Abstract
Changes in functional brain connectivity (FBC) may indicate how lifestyle modifications can prevent the progression to dementia; FBC identifies areas that are spatially separate but temporally synchronized in their activation and is altered in those with mild cognitive impairment (MCI), a prodromal state between healthy cognitive aging and dementia. Participants with MCI were randomly assigned to one of five study arms. Three times per week for 20-weeks, participants performed 30-min of (control) cognitive training, followed by 60-min of (control) physical exercise. Additionally, a vitamin D3 (10,000 IU/pill) or a placebo capsule was ingested three times per week for 20-weeks. Using the CONN toolbox, we measured FBC change (Post-Pre) across four statistical models that collapsed for and/or included some or all study arms. We conducted Pearson correlations between FBC change and changes in physical and cognitive functioning. Our sample included 120 participants (mean age: 73.89 ± 6.50). Compared to the pure control, physical exercise (model one; p-False Discovery Rate (FDR) < 0.01 & < 0.05) with cognitive training (model two; p-FDR = < 0.001), and all three interventions combined (model four; p-FDR = < 0.01) demonstrated an increase in FBC between regions of the Default-Mode Network (i.e., hippocampus and angular gyrus). After controlling for false discovery rate, there were no significant correlations between change in connectivity and change in cognitive or physical function. Physical exercise alone appears to be as efficacious as combined interventional strategies in altering FBC, but implications for behavioral outcomes remain unclear.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00805-6.
Keywords: fMRI, Mild Cognitive Impairment, Intervention, Physical Exercise, Randomized Controlled Trial
Introduction
Brain health deteriorates with aging [1-4], but it occurs much more rapidly in those with mild cognitive impairment (MCI) [5, 6], a prodromal state between healthy cognitive aging and dementia syndromes, including Alzheimer's disease [7, 8]. Changes in brain function represent one of the earliest biomarkers in those at risk for dementia syndromes as they precede structural atrophy and occur years before clinical manifestation [9]. Functional magnetic resonance imaging (fMRI) provides one avenue to measure brain function via the blood oxygen level-dependent signal [10]. Subsequently, this permits the measurement of functional brain connectivity (FBC) or brain regions and, by extension, networks that are spatially separated but temporally synchronized in their activation [11]; FBC is believed to enable efficient information processing and the completion of complex functions [12]. Indeed, individuals with MCI show alterations (i.e., increases and decreases) in FBC beyond those of their normal aging counterparts [13-15].
The rising aging population and subsequent increase in absolute cases, as well as the ineffectiveness of pharmacological interventions, has created a fundamental shift to early identification and prevention as a treatment for those at risk of dementia syndromes [16]. Lifestyle modification involves altering long-term routines and might delay or prevent up to 40% of all dementias [17]. As a result, there has been a significant uptick in the number of intervention trials evaluating the efficacy of lifestyle modification in dementia-related outcomes. For example, a PubMed search using subject headings and title/abstract keywords for dementia and physical exercise indicates that 87 clinical trials were published in 2022.
Our recent systematic review found that within-network connectivity of the Default-Mode and Frontoparietal increased following an intervention that focused on or included physical exercise in older adults, regardless of cognitive status [18]. Two similar and recent reviews found that exercise may increase functional activation or connectivity within the Default-Mode Network [19] and strengthen connectivity between hemispheres [20]. A meta-analysis on the effects of cognitive training in aging also found an increase in connectivity within the Default-Mode, Frontoparietal, and Attention Networks, as well as specific regions of interest, such as the hippocampus [21]. No previous intervention has examined the effectiveness of vitamin D supplementation on an fMRI-related outcome. However, vitamin D deficiency is associated with reduced hippocampal volume and altered structural connectivity [22], as well as cortical thinning [23] and a higher risk of Alzheimer's disease [24]. Despite being an active area of research, vitamin D has been theorized to improve brain health by inhibiting the plaques associated with aging and Alzheimer’s disease, regulating neuronal calcium channels to prevent cell death, and modulating mitochondrial activity to reduce oxidative stress [25].
The purpose of this study was to evaluate the effect of combined physical exercise (PE) modalities (aerobic and resistance training) separately and synergistically with cognitive training (CT) and/or vitamin D (VD) supplementation on FBC in older adults with MCI. Despite recent criticism [26], which subsequently sparked multiple counter-opinion pieces [27-29], we also explored if a change in FBC correlated with a change in behavioral (physical and cognitive performance) outcomes. We hypothesized that: 1) compared to the control arm, the intervention strategies would show significant increases in within-network FBC; and 2) changes in FBC would be significantly correlated with changes in physical and cognitive performance.
Methods
Design & participants
The SYNERGIC (SYNchronizing Exercises, Remedies in GaIt and Cognition) trial [30] (NCT02808676: https://www.clinicaltrials.gov/ct2/show/NCT02808676) was a multi-site, randomized, phase II, fractional-factorial, double-blind controlled study evaluating the effect of combined PE with and without CT and/or VD supplementation in older adults (60 to 85 years) with MCI. All SYN sites were in Canada and included: Western University (London, ON; lead site), University of Waterloo (Waterloo, ON), Wilfrid Laurier University (Waterloo, ON), University of Montreal (Montreal, QC), and University of British Columbia (Vancouver, BC). Potential participants were diagnosed with MCI as per existing guidelines [31]. Other inclusion criteria included: proficiency in English or French (Montreal site), ability to ambulate at least 10 m independently, possessing (corrected) normal vision, being considered sufficiently healthy as per the Physical Activity Readiness Questionnaire-Plus (PAR-Q +) [32], and ability to comply with trial procedures.
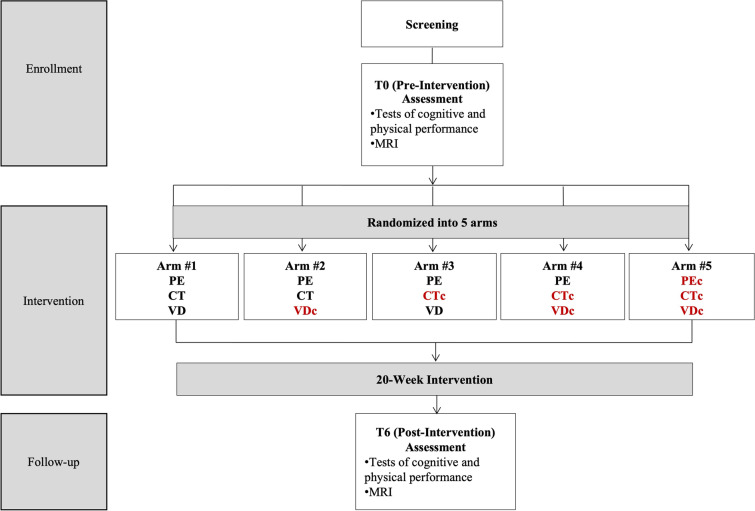
In addition to a clinical screening, SYN participants completed three in-person assessments, including pre-intervention (T0), post-intervention (T6), and follow-up (T12). T0 and T6 occurred immediately before and after a 20-week intervention, while T12 occurred 6-months after T6. All in-person assessments included collecting demographic information and a battery of neuropsychological, physical, and metabolic tests. Brain imaging was a secondary outcome of the SYN trial and was only conducted at T0 and T6; therefore, these are the only time points of interest for the present study (Fig. 1). The present study's inclusion and exclusion criteria were identical to the parent trial, except for the following two additions to the exclusion criteria: 1) Did not complete an MRI assessment at both T0 and T6; and 2) Participants consider their left hand to be dominant.
Fig. 1.
Overview of the timeline, assessment periods, and study arms. Red font helps to identify control arms. c = control; CT = cognitive training; PC = pure control; PE = physical exercise; SYN = synergic; VD = vitamin D
Randomization for SYN was generated centrally by a research pharmacist for each study site using a web-based randomization service (www.randomizer.org). Block randomization by five was applied to ensure an appropriate balance of participant characteristics. Permuted blocks were employed to ensure balance over time. After the T0 assessment, research personnel not involved in measuring outcomes or administering the intervention accessed the randomization list to determine arm allocation. Research personnel conducting assessments and/or analyzing data were blinded to arm allocation. Participants were blind to the active intervention and study hypotheses. The sponsor site obtained approval from the Research Ethics Board at Western University (REB# 107670), the Lawson Health Research Institute’s Clinical Research Impact Committee (R-15–038), and Health Canada (HC file—HC6–24-c195918 / HC protocol #201619) before initiating study-related activities. Each site also obtained local ethical approval. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Intervention
Overview
Regardless of the intervention arm, all participants completed group-training sessions three times per week for 20-weeks. Each training session included 30-min of CT or cognitive training control (CTc), followed by 60-min of combined PE or physical exercise control (PEc). To ensure a 1(trainer):4 ratio, no more than eight individuals participated in a single exercise session.
Active & control cognitive training
Participants performed CT and CTc on a tablet (iPad®). CT included two different visuomotor tasks that targeted working memory and attention. The CT program was custom-written, previously utilized for neurorehabilitation [33, 34], and individually tailored to the participant. In brief, the application provides individual tailoring via visual feedback bars that change colours (i.e., green, yellow, and red) according to performance in the two previous trials; participants are instructed to keep the bars in the green zone and prevent them from turning red. Additionally, at the end of a session, participants are informed of their mean reaction time and the accuracy achieved via histograms [35, 36]. CTc alternated between performing a pre-defined touristic search of a foreign city and watching a National Geographic video. Each session included a new city or video. The pre-defined touristic search required participants to find three hotels, tourist attractions, and restaurants. After watching the National Geographic video, the participant answered three questions about the video (Supplemental Material A).
Combined & control physical exercise
PE began with a 10-min warm-up on either a treadmill, stationary bike, or elliptical at a self-selected pace that would increase heart rate. Resistance training incorporated two lower (leg press and hamstring curl) and three upper body (chest press, seated row, and latissimus dorsi pull) exercises. For each session, participants began with an upper-body exercise of their choosing and then alternated between a lower- and upper-body exercise. Resistance training followed a pre-determined and standardized intensity, volume, and progression (Supplemental Material B). Within each session, trainers prescribed resistance training so that participants reached exhaustion at or near the last prescribed repetition of the final set of every exercise. For example, trainers increased the resistance training weight when it became “too easy,” as per a rating of perceived exertion of 8 or less [37, 38]. Following resistance training, participants performed 2 × 10-min of aerobic exercise on the same ergometers used to warm-up. Like resistance training, aerobic training intensity systematically increased throughout the program (Supplemental Material B). PEc included stretching, balance, and toning exercises that did not improve muscle strength or endurance nor progress in volume, intensity, and rest periods. To keep participants engaged, PEc exercises alternated every three weeks according to a pre-determined schedule.
Active & control vitamin D3
The VD intervention required participants to ingest one tablet of 10,000 IU of vitamin D3 three times per week for 20-weeks. Our previous work demonstrating safety and efficacy provided the rationale for the dosing strategy [39]. Vitamin D control (VDc) followed the same dosing timeline, but participants ingested a placebo pill that was visually identical to the VD capsule. At the intervention’s start, participants were given a four-week supply (i.e., 12 pills) of vitamin D3/placebo in a pill bottle. Participants returned their bottles every 5th week, and any remaining pills identified a day/pill missed. The number of days/pills missed was recorded, and then participants were given a new four-week supply. This process was repeated until the end of the intervention.
Overall, PE, CT, and VD interventions were combined within arm1 and represented the SYN group. Conversely, PEc, CTc, and VDc were combined within arm5 and represented the pure control (PC) group. In addition to PE, arms 2, 3, and 4 included CT and VDc, CTc and VD, and CTc and VDc, respectively (Fig. 1).
Primary outcome: FBC
Acquisition
We conducted imaging for Ontario, Montreal, and British Columbia at Robarts Research Institute, Centre de Recherche de l'Institut Universitaire de Gériatrie de Montréal, and UBC MRI Research Centre, respectively. Both Ontario and Montreal sites utilized a Siemens Magnetom Prisma Fit 3 Tesla MRI scanner (Siemens AG, Munich, Germany). The British Columbia site conducted imaging on a Philips Achieva 3 Tesla MRI Scanner (Koninklijke Philips N.V., Amsterdam, The Netherlands). MRIs followed version 3.8 of the Canadian Dementia Imaging Protocol, a standardized protocol for multicentric research on dementia linked to neurodegeneration in aging, harmonized on all three major vendor platforms [40]. Imaging included a variety of modalities with a total scan time of 1.3 h, but for the present study, we utilized only T1W (Siemens: 20-channel head coil; sequence: MPRAGE; acceleration factor: 2; TR/TE: 2300/2.98 ms; flip angle: 9°; slice thickness: 1.0 mm without gap; acquisition matrix: 256 × 256 mm; resolution: 1.0 × 1.0 mm; bandwidth: 240 Hz. Phillips: 8-channel Sense head coil; sequence: MPRAGE; acceleration factor: 2; TR/TE: 7.3/3.3 ms; flip angle: 9°; slice thickness: 1.0 mm without gap; acquisition matrix: 248(AP) × 256 (FH) mm; resolution: 1.0 × 1.0 mm; bandwidth: 228.6 Hz) and resting state-fMRI (Siemens: 20-channel head coil; sequence: EPI BOLD; acceleration factor: 2; TR/TE: 2130/30 ms; flip angle: 70°; slice thickness: 3.5 mm without gap; acquisition matrix: 64 × 64 mm; volumes: 250; resolution: 3.5 × 3.5 mm; bandwidth: 2442 Hz; eyes: open. Phillips:8-channel Sense head coil; EPI (Fast Field Echo); acceleration factor: 2; TR/TE: 2110/30 ms; flip angle: 70°; slice thickness: 3.5 mm without gap; acquisition matrix: 64 × 64 mm; volumes: 250; resolution: 3.5 × 3.5 mm; bandwidth: 2371.7 Hz; eyes: open).
Preprocessing
We visually inspected all raw data before using in-house tools [41-43] to organize files according to the brain imaging data structure [44] and then preprocessed them using fMRIPrep (version 20.2.0) [45] (Supplemental Material C). Post-fMRIPrep, the data was skull-stripped using FMRIB Software Library (version 6.0.4) [46] Brain Extraction Tool [47] and then uploaded to the CONN Functional Connectivity Toolbox (version 20.b) [48]. CONN is an open-source MATLAB (version R2020b) and Statistical Parametric Mapping (version SPM12) based cross-platform software. As per recommendations [49] and as a final preprocessing step, an 8 mm FWHM Gaussian kernel smoothed the functional volumes. Similar to previous publications using CONN [50], denoising regressed out signal contributions of white matter (five parameters) and cerebrospinal fluid (five parameters), as well as motion realignment parameters and their first-order derivatives (12 parameters). Intermediate (0.5 mm, three sd) ART-based scrubbing detected and removed outlier volumes. Additional denoising steps included linear detrending and band-pass filtering (0.008 Hz to 0.09 Hz) after regression (RegBP) [50]. CONN's quality assurance plots [49], including variables related to motion, global signal change, and valid scans, should score > 95%; after completing the original denoising, the quality assurance plots achieved this 95% goal. Therefore, no further preprocessing was completed.
Region of interest-to-region of interest analysis
A bivariate correlations coefficient (Fisher's transformed) with a hemodynamic response function weighting calculated a functional connectivity map between each region of interest. We included the following networks and their respective regions of interest because of their inclusion in a prior publication [51], susceptibility to aging [52, 53], and relation to behavioral outcomes: Default-Mode, Dorsal Attention, Salience, Frontoparietal, and Sensorimotor (Supplemental Material D). The CONN Toolbox automatically includes the selected networks and their corresponding regions of interest based upon a CONN Independent Component Analysis of 497 subjects from the Human Connectome Project [54] (Supplemental Material E).
Seed-to-voxel (Whole Brain) analysis
Other researchers have conducted multiple FBC analyses within the same study. Therefore, we also conducted a seed-to-voxel analysis [55, 56]. We selected the left and right hippocampus as seeds because of their susceptibility to dementia-related syndromes and usage in similar studies [57, 58]. The seed-to-voxel analysis was identical to the region of interest-to-region of interest analysis, except a functional connectivity map was generated for the seed(s) and every other voxel in the brain (Supplemental Material E). Notably, although resting state functional connectivity was identified in the trial registration (NCT02808676) and protocol paper [30], the region of interest and seed-to-voxel analyses were not detailed. Such analyses should, therefore, not be considered apriori.
Secondary outcome: FBC and cognitive/physical function
To determine the behavioral or clinical implications of FBC change, we conducted a correlation analysis between change in FBC and change in seven cognitive and physical performance outcomes.
The seven behavioral outcomes represented a broad range of functions. Alzheimer's Disease Assessment Scale-Cog 13 [59], a global measure of cognition, was the SYN's primary outcome. We used the number of items missed on delayed recall of the Alzheimer's Disease Assessment Scale-Cog 13 [59] and trail-making test normalized ((B-A)/A) [60] to evaluate memory and executive function, respectively. Importantly, trail-making test was used more than any other cognitive test in previous studies assessing brain connectivity and behavioral outcomes [18]. The physical performance outcomes included grip strength, as it is a commonly used clinical assessment of strength [61], as well as sit-to-stand time (lower body power) and usual gait speed because of their sensitivity to aging [62, 63]. We included muscle strength and power measures because no previous study has examined their relationship with fMRI data, despite a call to do so [64]. Importantly, cardiovascular fitness (i.e., six-minute walk distance) was used more than any other physical performance test in previous studies assessing brain connectivity and behavioral outcomes [18].
All cognitive assessments and the six-minute walk test followed standardized instructions [65]. As previously described in another publication [39], we assessed maximum grip strength and usual gait speed via three attempts, with the average used for data analysis. Sit-to-stand testing followed instructions from the short physical performance battery protocol [66]. For a complete description of all cognitive and physical performance outcomes included in the SYNERGIC trial, the interested reader is encouraged to see the protocol paper [30].
Statistical analyses
Pre-intervention (T0) characteristics
Except for sex reported as sample size, demographics and clinical characteristics for each study arm are summarized using means and standard deviations. To identify differences in characteristics regarding MRI status and, thus, provide insight into who or why specific individuals chose to forgo MRIs, we also compared T0 characteristics of participants that completed: 1) both T0 and T6 imaging; 2) only T0 imaging; and 3) no imaging. A one-way analysis of variance (ANOVA) or Pearson chi-square assessed differences between arms/groups for all participant characteristics.
Models of analyses
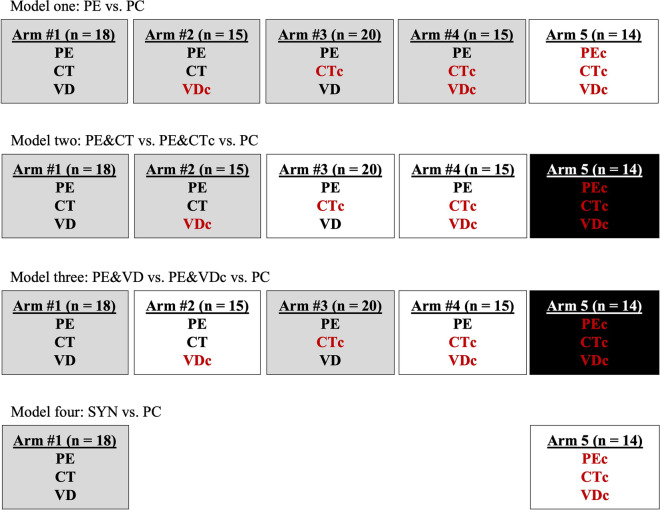
Within the present study, we conducted four separate models of analyses by pooling the study arms, as per our protocol [30], as follows:
Model one (effects of PE) = PE (arm1 + 2 + 3 + 4) vs. PC (arm5)
Model two (effect of CT and PE) = PE & CT (arm1 + 2) vs. PE & CTc (arm3 + 4) vs. PC (arm5)
Model three (effect of VD and PE) = PE & VD (arm1 + 3) vs. PE & VDc (arm2 + 4) vs. PC (arm5)
Model four (full synergistic effect): PE & CT & VD (SYN; arm1) vs. PEc & CTc & VDc (PC; arm5)
These comparisons align with the recommended analysis and reporting of factorial trials and analyses for different comparisons [67]. The pooling of arms in pre-specified combinations is used to examine potential synergism. Ultimately, such analyses allowed us to first explore the efficacy of combined (aerobic and resistance training) PE on FBC; to our knowledge, no previous randomized controlled trial has explored this in a demographic living with MCI. Subsequent analyses (models 2–4) aimed to determine the value of adding other lifestyle intervention strategies (CT and/or VD) to PE. See Fig. 2 for an aerial view of the statistical model.
Fig. 2.
Overview of the four models used for statistical analyses. Shading (white, grey, and black) identifies arms that are collapsed within each model. Red font helps to identify control arms. c = control; CT = cognitive training; PC = pure control; PE = physical exercise; SYN = synergic; VD = vitamin D. Post-hoc testing in model two: A) Collapsed Arms3-4 vs Arm5; B) Collapsed Arms1-2 vs Arm5; C) Collapsed Arms1-2 vs Collapsed Arms3-4. No post-hoc test in model 3 as comparison of all three groups identified no significant differences
FBC
To determine between-arm differences at T0 (main effect), we conducted a t-test in models one and four and a one-way ANOVA in models two and three. We analyzed average change (T6-T0 or Post-pre) in FBC using a 2 × 2 mixed ANOVA in models one and four and a 3 × 2 mixed ANOVA in models two and three. If model two or three identified a significant difference between the three study arms, we conducted post-hoc testing via a 2 × 2 mixed ANOVA [49].
Covariates & false discovery rate
We adjusted all FBC analyses for the covariates of age, sex, self-reported years of education, and self-reported number of comorbidities. Change in FBC was considered statistically significant based upon standard settings for cluster-based inferences of region of interest-to-region of interest (cluster threshold: p < 0.05 cluster-level p-false discovery rate (FDR) corrected; connection threshold: p < 0.05 p-uncorrected) [68], and seed-to-voxel analyses (cluster threshold: p < 0.05 cluster-size p-FDR corrected; voxel threshold: p < 0.001 p-uncorrected) [69].
Pearson’s correlations
Using the Statistical Package for the Social Sciences (version 27; IBM Canada Ltd. Markham, Ontario), change score (T6-T0) was calculated for all physical and cognitive performance outcomes and then converted to a z-score of standardized residuals via linear regression to control for the same covariates used in the FBC analysis (age, sex, number of comorbidities, and years of education). Prior to assessing correlations between connectivity change (T6-T0) scores and behavioural change scores, we confirmed the required assumptions (i.e., linearity, outliers, and normality) were satisfied within all models. Linearity was assessed via visual inspections, normality was assessed via a Shapiro–Wilk test, and data points three times the interquartile range (i.e., extreme outliers) were removed. Pearson's correlation is robust to deviations from normality but is susceptible to outliers; this provided the rationale for excluding extreme outliers. Correlation analyses aimed to determine the relationship between FBC change (T6-T0) and physical and cognitive performance change (T6-T0) in each model's intervention and control arms. In alignment with our previous systematic review [18], we restricted correlation to only FBC connections that survived the most conservative standards (correcting for FDR and adjusting for all covariates). Correlations were two-tailed, and a p ≤ 0.05 controlling for FDR (Supplemental Material F) indicated statistical significance.
Data availability
The data supporting this study’s findings are available from the corresponding authors upon reasonable request.
Results
Demographic information
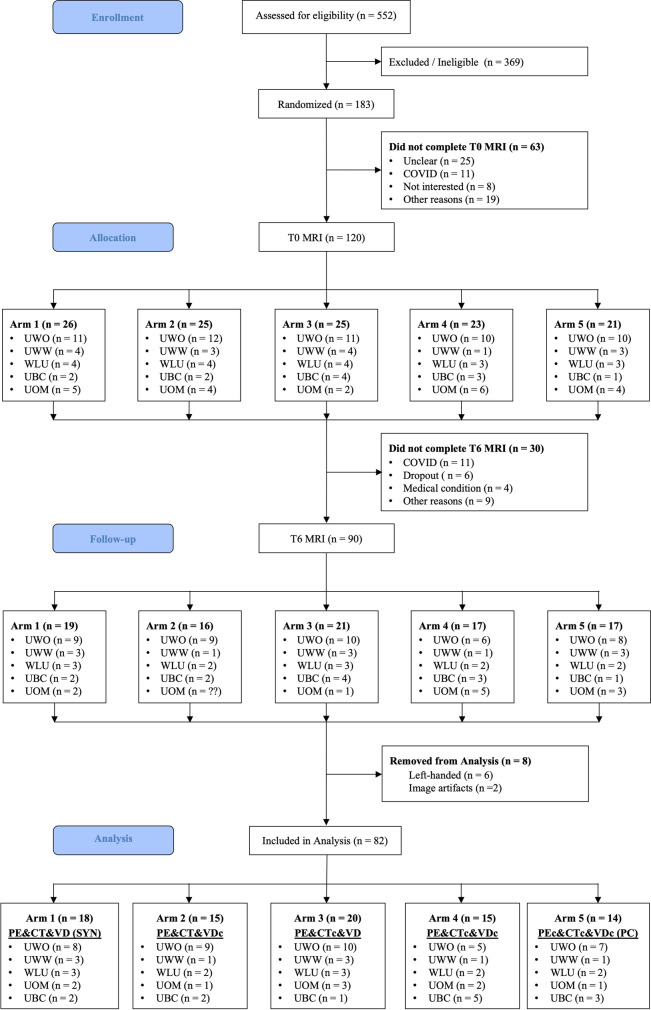
SYN recruited potential participants from the community and clinics serving MCI populations from September 2016 – March 2020; the trial was terminated early due to the COVID-19 pandemic. 183 participants were randomized into the five study arms. 120 and 90 participants completed T0 and T6 MRI, respectively, but eight were excluded (left-handedness, n = 6; and image artifacts, n = 2) (Fig. 3) from the following arms: arm1 (SYN), n = 1; arm2 (PE&CT&VDc), n = 1; arm3 (PE&CTc&VD), n = 1; arm4 (PE&CTc&VDc), n = 2; and arm5 (PC), n = 3. Therefore, our final analysis included 82 participants. Participants abstained from T0 MRI for various reasons (i.e., not interested, claustrophobia, etc.), and most participants missed their T6 MRI due to restrictions surrounding the COVID-19 pandemic. The average days between T0 MRI and start of the intervention, and end of the intervention and T6 MRI were 27 and 22, respectively.
Fig. 3.
Study flowchart for participants included in imaging analysis, stratified by arms. c = control; CT = cognitive training; PC = pure control; PE = physical exercise; SYN = synergic; VD = vitamin D. UWO = University of Western Ontario; UWW = University of Waterloo; WLU = Wilfrid Laurier University; UOM = University of Montreal; UBC = University of British Columbia
Despite being a randomized controlled trial, there was a significant between-arm difference in sex distribution and height (cm) at T0 (Table 1) for arm4 and arm5. However, there were no significant between-group differences for those that completed imaging versus those that completed only T0 imaging or no imaging (Supplemental Material G).
Table 1.
T0 (Pre-intervention) characteristics for participants included in imaging analysis, stratified by SYNERGIC arms
| Characteristic | Total (n = 90) |
Arm 1 PE&CT&VD (SYN) (n = 19) |
Arm 2 PE&CT&VDc (n = 16) |
Arm 3 PE&CTc&VD (n = 21) |
Arm 4 PE&CTc&VDc (n = 17) |
Arm 5 PEc&CTc&VDc (PC) (n = 17) |
p-value |
|---|---|---|---|---|---|---|---|
| Age | 73.89 ± 6.50 | 73.68 ± 6.86 | 73.25 ± 7.39 | 75.90 ± 7.55 | 72.12 ± 4.15 | 74.00 ± 5.77 | 0.491 |
| # of Males (Females) | 47 (43) | 9 (10) | 11 (5) | 11 (10) | 12 (5) | 4 (13) | 0.043* |
| # of Comorbidities | 4.77 ± 2.46 | 5.21 ± 2.62 | 5.00 ± 2.10 | 4.90 ± 3.03 | 4.18 ± 2.51 | 4.47 ± 1.84 | 0.735 |
| Years of Education | 15.35 ± 3.66 | 14.21 ± 2.76 | 15.75 ± 3.28 | 14.81 ± 2.98 | 15.38 ± 2.98 | 16.88 ± 5.60 | 0.244 |
| Height (cm) | 167.61 ± 10.03 | 169.33 ± 10.04 | 168.13 ± 10.68 | 168.68 ± 7.73 | 170.74 ± 10.84 | 160.74 ± 9.02 | 0.029* |
| Weight (kg) | 76.39 ± 14.61 | 76.79 ± 16.55 | 77.89 ± 12.52 | 77.08 ± 12.67 | 79.77 ± 15.78 | 70.31 ± 15.11 | 0.401 |
| Body Mass Index | 27.13 ± 4.39 | 26.66 ± 4.59 | 27.62 ± 4.11 | 27.04 ± 3.75 | 27.29 ± 4.58 | 27.17 ± 5.33 | 0.979 |
| MoCA | 22.94 ± 2.96 | 23.68 ± 4.03 | 23.13 ± 2.45 | 22.90 ± 3.05 | 22.82 ± 1.81 | 22.12 ± 2.91 | 0.634 |
| Vitamin D | |||||||
| Serum Levels (nmol/L) | 81.95 ± 25.14a | 86.92 ± 18.47b | 85.23 ± 16.42c | 82.87 ± 26.78d | 80.36 ± 35.12e | 74.00 ± 29.03f | 0.731 |
| Deficient (≤ 75 nmol/L) | 41.54%a | 30.77%b | 38.46%c | 40.00%d | 45.45%e | 53.85%f | 0.815 |
All values are mean ± standard deviation, except sex shows the sample size. ANOVA or Pearson chi-square analysis as appropriate. MoCA Montreal Cognitive Assessment; # number; cm centimeters; kg kilograms. c control; CT cognitive training; PC pure control; PE physical exercise; nmol/L nanomoles per litre; SYN synergic; VD vitamin D; * p-value < 0.05. The threshold for deficient vitamin D levels was based on our previous work [39]. Reasons for not completing blood withdrawal included the phlebotomist's inability to find an adequate vein or failure to draw blood after two attempts and the participant declining the blood withdrawal portion of the study; therefore, the sample size for serum levels is less than the sample for every other variable: a = 65; b = 13; c = 13; d = 15; e = 11; f = 13
FBC region of interest-to-region of interest
There was a significant between-arm difference in T0 Salience Network connectivity in model four (SYN vs. PC). However, this difference no longer existed at T6 (Supplemental Material H). There were no significant between-arm changes (T6-T0) in FBC in models one (PE vs. PC), two (PE&CT vs. PE&CTc vs. PC), or three (PE&VD vs. PE&VDc vs. PC). The intervention arm demonstrated a significant between-arm increase (T6-T0) in connectivity for a single cluster in model four (SYN vs. PC), (F(2,27) = 6.57; p-FDR < 0.05; Supplemental Material I). However, the cluster only survived controlling for years of education and sex.
FBC seed-to-voxel (Seed = Right hippocampus)
There were no between-arm differences at T0. Intervention arms demonstrated a significant between-arm increase (T6-T0) in model one (PE vs. PC; Cluster: size = 376 & 215 p-FDR < 0.01 & < 0.05), two (PE&CT vs. PE&CTc vs. PC; Cluster: size = 535 p-FDR = < 0.001), and four (SYN vs. PC; Cluster: size = 381 p-FDR = < 0.01). In all models, the cluster included the left superior division of the lateral occipital cortex and left angular gyrus. For model two, post-hoc tests showed that the significant between-arm increases (T6-T0) were for both intervention arms relative to the PC (PE&CT; Cluster: size = 265 p-FDR < 0.05 / PE&CTc; Cluster: size = 334 p-FDR < 0.01). All connections maintained significance after controlling for all covariates (Table 2).
Table 2.
Clusters with a significant increase (T6-T0) in connectivity with the Hippocampus
| Model | Cluster | size p-FDR | peak p-unc | Anatomical area | Covering | ||
|---|---|---|---|---|---|---|---|
| x, y, z | Size | % | Voxels | ||||
| 1 (PE vs. PC) | -50, -64, 36 | 376 | 0.008716 | 0.000004 | Lateral Occipital Cortex, superior division Left | 60 | 225 |
| Angular Gyrus Left | 38 | 141 | |||||
| Not Labelled | 3 | 10 | |||||
| -30, -70, 22 | 215 | 0.046003 | 0.000003 | Lateral Occipital Cortex, superior division Left | 33 | 71 | |
| Angular Gyrus Left | 4 | 8 | |||||
| Not Labelled | 63 | 136 | |||||
| 2 (PE&CT vs. PE&CTc vs. PC) | -50, -64, 32 | 535 | 0.0003 | 0.000008 | Lateral Occipital Cortex, superior division Left | 57 | 303 |
| Angular Gyrus Left | 16 | 87 | |||||
| Not Labelled | 27 | 145 | |||||
|
Post-hoc for 2 (PE&CTc vs. PC) |
-50, -64, 44 | 265 | 0.035973 | 0.000023 | Lateral Occipital Cortex, superior division Left | 60 | 158 |
| Angular Gyrus Left | 37 | 98 | |||||
| Not Labelled | 3 | 9 | |||||
|
Post-hoc for 2 (PE&CT vs. PC) |
-30, -72, 20 | 334 | 0.003614 | 0.000002 | Lateral Occipital Cortex, superior division Left | 53 | 177 |
| Angular Gyrus Left | 7 | 25 | |||||
| Not Labelled | 40 | 132 | |||||
| 4 (SYN vs. PC) | -38, -60, 32 | 381 | 0.001073 | 0.000007 | Lateral Occipital Cortex, superior division Left | 51 | 194 |
| Angular Gyrus Left | 10 | 38 | |||||
| Not Labelled | 39 | 149 | |||||
| 4L (SYN vs. PC) | -46, -66, 42 | 297 | 0.00562 | 0.000012 | Lateral Occipital Cortex, superior division Left | 84 | 248 |
| Not Labelled | 16 | 49 | |||||
All from the seed-voxel analysis. With the exception of model 4L, the significant clusters within each model demonstrated increased connectivity with the right Hippocampus. c control; CT cognitive training; PC pure control; PE physical exercise; SYN synergic; VD vitamin D; vs. versus
FBC seed-to-voxel (Seed = Left hippocampus)
There was a significant between-arm difference in connectivity with the left inferior frontal and precentral gyrus at T0 in model four (SYN vs. PC). However, this difference no longer existed at T6 (Supplemental Material H). The intervention arm demonstrated a significant between-arm increase (T6-T0) in only model four (SYN vs. PC; Cluster: size = 297 p-FDR < 0.01). The cluster included the left superior division of the lateral occipital cortex. The connection maintained significance after controlling for all covariates (Table 2).
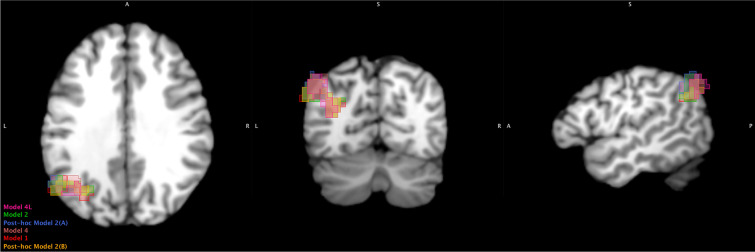
Despite slight differences in the coordinates of clusters that demonstrated a significant change in connectivity with the seed(s), they displayed general overlap when reviewed in Multi-image Analysis GUI (MANGO; version 4.1) (Fig. 4). Given the field’s inconsistency in anatomical labelling of the brain [70] and because the seed-to-voxel clusters included two different but significant anatomical regions, we used xjView (version 9.7) [71] to review anatomical labelling. In brief, only the left angular gyrus possessed a high number (~ 40–70%) of active voxels in all clusters and across all models.
Fig. 4.
Overlay of all clusters (see Table 2) that showed a significant increase (T6-T0) with the Hippocampus. Clusters are from the seed-voxel analysis. Clusters extend outside the brain but this is due to the combination of slightly different coordinates for each cluster being overlayed onto a single image. Created using Multi-image Analysis GUI (MANGO) version 4.1. Left to right = axial, coronal, and sagittal view. Slice coordinates (x, y, z) = -49, -65, 31. A = anterior; L = left; P = posterior; R = right; S = superior
Correlation
We restricted our Pearson’s correlation of change (T6-T0) scores to only the seed-to-voxel analyses because it survived controlling for all covariates. Specific to sit-to-stand time, we removed four participants from the analysis who could not perform the task at either T0 or T6. We also removed outliers from the Alzheimer’s Disease Assessment Scale-Cog overall (n = 1), grip strength (n = 1), and six-minute walk test (n = 4). Supplemental Material J includes average scores for all cognitive and physical performance outcomes at T0, T6, and T6-T0.
Only connectivity change (T6-T0) in model two (PE&CT x PE&CTc x PC) demonstrated statistically significant correlations at p < 0.05. PE&CTc cluster connectivity change showed a small-moderate negative correlation with usual gait speed change (r(30) = -0.365, p = 0.04); this means that an increase in connectivity correlated with a decrease (decline) in gait speed. However, this correlation did not survive correction for FDR. Other correlations across multiple models were trending towards but ultimately failed to reach a p < 0.05 (Supplemental Material K). For example, sit-to-stand time change for PE of model one (PE vs. PC) showed a small-moderate negative correlation with cluster connectivity change (r(59) = -0.226 p = 0.08); this means that connectivity increased as sit-to-stand time decreased (improved).
Discussion
We investigated the effect of PE with and without CT and/or VD supplementation on FBC, as assessed by fMRI, in older adults with MCI. In support of our hypothesis, we found significant increases in FBC between the hippocampus and angular gyrus in interventions that included PE (model one) with CT (model two) and VD (model four). Contrary to our hypothesis, there was no significant correlation between FBC change (T6-T0) and change in behavioral outcomes of physical and cognitive performance after controlling for FDR.
Our results suggest that CT with and without VD may provide some additional increases or alterations in FBC, but based on its involvement in all models and the general overlap of all significant clusters, PE appears to be primarily responsible for the observed changes post-intervention. Such results suggest that combined PE executed at sufficient length and intensity may be as efficacious as multimodal interventions at improving brain function in those living with MCI. Indeed, type, length, and intensity are essential parameters in the design of an exercise prescription and vary based on the overarching goals of the program. The lack of significant change in FBC when we added just VD to PE (model three) inevitably brings into question the efficacy of VD. VD is an essential nutrient, and work by our group [39], as well as another [72], has suggested that high doses provide benefits to physical but not cognitive performance in those with the most significant deficits (i.e., physically frail and/or insufficient vitamin D serum levels). Therefore, the lack of findings in model three may reflect our failure to account for these variables; ~ 40% of our participants were identified as having insufficient (≤ 75 nmol/L) vitamin D serum levels at T0 [39].
Research has suggested a linkage between the hippocampus and angular gyrus [73, 74]. Although not included in CONN's predetermined regions of interest, previous research indicates that both the hippocampus and angular gyrus are part of the Default-Mode Network [75, 76], albeit with some evidence of lateralization [77, 78]. The hippocampus and angular gyrus also play a role in the "Core" [78] and "Core Recollection Network" [79], which overlap but differ from the Default-Mode [80]. The Default-Mode Network is one of the first networks to demonstrate alterations (decrease in within-network connectivity) in those at risk of Alzheimer's disease [81]. Anatomically, the Default-Mode Network overlaps with regions that accumulate the highest degree of Alzheimer's pathology [82, 83]. Therefore, our intervention-induced increases in hippocampus to angular gyrus connectivity may help counter some of the initial changes in FBC for those at risk of dementia syndromes and, in doing so, reflect a return to brain function that may be considered more typical of biological or healthy aging.
As part of the Default-Mode Network, the hippocampus and angular gyrus play a role in self-referential thought, such as remembering the past and planning for the future [84]. Hippocampus to angular gyrus connectivity may also be crucial to spatial navigation or knowing where you are and how to get to places [85, 86]. Despite not reaching significance once corrected for FDR, our findings showed that change in usual gait speed and sit-to-stand time held small-moderate effect sizes with a change in hippocampus to angular gyrus connectivity. Therefore, such changes in connectivity may influence the completion of physical performance or everyday functional tasks like walking and sitting. Notably, the changes observed in gait speed and sit-to-stand time and their relationship with connectivity were conflicting, as the former was associated with decreasing performance, while the latter was associated with improvement. Research must continue to examine the (longitudinal) relationship between FBC and behavioral outcomes, as it will help delineate increases and decreases in connectivity. Relative to sit-to-stand time, future research should address if increases in hippocampus to angular gyrus connectivity impact alterations in spatial navigation or the ability to exert muscle power, for which sit-to-stand time is considered a proxy measure. Such information could have important implications for interventional strategies.
The present study supports our recent systematic review [18] and others [19, 21] showing that RCTs can increase within-network FBC, regardless of older adults' cognitive status. Our systematic review included five studies on older adults with MCI, three [57, 58, 87] of which included the hippocampus as a seed. Except for one, the hippocampus demonstrated an increase in connectivity to the posterior cingulate cortex, middle frontal cortex, and like the present study, angular gyrus; two of these three studies also identified their anatomical areas as belonging to the Default-Mode Network. The remaining two studies in our recent systematic review [18] did not conduct an independent statistical analysis of FBC [88] or focused on the Frontoparietal Network [89]. Similar to our findings, no previous studies demonstrated a significant correlation between FBC change and change in physical or cognitive performance in just the intervention group after controlling for covariates and/or correcting for FDR. Therefore, and as previously stated, implications for behavioral outcomes at this current time are unclear. The effect sizes are interesting and, at the very least, warrant further examination in future work with an adequately powered sample size.
Changes in FBC are believed to reflect a cascade of physiological changes [90]. Potential downstream effects on behavioral outcomes are likely to be moderated by various factors. For example, individuals with higher (worse) frailty status demonstrate more significant clinical impairment despite lower Alzheimer's pathology [91]. Further, frailty may explain why some individuals progress from MCI to Alzheimer’s while others remain stable or revert to cognitively healthy [92]. Ultimately, how exactly interventions alter FBC and the resulting change, if any, in behavioral outcomes is complex given the variety of biological, cellular, genetic, and lifestyle factors involved, further complicated by their interaction with neurodegeneration. Given that fMRI is a proxy measure of neural activation, one particularly insightful avenue for exploration would be determining if post-exercise changes in connectivity reflect alterations in neural function, vasculature, or some combination of the two. It is tempting to suggest that behavioural changes, particularly cognition, reflect neural alterations, but modifications in blood flow have also been associated with improved cognitive performance [93]. Combining fMRI with more direct measures of the vasculature, such as arterial spin labelling or transcranial Doppler ultrasound, as well as more direct measures of neural activity, such as electroencephalography, would help delineate neurovascular changes post-intervention. Further, it would provide a deeper understanding of the underlying physiology and, therefore, create more targeted and effective therapies.
The current study is the first randomized controlled trial to examine the effect of combined PE, with and without CT, and/or VD supplementation in older adults with MCI, but it is not without limitation. Only our seed-to-voxel analysis showed a significant change in connectivity and none of our seven behavioral outcomes correlated with a change in connectivity after correcting for FDR. Therefore, most of our analyses demonstrated no statistically significant results. Furthermore, the present study includes just two (region of interest-to-region of interest and seed-to-voxel) of the many analyses available; network, graph, dynamic connectivity, and low-frequency fluctuations are all alternative measures of functional connectivity that go beyond and/or provide additional insight than just the strength of a connection. To this end, previous work has demonstrated that researchers may take a different approach to answer identical questions [94]. Statistical models (1–4) reflect a fractional-factorial design, replicating the parent trial's primary statistical approach [30] and following recommended guidelines [67]. Such an approach helps increase statistical power but does not account for a potential interaction between different interventions within collapsed arms. For example, Model 3 collapsed arm1 and arm3 to create a PE and VD group. However, arm1 received CT, whereas arm3 received CTc. Theoretically, individuals that received CTc neutralize those that received CT. If we were to create a true PE and VD group, then we would utilize just arm3, but this would reduce the sample size by half. Ultimately, both approaches have their positives and drawbacks. We did not include site, scanner, body mass index, etc., as covariates, but similar to the selection of MRI preprocessing methods [95], covariate selection in neuroimaging is an active area of research [96]. We did not include a comparator group experiencing biological or healthy aging, which would likely enhance the interpretation of the present results. Specifically, it would help clarify if the observed improvements in connectivity reflect restoration or normalization to brain function experienced during healthy aging. Although a multi-site trial, we only included participants from central and western Canada. The Canadian Atlantic provinces hold some of the highest obesity rates [97], likely reflecting an alternative lifestyle. As such, our sample should not be considered indicative of all Canadians. As with all exercise interventions and despite excluding individuals that participated in a structured exercise program for the six months before their T0 assessment, we cannot rule out that our current sample reflects those with a high affinity for exercise. Given Canada's low rates of physical activity and exercise, our participants should be considered atypical [98]. Finally, and given the already small (~ 20) number of participants per study arm, we did not conduct a separate analysis for sex, gender, race, and/or ethnicity.
In addition to the previous suggestions, future research in FBC must be adequately powered to detect true post-exercise intervention differences, despite MRI being described as the least enjoyable component of tested outcomes [99]. Alternative techniques, such as electroencephalography, are a portable, cost-effective measure of FBC but lack the spatial resolution of fMRI. Ultimately, each neuroimaging tool possesses positives and drawbacks. Future work should also consider vitamin D serum levels pre-intervention as it could provide additional insight; for the present study, it may help explain why vitamin D (model 3) was the only model not to impose a significant change on FBC. Researchers should also examine factors that moderate the impact of PE on FBC. Given recent work, frailty status [91, 92, 100] and vascular properties (i.e., flow, reserve, etc.) [101, 102] represent fruitful avenues. PE is a therapeutic intervention with a multitude of benefits or “sledgehammer effect” [103, 104]. Still, detailed reporting of exercise parameters (i.e., frequency, intensity, time, type, etc.) [105] is sometimes inadequate [18, 106] despite being critical to replication and clinical uptake. Similarly, future work must compare different exercise parameters if we are to identify an optimal approach. For example, machine versus free-weight resistance training, specific exercise selection, and different intensity ranges (i.e., high-intensity aerobic interventions may be more beneficial [107, 108] and equally or more enjoyable than low-intensity [109, 110]).
The present study examined the impact of combined PE separately and synergistically with CT and/or VD on FBC, assessed via fMRI, in older adults with MCI. In support of our hypothesis, we demonstrated that PE increased FBC between the hippocampus and the angular gyrus, representing regions of the Default-Mode Network. CT with and without VD appeared to add very little to PE-induced FBC. No intervention or control arms demonstrated a significant correlation between FBC change and change in behavioral outcomes of physical and cognitive performance after controlling for FDR, but some effect sizes were in the small-moderate range. These findings support previous research suggesting that PE is efficacious in altering connectivity of the Default-Mode Network, one of the initial networks compromised in MCI. Further, appropriately prescribed PE may be as beneficial as combined interventional strategies for inducing FBC change.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr. Yanina Sarquis-Adamson and Nellie Kamkar for their help with this manuscript.
Abbreviations
- CT(c)
Cognitive training (control)
- FBC
Functional brain connectivity
- FDR
False discovery rate
- fMRI
Functional magnetic resonance imaging
- MCI
Mild cognitive impairment
- PE(c)
Physical exercise (control)
- PC
Pure control
- SYN or SYNERGIC
SYNchronizing Exercises, Remedies in GaIt and Cognition
- T0/T6
Pre/post-intervention
- T12
Follow-up
- VD(c)
Vitamin D (control)
Author contribution
Nick W. Bray: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Visualization, Roles/Writing—original draft; Frederico Pieruccini-Faria: Formal analysis, Investigation, Roles/Writing—original draft; Suzanne Witt: Formal analysis, Investigation, Roles/Writing—original draft; Robert Bartha: Conceptualization, Supervision, Writing—review & editing; Timothy J. Doherty: Conceptualization, Supervision, Writing—review & editing; Lindsay S. Nagamatsu: Conceptualization, Supervision, Writing—review & editing; Quincy J. Almeida: Conceptualization, Writing—review & editing; Teresa Liu-Ambrose: Conceptualization, Writing—review & editing; Laura E. Middleton: Conceptualization, Writing—review & editing; Louis Bherer: Conceptualization, Writing—review & editing; Manuel Montero-Odasso: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing—review & editing.
Funding
The SYNERGIC Trial is funded by Canadian Consortium on Neurodegeneration in Aging (CCNA Grant# “FRN” CNA 137794). The CCNA is supported by a grant from the Canadian Institutes of Health Research with funding from several partners. Dr. Nick W. Bray was supported by a 2018–2021 Ontario Graduate Scholarship (OGS). Dr. Frederico Pieruccini-Faria was supported by the “Fellowship in Care of the Older Adult” from the St. Joseph’s Healthcare Foundation, Parkwood Institute, Division of Geriatric Medicine, University of Western Ontario; and the Canadian Consortium on Neurodegeneration in Aging (FRN CNA 137794). Teresa Liu-Ambrose is a Canada Research Chair (Tier I) in Healthy Aging. Dr. Montero-Odasso’s program in Gait and Brain Health is supported by grants from the Canadian Institute of Health Research (MOP 211220; PJT 153100), the Ontario Ministry of Research and Innovation (ER11–08–101), the Ontario Neurodegenerative Diseases Research Initiative (OBI 34739), the Canadian Consortium on Neurodegeneration in Aging (FRN CNA 137794), and Department of Medicine Program of Experimental Medicine Research Award (POEM 768915), University of Western Ontario. He is the first recipient of the Schulich Clinician-Scientist Award.
Declarations
Disclosures
The authors report no competing interests.
Disclaimer
The sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Trial registration
NCT02808676. https://www.clinicaltrials.gov/ct2/show/NCT02808676.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nick W. Bray, Email: nicholas.bray@ucalgary.ca
Frederico Pieruccini-Faria, Email: frederico.faria@sjhc.london.on.ca.
Suzanne T. Witt, Email: switt4@uwo.ca
Robert Bartha, Email: rbartha@uwo.ca.
Timothy J. Doherty, Email: tim.doherty@lhsc.on.ca
Lindsay S. Nagamatsu, Email: lnagamat@uwo.ca
Quincy J. Almeida, Email: qalmeida@wlu.ca
Teresa Liu-Ambrose, Email: teresa.ambrose@ubc.ca.
Laura E. Middleton, Email: laura.middleton@uwaterloo.ca
Louis Bherer, Email: louis.bherer@umontreal.ca.
Manuel Montero-Odasso, Email: mmontero@uwo.ca.
References
- 1.Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: A quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34(8):1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, et al. White matter changes with normal aging. Neurology. 1998;50(4):972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- 4.Bagarinao E, Watanabe H, Maesawa S, Mori D, Hara K, Kawabata K, et al. Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Sci Rep. 2019;9(1):11352-. doi: 10.1038/s41598-019-47922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2005;26(2):165–171. doi: 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23(2):708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice Guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3). 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed]
- 8.Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal alzheimer's disease to alzheimer's dementia: A systematic review of the literature. Dement Geriatr Cogn Disord Extra. 2013;3(1). 10.1159/000354370 [DOI] [PMC free article] [PubMed]
- 9.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiat. 2013;74(5):340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 12.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Chiesa PA, Cavedo E, Vergallo A, Lista S, Potier M-C, Habert M-O, et al. Differential default mode network trajectories in asymptomatic individuals at risk for Alzheimer's disease. Alzheimers Dement. 2019;15(7):940–950. doi: 10.1016/j.jalz.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77(16):1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/J.NEURON.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2022 Alzheimer's disease facts and figures. Alzheimer's Dement J Alzheimer's Assoc. 2022;18(4):700–89. 10.1002/alz.12638. [DOI] [PubMed]
- 17.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England) 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bray NW, Pieruccini-Faria F, Bartha R, Doherty TJ, Nagamatsu LS, Montero-Odasso M. The effect of physical exercise on functional brain network connectivity in older adults with and without cognitive impairment. A systematic review. Mech Ageing Dev. 2021; 196:111493. doi: 10.1016/j.mad.2021.111493. [DOI] [PubMed] [Google Scholar]
- 19.Li M-y, Huang M-m, Li S-z, Tao J, Zheng G-h, Chen L-d. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int J Neurosci. 2017;127(7):634–49. doi: 10.1080/00207454.2016.1212855. [DOI] [PubMed] [Google Scholar]
- 20.Teixeira-Machado L, Arida RM, de Jesus MJ. Dance for neuroplasticity: A descriptive systematic review. Neurosci Biobehav Rev. 2019;96:232–240. doi: 10.1016/j.neubiorev.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 21.van Balkom TD, van den Heuvel OA, Berendse HW, van der Werf YD, Vriend C. The effects of cognitive training on brain network activity and connectivity in aging and neurodegenerative diseases: a systematic review. Neuropsychol Rev. 2020;30(2):267–286. doi: 10.1007/s11065-020-09440-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Amin M, Bradford D, Sullivan RKP, Kurniawan ND, Moon Y, Han S-H, et al. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum Brain Mapp. 2019;40(2):394–406. doi: 10.1002/hbm.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foucault G, Duval GT, Simon R, Beauchet O, Dinomais M, Annweiler C, et al. Serum vitamin D and cingulate cortex thickness in older adults: quantitative MRI of the brain. Curr Alzheimer Res. 2019;16(11):1063–1071. doi: 10.2174/1567205016666191113124356. [DOI] [PubMed] [Google Scholar]
- 24.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morley JE. Dementia: Does vitamin D modulate cognition? Nat Rev Neurol. 2014;10(11):613–614. doi: 10.1038/nrneurol.2014.193. [DOI] [PubMed] [Google Scholar]
- 26.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gratton C, Nelson SM, Gordon EM. Brain-behavior correlations: Two paths toward reliability. Neuron. 2022;110(9):1446–1449. doi: 10.1016/j.neuron.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg MD, Finn ES. How to establish robust brain-behavior relationships without thousands of individuals. Nat Neurosci. 2022;25(7):835–837. doi: 10.1038/s41593-022-01110-9. [DOI] [PubMed] [Google Scholar]
- 29.Revisiting doubt in neuroimaging research. Nat Neurosci. 2022;25(7):833–4. 10.1038/s41593-022-01125-2. [DOI] [PubMed]
- 30.Montero-Odasso M, Almeida QJ, Burhan AM, Camicioli R, Doyon J, Fraser S, et al. SYNERGIC TRIAL (SYNchronizing Exercises, Remedies in Gait and Cognition) a multi-Centre randomized controlled double blind trial to improve gait and cognition in mild cognitive impairment. BMC Geriatr. 2018;18(1):93-. doi: 10.1186/s12877-018-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warburton DER, Bredin SSD, Jamnik VK. Gledhill N. Validation of the PAR-Q+ and ePARmed-X+ Health Fit J Canada. 2011;4(2):38–46. doi: 10.14288/hfjc.v4i2.151. [DOI] [Google Scholar]
- 33.Kueider AM, Parisi JM, Gross AL, Rebok GW. Computerized cognitive training with older adults: a systematic review. PloS one. 2012;7(7):e40588-e. doi: 10.1371/journal.pone.0040588;10.1371/journal.pone.0040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res Rev. 2013;12(1):263–275. doi: 10.1016/j.arr.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Lussier M, Brouillard P, Bherer L. Limited benefits of heterogeneous dual-task training on transfer effects in older adults. J Gerontol B Psychol Sci Soc Sci. 2017;72(5):801–812. doi: 10.1093/geronb/gbv105. [DOI] [PubMed] [Google Scholar]
- 36.Lussier M, Saillant K, Vrinceanu T, Hudon C, Bherer L. Normative data for a tablet-based dual-task assessment in healthy older adults. Arch Clin Neuropsychol. 2021;36(7):1316–1325. doi: 10.1093/arclin/acaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Zourdos MC, Klemp A, Dolan C, Quiles JM, Schau KA, Jo E, et al. Novel resistance training-specific rating of perceived exertion scale measuring repetitions in reserve. J Strength Cond Res. 2016 doi: 10.1519/JSC.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 39.Bray NW, Doherty TJ, Montero-Odasso M. The effect of high dose vitamin D3 on physical performance in frail older adults. A feasibility study. J Frailty Aging. 2018;7(3). 10.14283/jfa.2018.18. [DOI] [PubMed]
- 40.Duchesne S, Chouinard I, Potvin O, Fonov VS, Khademi A, Bartha R, et al. The Canadian dementia imaging protocol: harmonizing national cohorts. J Magn Reson Imaging. 2019;49(2):456–465. doi: 10.1002/jmri.26197. [DOI] [PubMed] [Google Scholar]
- 41.Khan A. khanlab/neuroglia-helpers. Date Accessed: 03 March 2020. Retrieved from: https://github.com/khanlab/neuroglia-helpers.
- 42.Khan A. khanlab/tar2bids. Date Accessed: 03 March 2020. Retrieved from: https://github.com/khanlab/tar2bids.
- 43.Khan A. khanlab/dicom2tar. Date Accessed: 03 March 2020. Retrieved from: https://github.com/khanlab/dicom2tar.
- 44.Gorgolewski KJ, Auer T, Calhoun VD, Craddock RC, Das S, Duff EP, et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data. 2016;3(1):160044-. doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/J.NEUROIMAGE.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 49.Nieto-Castanon A. Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN. Hilbert Press; 2020. [Google Scholar]
- 50.Van der Gucht K, Ahmadoun S, Melis M, de Cloe E, Sleurs C, Radwan A, et al. Effects of a mindfulness-based intervention on cancer-related cognitive impairment: results of a randomized controlled functional magnetic resonance imaging pilot study. Cancer. 2020;126(18):4246–4255. doi: 10.1002/cncr.33074. [DOI] [PubMed] [Google Scholar]
- 51.Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci Off J Soc Neurosci. 2012;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle Marcus E, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100\r2235925100[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K, et al. The WU-Minn human connectome project: an overview. Neuroimage. 2013;80:62–79. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flodin P, Jonasson LS, Riklund K, Nyberg L, Boraxbekk CJ. Does aerobic exercise influence intrinsic brain activity? An aerobic exercise intervention among healthy old adults. Front Aging Neurosci. 2017;9:267-. doi: 10.3389/fnagi.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li R, Zhu X, Yin S, Niu Y, Zheng Z, Huang X, et al. Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobeâ€. Front Aging Neurosci. 2014;6:39-. doi: 10.3389/fnagi.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21(11):1633–1642. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao J, Liu J, Chen X, Xia R, Li M, Huang M, et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. NeuroImage Clin. 2019;23:101834-. doi: 10.1016/J.NICL.2019.101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner J, Carvalho JO, Potter GG, Thames A, Zelinski E, Crane PK, et al. The Alzheimer's Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus): An expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging Behav. 2012;6(4):489–501. doi: 10.1007/s11682-012-9166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- 61.Roberts HCHC, Denison HJHJ, Martin HJHJ, Patel HPHP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/AGEING/AFR051. [DOI] [PubMed] [Google Scholar]
- 62.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan RR, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 63.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borzuola R, Giombini A, Torre G, Campi S, Albo E, Bravi M, et al. Central and peripheral neuromuscular adaptations to ageing. J Clin Med. 2020;9(3). 10.3390/JCM9030741. [DOI] [PMC free article] [PubMed]
- 65.Enright PL. The six-minute walk test. Respir Care. 2003;48(8):783–5. [PubMed]
- 66.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 67.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA. 2003;289(19):2545–2553. doi: 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]
- 68.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 70.Bohland JW, Bokil H, Allen CB, Mitra PP. The brain atlas concordance problem: quantitative comparison of anatomical parcellations. PloS one. 2009;4(9):e7200-e. doi: 10.1371/journal.pone.0007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui X, et al. xjView. Date Accessed: 03 April 2021. Retrieved from: https://www.alivelearn.net/xjview/.
- 72.Drey M, Zech A, Freiberger E, Bertsch T, Uter W, Sieber CC, et al. Effects of strength training versus power training on physical performance in prefrail community-dwelling older adults. Gerontology. 2012;58(3):197–204. doi: 10.1159/000332207. [DOI] [PubMed] [Google Scholar]
- 73.Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex (New York, NY : 1991) 2010;20(11):2636–46. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng Q, Wang M, Song Q, Wu Z, Jiang H, Pang P, et al. Correlation between hippocampus MRI radiomic features and resting-state intrahippocampal functional connectivity in alzheimer's disease. Front Neurosci. 2019;13:435-. doi: 10.3389/fnins.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villena-Gonzalez M, Wang H-T, Sormaz M, Mollo G, Margulies DS, Jefferies EA, et al. Individual variation in the propensity for prospective thought is associated with functional integration between visual and retrosplenial cortex. Cortex J Study Nervous Syst Behav. 2018;99:224–234. doi: 10.1016/j.cortex.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham SI, Tomasi D, Volkow ND. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp. 2017;38(2):938–956. doi: 10.1002/hbm.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng D, Xia W, Yi ZQ, Zhao PW, Zhong JG, Shi HC, et al. Alterations of brain local functional connectivity in amnestic mild cognitive impairment. Transl Neurodegener. 2018;7(1):26-. doi: 10.1186/s40035-018-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thakral PP, Madore KP, Schacter DL. A role for the left angular gyrus in episodic simulation and memory. J Neurosci Off J Soc Neurosci. 2017;37(34):8142–8149. doi: 10.1523/JNEUROSCI.1319-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheke LG, Bonnici HM, Clayton NS, Simons JS. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia. 2017;96:137–149. doi: 10.1016/j.neuropsychologia.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thakral PP, Wang TH, Rugg MD. Decoding the content of recollection within the core recollection network and beyond. Cortex J Study Nervous Syst Behav. 2017;91:101–113. doi: 10.1016/j.cortex.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 83.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci Off J Soc Neurosci. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raichle ME. The brain's default mode network. Annu Rev Neurosci. 2015;38(1):433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 85.Boccia M, Sulpizio V, Nemmi F, Guariglia C, Galati G. Direct and indirect parieto-medial temporal pathways for spatial navigation in humans: evidence from resting-state functional connectivity. Brain Struct Funct. 2017;222(4):1945–1957. doi: 10.1007/s00429-016-1318-6. [DOI] [PubMed] [Google Scholar]
- 86.Kuhns AB, Dombert PL, Mengotti P, Fink GR, Vossel S. Spatial Attention, Motor Intention, and Bayesian Cue Predictability in the Human Brain. J Neurosci Off J Soc Neurosci. 2017;37(21):5334–5344. doi: 10.1523/JNEUROSCI.3255-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wells RE, Yeh GY, Kerr CE, Wolkin J, Davis RB, Tan Y, et al. Meditation's impact on default mode network and hippocampus in mild cognitive impairment: A pilot study. Neurosci Lett. 2013;556:15–19. doi: 10.1016/j.neulet.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eyre HA, Acevedo B, Yang H, Siddarth P, Van Dyk K, Ercoli L, et al. Changes in neural connectivity and memory following a Yoga intervention for older adults: a pilot study. J Alzheimers Dis. 2016;52(2):673–684. doi: 10.3233/JAD-150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu CL, Best JR, Wang S, Voss MW, Hsiung RGY, Munkacsy M, et al. The impact of aerobic exercise on fronto-parietal network connectivity and its relation to mobility: an exploratory analysis of a 6-month randomized controlled trial. Front Human Neurosci. 2017;11:344-. doi: 10.3389/fnhum.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist Rev J Neurobiol Neurol Psychiatry. 2019;25(1):65–85. doi: 10.1177/1073858418771538. [DOI] [PubMed] [Google Scholar]
- 91.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer's disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18(2):177–184. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bray NW, Pieruccini-Faria F, Witt ST, Rockwood K, Bartha R, Doherty TJ, et al. Frailty and functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI): baseline results from the SYNERGIC Trial. Geroscience. 2022:1–16. 10.1007/s11357-022-00702-4. [DOI] [PMC free article] [PubMed]
- 93.Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC. Resting cerebral blood flow after exercise training in mild cognitive impairment. J Alzheimers Dis. 2019;67(2):671–684. doi: 10.3233/JAD-180728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Botvinik-Nezer R, Holzmeister F, Camerer CF, Dreber A, Huber J, Johannesson M, et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582(7810):84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hyatt CS, Owens MM, Crowe ML, Carter NT, Lynam DR, Miller JD. The quandary of covarying: A brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. NeuroImage. 2020;205:116225-. doi: 10.1016/J.NEUROIMAGE.2019.116225. [DOI] [PubMed] [Google Scholar]
- 97.Statistics Canada. Obesity in Canadian Adults, 2016 and 2017. Date Accessed: 03 April 2021. Retrieved from: https://www150.statcan.gc.ca/n1/pub/11-627-m/11-627-m2018033-eng.htm.
- 98.ParticipACTION. The 2019 ParticpACTION Report Card on Physical Activity for Adults. Date Accessed: 03 April 2021. Retrieved from: https://www.participaction.com/en-ca/resources/adult-report-card.
- 99.Furlano JA, Nagamatsu LS. Feasibility of a 26-week exercise program to improve brain health in older adults at risk for type 2 diabetes: a pilot study. Can J Diabetes. 2020 doi: 10.1016/j.jcjd.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Wallace L, Hunter S, Theou O, Fleming J, Rockwood K, Brayne C. Frailty and neuropathology in relation to dementia status: the Cambridge City over-75s Cohort study. Int Psychogeriatr. 2021:1–9. 10.1017/S1041610220003932. [DOI] [PubMed]
- 101.Lamar M, Boots EA, Arfanakis K, Barnes LL, Schneider JA. Common brain structural alterations associated with cardiovascular disease risk factors and alzheimer's dementia: future directions and implications. Neuropsychol Rev. 2020;30(4):546–557. doi: 10.1007/s11065-020-09460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Badji A, Westman E. Cerebrovascular pathology in Alzheimer's disease: Hopes and gaps. Psychiatry Res Neuroimaging. 2020;306:111184-. doi: 10.1016/J.PSCYCHRESNS.2020.111184. [DOI] [PubMed] [Google Scholar]
- 103.Erickson KI, Donofry SD, Sewell KR, Brown BM, Stillman CM. Cognitive aging and the promise of physical activity. Annu Rev Clin Psychol. 2022;18:417–442. doi: 10.1146/annurev-clinpsy-072720-014213. [DOI] [PubMed] [Google Scholar]
- 104.Bray NW, Jones GJ, Rush KL, Jones CA, Jakobi JM. Multi-component exercise with high-intensity, free-weight, functional resistance training in pre-frail females: a quasi-experimental, pilot study. J Frailty Aging. 2020;9(2):111–7. doi: 10.14283/jfa.2020.13. [DOI] [PubMed] [Google Scholar]
- 105.Liguori G, American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins; 2020.
- 106.Titus J, Bray NW, Kamkar N, Camicioli R, Nagamatsu LS, Speechley M, et al. The role of physical exercise in modulating peripheral inflammatory and neurotrophic biomarkers in older adults: A systematic review and meta-analysis. Mech Ageing Dev. 2021;194:111431-. doi: 10.1016/J.MAD.2021.111431. [DOI] [PubMed] [Google Scholar]
- 107.Mekari S, Neyedli HF, Fraser S, O'Brien MW, Martins R, Evans K, et al. High-intensity interval training improves cognitive flexibility in older adults. Brain Sci. 2020;10(11). 10.3390/brainsci10110796. [DOI] [PMC free article] [PubMed]
- 108.O'Brien MW, Johns JA, Robinson SA, Bungay A, Mekary S, Kimmerly DS. Impact of high-intensity interval training, moderate-intensity continuous training, and resistance training on endothelial function in older adults. Med Sci Sports Exerc. 2020;52(5):1057–1067. doi: 10.1249/MSS.0000000000002226. [DOI] [PubMed] [Google Scholar]
- 109.Heisz JJ, Tejada MGM, Paolucci EM, Muir C. Enjoyment for high-intensity interval exercise increases during the first six weeks of training: implications for promoting exercise adherence in sedentary adults. PloS one. 2016;11(12):e0168534-e. doi: 10.1371/journal.pone.0168534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stork MJ, Banfield LE, Gibala MJ, Martin Ginis KA. A scoping review of the psychological responses to interval exercise: is interval exercise a viable alternative to traditional exercise? Health Psychol Rev. 2017;11(4):324–344. doi: 10.1080/17437199.2017.1326011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available from the corresponding authors upon reasonable request.