Abstract
Background:
Muscle atrophy is common after an injury to the knee and anterior cruciate ligament reconstruction (ACLR). Blood flow restriction therapy (BFR) combined with low-load resistance exercise may help mitigate muscle loss and improve the overall condition of the lower extremity (LE).
Purpose:
To determine whether BFR decreases the loss of LE lean mass (LM), bone mass, and bone mineral density (BMD) while improving function compared with standard rehabilitation after ACLR.
Study Design:
Randomized controlled clinical trial
Methods:
A total of 32 patients undergoing ACLR with bone-patellar tendon-bone autograft were randomized into 2 groups (CONTROL: N = 15 [male = 7, female = 8; age = 24.1 ± 7.2 years; body mass index [BMI] = 26.9 ± 5.3 kg/m2] and BFR: N = 17 [male = 12, female = 5; age = 28.1 ± 7.4 years; BMI = 25.2 ± 2.8 kg/m2]) and performed 12 weeks of postsurgery rehabilitation with an average follow-up of 2.3 ± 1.0 years. Both groups performed the same rehabilitation protocol. During select exercises, the BFR group exercised under 80% arterial occlusion of the postoperative limb (Delfi tourniquet system). BMD, bone mass, and LM were measured using DEXA (iDXA, GE) at presurgery, week 6, and week 12 of rehabilitation. Functional measures were recorded at week 8 and week 12. Return to sport (RTS) was defined as the timepoint at which ACLR-specific objective functional testing was passed at physical therapy. A group-by-time analysis of covariance followed by a Tukey’s post hoc test were used to detect within- and between-group changes. Type I error; α = 0.05.
Results:
Compared with presurgery, only the CONTROL group experienced decreases in LE-LM at week 6 (−0.61 ± 0.19 kg, −6.64 ± 1.86%; P < 0.01) and week 12 (−0.39 ± 0.15 kg, −4.67 ± 1.58%; P = 0.01) of rehabilitation. LE bone mass was decreased only in the CONTROL group at week 6 (−12.87 ± 3.02 g, −2.11 ± 0.47%; P < 0.01) and week 12 (−16.95 ± 4.32 g,−2.58 ± 0.64%; P < 0.01). Overall, loss of site-specific BMD was greater in the CONTROL group (P < 0.05). Only the CONTROL group experienced reductions in proximal tibia (−8.00 ± 1.10%; P < 0.01) and proximal fibula (−15.0±2.50%,P < 0.01) at week 12 compared with presurgery measures. There were no complications. Functional measures were similar between groups. RTS time was reduced in the BFR group (6.4 ± 0.3 months) compared with the CONTROL group (8.3 ± 0.5 months; P = 0.01).
Conclusion:
After ACLR, BFR may decrease muscle and bone loss for up to 12 weeks postoperatively and may improve time to RTS with functional outcomes comparable with those of standard rehabilitation.
Keywords: ACL, anterior cruciate ligament, blood flow restriction, rehabilitation
Anterior cruciate ligament (ACL) tears are common sports injuries whereby quadriceps weakness and atrophy are of primary concern after surgery. 25 Muscular strength imbalance and deficiencies in the knee extensors increase the probability of an unsuccessful return to sport (RTS) and/or reinjury.5,34,44 Loss of bone mineral density (BMD) in the immobilized limb may also contribute to postoperative periarticular or patellar fractures.4,43 Because of functional limitations immediately after surgery, therapists are challenged to minimize the deleterious effects of unloading while promoting recovery.
Blood flow restriction therapy (BFR) uses a specialized cuff applied around the proximal limb that restricts vascular flow via either automated or manual compression. 36 Modified from a form of resistance training known as “Kaastu training,” therapies employing BFR have been shown to stimulate muscle anabolism. 29 When combined with low-intensity resistance exercise (LIX; <30% of maximal strength, 30% 1-repetition maximum [1RM]), BFR has been reported to increase fatigue resistance and strength in a manner comparable with that of high-intensity resistance exercise (HIX) in healthy adults,10,37,57 via mechanisms associated with metabolic and mechanical stress sensing. 29 As a result, BFR-LIX has become popular in rehabilitation to reduce muscle atrophy while preserving strength and function at safe resistance loads.24,45 However, the impact of BFR-LIX on clinical outcomes such as functional measures and RTS timelines after ACL reconstruction (ACLR) are not well known. While BFR does not prevent postoperative reductions in mechanical loading of bone, exercise intensity-dependent effectors involved in the regulation of bone metabolism may still play a role in bone preservation after surgery.21,22 Information regarding the efficacy of BFR-LIX for bone preservation during chronic postsurgery rehabilitation would provide insight with regard to reducing fracture risk or improving bone-patellar tendon-bone (BTB) autograft healing.
In light of previous observations regarding the therapeutic efficacy of BFR-LIX, the authors hypothesized that the use of BFR would decrease the loss of lower extremity lean muscle mass (LE-LM), bone mass, and BMD, and would decrease RTS time and improve functional measures.
Methods
The institutional review board for research involving human subjects approved all protocols presented here. Before beginning the investigation, data from previous training investigations,7,26,30 as well as from pilot BFR investigations, 27 were analyzed. Based on a power of 0.80 at α = 0.05 with a minimum between-group detectable effect size (ES) of 0.5 and within-group detectable difference (pre- to postrehabilitation) of 5% in leg LM and BMD at the distal femur and proximal tibia, it was determined that a minimum of 15 participants per group would be required. Therefore, a target of 20 participants per group was set to account for potential dropouts.
Patients between the ages of 16 and 39 years who sustained a primary tear of the ACL scheduled to undergo ACLR with a BTB autograft were recruited. They were required to complete a minimum of 6 months of supervised outpatient physical therapy at the designated study site. After consent, patients were randomized into a standard rehabilitation group (CONTROL) or a standard rehabilitation group with the addition of BFR (CONSORT Diagram; Figure 1). Both groups performed12 weeks of progressive rehabilitation (rehab) (twice weekly) following the same protocol with a licensed physical therapist beginning within 7 days postsurgery. ACLR was performed by 1 of 2 sports medicine fellowship-trained surgeons at 1 institution.
Figure 1.
CONSORT diagram. BFR, blood flow restriction therapy; Post-Op, postoperatively; Pre-Op, preoperatively; rehab, rehabilitation.
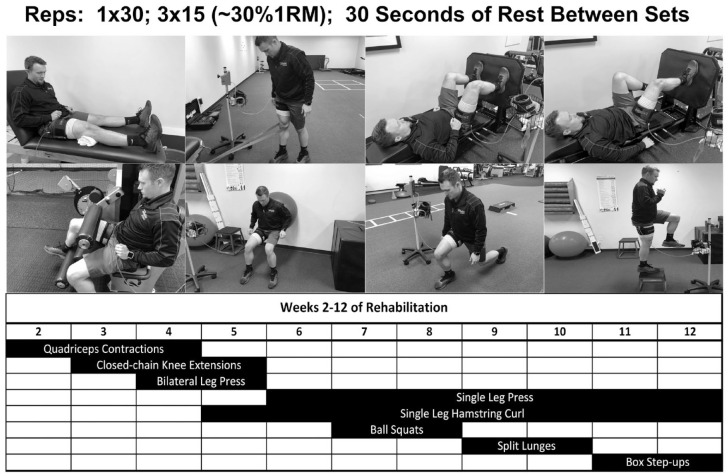
Baseline demographics and concomitant procedures are shown in Table 1 with no differences detected between group or between limbs within group. In addition, time between initial clinic visit and date of surgery did not differ between groups (BFR, 65 ± 30 days; CONTROL, 65 ± 36 days). During select exercises within the rehab protocol, the BFR group exercised under conditions of 80% arterial limb occlusion pressure (80%LOP) (Figure 2). LOP was determined and applied using an automated tourniquet around the proximal thigh (Delfi Medical). LOP was maintained and adjusted in real time with each contraction. Resistance was set at 20%1RM assessed in the contralateral limb. Exercises were performed for 4 sets of 30-15-15-15 repetitions separated by 30 seconds of rest as described in previous investigations. 29
Table 1.
Baseline patient demographics and concomitant procedures a
| Demographics | |||||
|---|---|---|---|---|---|
| Group | Age, years | Height, cm | Weight, kg | BMI, kg/m2 | Lean Mass, kg |
| BFR (m12, f5) | 28.1 ± 7.4 | 173.6 ± 9.2 | 76.6 ± 15.5 | 25.2 ± 2.8 | 52.5 ± 9.0 |
| CONTROL (m7, f8) | 24.1 ± 7.2 | 170.9 ± 12.4 | 79.3 ± 22.0 | 26.9 ± 5.3 | 52.6 ± 13.1 |
| Lower Extremity Lean Mass and Bone Mass | |||||
| Group | Lean Mass, kg | Bone Mass, g | |||
| Whole Limb | Thigh (Distal 2/3) |
Whole Limb | Femur (Distal 2/3) |
||
| BFR | |||||
| Injured limb | 8.9 ± 1.5 | 3.2 ± 0.6 | 574.7 ± 101.1 | 158.2 ± 26.6 | |
| Healthy limb | 9.1 ± 1.7 | 3.3 ± 0.7 | 588.9 ± 106.6 | 163.6 ± 28.4 | |
| CONTROL | |||||
| Injured limb | 9.1 ± 1.7 | 3.2 ± 0.9 | 560.6 ± 132.1 | 154.3 ± 36.4 | |
| Healthy limb | 9.3 ± 1.4 | 3.3 ± 1.0 | 562.0 ± 130.9 | 155.1 ± 37.4 | |
| Site-specific Bone Mineral Density (g/cm2) | |||||
| Group | Distal Femur | Proximal Tibia | Proximal Fibula | ||
| BFR | |||||
| Injured limb | 1.19 ± 0.20 | 1.47 ± 0.16 | 0.45 ± 0.11 | ||
| Healthy limb | 1.21 ± 0.19 | 1.47 ± 0.15 | 0.47 ± 0.10 | ||
| CONTROL | |||||
| Injured limb | 1.20 ± 0.22 | 1.47 ± 0.21 | 0.47 ± 0.11 | ||
| Healthy limb | 1.21 ± 0.25 | 1.46 ± 0.20 | 0.47 ± 0.12 | ||
| Concomitant Procedures | |||||
| Group | None | PMMx | PLMx | MMR | LMR |
| BFR | 9 | 1 | 0 | 4 | 4 |
| CONTROL | 9 | 0 | 1 | 4 | 2 |
BFR, blood flow restriction therapy; BMI, body mass index; LMR, lateral meniscal repair; MMR, medial meniscal repair; PLMx, partial lateral meniscectomy; PMMx, partial medial meniscectomy.
Data are presented as mean ± SD for baseline patient characteristics. No significant differences detected between groups or between limbs within group at baseline.
Figure 2.
Exercises and exercise progression chart. 1RM, 1-repetition maximum.
Functional assessments were performed at 8 and 12 weeks postsurgery in accordance with standardized therapy protocols for the following: single-leg (SL) squat (best of 3 attempts), SL eccentric step-down (reps to fatigue), Y-balance (best of 3 attempts),11,51 SL leg press (1RM), and SL hamstring curl (1RM). BMD, bone mass, and LM were measured using dual-energy-x-ray-absorptiometry (DXA; iDXA, GE) at presurgery as well as week 6 and week 12 of rehabilitation. All scans were performed by a licensed radiation technician blinded to study group assignment. Additionally, using techniques similar to those reported by Lambert et al 26 and Chapeleau et al 9 , site-specific DXA knee scans were also used for site-specific measurements of BMD at the knee taken at 3 cm proximal to the end of the distal femur, 3 cm distal to the tibial plateau, and in the center of the head of the fibula.6,54 Previous investigations have reported the accuracy of segmented regional soft tissue and bone analysis via DEXA to be within 1% to 6% error, with excellent reliability and repeatability between measurements (intraclass correlation coefficient [ICC] 0.99). 8 For the specific measures performed in this investigation, analysis performed in triplicate revealed similar results, with an ICC >0.96 for all measurements. In a subset of patients where data were available (BFR, n = 12; CONTROL, n = 10), physician clearance for time to return to full activity (RTS) was also recorded (physicians blinded to group assignment). Physician clearance was defined as the date seen in clinic after successful completion of functional testing specific to ACLR. Patients were not cleared to RTS without completion of ACLR-specific testing.
For primary outcome variables, a 2(group) × 3(time) analysis of covariance (ANCOVA) (covaried on presurgery measures and sex) was used to detect changes in muscle and bone measures from presurgery to week 6 and week 12 of rehab within and between groups. For secondary outcome variables, a 2(group) × 2(time) ANCOVA (covaried on sex) was used to detect and compare changes in functional outcomes tested at week 8 and week 12 between groups. Significant interactions indicated by Type-III tests of fixed effects were followed with a Bonferroni post hoc test for pairwise comparisons. These statistical models were selected based on previous investigations regarding musculoskeletal and cardiovascular responses to 12 weeks of physical training.28,30,42 An independent samples t test was used to compare time to RTS and time between initial clinic visit and date of surgery between groups. For all significant pairwise comparisons between groups, ES was calculated using a Cohen d statistic, 48 and interpreted as follows: ES <0.1, negligible; 0.1 to 0.3, small; 0.3 to 0.5 moderate; 0.5 to 0.7, large; and +0.7, very large (VL). Type-I error was set atα = 0.05.
Results
The average patient follow up was 2.3 ± 1.0 years. There were no surgical complications or adverse events in rehabilitation from the use of the BFR cuff. For all group-by-time comparisons, sex was not observed to be a significant covariate and was therefore excluded from the final analysis.
Bone Mass and Site-Specific BMD
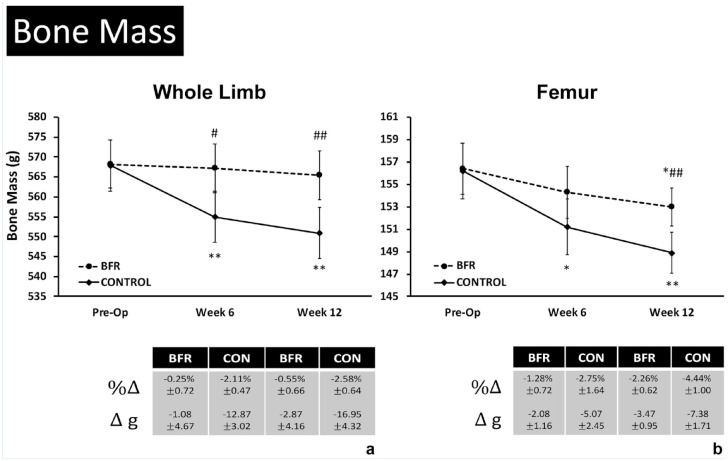
Measures of LE bone mass in the injured/operative limb are shown in Figure 3, where loss of whole limb and femur bone mass was attenuated in the BFR group compared with the CONTROL group (P < 0.05). Only the CONTROL group experienced decreases in LE bone mass relative to presurgery (Pre-Op) measures following weeks 6 (P = 0.01) and 12 (P < 0.01) of rehab (Figure 3a), resulting in reduced injured/healthy limb ratios in the CONTROL group at both timepoints relative to Pre-Op (P < 0.05). No change in LE bone mass was observed in the BFR group over time. Similarly, femur bone mass was preserved in the BFR group compared with the CONTROL group, where a decrease in bone mass was observed following 6 (P = 0.03) and 12 (P < 0.01) weeks of rehabilitation, resulting in reduced injured/healthy limb ratios (week 6: −4.21 ± 0.92%; week 12: −4.44 ± 0.88%; P < 0.01) (Figure 3b). Femur bone mass was observed to be decreased only in the BFR group at week 12 (P = 0.02) (Figure 3b).
Figure 3.
Changes in site-specific bone mass after 6 and 12 weeks of rehabilitation. BFR, blood flow restriction therapy; CON, CONTROL.
Data are presented as adjusted means ± 95% CI for site specific bone mineral density (BMD, g/cm2) measures in the injured limb before surgery (Pre-Op) and following 6 and 12 weeks of rehabilitation for the A) distal femur, B) proximal tibia, and C) proximal fibula. *,**Significant change from Pre-Op baseline within group at p < 0.05 and p < 0.01, respectively. #,##Significant difference between groups at the same measurement timepoint at p < 0.05 and p < 0.01, respectively.
Similar to measures of whole limb and femur bone mass, loss in site-specific BMD around the knee was attenuated overall in the BFR group compared with the CONTROL group (Figure 4) over time for the distal femur (Figure 4a), proximal tibia (Figure 4b), and proximal fibula (Figure 4c).
Figure 4.
Changes in site-specific BMD after 6 and 12 weeks of rehabilitation.
Data are presented as adjusted mean ± 95% CI for site-specific (BMD, g/cm2) measures in the injured limb before surgery (Pre-Op) and after 6 and 12 weeks of rehabilitation for the (a) distal femur, (b) proximal tibia, and (c) proximal fibula. *,**Significant change from Pre-Op baseline within group at P < 0.05 and P < 0.01, respectively. #,##Significant difference between groups at the same measurement timepoint at P < 0.05 and P < 0.01, respectively. BFR, blood flow restriction therapy; BMD, bone mineral density; CON, CONTROL.
Lean Mass
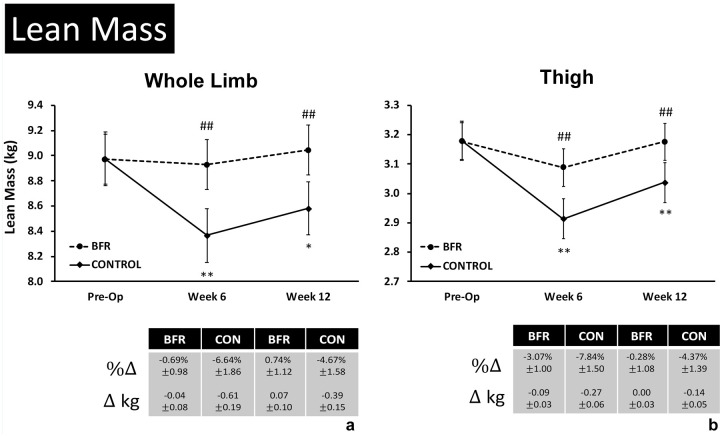
Only the CONTROL group was observed to experience a change in total-LM at week 6 (−1.27 ± 0.53 kg; P = 0.04) with both groups not differing from baseline by week 12. Measures of LE-LM in the injured/operative limb are shown in Figure 5. Only the CONTROL group experienced decreases in LE-LM relative to presurgery (Pre-Op) measures after weeks 6 (P < 0.01) and 12 (P = 0.01) of rehabilitation (Figure 5a). This resulted in reduced injured/healthy limb ratios in the CONTROL group at both timepoints relative to Pre-Op (week 6: −5.65 ± 1.38%; week 12: −4.06 ± 0.84%; P < 0.01). No change was observed in the BFR group over time, which resulted in greater LE-LM measures in the operative limb at 6 (P < 0.01; ES = 1.35, VL) and 12 (P < 0.01; ES = 1.10, VL) weeks of rehabilitation compared with the CONTROL group (Figure 5a). Similarly, thigh LM was observed to decrease only in the CONTROL group relative to presurgery after 6 (P < 0.01) and 12 (P < 0.01) weeks of rehabilitation resulting in reduced injured/healthy limb ratios (week 6: −7.14 ± 1.47%; week 12: −5.42 ± 1.10%; P < 0.01) as well as reduced thigh LM compared with the BFR group (week 6: P < 0.01; ES = 1.31,VL; week 12: P < 0.01; ES = 1.04,VL) (Figure 5b). Lastly, gluteal LM was decreased at week 6 only in the CONTROL group (-0.38 ± 0.11 kg; P < 0.01).
Figure 5.
Changes in lower extremity lean mass after 6 and 12 weeks of rehabilitation.
Data are presented as adjusted mean ± 95% CI for lean mass measures in the injured limb before surgery (Pre-Op) and after 6 and 12 weeks of rehabilitation for (a) whole limb lean mass and (b) thigh lean mass (kg, templated to distal 2/3 of the thigh for each patient). *, **Significant change from Pre-Op baseline within group at P < 0.05 and P < 0.01, respectively; #,##Significant difference between groups at the same measurement timepoint at P < 0.05 and P < 0.01, respectively. BFR, blood flow restriction therapy; CON, CONTROL.
Functional Measures and RTS/Return to Activity
For functional measurements, both groups were found to have similar improvements in all measures across the 4-week interval with the exception of the anterior Y-balance measurement, where only the BFR group was observed to have a significant improvement between 8 and 12 weeks (Table 2). Physician clearance for RTS is shown in Figure 6 in addition to sport participation frequencies for each group. Of the patients who were either actively involved in organized competitive sports or classified themselves as recreational athletes (BFR, n = 12; CONTROL, n = 10), time to RTS was lower in the BFR group compared with the CONTROL group (P = 0.01, ES = 1.34,VL).
Table 2.
Change in functional outcomes between weeks 8 and 12 of rehabilitation a
| Independent Variables | BFR | CONTROL | |
|---|---|---|---|
| SL squat, cm | Week 8 | 33.4 ± 5.1 | 34.3 ± 6.3 |
| Week 12 | 43.0 ± 5.5* | 40.9 ± 5.4* | |
| Δ | 9.6 ± 5.3 | 6.7 ± 2.6 | |
| P value (wig) | <0.01 | <0.01 | |
| SL eccentric step-down, reps | Week 8 | 45.1 ± 13.9 | 44.3 ± 14.7 |
| Week 12 | 62.7 ± 18.0* | 61.0 ± 18.6* | |
| Δ | 17.6 ± 10.3 | 17.1 ± 11.7 | |
| P value (wig) | <0.01 | 0.01 | |
| Y-balance, cm | |||
| Anterior | Week 8 | 50.0 ± 2.2 | 48.7 ± 14.3 |
| Week 12 | 54.6 ± 3.5* | 55.9 ± 18.6 | |
| Δ | 4.6 ± 2.8 | 7.2 ± 6.7 | |
| P value (wig) | 0.01 | 0.29 | |
| Posteromedial | Week 8 | 93.9 ± 7.4 | 93.3 ± 8.6 |
| Week 12 | 100.0 ± 8.5* | 104.4 ± 9.2* | |
| Δ | 5.9 ± 5.1 | 11.1 ± 6.4 | |
| P value (wig) | 0.03 | <0.01 | |
| Posterolateral | Week 8 | 91.9 ± 4.6 | 90.3 ± 10.3 |
| Week 12 | 99.7 ± 8.5* | 104.5 ± 9.2* | |
| Δ | 7.8 ± 7.6 | 14.1 ± 7.7 | |
| P value (wig) | 0.03 | <0.01 | |
| SL leg press, kg |
Week 8 | 59.3 ± 10.0 | 58.6 ± 8.0 |
| Week 12 | 72.7 ± 8.7* | 76.5 ± 9.0* | |
| Δ | 13.3 ± 5.2 | 14.3 ± 5.7 | |
| P value (wig) | <0.01 | <0.01 | |
| SL hamstring curl, kg | Week 8 | 28.2 ± 4.2 | 29.9 ± 5.1 |
| Week 12 | 37.7 ± 4.9* | 38.5 ± 4.5* | |
| Δ | 9.5 ± 2.1 | 8.6 ± 2.9 | |
| P value (wig) | <0.01 | <0.01 | |
BFR, blood flow restriction therapy; CON, CONTROL; 1RM, 1-repetition maximum; SL, single leg, wig, within group.
Data are presented as mean ± 95% CI for functional outcome measures assessed at weeks 8 and 12 of rehabilitation for the following exercises: SL squat depth, eccentric step-down (repetitions to fatigue), Y-balance, leg curl (1RM), and hamstring curl (1RM).
Significant change wig from baseline (P < 0.05). No between-group interactions detected.
Figure 6.
Return to sport.
Data are presented as mean ± CI for time to RTS (months) as cleared by a physician. Frequencies of sport participation for each group are also shown. Patients not actively participating in an organized competitive sport but who regularly perform exercise training and play sports were classified as recreational athletes. BFR, blood flow restriction therapy; RTS, return to sport.
*Significantly different from the BFR group at P < 0.05.
Discussion
In the present study, the use of BFR was observed to decrease the loss of LE-LM, LE body mass, and LE-BMD during 12 weeks of rehab from ACLR as well as decrease time to RTS compared with standard rehab alone. The findings, in addition to others,15,58 provide considerable support for the efficacy of BFR augmented rehabilitation after ACLR. In a novel finding, the use of BFR resulted in preservation of LE body mass and BMD. These observations were made in conjunction with a reduced time to RTS. Therefore, these results not only support the therapeutic use of BFR after ACLR but also provide new potential target populations in those with osteopenia and/or requiring prolonged unloading.
Bone Preservation
A novel finding from this investigation was that the BFR group was observed to have preserved whole-limb and site-specific bone compared with the CONTROL group (Figures 3 and 4). Because bone is highly responsive to loading, 32 patients who are completely or partially immobilized may be at risk of osteopenia or subsequent fracture. The finding of decreased BMD in the proximal tibia of the CONTROL group is similar to the findings of Mundermann et al 41 (and others),6,54 where patients were observed to have an average BMD decrease of ~12% in the tibial plateau after 3 months of postoperative rehab in an injured limb. The mechanism(s) by which BFR may have stimulated the preservative responses observed here are not well understood. It has been hypothesized that venous occlusion may lead to fluid shifts causing increased intramedullary pressure and interstitial fluid flow within the bone. 38 Previous observations suggest that the response of bone to chronic exercise is potentially interlinked with skeletal muscle with regard to mechanical, systemic, and local signaling factors.21,22,31 Swift et al 52 demonstrated in a rodent model that simulated HIX using electrical stimulation was able to preserve bone under conditions of unloading, which suggests that muscle contractions alone can partially mitigate bone loss during disuse. 52 Relatedly, myokines are secreted from muscle during exercise in an intensity-stress-dependent manner and are known to act on bone metabolism in either proformation or resorption capacities.22,47,56 Both systemic and muscle-derived insulin-like growth factors (IGFs) and fibroblast growth factors are also known to act directly at the muscle-bone interface.13,22 Lastly, inhibition of myostatin, a negative regulator of muscle mass and bone repair, 22 has been observed to improve muscle and fracture healing after trauma. 17 In a group of 29 active men, Laurentino et al 33 observed reductions in myostatin expression after 8 weeks of BFR-LIX that were similar to responses found with HIX. While further research will be required to determine cause and effect, the present data provide impactful evidence that BFR may be a suitable tool for combatting postoperative bone loss. Perhaps most importantly for ACLR using BTB grafts is to determine whether BFR expedites graft integration, which is currently thought to be complete at 5 months. 40 Given the novel findings of this study with regard to bone preservation, further study should be focused on the rate of graft integration with BFR.
Skeletal Muscle Mass/Prevention of Atrophy
LE-LM and thigh LM were more well-preserved in the BFR group (Figure 5). This finding is supported by previous literature suggesting BFR-LIX is effective at minimizing atrophy in the early stages of unloading (1-4 weeks) after surgery.15,29,58 However, few have investigated more long-term postoperative effects of BFR on preserving total body-LM, LE-LM, and site-specific LM (>8 weeks). This suggests that BFR may be used as part of an effective rehab strategy even as patients begin to progress back into high-intensity or sport-specific exercises.
Support for longer-term interventions with BFR may be drawn from previous studies in healthy populations.1,2 Vechin et al 55 observed that BFR-LIX produced similar improvements in quadriceps cross sectional area compared with HIX in older adults. In young healthy adults, Lowery et al 39 observed that, similar to HIX alone, 8 weeks of BFR-LIX resulted in increased muscle thickness. However, in a study by Curran et al, 12 the use of BFR with HIX beginning 10 weeks after ACL surgery did not produce additional gains compared with HIX alone. Therefore, it seems the greatest benefit may be early in rehab or after injury when HIX is not possible or recommended.
Potential Proximal Responses to BFR
Prevention of muscle loss in the BFR group was not limited to the musculature distal to the site of occlusion, which may indicate proximal benefit. A perceived limitation of BFR is that the benefits are isolated to musculature distal to the occlusion site. Currently, the impact of LE BFR on hip/trunk musculature is not well known. However, previous investigations have reported size and strength improvements of shoulder and back musculature with upper extremity occlusion. 14 These findings have been indirectly attributed to factors such as increased motor unit recruitment as well as systemic metabolic effects mimicking the responses to HIX that influence skeletal muscle metabolism. 29 In a recent investigation on the effects of BFR-LIX for rotator cuff strengthening across 8 weeks of training in healthy adults, Lambert et al 26 observed significantly greater improvements in shoulder region muscle mass (BFR-LIX: +175 ± 54 g; LIX alone: +96 ± 61 g; P < 0.05) and work capacity compared with LIX alone. During fatigue testing, it was also observed that, while under occlusion, BFR significantly increased deltoid and rotator cuff EMG amplitude compared with the unoccluded state.
In the present study, the BFR group performed exercises under conditions of 80%LOP previously shown to result in an accumulation of metabolites due to increased hypoxic conditions in the limb.29,50 Under these conditions, BFR-LIX yields increases in systemic blood lactate, calcium, muscle-derived myokines, and blood acidity that are similar to HIX.18,19,29 These metabolically stressful conditions and mechanical stress resulting from cell swelling during occlusion have been hypothesized to play a role in acute stimulation of muscle anabolism via multiple cell signaling pathways.18-20,35,46 BFR-LIX has also been observed to rapidly increase systemic growth hormone release 29 and to chronically increase systemic IGFs,3,53 known to play a role in satellite cell proliferation and differentiation and bone anabolism during recovery from exercise.16,49 Therefore, it is possible that the present findings may result from both increased motor unit recruitment and an increased presence of systemic anabolic effectors. However, further study is needed to determine direct cause and effect.
Functional Outcomes and RTS
Here, BFR proved to be a safe intervention as there were no short-term complications including superficial or deep infections and deep or superficial venous thrombosis. BFR was also safely integrated into existing rehab protocols without difficulty. RTS time was also decreased in the BFR group compared with the CONTROL group in this study. Notably, RTS in this study was defined as a clearance to return to full activity by the treating surgeon with successful completion of functional testing specific to ACLR (similar to other studies). 23 A decrease of 1.4 months in the BFR group compared with the CONTROL group is clinically significant and important for professional and recreational athletes alike.
Interestingly, functional outcome measures were similar between groups (Table 2). There are many possible contributing factors, but the most likely explanation is within the timing of testing. Due to the nature of rehab protocols for ACLR, the earliest the select functional tests can be safely done is 8 weeks postsurgery. Therefore, early functional gains due to BFR may be “hidden” due to the lack of evaluations during the first 2 months. In addition, the second evaluation was done at week 12 postsurgery. In this study, only a 4-week window was captured, which was already 2 months into rehab and, as such, the timeframe used may not have been optimal to identify differences. Nevertheless, it can be concluded that BFR did not have a negative effect on patients’ functional outcomes.
Limitations and Conclusion
The present study has several major limitations. Although powered to detect the present findings, the small patient sample size from a single institution should be considered. Next, although participants were screened for supplement use before entry into the study, nutritional intake was not monitored. However, because subjects were randomized to each group, it is unlikely that such dietary modifications would have been made to favor one group more than another. Patients did not undergo functional testing until week 8 of the rehab program in accordance with protocols and to avoid graft injury. Therefore, the rate of functional progress from surgery to the week 8 testing is unknown. Conclusions for complications including graft tear/failure are limited due to the lack of long-term follow up. Patient-reported outcomes, overall patient satisfaction, and performance metrics were not evaluated. Although DXA allows for precise assessments of soft tissue mass/distribution, bone mineral content, and bone density, we caution the reader that it is not possible to differentiate between knee flexors and extensor muscles, which presents a limitation regarding which specific muscles of the LE may have been most impacted by the rehab protocol. Regarding the DEXA measures performed here, further studies requiring longer follow-up timelines (>6 months) and larger patient sample sizes will be required to confirm the present findings related to preservation of whole limb bone mass and site-specific BMD. As changes in bone assessed via densitometry are often tracked over the course of 6-months to multiple years, such information will be valuable to further characterize chronic patient responses to rehabilitation protocols involving BFR. Lastly, tissue collection was not performed which precludes insight on systemic and local effectors in this study.
In light of the findings presented here, when added to a standardized rehabilitation protocol, BFR may decrease muscle and bone loss up to 12 weeks postoperatively and may improve time to RTS after ACLR. These findings are of clinical significance for active individuals after surgery for accelerating recovery. The findings regarding bone preservation are of great interest and warrant further investigation with regard to the mechanisms responsible as well as applied application for other operative and nonoperative injuries of the extremities.
Footnotes
The results of the present study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460-1466. [DOI] [PubMed] [Google Scholar]
- 2.Abe T, Sakamaki M, Fujita S, et al. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. J Ger Phys Ther. 2010;33:34-40. [PubMed] [Google Scholar]
- 3.Abe T, Yasuda T, Midorikawa T, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int J KAATSU Train Res. 2005;1:6-12. [Google Scholar]
- 4.Anderson AW, Smith JJ. Proximal tibial fracture after patellar tendon autograft for ipsilateral ACL reconstruction. J Knee Surg. 2009;22:142-144. [DOI] [PubMed] [Google Scholar]
- 5.Ardern CL, Webster KE, Taylor NF, Feller JA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med. 2011;39:538-543. [DOI] [PubMed] [Google Scholar]
- 6.Bayar A, Sarıkaya S, Keser S, et al. Regional bone density changes in anterior cruciate ligament deficient knees: a DEXA study. Knee. 2008;15:373-377. [DOI] [PubMed] [Google Scholar]
- 7.Bowman EN, El-shaar R, Milligan H, et al. The proximal and distal effects of blood flow restriction therapy on upper and lower extremity strengthening: a randomized controlled trial. Orthop J Sports Med. 2019;7:2325967119S2325900337. [Google Scholar]
- 8.Burkhart TA, Arthurs KL, Andrews DM. Manual segmentation of DXA scan images results in reliable upper and lower extremity soft and rigid tissue mass estimates. J Biomech. 2009;42:1138-1142. [DOI] [PubMed] [Google Scholar]
- 9.Chapleau J, Lambert BS, Sullivan TC, et al. Impact of valgus vs varus mechanical axis correction during primary total knee arthroplasty on postoperative periarticular bone mineral density. J Arthroplasty. 2021;36:1792-1798. [DOI] [PubMed] [Google Scholar]
- 10.Cook SB, Clark BC, Ploutz-Snyder LL. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med Sci Sports Exerc. 2007;39:1708. [DOI] [PubMed] [Google Scholar]
- 11.Coughlan GF, Fullam K, Delahunt E, Gissane C, Caulfield BM. A comparison between performance on selected directions of the star excursion balance test and the Y balance test. J Athl Train. 2012;47:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran MT, Bedi A, Mendias CL, et al. Blood flow restriction training applied with high-intensity exercise does not improve quadriceps muscle function after anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med. 2020;48:825-837. [DOI] [PubMed] [Google Scholar]
- 13.D’Amore PA, Brown RH, Jr, Ku PT, et al. Elevated basic fibroblast growth factor in the serum of patients with Duchenne muscular dystrophy. Ann Neurol. 1994;35:362-365. [DOI] [PubMed] [Google Scholar]
- 14.Dankel SJ, Jessee MB, Abe T, Loenneke JP. The effects of blood flow restriction on upper-body musculature located distal and proximal to applied pressure. Sports Med. 2016;4:23-33. [DOI] [PubMed] [Google Scholar]
- 15.DePhillipo NN, Kennedy MI, Aman ZS, et al. The role of blood flow restriction therapy following knee surgery: expert opinion. Arthroscopy. 2018;34:2506-2510. [DOI] [PubMed] [Google Scholar]
- 16.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167:344-351. [DOI] [PubMed] [Google Scholar]
- 17.Elkasrawy M, Immel D, Wen X, et al. Immunolocalization of myostatin (GDF-8) following musculoskeletal injury and the effects of exogenous myostatin on muscle and bone healing. J Histochem Cytochem. 2012;60:22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry CS, Glynn EL, Drummond MJ, et al. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol. 2010;108:1199-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita S, Abe T, Drummond MJ, et al. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol. 2007;103:903-910. [DOI] [PubMed] [Google Scholar]
- 20.Gundermann DM, Fry CS, Dickinson JM, et al. Reactive hyperemia is not responsible for stimulating muscle protein synthesis following blood flow restriction exercise. J Appl Physiol. 2012;112:1520-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamrick MW. A role for myokines in muscle-bone interactions. Exerc Sport Sci Rev. 2011;39:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. BoneKEy Rep. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris JD, Abrams GD, Bach BR, et al. Return to sport after ACL reconstruction. Orthopedics. 2014;37:e103-e108. [DOI] [PubMed] [Google Scholar]
- 24.Korakakis V, Whiteley R, Epameinontidis K. Blood flow restriction-induced analgesia in patients with anterior knee pain. J Sci Med Sport. 2017;20:e100. [DOI] [PubMed] [Google Scholar]
- 25.Kuenze CM, Blemker SS, Hart JM. Quadriceps function relates to muscle size following ACL reconstruction. J Orthop Res. 2016;34:1656-1662. [DOI] [PubMed] [Google Scholar]
- 26.Lambert B, Hedt C, Daum J, et al. Blood flow restriction training for the shoulder: a case for proximal benefit. Am J Sports Med. 2021;49:2716-2728. [DOI] [PubMed] [Google Scholar]
- 27.Lambert B, Hedt CA, Jack RA, et al. Blood flow restriction therapy preserves whole limb bone and muscle following ACL reconstruction. Orthop J Sports Med. 2019;7:2325967119S2325900196. [Google Scholar]
- 28.Lambert BS, Greene NP, Carradine AT, et al. Aquatic treadmill training reduces blood pressure reactivity to physical stress. Med Sci Sports Exerc. 2014;46:809-816. [DOI] [PubMed] [Google Scholar]
- 29.Lambert BS, Hedt C, Moreno M, Harris JD, McCulloch P. Blood flow restriction therapy for stimulating skeletal muscle growth: practical considerations for maximizing recovery in clinical rehabilitation settings. Tech Orthop. 2018;33:89-97. [Google Scholar]
- 30.Lambert BS, Shimkus KL, Fluckey JD, et al. Anabolic responses to acute and chronic resistance exercise are enhanced when combined with aquatic treadmill exercise. Am J Physiol Endo Metab. 2015;308:E192-E200. [DOI] [PubMed] [Google Scholar]
- 31.Lang TF. The bone-muscle relationship in men and women. J Osteoporos. 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lara-Castillo N, Kim-Weroha NA, Kamel MA, et al. In vivo mechanical loading rapidly activates β-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone. 2015;76:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurentino GC, Ugrinowitsch C, Roschel H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012;44:406-412. [DOI] [PubMed] [Google Scholar]
- 34.Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40:595-605. [DOI] [PubMed] [Google Scholar]
- 35.Loenneke J, Fahs C, Thiebaud R, et al. The acute muscle swelling effects of blood flow restriction. Acta Physiol. 2012;99:400-410. [DOI] [PubMed] [Google Scholar]
- 36.Loenneke JP, Kim D, Fahs CA, et al. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve. 2015;51:713-721. [DOI] [PubMed] [Google Scholar]
- 37.Loenneke JP, Thiebaud RS, Fahs CA, et al. Blood flow restriction does not result in prolonged decrements in torque. Eur J Appl Physiol. 2013;113:923-931. [DOI] [PubMed] [Google Scholar]
- 38.Loenneke JP, Young KC, Fahs CA, et al. Blood flow restriction: rationale for improving bone. Med Hypotheses. 2012;78:523-527. [DOI] [PubMed] [Google Scholar]
- 39.Lowery RP, Joy JM, Loenneke JP, et al. Practical blood flow restriction training increases muscle hypertrophy during a periodized resistance training programme. Clin Physiol Funct Imag. 2014;34:317-321. [DOI] [PubMed] [Google Scholar]
- 40.Masuda H, Taketomi S, Inui H, et al. Bone-to-bone integrations were complete within 5 months after anatomical rectangular tunnel anterior cruciate ligament reconstruction using a bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc. 2018;26:3660-3666. [DOI] [PubMed] [Google Scholar]
- 41.Mündermann A, Payer N, Felmet G, Riehle H. Comparison of volumetric bone mineral density in the operated and contralateral knee after anterior cruciate ligament and reconstruction: a 1-year follow-up study using peripheral quantitative computed tomography. J Orthop Res. 2015;33:1804-1810. [DOI] [PubMed] [Google Scholar]
- 42.Oliver JM, Jagim AR, Sanchez AC, et al. Greater gains in strength and power with intraset rest intervals in hypertrophic training. J Strength Cond Res. 2013;27:3116-3131. [DOI] [PubMed] [Google Scholar]
- 43.Palazzolo A, Rosso F, Bonasia DE, Saccia F, Rossi R. Uncommon complications after anterior cruciate ligament reconstruction. Joints. 2018;6:188-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson SD, Brandner CR. The role of blood flow restriction training for applied practitioners: a questionnaire-based survey. J Sports Sci. 2017:1-8. [DOI] [PubMed] [Google Scholar]
- 46.Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187-200. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379-1406. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation; 1994:231-244. [Google Scholar]
- 49.Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013;43:179-194. [DOI] [PubMed] [Google Scholar]
- 50.Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313-325. [DOI] [PubMed] [Google Scholar]
- 51.Smith CA, Chimera NJ, Warren M. Association of Y balance test reach asymmetry and injury in Division I athletes. Med Sci Sports Exerc. 2015;47:136-141. [DOI] [PubMed] [Google Scholar]
- 52.Swift JM, Nilsson MI, Hogan HA, Sumner LR, Bloomfield SA. Simulated resistance training during hindlimb unloading abolishes disuse bone loss and maintains muscle strength. J Bone Min Res. 2010;25:564-574. [DOI] [PubMed] [Google Scholar]
- 53.Takano H, Morita T, Iida H, et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Euro J Appl Physiol. 2005;95:65-73. [DOI] [PubMed] [Google Scholar]
- 54.Van Meer B, Waarsing J, van Eijsden W, et al. Bone mineral density changes in the knee following anterior cruciate ligament rupture. Osteoarth Cartilage. 2014;22:154-161. [DOI] [PubMed] [Google Scholar]
- 55.Vechin FC, Libardi CA, Conceição MS, et al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J Strength Cond Res. 2015;29:1071-1076. [DOI] [PubMed] [Google Scholar]
- 56.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13-18. [DOI] [PubMed] [Google Scholar]
- 57.Yamanaka T, Farley RS, Caputo JL. Occlusion training increases muscular strength in division IA football players. J Strength Cond Res. 2012;26:2523-2529. [DOI] [PubMed] [Google Scholar]
- 58.Žargi T, Drobnič M, Stražar K, Kacin A. Short-term preconditioning with blood flow restricted exercise preserves quadriceps muscle endurance in patients after anterior cruciate ligament reconstruction. Front Physiol. 2018;9:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]