Abstract
Purpose of Review
Despite single-photon emission computerized tomography (SPECT) being the most used nuclear imaging technique for diagnosis of coronary artery disease (CAD), many now consider positron emission tomography (PET) as a superior modality. This review will focus on the advances of cardiac PET in recent years and its advantages compared to SPECT in diagnosis and prognosis of CAD.
Recent Findings
PET’s higher resolution and enhanced diagnostic accuracy, as well as lower radiation exposure, all help explain the rationale for its wider spread and use. PET also allows for measurement of myocardial blood flow (MBF) and myocardial flow reserve (MFR), which aids in several different clinical scenarios, such as diagnosing multivessel disease or identifying non-responders. PET has also been shown to be useful in diagnosing CAD in various specific populations, such as patients with prior COVID-19 infection, cardiac transplant, and other comorbidities.
Keywords: Positron emission tomography, Myocardial perfusion, Ischemia, Myocardial flow reserve
Introduction
Heart disease remains the number one cause of mortality in the USA according to data from 2019, with ischemic heart disease (IHD) accounting for more than 12% of all deaths in the USA [1]. Incidence remains high with studies showing that more than one-third of middle-aged adults in the USA are expected to develop coronary artery disease (CAD) throughout their lifetime [2]. With the availability of effective medical and interventional management approaches, the choice of optimal imaging modality to diagnose CAD, even before clinical manifestation, becomes a crucial step in the care of patients. Up until now, single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) continues to be the most used nuclear imaging modality for CAD assessment. However, use of positron emission tomography (PET) has recently been increasing due to its superior utility and accuracy. Similar to SPECT, PET is a nuclear imaging technique that employs radionuclide tracers to produce images using an external detector system. It allows qualitative interpretation of MPI, as well as quantitative measurements of myocardial blood flow, in both absolute and relative terms. These features make it a unique tool to assist in diagnosis and prognosis of patients with suspected ischemic coronary disease. In fact, among non-invasive cardiac imaging modalities, PET has seen the largest increase in use among Medicare beneficiaries with an increase of 146% between 2010 and 2019, while SPECT has decreased by 36% in that same period [3, 4]. This review aims to explain the advantages that PET holds over SPECT. It also presents evidence of PET superiority in different groups of patients with suspected or confirmed ischemic heart disease.
Advantages of Cardiac PET vs SPECT
High Resolution
In imaging, there are two types of resolutions, both considered equally important when assessing an imaging modality. Spatial resolution denotes the ability to differentiate two adjacent objects as being distinct from one another. On the other hand, temporal resolution refers to the time gap between adjacent images, similar to the shutter speed on a camera [5]. PET provides a substantial advantage over SPECT with regard to both these parameters. PET has a spatial resolution of 2 to 3 mm as opposed to SPECT which has a spatial resolution of 6 to 8 mm. This is in part due improved image resolution and electronics in PET vs SPECT machines. [6, 7] PET also has better temporal resolution as it can register events faster than SPECT, and the gantry in PET is stationary, compared to that in SPECT which is rotational. Temporal resolution is also closely related to the sensitivity of the imaging system, which confers PET an advantage [8, 9].
Low Radiation Dose
One of the pitfalls of nuclear imaging is radiation exposure. The as-low-as-reasonably-achievable principle has been widely accepted as mainstay when it comes to procedures requiring ionizing radiation. The radiotracers used in PET (Rubidium-82 or 13N-Ammonia) have a photon energy of 511 keV versus those used in SPECT whose energy is 80 to 140 keV, which leads to lower requirements of radiotracer injection in PET [6]. The PET radiotracers also have a shorter half-life than those used in SPECT, which leads to a shorter study time and less radiation exposure. A study by Desiderio et al. in 2018 found that the average PET myocardial perfusion imaging (MPI) radiation exposure was 3.7 (3.2–4.1) mSv per study, as opposed to the average SPECT MPI radiation exposure which was 12.8 (12.2–14.3) mSv [8]. This is reduced even further as newer digital PET cameras allow for usage of lower doses of radiotracers (routine Sub-milliSievert PET scans can be performed among patients with BMI < 30 kg/m2) [9].
High Diagnostic Accuracy
Diagnostic yield is often summarized using accuracy, sensitivity, and specificity, making them important parameters when evaluating an imaging modality. Several studies have assessed this by comparing PET and SPECT, specifically in the setting of coronary artery disease. One such study of 224 patients who received either SPECT technetium-99 m sestamibi or 112 PET rubidium-82 MPI electrocardiography (ECG)-gated rest/pharmacologic stress found diagnostic accuracy to be higher in PET for both stenosis severity thresholds of 70% and 50% [10]. Multiple other studies directly comparing the accuracy of PET vs SPECT in diagnosing coronary artery disease all favor the former [11, 12]. The PACIFIC I study that looked at patients with no known CAD showed that PET exhibited the highest accuracy for diagnosis of myocardial ischemia when compared to other non-invasive modalities [13]. The PACIFIC II study on the other hand looked at patients with known CAD and showed that PET had better sensitivity for detection of ischemia [9]. In addition, two systematic reviews and meta-analyses also found PET MPI demonstrated higher sensitivity in detecting coronary artery disease as compared to SPECT MPI [14]. Similar results were shown in studies targeting special populations, such as in a study by Vidula et al. that showed PET to be more accurate than SPECT at detecting obstructive CAD in patients with left bundle branch block (LBBB) or ventricular-paced rhythm (VPR) [15].
Assessment of True Peak EF
One major advantage of PET over SPECT MPI is the ability to measure the ejection fraction during peak stress. This is not possible in SPECT as the study is usually done 15 to 45 min after inducing cardiac stress [4]. Peak EF, therefore, allows derivation of left ventricular ejection fraction (LVEF) reserve, which is the difference between peak EF and rest EF. Several studies have assessed the utility of LVEF reserve, most notable one by Dorbala et al. in 2009 [16]. In this study, 1432 consecutive patients undergoing gated rest/vasodilator stress rubidium-82 PET were followed up over time and assessed for outcomes, which included cardiac events (CE) and all-cause mortality. Results showed that patients with LVEF reserve < 0% had higher annualized rates of both CE (2.1% vs 5.3%, p < 0.001) and all-cause death (4.3% vs 9.2%, p < 0.001) as compared with patients with LVEF reserve ≥ 0%. In addition, Cox analysis showed that LVEF reserve can provide valuable independent and incremental value to studies using RB-82 in predicting risk of future cardiac events [16]. However, the only PET radiotracer for which data is available for regarding peak EF is Rubidium-82.
Quantification of MBF
Perhaps the most significant aspect of PET is its ability to quantify myocardial blood flow (MBF) in absolute units (ml/min/g). Measurements are made at rest and peak stress, after which calculation of the myocardial flow reserve (MFR) can be made. MFR constitutes the ratio of MBF during maximal stress over rest and therefore represents the reserve of coronary circulation [14]. These parameters allow us to better categorize patients with CAD, such as in a study by Ziadi et al., where MFR was shown to be an independent predictor of triple coronary vessel disease in patients with known CAD [17]. MBF and MFR also play an important role in diagnosing microvascular disease in the absence of obstructive coronary lesions. This was assessed in a study by Geltman et al., in which patients with chest pain and angiographically normal coronaries, as well as normal participants, underwent PET perfusion studies. Results showed that about 50% of patients in the chest pain group had perfusion abnormalities on PET [18]. Finally, some studies show that MFR can play an important role in predicting outcomes in patients with suspected or known CAD. One such study involving 3534 patients with known or suspected CAD and clinically indicated PET MPI showed that MFR < 2 was significantly associated with their primary outcome (which was composite of all-cause death, myocardial infarction, and percutaneous intervention or coronary artery bypass graft that occurs > 90 days after PET imaging) [19, 20]. MFR can even help in determining which patients will benefit from therapy, as was showed in the large study by Patel et al., involving 12,594 patients undergoing 82-Rb rest/stress PET MPI. Results showed that patients with MFR ≤ 1.8 exhibited survival benefit with early revascularization, regardless of level of ischemia or type of revascularization [21].
Clinical Scenarios
PET in Patients with Suboptimal SPECT
Despite the multiple advantages that PET holds over SPECT, the latter remains the most used modality, partly due to the abundance of machines available compared to its counterpart. Therefore, it is necessary to consider the usefulness of PET in patients who have already received SPECT. The PACIFIC trial is the largest study to date in which the same patients received CCTA, PET, and SPECT. Results showed PET to have the highest diagnostic accuracy (85%; 95% CI, 80–90%) in detecting hemodynamically significant stenosis in at least one coronary artery [13]. A similar study was done on a smaller group of patients (N = 27) with suspected CAD that first received SPECT followed by PET as a confirmatory test. It found both image quality and interpretive confidence to be higher with PET, as well as interpretive confidence and inter-reader agreement [22]. Therefore, there appears to be value in PET in patients with previous suboptimal or inconclusive SPECT findings for ischemic disease in different patient populations, such as in morbidly obese patients [23, 24].
MBF Identifying Multivessel Disease
In addition to their role in diagnosing CAD, MBF and MFR can also help differentiate between high and low risk patients. High-risk CAD includes 2 vessel disease including proximal LAD, 3-vessel disease, or left main disease (≥ 50%) [25]. Identifying these patients by non-invasive testing is crucial as it can help the clinician decide on the best next step (pharmacological treatment vs invasive testing). In a study by Naya et al., MFR was shown to possess a high negative predictive value for ruling out high-risk CAD on angiography. However, abnormal MFR values were not able to distinguish between severe obstructive epicardial stenosis and other types of coronary disease (diffuse, nonobstructive epicardial, microvascular dysfunction) [26]. Other studies also showed MFR to be an independent predictor of 3-vessel disease in patients with known or suspected CAD [17]. Therefore, there is evidence for the use of MBF and MFR to assist in stratifying CAD patients into high-risk and low-risk groups.
MBF Identifying Balanced Ischemia
Although a normal perfusion study by PET usually signifies absence of physiologically significant coronary artery narrowing, it is important to remember that it is based on relative tracer uptake. Therefore, if there is reduction of blood flow in all three territories, such as in left main or triple-vessel disease, perfusion study can appear as falsely negative. This phenomenon is referred to as “balanced ischemia” (Fig. 1) [27]. A study from the Netherlands that included patients who had undergone cardiac SPECT between 2006 and 2010 found that 17% of patients had balanced ischemia (normal MPI and significant CAD due to triple-vessel or left main disease), demonstrating that its prevalence is significant [28]. Since MBF is an absolute, rather than a relative, measure, it can unmask ischemia in these patients. This is demonstrated by a study from Finland, in which 286 symptomatic patients with previous computed tomography angiography (CTA) underwent 15-O water PET MPI. Among those found to have a reduced absolute quantified MBF in all three territories (triple-vessel or left main disease), 28% had normal relative MPI values. These patients likely have balanced ischemia and would have been missed if only assessed by relative MPI.
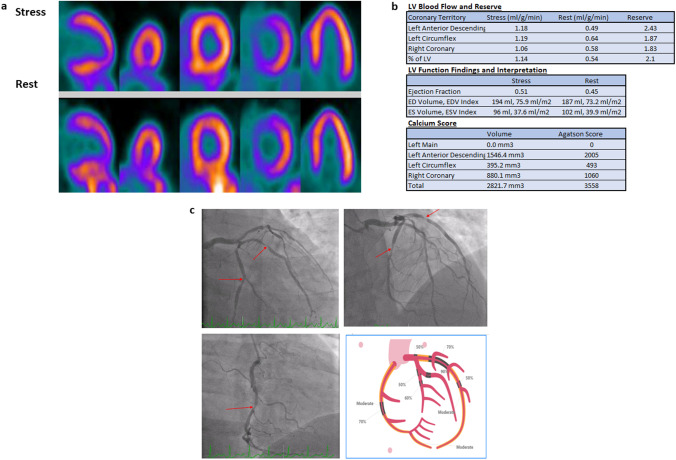
Fig. 1.
A 53-year-old male with hypertension and dyslipidemia presented with chest pain with exertion. PET relative perfusion imaging showed no defect. a The ejection fraction was 45% at rest and increased to 51% at stress. However, the total coronary artery calcium scoring was 3558, with calcifications noted in the LAD, LCx, and RCA. The global myocardial flow reserve was borderline (2.1), primarily because of reduced stress flow (1.14 ml/g/min). b Balanced ischemia was suspected, and the patient was referred for Invasive angiography, which showed multivessel disease with obstructive stenosis in the distal LM, proximal-to-mid LAD, proximal-to-mid LCx, and mid-RCA (red arrows). The patient subsequently underwent triple vessel CABG (LIMA to LAD, SVG to PDA, and SVG to OM). c On a recent follow-up visit 1.5 years from CABG, the patient continued his guideline-directed therapy, experienced no incident cardiovascular events, and reported no symptoms
MBF Identifying Non-responders
One of the most used vasodilators for MPI imaging is adenosine, mostly due to its rapid onset of action, short half-life, and good safety profile. Achieving maximal vasodilation is crucial to obtain accurate MFR measurements, and therefore, failure to achieve it might lead to false-negative results [20]. Although changes in vital signs (such as blood pressure and heart rate) were once thought to be good predictors to assess the effectiveness of vasodilator stress, studies have shown that they fail to do so [29]. MBF can potentially play a role in helping unmask coronary disease in these patients. Flow is expected to increase after adenosine administration, and therefore, the absence of or smaller than expected change in MBF, coupled with absence of change in vital signs, might be an indicator of unresponsiveness. Another indicator that has been used to assess response is splenic switch-off (SSO) sign, which refers to a visible decrease in splenic signal intensity during stress as compared to rest. It is hypothesized that sympathetic vasoconstriction occurs after vasodilator-induced hypotension, which leads to reduced splenic blood flow [30]. Most studies have been done using dipyridamole or adenosine, but new data suggests that this may also be seen using regadenoson [31].
INOCA
Ischemic heart disease with no obstructed coronary arteries (INOCA) has become a common and recognized disease. These are a subset of patients who usually present with signs and symptoms suggestive of ischemia, but upon coronary angiography, are found to have no obstructive lesions (> 50%). Not only is INOCA prevalent, but it is also associated with increase in cardiac outcomes, such as MI and mortality [32]. Cardiac PET is considered one of the most reliable non-invasive imaging modalities to help with diagnosis of INOCA, particularly due to its ability to measure MBF and evaluate MFR [33].
Special Population
Post-COVID
Coronary microvascular dysfunction (CMD) refers to structural and functional abnormalities that occur in the coronary microvasculature. Several mechanisms are thought to be involved in the pathophysiology of CMD including inflammation, coronary vasospasm, and impaired vasodilation [29].
SARS-COV-2 infection has been shown to potentially lead to cardiovascular injury [34]. In fact, it has been linked to coronary disease, arrhythmias, heart failure, and hyper-coagulation [35-38]. Considering the high prevalence of ischemic heart disease in the world, many studies have focused on the cardiovascular involvement of SARS-COV-2 infection. Most recently, one study looked at 101 patients with clinically indicated cardiac PET scans who previously contracted COVID-19 (matched to 292 controls with no COVID-19 infection). A higher proportion of cases had a MFR < 2 (58% vs 28%; p < 0.001), which can indicate the presence of coronary microvascular dysfunction likely related to endothelial dysfunction [39]. Other studies performed on smaller sample sizes have been published since then and have shown similar results [40]. Therefore, it appears COVID-19 can lead to exacerbation of previously existing or development of new coronary microvascular disease, and PET imaging may play an important role in managing high-risk COVID-19 patients.
Heart Transplant
Cardiac allograft vasculopathy (CAV) remains one of the major limiting factors in the long-term success of cardiac transplantation [41]. Although invasive angiography with intravascular ultrasound is currently considered the gold standard for detecting CAV, it is quite costly and possesses an increased risk of complications. Angiography is also considered suboptimal when detecting non-obstructive and microvascular disease [42]. Therefore, cardiac PET has been investigated as a potential non-invasive alternative diagnostic modality. PET was shown to have significant correlation with invasive coronary flow indices (r = 0.28, relative flow reserve versus fractional flow reserve) [43]. Studies assessing diagnostic accuracy have found that PET derived MBF and MFR possess independent value in diagnosing CAV and predicting clinical outcomes such as adverse cardiovascular events or mortality in heart transplant patients [44-47]. A recent study by Clerkin et al. showed microvascular CAV (MFR ≤ 2.0 and no epicardial CAV detected by PET or angiography) to be independently associated with increased mortality or the need for re-transplantation [48].
Diabetic Patients
Diabetes is a complex metabolic disorder associated with an increased risk of cardiovascular disease, especially coronary artery disease [49]. Therefore, early detection of CAD is crucial for patient outcomes. PET MPI is currently considered as the optimal modality to diagnose patients with suspected CAD. However, evidence suggests that diabetic patients are more likely to first develop coronary microvascular dysfunction [50]. Therefore, PET-derived MBF and MFR may help in identifying such cases and further stratify them based on risk of future adverse events. One large single-center study with a total of 2783 consecutive patients assessed MFR and clinical outcomes in diabetic patients vs controls. Results showed that among patients with an impaired MFR (defined as MFR below the median), those with prior CAD had similar event rates to those without prior CAD. Therefore, detecting an impaired MFR was more predictive of adverse cardiovascular events than the presence or absence of known CAD, primarily due to the presence of microvascular dysfunction [51]. Similarly, among patients with a preserved MFR, there was no statistically significant difference in event rates between diabetics and non-diabetics. Another study on 902 patients also found that MFR < 2 was an independent predictor of cardiac events in diabetic patients [52]. However, contrary to the previous study, patients with diabetes and preserved MFR had similar cardiac event rates as those without diabetes and an impaired MFR. Further studies are needed to assess whether targeting early diabetic treatment that improves MFR would lead to improved clinical outcomes.
Target Population
From a clinician’s point of view, it is essential to know who are the patients that will benefit the most from a cardiac PET. Dilsizian et al. shed some clarity on this in the 2016 PET guidelines in the Journal of Nuclear Cardiology. PET utility is optimal for patients with no known history of heart disease that are presenting with symptoms suggestive of CAD. PET-MPI is also helpful in patients with known CAD and for which we would like to have a more thorough physiological assessment. Cardiac PET is also a useful tool for evaluation of patients with suspected multivessel disease or microvascular dysfunction (abnormal SPECT with normal angiography). In addition, cardiac PET plays an important role in diagnosing CAV in cardiac transplant patients. However, there is a subset of patients in which the use of MBF/MFR needs further evaluation. These include patients post coronary artery bypass graft (CABG), who might have a diffuse reduction in MBF despite patent grafts. Similarly, in patients with severe left ventricular dysfunction, severe advanced renal disease, or large transmural infarcts, the added value of MFR is not clear [48].
Limitation of Cardiac PET
Despite all the advantages it possesses, cardiac PET is not the most used imaging modality for assessing suspected ischemic heart disease. One reason for that is due to the limited number of available PET machines. In fact, there are only about 300 centers that offer cardiac PET in the USA. Another cause for limited PET use is its cost when compared to its counterparts.
Conclusion
Cardiac PET has become an invaluable tool in the diagnosis and management of ischemic heart disease. The complimentary information gained from relative myocardial perfusion paired with quantification of atherosclerotic burden enables accurate diagnosis at lower radiation dose as compared with SPECT. Furthermore, a unique feature of PET is its ability to quantify blood flow at the level of the coronary microvasculature. This translates into an improved diagnostic and prognostic accuracy in several unique clinical scenarios where other imaging modalities would fall short.
Declarations
Conflict of Interest
Dr. Mouaz Al-Mallah receives research support from Siemens unrelated to this study. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/cir.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves RA, Halpern EJ, Rao VM. Cardiac imaging trends from 2010 to 2019 in the Medicare population. Radiol Cardiothorac Imaging. 2021;3(5):e210156. doi: 10.1148/ryct.2021210156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2(4):343–358. doi: 10.1007/s40336-014-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin E, Alessio A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J Cardiovasc Comput Tomogr. 2009;3(6):403–408. doi: 10.1016/j.jcct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Tallawi KC, Aljizeeri A, Nabi F, Al-Mallah MH. Myocardial perfusion imaging using positron emission tomography. Methodist Debakey Cardiovasc J. 2020;16(2):114–121. doi: 10.14797/mdcj-16-2-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009;2(5):412–424. doi: 10.1161/circimaging.109.854893. [DOI] [PubMed] [Google Scholar]
- 8.Desiderio MC, Lundbye JB, Baker WL, Farrell MB, Jerome SD, Heller GV. Current status of patient radiation exposure of cardiac positron emission tomography and single-photon emission computed tomographic myocardial perfusion imaging. Circ Cardiovasc Imaging. 2018;11(12):e007565. doi: 10.1161/circimaging.118.007565. [DOI] [PubMed] [Google Scholar]
- 9.Driessen RS, van Diemen PA, Raijmakers PG, Knuuti J, Maaniitty T, Underwood SR, et al. Functional stress imaging to predict abnormal coronary fractional flow reserve: the PACIFIC 2 study. Eur Heart J. 2022;43(33):3118–3128. doi: 10.1093/eurheartj/ehac286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman TM, Heller GV, McGhie AI, Friedman JD, Case JA, Bryngelson JR, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Marwick TH, Go RT, MacIntyre WJ, Saha GB, Underwood DA. Myocardial perfusion imaging with positron emission tomography and single photon emission computed tomography: frequency and causes of disparate results. Eur Heart J. 1991;12(10):1064–1069. doi: 10.1093/oxfordjournals.eurheartj.a059838. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki N, Yonekura Y, Senda M, Yamashita K, Koide H, Saji H, et al. Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med. 1988;29(7):1181–1188. [PubMed] [Google Scholar]
- 13.Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, et al. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol. 2017;2(10):1100–1107. doi: 10.1001/jamacardio.2017.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziadi MC. Myocardial flow reserve (MFR) with positron emission tomography (PET)/computed tomography (CT): clinical impact in diagnosis and prognosis. Cardiovasc Diagn Ther. 2017;7(2):206–218. doi: 10.21037/cdt.2017.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidula MK, Wiener P, Selvaraj S, Khan MS, Salam UA, Rojulpote C, et al. Diagnostic accuracy of SPECT and PET myocardial perfusion imaging in patients with left bundle branch block or ventricular-paced rhythm. J Nucl Cardiol. 2021;28(3):981–988. doi: 10.1007/s12350-020-02398-5. [DOI] [PubMed] [Google Scholar]
- 16.Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2(7):846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziadi MC, Dekemp RA, Williams K, Guo A, Renaud JM, Chow BJ, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19(4):670–680. doi: 10.1007/s12350-011-9506-5. [DOI] [PubMed] [Google Scholar]
- 18.Geltman EM, Henes CG, Senneff MJ, Sobel BE, Bergmann SR. Increased myocardial perfusion at rest and diminished perfusion reserve in patients with angina and angiographically normal coronary arteries. J Am Coll Cardiol. 1990;16(3):586–595. doi: 10.1016/0735-1097(90)90347-r. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed AI, Al Rifai M, Alahdab F, Saad JM, Han Y, Alfawara MS, et al. Incremental prognostic value of digital positron emission tomography derived myocardial flow reserve: a prospective cohort study. Int J Cardiol. 2023;371:465–471. doi: 10.1016/j.ijcard.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Aljizeeri A, Ahmed AI, Alfaris MA, Ahmed D, Farea J, Elneama A, et al. Myocardial flow reserve and coronary calcification in prognosis of patients with suspected coronary artery disease. JACC Cardiovasc Imaging. 2021;14(12):2443–2452. doi: 10.1016/j.jcmg.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease: a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5(6):700–707. doi: 10.1161/circimaging.112.978270. [DOI] [PubMed] [Google Scholar]
- 22.Flotats A, Bravo PE, Fukushima K, Chaudhry MA, Merrill J, Bengel FM. Rb PET myocardial perfusion imaging is superior to mTc-labelled agent SPECT in patients with known or suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39(8):1233–9. doi: 10.1007/s00259-012-2140-x. [DOI] [PubMed] [Google Scholar]
- 23.Chow BJW, Dorbala S, Carli MFD, Merhige ME, Williams BA, Veledar E, et al. Prognostic value of PET myocardial perfusion imaging in obese patients. JACC Cardiovascular Imaging. 2014;7(3):278–87. doi: 10.1016/j.jcmg.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Harnett DT, Hazra S, Maze R, Mc Ardle BA, Alenazy A, Simard T, et al. Clinical performance of Rb-82 myocardial perfusion PET and Tc-99m-based SPECT in patients with extreme obesity. J Nucl Cardiol. 2019;26(1):275–283. doi: 10.1007/s12350-017-0855-6. [DOI] [PubMed] [Google Scholar]
- 25.Jang JJ, Bhapkar M, Coles A, Vemulapalli S, Fordyce CB, Lee KL, et al. Predictive model for high-risk coronary artery disease. Circ Cardiovasc Imaging. 2019;12(2):e007940. doi: 10.1161/circimaging.118.007940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55(2):248–255. doi: 10.2967/jnumed.113.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 28.Yokota S, Ottervanger JP, Mouden M, Timmer JR, Knollema S, Jager PL. Prevalence, location, and extent of significant coronary artery disease in patients with normal myocardial perfusion imaging. J Nucl Cardiol. 2014;21(2):284–290. doi: 10.1007/s12350-013-9837-5. [DOI] [PubMed] [Google Scholar]
- 29.Bravo PE, Di Carli MF, Dorbala S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev. 2017;22(4):455–464. doi: 10.1007/s10741-017-9628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patriki D, von Felten E, Bakula A, Giannopoulos AA, Kamani CH, Schwyzer M, et al. Splenic switch-off as a predictor for coronary adenosine response: validation against 13N-ammonia during co-injection myocardial perfusion imaging on a hybrid PET/CMR scanner. J Cardiovasc Magn Reson. 2021;23(1):3. doi: 10.1186/s12968-020-00696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad JM, Ahmed AI, Han Y, El Nihum LI, Alahdab F, Nabi F, et al. Splenic switch-off in regadenoson 82Rb-PET myocardial perfusion imaging: assessment of clinical utility. J Nucl Cardiol. 2023 doi: 10.1007/s12350-022-03158-3. [DOI] [PubMed] [Google Scholar]
- 32.Patel KK, Spertus JA, Chan PS, Sperry BW, Al Badarin F, Kennedy KF, et al. Myocardial blood flow reserve assessed by positron emission tomography myocardial perfusion imaging identifies patients with a survival benefit from early revascularization. Eur Heart J. 2020;41(6):759–768. doi: 10.1093/eurheartj/ehz389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BaireyMerz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135(11):1075–1092. doi: 10.1161/circulationaha.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol. 2022;19(5):332–341. doi: 10.1038/s41569-021-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Saguner AM, An J, Ning Y, Yan Y, Li G. Dysfunctional coagulation in COVID-19: from cell to bedside. Adv Ther. 2020;37(7):3033–3039. doi: 10.1007/s12325-020-01399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed AI, Saad JM, Han Y, Alahdab F, Malahfji M, Nabi F, et al. Coronary microvascular health in patients with prior COVID-19 infection. JACC Cardiovasc Imaging. 2022;15(12):2153–2155. doi: 10.1016/j.jcmg.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber B, Parks S, Huck DM, Kim A, Bay C, Brown JM, et al. Prior SARS-CoV-2 infection is associated with coronary vasomotor dysfunction as assessed by coronary flow reserve from cardiac positron emission tomography. J Am Heart Assoc. 2022;11(20):e025844. doi: 10.1161/jaha.122.025844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Waltz DA, Keck BM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult heart transplantation report–2006. J Heart Lung Transplant. 2006;25(8):869–879. doi: 10.1016/j.healun.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 43.Chih S, Chong AY, Erthal F, deKemp RA, Davies RA, Stadnick E, et al. PET Assessment of epicardial intimal disease and microvascular dysfunction in cardiac allograft vasculopathy. J Am Coll Cardiol. 2018;71(13):1444–1456. doi: 10.1016/j.jacc.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 44.Konerman MC, Lazarus JJ, Weinberg RL, Shah RV, Ghannam M, Hummel SL, et al. Reduced myocardial flow reserve by positron emission tomography predicts cardiovascular events after cardiac transplantation. Circ Heart Fail. 2018;11(6):e004473. doi: 10.1161/circheartfailure.117.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller RJH, Manabe O, Tamarappoo B, Hayes S, Friedman JD, Slomka PJ, et al. Comparative prognostic and diagnostic value of myocardial blood flow and myocardial flow reserve after cardiac transplantation. J Nucl Med. 2020;61(2):249–255. doi: 10.2967/jnumed.119.229625. [DOI] [PubMed] [Google Scholar]
- 46.Feher A, Srivastava A, Quail MA, Boutagy NE, Khanna P, Wilson L, et al. Serial assessment of coronary flow reserve by rubidium-82 positron emission tomography predicts mortality in heart transplant recipients. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):109–120. doi: 10.1016/j.jcmg.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajluni SC, Jr, Mously H, Chami T, Hajjari J, Stout A, Zacharias M, et al. Non-invasive imaging in the evaluation of cardiac allograft vasculopathy in heart transplantation: a systematic review. Curr Probl Cardiol. 2022;47(8):101103. doi: 10.1016/j.cpcardiol.2022.101103. [DOI] [PubMed] [Google Scholar]
- 48.Clerkin KJ, Topkara VK, Farr MA, Jain R, Colombo PC, Restaino S, et al. Noninvasive physiologic assessment of cardiac allograft vasculopathy is prognostic for post-transplant events. J Am Coll Cardiol. 2022;80(17):1617–1628. doi: 10.1016/j.jacc.2022.08.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poothullil JM. Diabetes and decline in heart disease mortality. JAMA. 1999;282(12):1132-. doi: 10–1001/pubs.JAMA-ISSN-0098–7484–282–12-jbk0922. [DOI] [PubMed]
- 50.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41(8):1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 51.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858–1868. doi: 10.1161/circulationaha.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assante R, Mainolfi CG, Zampella E, Gaudieri V, Nappi C, Mannarino T, et al. Relation between myocardial blood flow and cardiac events in diabetic patients with suspected coronary artery disease and normal myocardial perfusion imaging. J Nucl Cardiol. 2021;28(4):1222–1233. doi: 10.1007/s12350-021-02533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]