Abstract
On a global scale, organisms face significant challenges due to climate change and anthropogenic disturbance. In many ectotherms, developmental and physiological processes are sensitive to changes in temperature and resources. Developmental plasticity in thermal physiology may provide adaptive advantages to environmental extremes if early environmental conditions are predictive of late-life environments. Here, we conducted a laboratory experiment to test how developmental temperature and maternal resource investment influence thermal physiological traits (critical thermal maximum: CTmax and thermal preference: Tpref) in a common skink (Lampropholis delicata). We then compared our experimental findings more broadly across reptiles (snakes, lizards and turtles) using meta-analysis. In both our experimental study and meta-analysis, we did not find evidence that developmental environments influence CTmax or Tpref. Furthermore, the effects of developmental environments on thermal physiology did not vary by age, taxon or climate zone (temperate/tropical). Overall, the magnitude of developmental plasticity on thermal physiology appears to be limited across reptile taxa suggesting that behavioural or evolutionary processes may be more important. However, there is a paucity of information across most reptile taxa, and a broader focus on thermal performance curves themselves will be critical in understanding the impacts of changing thermal conditions on reptiles in the future.
Keywords: meta-analysis, developmental, environments, thermal, physiology
1. Introduction
Climate warming and anthropogenic stressors pose significant challenges to organisms on a global scale [1,2]. Rapidly increasing temperatures are a particularly significant threat for ectothermic species. Indeed, increasing temperatures can drive fitness declines due to physiological intolerance [3] and alter the distribution of species [4]. Inevitably, these impacts are primarily mediated by how organisms change their behaviour and physiology through development and evolutionary time in response to shifting environments. Phenotypic changes that occur during an animal's lifetime in response to changing environments (i.e. phenotypic plasticity) are important mechanisms by which ectotherms can cope with climate change over short timescales [5]. However, the magnitude of plastic responses is widely trait- and species-specific [5–7]
Temperature can also have transgenerational effects by impacting parental generations [8,9]. For instance, recent evidence indicates that some ectotherms can tolerate heat events for long periods [5,10]. Thermal ecology of ectotherms can also be shaped by other factors, such as diet or maternal investment, which can influence physiological traits that are temperature dependent [11–13]. For example, a diet high in nutrients (carbohydrate or protein) leads to higher metabolic rates and critical thermal maximum (CTmax), while a diet low in these nutrients can result in lower physiological trait values [14–16]. Additionally, the resources a mother invests in her offspring (i.e. the energetic provisioning of eggs) can influence metabolic processes like growth and development [17]. Determining how thermal and resource environments during development affect key thermal physiological traits in various taxa may provide an understanding of how species are likely to cope with changing environments.
While phenotypic plasticity can adjust phenotypes throughout life, developmental plasticity—plasticity occurring during early embryonic development—can have organizational effects on phenotypes that can affect responses later in life [6]. For vertebrates in particular, such effects may be adaptive or maladaptive depending on whether early-life environments are predictive of late-life environments. While temperature and early resource provisioning can influence thermal traits in ectotherms [18], most research effort has focused on temperature, which is known to have a profound effect on fitness [19,20]. In reptiles, temperatures during embryonic development are known to affect phenotypes throughout ontogeny [7]. For example, incubation conditions of developing reptile embryos can impact a variety of traits including sex, growth rate, morphology, behaviour and cognition [7,20,21]. However, there is a dearth of evidence linking developmental factors more generally to thermal traits, and whether these differences persist through various stages of ontogeny in reptiles [22,23].
Here, we aim to determine how early developmental environments affect thermal physiology (CTmax and thermal preference: Tpref) in reptiles. CTmax and Tpref are two common thermal indices used as proxies for how the environment influences individual fitness and are used to predict how species distributions are predicted to shift with climate change [3,24,25]. We first conduct a laboratory experiment to test how maternal investment and developmental temperature both influence CTmax and Tpref in a common skink (Lampropholis delicata). We then compare our experimental findings with quantitative results testing this same question more broadly in reptiles using a meta-analysis.
2. Method and materials
(a) . Consequences of incubation temperature and resource allocation on thermal physiology: an experimental manipulation
We collected gravid Lampropholis delicata (common garden skink, n = 100) from populations in Sydney (Australia) and transported them back to the Australian National University, where females were housed until eggs (n = 40) were laid. We then pseudo-randomly (to ensure equal sample sizes) assigned eggs (n = 20) to both a resource allocation treatment (‘R’—yolk removal or ‘C’—control) and an incubation temperature (23°C or 28°C s.d. ± 3.0) treatment (see electronic supplementary material for details on husbandry of hatchlings). Egg incubation temperatures were chosen to mimic conditions experienced at extremes of natural nest temperatures in nature while also showing natural thermal fluctuations throughout the day [26]. Yolk removal treatments followed Sinervo [16], with 15–20% of the total egg mass being removed via a sterilized syringe. Control treatments were punctured with the syringe without any yolk removal. For further description of husbandry conditions of adults and incubation details, see Kar et al. [27].
Hatchlings from their respective treatment were housed in mixed treatment groups of 5–6 within 20 plastic enclosures [40 cm (l) × 29.5 cm (w) × 20.5 cm (h)], with UVA/UVB lighting and a 20 W heat lamp in each enclosure. Water was provided ad libitum, with enclosures misted daily. Lizards were fed calcium and vitamin-dusted crickets (Acheta domesticus) every second day. At eight to eleven months post-hatching, lizards were selected at random, and thermal traits (CTmax and Tpref) were measured. Briefly, after undergoing a 24 h fasting period, animals were transferred into individual lanes of a thermal gradient (5°C to 55°C) to measure Tpref. A FLIR T640 thermal camera was used to take thermal images of all lanes every 15 min over an 8 h observation period. Tpref was defined as the mean skin surface temperature (on the neck) over the 8 h observation period. Given the small size of lizards (i.e. 1.3 g), we assumed skin surface temperature reflected body temperature, which has been shown for many small lizards [28]. For CTmax, we followed the same fasting period used for Tpref experiments. Here, lizards were placed in Falcon tubes in a water bath for 5 min at a temperature of 30°C. The water temperature was increased to 38°C at a rate of 1°C min−1. We used a control Falcon tube with a thermal couple attached to the bottom of the tub where lizards were positioned to record the temperature of the tube surface, which we took to be the temperature experienced by the lizards. This approach was needed because it was not possible to have a thermal couple in each lizards Falcon tube when measuring righting responses in the CTmax procedure [29]. CTmax was defined as the temperature at which an individual lost their righting reflex (for further details in collection methods, see electronic supplementary material).
All statistical analyses were conducted using the R environment, ver. 4.1.0 (https://www.r-project.org/). We used linear mixed-effects models to analyse thermal traits (Tpref and CTmax). We constructed models that contained the main effects of body mass, sex, incubation temperature and resource treatment. We also tested for the interaction between incubation temperature and resource treatment (see electronic supplementary material for more details). If the interaction was not significant, we removed it and presented the full main effects model.
(b) . Meta-analysis of early thermal effects on thermal physiology in reptiles
To understand more broadly the impact of developmental environments on thermal physiology, we systematically searched for studies manipulating early developmental environments and subsequently measuring thermal physiological traits. Unfortunately, few studies manipulated egg resource investment and measured thermal tolerance. As such, it was only possible to focus on developmental temperature manipulations. Our meta-analysis collected data on offspring Tpref and CTmax in lizards, snakes, tortoises, turtles and tuatara. Our search string included cold tolerance (i.e. critical thermal minimum, CTmin), but there were too few studies that manipulated developmental environments and measured this trait to conduct a formal meta-analysis. As such, we focus on Tpref and CTmax.
In brief, we conducted a systematic literature search in Scopus, ISI Web of Science (core collection), and ProQuest (dissertations and thesis) and did not apply a timespan limit. We followed the PRISMA-EcoEvo (Preferred Reporting Items for Systematic Reviews & Meta-Analyses in Ecology and Evolutionary biology) guidelines for reporting [30]. Full search strings, search methods and selection criteria are described in detail in electronic supplementary material, figures S1 and S2. We obtained 485 original records, and 15 articles satisfied our selection criteria [31–42].
Multi-level meta-analytic models were constructed using the rma.mv function in the metafor package (version 3.8) [43]. To determine the ability of an organism to acclimate to changes in the environment, we used the acclimation response ratio (ARR) as our effect size [44]. Sampling variance for the ARR was derived in Pottier et al. [45]. Study, phylogeny and species were designated as random effects and we included an observation-level random effect (effect size ID). A model that included only study, species and effect size ID was best supported over one with phylogeny, so we present meta-analytic results from a model without phylogeny. Studies often had more than two temperature treatments. As such, we derived all pairwise effect size comparisons. This, however, does induce a correlation between effect size sampling errors, which we controlled for through the inclusion of a sampling (co)variance matrix derived by assuming effect sizes are correlated by r = 0.5 [46]. Thermal trait (Tpref or CTmax), life stage at measurement (hatchling, juvenile or adult), climate zone (temperate or tropical) and major taxonomic group (lizard, snake, tuatara or turtle) were included as fixed factors in separate multi-level meta-regression (MLMR) models. We also tested for publication bias using a MLMR model with sampling variance and standard error as predictors [47] and was visually inspected using a funnel plot (see electronic supplementary material for more details). We present effect size heterogeneity by constructing prediction intervals [48] and presenting I2 using the orchaRd package (version 2.0) [49].
3. Results
(a) . Incubation temperature and resource allocation consequences on thermal preference and critical thermal maximum
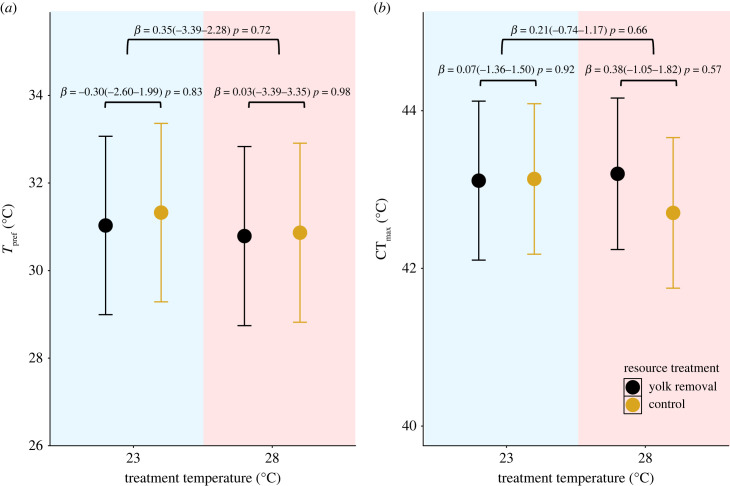
Mean Tpref was 31°C ± 0.47 (mean ± s.e.) and ranged from 20.99 to 34.26°C. Mean CTmax was 43.04°C ± 0.23 and ranged from 38.6 to 45.2°C. We did not detect any effect of incubation temperature, yolk treatment, sex or body mass on Tpref or CTmax (figure 1a,b; table 1).
Figure 1.
Thermal indices across different incubation temperatures and resource treatments for hatchling Lampropholis delicata (n = 10 per temperature and treatment). (a) Thermal preference (Tpref) in lizards incubated at 23 and 28°C for each resource treatment (yolk ablation and control). (b) Critical thermal maximum (CTmax) in lizards incubated at 23 and 28°C for each resource treatment. Bars above plots indicate pairwise comparisons of thermal indices between temperature and resource treatments. Means and 95% confidence intervals are provided along with the p-value for each contrast.
Table 1.
Model outputs coefficients for testing whether sex, body mass, incubation temperature, resource or the interaction between resource and temperature had an effect on Tpref or CTmax in hatchling Lampropholis delicata. Estimate value describes the estimated coefficient value and 95% CI describes the lower and upper bound of the 95% credible interval for each coefficient value. Intercept is the estimated mean of each thermal trait from the null model. Italics indicate coefficients that are significant at p < 0.05.
| thermal index | covariate | estimate | l–95% CI | u-95% CI | p-value |
|---|---|---|---|---|---|
| Tpref | (intercept) | 30.94 | 28.67 | 33.20 | <0.01 |
| body mass | 0.44 | −0.97 | 1.86 | 0.53 | |
| sex | 0.30 | −2.50 | 3.09 | 0.83 | |
| incubation temperature | −0.35 | −2.36 | 1.66 | 0.72 | |
| resource | 0.19 | −1.83 | 2.20 | 0.85 | |
| incubation temperature × resource | −0.22 | −4.31 | 3.87 | 0.91 | |
| CTmax | (intercept) | 43.27 | 42.17 | 44.37 | <0.01 |
| body mass | −0.41 | −1.08 | 0.25 | 0.21 | |
| sex | −0.03 | −1.35 | 1.28 | 0.96 | |
| incubation temperature | −0.18 | −1.14 | 0.78 | 0.70 | |
| resource | −0.24 | −1.20 | 0.71 | 0.61 | |
| incubation temperature × resource | −0.52 | −2.47 | 1.44 | 0.59 |
(b) . Meta-analysis of early thermal effects on thermal physiology in reptiles
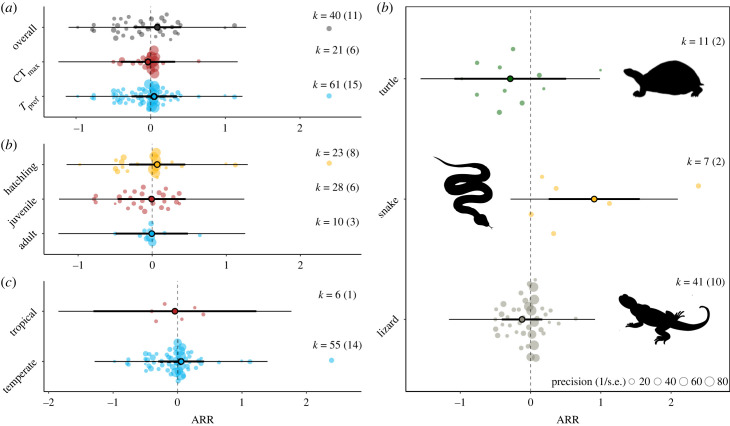
Across reptiles, developmental temperatures did not influence thermal traits (Tpref or CTmax), but heterogeneity was high (ARR = 0.05, 95% CI: −0.28–0.37; , prediction interval: −1.23–1.32; figure 2a, n = 69 effects from 14 species). Overall, we found no evidence for publication biases (β = −0.81, 95% CI = −1.92–0.3, p = 0.15; electronic supplementary material, figure S3; for further details see electronic supplementary material). Species effects () drove most of the heterogeneity in ARR, but thermal traits were not influenced by life stage, climate zone or major taxonomic group (i.e. snakes, turtles and lizards) (figure 2b,c). While there was a significant increase in thermal traits in snakes (figure 2d), this was driven by a single species (Nerodia sipdedon), and given the small sample sizes, we need to caution whether any true differences between snakes and other groups exist.
Figure 2.
The magnitude of the effect of developmental temperature on thermal indices (Tpref and CTmax) in reptiles (a) concerning age class of thermal physiological measurement (b), climate zone (c) and taxon (d). Mean meta-analytic ARR estimates (circles) with their 95% confidence intervals (thicker error bars) and prediction intervals (thinner error bars). Data points from each study from the meta-analysis are scaled by precision (inverse of s.e.), and k is the number of effect sizes with the number of species in brackets. ARR is the acclimation response ratio. Ninety-five per cent confidence intervals not overlapping 0 are statistically significant. Graphs were constructed using the orchaRd package [50]. Tuatara was removed for visual purposes due to the small number of effect sizes (n = 3).
4. Discussion
Genetic adaptation and phenotypic plasticity are two hypotheses for how ectotherms can cope with warming temperatures associated with anthropogenic climate change [3,51–53]. Plastic responses occurring early in development can have long-lasting effects on organisms, with significant implications for how they cope with environmental stressors.
We show that early developmental environments do little to modify thermal physiological traits (CTmax and Tpref) in most reptile taxa. Both our experimental and meta-analytic approaches suggest that the magnitude of developmental plasticity on thermal indices appears to be canalized. For example, our meta-analysis indicated that for every 1°C change in developmental temperature, we only expect a 0.05°C change in thermal physiological response. Our findings are consistent with those of other ectotherm systems, which show that developmental plasticity has little impact on adult heat tolerance [6,54–56]. Nonetheless, we detected significant species-specific heterogeneity (), suggesting substantial differences across species that cannot be ignored. Such variability may be driven by species differences in: (1) micro-habitat selection of nests; (2) nesting phenology; (3) the propensity for local adaptation in the wild and/or (4) the different conditions chosen for laboratory experiments across species. It has been indicated in other studies [57–60] that differences in nest depth, nest location, clutch density or maternal condition may select for developmentally plastic responses in offspring. Together, these data highlight that further ecological data on developmental environments in nature is needed to test if static manipulations in the laboratory provide a functional link to how species can cope with environmental change.
While there are still limited empirical studies, across reptile taxa, plasticity in thermal physiology did not differ by age, taxon or climate zone. We expected that the earlier the age at which thermal traits were measured would make it more likely that effects of early environments would be detected because of the tighter connection between egg and post-hatchling experiences. In addition, tropical species are expected to maintain body temperatures near their thermal limits, and an increase in temperature can push these species to physiological extremes compared to temperate species [3,53,61]. Greater thermal variability in temperate regions may also select for greater plasticity. However, our meta-analysis does not support these hypotheses. Instead, the microthermal environments chosen, along with behavioural flexibility (or lack thereof) in nest site selection, may be more important driving mechanisms in determining whether species respond plastically to developmental environments or not [3,51]. Future studies looking at the autocorrelation between early and late developmental environments would be fruitful in helping elucidate species-specific responses to thermal environments.
Overall, our results suggest that many reptiles may have limited developmental plasticity in thermal traits, relying instead on energetically expensive behaviours (i.e. thermoregulation) [3,62] or responses that operate on slower timescales (i.e. local adaptation) [52,63]. Given the small effect sizes we observed, statistical power is likely an issue in ours and others' empirical work. However, ethical constraints in measuring thermal limits in large numbers of animals will mean such studies are likely to be common. As such, we will need to rely on meta-analysis to help circumvent power limitations in individual studies (as we have done here) [64]. We have also identified clear gaps in the literature that should help pave the way for future research. First, we encourage measuring thermal physiology under different developmental manipulations across a greater diversity of reptile taxa. Greater taxonomic diversity will clarify when developmental environments matter and allow us to explore the reasons for this heterogeneity. Second, we encourage measuring CTmin, in addition to other thermal physiological traits (i.e. CTmax, Tpref, etc.), as it is often more environmentally flexible than upper thermal limits. Despite these gaps, our results provide valuable insights into possible responses that are plausible under changing thermal conditions.
Contributor Information
Kristoffer H. Wild, Email: kristoffer.wild@anu.edu.au.
Daniel W. A. Noble, Email: daniel.noble@anu.edu.au.
Ethics
All experimental procedures followed approved protocols by the ANU Animal Ethics Committee (ARA2019/17). Lizards were caught under NPWS permit LT201917.
Data accessibility
Experimental and meta-analytic datasets and code are available from the Zenodo repository: https://doi.org/10.5281/zenodo.7700383 [65].
The data are provided in the electronic supplementary material [66].
Authors' contributions
R.Y.Z.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, writing—original draft and writing—review and editing; K.H.W.: data curation, formal analysis, investigation, methodology, writing—original draft and writing—review and editing; P.P.: conceptualization, data curation, formal analysis, investigation, methodology and writing—review and editing; M.I.C.: conceptualization, data curation, investigation, methodology, supervision and writing—review and editing; S.N.: conceptualization, funding acquisition, methodology, resources, validation and writing—review and editing; D.W.A.N.: conceptualization, data curation, formal analysis, funding acquisition, project administration, supervision, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received funding for this study from an Australian Research Council (ARC) Discovery grant to D.W.A.N. (grant no. DP210101152).
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637-669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770-1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 3.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665-1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peralta-Maraver I, Rezende EL. 2021. Heat tolerance in ectotherms scales predictably with body size. Nat. Clim. Chang. 11, 58-63. ( 10.1038/s41558-020-00938-y) [DOI] [Google Scholar]
- 5.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Chang. 5, 61-66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 6.Pottier P, Burke S, Zhang RY, Noble DWA, Schwanz LE, Drobniak SM, Nakagawa S. 2022. Developmental plasticity in thermal tolerance: ontogenetic variation, persistence, and future directions. Ecol. Lett. 25, 2245-2268. ( 10.1111/ele.14083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noble DWA, Stenhouse V, Schwanz LE. 2018. Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta-analysis. Biol. Rev. 93, 72-97. ( 10.1111/brv.12333) [DOI] [PubMed] [Google Scholar]
- 8.Sales K, et al. 2018. Experimental heatwaves compromise sperm function and cause transgenerational damage in a model insect. Nat. Commun. 9, 4771. ( 10.1038/s41467-018-07273-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159-163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof S, et al. 2017. Thermoregulatory behavior and high thermal preference buffer impact of climate change in a Namib Desert lizard. Ecosphere 8, e02033. ( 10.1002/ecs2.2033) [DOI] [Google Scholar]
- 11.Burton T, Killen SS, Armstrong JD, Metcalfe NB. 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 278, 3465-3473. ( 10.1098/rspb.2011.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobler M, Nilsson JÅ, Nilsson JF. 2007. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol. Lett. 3, 408-410. ( 10.1098/rsbl.2007.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao CL, et al. 2022. Temperature and diet acclimation modify the acute thermal performance of the largest extant amphibian. Animals 12, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardison EA, Kraskura K, van Wert J, Nguyen T, Eliason EJ. 2021. Diet mediates thermal performance traits: implications for marine ectotherms. J. Exp. Biol. 224, jeb242846. ( 10.1242/jeb.242846) [DOI] [PubMed] [Google Scholar]
- 15.Bujan J, Kaspari M. 2017. Nutrition modifies critical thermal maximum of a dominant canopy ant. J. Insect. Physiol. 102, 1-6. ( 10.1016/j.jinsphys.2017.08.007) [DOI] [PubMed] [Google Scholar]
- 16.Sinervo B. 1990. The evolution of maternal investment in lizards: an experimental and comparative analysis of egg size and its effects on offspring performance. Evolution 44, 279-294. ( 10.2307/2409407) [DOI] [PubMed] [Google Scholar]
- 17.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403-407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 18.Angilletta MJ Jr, Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. New York, NY: Oxford University Press. [Google Scholar]
- 19.Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am. Naturalists 2, 158-210. ( 10.1086/321314) [DOI] [PubMed] [Google Scholar]
- 20.Sibly RM, Atkinson D. 1994. How rearing temperature affects optimal adult size in ectotherms. Ecology 8, 486-493. [Google Scholar]
- 21.Bull JJ. 1980. Sex determination in reptiles. Q. Rev. Biol. 55, 3-21. ( 10.1086/411613) [DOI] [Google Scholar]
- 22.Refsnider JM, Clifton IT, Vazquez TK. 2019. Developmental plasticity of thermal ecology traits in reptiles: trends, potential benefits, and research needs. J. Therm. Biol. 84, 74-82. ( 10.1016/j.jtherbio.2019.06.005) [DOI] [PubMed] [Google Scholar]
- 23.Bodensteiner BL, et al. 2021. Thermal adaptation revisited: how conserved are thermal traits of reptiles and amphibians? J. Exp. Zool. A Ecol. Integr. Physiol. 335, 173-194. ( 10.1002/jez.2414) [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann AA, Chown SL, Clusella-Trullas S. 2013. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934-949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 25.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894-899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 26.Cheetham E, Doody JS, Stewart B, Harlow P. 2011. Embryonic mortality as a cost of communal nesting in the delicate skink. J. Zool. 283, 234-242. ( 10.1111/j.1469-7998.2010.00764.x) [DOI] [Google Scholar]
- 27.Kar F, Nakagawa S, Noble DWA. 2022. Impact of developmental temperatures on thermal plasticity and repeatability of metabolic rate. Evol. Ecol. 36, 199-216. ( 10.1007/s10682-022-10160-1) [DOI] [Google Scholar]
- 28.Garrick D. 2008. Body surface temperature and length in relation to the thermal biology of lizards. Biosci. Horizons 1, 136-142. ( 10.1093/biohorizons/hzn014) [DOI] [Google Scholar]
- 29.Llewelyn J, Macdonald SL, Hatcher A, Moritz C, Phillips BL. 2016. Intraspecific variation in climate-relevant traits in a tropical rainforest lizard. Divers. Distrib. 1, 1000-1012. ( 10.1111/ddi.12466) [DOI] [Google Scholar]
- 30.O'Dea RE, et al. 2021. Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol. Rev. 96, 1695-1722. ( 10.1111/brv.12721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abayarathna MG. The effect of incubation temperature on offspring phenotypes and survival of velvet gecko, Amalosia lesueurii. PhD thesis, University of Technology, Sydney, Australia. [Google Scholar]
- 32.Alberts AC, Perry AM, Lemm JM, Phillips JA. 1997. Effects of incubation temperature and water potential on growth and thermoregulatory behavior of hatchling Cuban rock iguanas (Cyclura nubila). Copeia 1997, 766-776. ( 10.2307/1447294) [DOI] [Google Scholar]
- 33.Arnold SJ, Peterson CR, Gladstone J. 1995. Behavioural variation in natural populations. VII. Maternal body temperature does not affect juvenile thermoregulation in a garter snake. Anim. Behav. 50, 623-633. ( 10.1016/0003-3472(95)80124-3) [DOI] [Google Scholar]
- 34.Beltrán I, Durand V, Loiseleur R, Whiting MJ. 2020. Effect of early thermal environment on the morphology and performance of a lizard species with bimodal reproduction. J. Comp. Physiol. B 190, 795-809. ( 10.1007/s00360-020-01312-2) [DOI] [PubMed] [Google Scholar]
- 35.Blouin-Demers G, Kissner KJ, Weatherhead PJ. 2000. Plasticity in preferred body temperature of young snakes in response to temperature during development. Copeia 2000, 841-845. ( 10.1643/0045-8511(2000)000[0841:PIPBTO]2.0.CO;2) [DOI] [Google Scholar]
- 36.Dayananda B, Murray BR, Webb JK. 2017. Hotter nests produce hatchling lizards with lower thermal tolerance. J. Exp. Biol. 220, 2159-2165. ( 10.1242/jeb.152272) [DOI] [PubMed] [Google Scholar]
- 37.Goodman RM, Walguarnery JW. 2007. Incubation temperature modifies neonatal thermoregulation in the lizard Anolis carolinensis. J. Exp. Zool. Part A 307, 439-448. ( 10.1002/jez.397) [DOI] [PubMed] [Google Scholar]
- 38.Llewelyn J, Macdonald SL, Moritz C, Martins F, Hatcher A, Phillips BL. 2018. Adjusting to climate: acclimation, adaptation and developmental plasticity in physiological traits of a tropical rainforest lizard. Integr. Zool. 13, 411-427. ( 10.1111/1749-4877.12309) [DOI] [PubMed] [Google Scholar]
- 39.Nelson NJ, Keall SN, Hare KM. 2017. Temperature selection by juvenile tuatara (Sphenodon punctatus) is not influenced by temperatures experienced as embryos. J. Therm. Biol. 69, 261-266. ( 10.1016/j.jtherbio.2017.08.008) [DOI] [PubMed] [Google Scholar]
- 40.O'Steen SH. 1998. Embryonic temperature influences juvenile temperature choice and growth rate in snapping turtles Chelydra serpentina. J. Exp. Biol. 201, 439-449. ( 10.1242/jeb.201.3.439) [DOI] [PubMed] [Google Scholar]
- 41.Qualls CP, Andrews RM. 1999. Cold climates and the evolution of viviparity in reptiles: cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol. J. Linn. Soc. 67, 353-376. [Google Scholar]
- 42.Spotila JR, Zimmerman LC, Binckley CA, Grumbles JS, Rostal DC, List A Jr, Beyer EC, Phillips KM, Kemp SJ. 1994. Effects of incubation conditions on sex determination, hatching success, and growth of hatchling desert tortoises, Gopherus agassizii. Herpetol. Monogr. 1, 103-116. ( 10.2307/1467074) [DOI] [Google Scholar]
- 43.Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J. Stat. Softw. 2010; 36, 1-48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 44.Claussen DL. 1977. Thermal acclimation in ambystomatid salamanders. Comp. Biochem. Physiol. 58, 333-340. ( 10.1016/0300-9629(77)90150-5) [DOI] [Google Scholar]
- 45.Pottier P, Burke S, Drobniak SM, Lagisz M, Nakagawa S. 2021. Sexual (in)equality? A meta-analysis of sex differences in thermal acclimation capacity across ectotherms. Funct. Ecol. 35, 2663-2678. ( 10.1111/1365-2435.13899) [DOI] [Google Scholar]
- 46.Noble DWA, Lagisz M, O'Dea RE, Nakagawa S. 2017. Nonindependence and sensitivity analyses in ecological and evolutionary meta-analyses. Mol. Ecol. 26, 2410-2425. ( 10.1111/mec.14031) [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa S, Lagisz M, Jennions MD, Koricheva J, Noble DWA, Parker TH, Sánchez-Tójar A, Yang Y, O'Dea RE. 2022. Methods for testing publication bias in ecological and evolutionary meta-analyses. Methods Ecol. Evol. 13, 4-21. ( 10.1111/2041-210X.13724) [DOI] [Google Scholar]
- 48.Noble DWA, Pottier P, Lagisz M, Burke S, Drobniak SM, O'Dea RE, Nakagawa S. 2022. Meta-analytic approaches and effect sizes to account for ‘nuisance heterogeneity’ in comparative physiology. J. Exp. Biol. 225, Jeb243225. ( 10.1242/jeb.243225) [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa S, Lagisz M, O'Dea RE, Pottier P, Rutkowska J, Senior AM, Yang Y, Noble DW. 2023. orchaRd 2.0: An R package for visualizing meta-analyses with 2 orchard plots. EcoEvoRxiv. ( 10.32942/X2QC7K) [DOI] [Google Scholar]
- 50.Nakagawa S, Lagisz M, O'Dea RE, Rutkowska J, Yang Y, Noble DWA, Senior AM. 2021. The orchard plot: cultivating a forest plot for use in ecology, evolution, and beyond. Res. Synth. Methods 12, 4-12. ( 10.1002/jrsm.1424) [DOI] [PubMed] [Google Scholar]
- 51.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610-5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kellermann V, van Heerwaarden B, Sgrò CM, Hoffmann AA. 2009. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325, 1244-1246. [DOI] [PubMed] [Google Scholar]
- 53.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668-6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacLean HJ, Sørensen JG, Kristensen TN, Loeschcke V, Beedholm K, Kellermann V, Overgaard J. 2019. Evolution and plasticity of thermal performance: an analysis of variation in thermal tolerance and fitness in 22 Drosophila species. Phil. Trans. R. Soc. B 374, 20180548. ( 10.1098/rstb.2018.0548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enriquez-Urzelai U, Sacco M, Palacio AS, Pintanel P, Tejedo M, Nicieza AG. 2019. Ontogenetic reduction in thermal tolerance is not alleviated by earlier developmental acclimation in Rana temporaria. Oecologia 189, 385-394. ( 10.1007/s00442-019-04342-y) [DOI] [PubMed] [Google Scholar]
- 56.Gunderson AR, Fargevieille A, Warner DA. 2020. Egg incubation temperature does not influence adult heat tolerance in the lizard Anolis sagrei. Biol. Lett. 16, 20190716. ( 10.1098/rsbl.2019.0716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shine R, Harlow PS. 1996. Maternal manipulation of offspring phenotypes via nest-site selection in an oviparous lizard. Ecology 77, 1808-1817. ( 10.2307/2265785) [DOI] [Google Scholar]
- 58.Mitchell TS, Warner DA, Janzen FJ. 2013. Phenotypic and fitness consequences of maternal nest-site choice across multiple early life stages. Ecology 94, 336-345. ( 10.1890/12-0343.1) [DOI] [PubMed] [Google Scholar]
- 59.Bonduriansky R, Head M. 2007. Maternal and paternal condition effects on offspring phenotype in Telostylinus angusticollis (Diptera: Neriidae). J. Evol. Biol. 20, 2379-2388. ( 10.1111/j.1420-9101.2007.01419.x) [DOI] [PubMed] [Google Scholar]
- 60.Parker GA, Begon M. 1986. Optimal egg size and clutch size: effects of environment and maternal phenotype. Am. Nat. 128, 573-592. ( 10.1086/284589) [DOI] [Google Scholar]
- 61.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233-249. ( 10.1086/282487) [DOI] [Google Scholar]
- 62.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘‘cold-blooded’’ animals against climate warming. Proc. Natl Acad. Sci. USA 10, 3835-3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401. ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa S, Noble DWA, Senior AM, Lagisz M. 2017. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biol. 15, 1-14. ( 10.1186/s12915-016-0343-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang RY, Wild KH, Pottier P, Carrasco MI, Nakagawa S, Noble DWA. 2023. Code for: Developmental environments do not affect thermal physiology in reptiles: an experimental test and meta-analysis. Zenodo. ( 10.5281/zenodo.7700383) [DOI] [PMC free article] [PubMed]
- 66.Zhang RY, Wild KH, Pottier P, Carrasco MI, Nakagawa S, Noble DWA. 2023. Developmental environments do not affect thermal physiology in reptiles: an experimental test and meta-analysis. Figshare. ( 10.6084/m9.figshare.c.6620541) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhang RY, Wild KH, Pottier P, Carrasco MI, Nakagawa S, Noble DWA. 2023. Code for: Developmental environments do not affect thermal physiology in reptiles: an experimental test and meta-analysis. Zenodo. ( 10.5281/zenodo.7700383) [DOI] [PMC free article] [PubMed]
- Zhang RY, Wild KH, Pottier P, Carrasco MI, Nakagawa S, Noble DWA. 2023. Developmental environments do not affect thermal physiology in reptiles: an experimental test and meta-analysis. Figshare. ( 10.6084/m9.figshare.c.6620541) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Experimental and meta-analytic datasets and code are available from the Zenodo repository: https://doi.org/10.5281/zenodo.7700383 [65].
The data are provided in the electronic supplementary material [66].