44. doi: 10.1002/gps.5556. [DOI] [PubMed] [Google Scholar]
- 176.Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, et al. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83:702–9. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3:628–35. doi: 10.1016/S2215-0366(16)00097-3. [DOI] [PubMed] [Google Scholar]
- 178.Krishnan KR, Tupler LA, Ritchie JCJ, McDonald WM, Knight DL, Nemeroff CB, et al. Apolipoprotein E-e4 frequency in geriatric depression. Biol Psychiatry. 1996;40:69–71. doi: 10.1016/0006-3223(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 179.Steffens DC, Norton MC, Hart AD, Skoog I, Corcoran C, Breitner JC. Apolipoprotein E genotype and major depression in a community of older adults. The Cache County Study. Psychol Med. 2003;33:541–7. doi: 10.1017/S0033291702007201. [DOI] [PubMed] [Google Scholar]
- 180.Harerimana NV, Liu Y, Gerasimov ES, Duong D, Beach TG, Reiman EM, et al. Genetic evidence supporting a causal role of depression in Alzheimer’s disease. Biol Psychiatry. 2022;92:25–33. doi: 10.1016/j.biopsych.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mahgoub N, Alexopoulos GS. Amyloid hypothesis: is there a role for antiamyloid treatment in late-life depression? Am J Geriatr Psychiatry. 2016;24:239–47. doi: 10.1016/j.jagp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu KY, Hsiao IT, Chen CS, Chen CH, Hsieh CJ, Wai YY, et al. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:714–22. doi: 10.1007/s00259-013-2627-0. [DOI] [PubMed] [Google Scholar]
- 183.Wu KY, Liu CY, Chen CS, Chen CH, Hsiao IT, Hsieh CJ, et al. Beta-amyloid deposition and cognitive function in patients with major depressive disorder with different subtypes of mild cognitive impairment: (18)F-florbetapir (AV-45/Amyvid) PET study. Eur J Nucl Med Mol Imaging. 2016;43:1067–76. doi: 10.1007/s00259-015-3291-3. [DOI] [PubMed] [Google Scholar]
- 184.Hyung WSW, Kang J, Kim J, Lee S, Youn H, Ham BJ, et al. Cerebral amyloid accumulation is associated with distinct structural and functional alterations in the brain of depressed elders with mild cognitive impairment. J Affect Disord. 2021;281:459–66. doi: 10.1016/j.jad.2020.12.049. [DOI] [PubMed] [Google Scholar]
- 185.Wang SM, Kim NY, Um YH, Kang DW, Na HR, Lee CU, et al. Default mode network dissociation linking cerebral beta amyloid retention and depression in cognitively normal older adults. Neuropsychopharmacology. 2021;46:2180–7. doi: 10.1038/s41386-021-01072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mackin RS, Insel PS, Landau S, Bickford D, Morin R, Rhodes E, et al. Late-life depression is associated with reduced cortical amyloid burden: findings from the Alzheimer’s disease neuroimaging initiative depression project. Biol Psychiatry. 2021;89:757–65. doi: 10.1016/j.biopsych.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Babulal GM, Roe CM, Stout SH, Rajasekar G, Wisch JK, Benzinger TLS, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimers Dis. 2020;74:1045–55. doi: 10.3233/JAD-191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58:204–10. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 189.Rosenberg PB, Drye LT, Martin BK, Frangakis C, Mintzer JE, Weintraub D, et al. Sertraline for the treatment of depression in Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:136–45. doi: 10.1097/JGP.0b013e3181c796eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Taylor WD, Boyd BD, Elson D, Andrews P, Albert K, Vega J, et al. Preliminary evidence that cortical amyloid burden predicts poor response to antidepressant medication treatment in cognitively intact individuals with late-life depression. Am J Geriatr Psychiatry. 2021;29:448–57. doi: 10.1016/j.jagp.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Li P, Hsiao IT, Liu CY, Chen CH, Huang SY, Yen TC, et al. Beta-amyloid deposition in patients with major depressive disorder with differing levels of treatment resistance: a pilot study. EJNMMI Res. 2017;7:24. doi: 10.1186/s13550-017-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Morimoto SS, Altizer RA, Gunning FM, Hu W, Liu J, Cote SE, et al. Targeting cognitive control deficits with neuroplasticity-based computerized cognitive remediation in patients with geriatric major depression: a randomized, double-blind, controlled trial. Am J Geriatr Psychiatry. 2020;28:971–80. doi: 10.1016/j.jagp.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Weintraub D, Aarsland D, Chaudhuri KR, Dobkin RD, Leentjens AF, Rodriguez-Violante M, et al. The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurol. 2022;21:89–102. doi: 10.1016/S1474-4422(21)00330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang S, Mao S, Xiang D, Fang C. Association between depression and the subsequent risk of Parkinson’s disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:186–92. doi: 10.1016/j.pnpbp.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 195.Rotter A, Lenz B, Pitsch R, Richter-Schmidinger T, Kornhuber J, Rhein C. Alpha-synuclein RNA expression is increased in major depression. Int J Mol Sci. 2019;20:2029. doi: 10.3390/ijms20082029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bruno D, Reichert Plaska C, Clark DPA, Zetterberg H, Blennow K, Verbeek MM, et al. CSF alpha-synuclein correlates with CSF neurogranin in late-life depression. Int J Neurosci. 2021;131:357–61. doi: 10.1080/00207454.2020.1744596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16:624–33. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Christman S, Bermudez C, Hao L, Landman BA, Boyd B, Albert K, et al. Accelerated brain aging predicts impaired cognitive performance and greater disability in geriatric but not midlife adult depression. Transl Psychiatry. 2020;10:317. doi: 10.1038/s41398-020-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2019;24:266–81. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19:1156–62. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Han LKM, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175:774–82. doi: 10.1176/appi.ajp.2018.17060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Robinson O, Chadeau Hyam M, Karaman I, Climaco Pinto R, Ala-Korpela M, Handakas E, et al. Determinants of accelerated metabolomic and epigenetic aging in a UK cohort. Aging Cell. 2020;19:e13149. doi: 10.1111/acel.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dunlop K, Victoria LW, Downar J, Gunning FM, Liston C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacology. 2021;46:911–9. doi: 10.1038/s41386-021-00967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2021;26:5124–39. doi: 10.1038/s41380-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Han LKM, Dinga R, Leenings R, Hahn T, Cole JH, Aftanas LI, et al. A large-scale ENIGMA multisite replication study of brain age in depression. Neuroimage Rep. 2022;2:100149. doi: 10.1016/j.ynirp.2022.100149. [DOI] [Google Scholar]
- 206.Taylor WD. Coexisting depression and frailty as an accelerated aging phenotype of late-life depression. Int Psychogeriatr. 2023;1–7. 10.1017/S1041610223000170. [DOI] [PMC free article] [PubMed]
- 207.Brown PJ, Wall MM, Chen C, Levine ME, Yaffe K, Roose SP, et al. Biological age, not chronological age, is associated with late-life depression. J Gerontol A Biol Sci Med Sci. 2018;73:1370–6. doi: 10.1093/gerona/glx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Diniz BS, Mulsant BH, Reynolds CF, 3rd, Blumberger DM, Karp JF, Butters MA, et al. Association of molecular senescence markers in late-life depression with clinical characteristics and treatment outcome. JAMA Netw Open. 2022;5:e2219678. doi: 10.1001/jamanetworkopen.2022.19678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ballester PL, Suh JS, Nogovitsyn N, Hassel S, Strother SC, Arnott SR, et al. Accelerated brain aging in major depressive disorder and antidepressant treatment response: a CAN-BIND report. Neuroimage Clin. 2021;32:102864. doi: 10.1016/j.nicl.2021.102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Han LKM, Verhoeven JE, Tyrka AR, Penninx B, Wolkowitz OM, Mansson KNT, et al. Accelerating research on biological aging and mental health: current challenges and future directions. Psychoneuroendocrinology. 2019;106:293–311. doi: 10.1016/j.psyneuen.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elliott ML, Caspi A, Houts RM, Ambler A, Broadbent JM, Hancox RJ, et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1:295–308. doi: 10.1038/s43587-021-00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36–46. doi: 10.1038/s43587-020-00017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brown PJ, Roose SP, Zhang J, Wall M, Rutherford BR, Ayonayon HN, et al. Inflammation, depression, and slow gait: a high mortality phenotype in later life. J Gerontol A Biol Sci Med Sci. 2016;71:221–7. doi: 10.1093/gerona/glv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Brown PJ, Ciarleglio A, Roose SP, Montes Garcia C, Chung S, Fernandes S, et al. Frailty and depression in late life: a high-risk comorbidity with distinctive clinical presentation and poor antidepressant response. J Gerontol A Biol Sci Med Sci. 2022;77:1055–62. doi: 10.1093/gerona/glab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Brown PJ, Roose SP, O’Boyle KR, Ciarleglio A, Maas B, Igwe KC, et al. Frailty and its correlates in adults with late life depression. Am J Geriatr Psychiatry. 2020;28:145–54. doi: 10.1016/j.jagp.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lever-van Milligen BA, Lamers F, Smit JH, Penninx BW. Six-year trajectory of objective physical function in persons with depressive and anxiety disorders. Depress Anxiety. 2017;34:188–97. doi: 10.1002/da.22557. [DOI] [PubMed] [Google Scholar]
- 217.Rutherford BR, Taylor WD, Brown PJ, Sneed JR, Roose SP. Biological aging and the future of geriatric psychiatry. J Gerontol A Biol Sci Med Sci. 2017;72:343–52. doi: 10.1093/gerona/glw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McEwen BS, Karatsoreos IN. Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep Med Clin. 2015;10:1–10. doi: 10.1016/j.jsmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Belvederi Murri M, Pariante C, Mondelli V, Masotti M, Atti AR, Mellacqua Z, et al. HPA axis and aging in depression: systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62. doi: 10.1016/j.psyneuen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 220.Gianaros PJ, Sheu LK, Uyar F, Koushik J, Jennings JR, Wager TD, et al. A brain phenotype for stressor-evoked blood pressure reactivity. J Am Heart Assoc. 2017;6:e006053. doi: 10.1161/JAHA.117.006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bagley SL, Weaver TL, Buchanan TW. Sex differences in physiological and affective responses to stress in remitted depression. Physiol Behav. 2011;104:180–6. doi: 10.1016/j.physbeh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 222.Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, et al. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry. 2015;78:67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88:233–42. doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 224.Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom Med. 2008;70:461–7. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- 225.McEwen BS, Akil H. Revisiting the stress concept: implications for affective disorders. J Neurosci. 2020;40:12–21. doi: 10.1523/JNEUROSCI.0733-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Gudnason V, et al. Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology. 2015;85:976–83. [DOI] [PMC free article] [PubMed]
- 227.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 228.Cox SR, Bastin ME, Ferguson KJ, Maniega SM, MacPherson SE, Deary IJ, et al. Brain white matter integrity and cortisol in older men: the Lothian Birth Cohort 1936. Neurobiol Aging. 2015;36:257–64. doi: 10.1016/j.neurobiolaging.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22:1504–12. doi: 10.1016/j.jagp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wertz J, Caspi A, Ambler A, Broadbent J, Hancox RJ, Harrington H, et al. Association of history of psychopathology with accelerated aging at midlife. JAMA Psychiatry. 2021;78:530–9. doi: 10.1001/jamapsychiatry.2020.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Patel K, Abdool PS, Rajji TK, Mulsant BH. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18:599–609. doi: 10.1080/14656566.2017.1308484. [DOI] [PubMed] [Google Scholar]
- 232.Tedeschini E, Levkovitz Y, Iovieno N, Ameral VE, Nelson JC, Papakostas GI. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72:1660–8. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- 233.Calati R, Salvina Signorelli M, Balestri M, Marsano A, De Ronchi D, Aguglia E, et al. Antidepressants in elderly: metaregression of double-blind, randomized clinical trials. J Affect Disord. 2013;147:1–8. doi: 10.1016/j.jad.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 234.Kok RM, Nolen WA, Heeren TJ. Efficacy of treatment in older depressed patients: a systematic review and meta-analysis of double-blind randomized controlled trials with antidepressants. J Affect Disord. 2012;141:103–15. doi: 10.1016/j.jad.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 235.Karim HT, Andreescu C, Tudorascu D, Smagula SF, Butters MA, Karp JF, et al. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol Psychiatry. 2017;22:450–7. doi: 10.1038/mp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Disabato BM, Morris C, Hranilovich J, D’Angelo GM, Zhou G, Wu N, et al. Comparison of brain structural variables, neuropsychological factors, and treatment outcome in early-onset versus late-onset late-life depression. Am J Geriatr Psychiatry. 2014;22:1039–46. doi: 10.1016/j.jagp.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reynolds CF, 3rd, Dew MA, Frank E, Begley AE, Miller MD, Cornes C, et al. Effects of age at onset of first lifetime episode of recurrent major depression on treatment response and illness course in elderly patients. Am J Psychiatry. 1998;155:795–9. doi: 10.1176/ajp.155.6.795. [DOI] [PubMed] [Google Scholar]
- 238.Kok RM, Reynolds CF., 3rd Management of depression in older adults: a review. J Am Med Assoc. 2017;317:2114–22. doi: 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 239.Lenze EJ, Mulsant BH, Roose SP, Lavretsky H, Reynolds CF, 3rd, Blumberger DM, et al. Antidepressant augmentation versus switch in treatment-resistant geriatric depression. N Engl J Med. 2023;388:1067–79. doi: 10.1056/NEJMoa2204462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172:561–9. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cooper C, Katona C, Lyketsos K, Blazer D, Brodaty H, Rabins P, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168:681–8. doi: 10.1176/appi.ajp.2011.10081165. [DOI] [PubMed] [Google Scholar]
- 242.Lenze EJ, Mulsant BH, Blumberger DM, Karp JF, Newcomer JW, Anderson SJ, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–12. doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Buchalter ELF, Oughli HA, Lenze EJ, Dixon D, Miller JP, Blumberger DM, et al. Predicting remission in late-life major depression: a clinical algorithm based upon past treatment history. J Clin Psychiatry. 2019;80:18m12483. doi: 10.4088/JCP.18m12483. [DOI] [PubMed] [Google Scholar]
- 244.George D, Galvez V, Martin D, Kumar D, Leyden J, Hadzi-Pavlovic D, et al. Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry. 2017;25:1199–209. doi: 10.1016/j.jagp.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 245.Lipsitz O, Di Vincenzo JD, Rodrigues NB, Cha DS, Lee Y, Greenberg D, et al. Safety, tolerability, and real-world effectiveness of intravenous ketamine in older adults with treatment-resistant depression: a case series. Am J Geriatr Psychiatry. 2021;29:899–913. doi: 10.1016/j.jagp.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 246.Subramanian S, Lenze EJ. Ketamine for depression in older adults. Am J Geriatr Psychiatry. 2021;29:914–6. doi: 10.1016/j.jagp.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 247.Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28:121–41. doi: 10.1016/j.jagp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 248.Prudic J, Olfson M, Marcus SC, Fuller RB, Sackeim HA. Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry. 2004;55:301–12. doi: 10.1016/j.biopsych.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 249.Dols A, Bouckaert F, Sienaert P, Rhebergen D, Vansteelandt K, Ten Kate M, et al. Early- and late-onset depression in late life: a prospective study on clinical and structural brain characteristics and response to electroconvulsive therapy. Am J Geriatr Psychiatry. 2017;25:178–89. doi: 10.1016/j.jagp.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 250.Prudic J, Haskett RF, McCall WV, Isenberg K, Cooper T, Rosenquist PB, et al. Pharmacological strategies in the prevention of relapse after electroconvulsive therapy. J ECT. 2013;29:3–12. doi: 10.1097/YCT.0b013e31826ea8c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kumar S, Mulsant BH, Liu AY, Blumberger DM, Daskalakis ZJ, Rajji TK. Systematic review of cognitive effects of electroconvulsive therapy in late-life depression. Am J Geriatr Psychiatry. 2016;24:547–65. doi: 10.1016/j.jagp.2016.02.053. [DOI] [PubMed] [Google Scholar]
- 252.Tor PC, Bautovich A, Wang MJ, Martin D, Harvey SB, Loo C. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry. 2015;76:e1092–8. doi: 10.4088/JCP.14r09145. [DOI] [PubMed] [Google Scholar]
- 253.Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am J Psychiatry. 2016;173:1101–9. doi: 10.1176/appi.ajp.2016.15081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lisanby SH, McClintock SM, Alexopoulos G, Bailine SH, Bernhardt E, Briggs MC, et al. Neurocognitive effects of combined electroconvulsive therapy (ECT) and venlafaxine in geriatric depression: phase 1 of the PRIDE study. Am J Geriatr Psychiatry. 2020;28:304–16. doi: 10.1016/j.jagp.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry. 2016;173:1110–8. doi: 10.1176/appi.ajp.2016.16010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.McCall WV, Lisanby SH, Rosenquist PB, Dooley M, Husain MM, Knapp RG, et al. Effects of continuation electroconvulsive therapy on quality of life in elderly depressed patients: a randomized clinical trial. J Psychiatr Res. 2018;97:65–9. doi: 10.1016/j.jpsychires.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Trevizol AP, Goldberger KW, Mulsant BH, Rajji TK, Downar J, Daskalakis ZJ, et al. Unilateral and bilateral repetitive transcranial magnetic stimulation for treatment-resistant late-life depression. Int J Geriatr Psychiatry. 2019;34:822–7. doi: 10.1002/gps.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–26. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.van Rooij SJH, Riva-Posse P, McDonald WM. The efficacy and safety of neuromodulation treatments in late-life depression. Curr Treat Options Psychiatry. 2020;7:337–48. doi: 10.1007/s40501-020-00216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jorge RE, Moser DJ, Acion L, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65:268–76. doi: 10.1001/archgenpsychiatry.2007.45. [DOI] [PubMed] [Google Scholar]
- 261.Overvliet GM, Jansen RAC, van Balkom A, van Campen DC, Oudega ML, van der Werf YD, et al. Adverse events of repetitive transcranial magnetic stimulation in older adults with depression, a systematic review of the literature. Int J Geriatr Psychiatry. 2021;36:383–92. doi: 10.1002/gps.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology. 2018;43:2231–8. doi: 10.1038/s41386-018-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mosimann UP, Marre SC, Werlen S, Schmitt W, Hess CW, Fisch HU, et al. Antidepressant effects of repetitive transcranial magnetic stimulation in the elderly: correlation between effect size and coil-cortex distance. Arch Gen Psychiatry. 2002;59:560–1. doi: 10.1001/archpsyc.59.6.560. [DOI] [PubMed] [Google Scholar]
- 264.Blumberger DM, Mulsant BH, Thorpe KE, McClintock SM, Konstantinou GN, Lee HH, et al. Effectiveness of standard sequential bilateral repetitive transcranial magnetic stimulation vs bilateral theta burst stimulation in older adults with depression: the FOUR-D randomized noninferiority clinical trial. JAMA Psychiatry. 2022;79:1065–73. [DOI] [PMC free article] [PubMed]
- 265.Ryff CD, Friedman EM, Morozink JA, Tsenkova V. Psychological resilience in adulthood and later life: implications for health. Annu Rev Gerontol Geriatr. 2012;32:73–92.
- 266.Pfau ML, Russo SJ. Peripheral and central mechanisms of stress resilience. Neurobiol Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Laird KT, Krause B, Funes C, Lavretsky H. Psychobiological factors of resilience and depression in late life. Transl Psychiatry. 2019;9:88. doi: 10.1038/s41398-019-0424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Laird KT, Lavretsky H, St Cyr N, Siddarth P. Resilience predicts remission in antidepressant treatment of geriatric depression. Int J Geriatr Psychiatry. 2018;33:1596–603. doi: 10.1002/gps.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Struijs SY, de Jong PJ, Jeronimus BF, van der Does W, Riese H, Spinhoven P. Psychological risk factors and the course of depression and anxiety disorders: a review of 15 years NESDA research. J Affect Disord. 2021;295:1347–59. doi: 10.1016/j.jad.2021.08.086. [DOI] [PubMed] [Google Scholar]
- 270.Duckworth AL, Peterson C, Matthews MD, Kelly DR. Grit: perseverance and passion for long-term goals. J Pers Soc Psychol. 2007;92:1087–101. doi: 10.1037/0022-3514.92.6.1087. [DOI] [PubMed] [Google Scholar]
- 271.Windsor TD, Curtis RG, Luszcz MA. Sense of purpose as a psychological resource for aging well. Dev Psychol. 2015;51:975–86. doi: 10.1037/dev0000023. [DOI] [PubMed] [Google Scholar]
- 272.Sowislo JF, Orth U. Does low self-esteem predict depression and anxiety? A meta-analysis of longitudinal studies. Psychol Bull. 2013;139:213–40. doi: 10.1037/a0028931. [DOI] [PubMed] [Google Scholar]
- 273.Laird KT, Lavretsky H, Paholpak P, Vlasova RM, Roman M, St Cyr N, et al. Clinical correlates of resilience factors in geriatric depression. Int Psychogeriatr. 2019;31:193–202. doi: 10.1017/S1041610217002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cheng C, Lau HP, Chan MP. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psychol Bull. 2014;140:1582–607. doi: 10.1037/a0037913. [DOI] [PubMed] [Google Scholar]
- 275.Cuijpers P, Karyotaki E, Pot AM, Park M, Reynolds CF., 3rd Managing depression in older age: psychological interventions. Maturitas. 2014;79:160–9. doi: 10.1016/j.maturitas.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kiosses DN, Leon AC, Arean PA. Psychosocial interventions for late-life major depression: evidence-based treatments, predictors of treatment outcomes, and moderators of treatment effects. Psychiatr Clin North Am. 2011;34:377–401. doi: 10.1016/j.psc.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shou H, Yang Z, Satterthwaite TD, Cook PA, Bruce SE, Shinohara RT, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin. 2017;14:464–70. doi: 10.1016/j.nicl.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pinquart M, Duberstein PR, Lyness JM. Treatments for later-life depressive conditions: a meta-analytic comparison of pharmacotherapy and psychotherapy. Am J Psychiatry. 2006;163:1493–501. doi: 10.1176/ajp.2006.163.9.1493. [DOI] [PubMed] [Google Scholar]
- 279.Alexopoulos GS, Arean P. A model for streamlining psychotherapy in the RDoC era: the example of ‘Engage’. Mol Psychiatry. 2014;19:14–9. doi: 10.1038/mp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Alexopoulos GS, Raue PJ, Banerjee S, Marino P, Renn BN, Solomonov N, et al. Comparing the streamlined psychotherapy “Engage” with problem-solving therapy in late-life major depression. A randomized clinical trial. Mol Psychiatry. 2021;26:5180–9. doi: 10.1038/s41380-020-0832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fung HH, Carstensen LL, Lang FR. Age-related patterns in social networks among European Americans and African Americans: implications for socioemotional selectivity across the life span. Int J Aging Hum Dev. 2001;52:185–206. doi: 10.2190/1ABL-9BE5-M0X2-LR9V. [DOI] [PubMed] [Google Scholar]
- 282.Schwarzbach M, Luppa M, Forstmeier S, Konig HH, Riedel-Heller SG. Social relations and depression in late life-a systematic review. Int J Geriatr Psychiatry. 2014;29:1–21. doi: 10.1002/gps.3971. [DOI] [PubMed] [Google Scholar]
- 283.Woods A, Solomonov N, Liles B, Guillod A, Kales HC, Sirey JA. Perceived social support and interpersonal functioning as predictors of treatment response among depressed older adults. Am J Geriatr Psychiatry. 2021;29:843–52. doi: 10.1016/j.jagp.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bosworth HB, McQuoid DR, George LK, Steffens DC. Time-to-remission from geriatric depression: psychosocial and clinical factors. Am J Geriatr Psychiatry. 2002;10:551–9. doi: 10.1097/00019442-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 285.King Johnson ML, Roche AI, Markon K, Denburg NL. Emotion regulation in older adulthood: roles of executive functioning and social relationships. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2023;30:336–53. doi: 10.1080/13825585.2022.2027331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, Glymour MM. Loneliness, depression and cognitive function in older U.S. adults. Int J Geriatr Psychiatry. 2017;32:564–73. doi: 10.1002/gps.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Perissinotto CM, Stijacic Cenzer I, Covinsky KE. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med. 2012;172:1078–83. doi: 10.1001/archinternmed.2012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Van Orden KA. Addressing loneliness in older adults during the COVID-19 pandemic: a commentary on “understanding psychological distress and protective factors among older adults during the COVID-19 pandemic”. Am J Geriatr Psychiatry. 2021;29:895–8. doi: 10.1016/j.jagp.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 289.Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–40. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 290.d’Oleire Uquillas F, Jacobs HIL, Biddle KD, Properzi M, Hanseeuw B, Schultz AP, et al. Regional tau pathology and loneliness in cognitively normal older adults. Transl Psychiatry. 2018;8:282. doi: 10.1038/s41398-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Donovan NJ, Okereke OI, Vannini P, Amariglio RE, Rentz DM, Marshall GA, et al. Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 2016;73:1230–7. doi: 10.1001/jamapsychiatry.2016.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chen JT, Wuthrich VM, Rapee RM, Draper B, Brodaty H, Cutler H, et al. Improving mental health and social participation outcomes in older adults with depression and anxiety: Study protocol for a randomised controlled trial. PLoS ONE. 2022;17:e0269981. doi: 10.1371/journal.pone.0269981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tavares LR, Barbosa MR. Efficacy of group psychotherapy for geriatric depression: a systematic review. Arch Gerontol Geriatr. 2018;78:71–80. doi: 10.1016/j.archger.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 294.Van Orden KA, Arean PA, Conwell Y. A pilot randomized trial of engage psychotherapy to increase social connection and reduce suicide risk in later life. Am J Geriatr Psychiatry. 2021;29:789–800. doi: 10.1016/j.jagp.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Choi NG, Pepin R, Marti CN, Stevens CJ, Bruce ML. Improving social connectedness for homebound older adults: randomized controlled trial of tele-delivered behavioral activation versus tele-delivered friendly visits. Am J Geriatr Psychiatry. 2020;28:698–708. doi: 10.1016/j.jagp.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bruce ML, Pepin R, Marti CN, Stevens CJ, Choi NG. One year impact on social connectedness for homebound older adults: randomized controlled trial of tele-delivered behavioral activation versus tele-delivered friendly visits. Am J Geriatr Psychiatry. 2021;29:771–6. doi: 10.1016/j.jagp.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kahlon MK, Aksan N, Aubrey R, Clark N, Cowley-Morillo M, Jacobs EA, et al. Effect of layperson-delivered, empathy-focused program of telephone calls on loneliness, depression, and anxiety among adults during the COVID-19 pandemic: a randomized clinical trial. JAMA Psychiatry. 2021;78:616–22. doi: 10.1001/jamapsychiatry.2021.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Szymkowicz SM, Ryan C, Elson DM, Kang H, Taylor WD. Cognitive phenotypes in late-life depression. Int Psychogeriatr. 2023;35:193–205. [DOI] [PMC free article] [PubMed]
- 299.Dotson VM. Can we promote cognitive resilience in late-life depression? Int Psychogeriatr. 2023;35:171–3. [DOI] [PMC free article] [PubMed]
- 300.Morimoto SS, Wexler BE, Alexopoulos GS. Neuroplasticity-based computerized cognitive remediation for geriatric depression. Int J Geriatr Psychiatry. 2012;27:1239–47. [DOI] [PMC free article] [PubMed]
- 301.Morimoto SS, Gunning FM, Wexler BE, Hu W, Ilieva I, Liu J, et al. Executive dysfunction predicts treatment response to neuroplasticity-based computerized cognitive remediation (nCCR-GD) in elderly patients with major depression. Am J Geriatr Psychiatry. 2016;24:816–20. [DOI] [PMC free article] [PubMed]
- 302.Gunning FM, Anguera JA, Victoria LW, Arean PA. A digital intervention targeting cognitive control network dysfunction in middle age and older adults with major depression. Transl Psychiatry. 2021;11:269. doi: 10.1038/s41398-021-01386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Oberlin LE, Jaywant A, Wolff A, Gunning FM. Strategies to promote cognitive health in aging: recent evidence and innovations. Curr Psychiatry Rep. 2022;24:441–50. doi: 10.1007/s11920-022-01348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cao W, Cao X, Hou C, Li T, Cheng Y, Jiang L, et al. Effects of cognitive training on resting-state functional connectivity of default mode, salience, and central executive networks. Front Aging Neurosci. 2016;8:70. doi: 10.3389/fnagi.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Locke DEC, Khayoun R, Shandera-Ochsner AL, Cuc A, Eilertsen J, Caselli M, et al. Innovation inspired by COVID: a virtual treatment program for patients with mild cognitive impairment at Mayo Clinic. Mayo Clin Proc Innov Qual Outcomes. 2021;5:820–6. doi: 10.1016/j.mayocpiqo.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Szymkowicz SM, Taylor WD, Woods AJ. Augmenting cognitive training with bifrontal tDCS decreases subclinical depressive symptoms in older adults: preliminary findings. Brain Stimul. 2022;15:1037–9. doi: 10.1016/j.brs.2022.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. J Am Med Assoc. 1989;261:2663–8. doi: 10.1001/jama.1989.03420180087036. [DOI] [PubMed] [Google Scholar]
- 309.Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Mot Control. 2002;6:19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- 310.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–7. doi: 10.1093/gerona/55.11.M691. [DOI] [PubMed] [Google Scholar]
- 311.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 312.White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–64. doi: 10.1093/gerona/gls197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–31. doi: 10.1093/gerona/55.4.M221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–9. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 316.Zheng JJ, Lord SR, Close JC, Sachdev PS, Wen W, Brodaty H, et al. Brain white matter hyperintensities, executive dysfunction, instability, and falls in older people: a prospective cohort study. J Gerontol A Biol Sci Med Sci. 2012;67:1085–91. doi: 10.1093/gerona/gls063. [DOI] [PubMed] [Google Scholar]
- 317.Crockett RA, Hsu CL, Dao E, Tam R, Alkeridy W, Eng JJ, et al. Mind the gaps: functional networks disrupted by white matter hyperintensities are associated with greater falls risk. Neurobiol Aging. 2022;109:166–75. doi: 10.1016/j.neurobiolaging.2021.09.023. [DOI] [PubMed] [Google Scholar]
- 318.Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta-analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13:1002–14. doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- 319.Klil-Drori S, Klil-Drori AJ, Pira S, Rej S. Exercise intervention for late-life depression: a meta-analysis. J Clin Psychiatry. 2020;81:19r12877. doi: 10.4088/JCP.19r12877. [DOI] [PubMed] [Google Scholar]
- 320.Rhyner KT, Watts A. Exercise and depressive symptoms in older adults: a systematic meta-analytic review. J Aging Phys Act. 2016;24:234–46. doi: 10.1123/japa.2015-0146. [DOI] [PubMed] [Google Scholar]
- 321.Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–8. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 322.Kilpatrick LA, Siddarth P, Milillo MM, Krause-Sorio B, Ercoli L, Narr KL, et al. Impact of Tai Chi as an adjunct treatment on brain connectivity in geriatric depression. J Affect Disord. 2022;315:1–6. doi: 10.1016/j.jad.2022.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Prehn K, Lesemann A, Krey G, Witte AV, Kobe T, Grittner U, et al. Using resting-state fMRI to assess the effect of aerobic exercise on functional connectivity of the DLPFC in older overweight adults. Brain Cogn. 2019;131:34–44. doi: 10.1016/j.bandc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 324.Belvederi Murri M, Amore M, Menchetti M, Toni G, Neviani F, Cerri M, et al. Physical exercise for late-life major depression. Br J Psychiatry. 2015;207:235–42. doi: 10.1192/bjp.bp.114.150516. [DOI] [PubMed] [Google Scholar]
- 325.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51:1242–51. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52:154–60. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 327.Sanders LMJ, Hortobagyi T, la Bastide-van Gemert S, van der Zee EA, van Heuvelen MJG. Dose-response relationship between exercise and cognitive function in older adults with and without cognitive impairment: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0210036. doi: 10.1371/journal.pone.0210036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hall AJ, Febrey S, Goodwin VA. Physical interventions for people with more advanced dementia—a scoping review. BMC Geriatr. 2021;21:675. doi: 10.1186/s12877-021-02577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Laird KT, Paholpak P, Roman M, Rahi B, Lavretsky H. Mind-body therapies for late-life mental and cognitive health. Curr Psychiatry Rep. 2018;20:2. doi: 10.1007/s11920-018-0864-4. [DOI] [PubMed] [Google Scholar]
- 330.Chou KL. Combined effect of vision and hearing impairment on depression in older adults: evidence from the English Longitudinal Study of Ageing. J Affect Disord. 2008;106:191–6. doi: 10.1016/j.jad.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 331.Brewster KK, Ciarleglio A, Brown PJ, Chen C, Kim HO, Roose SP, et al. Age-related hearing loss and its association with depression in later life. Am J Geriatr Psychiatry. 2018;26:788–96. doi: 10.1016/j.jagp.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Simning A, Fox ML, Barnett SL, Sorensen S, Conwell Y. Depressive and anxiety symptoms in older adults with auditory, vision, and dual sensory impairment. J Aging Health. 2019;31:1353–75. doi: 10.1177/0898264318781123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Casten RJ, Rovner BW. Update on depression and age-related macular degeneration. Curr Opin Ophthalmol. 2013;24:239–43. doi: 10.1097/ICU.0b013e32835f8e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Horowitz A, Reinhardt JP, Kennedy GJ. Major and subthreshold depression among older adults seeking vision rehabilitation services. Am J Geriatr Psychiatry. 2005;13:180–7. doi: 10.1097/00019442-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 335.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2018;175:215–24. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Heine C, Browning CJ. Mental health and dual sensory loss in older adults: a systematic review. Front Aging Neurosci. 2014;6:83. doi: 10.3389/fnagi.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Vink D, Aartsen MJ, Schoevers RA. Risk factors for anxiety and depression in the elderly: a review. J Affect Disord. 2008;106:29–44. doi: 10.1016/j.jad.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 338.Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:654–61. doi: 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Brewster KK, Hu MC, Zilcha-Mano S, Stein A, Brown PJ, Wall MM, et al. Age-related hearing loss, late-life depression, and risk for incident dementia in older adults. J Gerontol A Biol Sci Med Sci. 2021;76:827–34. doi: 10.1093/gerona/glaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Horowitz A, Brennan M, Reinhardt JP, Macmillan T. The impact of assistive device use on disability and depression among older adults with age-related vision impairments. J Gerontol B Psychol Sci Soc Sci. 2006;61:S274–80. doi: 10.1093/geronb/61.5.S274. [DOI] [PubMed] [Google Scholar]
- 341.Brody BL, Roch-Levecq AC, Kaplan RM, Moutier CY, Brown SI. Age-related macular degeneration: self-management and reduction of depressive symptoms in a randomized, controlled study. J Am Geriatr Soc. 2006;54:1557–62. doi: 10.1111/j.1532-5415.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 342.Rovner BW, Casten RJ, Hegel MT, Massof RW, Leiby BE, Ho AC, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2014;121:2204–11. doi: 10.1016/j.ophtha.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Brewster K, Choi CJ, He X, Kim AH, Golub JS, Brown PJ, et al. Hearing rehabilitative treatment for older adults with comorbid hearing loss and depression: effects on depressive symptoms and executive function. Am J Geriatr Psychiatry. 2022;30:448–58. doi: 10.1016/j.jagp.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Brewster KK, Pavlicova M, Stein A, Chen M, Chen C, Brown PJ, et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry. 2020;35:842–50. doi: 10.1002/gps.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mulrow CD, Aguilar C, Endicott JE, Tuley MR, Velez R, Charlip WS, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. 1990;113:188–94. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]