Abstract
Purpose
Tocilizumab, a monoclonal IL-6 receptor blocker, is an effective agent for severe-to-critical cases of COVID-19; however, its target patients for the optimum use need to be detailed. We performed a systematic review and meta-analysis to define its effect among severely ill but non-intubated cases with COVID-19.
Methods
We searched PubMed, Scopus, Web of Science, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Medrxiv, and Biorxiv until February 13, 2022, for non-intubated cases, and included randomized-controlled trials (RCT) based on bias assessment. The primary outcomes were the requirement of invasive mechanical ventilation and mortality. Random effect and fixed-effect models were used. The heterogeneity was measured using the χ2 and I2 statistics, with χ2 p ≤ 0.05 and I2 ≥ 50% indicating the presence of significant heterogeneity. We registered the study to the International Prospective Register of Systematic Reviews (PROSPERO) with the registration number CRD42021232575.
Results
Among 261 articles, 11 RCTs were included. The pooled analysis of the 11 RCTs demonstrated that the rate of mortality was significantly lower in the tocilizumab group than in the control group (20.0% and 24.2%, OR: 0.84, 95% CI 0.73–0.96, and heterogeneity I2 = 0%. p = 0.82.). The mechanical ventilation rate was lower in the tocilizumab group than the control group (27% vs 35.2%, OR: 0.76, 95% CI 0.67–0.86, and heterogeneity I2 = 6%. p = 0.39).
Conclusion
Among non-intubated severe COVID-19 cases, tocilizumab reduces the risk of invasive mechanical ventilation and mortality compared to standard-of-care treatment.
Keywords: Tocilizumab, COVID-19, Randomized-controlled trials, Pneumonia, Non-intubated
Introduction
Novel Coronavirus disease (COVID-19) is a severe respiratory system infection caused by SARS CoV-2 [1, 2]. The majority of the cases are mild and moderate and recover without any specific treatment. However, 5–10% of the cases require hospitalization and may progress to severe COVID-19 [3, 4]. The morbidity and mortality are mainly caused by hyperinflammation among the patients, who have high-risk factors.
Immunomodulatory treatment including tocilizumab, an IL-6 receptor blocker, was defined as an effective measure in different studies. However, the appropriate timing of administration of tocilizumab has not been clearly defined yet. There are a wide range of administration time of tocilizumab from moderate to critical cases in different studies including randomized control trials (RCTs) and cohort studies [5–11]. It was shown that cytokine levels increase sharply 10 days after the onset of symptoms in COVID-19 [12]. Accordingly, IL-6 blockers might be more effective if given soon after hypoxia and before the need for invasive mechanical ventilation. This is an earlier timing than many studies, and compatible with our clinical practice. Therefore, we performed a meta-analysis including severe cases rather than critical cases to describe the appropriate time of tocilizumab use in COVID-19.
Materials and methods
This is a systematic review and meta-analysis which was registered at the International Prospective Register of Systematic Reviews (PROSPERO) with a registration number CRD42021232575. We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. We searched PubMed, Scopus, Web of Science, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Medrxiv, and Biorxiv between January 01, 2020, and February 13, 2022. “COVID-19, tocilizumab” and “SARS CoV-2, tocilizumab” keyword combinations were used in defined databases.
Inclusion criteria
Adult patients with COVID-19;
Studies that compare tocilizumab to control groups which consist of patients receiving standard-of-care (SOC) treatment or placebo;
Only randomized-controlled trials;
Studies that include only non-intubated cases
A severe case was defined as a case who has hypoxia (oxygen saturation < 90% in room air) or has a respiratory rate of > 30 breaths/min or signs of severe respiratory distress [14]. However, depending on the design of the studies, there were some minor differences in the definition of the “severe case”. Early severe was defined as oxygen supplementation with a nasal cannula or simple mask, while progressive severe was defined as oxygen supplementation with high-flow or non-invasive ventilation. The outcomes were mortality and the requirement for invasive mechanical ventilation. Review articles, case studies, case series, letters, and studies without control groups were excluded. The RCTs that included the intubated cases were excluded.
Data extraction
Based on the PRISMA Checklist, papers were retrieved from different data sources. After duplicate files were removed, the information, including the type of study, country, baseline characteristics such as age and gender, the total number of cases, the severity of the infection (severe or critical) in case of receiving tocilizumab, number of cases who received tocilizumab, number of cases who received standard-of-care or no treatment, and outcome measures, were extracted from relevant studies.
Quality assessment
Quality assessment and scoring of included studies were performed based on the study design, intervention type, study population, stage of the disease, and outcome measures. Risk-of-bias assessment was carried out using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [15]. RoB 2 consists of the following six components: risk of bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Four reviewers independently evaluated the risk of bias, and disagreements were solved by another reviewer.
Statistical analysis
Data were summarized across treatment arms using the odds ratio (OR). Random effect and fixed-effect models were used. Statistical significance was set as p ≤ 0.05. The heterogeneity was measured using the χ2 and I2 statistics, with χ2 p ≤ 0.05 and I2 ≥ 50% indicating the presence of significant heterogeneity. The results were displayed using Forest plots. Funnel plots were used to visually evaluate publication bias, while Egger’s regression test and Begg’s test were used to statistically evaluate publication bias. Statistical analysis was performed using R.
Results
Assessment of the studies
After the removal of duplications, 261 articles were identified from PubMed (n = 22), Scopus (n = 53), Web of Science (n = 29), and Cochrane Library (n = 114). Twenty articles remained after the title and abstract screening. A total of 11 RCTs [7–11, 16–21] were included after the full texts of 20 articles were reviewed (Fig. 1).
Fig. 1.
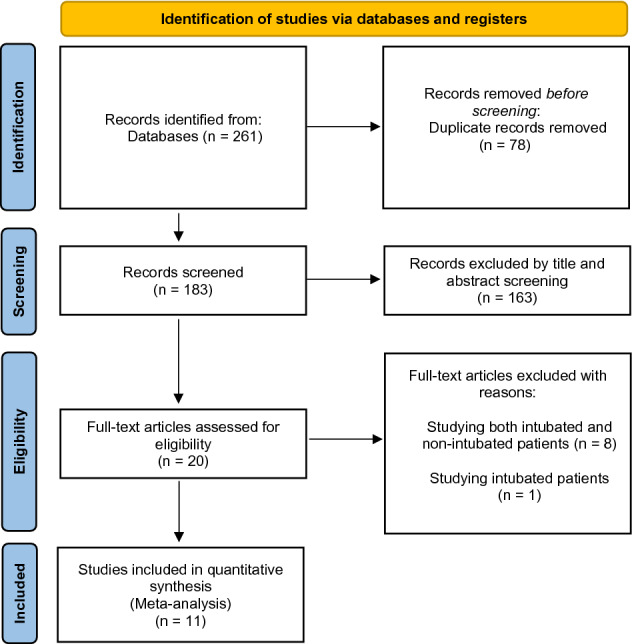
Flow diagram for selection of the studies (PRISMA). From: Page et al. [13]. For more information, visit: http://www.prisma-statement.org/
The features of the studies
We included 11 RCTs, two of them were multi-center global studies, and the other trials were conducted in France, Italy, USA, United Arab Emirates, India, China, and UK. In total, 5472 patients were included of which 2864 were assigned to the tocilizumab group and 2608 were assigned to the control group (Table 1).
Table 1.
Randomized-controlled trials included in the study
| Authors | Study design | Country | Study period | Inclusion criteria | Study population (TCZ versus control) n | Intubation (TCZ versus control) n | Mortality (TCZ versus control) n | Concomitant antiviral use (TCZ versus control) % | Concomitant anti-inflammatory use (TCZ versus control) % | Timing of onset of TCZ versus placebo/standard of care |
|---|---|---|---|---|---|---|---|---|---|---|
| Hermine et al. [8] | Randomized, open-label | France | 31 March 2020 to 18 April 2020 |
1. Moderate or severe pneumonia and with WHO 10-point Clinical Progression Scale (WHO-CPS) score of 5 receiving at least 3L/min oxygen (O2) 2. Without high-flow oxygen, non-invasive ventilation or mechanical ventilation |
63–67 | 5–14 | 7–8 | Remdesivir: 0% and 1.5% |
Dexamethasone: 14%–28% Anakinra: 2%–4.5% Corticosteroids: 30%–55% |
No cases received non-invasive ventilation or high flow Comment: Early severe |
| Rosas et al. [10] | Randomized, double-blind, placebo-controlled, phase 3 trial | Global, multi-center | 3 April 2020 to 28 May 2020 |
1. Adults ≥ 18 years of age with severe COVID-19 pneumonia 2. A blood oxygen saturation of 93% or less or a ratio of the partial pressure of oxygen to the fraction of inspired oxygen of less than 300 mm Hg |
183–90 | 39–21 | 27–15 |
24.1%–29.2% *The details of the antiviral was not noted |
Corticosteroids: 19.4%–28.5% |
Early severe 29.6%–34.8% Progressive severe: 70.4%–65.2% |
| Salama et al. [16] | Randomized, double-blind, placebo-controlled, phase 3 trial | Global, multi-center | The data cutoff date was 30 September 2020 |
1. Patients who were 18 years of age or older 2. Hospitalized with COVID-19 pneumonia 3. A blood oxygen saturation below 94% while breathing ambient air |
249–128 | 20–16 | 29–15 | Remdesivir: 52.6%–58.6% |
Corticosteroid: 80.3%–87.5% Dexamethasone: 55.4%–67.2% |
Early severe: 74.3%–71.9%) Progressive severe: 25.7%–28.1% |
| Salvarani et al. [7] | Prospective, open-label, randomized | Italy | 31 March 2020 to 11 June 2020 |
1. Patients with COVID-19 2. On oxygen therapy except invasive or non-invasive mechanical ventilation 3. Temperature greater than 38 °C during the last 2 days, and/or CRP levels of 10 mg/dL or greater and/or CRP levels increased to at least twice the admission measurement 4. Parameters: IL6 > 49 pg/ml, increasing LDH or LDH > twice the upper limit of normal, increasing CRP, D-dimer > 1500 ng/ml, lymphocytes < 1200/µl, ferritin > 500 ng/ml |
60–63 | 6–5 | 2–1 | N/A | Corticosteroids: 10%–11.1% | Patients receiving oxygen therapy with Venturi mask or high-flow nasal cannula, but not invasive or non- invasive mechanical ventilation were included |
| Stone et al. [9] | Randomized, double-blind, placebo-controlled | USA | 20 April 2020 to 15 June 2020 |
1. Hyperinflammatory states 2. At least two of the following signs: fever (> 38 °C), pulmonary infiltrates, or the need for supplemental oxygen in order to maintain an oxygen saturation greater than > 92 |
161–81 | 11–8 | 9–3 | Remdesivir: 33%–29% | Corticosteroids: 11%–6% |
Early Severe: 97%–92% Progressive severe: 3%–8% |
| Horby et al. | Randomized, open-label | UK | 23 April 2020 to 24 January 2021 |
1. Participants with hypoxia (oxygen saturation < 92% on air or requiring oxygen therapy) 2. C-reactive protein ≥ 75 mg/L |
1754–1800 | 686–825 | 471–552 | No effective antiviral | Corticosteroids: 82%–82% |
Early Severe 46%–45% Progressive severe: 54%–55% |
| Hamed et al. [17] | Randomized, open-label | United Arab Emirates | June 2020 to July 2020 |
1. SARS-CoV-2 infection shown by positive results on real-time PCR (RT-PCR) of nasopharyngeal swab samples 2. Development lung infiltrates involving > 50% of the lung fields within the 48 h of admission 3. O2 saturation < 93% at rest on room air |
26–23 | 6–2 | 2–1 | N/A | Methylprednisolone: 100%–100% | N/A |
| Soin et al. [18] | Randomized, open-label | India | April 2020 to September 2020 |
1. Moderate to severe patients defined according to the Indian MoHFW clinical management protocol for COVID (moderate defined as respiratory rate 15–30 per min and blood oxygen saturation [SpO2] 90–94%; and severe defined as respiratory rate ≥ 30 per min or SpO2 < 90% in ambient air, or ARDS or septic shock) |
91–89* *89 patients included in the safety analysis, 88 patients in the intention-to-treat analysis |
5–4 | 11–15 | Remdesivir: 43%-41% | Corticosteroids: 91%-91% |
Early severe: 45%–53% Progressive severe: 55%–47% |
| Wang et al. [19] | Randomized, open-label | China | February 2020 to March 2020 |
1. Confirmed SARS-CoV-2 infection through reverse transcriptase polymerase chain reaction positivity 2. 18–85 years old 3. Elevated plasma IL-6 levels 4. Moderate disease (fever or other respiratory symptoms, bilateral pulmonary lesions confirmed by chest imaging) or severe disease (respiratory rate ≥ 30 breaths per min or SpO2 ≤ 93% while breathing room air or PaO2/FiO2 ≤ 300 mmHg.) |
34–31 | 0–0 | 0–0 | N/A | Corticosteroids: 14.7%–6.45% |
Early severe: 67.6%–61.3% Progressve severe: 32.4%–38.7% |
| Zhao et al. [20] | Randomized, open-label | China | February 2020 to March 2020 |
1. Laboratory-confirmed cases according to Chinese guidelines of COVID-19 2. Increased interleukin-6 |
19–7 | 0–2 | 0–2 | No effective antiviral | N/A |
Early severe: 92.8%–100% Progressive severe: 7.2%–0% |
| Hermine et al. [21] | Randomized, open-label | France | July 2020 to May 2021 |
1. Confirmed SARS-CoV-2 infection (PCR test or chest CT-scan) 2. Moderate and severe pneumopathy requiring oxygen (> 3 L/ min) but without ventilation support (NIV), high flow or MV, WHO class 5 according to the WHO 10 points-Clinical Progression Scale (CPS) for COVID-19 pneumopathy |
224–226 |
15–20* *Mechanical Ventilation up to Day 14 |
15–19 |
1%–0.5% *The details of the antiviral was not noted |
Dexamethasone: 100%–100% | All the patients were early severe |
Quality assessment
The risk of bias was detected as low among all 11 included RCTs.
Outcomes of the tocilizumab
Mortality
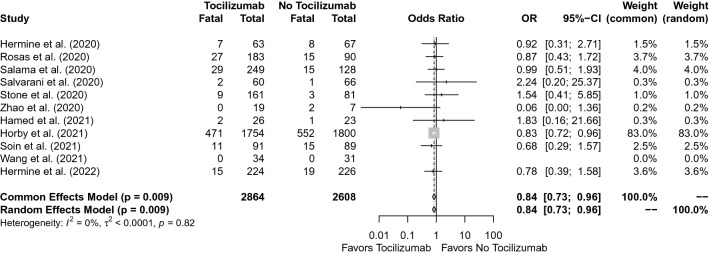
In the pooled analysis of the 11 RCTs, the rate of mortality was 20.0% in the tocilizumab group and 24.2% in the control group (OR, 0.84; 95% CI 0.73–0.96, p = 0.009). In the test for heterogeneity, I2 = 0%, and the p value was detected as 0.82 (Fig. 2).
Fig. 2.
Forest plot for mortality
Invasive mechanical ventilation
The pooled analysis of the 11 RCTs revealed that the invasive mechanical ventilation rate was 27.0% in the tocilizumab group and 35.2% in the control group (OR, 0.76; 95% CI 0.67–0.86, p < 0.001). In the heterogeneity test, I2 = 6% and p = 0.39 (Fig. 3).
Fig. 3.
Forest plot for invasive mechanical ventilation
The use of concomitant drugs
Based on the different nature of the studies, the use of concomitant drugs such as remdesivir and glucocorticoids that might affect the primary outcomes was evaluated (Table 1). The only effective antiviral was remdesivir in four studies. The use of remdesivir was detected as similar between the case and control groups. The use of corticosteroids was described as being similar in ten studies. Anakinra was used in only one study.
Discussion
Through this systematic review and meta-analysis, we described the effectiveness of using tocilizumab in severe COVID-19 patients before receiving invasive mechanical ventilation. Our meta-analysis included only severe COVID-19 cases and showed that tocilizumab decreased the mortality (OR, 0.84; 95% CI 0.73–0.96, p = 0.009, and for the heterogeneity I2 = 0%. p = 0.82, Fig. 2) and decreased the need for invasive mechanical ventilation (OR, 0.76; 95% CI 0.67–0.86, p < 0.001 and for the heterogeneity I2 = 6%. p = 0.39, Fig. 3). In our meta-analysis, no significant heterogeneity was detected (I2 = 0%. p = 0.82, Fig. 2 and I2 = 6%. p = 0.39, Fig. 3), and therefore, included studies were accepted as convenient for the meta-analysis. In terms of clinical impact, tocilizumab use decreased the mortality and the need for mechanical ventilation among non-intubated severe COVID-19 cases.
These findings were consistent with our clinical practice. Our results will be useful in the appropriate use of tocilizumab in clinical practice. Previously, the studies with a small sample size [7, 8] could not detect the effectiveness of tocilizumab until the report of the RECOVERY trial [11] which had > 3500 cases. However, the RECOVERY trial and other RCTs [8, 10, 11, 22] included moderate, severe, and critical cases, and, therefore, could not help us to point out the appropriate timing of the use of tocilizumab in clinical practice. We included the severe patients and excluded the patients who received tocilizumab after intubation. It was shown that inflammation markers including IL-6 increase sharply at about the 10th day of onset of symptoms [12]. Although REMAP-CAP [22] showed that tocilizumab is effective in critical cases, the development of hypoxia soon after the sharp increase in IL-6 on the 10th day makes us think that IL-6 inhibitors might be effective at this stage.
Concomitant drug use, such as glucocorticoids and remdesivir, may also influence the effectiveness of tocilizumab. After the RECOVERY trial’s early results were reported in June 2020, glucocorticoids have been used among patients with hypoxia [22]. It was not possible to extract the ratio of glucocorticoid use among tocilizumab and control group arms in severe cases. Thus, we could not do a pooled analysis of steroid use; however, the frequency of steroid use was similar among tocilizumab and control groups in RCTs. The indications of glucocorticoid use should be clear as it might have an effect on the outcomes, doses, and duration of administration of tocilizumab.
The strongest and unique point of our systematic review and meta-analysis is including RCTs composed of non-intubated severe cases, but not critical cases. The major limitation of the previous similar meta-analyses was the inclusion of both critical and non-critical cases [23–26]. Country-level differences, different time intervals among studies, and different inclusion criteria are the most important limitations of our pooled analysis. We evaluated the time of onset of tocilizumab among studies as stated in Table 1. However, the primary outcomes for different timing of tocilizumab (early versus progressive severe stage) were not available. The pooling of double-blinded and open-label studies was another limitation.
In conclusion, tocilizumab reduces the risk of invasive mechanical ventilation and mortality when started in severe and non-intubated patients, before they become critically ill.
Acknowledgements
We are thankful to Ertaç Nebioğlu from Koç University Health Sciences Library for his detailed literature search.
Author contributions
The idea of the article: SK, BS, and OE. Literature search: CT, MA, REA, BK, UG, and BÖ. Data analysis: MG. Draft: SK, OE, MG, CT, BÖ, and MA.
Funding
No funding received. The authors did not receive support from any organization for the submitted work.
Availability of data and materials
This declaration is “not applicable”. However, Table 1 shows the data of the studies which were used in this meta-analysis. This declaration is “not applicable”.
Declarations
Conflict of interest
There is no competing interest. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This declaration is “not applicable”.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keske S, Tekin S, Sait B, Irkoren P, Kapmaz M, Cimen C, et al. Appropriate use of tocilizumab in COVID-19 infection. Int J Infect Dis. 2020;99:338–343. doi: 10.1016/j.ijid.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Wang W, Hayek SS, Chan LL, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group RC Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therapeutics and COVID-19: living guideline. 2020. https://app.magicapp.org/#/guideline/5058. Accessed 17 Dec 2020.
- 15.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamed DM, Belhoul KM, Al Maazmi NA, Ghayoor F, Moin M, Al Suwaidi M, et al. Intravenous methylprednisolone with or without tocilizumab in patients with severe COVID-19 pneumonia requiring oxygen support: a prospective comparison. J Infect Public Health. 2021;14(8):985–989. doi: 10.1016/j.jiph.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511–521. doi: 10.1016/s2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Fu B, Peng Z, Yang D, Han M, Li M, et al. Tocilizumab in patients with moderate or severe COVID-19: a randomized, controlled, open-label, multi-center trial. Front Med. 2021;15(3):486–494. doi: 10.1007/s11684-020-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Zhu Q, Zhang C, Li J, Wei M, Qin Y, et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: a multi-center trial in a small sample size. Biomed Pharmacother. 2021;133:110825. doi: 10.1016/j.biopha.2020.110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermine O, Mariette X, Porcher R, Djossou F, Nguyen Y, Arlet JB, et al. Tocilizumab plus dexamethasone versus dexamethasone in patients with moderate-to-severe COVID-19 pneumonia: a randomised clinical trial from the CORIMUNO-19 study group. EClinicalMedicine. 2022;46:101362. doi: 10.1016/j.eclinm.2022.101362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berardicurti O, Ruscitti P, Ursini F, D'Andrea S, Ciaffi J, Meliconi R, et al. Mortality in tocilizumab-treated patients with COVID-19: a systematic review and meta-analysis. Clin Exp Rheumatol. 2020;38(6):1247–1254. [PubMed] [Google Scholar]
- 24.Aziz M, Haghbin H, Abu Sitta E, Nawras Y, Fatima R, Sharma S, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–1630. doi: 10.1002/jmv.26509. [DOI] [PubMed] [Google Scholar]
- 25.Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alkofide H, Almohaizeie A, Almuhaini S, Alotaibi B, Alkharfy KM. Tocilizumab and systemic corticosteroids in the management of patients with COVID-19: a systematic review and meta-analysis. Int J Infect Dis. 2021;110:320–329. doi: 10.1016/j.ijid.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This declaration is “not applicable”. However, Table 1 shows the data of the studies which were used in this meta-analysis. This declaration is “not applicable”.