Abstract
Single atoms of platinum group metals on CeO2 represent a potential approach to lower precious metal requirements for automobile exhaust treatment catalysts. Here we show the dynamic evolution of two types of single-atom Pt (Pt1) on CeO2, i.e., adsorbed Pt1 in Pt/CeO2 and square planar Pt1 in PtATCeO2, fabricated at 500 °C and by atom-trapping method at 800 °C, respectively. Adsorbed Pt1 in Pt/CeO2 is mobile with the in situ formation of few-atom Pt clusters during CO oxidation, contributing to high reactivity with near-zero reaction order in CO. In contrast, square planar Pt1 in PtATCeO2 is strongly anchored to the support during CO oxidation leading to relatively low reactivity with a positive reaction order in CO. Reduction of both Pt/CeO2 and PtATCeO2 in CO transforms Pt1 to Pt nanoparticles. However, both catalysts retain the memory of their initial Pt1 state after reoxidative treatments, which illustrates the importance of the initial single-atom structure in practical applications.
Subject terms: Chemical engineering, Heterogeneous catalysis
The use of single atoms of platinum group metals on CeO2 is a promising approach to reduce precious metal requirements for automobile exhaust treatment catalysts. Here, the authors discovered that by manipulating the calcination temperatures, they could control the configuration of Pt1 on the CeO2 surface, leading to differences in CO oxidation activity.
Introduction
Single-atom catalysts (SACs) have been attracting widespread attention in the catalysis community for the past decade1,2. Among them, CeO2-supported SACs are particularly interesting because of the oxygen storage capacity of CeO2 and the ability of CeO2 to intrinsically trap platinum group metals (PGMs: Pt, Pd, Rh, etc.) under high-temperature oxidative condition3–6. CeO2-supported PGMs prepared by atom-trapping (AT) method at 800 °C have recently been reported to be promising sintering-resistant catalysts for the removal of vehicle criteria pollutants (e.g., CO, NOx, and hydrocarbons)7–9. While the maximum atomic utilization can be realized for isolated PGMs (e.g., Pt1), the intrinsic activity of Pt1 is usually lower than Pt aggregates10–12. To circumvent this issue, Pt1 on CeO2 was transformed to more active Pt nanoparticles (NPs, <2 nm) via the treatment in reducing atmospheres (CO, H2, or HCs) at elevated temperatures10,13,14. However, these agglomerated Pt NPs redisperse into less-active Pt1 under an additional treatment in O2 or even in a lean reaction condition at temperatures higher than 400 °C15,16, which complicates their applications in practical exhaust gas treatment.
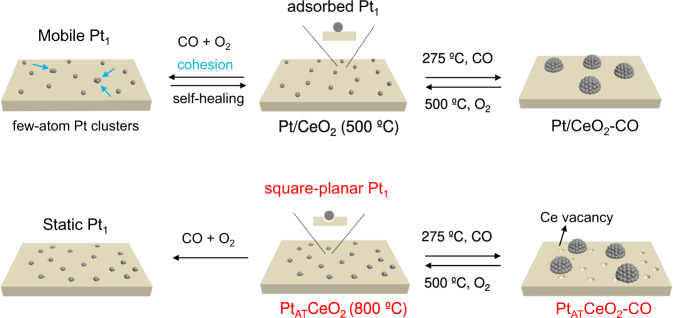
It has been reported that single-atom Pt, Pd, or Cu on CeO2 fabricated by different annealing temperatures show various catalytic performances13,17,18. However, the origin of significant reactivity difference induced by different annealing temperatures is still unknown4,7–10. Although the dynamics of Pt1 under reductive and oxidative conditions are both studied, the dynamic evolution of different types of Pt1 under a real reaction condition is still missing. Understanding the initial Pt1 structure and its dynamics under reaction conditions is of great importance to design more efficient Pt1 or its derived active site for practical exhaust gas treatment. Therefore, two types of Pt1 on CeO2 catalysts were fabricated, one via treatment at 500 °C (Pt/CeO2) and the second by atom-trapping method at 800 °C (PtATCeO2). The local structure and dynamic behavior of the two Pt1 structures under CO oxidation condition were studied by in situ X-ray absorption spectroscopy (XAS), in situ infrared spectroscopy, quasi in situ X-ray photoelectron spectroscopy (XPS), and density functional theory (DFT) calculations. Both types of Pt1 structures were studied under treatment in CO at 275 °C which led to the formation of Pt NPs in both Pt/CeO2-CO and PtATCeO2-CO, followed by a reoxidative treatment at 500 °C to disintegrate the as-formed Pt NPs to form Pt1 again in Pt/CeO2-CO-O2 and PtATCeO2-CO-O2 (Fig. 1). The dynamics of Pt1 under CO oxidation, reductive and oxidative treatments are investigated by comparing their CO oxidation activity, reaction kinetics, characterization results, and theoretical calculations.
Fig. 1. Dynamics of Pt1 under different conditions.
Schematic illustration of dynamic behaviors of single-atom Pt1 in Pt/CeO2 and PtATCeO2 under CO oxidation, reductive, and oxidative conditions at elevated temperatures.
Results and discussion
Single-atom Pt1 structure in fresh Pt/CeO2 and PtATCeO2
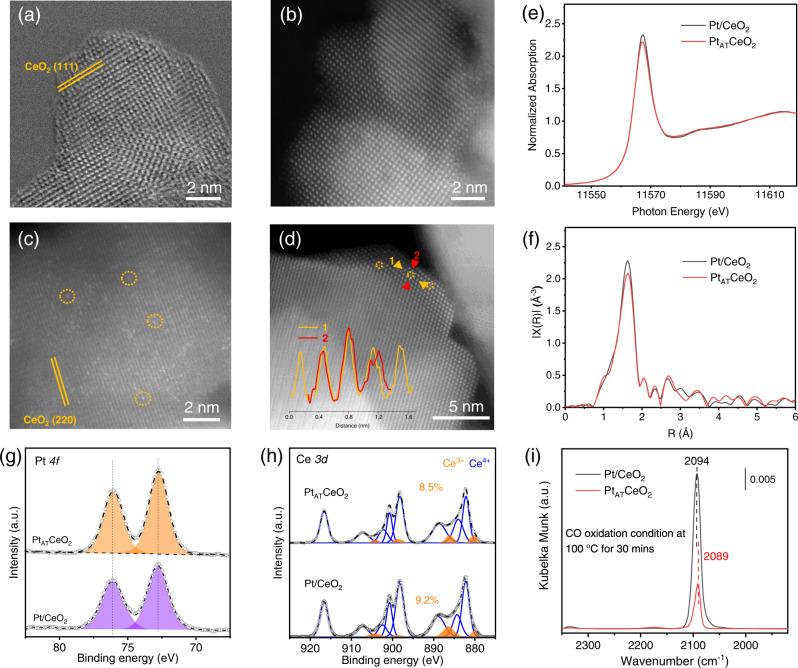
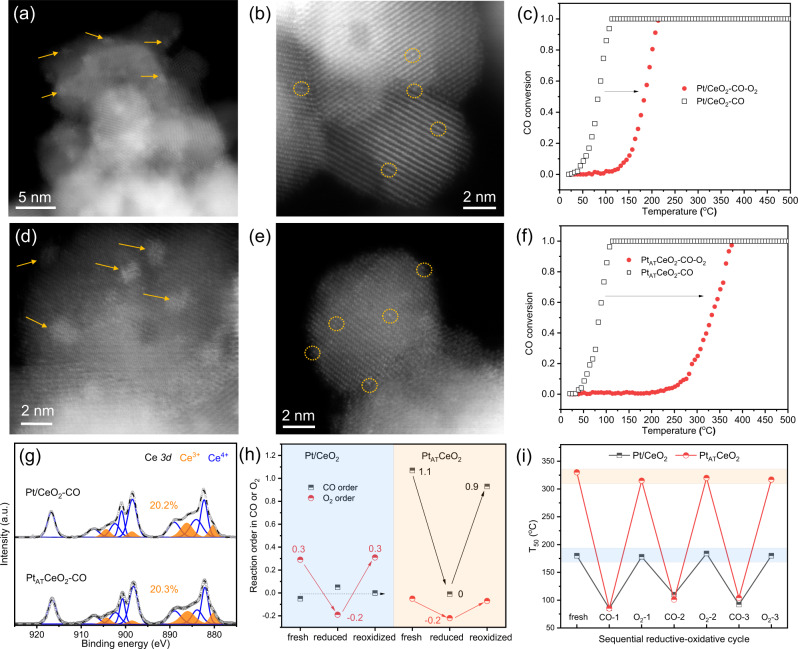
Pt/CeO2 and PtATCeO2 with ~1 wt% Pt loading (Supplementary Table S1) were synthesized by two post-calcination temperatures of 500 and 800 °C in air, in which the calcination temperature of 800 °C represents a previously reported atom-trapping method8. Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images in Fig. 2a–d and Supplementary Fig. S1, and line-scanning results in the inset of Fig. 2d display that isolated Pt1 atoms are present in Pt/CeO2 and PtATCeO2. The powder X-ray diffraction (XRD) patterns of Pt/CeO2 and PtATCeO2 in Supplementary Fig. S2 show only the diffraction peaks for fluorite CeO2. Pt L3-edge X-ray absorption near edge structure (XANES) spectroscopy in Fig. 2e, Supplementary Fig. S3 exhibits a white line intensity slightly lower than the PtO2 (Pt4+) reference, indicating a cationic Ptσ+ nature (σ < 4)19. In contrast, Pt 4f X-ray photoelectron spectroscopy (XPS) in Fig. 2g displays a similar characteristic of Pt2+ for two fresh samples3. The observed different Pt valences (Pt2+ in XPS, near Pt4+ in XANES) are mainly ascribed to various oxygen partial pressures in XANES (ambient air) and XPS (vacuum) measurement conditions20. Both the percentage of surface Ce3+ and the defect-related O in Pt/CeO2 and PtATCeO2 are similar (Fig. 2h, Supplementary Fig. S4). The extended X-ray absorption fine structure (EXAFS) results in Fig. 2f, Supplementary Fig. S3, display that the two samples are dominated by the first-shell Pt-O contribution, and the corresponding coordination number (CN) is 5.0 ± 0.43 for Pt/CeO2, and 4.9 ± 0.52 for PtATCeO2 (Supplementary Table S2, Supplementary Figs. S5 and S6). The above ex situ characterizations suggest that the two fresh catalysts have the same atomically dispersed nature, similar Pt valence, similar Pt-O local coordination, and similar Ce3+ and defect-related O percentage. However, their difference can be revealed by diffuse-reflectance infrared Fourier transform spectra with CO as a probe molecule (CO-DRIFTS) (Fig. 2i, Supplementary Fig. S7), which reveals a single IR band at ~2094 for Pt/CeO2 and ~2089 cm−1 for PtATCeO2 at 100 °C under CO oxidation condition, ascribed to CO linearly adsorbed on ionic Pt21. The different vibration frequencies of adsorbed CO molecules can be tentatively assigned to their different CO-Pt1 interactions under CO oxidation condition22. This implies the possible structural change of Pt1 from ambient air to CO oxidation condition for Pt/CeO2 or PtATCeO2. Previous studies reported that Pt1 on CeO2 synthesized by atom-trapping method holds a square planar structure7,21,23; however, Pt1 structure synthesized at low calcination temperature is less discussed.
Fig. 2. Ex situ characterizations.
HAADF-STEM images of a, b Pt/CeO2 and c, d PtATCeO2 (Pt1 is marked as cycles, and line-scanning of a single Pt1 is shown in the inset of (d). e Pt L3-edge XANES and f the corresponding magnitude of the Fourier transform of the EXAFS spectra of Pt/CeO2 and PtATCeO2. g, h Pt 4f and Ce 3d XPS spectra of Pt/CeO2 and PtATCeO2. i In situ CO diffuse-reflectance infrared Fourier transform spectra (CO-DRIFTS) for Pt/CeO2 and PtATCeO2 under CO oxidation condition at 100 °C for 30 min.
CO oxidation activity and reaction kinetics
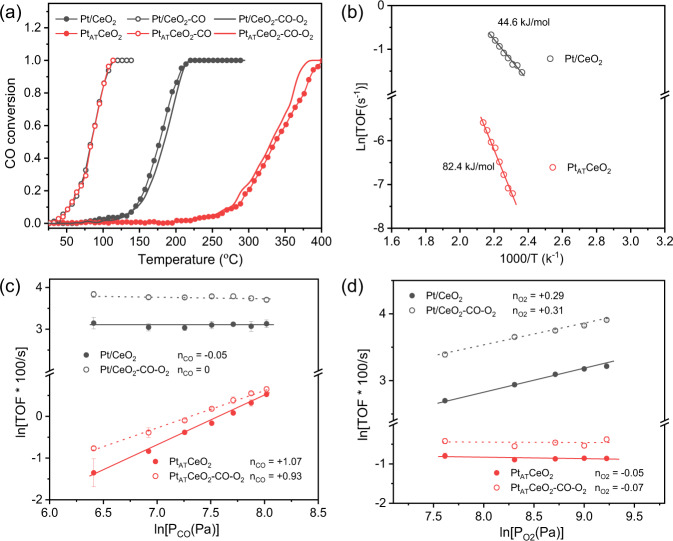
Pt/CeO2 and PtATCeO2 were then evaluated for CO oxidation under O2-rich (lean) conditions with a weight hourly space velocity (WHSV) of 300 L/g*h. The light-off curves and corresponding Arrhenius plots in Fig. 3a and Supplementary Fig. S8 show that Pt/CeO2 is more active than PtATCeO2. For instance, T50 (temperature for 50% CO conversion) for Pt/CeO2 and PtATCeO2 are 180 and 335 °C, respectively. Five repeated light-off curves (Supplementary Fig. S9) display that two catalysts show stable catalytic performance and are both more active than the pristine CeO2 (Supplementary Fig. S10). The obtained apparent activation energies (Ea) of Pt/CeO2 and PtATCeO2 in the same temperature region (160–215 °C) by changing the WHSV are 44.6 and 82.4 kJ/mol (Fig. 3b), suggesting the reaction energy barrier in Pt/CeO2 is distinctly lower than that in PtATCeO2. Moreover, the reaction order at ~200 °C in CO is ~0 for Pt/CeO2 but +1 for PtATCeO2 (Fig. 3c). The near-zero reaction order in CO suggests the more favorable CO adsorption on Pt/CeO2, which can be confirmed by a higher intensity of adsorbed CO peak in CO-DRIFTS (Fig. 2i) and more CO2 evolution in temperature-programmed desorption of CO (CO-TPD, Supplementary Fig. S11). The kinetic feature of Pt/CeO2 is also similar to that of reduced Pt/CeO2 and PtATCeO2 samples obtained after a reduction in CO at 275 °C (Supplementary Fig. S12), as well as the Pt or Pd clusters on CeO211,24. This indicates that Pt1 might sinter in Pt/CeO2 under CO oxidation conditions. Increasing the surface coverage of Pt1 is observed for two SACs with increasing CO partial pressure in CO-DRIFTS experiments at 100 °C (Supplementary Fig. S13a, b). However, the surface CO coverage in Pt/CeO2 is higher than that in PtATCeO2 under the same condition, suggesting CO adsorption on Pt/CeO2 is more kinetic-irrelevant, in agreement with the results in Fig. 3c. Moreover, the reaction orders in O2 (Fig. 3d) over the two catalysts are also different, i.e., +0.3 for Pt/CeO2 and ~0 for PtATCeO2. Based on O2-dependent CO-DRIFTS results (Supplementary Fig. S13c, d), higher O2 partial pressure leads to a higher surface CO coverage on Pt/CeO2; however, O2 partial pressure does not have a noticeable effect on CO coverage on PtATCeO2. The above activity and kinetics suggest different dynamic behaviors of Pt1 in Pt/CeO2 and PtATCeO2 under CO oxidation condition. Both CO-DRIFTS (Fig. 2i) and CO oxidation kinetics suggest that two SACs hold different Pt1 structures under CO oxidation condition.
Fig. 3. Catalytic evaluation.
a CO oxidation performance (light-off curve) with 20 mg catalyst, and b Arrhenius plots of Pt/CeO2 and PtATCeO2 with different catalyst loadings (4 mg Pt/CeO2, 300 mg PtATCeO2). Reaction conditions: 1% CO and 4% O2 balanced with Ar, catalyst diluted with SiC to 400 mg, total flow rate = 100 mL/min. Effect of c CO and d O2 partial pressure on the reaction rate (TOF). Measurement conditions: PCO = 0.6–3 kPa, PO2 = 4 kPa in (c); PCO = 1 kPa, PO2 = 2–10 kPa in (d). The operating temperatures for Pt/CeO2, PtATCeO2, Pt/CeO2-CO-O2, and PtATCeO2-CO-O2 are 210, 210, 220, and 215 °C, respectively, in (c, d).
Additionally, Pt/CeO2 and PtATCeO2 with a lower Pt loading (~0.1 wt%) also display a similar activity trend (Supplementary Figs. S14–S18). The detailed discussion is provided in Supplementary Information after Supplementary Figs. S14 and S15. It has been reported that surface reconstruction of CeO2 at different calcination temperatures would affect the catalytic activity25. To minimize these effects, the CeO2 support was pre-calcined at 800 °C for 10 h to yield 800CeO2, followed by deposition of 0.1 wt% Pt (to maintain the atomically dispersed nature) at 500 and 800 °C to yield 0.1Pt/800CeO2 and 0.1PtAT800CeO2, respectively. Since the support was pre-calcined at 800 °C, these two samples exhibited similar porosity properties (Supplementary Fig. S17, Supplementary Table S3) and CeO2 particle size (Supplementary Fig. S18) as the 800CeO2 support. We found that the activity of 0.1Pt/800CeO2 was still significantly higher than that of 0.1PtAT800CeO2 (Supplementary Fig. S14), similar to Pt/CeO2 and PtATCeO2 (Fig. 3a).
Dynamic evolution under CO oxidation condition
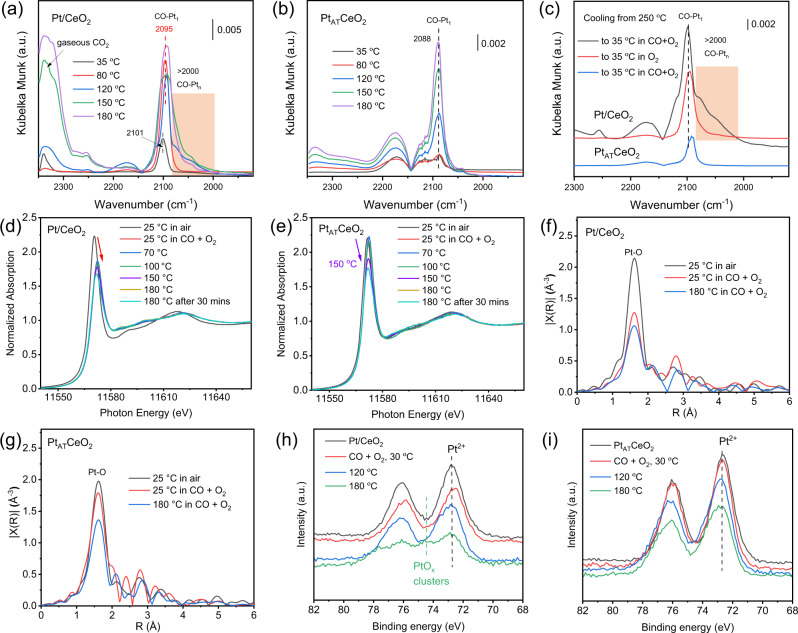
To probe the activity origin of Pt/CeO2 and PtATCeO2, in situ CO-DRIFTS was first performed under CO oxidation conditions at different temperatures. At 35 or 80 °C, PtATCeO2 shows a similar weak IR peak centered at ~2088 cm−1 (Fig. 4b)12,19, which becomes increasingly apparent at 120 °C. It has been reported that square planar Pt1 hardly chemisorbs CO26,27, and high-temperature treatment in CO + O2 reconstructs new Pt1 cations, which are capable of adsorbing CO22, in agreement with our PtATCeO2 results. In contrast, Pt/CeO2 shows a stronger adsorbed CO-Pt1 peak (~2101 cm−1) at 35 °C (Fig. 4a), which becomes more intense at 80 °C with a red shift, suggesting a possible reduction of Pt1. An obvious shoulder (2000–2060 cm−1) is observed in Pt/CeO2 above 120 °C, along with an increased intensity of gaseous CO2 in the IR cell, indicating that the reaction has begun and a new Pt species has formed. This shoulder is a typical characteristic of Pt clusters10,11. The above results show the reduction and sintering of Pt1 in Pt/CeO2 under the elevated reaction temperature. However, the features (<2000 cm−1) ascribed to bridge adsorbed CO on the traditional large Pt NPs28–30 are not observed, which can be seen in the reduced Pt/CeO2 and PtATCeO2 (Supplementary Fig. S19). After cooling down to 35 °C in CO + O2 from 250 °C, the Pt clusters feature can still be found (Fig. 4c). This feature disappears after cooling down in O2, suggesting as-formed Pt clusters completely redisperse on CeO2 in O2. These suggest that Pt1 in Pt/CeO2 may only sinter into few-atom Pt clusters under reaction conditions due to the presence of self-healing of Pt clusters into Pt1 under O2-rich condition. The co-existence of Pt1 cohesion and self-healing of Pt clusters in Pt/CeO2 is crucial to maintain fully exposed Pt clusters under CO oxidation condition31. It should be emphasized that a gaseous CO2 signal shows up at 35 °C and then disappears at 80 °C for Pt/CeO2 in CO-DRIFTS (Fig. 4a). To validate this phenomenon, we then performed the temperature-programmed surface reaction (Supplementary Fig. S20). Once CO is introduced in O2-treated samples at 35 °C, an immediate and ephemeral CO2 evolution together with CO consumption is found only in Pt/CeO2, suggesting the active O (or weakly bonded O) in Pt/CeO2 can react with CO to form CO2 at 35 °C. Meanwhile, a surface Pt1 reconstruction in Pt/CeO2 must occur due to the loss of surface O.
Fig. 4. In situ characterizations.
In situ CO-DRIFTS for a Pt/CeO2 and b PtATCeO2 in CO and O2 mixture as varying the temperature from 35 to 180 °C, as well as c the CO-DRIFTS after reaction at 250 °C, and cooling down to 35 °C in CO + O2 or O2. Pt L3-edge in situ XANES of d Pt/CeO2, and e PtATCeO2 at 25 °C in ambient air and different reaction temperatures (25, 70, 100, 150, 180 °C) in CO oxidation condition (CO/O2 ratio is 1:4). the corresponding magnitude of the Fourier transform of the EXAFS of f Pt/CeO2 and g PtATCeO2 at 25 °C in ambient air, and at 25 °C, 180 °C under reaction condition, k = 3–12.5 Å−1 for the Fourier transform. Quasi in situ Pt 4f XPS spectra for h Pt/CeO2 and i PtATCeO2 without or with treatment at different reaction temperatures (CO/O2 ratio is 1:4) for 20 min. After treatment, gases were pumped for the XPS test.
To further study the dynamic evolution of Pt1, in situ XANES data were collected under CO oxidation condition. For Pt/CeO2, an obvious decrease of the white line intensity is observed while switching the exposed atmosphere from ambient air to CO and O2 at 25 °C (Fig. 4d), indicating Pt1 transforms from near Pt4+ in ambient air to ~Pt2+ in CO and O2, as compared with the Pt reference (Supplementary Fig. S21). This finding explains why a CO2 signal is observed at 35 °C in Pt/CeO2 after introducing CO + O2 (Fig. 4a, Supplementary Fig. S20). The decreased Pt valence can also be evidenced by the decreased first-shell Pt-O CN (5 to 3.1) from in situ EXAFS (Fig. 4f, Supplementary Table S2), clearly indicating the abovementioned active O in Pt/CeO2 directly bonds with Pt1. As further increasing temperature to 180 °C in CO + O2, Pt valence in Pt/CeO2 descends slowly (Fig. 4d). In contrast, white line intensity in PtATCeO2 is stable after flowing CO and O2 at 25 °C or even at 100 °C, and it decreases only at 150 °C (Fig. 4e). The Pt-O CN decreases from 4.9 at 25 °C to 3.2 at 180 °C (Fig. 4g), indicating Pt1 in PtATCeO2 reconstructs into lower-valence Pt1 at increased temperature. Nonetheless, Pt-O CN in PtATCeO2 at 180 °C is still higher than 2.8 found in Pt/CeO2 (Supplementary Table S2). After CO oxidation treatment at different temperatures, XPS data were collected quasi in situ. For Pt/CeO2, the mild treatment at 30 °C does not influence the XPS signal, but a new feature appears at 180 °C, as seen in Fig. 4h. This suggests the formation of Pt species with the valence higher than 2. Based on the previous studies30, the oxidation of Pt NPs to PtO2/PtO cluster mixture or the formation of thin PtOx oxide film can induce the formation of Pt cations (>2+). In comparison, this new Pt feature is not observed in PtATCeO2 under the same treatment condition (Fig. 4i). Therefore, we ascribe the newly formed Pt species under CO oxidation condition in Pt/CeO2 as few-atom Pt clusters (Fig. 1). Moreover, the relatively lower Pt-O CN in Pt/CeO2 at 180 °C is ascribed to the formation of few-atom Pt clusters under reaction condition by combining with CO-DRIFTS, XPS, and kinetics studies.
Dynamic evolution under reductive-oxidative cycle and structural memory
To further investigate the difference between the two Pt1 configurations, we designed a cohesion-redispersion cycle experiment. Two SACs are first treated in CO at 275 °C to form Pt/CeO2-CO and PtATCeO2-CO. Pt NPs (1–2 nm in size) in reduced samples can be evidenced by HAADF-STEM images (Fig. 5a, d, Supplementary Fig. S22), XPS32–34 (Supplementary Fig. S23), CO-DRIFTS (Supplementary Fig. S19), and Raman spectroscopy (Supplementary Fig. S24). Pt/CeO2-CO and PtATCeO2-CO show similar enhanced CO oxidation reactivity (Fig. 3a) and similar reaction orders (Supplementary Fig. S12), ascribed to the presence of Pt clusters13. The percentage of Ce3+ and surface defect-related O also increases after CO reduction (Fig. 5g, Supplementary Fig. S25). However, the increased activity is lost during the repeated CO oxidation experiments from 25 to 500 °C for both reduced catalysts (Supplementary Fig. S26). If we treat Pt/CeO2-CO and PtATCeO2-CO in O2 at 500 °C, both enhanced activities will also decrease and become similar to their respective initial activity (Fig. 5c, f). The activity loss is due to the redispersion of Pt NPs into Pt1 evidenced by HAADF-STEM images (Fig. 5b, e, Supplementary Fig. S27) and CO-DRIFTS (Supplementary Figs. S28 and S29) results. Moreover, adsorbed CO-Pt1 peak (Supplementary Figs. S28 and S29) in reoxidized Pt/CeO2-CO-O2 and PtATCeO2-CO-O2 is located at ~2095 and ~2089 cm−1, respectively, that is consistent with their respective fresh sample (Fig. 2i). This implies that two kinds of Pt1 appear to have memory back to their initial state after a cohesion-redispersion cycle. More interestingly, the reaction kinetics also shows a similar memory behavior. Specifically, the reaction orders in CO for Pt/CeO2 in three states (fresh-reduced-reoxidized) are all closer to 0 but change from 0.3 through −0.2 to 0.3 in O2 (Fig. 5h). In PtATCeO2, the reaction order changes from 1.1 through 0 to 0.9 in CO, and from 0 through −0.2 to 0 in O2. We also reduced Pt1 in Pt/CeO2 and PtATCeO2 with H2 instead of CO, and the enhanced reactivity was also lost after a further reoxidation treatment at 500 °C (Supplementary Fig. S30). This indicates two catalysts have structural memory after both CO-O2 and H2-O2 treatment cycles. Furthermore, T50 of Pt/CeO2 and PtATCeO2 after the sequential reductive-oxidative cycle (Fig. 5i) show that the cohesion-redispersion behavior of Pt1 can be repeated many times. Therefore, we believe that after a reduction-reoxidation cycle, two Pt1 configurations in Pt/CeO2 and PtATCeO2 both return to their initial structure.
Fig. 5. Structural memory under reductive-oxidative cycle.
STEM images of a Pt/CeO2-CO, b Pt/CeO2-CO-O2, d PtATCeO2-CO, and e PtATCeO2-CO-O2. Light-off curves of reduced and reoxidized c Pt/CeO2 and f PtATCeO2. g Ce 3d XPS spectra of Pt/CeO2-CO and PtATCeO2-CO; XPS data are collected after treatment without exposure to air. h Reaction orders in CO and O2 for Pt/CeO2 and PtATCeO2 in three states. i T50 results of Pt/CeO2 and PtATCeO2 after a sequential reductive-oxidative cycle. Reaction conditions in (c, f, i) are the same as that in Fig. 3a.
Theoretical insight into the dynamic behaviors
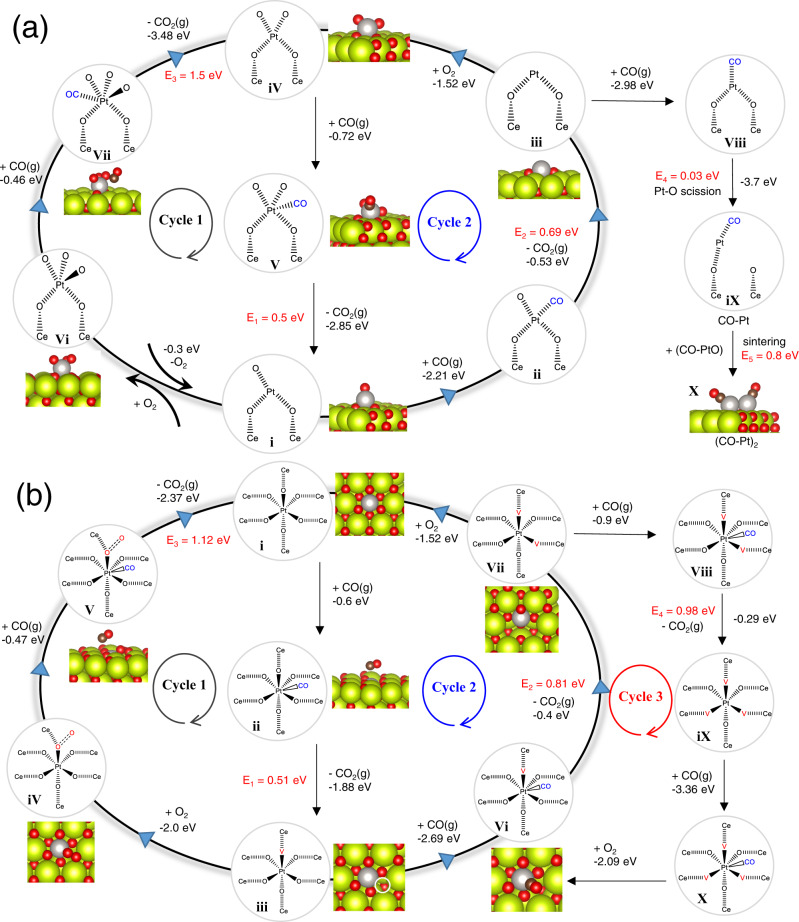
To explain the above dynamic behaviors, the nascent Pt1 structures of Pt/CeO2 and PtATCeO2 are identified first. Based on the previous studies7,21–23, PtATCeO2 is dominated by square planar Pt1 structure on CeO2(111) terrace (Supplementary Fig. S31b) or step site (Supplementary Fig. S31c). However, Pt1 configuration in Pt/CeO2 is still unknown. To understand if Pt1 in Pt/CeO2 is another reported single-atom structure–adsorbed Pt1 (Supplementary Fig. S31a)35,36, we first compare EXAFS fitting results of Pt/CeO2 and adsorbed Pt1 models (Supplementary Fig. S31d), and the adsorbed PtO5 model on CeO2 (111) fits well with Pt/CeO2. Then, we calculate the oxygen vacancy (VO) formation energy of neighboring O of both adsorbed Pt1 and square planar Pt1. It is found that the VO formation energy of adsorbed Pt1 is significantly lower than that of square planar Pt1 (Supplementary Fig. S32). This indicates that neighboring O atoms of adsorbed Pt1 are easier to remove, consistent with previous results (Fig. 4a, d, Supplementary Fig. S20), which further justifies our proposed adsorbed Pt1 model for Pt/CeO2. Therefore, we assume that our Pt/CeO2 is mainly composed of adsorbed PtO5 structure in air (Supplementary Fig. S31a). Under CO oxidation, the adsorbed PtO5 adsorbs CO with the adsorption energy of −0.46 eV (Vi to Vii, Fig. 6a), but the adsorbed CO-PtO5 is difficult to release CO2 with an energy barrier of 1.5 eV (Vii to iV). Therefore, cycle 1 in Fig. 6a is unlikely to occur. Instead, the PtO5 structure can easily transform into a PtO3 structure (Vi to i) with an exothermic energy of −0.3 eV, consistent with in situ XAS result (Fig. 4d). The formed PtO3 adsorbs CO strongly (i to ii), then releases CO2 to form PtO2 with an energy barrier of 0.69 eV (ii to iii). O2 fills the Ov around PtO2 to form PtO4 with an exothermic energy of −1.52 eV, followed by a CO adsorption (iii to iV to V). The adsorbed CO-PtO4 loses CO2 to form PtO3 with the energy barrier of 0.5 eV to complete cycle 2. PtO2 can also adsorb CO strongly to form CO-PtO2; however, Pt-O scission occurs spontaneously with a strong exothermic energy of −3.7 eV (Viii to iX) to form CO-PtO structure (iX). Assuming that there are two CO-PtO on the CeO2 surface, the calculated Pt-Pt cohesion energy barrier is 0.8 eV (iX to X), which indicates the possible Pt-Pt cohesion under reaction condition in Pt/CeO2.
Fig. 6. DFT calculation.
CO oxidation reaction mechanism on a adsorbed Pt1 and b square planar Pt1 on CeO2 (111) terrace. The main model structures are shown in the reaction cycle.
In Fig. 6b, square planar Pt1 on CeO2(111) terrace is adpoted23. First, CO adsorbs on square planar Pt1 (PtO6) with an adsorption energy of −0.6 eV (i to ii), much lower than −2.21 eV observed on PtO3 in Pt/CeO2. This is consistent with the stronger IR signal found in Pt/CeO2 at 35 and 80 °C (Fig. 4a, b). Thereafter, CO-PtO6 requires a moderate energy barrier of 0.5 eV to release CO2 to form PtO5 (ii to iii), which is why the white line intensity of PtATCeO2 only decreases at 150 °C (Fig. 4e). PtO5 can either adsorb O2 or CO to form PtO5(O2) (iii to iV) or CO-PtO5 (iii to Vi). However, CO adsorbed on PtO5(O2) requires an energy barrier of 1.12 eV to release CO2 (V to i), which makes cycle 1 less favorable. In contrast, CO-PtO5 loses CO2 to form PtO4 with an energy barrier of 0.81 eV. It should be noted that the formed PtO4 here has a similar structure as square planar Pt1 on the CeO2 step site (Vii), so the step-site situation is not considered individually. PtO4 can either adsorb O2 to close cycle 2 or adsorb CO to form CO-PtO4, which will further transform to PtO3 after CO2 removal. PtO3 then adsorbs CO strongly, but adsorbed CO-PtO3 is unlikely to transform to PtO2 due to its endothermic nature. Instead, it adsorbs O2 with an adsorption energy of −2.09 eV to complete cycle 3, which prevents the sintering of Pt1. The overall energy barriers of relatively favorable cycle 2 in adsorbed Pt1 in Pt/CeO2 and square planar Pt1 in PtATCeO2 are 0.69 and 0.81 eV, respectively. Such a small difference should not induce the huge activity difference in Fig. 3, which also implies parts of Pt1 in Pt/CeO2 sinter under CO oxidation condition. Supplementary Fig. S33 shows the simulated CO vibrational frequencies on both adsorbed and square planar Pt1. The calculated vibrational frequencies of adsorbed CO on PtO3 for both Pt1 structures are consistent with the CO-DRIFTS results, indicating that the observed CO-Pt1 band in CO-DRIFTS can be attributed to the adsorbed CO on PtO3. What sets Pt/CeO2 apart is that PtO3 further transforms into PtO and then sinters at higher temperatures. However, PtO3 is relatively stable in PtATCeO2.
It has been reported that Ce vacancy (VCe) is more difficult to generate compared to the VO37. However, we can speculate that the exsolution of square planar Pt1 on the CeO2 terrace in PtATCeO2 is a strategy to generate VCe. The possible reason for the formation of square planar Pt1 only at 800 °C is the lattice expansion of CeO2 at high temperatures, as seen in in-situ XRD (Supplementary Fig. S34) and the favorable migration of cerium cations to PGMs surface at higher temperatures in O238–40. These could facilitate the surface CeO2 reconstruction around the Pt atom or migration of cerium cations onto the Pt surface to form a local square planar Pt1 structure (Supplementary Fig. S35). We then construct two models for Pt/CeO2-CO and PtATCeO2-CO with a five-atom Pt NP on CeO2 (111) surface with and without VCe (Supplementary Fig. S36) to simulate their redispersion process. This process includes the oxidation of the five-atom Pt NP to the PtOx cluster and the redispersion of the top Pt atom (Supplementary Fig. S37). The results indicate that the redispersion of the top Pt atom into a surrounding VCe is energetically more favorable than the intact CeO2 surface (formation energy: −2.02 eV vs 0.26 eV). Therefore, from a thermodynamic point of view, VCe generated after the exsolution of square planar Pt1 could, in return, trap Pt atoms more readily during a reoxidation treatment to form a square planar structure again. In contrast, Pt clusters are formed without surface VCe after reducing the adsorbed Pt1 on CeO2, and then it redisperses into adsorbed form after a reoxidation treatment. This explains why two SACs have the memory to return to their respective native structure. The different dynamic evolution under CO oxidation condition and their structural memory behaviors under reductive-oxiditive treatment cycle is due to their various initial Pt1 location on CeO2 driven by different calcination temperatures. Therefore, designing SACs with tunable location is important to maximize their catalytic performance in the future.
In summary, Pt/CeO2 and PtATCeO2 were fabricated via two different annealing temperatures of 500 and 800 °C. Pt atoms are both atomically dispersed in nascent Pt/CeO2 and PtATCeO2, evidenced by the combined characterization results. These two catalysts display dramatically different catalytic activity toward CO oxidation and different apparent activation energies and reaction orders in CO and O2. These differences could be explained by the different initial Pt1 local configurations, where Pt1 in Pt/CeO2 and PtATCeO2 are dominated by adsorbed Pt1 and square planar Pt1, respectively. Under reaction condition, adsorbed Pt1 in Pt/CeO2 sinters into few-atom Pt clusters; however, square planar Pt1 in PtATCeO2 is strongly anchored to the support with a decrease in the Pt-O coordination number. After the treatment in CO at 275 °C, both types of Pt1 transform to Pt NPs, which inevitably redisperse at the elevated temperature in O2 or even under O2-rich reaction condition. What is more interesting is that the initial thermal treatment creates memory on the support where the Pt atoms return under CO oxidation or oxidative conditions, potentially providing a catalyst self-healing after severe catalyst sintering.
Methods
Synthesis of Pt/CeO2 and PtATCeO2
CeO2 powder was synthesized by the precipitation method with ammonia, followed by washing with DI water, drying, and calcination at 500 °C in air for 4 h. Tetraammineplatinum(II) nitrate was then impregnated on CeO2 powder by the incipient wetness impregnation, with the calculated Pt weight loadings of 1%. After impregnation, the samples were dried at 100 °C for 12 h, followed by calcination at 500 °C and 800 °C in air for 10 h to yield Pt/CeO2 and PtATCeO2 catalysts, respectively. Pt/CeO2-CO and PtATCeO2-CO were obtained after treating the fresh samples in CO/Ar (20 mL/min) at 275 °C for 20 min. Pt/CeO2-CO-O2 and PtATCeO2-CO-O2 were achieved after further treating the reduced samples in air at 500 °C for 10 h. The low-loading catalysts were synthesized by the same method.
Activity measurements
CO oxidation experiments of fresh Pt/CeO2 and PtATCeO2 were carried out in a fixed-bed flow reactor. Then, 20 mg of catalyst sieved between 40 and 80 mesh was diluted with 380 mg washed SiC powder and then loaded together into the reactor tube. The reaction temperature was ramped up from 20 to 500 °C with a heating rate of 3 °C /min in the mixture of 1 mL/min CO, 4 mL/min O2, and 95 mL/min Ar, with a weight hourly space velocity (WHSV) of 300 L/g*h. The reactor was cooled down to 20 °C in the above reaction mixture for the next light-off test. The product concentration was measured by a gas chromatograph Agilent 3000 Micro GC. The activity measurements of reduced and reoxidized catalysts were performed under the same reaction condition after the in situ pretreatment in the same fixed-bed flow reactor. CO oxidation kinetic measurements were carried out under different reaction conditions by controlling the CO conversion lower than 8%. The partial pressures of CO and O2 were adjusted by changing their flow rates. To study the effect of CO partial pressure on reaction rate, the partial pressure of O2 was kept at 4 kPa, and the partial pressure of CO changed between 0.6 and 3 kPa. To study the effect of O2 partial pressure on reaction rate, the partial pressure of CO was kept at 1 kPa, and the partial pressure of O2 changed between 1 and 10 kPa. The reported reaction rates were normalized by the total numbers of Pt, assuming that all Pt are accessible.
Characterization
Powder X-ray diffraction (XRD) patterns were collected using a Rigaku Miniflex 600 equipped with Cu Kα radiation, with an operating voltage of 40 kV and a current of 15 mA. All samples are collected from 15 to 65° with a speed of 0.5°/min. In situ XRD was carried out in an XRD cell on a PANalytical Empyrean X-ray diffractometer equipped with Cu Kα radiation, with an operating voltage of 45 kV and a current of 40 mA. Quasi in situ X-ray photoelectron spectroscopy measurements were carried out with a Physical Electronics Quantera SXM Scanning X-ray Microprobe with a focused monochromatic Al Kα X-ray (1486.7 eV) source and multi-channel detector. Prior to the test, the samples were pretreated in a preparation chamber under different temperatures and gases, i.e., 180 °C in CO and O2 or 275 °C in CO. After the pretreatment, the samples were directly transferred into the XPS detection chamber for the test without exposure to other gases. All spectra, including Pt, Ce, and O in binding energies, were charge corrected by shifting the Ce4+ 3d5/2 line to 916.7 eV41. Diffuse-reflectance infrared Fourier transform spectroscopy with CO as the probe molecule (CO-DRIFTS) was carried out on a Thermo Scientific IS-50R FTIR with the MCT/A detector. Prior to analysis, approximately 40 mg of the sample was pretreated at 200 °C for 30 min with O2/He flow in a DRIFTS reaction chamber. A spectral resolution of 4 cm−1 was used to collect spectra, and each spectrum in the work is an average of 32 scans. Ex situ XAS measurements were performed at X-ray Science Division bending-magnet beamline at sector 20 of the Advanced Photon Source operating at Argonne National Laboratory. In brief, the samples after calcination were pressed and covered into thin sheets in air before the test. In situ XAS measurements were carried out at the Stanford Synchrotron Radiation Light Source (SSRL) at beamline 9-3 in fluorescence mode. The catalysts were characterized by in situ XAS at the Pt L3-edge (11564 eV) using an in-house built cell with a 4-mm ID glassy carbon tube. The catalyst and standard samples were scanned simultaneously in transmission and fluorescence detection modes using ion chambers and a 100-element solid-state Ge monolith detector (Canberra). XANES and EXAFS data processing and analysis were performed using Athena and Artemis programs of the Demeter data analysis package42,43. The detailed measurement and analysis methods can be seen in our previous study21. The theoretical EXAFS signals for the Pt–O path of Pt1 adsorbed on CeO2 were generated using the FEFF6 code from a Pt doped on the CeO2 model. The theoretical EXAFS signals were fitted to the data in R-space using Artemis by varying the coordination numbers of the single scattering paths, the effective scattering lengths, the bond length disorder, and the correction to the threshold energy, ΔE0 (common for all paths since they are all from the same FEFF calculation). S02 (the passive electron reduction factor) was obtained by first analyzing the spectrum for the Pt oxide, and the best-fit value (0.90) was fixed in the fit. The k-range used for fitting was 3–14 Å−1 while the R-range was 1.2–2 Å for the model that only includes the Pt-O scattering shell. High-angle annular dark-field scanning transmission electron microscopy (HAAD-STEM) images were collected on a Nion UltraSTEM microscope operated at 100 keV. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) was performed using an Optima 2100 DV spectrometer (PerkinElmer Corporation). N2 adsorption-desorption isotherms were analyzed at 77 K on the Micromeritics gas adsorption apparatus (Quadrasorb-EVO, Quantachrome Corporation, America). Prior to analysis, all samples were pretreated at 200 °C for 4 h in a vacuum condition. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) equation. Temperature-programmed desorption of CO was performed on Micromeritics Autochem 2920 with a TCD detector and coupled mass spectroscopy (MS) detector. Prior to analysis, the sample was pretreated at 500 °C in He for 30 min, followed by cooling down to room temperature in He. The treated sample was then exposed to 10% CO/Ar before ramping in He. Temperature-programmed surface reaction (TPSR) was carried out with the same instrument. The visible Raman spectra (532 nm) were collected on a Horiba LabRAM HR Raman/FTIR microscope equipped with a Synapse Charge Coupled Device (CCD) camera and an in situ sample cell (Linkam CCR 1000). All Raman spectra were conducted at room temperature, including the one after CO pretreatment at 275 °C. No obvious changes upon extended laser exposure were observed in the sample.
DFT calculations
The periodic density function theory (DFT) calculations were carried out with the CP2K package44. The generalized-gradient approximation (GGA) with Perdew−Burke−Ernzerhof (PBE) functional was used to evaluate the exchange and correlation45. The wave functions were expanded in a molecularly optimized double-Gaussian basis set, with an auxiliary plane wave basis set with a cutoff energy of 500 Rydberg. The scalar relativistic norm-conserving pseudo-potentials were employed to model the core electrons46 with 18, 12, and 6 valence electrons for Pt, Ce, and O, respectively. The only Γ-point in the reciprocal space mesh was used for integrating the Brillouin zone. The DFT + U method47, based on the Mullikan 4f state population analysis, was used to describe the Ce 4f electrons. A U value was set at ~4.1 eV in line with the previous literature48, which ensures that the redox property is reproduced correctly49. Grimme’s third-generation DFT-D3 approach was used to describe dispersion corrections50. The CeO2(111) surfaces were used to model the CeO2 substrate, constructed with cell dimensions of 15.344 × 13.288 × 27.529 Å with 15-Å vacuum space to minimize the interaction between slabs. Geometry optimization was performed based on the BFGS method. The convergence criterion used for geometry optimizations was a maximum force of 0.01 eV Å−1. Spin polarization was considered in all calculations.
Supplementary information
Acknowledgements
This work was supported by the U.S. Department of Energy (DOE), Office of Basic Energy Sciences (SC), Division of Chemical Sciences (grant DE-FG02-05ER15712). We also acknowledge the U.S. Department of Energy (DOE) Energy Efficiency and Renewable Energy, Vehicle Technologies Office, for the support to Z.Z. and J.T. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. Co-ACCESS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division. A part of the research described in this paper was performed in the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE’s Office of Biological and Environmental Research and located at PNNL.We acknowledge the use of facilities within the Eyring Materials Center at Arizona State University supported in part by NNCI-ECCS-1542160.
Author contributions
Z.Z. and Y.W. conceived and planned the research. J.T. performed DFT computations. S.Y. carried out TEM measurements. S.R.B., J.H., and A.S.H. aided in the XAS experimental design and data collection. Y.B.L. performed the XAS modeling and CO-DRIFTS measurements. Y.X.L. and W.H. performed XRD measurements. D.J. performed Raman measurements. M.H.E. performed XPS measurements. Z.Z. synthesized the catalysts and performed other experimental and analytical studies. Z.Z., A.K.D., and Y.W. wrote the paper. All authors discussed the results and commented on the paper.
Peer review
Peer review information
Nature Communications thanks Yuemin Wang and the other, anonymous, reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data generated in this study are provided in the Supplementary Information. More detailed data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Zihao Zhang, Jinshu Tian.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-37776-3.
References
- 1.Qiao B, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011;3:634–641. doi: 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell S, Pérez-Ramírez J. Single atom catalysis: a decade of stunning progress and the promise for a bright future. Nat. Commun. 2020;11:4302. doi: 10.1038/s41467-020-18182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong H, et al. Thermally stable and regenerable platinum–tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. Engl. 2017;129:9114–9119. doi: 10.1002/ange.201701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurer F, et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 2020;3:824–833. doi: 10.1038/s41929-020-00508-7. [DOI] [Google Scholar]
- 5.Muravev V, et al. Interface dynamics of Pd–CeO2 single-atom catalysts during CO oxidation. Nat. Catal. 2021;4:469–478. doi: 10.1038/s41929-021-00621-1. [DOI] [Google Scholar]
- 6.Khivantsev K, et al. Economizing on precious metals in three-way catalysts: thermally stable and highly active single-atom rhodium on ceria for NO abatement under dry and industrially relevant conditions. Angew. Chem. Int. Ed. Engl. 2020;133:395–402. doi: 10.1002/ange.202010815. [DOI] [PubMed] [Google Scholar]
- 7.Nie L, et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science. 2017;358:1419–1423. doi: 10.1126/science.aao2109. [DOI] [PubMed] [Google Scholar]
- 8.Jones J, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science. 2016;353:150–154. doi: 10.1126/science.aaf8800. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Zhang Z, Lin F, Wang H, Wang Y. Single-atom automobile exhaust catalysts. ChemNanoMat. 2020;6:1659–1682. doi: 10.1002/cnma.202000407. [DOI] [Google Scholar]
- 10.Wang H, et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 2019;10:3808. doi: 10.1038/s41467-019-11856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, Thompson C, Kunwar D, Datye AK, Karim AM. Origin of the high CO oxidation activity on CeO2 supported Pt nanoparticles: weaker binding of CO or facile oxygen transfer from the support? ChemCatChem. 2020;12:1726–1733. doi: 10.1002/cctc.201901848. [DOI] [Google Scholar]
- 12.Ding K, et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science. 2015;350:189–192. doi: 10.1126/science.aac6368. [DOI] [PubMed] [Google Scholar]
- 13.Pereira-Hernández XI, et al. Tuning Pt-CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 2019;10:1358. doi: 10.1038/s41467-019-09308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong H, et al. Controlling the oxidation state of Pt single atoms for maximizing catalytic activity. Angew. Chem. Int. Ed. Engl. 2020;132:20872–20877. doi: 10.1002/ange.202009776. [DOI] [PubMed] [Google Scholar]
- 15.Bruix A, et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. Engl. 2014;53:10525–10530. doi: 10.1002/anie.201402342. [DOI] [PubMed] [Google Scholar]
- 16.Gänzler AM, et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. Engl. 2017;56:13078–13082. doi: 10.1002/anie.201707842. [DOI] [PubMed] [Google Scholar]
- 17.Jiang D, et al. Elucidation of the active sites in single-atom Pd1/CeO2 catalysts for low-temperature CO oxidation. ACS Catal. 2020;10:11356–11364. doi: 10.1021/acscatal.0c02480. [DOI] [Google Scholar]
- 18.García-Vargas CE, et al. Activation of lattice and adatom oxygen by highly stable ceria-supported Cu single atoms. ACS Catal. 2022;12:13649–13662. doi: 10.1021/acscatal.2c04001. [DOI] [Google Scholar]
- 19.Kunwar D, et al. Stabilizing high metal loadings of thermally stable platinum single atoms on an industrial catalyst support. ACS Catal. 2019;9:3978–3990. doi: 10.1021/acscatal.8b04885. [DOI] [Google Scholar]
- 20.Li X, et al. Temperature and reaction environment influence the nature of platinum species supported on ceria. ACS Catal. 2021;11:13041–13049. doi: 10.1021/acscatal.1c03165. [DOI] [Google Scholar]
- 21.Lu Y, et al. Unraveling the intermediate reaction complexes and critical role of support-derived oxygen atoms in CO oxidation on single-atom Pt/CeO2. ACS Catal. 2021;11:8701–8715. doi: 10.1021/acscatal.1c01900. [DOI] [Google Scholar]
- 22.Jiang D, et al. Tailoring the local environment of platinum in single‐atom Pt1/CeO2 catalysts for robust low‐temperature CO oxidation. Angew. Chem. Int. Ed. Engl. 2021;60:26054–26062. doi: 10.1002/anie.202108585. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, et al. Distinct role of surface hydroxyls in single-atom Pt1/CeO2 catalyst for room-temperature formaldehyde oxidation: acid–base versus redox. JACS Au. 2022;22:1651–1660. doi: 10.1021/jacsau.2c00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muravev V, et al. Operando spectroscopy unveils the catalytic role of different palladium oxidation states in CO oxidation on Pd/CeO2 catalysts. Angew. Chem. Int. Ed. Engl. 2022;61:e202200434. doi: 10.1002/anie.202200434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, et al. Surface refaceting mechanism on cubic ceria. J. Phys. Chem. Lett. 2020;11:7925–7931. doi: 10.1021/acs.jpclett.0c02409. [DOI] [PubMed] [Google Scholar]
- 26.Dvořák F, et al. Creating single-atom Pt-ceria catalysts by surface step decoration. Nat. Commun. 2016;7:10801. doi: 10.1038/ncomms10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Y, Wang YG, Li J. Theoretical investigations of Pt1@CeO2 single-atom catalyst for CO oxidation. J. Phys. Chem. C. 2017;121:11281–11289. doi: 10.1021/acs.jpcc.7b00313. [DOI] [Google Scholar]
- 28.Kottwitz M, et al. Local structure and electronic state of atomically dispersed Pt supported on nanosized CeO2. ACS Catal. 2019;9:8738–8748. doi: 10.1021/acscatal.9b02083. [DOI] [Google Scholar]
- 29.Xie P, et al. Nanoceria-supported single-atom platinum catalysts for direct methane conversion. ACS Catal. 2018;8:4044–4048. doi: 10.1021/acscatal.8b00004. [DOI] [Google Scholar]
- 30.Wan W, et al. Highly stable and reactive platinum single atoms on oxygen plasma-functionalized CeO2 surfaces: nanostructuring and peroxo effects. Angew. Chem. Int. Ed. Engl. 2022;61:e202112640. doi: 10.1002/anie.202112640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, et al. Dynamic structural evolution of ceria-supported Pt particles: a thorough spectroscopic study. J. Phys. Chem. C. 2022;126:9051–9058. doi: 10.1021/acs.jpcc.2c02420. [DOI] [Google Scholar]
- 32.Zhang Z, et al. Exceptional selectivity to olefins in the deoxygenation of fatty acids over an intermetallic platinum–zinc alloy. Angew. Chem. Int. Ed. Engl. 2022;134:e202202017. doi: 10.1002/anie.202202017. [DOI] [PubMed] [Google Scholar]
- 33.Huang N, et al. Assembly of platinum nanoparticles and single-atom bismuth for selective oxidation of glycerol. J. Mater. Chem. A. 2021;9:25576–25584. doi: 10.1039/D1TA07262E. [DOI] [Google Scholar]
- 34.Sápi A, et al. In situ DRIFTS and NAP-XPS exploration of the complexity of CO2 hydrogenation over size-controlled Pt nanoparticles supported on mesoporous NiO. J. Phys. Chem. C. 2018;122:5553–5565. doi: 10.1021/acs.jpcc.8b00061. [DOI] [Google Scholar]
- 35.Feng Y, et al. Correlating DFT calculations with CO oxidation reactivity on Ga-doped Pt/CeO2 single-atom catalysts. J. Phys. Chem. C. 2018;122:22460–22468. doi: 10.1021/acs.jpcc.8b05815. [DOI] [Google Scholar]
- 36.Ke J, et al. Strong local coordination structure effects on subnanometer PtOx clusters over CeO2 nanowires probed by low-temperature CO oxidation. ACS Catal. 2015;5:5164–5173. doi: 10.1021/acscatal.5b00832. [DOI] [Google Scholar]
- 37.Zhang C, Michaelides A, King DA, Jenkins SJ. Anchoring sites for initial Au nucleation on CeO2 {111}: O vacancy versus Ce vacancy. J. Phys. Chem. C. 2009;113:6411–6417. doi: 10.1021/jp810093a. [DOI] [Google Scholar]
- 38.Tang M, et al. Facet‐dependent oxidative strong metal‐support interactions of Pd‐TiO2 via in situ TEM. Angew. Chem. Int. Ed. Engl. 2021;133:22513–22518. doi: 10.1002/ange.202106805. [DOI] [PubMed] [Google Scholar]
- 39.Frey H, Beck A, Huang X, van Bokhoven JA, Willinger MG. Dynamic interplay between metal nanoparticles and oxide support under redox conditions. Science. 2022;376:982–987. doi: 10.1126/science.abm3371. [DOI] [PubMed] [Google Scholar]
- 40.Zhu M, et al. Combining in-situ TEM observations and theoretical calculation for revealing the thermal stability of CeO2 nanoflowers. Nano Res. 2022;15:1319–1326. doi: 10.1007/s12274-021-3659-6. [DOI] [Google Scholar]
- 41.Paparazzo E. Use and mis-use of X-ray photoemission spectroscopy Ce3d spectra of Ce2O3 and CeO2. J. Phys. Condens. Matter. 2018;30:343003. doi: 10.1088/1361-648X/aad248. [DOI] [PubMed] [Google Scholar]
- 42.Ravel B, Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005;12:537–541. doi: 10.1107/S0909049505012719. [DOI] [PubMed] [Google Scholar]
- 43.Newville M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001;8:322–324. doi: 10.1107/S0909049500016964. [DOI] [PubMed] [Google Scholar]
- 44.VandeVondele J, et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005;167:103–128. doi: 10.1016/j.cpc.2004.12.014. [DOI] [Google Scholar]
- 45.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 46.Goedecker S, Teter M, Hutter J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B. 1996;54:1703. doi: 10.1103/PhysRevB.54.1703. [DOI] [PubMed] [Google Scholar]
- 47.Dudarev S, Botton G, Savrasov S, Humphreys C, Sutton A. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+ U study. Phys. Rev. B. 1998;57:1505. doi: 10.1103/PhysRevB.57.1505. [DOI] [Google Scholar]
- 48.Wang YG, Mei D, Glezakou VA, Li J, Rousseau R. Dynamic formation of single-atom catalytic active sites on ceria-supported gold nanoparticles. Nat. Commun. 2015;6:6511. doi: 10.1038/ncomms7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YG, Yoon Y, Glezakou VA, Li J, Rousseau R. The role of reducible oxide–metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 2013;135:10673–10683. doi: 10.1021/ja402063v. [DOI] [PubMed] [Google Scholar]
- 50.Grimme S, Antony J, Ehrlich S, Krieg H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010;132:154104. doi: 10.1063/1.3382344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are provided in the Supplementary Information. More detailed data that support the findings of this study are available from the corresponding author upon reasonable request.