Abstract
Camouflage has been reported as a defensive strategy in plants, while our understanding of the evolution of such defensive coloration is still limited. In the present study, we tested the hypothesis that camouflaged plants are shorter than non-camouflaged ones in the same habitat. Based on a species list from the subnival zone from the Hengduan Mountains, SW China and the herbarium collection, we measured the plant heights of 2915 individuals from 621 species (either camouflaged or not), with elevation information as a reference. We show that camouflaged plants were significantly shorter than non-camouflaged ones, though the effects of phylogeny and elevation were considered. Interestingly, a negative correlation between plant height and elevation was found in non-camouflaged plants, but not in camouflaged ones. These results revealed the correlation between defensive coloration and plant height. Camouflage may have evolved from shorter ancestors because they may suffer stronger selection and provide a more efficient defence.
Keywords: adaptive evolution, alpine plant, camouflage, plant apparency, plant defence, plant height
1. Introduction
Height is a crucial trait that has multiple functions in plant growth and reproduction. Taller plants are favoured for many reasons. They may live longer [1], capture more light, attract more pollinators [2,3] or disperse their seeds farther [4]. However, being tall may also have drawbacks. The old Chinese saying ‘树大招风’ (Shù Dà Zhāo Fēng) describes two facts, a taller tree suffers more in heavy wind, and the high-profile attracts more attacks. Abiotically, plants in the Arctic and alpine zones appear shorter [5,6]. Biotically, taller plants may be more receptive to herbivory by chewing herbivores [7]. Distinct plant individuals surrounded by lower vegetation received more eggs from butterflies [8]. On the contrary, shorter plants are favoured in such circumstances by decreasing heat loss in cold environments [5] (but see [9] for higher risk of frost damage in certain conditions) or lowering conspicuousness to herbivores [7,10].
Defensive strategies often correlate with plant size or age. For example, trees are more spinescent in their sapling stages [11,12] but become far less spinescent when they grow up [13]. For chemical weapons, the plant apparency hypothesis [10] predicts that the apparent (tall and long-lived woody) plants produce more general compounds (quantitative defence, e.g. the tannin in oak trees), whereas the unapparent (small and short-lived herb) use a small amount of specialized compounds (qualitative defence, e.g. glucosinolates in Brassicaceae).
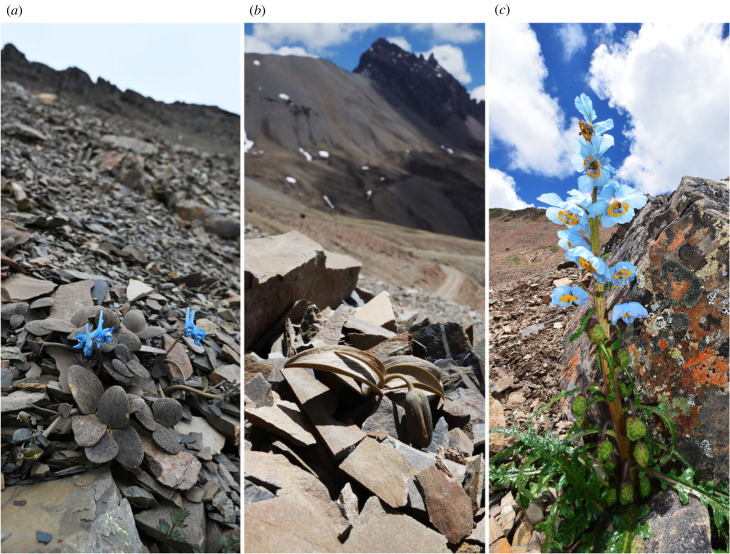
Alternatively, the often neglected first line of defence is to avoid being detected [14]. In the last decades, camouflage was reported in a number of plants worldwide as a defensive mechanism (reviewed in [15,16]). Cryptic coloration based on background matching, the most common camouflage strategy, was verified to decrease damage [17,18] and increase reproduction [19]. In the Hengduan Mountains., SW China, camouflage was found in different plant families in the subnival region, an alpine environment above the tree line but below permanent snowpack. The subnival region was characterized by low atmospheric pressure, strong temperature fluctuations and highly variable climates [5]. Vegetation in this region is sparse, and therefore food plants are much easier to be located by foraging herbivores [17,20,21]. The colour of camouflaged plants resembles the rock background of alpine screes, and some of them even show adaptive colour divergence among populations with different rock substrates (figure 1) [20]. A similar phenomenon was also found in the alpine region of New Zealand [22]. These camouflaged plants were suggested to have evolved under the selection by visual-searching herbivores, such as insects [17,18] and mammals [19] including humans [23].
Figure 1.
Examples of camouflaged and non-camouflaged plants in subnival region of Hengduan Mts, SW China. Dwarf camouflaged plant Corydalis hemidicentra (Papaveraceae) (a) and Fritillaria delavayi (Liliaceae) (b). Non-camouflaged herb Meconopsis speciosa (Papaveraceae) (c). Photo Credits: Yang Niu.
Based on field experiences, we realized that there may be an evolutionary correlation between defensive coloration and plant size (as suggested in [16]), but this has not been tested yet. First, camouflage may work more effectively in small-sized species than in taller ones [22], because they are easier to blend with the local substrates. Second, plants with smaller sizes are less likely to survive and reproduce after being attacked by herbivores, and therefore might suffer higher selection pressure to evolve camouflage [16]. Given that plant size may include several dimensions such as length, width, height, area and volume, it is difficult to estimate it as a general term. Plant height often (if not always) positively correlates with plant size and is more often reported in databases. Therefore, we hypothesize that camouflage plants are shorter than non-camouflage ones in similar habitats.
An obvious difficulty to test this hypothesis is that plant height is influenced by several abiotic factors, such as elevation [6,24,25]. For example, it is well known that plant size often decreases with increasing elevation [26,27]. Like other traits, plant height may also be influenced by phylogeny [28], which may affect the relationship between plant height and camouflage. However, these factors can be accounted for by incorporating elevation and phylogenetic structure into analyses. To test this prediction, we first compiled a list of camouflaged and non-camouflaged plants based on the flora of the subnival Hengduan Mts and field observations. We then measured plant height from herbarium specimens and tested whether camouflaged plants are shorter than non-camouflaged plants accounting for phylogeny or elevation.
2. Materials and methods
(a) . Plant list and herbarium specimens
To decrease the influence of factors other than coloration on plant height, we excluded plant species from other regions. For this, the flora Seed Plants of the Alpine Subnival Belt from the Hengduan Mountains, SW China [29] was used as the raw plant list, which includes 942 species (168 genera and 48 families). Compared with alpine flora in other regions, this flora used much stricter criteria to define the subnival region, which mainly includes the alpine screes and two other vegetation ‘types’, the rocky meadow and the cushion plant vegetation in the same elevation as alpine screes. We used an even stricter standard to collect data from herbarium specimens as follows.
Based on this list (excluding synonyms), we searched the digitalized specimens at the Chinese Virtual Herbarium (www.cvh.ac.cn/index.php) by setting four filtering keywords, i.e. locality, elevation, specimen condition and scale bars. To ensure the specimen was collected from the Hengduan Mts region, the collection location was set as ‘Yunnan | Sichuan | Qinghai | Tibet Autonomous Regions, China’. To ensure the specimen was collected from the subnival region, we set the lowest elevation as 4200 m.a.s.l., which is the approximate elevation of the highest tree line in this area. To exclude the influence of age on plant height, we only included adult specimens (with flowers) in the study. For height measurements, only specimens with roots and scale bars were used. Our final sampling included 621 species.
Elevations were averaged for specimens that only recorded elevation range (e.g. 4550 m for a record of ‘4500–4600 m’). When possible, we recorded plant height from 10 individuals from different specimens for each species (N = 42). For species with limited specimen numbers, all individuals were recorded (N = 579); 72% (N = 448) of species were sampled from at least three individuals. Species were scored as camouflaged/non-camouflaged, according to the living condition of the plants (instead of the dry specimen). Specifically, leaf colour that is very similar to the background as perceived by human eyes is defined as camouflage (figure 1). Ideally, reflectance spectra and animals' colour vision should be used to estimate plant colour, but given limited studies on this region, such data were only available in a few species, such as Corydalis benecincta [18], C. hemidicentra [20], C. bulbifera, Fritillaria delavayi [23], Saussurea medusa and Soroseris glomerata. Other camouflaged species were evaluated by human eyes. Although different from other animals, human colour vision has been proven to be robust enough to define camouflaged plants [23]. All published cases of camouflaged plants that were initially defined based on human eyes were then verified based on the analyses of reflectance spectra and animal colour vision (see [16]). Species that have both camouflage and non-camouflage morphs, such as Corydalis hemidicentra and Fritillria delavayi, were defined as camouflage.
(b) . Height measurement
We used ImageJ 1.44 [30] to measure plant height in the specimen image. Each individual was measured three times before averaging, accurate to 0.01 cm. Plant height was defined as the vertical length of the aboveground part, as this trait is the target of selection by visual-searching animals. It was measured as the vertical distance from the base of the stem to the top of the plant, leaving the underground stem or root excluded.
To avoid estimating plant height from incomplete specimens, we excluded those species where plants were larger than the herbarium sheet (N = 18). All these cases were non-camouflaged plants.
(c) . Statistical analyses
As the sample sizes vary among species (from one to 10, with a mean sample size of five), we used the mean plant height for each species (N = 621) for further analyses. Plant height was log-transformed to improve normality.
The general pattern of height differences between camouflaged and non-camouflaged plants was first compared by independent samples t-test. To decrease the influence of unequal sample size (Ncamouflaged = 45; Nnon-camouflaged = 576), 45 samples were randomly selected from the non-camouflaged group before comparing with the camouflaged group. This was repeated 1000 times and yielded 1000 p-values. We then examined the correlation between plant height and elevation using the Spearman rho method.
To account for possible effects of elevation and phylogeny, we used Bayesian phylogenetic mixed models in the MCMCglmm R packages [31]. Specifically, we ran four models as follows: (i) plant coloration (camouflage or not) and elevation as fixed variables; (ii) only plant coloration as the fixed variable; (iii) only elevation as the fixed variable and (iv) null model (only containing intercept). The phylogeny was set as a random effect in all models. Models with lower DIC values perform better. The phylogenetic tree of the 621 species was constructed based on the V.PhyloMaker [32] package. For species that do not match in the V.PhyloMaker database, the congenic clade was randomly inserted based on the genus name. This model was run for 300 000 iterations with 1000 burn-in and a thinning interval of 500. After running those models, we examined the effective sample size to make sure that it was above 200. ITOL v.6 [33] was used to visualize the phylogenetic tree (electronic supplementary material, figure S1). All the analyses were performed in R v. 4.1.1 [34].
3. Results
Collectively, we sampled 2915 individuals of 621 species (Nmean = 5, Nmin = 1, Nmax = 10, from 123 genera and 40 families) that were found in the subnival environment ranges from 4200 to 6500 m.a.s.l from the Hengduan Mountains. These include 45 camouflaged plant species and 576 non-camouflaged ones. Plant height ranges from 1.58 to 45.5 cm, with a mean value of 11.46 cm.
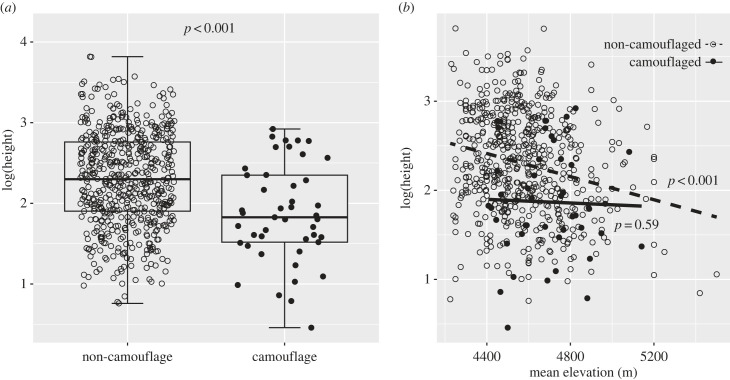
Camouflaged plants were significantly shorter than non-camouflaged ones (independent samples t-test, t = 4.739, d.f. = 619, p < 0.001, figure 2a). This pattern held when equal sample sizes were used by randomly selecting samples from the non-camouflage group. For 1000 times, random sampling followed by t-tests, p < 0.05 occurred 989 times (98%). In addition, we found a significant negative relationship between plant height and elevation in non-camouflaged plants (r = −0.2, d.f. = 574, p < 0.001), but not in camouflaged ones (r = −0.08, d.f. = 43, p = 0.59, figure 2b).
Figure 2.
The pattern of plant height in non-camouflaged and camouflaged plants (measures from multiple individuals were averaged for each species). (a) Mean plant height. (b) Correlation between plant height and elevation.
Based on MCMCglmm, plant height was found to be influenced by coloration (i.e. camouflaged or not, p < 0.002), elevation (p < 0.002) and phylogeny (λmean = 0.77), as shown in table 1. Coloration, with higher marginal R2 value (table 1), has a stronger effect on plant height than elevation.
Table 1.
The effect of coloration and elevation on plant height based on MCMCglmm including phylogeny as a random factor. Note: β is the regression coefficient. Marginal R2 describes the proportion of variance explained by the fixed effects only, while the conditional R2 describes variance explained by both the fixed and random effects. Both were calculated according to Nakagawa & Schielzeth [35]. Values in italics indicate significant differences; characters in bold indicate variables.
| β(95% CI) | marginal R2 | conditional R2 | pMCMC | |
|---|---|---|---|---|
| fixed effects | ||||
| coloration | −0.3622(−0.5224 to −0.2188) | 0.016 | 0.74 | <0.002 |
| elevation | −0.0005(−0.0007 to −0.0003) | 0.014 | 0.78 | <0.002 |
| random effects | ||||
| phylogeny | 0.6092(0.3927 to 0.8488) | _ | ||
4. Discussion
As the results show, plants in the subnival zone of Hengduan Mts are relatively short (with a mean height of 11.46 cm) and even shorter than plants in the Arctic tundra (ca 25 cm, see [6]). In general, this may be an adaptation to the alpine environment with extreme temperature fluctuations [36], strong winds [5,37], nutrient-poor soils [38] or low-temperatures that limit photosynthetic capacity [39]. We found 45 camouflaged plants (with cryptic coloration) in this region. As expected, camouflaged plants are indeed shorter than non-camouflaged plants that live in the same region. The results of MCMCglmm showed that when phylogenetic history was considered, both coloration and elevation have a significant relationship with plant height. The model including both factors, coloration and elevation, preforms better. Interestingly, coloration is an even greater explainer than elevation. It is well known that plant height decreases with increasing latitude or elevation because of climate differences (although exceptions do exist, see [1]). Our results suggest that herbivory may be a neglected factor that influences plant height. In open habitats such as alpine screes, vegetation is sparse and plants do not need to grow taller to compete for sunlight. Instead, considering the interaction between plants and animals, such a habitat forms a much simpler visually searching environment, which may result in stronger selection pressure from herbivores [21]. Taller plants may be selected against as they are likely to be more easily located by herbivores, while being short-statured may be favoured under such selection.
The shorter camouflaged plant pattern may have two non-exclusive evolutionary explanations, shorter plants become camouflaged or camouflaged plants become shorter. The former process seems more reasonable for at least three reasons. First, short or small organisms are suggested to be intrinsically less visually conspicuous and have a lower detection risk compared to larger ones [40,41]. Second, backgrounds are often more diverse at smaller size scales, providing complex visual environments that are known to facilitate camouflage [42]. Third, plants exhibit modular organization. When discovered and consumed, larger individuals possessing more modules may have an increased chance of surviving or reproducing, whereas smaller individuals are more vulnerable to death. Consequently, smaller individuals may experience greater selective pressures during herbivory [16]. On the contrary, camouflage may not be compatible with a large ancestor status because of inefficient concealment. Similarly, selection pressure for being camouflaged could be less intense for larger plants as they are more tolerant to herbivory [16]. The evolutionary sequences of plant height and coloration can be inferred through phylogenetic reconstruction of specific plant groups. For example, the Corydalis genus consists of approximately 530 species, a few of which are camouflaged, while most members have normal green leaves. Based on a recently reconstructed phylogeny of this genus, camouflaged species are found scattered among several alpine clades that contain many non-camouflaged, short-statured relatives [43]. This observation is consistent with the hypothetic scenario that the evolution of short stature preceded that of camouflage.
Consistent with the association between plant height and camouflage, we noticed that camouflage has often been found in small herbs [17–20], juveniles [22,44,45] and seeds [46–48]. For example, Corydalis benecincta and C. hemidicentra, two camouflaged species involved in the current study, have similar small trifoliate leaves and very short stature (5.00 and 6.55 cm, respectively). Another camouflaged congener C. bulbifera only has a single small leaf. These plants are the host plant of Parnassius butterflies, the larvae of which often consume the whole plant. As a comparison, a coexisting non-host Corydalis species, Corydalis conspersa, has many leaves and is taller (17.02 cm). In addition, before growing above 3 m, the leaves of Pseudoanax crassifolius from New Zealand have cryptic coloration and spinulous margins [44]. Camouflage is also reported in seeds of Acmispon wrangelianus (Fabaceae) [48] and Pinus halepensis (Pinaceae) [47].
The correlation between camouflage and short plant height parallels many animal systems. For example, smaller caterpillars tend to be concealed more, whereas larger caterpillars use large eyespots as defence more often [49]. Juvenile crabs Carcinus maenas have more camouflage variation than adults [50]. Cryptic morphs of the strawberry poison frog Oophaga pumilio are smaller than conspicuous ones [51]. In addition, the ontogenetic colour changes from camouflage to aposematism is common, such as in the larvae of moths and butterflies [52,53], but the reverse process is rare.
The present study also highlights the value of high-quality botanical specimens and herbaria in understanding plant evolution. Naturalists can indeed and should seek questions and explanations from the field, but herbarium specimens provide convenient and fast ways to examine general patterns.
Acknowledgements
We thank Q. Y. Zhang and W. Y. Dang for their assistance in plant height data collection.
Contributor Information
Hang Sun, Email: sunhang@mail.kib.ac.cn.
Yang Niu, Email: niuyang@mail.kib.ac.cn.
Data accessibility
The data are provided in the electronic supplementary material [54].
Authors' contributions
T.H.: resources, software, visualization, writing—original draft and writing—review and editing; Z.C.: methodology, resources, software and writing—review and editing; B.X.: data curation and writing—review and editing; H.S.: supervision, writing—original draft and writing—review and editing; Y.N.: conceptualization, investigation, methodology, supervision, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was funded by NSFC (grant no. 31971569 to Y.N.), The National Youth Talent Support Program and Yunnan Youth Talents Plan (grant no. YNWR-QNBJ-2018-183 to Y.N.), the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDA20050203) and the Second Tibetan Plateau Scientific Expedition and Research (STEP) programme (grant no. 2019QZKK0502).
References
- 1.Smith AP. 1980. The paradox of plant height in an Andean giant rosette species. J. Ecol. 68, 63-73. ( 10.2307/2259244) [DOI] [Google Scholar]
- 2.Donnelly SE, Lortie CJ, Aarssen LW. 1998. Pollination in Verbascum thapsus (Scrophulariaceae): the advantage of being tall. Am. J. Bot. 85, 1618-1625. ( 10.2307/2446490) [DOI] [PubMed] [Google Scholar]
- 3.Althoff DM, Segraves KA, Pellmyr O. 2005. Community context of an obligate mutualism: pollinator and florivore effects on Yucca filamentosa. Ecology 86, 905-913. ( 10.1890/04-1454) [DOI] [Google Scholar]
- 4.Thomson FJ, Moles AT, Auld TD, Kingsford RT. 2011. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 99, 1299-1307. ( 10.1111/j.1365-2745.2011.01867.x) [DOI] [Google Scholar]
- 5.Körner C. 2021. Alpine plant life: functional plant ecology of high mountain ecosystems. Cham, Switzerland: Springer. [Google Scholar]
- 6.Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR. 2009. Global patterns in plant height. J. Ecol. 97, 923-932. ( 10.1111/j.1365-2745.2009.01526.x) [DOI] [Google Scholar]
- 7.Castagneyrol B, Giffard B, Péré C, Jactel H. 2013. Plant apparency, an overlooked driver of associational resistance to insect herbivory. J. Ecol. 101, 418-429. ( 10.1111/1365-2745.12055) [DOI] [Google Scholar]
- 8.Valdés A, Ehrlén J. 2018. Direct and plant trait-mediated effects of the local environmental context on butterfly oviposition patterns. Oikos 127, 825-833. ( 10.1111/oik.04909) [DOI] [Google Scholar]
- 9.Jordan D, Smith W. 1994. Energy balance analysis of nighttime leaf temperatures and frost formation in a subalpine environment. Agric. For. Meteorol. 71, 359-372. ( 10.1016/0168-1923(94)90020-5) [DOI] [Google Scholar]
- 10.Feeny P. 1976. Plant apparency and chemical defense. In Biochemical interaction between plants and insects . Boston, MA: Springer. [Google Scholar]
- 11.Barton KE, Koricheva J. 2010. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am. Nat. 175, 481-493. ( 10.1086/650722) [DOI] [PubMed] [Google Scholar]
- 12.Ochoa-López S, Villamil N, Zedillo-Avelleyra P, Boege K. 2015. Plant defence as a complex and changing phenotype throughout ontogeny. Ann. Bot. 116, 797-806. ( 10.1093/aob/mcv113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark L, Burns K. 2015. The ontogeny of leaf spines: progressive versus retrogressive heteroblasty in two New Zealand plant species. N. Z. J. Bot. 53, 15-23. ( 10.1080/0028825X.2014.997254) [DOI] [Google Scholar]
- 14.Lev-Yadun S. 2019. Defensive (anti-herbivory) Batesian mimicry in plants. Isr. J. Plant Sci. 66, 34-51. ( 10.1163/22238980-00001044) [DOI] [Google Scholar]
- 15.Lev-Yadun S. 2016. Defensive (anti-herbivory) coloration in land plants. Cham, Switzerland: Springer. [Google Scholar]
- 16.Niu Y, Sun H, Stevens M. 2018. Plant camouflage: ecology, evolution, and implications. Trends Ecol. Evol. 33, 608-618. ( 10.1016/j.tree.2018.05.010) [DOI] [PubMed] [Google Scholar]
- 17.Strauss SY, Cacho NI. 2013. Nowhere to run, nowhere to hide: the importance of enemies and apparency in adaptation to harsh soil environments. Am. Nat. 182, E1-14. ( 10.1086/670754) [DOI] [PubMed] [Google Scholar]
- 18.Niu Y, Chen G, Peng DL, Song B, Yang Y, Li ZM, Sun H. 2014. Grey leaves in an alpine plant: a cryptic colouration to avoid attack? New Phytol. 203, 953-963. ( 10.1111/nph.12834) [DOI] [PubMed] [Google Scholar]
- 19.Klooster MR, Clark DL, Culley TM. 2009. Cryptic bracts facilitate herbivore avoidance in the mycoheterotrophic plant Monotropsis odorata (Ericaceae). Am. J. Bot. 96, 2197-2205. ( 10.3732/ajb.0900124) [DOI] [PubMed] [Google Scholar]
- 20.Niu Y, Chen Z, Stevens M, Sun H. 2017. Divergence in cryptic leaf colour provides local camouflage in an alpine plant. Proc. R. Soc. B 284, 20171654. ( 10.1098/rspb.2017.1654) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strauss SY, Cacho NI, Schwartz MW, Schwartz AC, Burns KC. 2015. Apparency revisited. Entomol. Exp. Appl. 157, 74-85. ( 10.1111/eea.12347) [DOI] [Google Scholar]
- 22.Fadzly N, Burns K. 2010. Hiding from the ghost of herbivory past: evidence for crypsis in an insular tree species. Int. J. Plant Sci. 171, 828-833. ( 10.1086/654850) [DOI] [Google Scholar]
- 23.Niu Y, Stevens M, Sun H. 2021. Commercial harvesting has driven the evolution of camouflage in an alpine plant. Curr. Biol. 31, 446-449. ( 10.1016/j.cub.2020.10.078) [DOI] [PubMed] [Google Scholar]
- 24.Kappelle M, Van Uffelen JG, Cleef AM. 1995. Altitudinal zonation of montane Quercus forests along two transects in Chirripó National Park, Costa Rica. Vegetatio 119, 119-153. ( 10.1007/BF00045594) [DOI] [Google Scholar]
- 25.Wilcke W, Oelmann Y, Schmitt A, Valarezo C, Zech W, Homeier J. 2008. Soil properties and tree growth along an altitudinal transect in Ecuadorian tropical montane forest. J. Plant Nutr. Soil Sci. 171, 220-230. ( 10.1002/jpln.200625210) [DOI] [Google Scholar]
- 26.Pellissier L, Fournier B, Guisan A, Vittoz P. 2010. Plant traits co-vary with altitude in grasslands and forests in the European Alps. Plant Ecol. 211, 351-365. ( 10.1007/s11258-010-9794-x) [DOI] [Google Scholar]
- 27.Körner C, Neumayer M, Menendez-Riedl SP, Smeets-Scheel A. 1989. Functional morphology of mountain plants. Flora 182, 353-383. ( 10.1016/S0367-2530(17)30426-7) [DOI] [Google Scholar]
- 28.Liu H, Xu Q, He P, Santiago LS, Yang K, Ye Q. 2015. Strong phylogenetic signals and phylogenetic niche conservatism in ecophysiological traits across divergent lineages of Magnoliaceae. Sci. Rep. 5, 1-12. ( 10.1038/srep12246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu B, Li ZM, Sun H. 2014. Seed plants of alpine subnival in hengduan mountains SW, China. Beijing, China: Science Press. [Google Scholar]
- 30.Ferreira T, Rasband W. 2012. ImageJ user guide. ImageJ/Fiji 1, 155-161. [Google Scholar]
- 31.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1-22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 32.Jin Y, Qian H. 2019. V.PhyloMaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353-1359. ( 10.1111/ecog.04434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letunic I, Bork P. 2021. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293-W296. ( 10.1093/nar/gkab301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.r-project.org/. [Google Scholar]
- 35.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133-142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 36.Lffler J, Pape R. 2020. Thermal niche predictors of alpine plant species. Ecology 101, 1-17. ( 10.1002/ecy.2891) [DOI] [PubMed] [Google Scholar]
- 37.Hadley JL, Smith WK. 1989. Wind erosion of leaf surface wax in alpine Timberline conifers. Arct. Alp. Res. 21, 392-398. ( 10.1080/00040851.1989.12002752) [DOI] [Google Scholar]
- 38.Hong J, Ma X, Yan Y, Zhang X, Wang X. 2018. Which root traits determine nitrogen uptake by alpine plant species on the Tibetan Plateau? Plant. Soil. 424, 63-72. ( 10.1007/s11104-017-3434-3) [DOI] [Google Scholar]
- 39.Hacker J, Neuner G. 2006. Photosynthetic capacity and PSII efficiency of the evergreen alpine cushion plant Saxifraga paniculata during winter at different altitudes. Arct. Antarct. Alp. Res. 38, 198-205. ( 10.1657/1523-0430(2006)38[198:PCAPEO]2.0.CO;2) [DOI] [Google Scholar]
- 40.Remmel T, Tammaru T. 2009. Size-dependent predation risk in tree-feeding insects with different colouration strategies: a field experiment. J. Anim. Ecol. 78, 973-980. ( 10.1111/j.1365-2656.2009.01566.x) [DOI] [PubMed] [Google Scholar]
- 41.Barnett JB, Yeager J, McEwen BL, Kinley I, Anderson HM, Guevara J. 2022. Size-dependent colouration balances conspicuous aposematism and camouflage. J. Evol. Biol. 00, 1-10. ( 10.1111/jeb.14143) [DOI] [PubMed] [Google Scholar]
- 42.Xiao F, Cuthill IC. 2016. Background complexity and the detectability of camouflaged targets by birds and humans. Proc. R. Soc. B 283, 20161527. ( 10.1098/rspb.2016.1527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J-T, et al. 2023. An updated classification for the hyper-diverse genus Corydalis (Papaveraceae: Fumarioideae) based on phylogenomic and morphological evidence. J. Integr. Plant Biol. ( 10.1111/jipb.13499) [DOI] [PubMed] [Google Scholar]
- 44.Fadzly N, Jack C, Schaefer HM, Burns K. 2009. Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds? New Phytol. 184, 495-501. ( 10.1111/j.1469-8137.2009.02926.x) [DOI] [PubMed] [Google Scholar]
- 45.Fadzly N, Zuharah WF, Mansor A, Zakaria R. 2016. Cryptic coloration of Macaranga bancana seedlings: a unique strategy for a pioneer species. Plant Signal. Behav. 11, e1197466. ( 10.1080/15592324.2016.1197466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nystrand O, Granström A. 1997. Post-dispersal predation on Pinus sylvestris seeds by Fringilla spp: ground substrate affects selection for seed color. Oecologia 110, 353-359. ( 10.1007/s004420050169) [DOI] [PubMed] [Google Scholar]
- 47.Lev-Yadun S, Ne'eman G. 2013. Bimodal colour pattern of individual Pinus halepensis Mill. seeds: a new type of camouflage. Biol. J. Linn. Soc. Lond. 109, 271-278. ( 10.1111/bij.12047) [DOI] [Google Scholar]
- 48.Porter SS. 2013. Adaptive divergence in seed color camouflage in contrasting soil environments. New Phytol. 197, 1311-1320. ( 10.1111/nph.12110) [DOI] [PubMed] [Google Scholar]
- 49.Hossie TJ, Skelhorn J, Breinholt JW, Kawahara AY, Sherratt TN. 2015. Body size affects the evolution of eyespots in caterpillars. Proc. Natl Acad. Sci. USA 112, 6664-6669. ( 10.1073/pnas.1415121112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens M, Lown AE, Wood LE. 2014. Camouflage and individual variation in shore crabs (Carcinus maenas) from different habitats. PLoS ONE 9, e115586. ( 10.1371/journal.pone.0115586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rudh A. 2013. Loss of conspicuous coloration has co-evolved with decreased body size in populations of poison dart frogs. Evol. Ecol. 27, 755-767. ( 10.1007/s10682-013-9649-8) [DOI] [Google Scholar]
- 52.Grant JB. 2007. Ontogenetic colour change and the evolution of aposematism: a case study in panic moth caterpillars. J. Anim. Ecol. 76, 439-447. ( 10.1111/j.1365-2656.2007.01216.x) [DOI] [PubMed] [Google Scholar]
- 53.Valkonen JK, Nokelainen O, JokimãKi M, Kuusinen E, Paloranta M, Peura M, Mappes J. 2014. From deception to frankness: benefits of ontogenetic shift in the anti-predator strategy of alder moth Acronicta alni larvae. Curr. Zool. 60, 114-122. ( 10.1093/czoolo/60.1.114) [DOI] [Google Scholar]
- 54.Huang T, Chen Z, Xu B, Sun H, Niu Y. 2023. Camouflaged plants are shorter than noncamouflaged plants in the alpine zone. Figshare. ( 10.6084/m9.figshare.c.6607501) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are provided in the electronic supplementary material [54].