Abstract
Objectives
To describe the first clinical implantation of the CorNeat™ keratoprosthesis, which utilizes a polymeric scaffold for biointegration within ocular tissue.
Methods
The CorNeat keratoprosthesis was implanted in the right eye of a patient with bilateral corneal opacification and neovascularization secondary to multiple failed grafts. The following surgical technique was used: 360 degree peritomy; epithelial scraping and corneal marking; pre-placement of three corneo-scleral sutures through the implant; central trephination using a 7 mm trephine and host cornea removal; keratoprosthesis placement and sutures tightening while fitting the corneal edge into the posterior groove of the CorNeat keratoprosthesis; and repositioning of the conjunctiva over the implant skirt and fixation with sutures and Fibrin sealant.
Results
Twelve months postoperatively visual acuity improved to 1/16 from hand movement. The keratoprosthesis was properly positioned. Tactile intraocular pressure was assessed as normal. Regional, mostly nasal, conjunctival retraction of 4–5 mm over the nano-fibre skirt was seen throughout follow-up. The anterior chamber was quiet and well-formed. No other postoperative complications were observed.
Conclusion
This initial case may imply a potential breakthrough in the treatment of corneal disease not amenable to standard corneal transplant. Long follow-up and additional implantations are desired to prove the long-term safety and efficacy of this device.
Subject terms: Outcomes research, Biological techniques
Introduction
Treatment of patients suffering from end stage corneal disease poses a great challenge as there are limited options available for visual restoration [1]. To date, the most successful solution is corneal transplantation [2]. However, the shortage of transplants and the lack of trained surgeons, limit the access to transplantations [2, 3]. In addition donor corneal tissue has limited applicability for some indications. These include multiple graft rejections, vascularized cornea and all forms of limbal stem cell disease [4–8].
The search for a replacement to the natural cornea has been going on for over 230 years, with the introduction of the first keratoprosthesis (KPro) by Pellier de Quengsy in 1789 [9]. The Boston KPro is the most widely used artificial cornea, but due to a high rate of complications, including retroprosthetic membrane formation, glaucoma, corneal melting and endophthalmitis, it has not scaled to meet the challenge [10–13]. Other options such as the Aurolab KPro and previously the AlphaCor KPro have a high rate of operative and postoperative complications limiting their clinical use [14, 15]. Another type of KPro uses autologous biological support of dentine tissue alveolar bone or tibia bone tissue [16]. These require serial surgical interventions and are also associated with unique surgical complications such as lamina-resorption and oral complications [17]. For these reasons, KPros are currently used as a last resort with a global implantation rate reaching only 1000–2000 cases annually [1].
Materials and methods
CorNeat keratoprosthesis
The CorNeat KPro (CorNeat Vision Ltd, Raanana, Israel) is a synthetic corneal implant designed to treat corneal blindness. The implant is composed of a central optical member and an external integrating skirt formed by electrospinning carbonated poly-urethane fibres, consequently assimilating synthetic optics within the ocular tissue (Fig. 1).
Fig. 1. CorNeat KPro’s design.
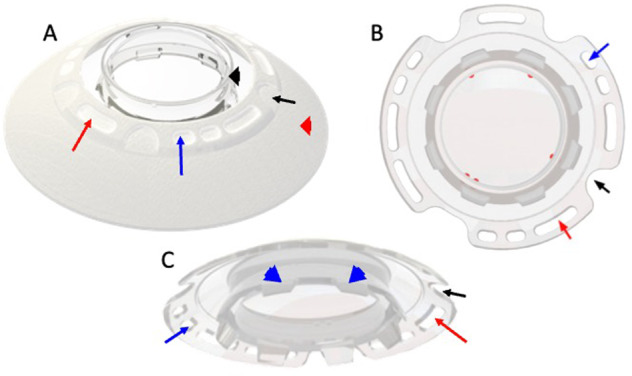
A final product form, (B) upper view, (C) bottom view. Black arrowhead indicates the lens component. Red arrowhead indicates the integrating skirt component. Red arrow indicates one of the five biostitching holes. Blue arrow indicates one of the six suturing holes (three pairs interspaced at 120 degrees apart). Black arrow indicates one of the four access ports which enable access into the anterior chamber for postoperative procedures. “Port indicators” are marked in red. Blue arrowhead indicate the posterior rim which is positioned into the corneal opening after trephination.
Its optical element is made of medical-grade Poly-methyl-methacrylate (PMMA). The lens is designed to provide a fixed optical power of 40.8 dioptres, which is equivalent to 42 dioptres at the plane of a normal cornea. The current design of the optics of the CorNeat KPro is suitable for phakic and pseudophakic patients. A matching IOL can be implanted before or during surgery for optimized visual acuity. Future versions of the CorNeat KPro are planned to support aphakic patients as well. To eliminate potential astigmatism due to device malposition or decentration, the CorNeat KPro lens’ surfaces are spherical. The lens, which spans almost the entire way to the limbus (10 mm in diameter), provides an effective optical zone of approximately 6.5 mm in diameter. The wide optical component potentially provides the patient a physiological visual field and enables a comprehensive ophthalmological examination similar to examining through the native cornea.
The CorNeat KPro’s design consists of the following features for ease of implantation and device retention: a corneal groove on the posterior side of the lens to accommodate the patient’s corneal stump, three pairs of suturing holes spaced at 120 degrees apart and a set of integration holes full of nanofibers to stimulate biointegration. In addition, the optical component’s perimeter has four access ports into the anterior chamber designed to allow future surgical interventions, one of them is 2.6 mm wide designed to enable IOL injection. A set of indicators visible under a microscope are positioned on the edge of the optical zone to assist the surgeon in locating them following implantation.
In contrast to previous KPros designed to integrate with the diseased corneal remnant, the CorNeat KPro’s integrating skirt is implanted under the conjunctiva. This highly vascularized tissue abundant with fibroblasts has better wound healing potential, likely resulting in long-term biointegration. In addition, its scaffold design promotes the migration of fibroblasts from Tenon’s capsule into the skirt, leading to long-term integration of the device––bypassing the prosthesis-host cornea interface that exists in other prostheses [18, 19]. The skirt is 250 microns thick and is easily placed under the conjunctiva. The skirt extends 5 mm from the edge of the lens transparent zone and ends approximately 1 mm from the closest insertion of the extraocular muscles. The skirt dimensions are designed to fit most eyes, yet it can be easily cut and adjusted by the surgeon to fit any eye. Finally, The CoreNeat KPro’s visible component resembles the natural cornea, allowing for favourable cosmetic results that mimic the native eye.
The surgical feasibility and biointegration of the CorNeat KPro were evaluated in a recent study of eight rabbits implanted unilaterally and observed for six months [18]. The results indicated a retention rate of 87.5% with no incidence of retroprosthetic membrane formation at the conclusion of the 6-month follow-up period. In addition histopathological evaluation revealed infiltration of fibroblasts with collagen deposition among the device’s fibrils, indicating the integration of the implant into surrounding tissue.
Clinical experiment
This experiment followed the tenets of the Declaration of Helsinki and was approved by the Israeli National Council for Clinical Experimentation in Humans (No. 201913872) and the Institutional Review Board Committee of the Rabin Medical Center (No. RMC-19-0776). An informed consent was obtained from the patient after explanation of the nature and possible complications of the procedure.
A patient with bilateral corneal opacification secondary to multiple failed grafts was examined at the cornea service of our medical centre. The patient had previously undergone bilateral Penetrating Keratoplasty due to pseudophakic bullous keratopathy following secondary bilateral anterior chamber intraocular lens implantation after cataract extraction surgeries. The patient subsequently underwent two Descemet’s Stripping Automated Endothelial Keratoplasty operations in the right eye, which ended up with graft failure. Other ocular history was positive for glaucoma. On examination, visual acuity was hand movement in the right eye and counting fingers in the left. The right eye had significant corneal opacification with corneal neovascularization and was therefore considered for a KPro implantation.
Surgical procedure
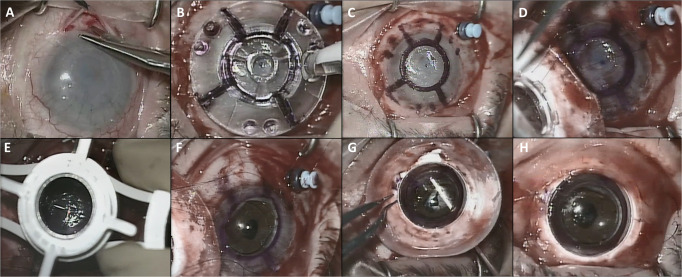
The surgery was performed under general anaesthesia. The CorNeat keratoprosthesis was implanted using the following technique: (1) 360 degrees peritomy was applied creating the intended space for placement of the CorNeat KPro’s skirt (Fig. 2A); (2) the epithelium was scraped; (3) the cornea was marked using the dedicated transparent marker tool, which has a central hole for alignment according to the central corneal mark, leaving a pattern on the cornea that includes three pairs of suturing marks, four lines to point to potential paracenteses and the trephination’s edge (Fig. 2B, C); (4) two clear corneal incisions of 1.1 mm were created where indicated by the paracentesis lines and the eye was filled with viscoelastic; (5) three non-degradable, 9-0 Nylon sutures (Ethilon, Johnson & Johnson, New Brunswick, New Jersey, U.S.), were pre-placed through the implant and the corneo-sclera, passing first through the dedicated holes on the rim of the KPro optics, then entering the suture hole marks on the cornea, passing radially through the cornea and sclera and exiting through the sclera (Fig. 2D); (6) trephination using a 7 mm trephine was performed and the opaque host cornea was removed (Fig. 2E, F); (7) the implant was placed and the sutures were tightened to seal the anterior chamber; (8) the corneal edge, which had been marked for better visualization, was manipulated into the posterior groove of the CorNeat KPro using a customized spatula tool, called the “Snapper”, inserted into the anterior chamber at the marked paracentesis lines. (Fig. 2G); (9) the conjunctiva was repositioned over the implant skirt to completely cover it and fixed with 8-0 Polyglactin 910 sutures (Vicryl, Johnson & Johnson, New Brunswick, New Jersey, U.S.) and Fibrin sealant (TISSEEL Lyo, Baxter International, Deerfield, Illinois, U.S.) (Fig. 2H).
Fig. 2. Photographs demonstrating the major steps of the CorNeat KPro surgical implantation procedure.
Conjunctival peritomy (A); Marking procedure using the dedicated Marker tool (B); Marking pattern which includes the mark of the three pairs of sutures and the trephination (C); Preplacing of three safety sutures at the marked spots and at the designated suturing holes in the CorNeat KPro (D); Trephination of 7 mm at the centre of the cornea according to the mark (E); An “open sky” after removal of the central 7 mm of the cornea (F); Suture fixation and insertion of the trephined corneal edge into the CorNeat KPro posterior undercut using the Snapper tool creating an interference fit for sealing the eye (G); Repositioning and suturing the conjunctiva over the CorNeat KPro’s integrating skirt (H).
Results
Postoperative follow-up was scheduled at one day after surgery, one week, two weeks, one month, two month, three months and then every three months after implantation of the KPro.
One day postoperatively visual acuity was 1/20. Tactile intraocular pressure was assessed as normal. Good conjunctival cover over the bio-integrating skirt with mild subconjunctival haemorrhage was observed. The implant was clear and well placed. Anterior chamber was deep, with cells++, flare++ and no fibrin or hypopyon. Anterior chamber intraocular lens was intact. Optic nerve pallor with attached retina and narrow blood vessels were visualized well through the KPro. Anterior segment optical coherence tomography (OCT) demonstrated proper location of the KPro at 360 degrees. Macular OCT showed thinning and disorganization of the inner retinal layers, with the outer retina and retinal pigment epithelium in the perifoveal area intact. In addition, sub-foveal disturbance in the ellipsoid zone as well as mild intraretinal cystic changes were noted. B scan ultrasonography findings were unremarkable.
On consecutive examinations, visual acuity ranged between 1/16 and 1/20. The KPro was well tolerated and properly positioned (Fig. 3). Intraocular pressure was within normal range. Mild conjunctival retraction of 4–5 mm over the nasal and superior part of the KPro skirt was seen (Fig. 4). The anterior chamber was quiet and well-formed. Posterior pole was visualized well. No postoperative complications were observed. Additional anterior and macular OCT images taken throughout the follow-up period showed similar findings to those of the first postoperative day evaluation. Six months postoperatively, a fine membrane over the inferior part of the anterior chamber intraocular lens was seen (Fig. 5). The membrane was successfully removed using Nd:YAG laser. At the last follow-up examination, twelve months postoperatively, visual acuity was stable at 1/16.
Fig. 3. Anterior segment optical coherence tomography demonstrating proper location of the CorNeat KPro at the 3-months postoperative visit.
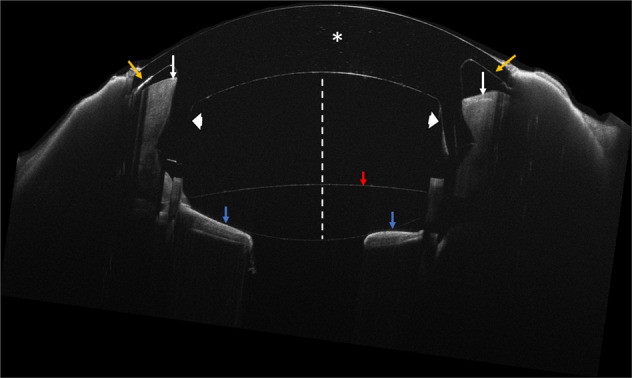
White asterisk indicates the centrally placed optical component. White arrows indicate a cross section of the corneal remnant seating securely in the dedicated undercut of the KPro (white arrowheads). Yellow arrows indicate the integrating skirt component located underneath the conjunctiva. Note the anterior chamber (white dotted line), anterior chamber intraocular lens (red arrow) and iris (blue arrows).
Fig. 4. An ocular photograph of the patient 6 month after the CorNeat KPro implantation.
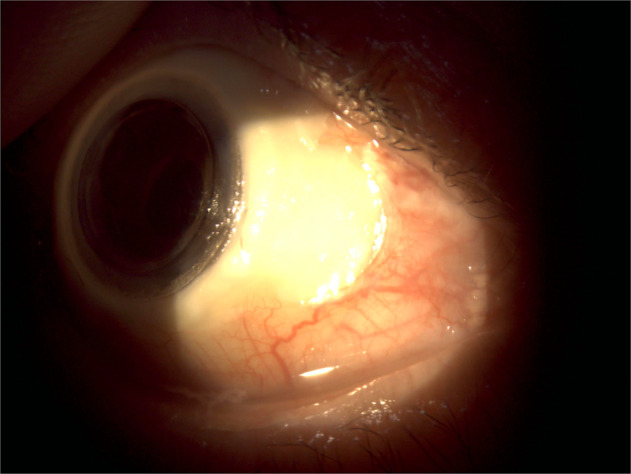
The well placed KPro and the conjunctival vitality over the implant with the nasal conjunctival retraction are demonstrated.
Fig. 5. An ocular photograph of the patient 6 month after the CorNeat KPro implantation.
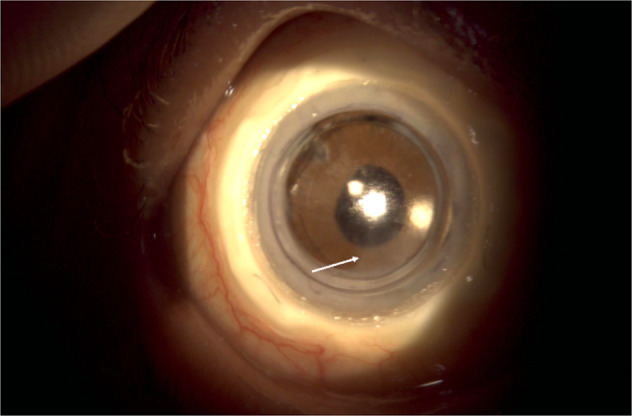
A fine membrane over the inferior part of the anterior chamber intraocular lens (white arrow) was obsereved.
Discussion
Treatment of corneal blindness is one of the most challenging tasks in ophthalmology [1]. In some patients, allogeneic corneal transplantation has an extremely guarded prognosis [4]. KPros are valid treatment options for eyes which are not suitable for therapy with tissue-based solutions. However, previous attempts at artificial corneas such as the Boston KPro have been mostly based on carrier tissue that anchor the implant to the corneal tissue, achieving poor and temporary integration [10, 14, 15]. This concern is especially noticeable in patients who are poor candidates for corneal transplantation. In addition, the commonly used Boston type 1 KPro, requires the use of corneal tissue, the availability of which is low [3, 10]. Another solution is KPros with an autologous biological haptic, such as the osteo-odonto-KPro or the tibia-KPro. However, these require major surgical intervention, serial procedures and are accompanied with unique surgical complications [16].
The CoreNeat KPro presents an alternative by a novel approach of subconjunctival integration, along with a microporous matrix that stimulates cellular growth. Previous studies have shown the feasibility of this integration concept. In a rabbit model study evaluating the CorNeat implant, histopathological samples demonstrated the presence of fibroblasts and abundance of collagen fibrils within the device’s integrating skirt and the biostitching openings, as well as foreign body reaction, composed of macrophages, multinucleated giant cells, and lymphocytes located mostly at the margins of the implanted device [18]. Comparable response was observed in the AVflo™ (Nicast Ltd, Israel) arteriovenous graft for haemodialysis, that uses similar polymers and manufacturing technology, showing rapid integration into the surrounding tissues, alongside favorable efficacy and safety clinical results over two years in comparison with other available implants in that field [20, 21]. This concept of using a skirt promoting biointegration, offers a potential for long term integration. In addition, it possibly expands treatment options available to patients with associated ocular surface disease such as Ocular Cicatricial Pemphigoid and Steven Johnson syndrome, who are considered poor candidates for the Boston type 1 KPro. Moreover, the CorNeat KPro provides a completely synthetic solution, resolving issues of tissue availability. Finally, The CorNeat KPro implantation procedure, based on fitting the device into the patient’s trephined cornea, using customized tools, and fastening it with only three sutures, is relatively short (approximately 45 min) and simple when compared to other KPros.
Our preliminary clinical evaluation indicates a feasible, one staged surgical procedure and good short-term integration of the CorNeat KPro within the ocular tissue while maintaining clarity of the visual axis, resulting in an improvement of visual acuity from hand movement to 1/16. It ought to be noted that the potential of visual recovery was limited in our patient due to other ocular conditions. It is likely that eyes with isolated corneal pathology would regain better visual recovery.
Nevertheless, not withholding its potential, several challenges need to be addressed. First, the insertion of the corneal stamp into the KPro’s posterior undercut requires highly skilful surgical technique. Difficulties in this step could lead to intraoperative complications such as bleeding or poor sealing of the eye. It is possible that changing the groove structure will facilitate this process. In addition, postoperative conjunctival retraction, possibly predisposing to infection and failure of long-term integration needs to be further studied.
In summary, the CorNeat KPro offers an alternative to current KPro procedures, with potential advantages including subconjunctival integration, wide visual field and favourable cosmetic results. This is the first-in-human implantation of the CorNeat KPro. This initial experience holds promise for a potential breakthrough in the treatment of corneal disease not amenable to standard corneal transplantation. Longer follow-up and additional implantations are necessary to better assess the long-term safety and efficacy of this device.
Summary
What was known before:
Previous attempts at an artificial cornea have been based on anchoring the implant to the corneal tissue, achieving poor and temporary integration. Previous keratoprosthesis implantation procedures have significant risks, including retroprosthetic membrane formation compromising clarity of the visual axis, severe glaucoma and melting of the surrounding tissue with secondary keratoprosthesis protrusion.
What this study adds:
This is the first-in-human implantation of the CorNeat keratoprosthesis. The synthetic CoreNeat keratoprosthesis utilizes a polymeric scaffold for biointegration, consequently assimilating synthetic optics within ocular tissue by a novel approach of subconjunctival integration. The CoreNeat keratoprosthesis has a wide optical component design that provides the patient an optimal visual field and allowing for favourable cosmetic results that mimic the native eye.
Author contributions
GL designed the model and the study framework. IB and EL performed the described experiment. All authors analysed the data. OR and IB wrote the manuscript. All authors provided critical feedback and helped shape the experiment, analysis and manuscript.
Funding
The study was funded by CorNeat Vision Ltd.
Data availability
All data generated in this case are included in this published article.
Competing interests
GL is an employee of CorNeat Vision Ltd and the inventor of the Corneat Kpro. No other author has any financial or proprietary interest in any material or method mentioned
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avadhanam VS, Smith HE, Liu C. Keratoprostheses for corneal blindness: a review of contemporary devices. Clin Ophthalmol (Auckl, N. Z) 2015;9:697–720. doi: 10.2147/OPTH.S27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37:1198–203. doi: 10.1097/ICO.0000000000001666. [DOI] [PubMed] [Google Scholar]
- 3.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167–73. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 4.Hicks C, Crawford G, Chirila T, Wiffen S, Vijayasekaran S, Lou X, et al. Development and clinical assessment of an artificial cornea. Prog retinal eye Res. 2000;19:149–70. doi: 10.1016/S1350-9462(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 5.Crawford GJ, Hicks CR, Lou X, Vijayasekaran S, Tan D, Mulholland B, et al. The Chirila Keratoprosthesis: phase I human clinical trial. Ophthalmology. 2002;109:883–9. doi: 10.1016/S0161-6420(02)00958-2. [DOI] [PubMed] [Google Scholar]
- 6.Hicks CR, Crawford GJ. Melting after keratoprosthesis implantation: the effects of medroxyprogesterone. Cornea. 2003;22:497–500. doi: 10.1097/00003226-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hicks CR, Crawford GJ, Dart JKG, Grabner G, Holland EJ, Stulting RD, et al. AlphaCor: clinical outcomes. Cornea. 2006;25:1034–42. doi: 10.1097/01.ico.0000229982.23334.6b. [DOI] [PubMed] [Google Scholar]
- 8.Ilhan-Sarac O, Akpek EK. Current concepts and techniques in keratoprosthesis. Curr Opin Ophthalmol. 2005;16:246–50. doi: 10.1097/01.icu.0000172829.33770.d3. [DOI] [PubMed] [Google Scholar]
- 9.Chirila TV, Hicks CR. The origins of the artificial cornea: Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus. 1999;56:96–106. [PubMed] [Google Scholar]
- 10.Nonpassopon M, Niparugs M, Cortina MS. Boston type 1 keratoprosthesis: updated perspectives. Clin Ophthalmol (Auckl, N. Z) 2020;14:1189–1200. doi: 10.2147/OPTH.S219270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-term visual outcomes and complications of boston keratoprosthesis type II implantation. Ophthalmology. 2017;124:27–35. doi: 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Sayegh RR, Avena Diaz L, Vargas-Martín F, Webb RH, Dohlman CH, Peli E. Optical functional properties of the boston keratoprosthesis. Investigative Opthalmology Vis Sci. 2010;51:857–63. doi: 10.1167/iovs.09-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aravena C, Yu F, Aldave AJ. Long-term visual outcomes, complications, and retention of the Boston Type I keratoprosthesis. Cornea. 2018;37:3–10. doi: 10.1097/ICO.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 14.Jirásková N, Rozsival P, Burova M, Kalfertova M. AlphaCor artificial cornea: clinical outcome. Eye. 2011;25:1138–46. doi: 10.1038/eye.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu S, Serna-Ojeda JC, Senthil S, Pappuru RR, Bagga B, Sangwan V. The Aurolab Keratoprosthesis (KPro) versus the Boston Type I Kpro: 5-year clinical outcomes in 134 cases of bilateral corneal blindness. Am J Ophthalmol. 2019;205:175–83. doi: 10.1016/j.ajo.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 16.de la Paz MF, Salvador-Culla B, Charoenrook V, Temprano J, Álvarez de Toledo J, Grabner G, et al. Osteo-odonto-, Tibial bone and Boston keratoprosthesis in clinically comparable cases of chemical injury and autoimmune disease. Ocul Surf. 2019;17:476–83. doi: 10.1016/j.jtos.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Paul B, Tandon R, Lee E, Fong K, Mavrikakis I, et al. The osteo-odonto-keratoprosthesis (OOKP) Semin Ophthalmol. 2005;20:113–28. doi: 10.1080/08820530590931386. [DOI] [PubMed] [Google Scholar]
- 18.Litvin G, Klein I, Litvin Y, Klaiman G, Nyska A. CorNeat KPro: ocular implantation study in rabbits. Cornea. 2021;40:1165–74. doi: 10.1097/ICO.0000000000002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordeiro MF, Chang L, Lim KS, Daniels JT, Pleass RD, Siriwardena D, et al. Modulating conjunctival wound healing. Eye. 2000;14:536–47. doi: 10.1038/eye.2000.141. [DOI] [PubMed] [Google Scholar]
- 20.Karatepe C, Altinay L, Yetim TD, Dagli C, Dursun S. A novel electrospun nano-fabric graft allows early cannulation access and reduces exposure to central venous catheters. The J Vasc Access. 2013;14:273–80. doi: 10.5301/jva.5000145. [DOI] [PubMed] [Google Scholar]
- 21.Ferraresso M, Bortolani EM, Amnon G. A two-year experience with a rapid access, self-sealing, polycarbonate urethane nanofiber vascular access graft for hemodialysis. J Vasc Access. 2016;17:210–4. doi: 10.5301/jva.5000541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated in this case are included in this published article.