Dear Editor,
The timing of spiking activity across neurons is believed to play an important role in information coding in brain circuits1[1]. At the cellular level, neurons can fire spikes with millisecond precision and the relative timing of pre- and postsynaptic spikes can determine the direction and extent of synaptic modification [2]. When such spike-timing-dependent plasticity (STDP) and other physiological and anatomical properties are implemented in theoretical models, the simulated networks of interconnected spiking neurons exhibit neuronal groups with stereotypical time-locked spatiotemporal firing patterns with millisecond temporal precision [3]. Combining STDP with the consideration of information coding in the spatiotemporal dynamics of neuronal populations, algorithms can be developed for spiking neural networks and energy-efficient neuromorphic devices to perform learning and problem-solving tasks [4, 5]. In a sense, such coding and learning schemes are in line with Hebb’s cell assembly theory, in which information is represented in the spatiotemporal pattern of reverberatory activity in the neuronal network [6]. However, direct experimental studies of reverberatory activity in cell assemblies in vivo have remained elusive. This is probably because neurons from a specific assembly are likely to be intermingled with many more neurons outside this assembly. Thus, even with the state-of-the-art technology capable of simultaneously recording from tens to hundreds of cells, it is still difficult to capture many neurons belonging to the same assembly [7]. In an attempt to explore the biophysical feasibility of population temporal coding, we took advantage of the accessibility of neuronal cultures that form self-organized networks exhibiting reverberatory activity [8]. We further evaluated the temporal precision of such population dynamics by combining electrophysiological recording with high-speed imaging to monitor the Ca2+ activity of all neurons in a network.
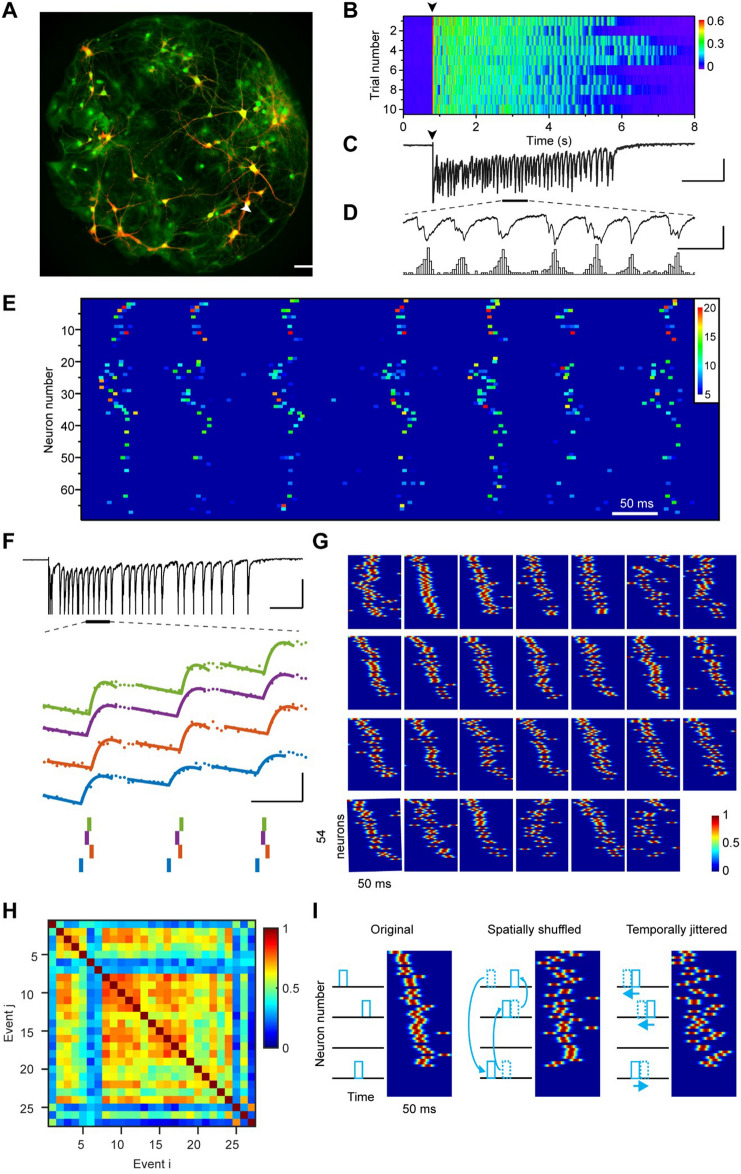
We grew networks of 30–100 hippocampal neurons together with glial cells to form isolated islands confined by microfabricated polydimethylsiloxane structures (Figs S1 and 1A). Consistent with previous reports [8], a brief 1-ms stimulus given to one glutamatergic neuron reliably elicited persistent network activity that lasted for several seconds (Fig. 1B, C). This activity was recorded as recurring polysynaptic current (PSC) clusters under voltage-clamp mode (Fig. 1D). Each of these clusters lasted for tens of milliseconds and consisted of multiple current components, indicating that a subpopulation of neurons in the network was firing in a bursting pattern with different time delays.
Fig. 1.
Spatiotemporal dynamics of reverberatory activity in neuronal networks. A Ca2+ imaging of a complete neuronal network. Green pseudocolor shows saturated Ca2+ fluorescence of all cells in the network activated by high K+ treatment at the end of the experiment. Red pseudocolor shows an average of background-subtracted Ca2+ images (ΔF) acquired within 100 ms after the stimulus, indicating neurons that were initially activated during this period. Scale bar, 200 µm. B Network reverberation evoked by single-pulse stimuli to a voltage-clamped neuron, indicated by an arrowhead in A. Stimulation pulses (100 mV, 1 ms) were given every 30 s at the time indicated by the black arrowhead. Color codes for the amplitude of polysynaptic currents (nA). C Polysynaptic current trace recorded from the reverberatory network (trial 1 in B). Scale bars: 1 s, 300 pA. D An enlarged segment of the trace in B to show seven periodic reverberatory events (upper) and a corresponding number of firing neurons (lower). Scale bars: 100 ms, 300 pA, 20 neurons. E Normalized activation signal (color-coded) of all 67 neurons in the network during the period shown in D. Similar firing patterns are visualized. F Example traces showing the inference of spike timing. In another network, during a reverberatory episode evoked by single-pulse stimulation (upper), a short segment of simultaneously recorded Ca2+ fluorescence signals (ΔF/F0) from four neurons (middle; colored dots), which are fitted locally (colored curves). Precise spike timings were determined from fitting results (lower). Short vertical bars below the traces show the corresponding timing of detected spikes. Scale bars: 500 ms, 0.5 nA (upper); 50 ms, 0.5 ΔF/F0 (middle). G All 27 consecutive reverberatory events from the reverberatory episode corresponding to the top panel shown in F sliced to 50-ms sections. Color codes for the normalized convolved spike trains of all neurons. H Similarity indexes (SI) of all reverberatory event pairs during the reverberatory episode. I Schematic showing how shuffling and jittering were applied. Spikes were shuffled or jittered before convolution was applied.
The well-defined culture system allows for close monitoring of the activity of all individual neurons in the reverberatory network by imaging the fluorescence signals of the Ca2+-sensitive dye Fluo-8 loaded into the cells (Fig. 1A). Upon elicitation of reverberation by a stimulus pulse applied to a single neuron through a patch-clamp recording electrode, many neurons exhibited stepwise increases in fluorescence signals that resulted from spike-triggered rapid Ca2+ influxes followed by a relatively slow clearance (Fig. S2A–C; Movie S1). The activation of individual neurons was then obtained by calculating changes in the fluorescence signal in adjacent frames of the raw fluorescence image series (Movie S2; also see Methods). Neuronal activation in this network aligns with simultaneously recorded PSCs (Fig. 1D).
In a typical reverberation episode (Fig. 1C, D), multiple bursts of network activation are punctuated by short, silent periods of around 50–200 ms (Fig. S3). We name these semi-rhythmic bursts “reverberation events”. After generating a raster plot of the activities of all identified neurons in the network during a period spanning several events, we found that six of the seven consecutive reverberation events displayed similar firing sequences that can be visually identified (Fig. 1E). In a reverberatory episode, only the first event was directly induced by an externally applied electrical stimulus, while all subsequent events were autonomously generated by intrinsic factors of cellular dynamics including asynchronous transmitter release at the presynaptic termini of these neurons. The existence of a conserved firing sequence across reverberation events indicates that the spatiotemporal pattern of network dynamics can be used for information encoding. Therefore, it would be of value to know how precisely the spatiotemporal pattern could be preserved, and whether the preserved pattern could be reactivated in later episodes.
To further assess the temporal precision of the conserved firing patterns across reverberation events, we took a template-matching approach to obtain “super-resolution” estimates of spike timing beyond the temporal resolution limited by the imaging frame rate (Figs 1F and S2D–F; also see Methods). When this approach was applied to our testing data set acquired from neurons under Ca2+ imaging with simultaneous patch-clamp recording and stimulation, the inferred spike-timing values had a temporal precision of ± 2.3 ms with a 95% confidence interval of 4.9 ms (Fig. S2F). After ranking the neurons according to their average spike timings, we found that many reverberation events have similar firing patterns (Fig. 1G).
We next quantitatively evaluated the similarity of spatiotemporal patterns. We defined a similarity index (SI) of a pair of reverberation events as the maximal cross-correlation coefficient of spike trains (convolved with a Gaussian kernel) from all active neurons in the events (Fig. 1H; also see Methods). Because this value can vary depending on the imaging speed and firing synchrony, we also calculated the SI for control data sets generated from the same experimental data after spatial shuffling and temporal jittering (Fig. 1I). In all the 26 reverberatory networks examined for the existence and temporal precision of spatiotemporal patterns, the SIs of the experimental data sets were significantly higher than the corresponding control datasets (Fig. S3). Thus, the neuronal networks with spontaneously-formed connectivity were capable of generating semi-rhythmic bursts of neuronal firing with truly conserved firing patterns at the millisecond level.
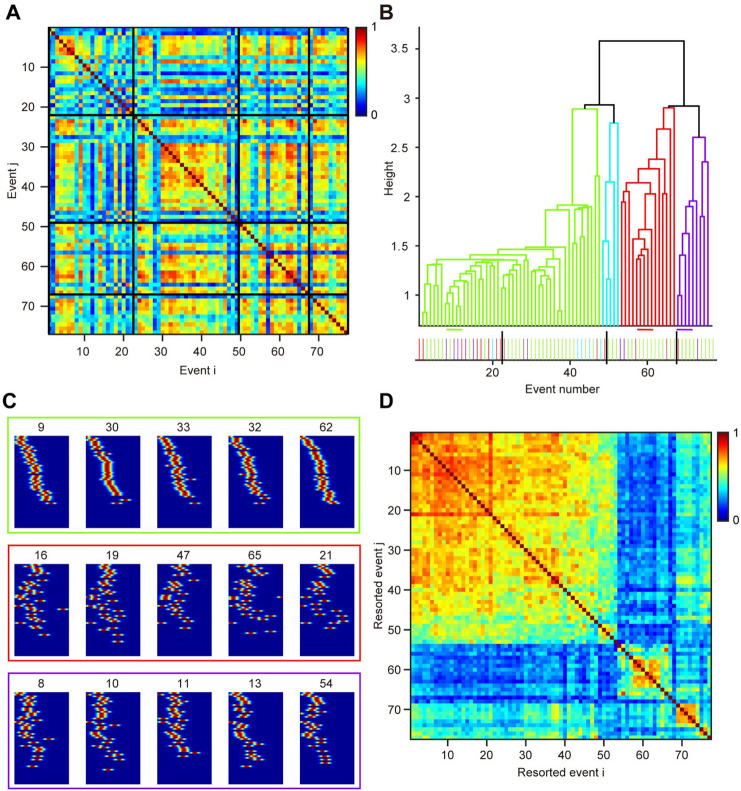
Intriguingly, not all reverberation events from a network share a common conserved activity pattern. An event can have high similarity with some other events, but low similarity with others in the same network (Fig. 2). Using cluster analysis with a standard dendrogram method [9], we found that multiple patterns of reverberation events coexisted in the same network, occurring in no particular temporal order during the reverberation episodes (Fig. 2A, B). And these different patterns involve the activation of largely the same group of neurons (Fig. 2C). To better visualize the similarity of these event patterns, the order of reverberation events in the similarity matrix was reorganized according to the dendrogram clustering results (Fig. 2D). From 29 networks examined, multiple clusters of reverberation patterns were found in nine networks, while the other 20 exhibited only one primary pattern.
Fig. 2.
Coexistence of multiple spatiotemporal patterns of reverberation events in a network. A SI of all event pairs from four distinct reverberatory episodes (interleaved by black horizontal or vertical lines) of an example network. B Dendrogram plot of the hierarchical binary cluster tree. Each leaf node corresponds to the reordered event number shown in D. The distances between two events are indicated by the height of n-shaped connection lines. Example events from three distinct clusters are indicated by horizontal lines and shown in C. Bottom: events colored by the clustering results are ranked in their temporal order to show that different patterns are mixed temporally. C Three groups of reverberatory spike trains showing patterns I, III, and IV. Neurons were sorted according to the first group and remained unchanged among all events. Values represent their event number in A. D As in A, but event numbers are reordered according to dendrogram results to visualize the cluster blocks.
In summary, by combining high-speed Ca2+ imaging and patch-clamp recordings, we have obtained direct experimental evidence that reverberatory activity with precise spatiotemporal patterns can exist and persist within networks of recurrently connected neurons. The reverberation we recorded contained conserved sequential firing at the millisecond level that rhythmically repeated itself at frequencies of 5–15 Hz, reminiscent of physiologically relevant theta oscillations in the brain that are highly conserved across many species [10, 11]. Such rhythmic activity could be due to the synergistic action of the firing property of recurrently-connected neurons and short-term synaptic plasticity as suggested in a previous modeling study [12]. Conserved neuronal firing has been reported in various native circuits from several brain regions in a broad range of behavioral contexts [13–15], indicating that neuronal networks of different scales in vivo can preserve precise spatiotemporal patterns, as seen in simpler culture systems. However, it is noted that these findings lack either high spatial or temporal resolution [15] or circuit integrity [13, 15] due to technical limitations and the complexity of in vivo circuits. These temporal structures are reminiscent of synfire chains [13]. However, instead of maintaining activity in the continuous feedforward propagation of neuronal firing along the synfire chain or loop, the reverberation we recorded contained conserved sequential firing that rhythmically repeated itself at theta frequency. Although not considered in classical models of network reverberation [16], the robustness and temporal precision of this unique dynamic mode suggest that it is capable of carrying information, as further indicated by the coexistence of multiple firing patterns in the same network. It is thus reasonable to speculate that for larger networks consisting of thousands or millions of neurons in vivo, the same biophysical properties may help increase the information coding capacity and support learning and intelligence in the system.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported in part by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32030200), the National Natural Science Foundation of China (U20A6005, 31621002, and 31070935), and 973 Program (2013CB835100). We thank X-Y. Tang for helping to develop the initial imaging analysis programs, B. Zhang for preparing the neuronal cell culture, and R. Gerkin, P-C. Zhou, and members of the Bi-Lau lab for helpful discussions.
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Dong-Qing Shi and Fang Xu contributed equally to this work.
Contributor Information
Guo-Qiang Bi, Email: gqbi@ustc.edu.cn.
Pak-Ming Lau, Email: plau@ustc.edu.cn.
References
- 1.Quiroga R, Panzeri S. Principles of Neural Coding. 1. Boca Raton: CRC Press; 2013. [Google Scholar]
- 2.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izhikevich EM, Gally JA, Edelman GM. Spike-timing dynamics of neuronal groups. Cereb Cortex. 2004;14:933–944. doi: 10.1093/cercor/bhh053. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen DA, Tran XT, Iacopi F. A review of algorithms and hardware implementations for spiking neural networks. J Low Power Electron Appl. 2021;11:23. doi: 10.3390/jlpea11020023. [DOI] [Google Scholar]
- 5.Tang J, Yuan F, Shen X, Wang Z, Rao M, He Y, et al. Bridging biological and artificial neural networks with emerging neuromorphic devices: Fundamentals, progress, and challenges. Adv Mater. 2019;31:e1902761. doi: 10.1002/adma.201902761. [DOI] [PubMed] [Google Scholar]
- 6.Hebb D. The Organization of Behavior. 1. New York: Wiley; 1949. [Google Scholar]
- 7.Sakurai Y, Osako Y, Tanisumi Y, Ishihara E, Hirokawa J, Manabe H. Multiple approaches to the investigation of cell assembly in memory research-present and future. Front Syst Neurosci. 2018;12:21. doi: 10.3389/fnsys.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau PM, Bi GQ. Synaptic mechanisms of persistent reverberatory activity in neuronal networks. Proc Natl Acad Sci U S A. 2005;102:10333–10338. doi: 10.1073/pnas.0500717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segev R, Baruchi I, Hulata E, Ben-Jacob E. Hidden neuronal correlations in cultured networks. Phys Rev Lett. 2004;92:118102. doi: 10.1103/PhysRevLett.92.118102. [DOI] [PubMed] [Google Scholar]
- 10.Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu QL, Xiao Y, Li S, Zhou D. Emergence of spatially periodic diffusive waves in small-world neuronal networks. Phys Rev E. 2019;100:042401. doi: 10.1103/PhysRevE.100.042401. [DOI] [PubMed] [Google Scholar]
- 12.Volman V, Gerkin RC, Lau PM, Ben-Jacob E, Bi GQ. Calcium and synaptic dynamics underlying reverberatory activity in neuronal networks. Phys Biol. 2007;4:91–103. doi: 10.1088/1478-3975/4/2/003. [DOI] [PubMed] [Google Scholar]
- 13.Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, et al. Synfire chains and cortical songs: Temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- 14.Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature. 2010;468:394–399. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemberger M, Shein-Idelson M, Pammer L, Laurent G. Reliable sequential activation of neural assemblies by single pyramidal cells in a three-layered cortex. Neuron. 2019;104:353–369. doi: 10.1016/j.neuron.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/S0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.