Abstract
Kombucha is a fermented tea with a combination of yeast and bacteria. Kombucha teas may have a variable microbiota based on geographic origin and cultural conditions. The microbial flora of kombucha has been studied with culture-dependent methods. But, the improvement of the metataxonomic approach has broadened our perspective on fermented foods. In this study, a kombucha mother was procured from an artisanal supplier in Türkiye. High-throughput new-generation sequencing (16S rRNA and Internal Transcribed Spacer (ITS)) was carried out to investigate the microbial communities of kombucha after 7 days of fermentation in both liquid tea (L) and pellicle (P). Microbial counts, pH (4.42 ± 0.01 and 3.50 ± 0.02), and TA% (0.26 ± 0.02 and 0.60 ± 0.04) were also detected on the first and 7th days of fermentation. According to metataxonomic results, the dominant bacteria were Komagataeibacter obediens (%21.13), an acetic acid-producing bacteria, and the dominant fungal genus was Pichia kudriavzevii (64.35%) in L while Romboutsia sp. CE17 was the dominant bacteria (7%) and Pichia kudriavzevii was also the dominant yeast in P. This study also revealed different species which were not common in kombucha including propionic acid and butyric acid-producing bacteria such as Anaerotignum propionicum and Butyrivibrio fibrisolvens, a butyrivibriocin producing bacteria. Accordingly, different yeast species were detected such as Tetrapisispora phaffii and Ogataea polimorpha.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05725-z.
Keywords: Metataxonomics, Kombucha, Pichia, Komagataeibacter
Introduction
Kombucha or kombucha tea is a traditional, sugared beverage consumed since ancient times and produced through fermentation of black tea by a complex microbial consortium consisting of yeasts, lactic acid bacteria (not always), and acetic acid bacteria (AAB) (Villarreal-Soto et al. 2018; Arıkan et al. 2020; Tran et al. 2020). The bacterial consortium of kombucha includes generally Komagataeibacter xylinum, Acetobacter xylinoides, Gluconobacter oxydans, Gluconacetobacter hansenii, Oenococcus oeni, Komagataeibacter europaeus, Lactobacillus sp., and yeast ecology consists of Brettanomyces/Dekkera, Candida, Kloeckera, Pichia, Saccharomyces, Saccharomycoides, Shizosaccharomyces, Torulospora, and Zygosaccharomyces (Teoh et al. 2004; Villarreal-Soto et al. 2018). Mainly, black tea (Camellia sinensis) is used for the fermentation of kombucha but alternatively, other tea types such as green and oolong tea have been used (Leal et al. 2018). Since Black tea infusion contains proteins, amino acids, volatile compounds, lipids, enzymes, and polyphenols, it is a good fermentation medium (Kumar and Joshi 2016). Kombucha tea originated in China but later spread to the rest of the world and recently has been very popular (Cvetković et al. 2019). This consortium leads to the form of a pellicle called different names such as “mother”, “tea fungus”, or Symbiotic Culture of Yeast and Bacteria (“SCOBY”) (Tran et al. 2020). The tea is inoculated with this cellulose pellicle to produce kombucha tea. During the fermentation process, periplasmic yeasts convert sucrose into glucose and fructose in a period from 7 to 10 days in which converted sugars are used to form a pellicle. Tea and sugar are converted to alcohol, organic acids, and CO2 thereby, pH drops around 2.5–3.5 (Leal et al. 2018). Additionally, vitamins C, B1, B2, B3, B6, B12, and folic acid are produced, thus, kombucha tea exhibits health-promoting characteristics through anti-microbial, antioxidant, anticarcinogenic, and antidiabetic effects (Cvetković et al. 2019). In addition, kombucha tea improves immune function and reduces cholesterol levels (Leal et al. 2018). In a study, apoptotic cell death of the hepatocytes was prevented with kombucha tea treatment (Bhattacharya et al. 2011).
Analysis of environmental samples with metataxonomic approaches has recently been a powerful tool to reveal both the non-culturable and culturable microbiota of samples (Kalamaki and Angelidis 2020). Indeed, metataxonomic analysis of fermentative foods has been a promising approach to understanding the microbial dynamics of this complex flora (Kalamaki and Angelidis 2020). Therefore, some fermentative foods such as cheese, kombucha, boza, and kimchi have been analyzed with a high-throughput metataxonomic approach in many studies (Castellanos-Rozo et al. 2020; Kamilari et al. 2020). Therefore, in this study, the microbial flora of kombucha tea and pellicle was determined with high-throughput sequencing. In addition, microbial enumeration, pH, and titratable acidity of kombucha were determined.
Materials and methods
Preparation of kombucha
A solid-phase SCOBY Kombucha cellulose pellicle (mat) sample was obtained from a local supplier in Ankara, Türkiye. Production of tea was slightly modified according to previous studies (Chen and Liu 2000; Cvetković et al. 2019). 2 spoons of black tea (“Rize”, Türkiye) were added and boiled in 1L of water and then allowed to infuse for 10 min. After removal of the tea, 250 ml of liquid tea was added to 1L of boiled and cooled sterile water. % 10 (w/v) of sucrose was added to the cooled black tea. Later on, a freshly grown Kombucha mat was used to inoculate the liquid tea medium. Kombucha tea was covered with a clean cloth and fixed with a rubber band. Kombucha was fermented at room temperature (at 25 ± 3ºC) and out of light for 7 days. A new Kombucha mat (daughter mat) was developed on the top of the tea surface during fermentation. 10 g of the pellicle (named P) and 10 ml of liquid (named L) samples were kept at + 4 °C for metataxonomic DNA extraction (Marsh et al. 2014; Jung et al. 2019).
Microbial enumeration of liquid and pellicle
The microbial population of liquid kombucha (L) and pellicle (P) was enumerated on the first and 7th days of fermentation. MRS agar (Merck, Germany) for lactobacilli and Dichloran Rose Bengal Chloramphenicol agar (Merck, Germany) for yeast/mold was used. 1 ml of the liquid tea sample was added to 9 ml of buffered peptone water and serially diluted between 10–2 and 10–7. 10 g of pellicles were measured and homogenized with 90 ml of buffered peptone water (Merck, Germany). After serially diluting until 10–7, 0.1 ml of each dilution was spread on plates. DRBCA plates were incubated at 25 °C for up to 7 days while MRS plates were incubated at 37 °C for 48 °C in an anaerobic jar. Analyses were repeated three times.
pH and TA % properties of kombucha properties of Kombucha
pH and titratable acidity of kombucha tea was measured on the first and 7th days of fermentation. pH was determined with a digital pH meter (WTW Inolab 720). For titratable acidity, A 10 ml aliquot of tea sample without CO2 was transferred to a beaker, 3 drops of phenolphthalein (0.1%) were added and then titrated with 0.1N NaOH (AOAC 2016). The value was calculated according to the volume consumed in milliliters of 0.1 mol/l NaOH per 100-ml sample and expressed as acetic acid % (Chen and Liu 2000). Analyses were repeated three times.
Total genomic DNA isolation from kombucha pellet and liquid tea
Zymo Research “Quick-DNA TM Fecal/Soil Microbe Miniprep, Cat. No.: D6010” genomic DNA isolation kit was used to isolate total DNA. Purity and quantitation of DNA were detected with Qubit 2.0 fluorometer DNA protocol (Thermo, USA). Reagent and buffer were mixed per sample (1:199) and a working solution was prepared. 10 µl of sample DNA was mixed with 190 µl of working solution. DNA purity and quantitation were read with a Qubit fluorometer.
PCR conditions of 16 s V3-V4 and 28 s rRNA D1/D2 regions
Universal primers 341F (5’-CCTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’) were used to amplify 16 s rRNA V3-V4 region while universal primers F63-F (5’ GCATATCAATAAGCGGAGGAAAAG-3’) and LR3-R (5’-GGTCCGTGTTTCAAGACGG-3’) were used to amplify 28 s rRNA D1/D2 region with SimpliAmp Thermal Cycler. PCR conditions were as follows: 95 °C for 10 min–initial denaturation—35 cycles: 95 °C for 45 s- denaturation, 50–55 °C for 45 s – annealing, 72 °C for 60 s–extension, and 72 °C for 3 min–final extension. Amplification results were determined on a 2% agarose gel (Ilıkkan and Bağdat 2021).
Preparing library and sequencing
PCR products were purified with AMPure XP beads (Beckman Coulter). The library was prepared with “Illumina, Nextera XT DNA Library Prep Kit, Cat. No.: FC-131–1096” for amplicon products. Indexing was carried out with “TG Nextera XT Index Kit v2 Set A (96 Indices, 384 Samples), Cat. No.: TG-131–2001”. Illumina, Miseq platform was utilized for sequencing with paired-end (PE) (2 × 150 bp paired-end run).
Bioinformatic analysis
The accuracy of microbial diversity was improved by quality check; therefore, raw sequence data (FastQ) were subjected to QC check. Sequencing artifacts, contaminating readings, and low-quality reads were trimmed using FastQC v0.10.1 (Ilıkkan and Bağdat 2021). Sequence data were clustered OTU classes with the Kraken Metagenomic system (Wood and Salzberg 2014). Krona charts and PCoA analysis were performed by the OmicsBox tool. The heatmaps were constructed with R studio, pheatmap package (Ilıkkan and Bağdat 2021). All the sample raw reads have been deposited at NCBI under the BioProject ID PRJNA742890.
The Shannon and Simpson diversity index
Shannon diversity indices were calculated at the species level for a-diversity. Shannon's Equitability is a value between 1.5 and 3.5. The higher this value is, the evenness is higher. The abundance and uniformity of OTUs were used to calculate Simpson indexes (Thukral 2017). Simpson's Index of Diversity (1-D) is a value between 0 and 1. 1 corresponds to complete evenness (Kalamaki and Angelidis 2020; Ilıkkan and Bağdat 2021).
Results and discussion
pH and TA % properties of kombucha
The pH of Kombucha tea was 4.42 ± 0.01 on the first day of fermentation and 3.50 ± 0.02 on the 7th day of fermentation. These results were consistent with other studies which find pH around 2.5–3.5 (Neffe-Skocińska et al. 2017; Leal et al. 2018; Yıkmış and Tuğgüm 2019). Generally, acetic acid bacteria dominate Kombucha (De Filippis et al. 2018). Therefore, the TA % value was determined as acetic acid value (Cvetković et al. 2019). TA% was 0.26 ± 0.02 on the first day of fermentation and 0.60 ± 0.04 on the 7th day of fermentation. However, the kombucha tea from this study had low acetic acid than other research after 7 days of fermentation while it was similar on the first day (Cvetković et al. 2019).
Microbial enumerations
Sample L had < 1 log CFU/ml lactobacilli on the first day while 3.30 log CFU/ml on the 7th day. P had 2.26 log CFU/g lactobacilli on the first day while 3.38 log CFU/g on the 7th day. In addition, sample L had 4.24 CFU/ml yeast/mold on the first day while 5.32 log CFU/ml yeast/mold on the 7th day. P had 4.25 CFU/g yeast/mold on the first day while 5.70 log CFU/g yeast/mold on the 7th day (Table 1). The general results showed that microbial counts in pellicle were slightly higher than in liquid beverage. In another study, LAB were not detected on the first day of fermentation in beverage, and yeast counts were close to the current study (Coton et al. 2017; Neffe-Skocińska et al. 2017). However, in another study, yeast counts were higher than current study (Cvetković et al. 2019).
Table 1.
Microbial enumeration of kombucha tea on the first and 7th days of fermentation
| Sample | Microorganism | Enumeration result (log CFU/ml-g) | |
|---|---|---|---|
| 1st day | 7th day | ||
| L | Lactobacilli | < 1 | 3.30 ± 0.00 |
| L | Yeast/Mold | 4.24 ± 0.05 | 5.32 ± 0.00 |
| P | Lactobacilli | 2.26 ± 0.00 | 3.38 ± 0.00 |
| P | Yeast/Mold | 4.25 ± 0.05 | 5.70 ± 0.00 |
L: Liquid P: Pellicle
Metataxonomic analysis of kombucha tea (L) and pellicle (P)
Bacterial diversity
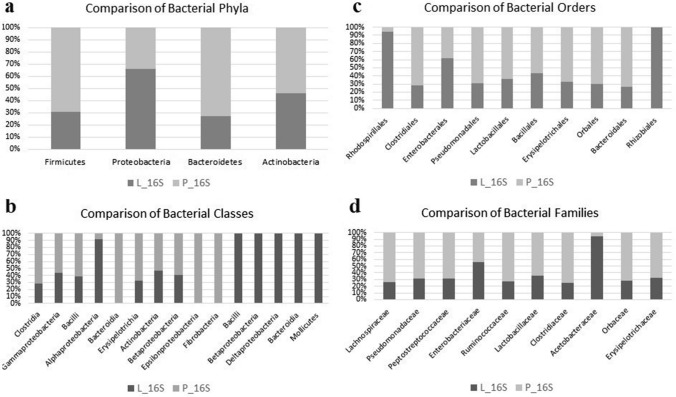
According to sequencing statistics, L_16S generated 28,153 reads/sample while P_16S generated 10,230 reads/sample. All classified reads were 100%. Relative abundances of bacterial OTUs were presented in Fig. 1, namely, four different phyla (Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria) (Fig. 1a), 15 classes (Fig. 1b), 10 orders (Fig. 1c), and 10 families (Fig. 1d). Proteobacteria was dominant (70%) in liquid phase samples while Firmicutes was dominant in pellicle (> 58%). At a family level, Acetobacteraceae was dominant (51%) in the liquid phase while Lachnospiraceae was dominant in pellicle (28%), a probiotic family group that produces short-chain fatty acids (Wu 2020). Members of this family have been known to produce butyric acid and thereby protect against colon cancer due to the characteristic of preventing the growth of some bacteria (Meehan and Beiko 2014). Lachnospiraceae has also been found in kombucha previously and in this study (Lavefve et al. 2021).
Fig.1.
Comparison of bacterial OTUs. Diversity of phyla (a), classes (b), orders (c), and Families (d) in Kombucha tea broth (L) and pellicle (P)
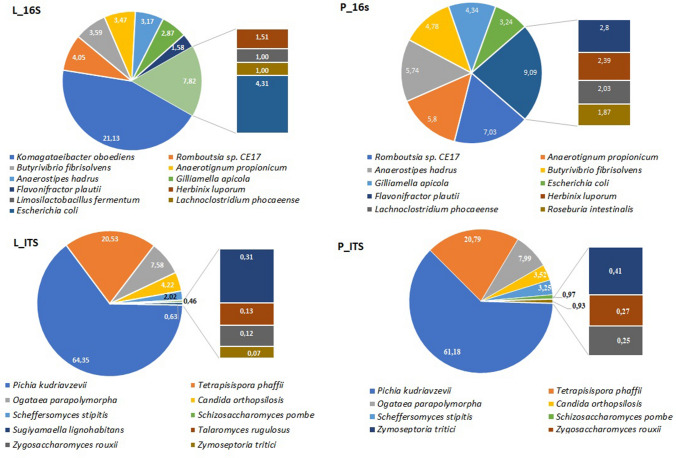
Komagataeibacter oboediens (syn, Acetobacter oboediens) was the dominant bacterium in L and this bacterium has not been seen in P (Fig. 3a and b). Komagataeibacter sp. is acetic acid and cellulose-producing bacterium in Acetobacteraceae. This genus has been found in other metataxonomic studies conducted with kombucha (Marsh et al. 2014; Arıkan et al. 2020; Harrison and Curtin 2021). Mostly found species of this genus in kombucha are K. xylinus, K. rhaeticus, K. saccharivorans, K. intermedius, and K. kombuchae (Harrison and Curtin 2021). Komagataeibacter oboediens has not been reported in kombucha before.
Fig.3.
Diversity piecharts of bacterial (a and b) and fungal (c and d) species found in Kombucha tea broth (L) and pellicle (P)
Butyrivibrio fibrisolvens was the third most abundant bacterium in L (3,59%) and fourth in P (4,78%). Butyrivibrio fibrisolvens is a butyrate and butyrivibriocin, a bacteriocin-producing ruminal bacterium (Kalmokoff et al. 2003). The probiotic potential has been assessed in a study and it has been shown to prevent colorectal cancer (Ohkawara et al. 2009). Anaerotignum propionicum (syn, Clostridium propionicum) was the fourth most abundant bacterium in L (3,47%) and second in P (5,8%). These latter two bacteria have not been found in kombucha in other studies. Sample P had a uniform distribution; however, Romboutsia sp. CE17 had a relative abundance of 7.03%. Other bacteria had a rate below 2% including Flavonifractor plautii, Herbinix luporum, Limosilactobacillus fermentum, and Lachnoclostridium phocaeense.
Krona charts representing the bacterial taxonomic composition of L and P were presented in Figure S1 and Figure S2, respectively. The heatmap of bacterial diversity was represented in Figure S3. Concerning bacterial diversity indexes, the Shannon index was 4.405 for L while it was 4.683 for P. Likewise, Simpson indexes (1-D) were 0.9441 for L and 0.9775 for P. According to diversity index results, the richness and evenness of P were higher than L as expected.
Fungal diversity
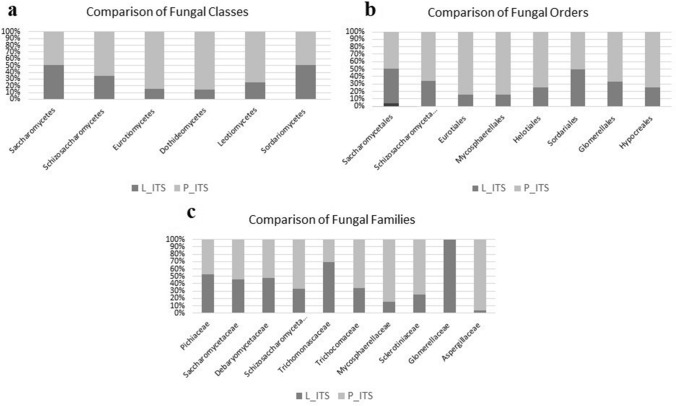
According to sequencing statistics, L_ITS generated 74,904 reads/sample while P_ITS generated 28,652 reads/sample. All classified reads were 100%. Relative abundances of fungal OTUs were presented in Fig. 2. One phylum, namely, Ascomycota was the dominant phylum in both samples (100%). 6 classes (Fig. 2a), 8 orders (Fig. 2b), and 10 families (Fig. 2c) were detected in samples. Pichiaceae was dominant in both samples (71.73% for L and 65.6% for P). Therefore, Pichia kudriavzevii (syn, Issatchenkia orientalis, Candida krusei) was also the dominant yeast in both L and P (64.35% and 61.18%, respectively) (Fig. 3c and d). The genus Pichia has been found in kombucha in previous studies (May et al. 2019). On the contrary, Pichia kudriavzevii has not been found in kombucha before. This yeast has been found to have probiotic potential (Greppi et al. 2017; Tolulope and Julius 2020). In addition, it is responsible for film formation in over-ripened Kimchi (Moon et al. 2014). The second dominant yeast was Tetrapisispora phaffii which produces a killer toxin preventing wine spoilage (Mehlomakulu et al. 2014). The third dominant yeast Ogataea polymorpha (syn, Pichia angusta), is methylotrophic and converts glucose to ethanol. This yeast has been used as a biocontrol yeast against postharvest decay of apple fruit (Fiori et al. 2008).
Fig.2.
Comparison of fungal OTUs. Diversity of classes (a), orders (b), and families (c) in Kombucha tea broth (L) and pellicle (P). All samples were 100% in Ascomycota phylum
Krona charts representing the fungal taxonomic composition of L and P were presented in Figure S4 and Figure S5, respectively. The heatmap of bacterial diversity was represented in Figure S6. Concerning fungal diversity indexes, the Shannon index was 1.099 for L while it was 1.251 for P. Likewise, Simpson indexes (1-D) were 0.5358 for L and 0.5737 for P. According to diversity index results, the richness and evenness of P were higher than L.
Principle coordinate analysis showed that the bacterial diversity of the L and P samples was very close as they overlapped. However, the fungal diversity of samples was different as they were placed on different sides of the plot (Figure S7).
Conclusion
Kombucha teas may have a variable microbiota based on geographic, climatic, and cultural conditions. To date, kombucha tea microbiota has been studied from different geographic origins and just one sample has been studied from Adana province, Türkiye with a metataxonomic approach. Therefore, in this study, an artisanal kombucha pellicle from Ankara was used to produce kombucha tea. The kombucha has been evaluated by lactobacilli and yeast/mold count, determination of pH and TA%, as well as microbiota analysis through a metataxonomic approach of tea liquid and pellicle. Microbiota results revealed that Komagataeibacter obediens was the dominant bacteria while Pichia kudriavzevii was the dominant yeast. Compared to other studies using metataxonomic approaches, Komagataeibacter and Pichia genera were the dominant members of kombucha but most of the other genera found in this study such as Butryvibrio, Tetrapisispora sp. are not common. Although this finding has seemed to support the idea that SCOBY geographic origin influences microbial community structure, more studies should be done from the same regions.
Supplementary Information
Below is the link to the electronic supplementary material.
The heatmap of L and P (16S) (PNG 26 kb)
The heatmap of L and P (ITS) (PNG 26 kb)
Acknowledgements
The author would like to thank local supplier of kombucha mother. This study was carried out in the Başkent University laboratory, the author would like to thank Başkent University for its technical support.
Abbreviations
- ITS
Internal Transcribed Spacer
- DRBCA
Dichloran Rose Bengal Chloramphenicol agar
- MRS
De Man, Rogosa and Sharpe
- PCR
Polymerase Chain Reaction
- DNA
Deoxyribonucleic Acid
- QC
Quality Control
- OTU
Operational taxonomic unit
- NCBI
The National Center for Biotechnology Information
Authors' contribution
OK-I was responsible for conceiving the idea, carrying out the work, and writing the MS.
Funding
Not applicable.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data supporting the findings of this research are available from the corresponding author, upon reasonable request. All the sample raw reads from metataxonomic research have been deposited at NCBI under the BioProject ID PRJNA742890.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC (2016) AOAC. Off Methods Anal 17th ed AOAC Int Washington, DC
- Arıkan M, Mitchell AL, Finn RD, Gürel F. Microbial composition of Kombucha determined using amplicon sequencing and shotgun metagenomics. J Food Sci. 2020;85:455–464. doi: 10.1111/1750-3841.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Manna P, Gachhui R, Sil PC. Protective effect of Kampuchea tea against tertiary butyl hydro peroxide induced cytotoxicity and cell death in murine hepatocytes. Indian J Exp Biol. 2011;49:511–524. [PubMed] [Google Scholar]
- Castellanos-Rozo J, Pulido RP, Grande MJ, et al. Analysis of the bacterial diversity of paipa cheese (A traditional raw cow’s milk cheese from Colombia) by high-throughput sequencing. Microorganisms. 2020;8(2):218. doi: 10.3390/microorganisms8020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu BY. Changes in major components of tea fungus metabolites during prolonged fermentation. J Appl Microbiol. 2000;89:834–839. doi: 10.1046/j.1365-2672.2000.01188.x. [DOI] [PubMed] [Google Scholar]
- Coton M, Pawtowski A, Taminiau B, et al. Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol Ecol. 2017;93:1–16. doi: 10.1093/femsec/fix048. [DOI] [PubMed] [Google Scholar]
- Cvetković D, Ranitović A, Savić D, et al. Survival of wild strains of lactobacilli during Kombucha fermentation and their contribution to functional characteristics of beverage. Polish J Food Nutr Sci. 2019;69:407–415. doi: 10.31883/pjfns/112276. [DOI] [Google Scholar]
- De Filippis F, Troise AD, Vitaglione P, Ercolini D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018;73:11–16. doi: 10.1016/j.fm.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Fiori S, Fadda A, Giobbe S, et al. Pichia angusta is an effective biocontrol yeast against postharvest decay of apple fruit caused by Botrytis cinerea and Monilia fructicola. FEMS Yeast Res. 2008;8:961–963. doi: 10.1111/j.1567-1364.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- Greppi A, Saubade F, Botta C, et al. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017;62:169–177. doi: 10.1016/j.fm.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Harrison K, Curtin C. Microbial composition of scoby starter cultures used by commercial kombucha brewers in North America. Microorganisms. 2021;9:1–21. doi: 10.3390/microorganisms9051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilıkkan ÖK, Bağdat EŞ. Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. Lwt. 2021;152:112375. doi: 10.1016/j.lwt.2021.112375. [DOI] [Google Scholar]
- Jung Y, Kim I, Mannaa M, et al. Effect of Kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci Biotechnol. 2019;28:261–267. doi: 10.1007/s10068-018-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamaki MS, Angelidis AS. High-throughput, sequence-based analysis of the microbiota of Greek kefir grains from two geographic regions. Food Technol Biotechnol. 2020;58:138–146. doi: 10.17113/FTB.58.02.20.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff ML, Cyr TD, Hefford MA, et al. Butyrivibriocin AR10, a new cyclic bacteriocin produced by the ruminal anaerobe Butyrivibrio fibrisolvens AR10: characterization of the gene and peptide. Can J Microbiol. 2003;49:763–773. doi: 10.1139/w03-101. [DOI] [PubMed] [Google Scholar]
- Kamilari E, Anagnostopoulos DA, Papademas P, et al. Characterizing Halloumi cheese’s bacterial communities through metagenomic analysis. Lwt. 2020;126:1–21. doi: 10.1016/j.lwt.2020.109298. [DOI] [Google Scholar]
- Kumar V, Joshi VK. Kombucha : technology, microbiology, production, composition and therapeutic value. Int J Food Ferment Technol. 2016;6:13. doi: 10.5958/2277-9396.2016.00022.2. [DOI] [Google Scholar]
- Lavefve L, Cureau N, Rodhouse L, et al. Microbiota profiles and dynamics in fermented plant-based products and preliminary assessment of their in vitro gut microbiota modulation. Food Frontiers. 2021;2(3):268–281. doi: 10.1002/fft2.75. [DOI] [Google Scholar]
- Leal JM, Suárez LV, Jayabalan R, et al. A review on health benefits of kombucha nutritional compounds and metabolites. CYTA - J Food. 2018;16:390–399. doi: 10.1080/19476337.2017.1410499. [DOI] [Google Scholar]
- Marsh AJ, O’Sullivan O, Hill C, et al. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014;38:171–178. doi: 10.1016/j.fm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- May A, Narayanan S, Alcock J, et al. Kombucha: A novel model system for cooperation and conflict in a complex multi-species microbial ecosystem. PeerJ. 2019;2019:1–22. doi: 10.7717/peerj.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlomakulu NN, Setati ME, Divol B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int J Food Microbiol. 2014;188:83–91. doi: 10.1016/j.ijfoodmicro.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Moon SH, Chang M, Kim HY, Chang HC. Pichia kudriavzevii is the major yeast involved in film-formation, off-odor production, and texture-softening in over-ripened Kimchi. Food Sci Biotechnol. 2014;23:489–497. doi: 10.1007/s10068-014-0067-7. [DOI] [Google Scholar]
- Neffe-Skocińska K, Sionek B, Ścibisz I, Kołożyn-Krajewska D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA - J Food. 2017;15:601–607. doi: 10.1080/19476337.2017.1321588. [DOI] [Google Scholar]
- Ohkawara S, Furuya H, Nagashima K, et al. Oral administration of butyrivibrio fibrisolvens, a butyrate-producing bacterium, decreases the formation of aberrant crypt foci in the colon and rectum of mice. J Nutr. 2009;139:194. doi: 10.3945/jn.108.102061. [DOI] [PubMed] [Google Scholar]
- Teoh AL, Heard G, Cox J. Yeast ecology of Kombucha fermentation. Int J Food Microbiol. 2004;95:119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Thukral AK. A review on measurement of Alpha diversity in biology. Agric Res J. 2017;54:1. doi: 10.5958/2395-146x.2017.00001.1. [DOI] [Google Scholar]
- Tolulope PA, Julius KO. Association of probiotic potential of strains of Pichia kudriavzevii isolated from ogi with the number of open reading frame (ORF) in the nucleotide sequences. African J Biotechnol. 2020;19:148–155. doi: 10.5897/ajb2019.16814. [DOI] [Google Scholar]
- Tran T, Grandvalet C, Verdier F, et al. Microbial dynamics between yeasts and acetic acid bacteria in kombucha: impacts on the chemical composition of the beverage. Foods. 2020;9(7):963. doi: 10.3390/foods9070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Soto SA, Beaufort S, Bouajila J, et al. Understanding Kombucha tea fermentation: a review. J Food Sci. 2018;83:580–588. doi: 10.1111/1750-3841.14068. [DOI] [PubMed] [Google Scholar]
- Wood DE, Salzberg SL. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;123(2):276–288. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M (2020) A potential probiotic- Lachnospiraceae NK4A136 group: Evidence from the restoration of the dietary pattern from a high-fat diet. Res Sq pp 1–24
- Yıkmış S, Tuğgüm S. Evaluation of microbiological, physicochemical and sensorial properties of purple basil kombucha beverage. Turkish J Agric - Food Sci Technol. 2019;7:1321. doi: 10.24925/turjaf.v7i9.1321-1327.2550. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The heatmap of L and P (16S) (PNG 26 kb)
The heatmap of L and P (ITS) (PNG 26 kb)
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data supporting the findings of this research are available from the corresponding author, upon reasonable request. All the sample raw reads from metataxonomic research have been deposited at NCBI under the BioProject ID PRJNA742890.