Abstract
Multiple sclerosis (MS) is an immune-driven disease that affects the central nervous system and is characterized by acute-on-chronic demyelination attacks. It is a major cause of global neurological disability, and its prevalence has increased in the United States. Conceptual understandings of MS have evolved over time, including the identification of B cells as key factors in its pathophysiology. The foundation of MS management involves preventing flares so as to avoid long-term functional decline. Treatments may be categorized into low-, middle-, and high-efficacy medications based on their efficacy in relapse prevention. With 24 FDA-approved treatments for MS, individual therapy is chosen based on distinct mechanisms and potential side effects. This review provides a detailed update on the epidemiology, diagnosis, treatment advances, and major ongoing research investigations in MS.
Keywords: multiple sclerosis, review, neuroimmunology, autoimmune neurology
INTRODUCTION
Multiple sclerosis (MS) is an immune-driven disease characterized by demyelination and axonal damage in the central nervous system (CNS). It is twice as common in females and most often diagnosed between 20–40 years of age.1 The MS prevalence in the United States has been steadily increasing over the past several decades, with the approximately 58 cases per 100,000 persons in 1975 increasing to 309.2 cases per 100,000 persons (450.1 per 100,000 for females) during 2010–2019.2 This roughly translates to 1 in 300 people in the United States living with MS, with the incidence being the highest for Black people (10.2 per 100,000 person-years versus 6.2 in white people).3 The increase in incidence may be related to increased awareness, improvements in magnetic resonance imaging (MRI), and increased sensitivity of diagnostic criteria.
MS RISK FACTORS AND PATHOGENESIS
MS mostly results from environmental factors including Epstein-Barr virus (EBV) infection, low vitamin D, obesity, and cigarette smoking.4,5,6 EBV infection of autoreactive-naïve B cells may be the first step in MS pathogenesis.4 These infected B cells remain in lymph nodes where they present antigens that have similarities to myelin. Autoreactive B cells and T lymphocytes (B and T cells) then enter the CNS and orchestrate immune attacks.4
Vitamin D typically promotes the development of T regulatory lymphocytes, and so low vitamin D may lead to an increased number of autoreactive T cells.7 Vitamin-D-dependent promoters are also responsible for the regulation of the human leukocyte antigen HLA-DRB1*1501 gene, which is a MS risk and susceptibility gene.8 Genetic risk factors for MS also exist, the strongest being major histocompatibility complex in chromosome 6p21.3, which contains six HLA genes, of which the HLA-DRB1*1501 allele has the strongest association with MS risk.9 MS risk is tenfold higher in a first-degree relative (0.3% population risk versus 3% family risk).
The prevalence of MS varies with latitude. There is less sun exposure at higher latitudes and therefore less vitamin D production. MS is consequently more prevalent at higher latitudes: in the southern United States, there are approximately 272.6 per 100,000 adults living with MS versus 377.4 in the northeastern United States.2 More-severe disease has also been associated with higher latitudes.10
MS DIAGNOSIS
Diagnostic criteria
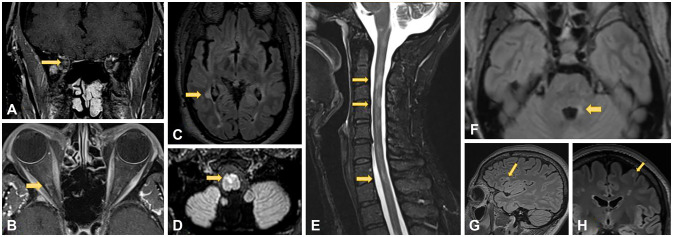
Criteria for MS diagnosis has undergone numerous changes over time. The original Schumacher criteria published in 1965 defined MS as spatial and temporal dissemination of focal neurological deficits. The current 2017 McDonald criteria still support for the use of surrogate markers to fulfill criteria for dissemination in space and time. For example, having simultaneous enhancing and nonenhancing lesions in the first attack fulfills the dissemination-in-time criteria without requiring a second clinical attack. Oligoclonal bands, a marker of inflammatory reaction chronicity, can also fulfill the dissemination-in-time criteria. The revised criteria aim to increase sensitivity in detecting MS to expedite treatment and prevent disability. For the current dissemination-inspace criteria, MS lesions must be seen in at least two of the following four locations: cortical/juxtacortical, periventricular, infratentorial, and spinal cord.11 Sample MS lesions in these regions are shown in Fig. 1. A more-thorough description with full details of the revised 2017 McDonald criteria is provided in Table 1.5
Fig. 1. Juxtacortical/cortical, periventricular, spinal cord, and brainstem lesions in multiple sclerosis. A (coronal) and B (axial): MRI T1-post contrast images show enhancement in the intraorbital segment of the optic nerve consistent with right optic neuritis (arrows). C: Right posterior periventricular lesion on axial T2-FLAIR (arrow). D: Ventral medullary demyelinating lesion on T2-FLAIR (arrow). E: MR cervical cord sagittal STIR demonstrating C2-3, C4, and C7 demyelinating lesions (arrows). F: Axial T2-FLAIR with demyelinating lesion in left brachium pontis (arrow). G (sagittal) and H (axial): T2-FLAIR images demonstrate left frontal juxtacortical demyelinating lesion (arrows).
Table 1. Revised 2017 McDonald criteria for the diagnosis of MS5.
| Number of attacks | Number of lesions | Additional requirements for MS diagnosis | |
|---|---|---|---|
| ≥2 | ≥2 | None | |
| ≥2 | 1 | Clear evidence of previous attack with a lesion in a distinct anatomical location | |
| ≥2 | 1 | Dissemination in space: | |
| 1) An additional clinical attack at a different CNS site OR | |||
| 2) MRI evidence of T2-weighted lesions in ≥2 of 4 areas (periventricular, cortical/juxtacortical, infratentorial, and spinal cord) | |||
| 1 | ≥2 | Dissemination in time: | |
| 1) An additional clinical attack OR | |||
| 2) MRI evidence of gadolinium enhancing and nongadolinium enhancing lesions OR | |||
| 3) CSF oligoclonal bands | |||
| 1 | 1 | Dissemination in space AND dissemination in time | |
| 0 | 1 | >1 year of disease progression AND 2 of the following: | |
| 1) T2-weighted hyperintense lesion in 1 of 3 brain areas (periventricular, cortical/juxtacortical, and infratentorial) | |||
| 2) T2-weighted hyperintense lesion in ≥2 spinal cord areas | |||
| 3) Oligoclonal bands | |||
Typical clinical syndromes and clinical features that are atypical of MS
Typical clinical syndromes for MS include optic neuritis, internuclear ophthalmoplegia, facial sensory loss or trigeminal neuralgia, ataxia, and partial transverse myelitis.12,13
Optic neuritis is characterized by reduced visual acuity, afferent pupillary defects, and impaired color vision, and is typically unilateral in MS.14 Visual acuity in optic neuritis is often better than no light perception. Pain during eye movement is common.14 Visual deficits regularly nadir at 2 weeks, and recover within 4 weeks.15
Weakness or numbness in MS typically presents over hours to days, unlike strokes that present within minutes. MS symptoms often last for days to months, with some symptoms becoming permanent. MS relapse typically lasts for at least 24 hours. When a fluctuation of prior MS symptoms occurs for less than 24 hours, it is considered a “pseudorelapse,” which is common after the acute inflammatory period. Risk factors for a pseudorelapse include infection, stress, and heat.16
The initial presentation of MS often impacts the disease course. Optic neuritis at the initial presentation is associated with a more favorable course.17 In contrast, cerebellar dysfunction at onset is associated with worse prognosis (shorter time to a score of 6 on the Expanded Disability Status Scale).18 Initial spinal cord involvement is similarly associated with faster disability progression, relapse risk, and poor treatment response.19,20
Mimics of MS include Lyme neuroborreliosis, neuromyelitis optica (NMO), myelin oligodendrocyte glycoprotein antibody disease (MOGAD), autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy, CNS vasculitis, and neurosarcoidosis. Neuroborreliosis should be considered in the clinical context of a patient with recent tick exposure that often occurs in woodland areas with skin manifestations such as rash or cranial neuropathy (neurological manifestations are often the second most common after dermatological symptoms).21 Testing may include a serum Lyme antibody index in the cerebrospinal fluid (CSF), since serum Lyme IgM testing can produce false positives, especially in cases of systemic autoimmune disease such as systemic lupus erythematosus (SLE).22 NMO and MOGAD are both demyelinating diseases that can mimic MS. When patients present with acute optic neuropathy, clinicians may distinguish between MS and these disorders by performing serum tests for water channel aquaporin-4 and myelin oligodendrocyte glycoprotein (MOG) in the CNS.23 Cell-based assays are more sensitive and specific for diagnosing NMO and MOGAD; testing should be performed in cases of optic neuritis, tumefactive lesions, or longitudinally extensive transverse myelitis. Care should be taken for low-positive MOG titers (e.g., 1:40), which can represent a false positive in MS.23 Other neuroinflammatory disorders such as GFAP astrocytopathy can present with longitudinal myelitis; in such cases, clinicians may perform a complete antineuronal antibody panel (of both the serum and CSF) to detect GFAP-IgG.24 CNS vasculitis can be primary or secondary to systemic rheumatological diseases such as SLE, and often presents with headaches, seizures, and encephalopathy.24 Strokes may be evident in MRI in cases of CNS vasculitis.24 Although patients with neurosarcoidosis present heterogeneously, in rare cases lesions present with scattered demyelinating lesions with or without leptomeningeal enhancement (“seed and spread;” for example, myelitis in one area moves up and down as it becomes more inflamed).25
MS pathology is isolated to the CNS, and systemic inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein are therefore nonspecific and unhelpful for MS diagnosis.26 Serum antinuclear antibody can be mildly elevated in patients with MS, but at rates similar to the general population.27 Anti-aquaporin-4 and anti-MOG tests are recommended for patients with optic neuritis,28 tumefactive brain lesions, or longitudinally extensive spinal cord lesions (>3 segments) to detect NMO and MOG-associated disorder. Current research has found that serum neurofilament light chain has potential efficacy as a biomarker for ongoing inflammatory activity, although this test currently has restricted availability.29
Clinical features atypical for MS include bilateral or severe optic neuritis with poor recovery, headache, acute or subacute cognitive impairment, dizziness or vertigo without brainstem or cerebellar findings, sensory loss in the extremities without clear CNS patterns, and complete transverse myelopathy.12
MRI in MS
MRI is the gold standard imaging procedure for MS. The current recommendation in the 2021 Consortium of Multiple Sclerosis Centers North American Imaging in MS Cooperative is to perform baseline brain MRI (with gadolinium if required according to the drug label) prior to initiating or switching disease-modifying therapy (DMT); a new baseline brain MRI scan without gadolinium contrast is then recommended at 3–6 months after the treatment onset, followed by annual brain MRI without gadolinium contrast while the patient is undergoing DMT.11 There are some important limitations to the existing guidelines. First, unlike in prior guidelines, gadolinium-based contrast agents are no longer routinely recommended, particularly during monitoring of the course of MS treatment.11 Second, spinal cord MRI is not recommended for routine follow-up monitoring of disease activity, however, 15% of spinal cord lesions can be asymptomatic and so many neurologists specializing in MS perform annual MRI monitoring of both the spinal cord and brain.11,30 Third, the current imaging guidelines do not recommend the implementation of quantitative MRI measures, new imaging features, or volumetric analysis in routine clinical practice.11 Fat suppression may be utilized on MRI, which is particularly helpful for acute optic neuritis diagnosis. Fat can appear hyperintense on T1-weighted MRI; fat suppression therefore allows for better visualization of contrast enhancement in acute optic neuritis.31 Furthermore, in the appropriate clinical context, MRI may be performed at the lumbosacral spine in the workup of patients with suspected MS to exclude mimic diseases such as MOGAD with conus involvement.32 Moreover, up to 8.2% of patients with MS may have one or multiple cranial nerves with root/entry zone enhancement; however, more longitudinal enhancement should prompt workup for neurosarcoidosis, infection, and neoplastic disorders.33
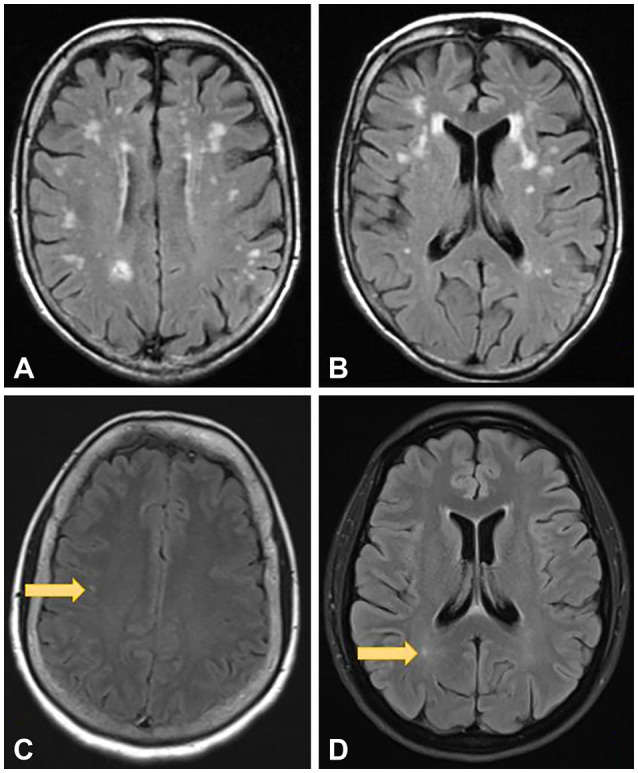
Several diseases and syndromes are routinely mistaken for MS in clinical settings. These include migraine (22% of cases), fibromyalgia (15% of cases), nonspecific or nonlocalizing neurological symptoms with abnormal MRI presentation (12% of cases), conversion disorder/functional neurological disorder (11% of cases), and NMO (6% of cases).34 Other diseases that may be considered in a differential diagnosis of MS include MOGAD,28 CNS vasculitis,35 neurosarcoidosis,36 CNS manifestations of autoimmune diseases such as Sjogren’s syndrome, SLE, and antiphospholipid antibody syndrome,37 chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids,38 and Beçhet’s syndrome.39 MRI is helpful for distinguishing MS from other disorders. MS lesions are typically 3 mm or larger. Migraines and chronic ischemic disease can present with subcortical and periventricular lesions, which should touch the ventricular surface. Moreover, chronic ischemic disease typically causes central brainstem hyperintensities on T2-weighted images while MS brainstem lesions are located peripherally. Demyelinating white-matter lesions (T2-weighted hyperintensities) are often associated with hypointensities in T1-weighted images.40 Sample lesions of axial brain MRI findings in migraine headache and chronic ischemic disease are shown in Fig. 2.
Fig. 2. Brain MRI findings show scattered T2-FLAIR hyperintensities in the subcortical distribution (A and B). While there is "ventricular capping" of white matter hyperintensity seen in image (B), there are no discrete, ovoid perpendicular periventricular lesions (ie. "Dawson's fingers") that one would expect to see in demyelinating disease. A similar pattern could also be seen in migraineurs. Images (C) and (D) point to subcortical <3 mm T2-FLAIR hyperintensities in a migraine patient (arrows). These are too small and in the wrong location for demyelinating disease.
CSF analysis in MS
CSF analysis in MS typically consists of the cell count, protein and glucose levels, oligoclonal bands, and the IgG index and synthesis rate.41 Oligoclonal bands are observed in 80% of first MS attacks and in more than 90% of patients with MS at some point. CSF tests that are considered less sensitive or specific for diagnosing MS mimics include angiotensin-converting enzyme42 and myelin basic protein (MBP); however, these tests can be considered in certain cases where necessary.43 A meningitis panel or bacterial or fungal cultures may be necessary to rule out infection. Atypical features in MS include absence of oligoclonal bands, cell count >50 WBC/mm3, and a protein concentration of >100 mg/dL.34 Oligoclonal band testing paired with (or replaced by) measurement of kappa free light chains (kFLC) in the CSF increases diagnostic yield. Comparative studies have found CSF kFLC concentrations to be highly sensitive and specific in all forms of MS without the need for a corresponding serum sample.44
Predictors of disability in MS
The median life expectancy of patients with MS is approximately 76 years, which is comparable to the average life expectancy of 77 years in the United States population.45 Comorbidities such as hyperlipidemia, hypertension, and vascular risk factors accelerate disability progression by at least approximately 6 years.46 More-severe disability and secondary progressive MS course are also associated with male sex (in relapse-onset MS),47 older age at onset (40 years or older),48 and spinal cord lesions.20
Socioeconomic consequences of MS
MS also has profound socioeconomic consequences, which are further amplified in low-income patients; one systematic review noted that, when compared with the general population, patients with MS have 15%–30% lower employment rates, lower earnings, higher absenteeism and presenteeism, increased work disability, and greater reliance on social welfare programs.49 Furthermore, patients living in areas with greater socioeconomic deprivation are often predisposed to a higher risk of disability due to disparities in access to and quality of care.50
MS TREATMENTS
Acute MS relapse management
Acute flares in a patient with MS are associated with new symptoms or old ones that have worsened. Most patients with MS flares will not require emergency care unless they present with strength, gait, or vision impairment.51 The use of intravenous (IV) corticosteroids (e.g., 1,000 mg of IV methylprednisolone daily for 3–5 days) may shorten the flare duration, but this does not impact the overall disability outcome.51 One exception is optic neuritis, for which the Optic Neuritis Treatment Trial17 found that patients treated with high-dose steroids had better visual outcomes at 6 months. Oral prednisone at 1,250 mg daily can be a substitute for 1 g of IV methylprednisolone. Plasma exchange can be considered for patients with symptoms that are refractory to IV steroids.51 IV immunoglobin monotherapy can be considered in cases of acute MS relapse in patients with contraindications to plasmapheresis and IV steroid therapy, although there is less evidence supporting this.52
Therapy for long-term MS management
As of March 2023, 24 different DMTs had been approved for MS treatment, including injectable, oral, and infused medications.53 Given this expansive list, this review focuses on the latest monoclonal antibody treatment regimens as well as representative treatments from other disease-modifying classes.
One area of practice in MS that has changed over the years is the early initiation of high-efficacy treatment. Recent research supported high-efficacy therapy that commences within 2 years of disease onset, since this is associated with lessened disability after 6–10 years compared with delaying such therapies.54 In one comparison study of patients who underwent high-efficacy therapy versus those who underwent moderate-efficacy therapy, 68% and 52% of patients achieved no evidence of disease activity (NEDA) at 1 year, respectively, and 52% and 19% achieved NEDA at 2 years.55 However, not all patients require high-efficacy therapy; clinically stable patients on mild- or moderate-efficacy therapy do not need to change their medication regimen. Similarly, patients with relatively few lesions or older patients with MS do not require such strong medications.
Low-, medium-, and high-efficacy treatments are compiled in Table 2.56
Table 2. Low-, moderate-, and high-efficacy treatments for multiple sclerosis56.
| Low-efficacy treatments | Moderate-efficacy treatments | High-efficacy treatments |
|---|---|---|
| • Interferons | • Cladribine* | • Ocrelizumab |
| • Glatiramer acetate | • s1p inhibitors* | • Ofatumumab |
| • Teriflunomide | • Fumarates | • Natalizumab |
| • Alemtuzumab |
*May be considered to have moderate-to-high efficacy.
Mild-efficacy therapies
Interferons
Interferons (IFNs) including beta-1α57 and beta-1β58,59,60 were developed during the 1990s. They work by binding to cell surface receptors and initiating signaling pathways that lead to increased antiviral, antiproliferative, and immunomodulatory gene products that ultimately inhibit proinflammatory cytokines and T-cell activation.61 Initially considered a platform therapy, tolerability restricts their use due to common flu-like side effects from injections.
Glatiramer acetate
GA functions via two mechanisms: 1) reducing interleukin-17 and IFN-γ production via autologous CD4+ T cells, thereby inhibiting proinflammatory cytokines, and 2) mimicking MBP regions, thereby functioning as a decoy receptor via molecular mimicry to be targeted by the immune system of the body.62,63 GA is the only MS drug that does not require laboratory monitoring.64 The main side effect is skin hardening at the injection site.64
Teriflunomide
Teriflunomide65,66,67 is an oral therapy that inhibits dihydroorotate dehydrogenase, which in turn reduces the levels of activated B and T lymphocytes. Teriflunomide is often administered to patients who have been treated with mild-efficacy injectables and who experience injection fatigue. There may also be a use for teriflunomide in older patients who wish to de-escalate treatment.
Moderate-to-high efficacy therapies
Cladribine
Cladribine (2-chlorodeoxyadenosine) works by incorporating into DNA and inhibiting DNA polymerase and ribonucleotide reductase (enzymes involved in DNA synthesis and transcription), thereby creating DNA strand breaks.68 Cladribine consists of two oral treatment courses administered approximately 1 year apart, and has been found to deplete the total T- and B-cell counts by 40%–50% and 80%, respectively.69 Cladribine may be considered a high-efficacy therapy after both courses. Side effects include lymphopenia (in 21.6% of cases) and herpetic infections.70 There is a black-box warning for malignancies, although more than 10 years of safety data had not indicated increased malignancy risk. However, additional cladribine treatment during the 2 years after the second treatment course is associated with increased malignancy incidence (0.91 events per 100 patient-years).71
s1p inhibitors
s1p inhibitors such as fingolimod, siponimod, ozanimod, and ponesimod72,73,74 block the egress of lymphocytes from lymph nodes. They are effective at preventing MS relapse, but also have the side effect of increased infection risk, including that of progressive multifocal leukoencephalopathy (PML) and cryptococcal meningitis, as well as the risk of rebound relapse after drug discontinuation; there have been 52 documented cases of severe MS rebound after fingolimod withdrawal.75
Fumarates
Fumarates (dimethyl, diroximel, and monomethyl fumarate) are oral medications that work via various pathways to both suppress proinflammatory cytokines (e.g., NF-kB) and activate the nuclear factor E2 pathway that leads to increased antioxidant enzyme synthesis.76 Taking fumarates twice daily has been found to approximately halve the frequency of MS relapses.77 Dimethyl fumarate78,79 was approved for relapsing-remitting MS (RRMS) in 2013. Up to 30% of patients on dimethyl fumarate can experience nausea or flushes. Nausea rates are lower for diroximel fumarate.80,81 This drug class is considered to have a lower infection risk than high-efficacy therapy.
High-efficacy therapies
Ocrelizumab
Ocrelizumab was approved for both RRMS and primary progressive MS (PPMS) in 2017.56,82,83 It works by depleting CD20 B cells and is administered via infusion every 6 months. Although MS is historically considered a T-cell disease, blockade of B-cell autoreactivity inhibits neuroinflammation through several pathways, including 1) preventing B cells from acting as antigen-presenting cells that activate autoreactive T cells, 2) preventing B cells from releasing proinflammatory cytokines, 3) preventing B cells from transforming into plasma cells that may produce myelin-directed autoantibodies, and 4) preventing B cells from forming meningeal lymphoid follicles.84 An interim analysis of an open-label phase IIIb study found that patients with MS treated using ocrelizumab demonstrated a 75% increase in NEDA after 1 year compared with an IFN β-1α group.85 Furthermore, a post-hoc analysis in the phase III ORATORIO study on PPMS found that ocrelizumab slowed the accumulation of T2-weighted lesions.86 The side effects of ocrelizumab include decreased responsiveness to vaccines and increased risks of mucosal and herpetic infections.87
Ofatumumab
Another anti-CD20 B-cell therapy, ofatumumab, was approved for RRMS in 2020 and is administered via monthly self-injection.88 Its side-effects profile is similar to that of ocrelizumab.89,90 Given the lack of a need for premedication, patients can inject ofatumumab at home.88,89
Natalizumab
Natalizumab is a monoclonal antibody that was approved for MS in 2004 and is administered via infusion every 4 weeks.91 The advantage of natalizumab over conventional DMTs is decreased peripheral immunosuppression due to a more-targeted effect. Natalizumab binds to α4β1 integrin to prevent vascular cell adhesion protein 1-mediated leukocyte transmigration across the blood–brain barrier. It should be noted that screening for JC virus (JCV) is necessary in patients taking natalizumab, since JCV can cause PML. Natalizumab was initially withdrawn from the market due to PML cases in 2005,92 but returned in 2006 with a Risk Evaluation and Mitigation Strategy program based on JCV serology. The current PML rate is <1:10,000 in JCV-negative patients.93 Monitoring laboratory tests include CBC with differential, CMP, and JC virus every 6 months.94
Alemtuzumab
Alemtuzumab is an anti-CD52 monoclonal antibody with high efficacy that is administered via injection.95 It is often utilized for RRMS and in MS cases that do not respond to multiple medications due to its side-effects profile that includes significant risks of secondary autoimmune thyroid disease, TB, HSV, and infusion reactions.95
Therapies with their recommended baseline and routine monitoring tests are summarized in Table 3.
Table 3. Selected MS DMTs with baseline and routine monitoring information.
| Drug (brand name) | Class | Efficacy | Dose | Screening tests | Additional information | Associated references |
|---|---|---|---|---|---|---|
| Ocrelizumab (Ocrevus) | Anti-CD20 monoclonal antibody | High | 300 mg IV×2 doses 2 weeks apart, then 600 mg every 24 weeks | • Screening laboratory tests: CBC, CMP, immunoglobulins, Quantiferon Gold, hepatitis B surface antigen and antibody • Optional laboratory tests include HIV, syphilis, hepatitis C, VZV IgG, baseline B and T cells • Monitoring laboratory tests include CBC, CMP, immunoglobulins, and CD20 counts every 6 months |
• Premedication with Tylenol, Benadryl, and IV Solumedrol • Infusions take 4 hours. Rapid protocol (2 hours) can be administered after the first year • Live vaccines at least 4 weeks prior to start of drug therapy |
82 83 84 85 86 89 |
| Ofatumumab (Kesimpta) | Anti-CD20 monoclonal antibody | High | 20 mg SQ weekly on weeks 0, 1, 2, then 20 mg every 4 weeks starting in week 4 | Screening and monitoring laboratory tests same as for ocrelizumab | • Side-effect profile similar to that of ocrelizumab • No need for premedication. Patients can self-administer via home injection |
83 88 89 90 |
| Natalizumab (Tysabri) | α4β1-integrin binder | High | 300 mg IV once a month. Option for every 6 weeks after 24 weeks on therapy | CBC, CMP, and JCV index every 3–6 months | • Risk of PML in JCV negative=1:10,000; 1:1,000 for patients with low titer • Monitor for liver dysfunction |
91 92 93 94 |
| Alemtuzumab (Lemtrada) | Anti-CD52 monoclonal antibody | High | Year 1:5 days of 12 mg IV daily with steroids Year 2:3 days of 12 mg IV daily with steroids |
• Baseline: CBC, creatinine, LFTs, UA, urine protein-to-creatine ratio, TSH, skin examination • Monthly laboratory tests (CBC with differential, CMP, and UA) for 48 months after the last treatment course of Lemtrada • TSH every 3 months • Yearly skin examinations |
• Only needs to be performed yearly, but requires monthly laboratory tests that continue 4 years after administration • Up to 40% risk of secondary autoimmune thyroid disease • Rare risk of ITP (2%), serious infusion reactions, TB, HSV, glomerulonephritis, thyroid malignancy, skin cancer (melanoma), and lymphoproliferative disorders |
95 |
| Fingolimod (Gilenya) | s1p inhibitor | Medium to high | 0.5 mg PO daily | • Baseline: CBC, CMP, Quantiferon Gold, hepatitis B surface antigen and antibody • EKG and first-dose monitoring due to bradycardia • Baseline ophthalmological and dermatological examinations • CBC with differential, CMP every 6 months • Ophthalmology examination to screen for macular edema 3 months after starting drug and then yearly • Annual dermatological examination |
• 6-hour first dose monitoring • Risk of PML up to 1:8,000 • Associated with increased risk of cryptococcal meningitis • Other side effects: infections (11%), bradycardia (3%), headache (25%), nausea/vomiting/diarrhea (13%), and macular edema (1.5%) • Stop 2 months before pregnancy • Lymphopenia is expected on s1p-inhibitor therapy and is not a reason for discontinuation |
72 74 75 103 104 105 |
| Siponimod (Mayzent) | s1p inhibitor | Medium to high | Initial: 0.25 mg PO once daily on days 1 and 2; 0.5 mg on day 3; 0.75 mg on day 4; 1.25 mg on day 5 Maintenance: 2 mg once daily, beginning on day 6 |
• Baseline: CBC, CMP, Quantiferon Gold, hepatitis B surface antigen and antibody, CYP2C9*3/*3, EKG • CBC with differential, CMP every 6 months • First-dose 6-hour monitoring is recommended for patients with certain preexisting cardiac conditions, including sinus bradycardia (HR <55 bpm), firstor second-degree (Mobitz type 1) AV block, or a history of MI or heart failure |
• Requires genetics testing to rule out CYP2C9*3/*3 before initiation • Side effects: hypertension (13%), headache (15%), falling (11%), increased serum transaminases (≤11%), peripheral edema (8%), bradycardia (4%–6%), first-degree atrioventricular block (5%), nausea (7%), diarrhea (6%), lymphocytopenia (<5%), and macular edema (2% to <5%) • Does not require first-dose monitoring, ophthalmological or dermatological examinations due to fewer off-target effects |
106 |
| Ozanimod (Zeposia) | s1p inhibitor | Medium to high | Initial: 0.23 mg once daily on days 1–4, 0.46 mg on days 5–7, then 0.92 mg daily | • CBC with differential every 6 months, EKG, CMP every 6 months, Quantiferon Gold, and hepatitis B surface antigen and antibody | • Does not require first-dose monitoring, ophthalmological or dermatological examinations due to fewer off-target effects • Risk of hypertension and liver enzyme elevation potentially lower than for other s1p inhibitors |
107 |
| Cladribine (Mavenclad) | Adenosine nucleoside analog | Medium to high | 2 oral treatment courses, each approximately 5 days each and 1 year apart Total dose of 3.5 mg/kg (1.75 mg/kg per treatment course) |
• LFTs and CBC with differential prior to initiating treatment • Annual CBC with differential to monitor for lymphopenia while receiving treatment • Screen patients for latent infections; vaccinate VZV-antibody-negative patients prior to treatment • If lymphocytes <200 cells/mm3, administer antiherpes prophylaxis |
• Side effects: lymphopenia (21.6%), herpetic infections, and teratogenicity • Additional cladribine beyond 2 years associated with slightly increased malignancy risk (0.91 events per 100 patient-years) |
71 |
| Dimethyl fumarate (Tecfidera) | Fumarate | Medium | Initial: 120 mg PO BID then after 7 days increase to maintenance dose of 240 mg BID Take with food |
CBC with differential, LFTs every 6 months | • Side effects: flushing (40%), abdominal pain (18%), diarrhea (14%), and nausea (12%) • Lymphopenia occurs in 2%. Withhold medication when lymphocyte count <500 |
79 81 |
| INF β-1α (Avonex, Rebif, Plegridy) and INF beta-1b (Betaseron) | Immunomodulator | Low | CBC, CMP at 3 months, 6 months, and then yearly | • Flu-like symptoms, depression, and injection-site irritation | 59 60 | |
| Teriflunomide (Aubagio) | Pyrimidine synthesis inhibitor | Low | 14 mg PO daily | CBC, CMP, Quantiferon Gold, LFTs monthly for first 6 months | • Hair thinning (10%); avoid in patients with liver disease • Category X in pregnancy. Long half-life, can stay in body for 1 year; must use cholestyramine elimination protocol |
67 |
| GA (Copaxone or Glatopa) | Receptor decoy | Low | 20 mg SQ daily or 40 mg TID | No laboratory monitoring required | • Flushing, chest pain after injection, and lipoatrophy | 64 |
CBC, complete blood count; CMP, comprehensive metabolic panel; HSV, herpes simplex virus; IM, intramuscular; ITP, immune thrombocytopenia; IV, intravenous; JCV, John Cunningham virus; LFT, liver function tests; PML, progressive multifocal leukoencephalopathy; PO, per oral; SQ, subcutaneous; TB, tuberculosis; UA, urinalysis; VZV, varicella zoster virus.
ONGOING RESEARCH AND TREATMENTS UNDERGOING INVESTIGATION FOR MS
Hematopoietic stem cell transplant
Autologous hematopoietic stem cell tranplantation (aHSCT) has been investigated for use in treatment-refractory relapsing MS since the 1990s. The available data have been obtained in four retrospective studies, five single-arm clinical trials, and two randomized controlled trials. Typical candidates for HSCT are younger patients with RRMS with high relapse rates (i.e., >2 relapses in 1 year) and the ability to walk without support from a cane or other assistive device.96 The theoretical basis of HSCT for RRMS is that it diminishes pathogenic clones of autoreactive lymphocytes, thereby regenerating the tolerance of the immune system to CD34+ hematopoietic stem cells.97
Uncertainty remains as to where aHSCT fits in the overall treatment protocol for RRMS. The various studies that have investigated its use in RRMS involved different study populations, control groups, therapeutic protocols, and outcome measures, with the treatment-related mortality rate ranging from 0% to 4% depending on the regimen.96
Bruton’s tyrosine kinase inhibitors
Bruton’s Tyrosine Kinase Inhibitor (BTKi) regimens remain under investigation regarding MS treatment, including the use of masitinib, fenebrutinib, tolebrutinib, evobrutinib, remibrutinib, and orelabrutinib.98 The putative benefit of BTKi over traditional CD20 depleters is that BTKi block an enzyme required for B-cell maturation, thereby more selectively removing autoreactive B cells while sparing more-tolerant B cells (vs. CD20 depleters that broadly diminish B-cell reserves).98 Autoreactive B cells depend more on Bruton’s tyrosine kinase signaling than normal B cells do.99 BTKi may also modulate proinflammatory macrophages and microglia, making them attractive for use in progressive MS. Masitinib has demonstrated favorable results in phase IIb/III studies, including slowed disability progression in patients with PPMS with no signs of active inflammation.100 Tolebrutinib, which is more brain-penetrant, demonstrated promising results for RRMS treatment in a phase IIb study.101 Evobrutinib and remibrutinib are currently being investigated for use in RRMS.
Remyelination
Remyelination involves the production of a new myelin sheath around CNS axons. It requires a complex set of processes including various cell differentiation, migration, and signaling pathways.102 A systematic review and meta-analysis of remyelination treatments revealed that 88 different therapies have been tested preclinically, 28% of which entered clinical trials and nearly all of which failed in phase II.102 Research into remyelination is ongoing and it presents potential opportunities for the future.
CONCLUSION
The prevalence of MS has increased over time. The diagnostic criteria for MS have become more sensitive to allow for earlier treatments. Early initiation of high-efficacy treatments may reduce or prevent disability accumulation. However, it remains important to recognize atypical MS syndromes in order to avoid unnecessary treatment in diseases that may mimic it.
Footnotes
- Supervision: Edith L Graham.
- Writing—original draft: Archit B Baskaran, Edith L Graham.
- Writing—review & editing: all authors.
Conflicts of Interest: Elena Grebenciucova: Advisory board member for Alexion, Genentech, Horizon Therapeutics, Prevail Therapeutics; research support from F. Hoffman-La Roche Ltd. Thomas Shoemaker: Advisory board member for Genentech and Genzyme, Speakers bureau for Biogen and Genentech. Edith Graham: Advisory board member for EMD Serono, Genentech, Horizon Therapeutics, and Novartis; research support from F. Hoffman-La Roche Ltd. Archit Baskaran has no disclosures.
Funding Statement: None
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
- 1.Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci. 1992;19:466–471. [PubMed] [Google Scholar]
- 2.Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029–e1040. doi: 10.1212/WNL.0000000000007035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013;80:1734–1739. doi: 10.1212/WNL.0b013e3182918cc2. [DOI] [PubMed] [Google Scholar]
- 4.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 5.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T, et al. Epstein-Barr virus in multiple sclerosis: theory and emerging immunotherapies: (trends in molecular medicine, 26:3 p:296-310, 2020) Trends Mol Med. 2021;27:410–411. doi: 10.1016/j.molmed.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolders J, Thewissen M, Peelen E, Menheere P, Tervaert JW, Damoiseaux J, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JS, James I, Qiu W, Castley A, Christiansen FT, Carroll WM, et al. HLA-DRB1 allele heterogeneity influences multiple sclerosis severity as well as risk in Western Australia. J Neuroimmunol. 2010;219:109–113. doi: 10.1016/j.jneuroim.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 10.Vitkova M, Diouf I, Malpas C, Horakova D, Kubala Havrdova E, Patti F, et al. Association of latitude and exposure to ultraviolet B radiation with severity of multiple sclerosis: an international registry study. Neurology. 2022;98:e2401–e2412. doi: 10.1212/WNL.0000000000200545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20:653–670. doi: 10.1016/S1474-4422(21)00095-8. [DOI] [PubMed] [Google Scholar]
- 12.Solomon AJ, Naismith RT, Cross AH. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019;92:26–33. doi: 10.1212/WNL.0000000000006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledesma J, Puttagunta PP, Torabi S, Berube K, Tamrazian E, Garcia D, et al. Presenting symptoms and disease severity in multiple sclerosis patients. Neurol Int. 2021;13:18–24. doi: 10.3390/neurolint13010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009;16:82–89. [PubMed] [Google Scholar]
- 15.Wilhelm H, Schabet M. The diagnosis and treatment of optic neuritis. Dtsch Arztebl Int. 2015;112:616–625. doi: 10.3238/arztebl.2015.0616. quiz 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez de Antonio LA, García Castañón I, Aguilar-Amat Prior MJ, Puertas I, González Suárez I, Oreja Guevara C. Non-inflammatory causes of emergency consultation in patients with multiple sclerosis. Neurologia (Engl Ed) 2021;36:403–411. doi: 10.1016/j.nrleng.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727–732. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinshenker BG, Rice GP, Noseworthy JH, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. 3. Multivariate analysis of predictive factors and models of outcome. Brain. 1991;114(Pt 2):1045–1056. doi: 10.1093/brain/114.2.1045. [DOI] [PubMed] [Google Scholar]
- 19.Zecca C, Disanto G, Sormani MP, Riccitelli GC, Cianfoni A, Del Grande F, et al. Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler. 2016;22:782–791. doi: 10.1177/1352458515599246. [DOI] [PubMed] [Google Scholar]
- 20.D’Amico E, Patti F, Leone C, Lo Fermo S, Zappia M. Negative prognostic impact of MRI spinal lesions in the early stages of relapsing-remitting multiple sclerosis. Mult Scler J Exp Transl Clin. 2016;2:2055217316631565. doi: 10.1177/2055217316631565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross Russell AL, Dryden MS, Pinto AA, Lovett JK. Lyme disease: diagnosis and management. Pract Neurol. 2018;18:455–464. doi: 10.1136/practneurol-2018-001998. [DOI] [PubMed] [Google Scholar]
- 22.Djukic M, Schmidt-Samoa C, Lange P, Spreer A, Neubieser K, Eiffert H, et al. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J Neurol. 2012;259:630–636. doi: 10.1007/s00415-011-6221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gospe SM, 3rd, Chen JJ, Bhatti MT. Neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disorder-optic neuritis: a comprehensive review of diagnosis and treatment. Eye (Lond) 2021;35:753–768. doi: 10.1038/s41433-020-01334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunchok A, Zekeridou A, McKeon A. Autoimmune glial fibrillary acidic protein astrocytopathy. Curr Opin Neurol. 2019;32:452–458. doi: 10.1097/WCO.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz D, van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol. 2016;16:220. doi: 10.1186/s12883-016-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitik B, Mercan R, Tufan A, Tezcan E, Küçük H, İlhan M, et al. Differential diagnosis of elevated erythrocyte sedimentation rate and C-reactive protein levels: a rheumatology perspective. Eur J Rheumatol. 2015;2:131–134. doi: 10.5152/eurjrheum.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alnajashi H, Alshamrani F. Prevalence of antinuclear antibody in patients with multiple sclerosis: a case-control study. Egypt J Neurol Psychiatry Neurosurg. 2021;57:27 [Google Scholar]
- 28.Filippatou AG, Mukharesh L, Saidha S, Calabresi PA, Sotirchos ES. AQP4-IgG and MOG-IgG related optic neuritis—prevalence, optical coherence tomography findings, and visual outcomes: a systematic review and meta-analysis. Front Neurol. 2020;11:540156. doi: 10.3389/fneur.2020.540156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chitnis T, Gonzalez C, Healy BC, Saxena S, Rosso M, Barro C, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol. 2018;5:1478–1491. doi: 10.1002/acn3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostini C, Bovis F, Disanto G, Ripellino P, Pravatà E, Sacco R, et al. Recurrence and prognostic value of asymptomatic spinal cord lesions in multiple sclerosis. J Clin Med. 2021;10:463. doi: 10.3390/jcm10030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney AM, Lohman BD, Sarikaya B, Benson M, Lee MS, Benson MT. Accuracy of routine fat-suppressed FLAIR and diffusion-weighted images in detecting clinically evident acute optic neuritis. Acta Radiol. 2013;54:455–461. doi: 10.1177/0284185113475444. [DOI] [PubMed] [Google Scholar]
- 32.Shor N, Deschamps R, Cobo Calvo A, Maillart E, Zephir H, Ciron J, et al. MRI characteristics of MOG-Ab associated disease in adults: an update. Rev Neurol (Paris) 2021;177:39–50. doi: 10.1016/j.neurol.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Haider L, Chan WE, Olbert E, Mangesius S, Dal-Bianco A, Leutmezer F, et al. Cranial nerve enhancement in multiple sclerosis is associated with younger age at onset and more severe disease. Front Neurol. 2019;10:1085. doi: 10.3389/fneur.2019.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon AJ, Bourdette DN, Cross AH, Applebee A, Skidd PM, Howard DB, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87:1393–1399. doi: 10.1212/WNL.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maggi P, Absinta M, Grammatico M, Vuolo L, Emmi G, Carlucci G, et al. Central vein sign differentiates multiple sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol. 2018;83:283–294. doi: 10.1002/ana.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto C, Wengert O, Unterwalder N, Meisel C, Ruprecht K. Analysis of soluble interleukin-2 receptor as CSF biomarker for neurosarcoidosis. Neurol Neuroimmunol Neuroinflamm. 2020;7:e725. doi: 10.1212/NXI.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11:762. doi: 10.3390/v11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dudesek A, Rimmele F, Tesar S, Kolbaske S, Rommer PS, Benecke R, et al. CLIPPERS: chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Review of an increasingly recognized entity within the spectrum of inflammatory central nervous system disorders. Clin Exp Immunol. 2014;175:385–396. doi: 10.1111/cei.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turker H, Terzi M, Bayrak O, Cengiz N, Onar M, Us O. Visual evoked potentials in differential diagnosis of multiple sclerosis and neurobehcet’s disease. Tohoku J Exp Med. 2008;216:109–116. doi: 10.1620/tjem.216.109. [DOI] [PubMed] [Google Scholar]
- 40.Kruit M. In: Neurovascular Imaging. 1st ed. Saba L, Raz E, editors. New York: Springer; 2016. Migraine; pp. 79–815. [Google Scholar]
- 41.Rammohan KW. Cerebrospinal fluid in multiple sclerosis. Ann Indian Acad Neurol. 2009;12:246–253. doi: 10.4103/0972-2327.58282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arun T, Pattison L, Palace J. Distinguishing neurosarcoidosis from multiple sclerosis based on CSF analysis: a retrospective study. Neurology. 2020;94:e2545–e2554. doi: 10.1212/WNL.0000000000009491. [DOI] [PubMed] [Google Scholar]
- 43.Greene DN, Schmidt RL, Wilson AR, Freedman MS, Grenache DG. Cerebrospinal fluid myelin basic protein is frequently ordered but has little value: a test utilization study. Am J Clin Pathol. 2012;138:262–272. doi: 10.1309/AJCPCYCH96QYPHJM. [DOI] [PubMed] [Google Scholar]
- 44.Hassan-Smith G, Durant L, Tsentemeidou A, Assi LK, Faint JM, Kalra S, et al. High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis. J Neuroimmunol. 2014;276:175–179. doi: 10.1016/j.jneuroim.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Scalfari A, Knappertz V, Cutter G, Goodin DS, Ashton R, Ebers GC. Mortality in patients with multiple sclerosis. Neurology. 2013;81:184–192. doi: 10.1212/WNL.0b013e31829a3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribbons KA, McElduff P, Boz C, Trojano M, Izquierdo G, Duquette P, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. 2015;10:e0122686. doi: 10.1371/journal.pone.0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillemin F, Baumann C, Epstein J, Kerschen P, Garot T, Mathey G, et al. Older age at multiple sclerosis onset is an independent factor of poor prognosis: a population-based cohort study. Neuroepidemiology. 2017;48:179–187. doi: 10.1159/000479516. [DOI] [PubMed] [Google Scholar]
- 49.Kavaliunas A, Danylaite Karrenbauer V, Hillert J. Socioeconomic consequences of multiple sclerosis-A systematic literature review. Acta Neurol Scand. 2021;143:587–601. doi: 10.1111/ane.13411. [DOI] [PubMed] [Google Scholar]
- 50.Calocer F, Dejardin O, Kwiatkowski A, Bourre B, Vermersch P, Hautecoeur P, et al. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Mult Scler Relat Disord. 2020;40:101930. doi: 10.1016/j.msard.2020.101930. [DOI] [PubMed] [Google Scholar]
- 51.Ontaneda D, Rae-Grant AD. Management of acute exacerbations in multiple sclerosis. Ann Indian Acad Neurol. 2009;12:264–272. doi: 10.4103/0972-2327.58283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elovaara I, Kuusisto H, Wu X, Rinta S, Dastidar P, Reipert B. Intravenous immunoglobulins are a therapeutic option in the treatment of multiple sclerosis relapse. Clin Neuropharmacol. 2011;34:84–89. doi: 10.1097/WNF.0b013e31820a17f3. [DOI] [PubMed] [Google Scholar]
- 53.National MS Society. Disease-modifying therapies for MS [Internet] National MS Society; 2023. [cited 2023 Feb 1]. Available from: https://www.nationalmssociety.org/Programs-and-Services/Resources/The-MS-Disease-Modifying-Medications-(-pdf) [Google Scholar]
- 54.He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19:307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 55.Simonsen CS, Flemmen HØ, Broch L, Brunborg C, Berg-Hansen P, Moen SM, et al. Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol. 2021;12:693017. doi: 10.3389/fneur.2021.693017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Häring DA, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10:495–507. doi: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 58.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 59.Biogen Idec. Avonex (interferon beta-1a) full prescribing information [Internet] Cambridge, MA: Biogen Idec; 1996. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103628s5189lbl.pdf . [Google Scholar]
- 60.Bayer. Betaseron (interferon beta-1b) full prescribing information [Internet] Whippany, NJ: Bayer; 1993. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103471s5193lbl.pdf . [Google Scholar]
- 61.Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology. 2002;58(8 Suppl 4):S3–S9. doi: 10.1212/wnl.58.8_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- 62.Melnikov M, Sharanova S, Sviridova A, Rogovskii V, Murugina N, Nikolaeva A, et al. The influence of glatiramer acetate on Th17-immune response in multiple sclerosis. PLoS One. 2020;15:e0240305. doi: 10.1371/journal.pone.0240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stapulionis R, Oliveira CL, Gjelstrup MC, Pedersen JS, Hokland ME, Hoffmann SV, et al. Structural insight into the function of myelin basic protein as a ligand for integrin alpha M beta 2. J Immunol. 2008;180:3946–3956. doi: 10.4049/jimmunol.180.6.3946. [DOI] [PubMed] [Google Scholar]
- 64.TEVA. Copaxone (glatiramer acetate) full prescribing information [Internet] North Wales, PA: TEVA; 1996. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020622s057lbl.pdf . [Google Scholar]
- 65.O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 66.Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 67.Genzyme Corporation. Aubagio (teriflunomide) full prescribing information [Internet] Cambridge, MA: Genzyme Corporation; 2012. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202992s000lbl.pdf . [Google Scholar]
- 68.Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34:28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 69.Leist TP, Comi G, Cree BA, Coyle PK, Freedman MS, Hartung HP, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13:257–267. doi: 10.1016/S1474-4422(14)70005-5. [DOI] [PubMed] [Google Scholar]
- 70.Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 71.EMD Serono, Inc. Mavenclad (cladribine) full prescribing information [Internet] Rockland, MA: EMD Serono, Inc; 2019. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf . [Google Scholar]
- 72.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 73.Janssen Pharmaceuticals, Inc. Ponvory (ponesimod) full prescribing information [Internet] Titusville, NJ: Janssen Pharmaceuticals, Inc; 2021. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213498s000lbl.pdf . [Google Scholar]
- 74.Singer BA. Initiating oral fingolimod treatment in patients with multiple sclerosis. Ther Adv Neurol Disord. 2013;6:269–275. doi: 10.1177/1756285613491520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fragoso YD, Adoni T, Gomes S, Goncalves MVM, Parolin LF, Rosa G, et al. Severe exacerbation of multiple sclerosis following withdrawal of fingolimod. Clin Drug Investig. 2019;39:909–913. doi: 10.1007/s40261-019-00804-6. [DOI] [PubMed] [Google Scholar]
- 76.Kasarełło K, Cudnoch-Jędrzejewska A, Członkowski A, Mirowska-Guzel D. Mechanism of action of three newly registered drugs for multiple sclerosis treatment. Pharmacol Rep. 2017;69:702–708. doi: 10.1016/j.pharep.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Prosperini L, Pontecorvo S. Dimethyl fumarate in the management of multiple sclerosis: appropriate patient selection and special considerations. Ther Clin Risk Manag. 2016;12:339–350. doi: 10.2147/TCRM.S85099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 79.Biogen Idec. Tecfidera (dimethyl fumarate) full prescribing information [Internet] Cambridge, MA: Biogen Idec; 2013. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204063lbl.pdf . [Google Scholar]
- 80.Naismith RT, Wundes A, Ziemssen T, Jasinska E, Freedman MS, Lembo AJ, et al. Diroximel fumarate demonstrates an improved gastrointestinal tolerability profile compared with dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. CNS Drugs. 2020;34:185–196. doi: 10.1007/s40263-020-00700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alkermes. Vumerity (diroximel fumarate) full prescribing information [Internet] Waltham, MA: Alkermes; 2013. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211855s000lbl.pdf . [Google Scholar]
- 82.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 83.Genentech. Ocrevus (ocrelizumab) full prescribing information [Internet] South San Francisco, CA: Genentech; 2017. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761053lbl.pdf . [Google Scholar]
- 84.Jain RW, Yong VW. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat Rev Immunol. 2022;22:513–524. doi: 10.1038/s41577-021-00652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Havrdová E, Arnold DL, Bar-Or A, Comi G, Hartung HP, Kappos L, et al. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018;4:2055217318760642. doi: 10.1177/2055217318760642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolinsky JS, Arnold DL, Brochet B, Hartung HP, Montalban X, Naismith RT, et al. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19:998–1009. doi: 10.1016/S1474-4422(20)30342-2. [DOI] [PubMed] [Google Scholar]
- 87.Golshani M, Hrdý J. Multiple sclerosis patients and disease modifying therapies: impact on immune responses against COVID-19 and SARS-CoV-2 vaccination. Vaccines (Basel) 2022;10:279. doi: 10.3390/vaccines10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.GlaxoSmithKline. Kesimpta (ofatumumab) full prescribing information [Internet] Brentford: GlaxoSmithKline; 2009. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf . [Google Scholar]
- 89.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 90.Florou D, Katsara M, Feehan J, Dardiotis E, Apostolopoulos V. Anti-CD20 agents for multiple sclerosis: spotlight on ocrelizumab and ofatumumab. Brain Sci. 2020;10:758. doi: 10.3390/brainsci10100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 92.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab--unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 93.Biogen, Idec. Medical information TYSABRI® (natalizumab): anti-JCV antibody index and PML risk [Internet] Cambridge, MA: Biogen, Idec; 2021. [cited 2023 Feb 1]. Available from: https://cdn.medinfo.biogen.com/50b0bd83-052a-4030-924d-600a1324bcee/9eedb9b8-9ae2-43a5-b8b9-e44d7151352d/9eedb9b8-9ae2-43a5-b8b9-e44d7151352d_viewable_rendition__v.pdf . [Google Scholar]
- 94.Biogen Idec. Tysabri (natalizumab) full prescribing information [Internet] Cambridge, MA: Biogen Idec; 2004. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125104s0576lbl.pdf. [Google Scholar]
- 95.Genzyme Corporation. Lemtrada (alemtuzumab) full prescribing information [Internet] Cambridge, MA: Genzyme Corporation; 2001. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103948s5158lbl.pdf . [Google Scholar]
- 96.Cohen JA, Baldassari LE, Atkins HL, Bowen JD, Bredeson C, Carpenter PA, et al. Autologous hematopoietic cell transplantation for treatment-refractory relapsing multiple sclerosis: position statement from the American Society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2019;25:845–854. doi: 10.1016/j.bbmt.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Muraro PA, Martin R, Mancardi GL, Nicholas R, Sormani MP, Saccardi R. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat Rev Neurol. 2017;13:391–405. doi: 10.1038/nrneurol.2017.81. [DOI] [PubMed] [Google Scholar]
- 98.Dolgin E. BTK blockers make headway in multiple sclerosis. Nat Biotechnol. 2021;39:3–5. doi: 10.1038/s41587-020-00790-7. [DOI] [PubMed] [Google Scholar]
- 99.Crofford LJ, Nyhoff LE, Sheehan JH, Kendall PL. The role of Bruton’s tyrosine kinase in autoimmunity and implications for therapy. Expert Rev Clin Immunol. 2016;12:763–773. doi: 10.1586/1744666X.2016.1152888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vermersch P, Brieva-Ruiz L, Fox RJ, Paul F, Ramio-Torrenta L, Schwab M, et al. Efficacy and safety of masitinib in progressive forms of multiple sclerosis: a randomized, phase 3, clinical trial. Neurol Neuroimmunol Neuroinflamm. 2022;9:e1148. doi: 10.1212/NXI.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reich DS, Arnold DL, Vermersch P, Bar-Or A, Fox RJ, Matta A, et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20:729–738. doi: 10.1016/S1474-4422(21)00237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooijmans CR, Hlavica M, Schuler FAF, Good N, Good A, Baumgartner L, et al. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta-analysis. Sci Rep. 2019;9:822. doi: 10.1038/s41598-018-35734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 104.Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J. Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs. 2021;81:985–1002. doi: 10.1007/s40265-021-01528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novartis Pharmaceuticals Corporation. Gilenya (fingolimod) full prescribing information [Internet] Basel: Novartis Pharmaceuticals Corporation; 2010. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022527s008lbl.pdf . [Google Scholar]
- 106.Novartis Pharmaceuticals Corporation. Mayzent (siponimod) full prescribing information [Internet] Basel: Novartis Pharmaceuticals Corporation; 2019. [cited 2023 Feb 1]. Available from: https://www.novartis.com/us-en/sites/novartis_us/files/mayzent.pdf . [Google Scholar]
- 107.Celgene Corporation. Zeposia (ozanimod) full prescribing information [Internet] Summit, NJ: Celgene Corporation; 2020. [cited 2023 Feb 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.