Abstract
Buprenorphine is an important medication for treating opioid use disorder, but medication adherence and treatment retention are key issues that can limit its impact, especially when patients have concurrent stimulant use. Contingency management is efficacious in promoting medication adherence and drug abstinence. Delivering contingency management via smartphones addresses practical barriers to its adoption and improves patient access. A single-group (n = 20) nonexperimental study was conducted to evaluate the feasibility of smartphone-based contingency management to promote adherence to buprenorphine treatment in people with opioid use disorder. Participants were recruited from outpatient treatment clinics. Over 12 weeks participants had access to a smartphone app that provided contingency management supported with peer recovery coaching. Adherence was confirmed daily either by GPS monitoring of clinic medication visits or self-recorded video, and salivary toxicology was conducted weekly. The overall rate of confirmed buprenorphine adherence was 76%, and visual inspection of individual participant outcomes shows consistent medication use for a large majority of participants. All participants were able to successfully use all app features and spend earnings. Participants rated the app and intervention highly on measures of likability, ease of use, and helpfulness. All participants (100%) were retained in buprenorphine treatment throughout the study period. Direct methods for confirming adherence appear superior to confirmation via salivary toxicology. This study shows that smartphone-based contingency management is a feasible means of promoting buprenorphine adherence. The potential efficacy of smartphone-based contingency management as a means of promoting buprenorphine adherence warrants evaluation in a randomized controlled trial.
Keywords: mHealth intervention, Medications for opioid use disorder, Medication-assisted treatment, Substance abuse treatment, Motivational incentives
Effective treatment for opioid use disorder is a critical part of the national response to the opioid crisis. Buprenorphine, the most widely used medication for opioid use disorder (MOUD), is an effective treatment component and means of preventing overdose (Mattick et al., 2014). However, many people drop out of medication assisted treatment (MAT) with buprenorphine over the first several months of enrollment (Hser et al., 2013; Timko et al., 2015). This is especially true of individuals with concurrent stimulant use (Tsui et al., 2021). Further, substance use disorders are chronic and relapse is common (Dennis et al., 2005), partly due to changes in the brain that persist well after drug abstinence is initiated (Koob & Le Moal, 2001). For example, preventing cocaine relapse during treatment has been characterized as more difficult than initiating it (Kampman, 2010). Thus, people with a history of stimulant use are at elevated risk of opioid relapse even if they are not currently using stimulants. In summary, behavioral interventions to promote adherence to and retention in buprenorphine treatment are potentially beneficial for all patients who are prescribed buprenorphine, and people who have a history of stimulant use are a population of special concern.
For individuals with substance use disorders, contingency management (CM) is among the most effective (Dutra et al., 2008; Prendergast et al., 2006) and cost-effective ( Washington State Institute for Public Policy, 2019) psychosocial interventions. In CM, material incentives are provided to patients contingent upon objective verification that they have engaged in one or more target behaviors. Drug abstinence is the most common behavioral target, and is verifiable via drug testing (Davis et al., 2016; Lussier et al., 2006). Incentives (i.e., putative reinforcers) are often monetary, but can conceivably include any goods, services, or privileges (e.g., take-home methadone; Stitzer et al., 1977).
There is also substantial evidence that CM can improve medication adherence (DeFulio & Silverman, 2012). For example, CM has proved highly effective in promoting adherence with and retention on naltrexone pharmacotherapy, whether delivered orally (Dunn et al., 2013; Preston et al., 1999), or in a long-acting injectable formulation (DeFulio et al., 2012; Jarvis et al., 2019). Likewise, providing access to take-home methadone contingent upon weekly submission of drug-negative urine samples and compliance with program requirements significantly enhanced retention in methadone maintenance treatment relative to daily supervised consumption alone or noncontingent take-home methadone (Gerra et al., 2011).
With respect to its role in enhancing buprenorphine treatment, there is some evidence that inclusion of CM in a treatment program improves treatment retention (Maricich et al., 2021). There is also preliminary evidence that CM can improve drug abstinence and attendance to treatment-related appointments in patients who are prescribed buprenorphine (DeFulio, Furgeson, et al., 2021b; DeFulio, Rzeszutek, et al., 2021c). Thus, buprenorphine adherence appears to be a reasonable and important behavioral target.
Despite a history of slow adoption of CM in practice (Petry et al., 2017) there has been substantial recent progress, with signs pointing toward substantial acceleration of the use of CM in practice in the near term (DeFulio, 2022). Much of this progress can be attributed to the development of CM as a remote digital intervention (see Dallery et al., 2019, for a review). Remote digital implementation, including smartphone-based CM, does not appear to reduce its efficacy (DeFulio et al., 2021a; Kurti et al., 2016). In addition, smartphone-based CM has been successful in promoting antiretroviral medication adherence in people living with HIV who have a history of drug use (DeFulio, Devoto, et al., 2021a). For these reasons, smartphone-based CM was used to deliver the CM medication adherence intervention in the present study.
In a study similar to the present study (Holtyn et al., 2021), people diagnosed with opioid use disorder who were not receiving treatment were recruited from needle exchange programs, as well as by peers already participating in the study. Participants were randomly assigned to an incentives group or a referral only control group. Incentive group participants were offered $70 in exchange for entering buprenorphine treatment and $10 for adhering to buprenorphine each day, verified through self-taken smartphone videos. Holtyn et al. found a large and statistically significant increase in buprenorphine treatment entry, but no differences in buprenorphine adherence or opioid use.
In the present study, participants were already enrolled in buprenorphine treatment and recruited directly from the substance abuse treatment clinics that were managing their pharmacotherapy. This is a critical difference, because the motivational operations that establish abstinence as a reinforcer are much more likely to be in place in people who have already enrolled in buprenorphine relative to people who are not currently treatment seeking. In addition, individuals who are out-of-treatment are at risk for experiencing opioid withdrawal when initiating buprenorphine. Holtyn et al. (2021) speculate that this may have been a critical barrier to adherence in their study. Thus, the purpose of the present study was to assess the feasibility, acceptability, and preliminary efficacy of CM as a means of promoting buprenorphine adherence in people diagnosed with opioid use disorder who have a history of stimulant use.
Methods
Participants and Setting
Participants were recruited by direct referral from MAT clinics that prescribe buprenorphine. Clinic staff provided potential participants with a flyer that described the study and encouraged them to call the study team. Those who called were given more information by phone and asked to complete informed consent prior to conducting a brief eligibility survey. To be included in this single-group, nonexperimental study, participants (n = 20) were required to (1) be diagnosed with opioid use disorder; (2) have a history of stimulant use; (3) be enrolled in a buprenorphine treatment program; and (4) own an operational smartphone with active service that used either Android or iOS operating systems. Participants were excluded if they were already participating in another substance abuse treatment study. Most participants were recruited from a BrightView clinic in Cincinnati, Ohio (n = 9), and from Victory Clinical Services in Battle Creek, Michigan (n = 7), and Kalamazoo, Michigan (n = 2). All participants were recruited between May and June of 2021.
Eleven participants (55%) were male and nine (45%) were female. Sixteen participants (80%) were white, two (10%) were multiracial Native American and white, one (5%) was Black and one (5%) was Asian. One participant (5%) was Hispanic. Mean age was 37.2 (SD = 7.6). Fifteen participants (75%) had completed high school or equivalent. For their usual employment pattern over the last three years, 11 participants (55%) reported full-time employment, five (25%) reported part-time employment, and four (20%) reported being unemployed. In the 30 days prior to enrollment, 11 participants (55%) had worked at least 1 day. Among that subset of participants, mean days of employment was 20.3 (SD = 8.1). At intake, two participants (10%) reported using an unprescribed opioid drug in the prior 30 days, two participants (10%) reported using an unprescribed stimulant drug in the prior 30 days, and one participant (5%) reported using unprescribed opioids and stimulants in the prior 30 days. Eight participants (40%) had a diagnosis of stimulant use disorder in their medical record.
Outcome Measures
Adherence and Retention
The primary dependent measure for this study was buprenorphine adherence. Buprenorphine adherence was measured in two ways. The primary method for measuring buprenorphine adherence was by review of remote self-recorded videos. Participants were trained to create a valid video of medication self-administration via instructions embedded in the video submission tool. Staff of the smartphone app vendor were trained to determine whether each video met the following requirements: (1) clear visibility of the participant’s face to confirm identity; (2) clear presentation of the bottle that contained the medication to match the prescription on record; (3) clear presentation of the appropriate sublingual strip or tablet to confirm the dose of the medication; and (4) appropriate consumption and duration for medication absorption with mouth check.
As an alternative, sometimes participants self-administered buprenorphine on-site during clinic visits under direct supervision of clinic staff who were trained in the identical observer procedures. In these cases, adherence at these was confirmed by GPS tracking that indicated the participant was at the clinic for a duration sufficient to take their medicine. Video were avoided in these cases to protect the privacy of other patients. In addition to confirming daily adherence via video or GPS, we assessed whether each participant remained in treatment at the end of the study (Y/N) by direct confirmation with the respective clinic. Note that participants’ clinics did not systematically measure buprenorphine adherence. Thus, preintervention data was not available from the clinics.
Secondary Measures
Surveys were used to collect basic demographic information. The Addiction Severity Index–Lite (McLellan et al., 1997) was used to collect information regarding participants’ drug use at study intake and at 4- and 12-week timepoints. Participant opinions regarding the intervention were collected at study weeks 4 and 12 using customized surveys. These surveys included assessments of ease of use of the app, liking of the app and intervention, and helpfulness of the intervention. The surveys featured a combination of five-point rating-scale questions and open-ended questions. Participants’ use of the app was recorded daily as a dichotomous measure of engagement. In addition, peer recovery coaches tracked participants’ use of and responsiveness to their coaching calls. Drug use was assessed via weekly salivary toxicology tests (OralTox, Premier Biotech, Minneapolis, MN). The 10-panel test assessed use of opioids, buprenorphine, oxycodone, methadone, fentanyl, tramadol, amphetamine, methamphetamine, cocaine, and benzodiazepines.
Procedures
All study procedures, including all informed consent procedures, were approved by the Western Michigan University Institutional Review Board. The consent form specified that participation in the CM program would have no effect on their care at the outpatient clinic. The smartphone-based CM program was delivered via a commercially available app (DynamiCare Health, Inc., Boston, MA). The app and related CM program had been previously evaluated as a means of promoting drug abstinence and clinic attendance in people diagnosed with opioid use disorder (DeFulio, Rzeszutek, et al., 2021c). Study participation lasted for 12 weeks, the duration of the CM program.
Assessment interviews were planned at study intake, week 4, and week 12. Participants were paid $40 for completing each assessment interview. Likewise, salivary drug tests were planned on a weekly basis as a study assessment. These tests were completed by participants using multipanel drug tests mailed to them by the app company, in conjunction with the video feature of the app. It is important to note that there was no drug abstinence contingency involving the salivary drug tests. Instead, participants received $10 for completing the test. For any given participant, toxicology tests were scheduled for the same day each week. The 4-hr testing window on the selected day was determined by the participant. A push notification indicating that a test was due was delivered at the beginning of each testing window, and at hourly intervals for 4 hr or until the test was completed, whichever came first.
All study payments and monetary CM incentives were delivered to participants via an anonymous reloadable gift card. This card could be used at retail outlets that accepted credit cards, but could not be used to obtain cash, and could not be used to make purchases at vendors that sold products inconsistent with the goals of treatment (e.g., liquor stores). Each participant received $20 for completing enrollment in the app and activating their account. Each day that a participant met their requirement for verifying buprenorphine adherence, they earned $3. For each week in which a participant verified their buprenorphine adherence as required on at least 6 days, they earned an additional $15 weekly adherence bonus. Thus, the maximum earnings for app enrollment and CM adherence combined was $452 over 12 weeks, and completion of all study assessments resulted in payments totaling $240. The app also sent push notifications prior to the beginning and ending of each participant’s buprenorphine adherence confirmation window. These 12-hr temporal windows began at a time set by the participant and specified the part of the day in which confirmation of consumption of prescribed buprenorphine resulted in the delivery of incentive payments. Videos were reviewed and all payments dispersed on a daily basis. Once dispersed, funds were available immediately.
In addition to facilitating the delivery of all aspects of the CM program, the app also facilitated SMS and phone access to a live, certified peer recovery coach who was supervised by an appropriately licensed professional (e.g. LMHC). Messages and conversations were designed to provide participants with an opportunity to ask questions and receive support, as well as to encourage intervention engagement and app usage (for details regarding the nature, history, and scope of peer recovery support services, see Gagne et al., 2018, and White, 2010). A specific peer recovery coach was assigned to each participant. Evidence in support of peer recovery coaching as a means of promoting drug abstinence is limited (Bassuk et al., 2016), but it appears to be helpful in supporting engagement in treatment (Byrne et al., 2020). Peer recovery coaching was included in the present study because it is incorporated into the standard services offered as part of a commercially available intervention package. As part of study intake procedures, participants were informed that a peer recovery coach who provides services as part of the app would reach out to them via phone, but that they could opt out of this service if they wished. On a weekly basis, peer recovery coaches attempted to initiate a phone call with any participant who had not opted out, and sent several SMS texts per week. The peer recovery coaches’ goal was to support the participants in recovery and encourage them to abstain from drug use and take their buprenorphine as prescribed.
Beyond the cost of the incentives, additional costs for conducting this intervention are incurred from the drug testing, the payment cards, the app, the peer recovery coaching services, and shipping (e.g., for delivering drug tests to participants’ homes). These products and services entail a monthly cost of $290 in addition to the cost of the incentives. Thus, the total cost for the intervention, including incentives, was approximately $110 per week.
Data Analysis
Analysis in the present study includes descriptive statistics and visual analysis of quantitative data. Most measures are characterized as means with standard deviations or as percentages as appropriate. Because the drug test panel included a test for buprenorphine, it was possible to compare directly observed adherence and the results of the buprenorphine salivary toxicology. To do this, results from weekly toxicology tests were matched to the results of the direct confirmation of adherence (whether by GPS or video) from the prior day. If direct confirmation data from the prior day were missing, then the sample was matched to adherence data from the day before that. If direct confirmation of adherence was not available on either day, then adherence was coded as unconfirmed. Thus, for any given collected toxicology sample, the final characterization could fall into four categories: (1) matched adherence, in which toxicology and directly observed confirmation indicate adherence; (2) observation-only adherence, in which toxicology is negative but video or GPS confirmation indicates adherence; (3) toxicology-only adherence, in which toxicology indicates adherence but no confirmation is available; and (4) matched nonadherence, in which toxicology indicates nonadherence and no confirmation of adherence is available.
Results
Adherence and Retention
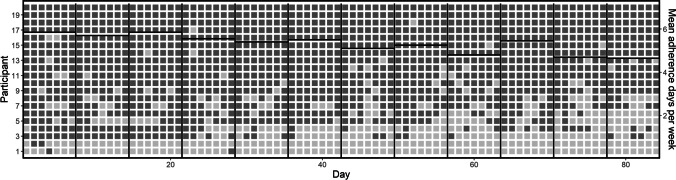
Figure 1 shows individual patterns of buprenorphine adherence for all participants throughout the study, as well as group means over time. Overall, there were 1,680 opportunities to observe buprenorphine adherence in this study sample. Adherence was observed in 1,277 cases (76%). In terms of individual outcomes, 11 participants (55%) adhered on over 90% of opportunities, 2 (10%) fell above 75% but below 90%, and another 3 (15%) fell above 50% but below 75%. Although adherence was generally high throughout the study, a slight decreasing trend in adherence is apparent over time. This trend is visible in Fig. 1, by attending to the mean adherence days per week as shown on the right y-axis. Likewise, during the first half of the study period, adherence was obtained in 81% of cases, compared to 71% in the second half of the study period.
Fig. 1.
Daily Buprenorphine Adherence for All Participants over 12 Weeks. Note. Each row shows outcomes from an individual participant, with each square representing one day of a participant’s expected treatment. Dark shaded squares indicate days in which buprenorphine adherence was directly confirmed via GPS or video. Vertical lines indicate weeks. Horizontal lines indicate mean days of confirmed adherence per participant in each week, across all participants
For GPS opportunities, 252 of 255 samples (98.8%) showed adherence. For video opportunities, 1,025 of 1,425 opportunities (71.9%) showed adherence. Of the 400 non-GPS-validated cases in which adherence was not observed via video, 382 cases (95.5%) were due to no video being submitted. Two participants accounted for 164 of the 382 cases of nonsubmission. Excluding these participants from the analysis increases the percentage of adherence among video submissions to 81.0% and increases the percentage of overall adherence to buprenorphine to 84.0%. Overall, 67.5% of possible weekly adherence bonuses were earned, and total payments for buprenorphine adherence averaged $313 (SD = $144) of a maximum possible of $432. At the start of the study, five participants were newly enrolled to buprenorphine treatment, three had been enrolled for fewer than 90 days, four had been enrolled for less than a year, and eight had been enrolled for longer than a year. All (100%) participants were retained in buprenorphine treatment throughout the study.
Figure 2 shows the relationship between buprenorphine adherence and two potential confounding variables—amount of peer recovery coaching contact (expressed as number of interactions over the course of the study), and duration in buprenorphine treatment prior to enrollment in the CM program (expressed in terms of the categories described in the participants section of the methods). Based on visual inspection, there appears to be a potential relationship between accessing peer recovery coaching and buprenorphine adherence. In particular, among people who accessed peer recovery coaching more than five times, 8 of 9 (89%) were verified as adherent on at least 75% of possible occasions, compared to 5 of 11 (45%) of people who accessed peer recovery coaching five or fewer times. In contrast, time in buprenorphine treatment appears less related to adherence. Five of 8 (63%) people with less than or equal to 3 months in treatment prior to enrolling in the CM program were verified as adherent on at least 75% of possible occasions, compared to 8 of 12 (67%) who were in treatment over 3 months prior to enrolling in the CM program.
Fig. 2.
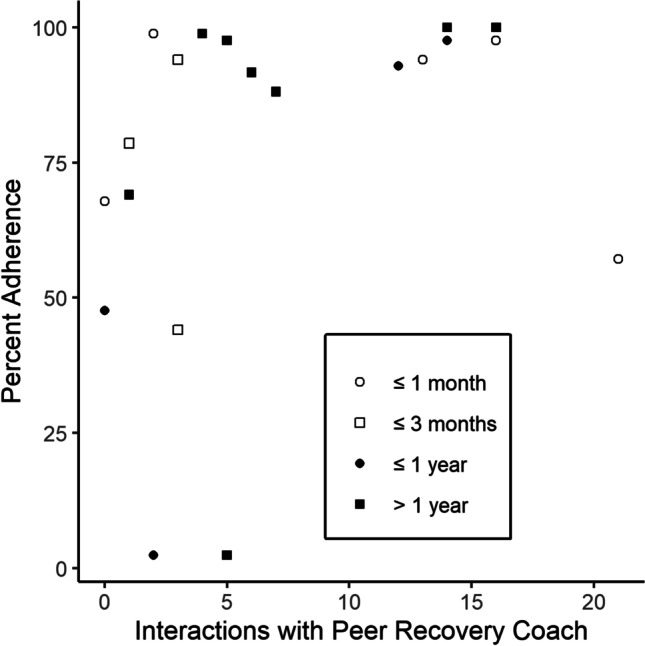
Overall Individual Percentage of Buprenorphine Adherence as a Function of the Number of Interactions with Peer Recovery Coaches. Note. Symbols indicate the number of days of buprenorphine treatment prior to the initiation of the contingency management program, across four categories
Usability, Acceptability, and Engagement
All participants were able to successfully submit videos and spend CM incentive earnings. Of the 40 planned post-intake assessments, 36 (90%) were completed. Table 1 shows participant ratings of usability, acceptability, and helpfulness at the 4- and 12-week timepoints. Overall, ratings on these measures were high at both timepoints. Mean days of app use during the 12-week intervention period was 60.8 days (SD 27.3), which is 72.4% of possible days. There were 191 instances of participant app use on a day that the participant did not submit a video. This suggests that individuals who did not need to interact with the app to confirm adherence (because it was confirmed via GPS) regularly used the app for other reasons.
Table 1.
Usability, acceptability, and helpfulness ratings (1–5 Scale) from assessments collected in study weeks 4 and 12
| Question | Week 4 Group Mean (SD) |
Week 12 Group Mean (SD) |
|---|---|---|
| Setting up a DynamiCare account was easy to do. | 4.78 (0.43) | 4.89 (0.32) |
| Navigating the application was simple. | 4.61 (0.98) | 4.78 (0.43) |
| Questions I had about how to use the app were answered promptly. | 4.56 (0.78) | 4.44 (0.78) |
| The DynamiCare application made the intervention easier. | 4.50 (0.51) | 4.28 (0.83) |
| I liked the intervention. | 4.56 (0.70) | 4.56 (0.51) |
| This intervention has helped me stay clean. | 4.56 (0.70) | 4.33 (0.69) |
| Overall, the intervention was helpful to me. | 4.67 (0.59) | 4.44 (0.62) |
| I would recommend this intervention to someone who is trying to get clean. | 4.78 (0.55) | 4.72 (0.46) |
| The intervention helped me attend treatment. | 4.39 (0.85) | 4.28 (0.46) |
Two participants never answered or initiated peer recovery coach calls and four more opted out of peer recovery coaching at some point during the study. The remaining 14 participants’ mean number of total interactions with peer recovery coaches (including SMS text message initiations and replies) was 9.3 (SD = 6.5) over the 12-week period.
Salivary Toxicology
Of the planned 240 salivary toxicology tests, 174 (72.5%) were completed. Of these, two were positive for cocaine and two were positive for amphetamine/methamphetamine. The remaining 170 (97.7%) were not positive for any tested drugs, with the possible exception of buprenorphine. Among matched comparisons, there were 90 instances (51.7%) of matched adherence, 74 instances (42.5%) cases of observation-only adherence, 5 instances (2.9%) of toxicology-only adherence, and 5 instances (2.9%) of matched nonadherence.
Discussion
This study indicates that smartphone-based CM is feasible in buprenorphine treatment and can serve as a means of promoting adherence and retention. The high participant ratings for ease of use and liking the intervention are indicators of intervention acceptability and usability. Usability is further supported by the successful use of all app functions and the reloadable gift cards by all 20 participants, and by high levels of app engagement for most participants throughout the study. It is also promising that all 20 participants remained in buprenorphine treatment throughout the study period. These results are consistent with prior studies of CM that indicate its efficacy as a means of promoting medication adherence (DeFulio & Silverman, 2012; DeFulio, Devoto, et al., 2021a). They are also consistent with the growing literature in support of remote delivery of CM more broadly (Dallery et al., 2019; Kurti et al., 2016). We also follow Dallery et al. (2019) in noting that although some patients who would benefit from CM cannot access remotely delivered CM because they do not own a smartphone, there are high levels of smartphone ownership across all economic strata and remote delivery can be viewed broadly as increasing access to CM services. For example, the ability for CM to reach underserved areas and reduce travel burden is evident in the high proportion of video confirmations of buprenorphine adherence in the present study.
The use of contingent monetary incentives appears to be critical to the success of remote intervention for buprenorphine adherence. Even when including participants who failed to engage in the present intervention, an overall adherence rate of 76% was obtained. This result appears in sharp contrast to the results of a study by Tsui et al. (2021). In that study, buprenorphine patients were randomly assigned to receive treatment as usual or treatment as usual plus a video-based directly observed therapy (DOT) intervention. DOT is like CM in that both involve direct observation of a relevant target behavior. However, DOT does not include incentives and has produced only limited success in promoting medication adherence to treat tuberculosis (Karumbi & Garner, 2015) and HIV (Hart et al., 2010). Tsui et al. (2021) showed that buprenorphine participants who received video-based DOT submitted 31% of possible videos. Thus, the combination of direct observation and monetary incentives appears to be a stronger approach that offers greater potential impact than direct observation alone.
It is possible that the actual rate of adherence is substantially higher than the rate of video confirmed adherence in the study by Tsui et al. (2021) as well as in the present study. For example, one participant who largely failed to engage in the CM buprenorphine adherence intervention in the present study nevertheless did submit three toxicology videos, all of which were positive for buprenorphine. However, most people who failed to submit adherence videos also failed to submit toxicology videos. Thus, the strongest evidence that adherence was likely higher than measured by direct observation in the present study comes from the especially high adherence rate when GPS was used as the measure of adherence (98.8%). This is important because unlike video confirmation of adherence, GPS confirmation requires no participant effort. Another key indicator that adherence was likely higher than observed by video in the present study is the retention of 100% of participants in buprenorphine treatment throughout the study period.
Based on the results of the toxicology-direct observation matching analysis, direct observation (whether by video or GPS) appears to be the superior method for measuring buprenorphine adherence in the context of remotely delivered CM. In the present study, there were many instances in which direct observation indicated adherence but salivary samples were assessed as buprenorphine negative. There could be several reasons for these discrepant results, including insufficient sensitivity or time window of the drug test, or improper use of the test by the participants. However, the salivary toxicology testing procedure was also directly observed, and delivery of the incentives was contingent on following appropriate testing procedures. Further, the tests include a control band to ensure a proper sample was collected. A confirmatory review of these videos was conducted after the matching analysis, and no inconsistencies or problems were found. To properly determine the factors relevant to the discrepancies between toxicology results and direct observation, a future study would need to incorporate a confirmatory testing method such as liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) in the study design, and also compare in-person and remote verification of medication consumption. Until buprenorphine salivary toxicology tests can be demonstrated to be highly accurate in practice, remote CM interventions should rely on direct observation of adherence as in the present study.
Limitations
The most critical limitations of the present study are its nonexperimental nature and small sample. Another equally critical limitation is that no baseline data were collected to measure buprenorphine adherence prior to the onset of the CM intervention. For these reasons, the present results should be interpreted cautiously and no causal inferences should be made. Another limitation is that 40% of the sample were enrolled in buprenorphine for over a year, and thus were not at high risk for treatment drop-out. Likewise, although all participants had a history of stimulant use, only three reported recent use at study intake, and only three participants submitted stimulant-positive saliva samples during the study (all different from those reporting recent stimulant use during the intake assessment). Thus, the overall level of stimulant use was low in the study sample. To maximize the impact of the intervention, it may be preferable to restrict the study sample to participants who recently enrolled in buprenorphine treatment and who have recent or ongoing stimulant use in future studies. Finally, another important limitation of this study is that interaction with peer recovery coaches was an uncontrolled variable that could confound the results. Indeed, there is preliminary evidence of a correlation between the amount of interaction with peer recovery coaches and buprenorphine adherence. Whether this is causal (e.g., peer recovery coaching promotes engagement and therefore facilitates adherence) or the result of a third variable (e.g. individuals who interact with peer recovery coaches are also more likely to adhere to buprenorphine for another reason) is unclear. A future study in which peer recovery coaching “dose” is controlled as an independent variable is necessary to properly evaluate the role and potential benefit of incorporating peer recovery coaching into digital CM interventions.
Conclusion
Buprenorphine treatment is a critical part of the response to the opioid epidemic. Adherence to and retention in buprenorphine treatment is an essential part of its effectiveness in practice. Technology can facilitate the delivery of high-fidelity behavioral interventions on a large scale, thereby easing patients’ access to the intervention. Smartphone-based CM is a feasible intervention for promoting adherence to buprenorphine. The overall high level of adherence observed in this study suggests that this intervention could promote adherence and retention in buprenorphine patients with a history of stimulant use.
Acknowledgments
We thank Zoey Golston and Sarah Morrissey for their assistance with data management and processing.
Author’s Contributions
Anthony DeFulio: Conceptualization, methodology, formal analysis, writing (original draft, review, and editing), visualization, supervision, project administration, and funding acquisition.
Hayley Brown: Formal analysis, investigation, data curation, writing (review and editing), visualization.
Rosemarie Davidson: Formal analysis, investigation, data curation, writing (review and editing).
Sean Regnier: Conceptualization, methodology, investigation, writing (review and editing).
Navdeep Kang: Methodology, resources, writing (review and editing).
Melissa Ehart: Methodology, resources, writing (review and editing).
Funding
This study was funded by the National Institute on Drug Abuse (R41DA049390, PI – DeFulio). The funding source had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Declarations
Conflicts of Interest
The first author has served as a research consultant for DynamiCare Health, Inc. The remaining authors declare no conflicts.
Ethical Approval
This study was approved by the Western Michigan University Human Subjects Institutional Review Board. All participants provided informed consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bassuk EL, Hanson J, Greene RN, Richard M, Laudet A. Peer-delivered recovery support services for addictions in the United States: A systematic review. Journal of Substance Abuse Treatment. 2016;63:1–9. doi: 10.1016/j.jsat.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Byrne KA, Roth PJ, Merchant K, Baginski B, Robinson K, Dumas K, Collie J, Ramsey B, Cull J, Cooper L, Churitch M, Rennert L, Heo M, Jones R. Inpatient link to peer recovery coaching: Results from a pilot randomized control trial. Drug & Alcohol Dependence. 2020;215:108234. doi: 10.1016/j.drugalcdep.2020.108234. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, Marsch LA. Technology-based contingency management in the treatment of substance-use disorders. Perspectives on Behavior Science. 2019;42(3):445–464. doi: 10.1007/s40614-019-00214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Preventive Medicine. 2016;92:36–46. doi: 10.1016/j.ypmed.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio, A. (2022). Dissemination of contingency management for the treatment of opioid use disorder. Perspectives on Behavior Science. Advance online publication. 10.1007/s40614-022-00328-z [DOI] [PMC free article] [PubMed]
- DeFulio A, Silverman K. The use of incentives to reinforce medication adherence. [Special issue] Preventive Medicine. 2012;55:S86–S94. doi: 10.1016/j.ypmed.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Everly JJ, Leoutsakos JMS, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: A randomized controlled trial. Drug & Alcohol Dependence. 2012;120(1–3):48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Devoto A, Traxler H, Cosottile D, Fingerhood M, Nuzzo P, Dallery J. Smartphone-based incentives for promoting adherence to antiretroviral therapy: A randomized controlled trial. Preventive Medicine Reports. 2021;21:101318. doi: 10.1016/j.pmedr.2021.101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio, A., Furgeson, J., Brown, H. D., & Ryan, S. (2021b). A smartphone–smartcard platform for implementing contingency management in buprenorphine maintenance patients with concurrent stimulant use disorder. Frontiers in Psychiatry, 12, Article 778992. 10.3389/fpsyt.2021.778992 [DOI] [PMC free article] [PubMed]
- DeFulio A, Rzeszutek MJ, Furgeson J, Ryan S, Rezania S. A smartphone-smartcard platform for contingency management in an inner-city substance use disorder outpatient program. Journal of Substance Abuse Treatment. 2021;120:108188. doi: 10.1016/j.jsat.2020.108188. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Scott CK, Funk R, Foss MA. The duration and correlates of addiction and treatment careers. Journal of Substance Abuse Treatment. 2005;28(2):S51–S62. doi: 10.1016/j.jsat.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Defulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, Leoutsakos JMS, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Experimental and Clinical Psychopharmacology. 2013;21(1):74–83. doi: 10.1037/a0030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008;165(2):179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Gagne CA, Finch WL, Myrick KJ, Davis LM. Peer workers in the behavioral and integrated health workforce: Opportunities and future directions. American Journal of Preventive Medicine. 2018;54(6):S258–S266. doi: 10.1016/j.amepre.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Gerra G, Saenz E, Busse A, Maremmani I, Ciccocioppo R, Zaimovic A, Gerra M, Amore M, Manfredini M, Donnini C, Somaini L. Supervised daily consumption, contingent take-home incentive and non-contingent take-home in methadone maintenance. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35(2):483–489. doi: 10.1016/j.pnpbp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hart JE, Jeon CY, Ivers LC, Behforouz HL, Caldas A, Drobac PC, Shin SS. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: A meta-analysis and systematic review. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;54(2):167–179. doi: 10.1097/qai.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Toegel F, Novak MD, Leoutsakos JM, Fingerhood M, Silverman K. Remotely delivered incentives to promote buprenorphine treatment engagement in out-of-treatment adults with opioid use disorder. Drug & Alcohol Dependence. 2021;225:108786. doi: 10.1016/j.drugalcdep.2021.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2013;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis BP, Holtyn AF, DeFulio A, Koffarnus MN, Leoutsakos JMS, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. The effects of extended-release injectable naltrexone and incentives for opiate abstinence in heroin-dependent adults in a model therapeutic workplace: A randomized trial. Drug & Alcohol Dependence. 2019;197:220–227. doi: 10.1016/j.drugalcdep.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM. What’s new in the treatment of cocaine addiction? Current Psychiatry Reports. 2010;12(5):441–447. doi: 10.1007/s11920-010-0143-5. [DOI] [PubMed] [Google Scholar]
- Karumbi, J., & Garner, P. (2015). Directly observed therapy for treating tuberculosis. Cochrane Database of Systematic Reviews, 2015(5), Article CD003343. 10.1002/14651858.cd003343.pub4 [DOI] [PMC free article] [PubMed]
- Koob, G., and Le Moal, M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. 10.1016/s0893-133x(00)00195-0 [DOI] [PubMed]
- Kurti AN, Davis D, Redner R, Jarvis B, Zvorsky I, Keith DR, Bolivar H, White TJ, Rippberger P, Markeish C, Atwood G, Higgins ST. A review of the literature on remote monitoring technology in incentive-based interventions for health-related behavior change. Translational Issues in Psychological Science. 2016;2(2):128–152. doi: 10.1037/tps0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Maricich, Y. A., Bickel, W. K., Marsch, L. A., Gatchalian, K., Botbyl, J., & Luderer, H. F. (2021). Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder. Current Medical Research and Opinion, 37(2), 167–173. 10.1080/03007995.2020.1846022 [DOI] [PMC free article] [PubMed]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JS, Zanis D. The Addiction Severity Index–“Lite” (ASI–“Lite”) Center for the Studies of Addiction, University of Pennsylvania/Philadelphia VA Medical Center; 1997. [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, Zajac K. Contingency management treatment for substance use disorders: How far has it come, and where does it need to go? Psychology of Addictive Behaviors. 2017;31(8):897–906. doi: 10.1037/adb0000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug & Alcohol Dependence. 1999;54(2):127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Stitzer M, Bigelow G, Lawrence C, Cohen J, D’Lugoff B, Hawthorne J. Medication take-home as a reinforcer in a methadone maintenance program. Addictive Behaviors. 1977;2(1):9–14. doi: 10.1016/0306-4603(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. Journal of Addictive Diseases. 2015;35(1):22–35. doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Leroux BG, Radick AC, Schramm ZA, Blalock K, Labelle C, Heerema M, Klein JW, Merrill JO, Saxon AJ, Samet JH, Kim TW. Video directly observed therapy for patients receiving office-based buprenorphine: A pilot randomized controlled trial. Drug & Alcohol Dependence. 2021;227:108917. doi: 10.1016/j.drugalcdep.2021.108917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington State Institute for Public Policy. (2019, December). Benefit-cost results: Substance use disorders. Retrieved March 3, 2022 from http://www.wsipp.wa.gov/BenefitCost?topicId=7
- White WL. Nonclinical addiction recovery support services: History, rationale, models, potentials, and pitfalls. Alcoholism Treatment Quarterly. 2010;28(3):256–272. doi: 10.1080/07347324.2010.488527. [DOI] [PMC free article] [PubMed] [Google Scholar]