Abstract
Context:
Oral contraceptives (OCs) manipulate hormonal fluctuations of the menstrual cycle and affect physical performance. Most investigations on the effect of OCs on physical performance did not discriminate between different types of OCs. Thus, the effects of monophasic OCs (MOCs) - the most common type of OCs - on muscle strength and recovery from exercise are largely unknown.
Objective:
To examine the effect of MOC use on muscle strength and markers of recovery after exercise-induced muscle damage (EIMD) in premenopausal women.
Data Sources:
Electronic databases Embase, PubMed, SportDiscus, and Web of Science were searched for studies examining the effect of MOCs on acute muscle strength and recovery.
Study Selection:
Keywords applied for the study selection were oral contraceptive* AND muscle strength or oral contraceptive* AND muscle damage.
Study Design:
Systematic review.
Level of Evidence:
Lowest quality assessed for an included study in this review was serious risk of bias using ROBINS-I tool made from Cochrane for nonrandomized studies.
Data Extraction:
A total of 104 studies on muscle strength were identified, of which 11 met the inclusion criteria. Concerning recovery, 51 studies were identified, of which 4 met the inclusion criteria.
Results:
Of the 11 studies included, 10 showed no effect of MOCs on acute muscle strength. Of the 4 studies on recovery, 2 found a greater decrease in muscle strength, and 3 found higher creatine kinase (CK) levels after EIMD in MOC users than in nonusers. The included studies were all rated with moderate-to-serious risk of bias.
Conclusion:
These findings suggest that MOCs may impair recovery from EIMD as indicated by lowered muscle strength and elevated CK levels. There is insufficient evidence to conclude whether MOCs acutely affect muscle strength. Moderate-to-serious risk of bias in studies makes interpretation challenging.
Keywords: exercise-induced muscle damage, muscle recovery, muscle strength, oral contraception, women
Fluctuations in female hormones during the menstrual cycle may potentially affect physical performance. 15 Oral contraceptives (OCs), containing ethinyl-estradiol and progestin, are widely used by female elite athletes, with an estimated prevalence of 83% and most of those using monophasic OCs (MOCs). 26 MOCs prevent ovulation and pregnancy by inhibiting endogenous estrogen and progesterone secretion. 6 Therefore, MOC users do not experience the same hormone fluctuations as nonusers. Because skeletal muscle expresses estrogen and progesterone receptors, 17 estrogen and progesterone may affect muscle strength, recovery processes,15,24,32 and training adaptations. 32 Although several studies have reviewed the effect of OCs on athletic performance,6,19,32 the effect of MOCs is unclear - particularly concerning their potential effects on muscle strength and recovery from exercise-induced muscle damage (EIMD). Given the high prevalence of MOC use among female athletes, it is crucial to clarify the potential impact of MOC use on these performance parameters.
Deciphering the effect of MOCs, based on estrogen levels, is challenging as ethinyl-estradiol has a higher estrogen receptor affinity and is more potent than endogenous estrogen. 10 In contrast to multiphasic OCs; however, MOCs consist of only 1 type of pill containing the same combination of ethinyl-estradiol and progestin throughout the cycle. Since variations in the combination of ethinyl-estradiol and progestin levels in OCs may affect muscle strength, 1 this could explain the conflicting results in studies not specifying the type of OC used. In addition, the concentration and androgenicity of progestin may reduce muscle strength in women using OCs, as some progestins have antiandrogenic effects. 6 Therefore, the effect of specific types of OCs, such as MOCs, on performance and skeletal muscle adaptations is preferred.
Studies indicate that anaerobic performance and muscle strength in eumenorrheic women are greater during the early follicular phase, characterized by low estrogen and progesterone levels than in the late follicular and luteal phase. 24 Furthermore, some but not all studies suggest that training-induced improvements in muscle strength are greater during the follicular phase than the luteal phase,27,31,35 while no such difference is evident across the menstrual cycle of OC users.25,28 A recent review found that OC use resulted in either increased, decreased, or unchanged muscle strength compared with nonusers. 19 However, the quality of the 12 included studies was low and no clear conclusions on the effect of OCs could be drawn. 19 Specifying the type of OCs, eg, MOCs, could potentially help draw more consistent conclusions.
There are reasons to believe that MOCs may affect recovery processes. While only sparsely investigated for OCs, some studies have found them to affect EIMD and recovery markers.3,29,33 Increased creatine kinase (CK) levels, muscle soreness, and muscle force decline are indirect markers of muscle damage.7,34 Thompson et al 33 showed no difference in CK levels or muscle force decline 48 hours post-EIMD between OC users and nonusers. However, OC users perceived less muscle soreness than nonusers. 33 In another study, OCs did not affect perceived muscle soreness, 29 but induced a larger muscle force decline 40 to 96 hours post-EIMD, indicating a slower recovery of muscle function. 29 In nonusers, muscle strength and soreness post-EIMD were affected more in the early follicular phase than midluteal phase, 3 and CK levels were shown to be higher 24 to 72 h post-EIMD in the early follicular phase than midluteal phase. 13 This indicates a protective effect of estrogen against EIMD. 3 However, it remains uncertain whether MOCs affect EIMD and the ability to recover post-EIMD. Thus, the purpose of this systematic review was to examine the effect of MOC use on muscle strength and markers of recovery post-EIMD in premenopausal women.
Methods
Literature Search
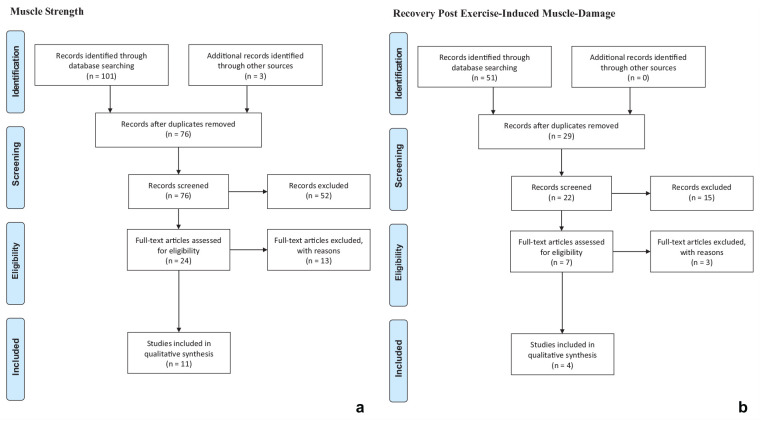
To investigate the effect of MOCs on muscle strength and recovery, an electronic literature search in the databases Embase, PubMed, SportDiscus, and Web of Science was performed. The search was completed on May 4, 2020. The process of the literature search is illustrated in Figure 1. Two separate literature searches were conducted: a search on muscle strength and another on recovery. On Web of Science, the category “topic” was used for the search, which included title, abstract, and keywords. In the other databases, the categories “title” and “abstract” were used. The keywords used for muscle strength were oral contraceptive* AND muscle strength, while the keywords used for recovery were oral contraceptive* AND muscle damage. The "AND" function was used to combine selected keywords. The search was limited to articles in English and only published full-text articles were included. The electronic search and subsequent selection were carried out by 2 reviewers. Duplicates and studies with titles and abstracts that did not meet the inclusion criteria were excluded. Studies that failed to meet the inclusion criteria after full-text reading were excluded. Additional articles meeting the inclusion criteria, found through the other literature search or the reference list of included studies, were included. Furthermore, articles meeting the criteria for both topics were included in the results of both topics.
Figure 1.
Flowcharts showing studies included and excluded in the electronic search in the databases Embase, PubMed, SportDiscus, and Web of Science for (a) muscle strength and (b) markers of recovery post-EIMD. EIMD, exercise-induced muscle-damage.
Inclusion Criteria
Only studies on healthy, premenopausal women comparing a group of MOC users with a group of nonusers were included. It was a criterion that the group of nonusers did not use any hormonal contraception. Studies on multiphasic and nonspecified OCs were excluded. Studies including a male control group were included, but results from men were not included in this review. In the search on muscle strength, studies including measures of muscle strength and statistical comparison of muscle strength between MOC users and nonusers were included. By contrast, studies with strength training interventions were excluded as these studies examined the relative change in muscle strength rather than absolute muscle strength. Concerning the recovery studies, it was a criterion that changes in muscle strength over time, CK levels, or perceived muscle soreness were measured postexercise. These parameters are common indicators of skeletal muscle damage, 19 and studies examining a minimum of 1 of the muscle-strength-related parameters were included in the review.
Quality Assessment
Study quality was assessed using the ROBINS-I tool from Cochrane made for nonrandomized studies, 30 as none of the included studies was randomized. The assessment was carried out by 2 of the authors cooperatively, and each study was evaluated individually. The studies were evaluated in 7 different domains, scoring either low, moderate, serious, or critical risk of bias and followed by an overall rating. Studies with a low risk of bias are expected to have no confounding. Studies with a moderate risk of bias may contribute to evidence, but confounding is expected although no serious residual confounding. Studies with a serious risk of bias have imperative shortcomings, and studies with a critical risk of bias cannot provide useful evidence on the effect of the intervention. 30
Results
Muscle Strength
A total of 104 studies were identified, of which 11 met the inclusion criteria. These studies evaluated the effect of MOC use on muscle strength in 245 premenopausal women aged 17 to 39 years with varying training backgrounds (Table 2).
Table 2.
Study characteristics a
| Muscle strength | ||||||
|---|---|---|---|---|---|---|
| Author(s), Year | Participants (n) | Training Volume | Test Days during the Cycle | Study Design | Performance Test | Results (P Value) |
| Bell et al, 4 2011 | MOC users: n = 15 Nonusers: n = 15 |
>20 min physical activity 3 times/week | MOC users: Days 3-5 and 15-17 Nonusers: Days 3-5 and ovulation |
Parallel group | MVC: knee flexion | No difference (P = 0.57) |
| Bryant et al, 5 2008 | MOC users: n = 20 Nonusers: n = 20 |
>20 km running/week | MOC users: Days 1 and 14 Nonusers: Day 1 and ovulation |
Parallel group | MVC: isometric plantarflexion | No difference (P > 0.05) |
| Drake et al, 9 2003 | MOC users: n = 6 Nonusers: n = 7 |
No involvement in any exercise program | MOC users: Days 1-3, 4-6, 9-11, 14-16 and 19-21 Nonusers: Days 1-3, 4-7, 9-11, ovulation and 5 days post ovulation |
Parallel group | MVC: knee extension | No difference (P > 0.05) |
| Ekenros et al, 10 2013 | MOC users: n = 8 Nonusers: n = 9 |
Moderate to highly recreationally active, physical activity ~2-3 times/week |
MOC users: Days 2-4, 7-8, and 14-15 Nonusers: Days 2-4, ovulation and 7-8 days postovulation |
Crossover | Maximal isokinetic muscle strength: knee
extensors Isometric handgrip strength |
No difference (P = 0.78) No difference (P = 0.76) |
| Elliott et al, 11 2005 | MOC users: n = 14 Nonusers: n = 7 |
Inactive | MOC users: Days 5, 14 and 21 Nonusers: Days 2 and 21 |
Parallel group | MVC: knee flexion MVC: knee extension MVC: FDI |
No difference (P > 0.05) |
| Gordon et al, 12 2013 | MOC users: n = 6 Nonusers: n = 11 |
Well-trained | MOC users and nonusers: Days 1-3, 9-11, 19-20, and 27-28 |
Parallel group | Maximal isokinetic torque: knee extensors and flexors | No difference (P > 0.05) |
| Hicks et al, 14 2017 | MOC users: n = 9 Nonusers: n = 9 |
Recreationally active. ≤1 h of moderate physical activity/week. |
MOC users and nonusers: Day 14 | Parallel group | MVC: knee extension | No difference (P > 0.05) |
| Mackay et al, 20 2019 | MOC users: n = 10 Nonusers (follicular): n = 10 Nonusers (ovulation): n = 10 |
No resistance or flexibility training the last 6 months. | MOC users: Days 1-2 Nonusers (follicular): Days 1-2 Nonusers (ovulation): Day 14 ± 2 |
Parallel group | MVC: knee extension | No difference (P = 0.09) |
| Minahan et al, 21 2015 | MOC users: n = 8 Nonusers: n = 8 |
30 min physical activity ~2-3 days/week, no resistance training. | MOC users and nonusers: Days 2-6 | Parallel group | Maximum isometric torque: knee extension | No difference (P = 0.38) |
| Morse et al, 22 2013 | MOC users: n = 12 Nonusers: n = 12 |
Recreationally active. <1 h physical activity/week. |
No specific test day | Parallel group | MVC torque: plantarflexion | No difference (P > 0.05) |
| Nicolay et al, 23 2008 | MOC users: n = 8 Nonusers: n = 11 |
Not specified | MOC users and nonusers: Days 4-6, 11-13, and 20-23. | Parallel group | MVC: single repetition grip strength 20-repetition grip test |
Nonusers stronger than MOC users (P =
0.02) Nonusers stronger than MOC users (P < 0.01) |
| Recovery Post-EIMD | ||||||
| Author(s), Year | Participants (n) | Training Volume | Test Days during the Cycle | Study Design | Markers of Muscle Damage | Results (P Value) |
| Hicks et al, 14 2017 | MOC users: n = 9 Nonusers: n = 9 |
Recreationally active .≤1 h of moderate physical activity/week. |
MOC users and nonusers: Day 14 .48, and 96, and 168 h post-EIMD. |
Parallel group | Decline in muscle strength (MVC), CK levels, and perceived muscle soreness | No difference in decreased > muscle strength (P = 0.06) .Higher CK in MOC users vs > nonusers (P = 0.02) .No difference in muscle > soreness (P = 0.60). |
| Joyce et al, 16 2014 | MOC users: n = 9 Nonusers: n = 9 |
30 min physical activity .~1-3 days/week No resistance training. |
MOC users and nonusers: Days 2-6 .48 h post-EIMD. |
Parallel group | CK levels and perceived muscle soreness | Higher CK in MOC users vs > nonusers (P = 0.01). No difference in muscle > soreness (P = 0.70). |
| Mackay et al, 20 2019 | MOC users: n = 10 Nonusers (follicular): n = 10 Nonusers (ovulation): n = 10 |
No resistance or flexibility training the last 6 months. | MOC users: Days 1-2 .Nonusers: Days 1-2 and ovulation .24, 48, 72, and 96 h post-EIMD. |
Parallel group | Decline in muscle strength (MVC), CK levels, and perceived muscle soreness | Greater decline in muscle strength in > MOC users vs nonusers follicular > 72 h post-EIMD (P = 0.02) and 96 h post-EIMD (P = 0.01) .No difference in CK-levels (P > 0.05) .More soreness in MOC users vs > nonusers ovulation (P < 0.05). |
| Minahan et al, 21 2015 | MOC users: n = 8 Nonusers: n = 8 |
30 min physical activity ~2-3 days/week .No resistance training |
MOC users and nonusers: Days 2-6 .6, 24, and 48 h post-EIMD. |
Parallel group | Decline in muscle strength (maximum isometric torque) and CK levels | Greater decline in muscle strength in MOC users vs nonusers
(P < 0.05) .Higher CK in MOC users vs nonusers 6 h post-EIMD (P = 0.04) and 24 h post-EIMD (P = 0.05). |
CK, creatine kinase; EIMD, exercise-induced muscle damage; FDI, first dorsal interosseous muscle; MOC users, users of monophasic oral contraceptives; MVC, maximum voluntary isometric contraction; Nonusers, no use of any hormonal contraception.
Characteristics of studies included in the systematic review. All studies compare MOC users with nonusers.
Of the 11 studies, 10 found no effect (P > 0.05) of MOCs on muscle strength as measured by a variety of tests, including maximal voluntary isometric contraction (MVC) and maximal isokinetic torque (Table 1).4,5,9,10,11,12,14,20,21,22 One study found that nonusers were 33% stronger than MOC users in a 20-repetition grip test (P < 0.01) and MVC grip strength (P = 0.02), 23 but had a serious risk of bias. Of the 9 studies that had a moderate risk of bias, which was the best rating of the studies included in this review, none found a difference (P > 0.05) in muscle strength between MOC users and nonusers (Table 1).
Table 1.
Study quality assessment a
| Author(s), Year | Bias due to Confounding | Bias in Selection of Participants into Study | Bias in Classification of Interventions | Bias due to Deviations from Intended Interventions | Bias due to Missing Data | Bias in Measurement of Outcome | Bias in Selection of Reported Results | Total Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Muscle strength | ||||||||
| Bell et al, 4 2011 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Bryant et al, 5 2008 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Drake et al, 9 2003 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Ekenros et al, 10 2013 | Moderate risk | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk |
| Elliott et al, 11 2005 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Gordon et al, 12 2013 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Hicks et al, 14 2017 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| Mackay et al, 20 2019 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Morse et al, 22 2013 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Minahan et al, 21 2015 | Moderate risk | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk |
| Nicolay et al, 23 2008 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| Recovery from EIMD | ||||||||
| Hicks et al, 14 2017 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| Joyce et al, 16 2014 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Mackay et al, 20 2019 | Moderate risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Low risk | Moderate risk |
| Minahan et al, 21 2015 | Moderate risk | Moderate risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Moderate risk |
EIMD, exercise-induced muscle damage.
Domain-level risk-of-bias judgment using ROBINS-I tool. The studies could be assessed with a low, moderate, serious, or critical risk of bias. Low-risk studies are comparable with well-performed randomized trials. Moderate-risk studies may contribute to evidence but are not comparable with well-performed randomized trials. Serious risk studies have imperative shortcomings. Critical risk studies cannot provide useful evidence on the effect of the intervention.
Recovery
A total of 51 studies were identified, of which 4 were included. The studies evaluated the effect of MOC use on recovery post-EIMD in 66 premenopausal women aged 18 to 35 years with varying training backgrounds (Table 2). Two studies included the same participants and were only included once in the total subject pool for this review.21,16 The indirect markers of recovery post-EIMD were muscle strength decline, increased serum CK levels, and greater perceived muscle soreness.
Of the 3 studies that examined decline in muscle strength post-EIMD, 2 found a greater decline in muscle strength in MOC users compared with nonusers,20,21 and 1 study found a tendency (P = 0.06) for MOC users to have a smaller decrease in muscle strength post-EIMD than nonusers. 14 However, the latter study showed a negative correlation between age and muscle strength (r = 0.58), and, after adjusting for this, the tendency no longer appeared (P = 0.18). 14 Three of 4 studies reported higher serum CK levels post-EIMD in MOC users compared with nonusers,14,16,21 whereas 1 study found no difference between MOC users and nonusers. 20 Three of the 4 studies investigated perceived muscle soreness, and 1 study found a greater perceived muscle soreness post-EIMD in MOC users that recovered slower than in nonusers, 20 whereas no studies found a greater perceived muscle soreness in nonusers with MOC users.14,16
Mackay et al 20 tested a group of nonusers in the follicular phase and another group of nonusers during ovulation, whereas the other studies tested only once during the menstrual cycle. Three of the studies tested at the beginning of the menstrual cycle (during menstruation) when the MOC users did not take OCs,16,20,21 whereas Hicks et al 14 tested on day 14 of the menstrual cycle (to mimic the time of ovulation). However, ovulation status was not verified, which was a shortcoming of the study. In addition, there were variations in frequency (1-4 times) and timing (48-168 h) in post-EIMD measurements.
In the studies on recovery included in the present review, at least 1 of the 3 markers of muscle damage post-EIMD (ie, decline in muscle strength, increases in CK levels, and/or perceived muscle soreness) indicated that the ability to recover from EIMD was reduced in MOC users compared with nonusers. Three of the studies were rated with a moderate risk of bias, whereas 1 study was rated with a serious risk of bias (Table 1).
Discussion
Exercise-Induced Alterations in Muscle Strength
Based on the 11 studies included in our analysis, MOC use does not appear to affect muscle strength. Findings were fairly consistent although 1 study, involving 19 participants, showed that nonusers were stronger than MOC users. 23 However, that study had a serious risk of bias as no criteria were set for the participants’ experience with resistance training, posing a risk that nonusers had greater experience with resistance training than MOC users. Furthermore, the participants were not matched for anthropometrics, such as palm width. The nonusers had wider palms than MOC users (P = 0.04), and, when adjusted for palm size, MVC grip strength was not different between the groups (P = 0.10). 23 Of the 10 studies showing no effect of MOC use on muscle strength, 2 stood out. Ekenros et al 10 utilized a nonrandomized crossover design, strengthening the validity of the results as the participants acted as their own controls, but suffered from a lack of blinding. In the study by Gordon et al, 12 the MOC use group was a control group with a smaller sample size than the nonuser group (n = 6 vs n = 11), which limits the power for between-group comparisons. Collectively, these findings suggest that maximal muscle strength is unaffected by MOC use. While most of the reviewed studies included small sample sizes, they totalled 245 women and pointed toward the same tendency for no effect of MOCs on muscle strength, hence supporting the validity of the findings. Given the moderate-to-serious risk of bias in the included studies, randomized controlled trials with a low risk of bias are warranted.
Two of the most common progestins in MOCs are levonorgestrel and norethindrone, which have antiandrogenic effects, 6 and the trivial androgenic effect of MOCs may explain why MOC use might not affect muscle strength. Indeed, Elliott et al 11 found no difference in muscle strength in MOC users between MOC consumption and pill withdrawal. Results on OCs’ effects on muscle strength have been conflicting,6,26,32 which may be due to variations in muscle strength during the menstrual cycle in nonusers, while muscle strength seems to be more constant in OC users.25,28 Therefore, the choice of testing days in the different studies is relevant to consider. One study did not test on specific days of the cycle, 22 while another study tested only in the early follicular phase (day 2-6 since the first day of bleeding), 21 corresponding to the pill withdrawal. Most of the included studies (7 of 11) tested muscle strength in at least 2 different phases of the menstrual cycle with no difference between phases or groups (MOC users vs nonusers). Thus, cycle-related differences in female sex hormones between MOC users and nonusers do not appear to affect maximal muscle strength.
Exercise-Induced Alterations in the Recovery Ability of Skeletal Muscle
With some inconsistencies, the included studies investigating the effect of MOCs on recovery from EIMD showed that recovery is impaired in MOC users compared with nonusers as indicated by a trend for lowered muscle strength, elevated CK levels, and greater perceived muscle soreness.
Concerning muscle force after EIMD, only 3 studies fulfilled our criteria for inclusion, of which 2 found that MOC use was associated with greater muscle force decline in recovery when tested during the early follicular phase (days 2-6 since the first day of bleeding),20,21 corresponding to pill withdrawal and the phase where both MOC users and nonusers have the lowest total estrogen levels. This coincides with the findings of Savage and Clarkson, 29 who reported prolonged recovery of muscle strength post-EIMD in OC users. Estrogen may have a protective effect on muscle damage, but whether ethinyl-estradiol has the same effect as endogenous estrogen remains debated.3,29 It is worth noticing that ethinyl-estradiol will bind with a higher affinity to estrogen receptors than endogenous estrogen, 10 and that higher MOC-derived ethinyl-estradiol levels lead to higher overall estrogen levels during MOC consumption than during pill withdrawal, which may induce a protective effect against muscle damage post-EIMD. This could explain why Hicks et al 14 observed a smaller decrease in muscle strength post-EIMD during periods of MOC use than that observed in studies testing MOC users during pill withdrawal.20,21 The greater decrease in muscle strength post-EIMD in MOC users, compared with nonusers in the early follicular phase, could be due to the reduction in ethinyl-estradiol levels during pill withdrawal. Collectively, these findings suggest that MOC use causes a greater decline in muscle strength post-EIMD during pill withdrawal, which may be due to low total estrogen levels compared with those observed in nonusers. 21 However, more studies are needed.
A general finding of the included studies was that MOC users had higher serum CK levels post-EIMD than nonusers.14,16,21 Only 1 of the 4 included studies showed no difference in CK levels post-EIMD between MOC users and nonusers. 20 However, in that study, CK levels increased markedly from before to 96 hours after EIMD (46 vs 250 UI/l) only in the MOC users and not in the nonusers. 20 Collectively, this suggests that MOCs increase CK levels post-EIMD. The results of the present review on serum CK levels post-EIMD indicate that MOC use decreases the ability to recover from EIMD.
The 3 studies on perceived muscle soreness yielded conflicting results. One study found greater muscle soreness post-EIMD in MOC users than in nonusers, 20 whereas 2 studies did not.14,16 Conflicting results are also evident in the existing literature, with reports of reduced or no differences in exercise-induced muscle soreness in MOC users compared with nonusers.29,33
Overall, the reviewed studies assessed with the highest quality found that MOC use is associated with impaired ability to recover from EIMD during pill withdrawal, while the effect of MOC use during the treatment period remains uncertain. Accordingly, the ability to recover is negatively affected by MOC use. This may be due to the antioxidant and membrane stabilizing properties of estrogen, which are shown to protect the skeletal muscle from damage in animals.2,8,18,34 Nevertheless, none of the studies included in the analysis had a low risk of bias and, therefore, the results must be interpreted with caution.
Methodological Considerations
Aside from the small number of participants in the studies included in this review, the studies all had a moderate-to-serious risk of bias. There was also substantial heterogeneity between the studies, including performance tests, participants (especially regarding training background), and menstrual cycle validation and testing. Differences in the ability to recover from EIMD were found primarily during pill withdrawal, which is associated with low levels of both endogenous estrogen and ethinyl-estradiol. Mackay et al 20 compared MOC users during pill withdrawal with nonusers in different phases of the cycle and showed that recovery depended on the phase of the cycle in nonusers. Hence, measures should preferably be conducted more than once and on verified menstrual cycle time-points, eg, early and late follicular and luteal phase. In this respect, Janse de Jonge et al 15 have established recommendations to optimize research on the menstrual cycle. However, there are no similar recommendations for studies on OCs or other hormonal contraceptives; although based on the present review, such are much needed. Participants should ideally use the same types of MOC to increase intra- and interstudy comparability, since there may be variations in the concentrations of ethinyl-estradiol and progestin within types of MOCs. 6 In this review, participants were required to use MOCs, but no criterion was established for a given type of MOC.
Conclusion
Most female athletes are MOC users, which makes the question of whether MOC use affects muscle strength and recovery from EIMD highly relevant. This systematic review provides an overview of the existing literature and contributes to a broad understanding of the effects of MOCs on muscle strength and recovery from EIMD in premenopausal women. Our findings indicate that MOC use may impair recovery processes after exercise as reflected by a greater muscle force decline and higher serum CK levels post-EIMD in MOC users compared with nonusers. The observation applies mainly during pill withdrawal when compared with the early follicular phase, where estrogen levels are low, and it remains uncertain whether MOC consumption affects recovery when compared with the other phases of the menstrual cycle. At present, the literature on the effects of MOCs on recovery is limited to the 4 studies included in this review. Our review also indicates that MOC use does not affect muscle strength, but a lack of high-quality studies makes it difficult to draw robust conclusions. To give female athletes the best prerequisites to choose contraception, future studies should investigate whether training background affects the potential negative effects of MOCs on recovery. Furthermore, studies examining whether the slower recovery in MOC users may negatively affect the performance of elite athletes with more than 1 competition per day (eg, sprinters, rowers, and swimmers) are warranted.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1.Allali F, El Mansouri L, Abourazzak FZ, et al. The effect of past use of oral contraceptive on bone mineral density, bone biochemical markers and muscle strength in healthy pre and post menopausal women. BMC Women’s Health. 2009;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amelink GJ, Koot RW, Erich WB, Van Gijn J, Bar PR. Sex-linked variation in creatine kinase release, and its dependence on oestradiol, can be demonstrated in an in-vitro rat skeletal muscle preparation. Acta Physiol Scand. 1990;138:115-124. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LJ, Baker LL, Schroeder ET. Blunted myoglobin and quadriceps soreness after electrical stimulation during the luteal phase or oral contraception. Res Quart Exer Sport. 2017;88:193-202. [DOI] [PubMed] [Google Scholar]
- 4.Bell DR, Blackburn JT, Ondrak KS, et al. The effects of oral contraceptive use on muscle stiffness across the menstrual cycle. Clin J Sport Med. 2011;21:467-473. [DOI] [PubMed] [Google Scholar]
- 5.Bryant A, Clark R, Bartold S, et al. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Phys. 2008;105:1035-1043. [DOI] [PubMed] [Google Scholar]
- 6.Burrows M, Peters CE. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007;37:557-574. [DOI] [PubMed] [Google Scholar]
- 7.Bär PR, Amelink GJ. Protection against muscle damage exerted by oestrogen: hormonal or antioxidant action? Biochem Soc Transactions. 1997;25:50-54. [DOI] [PubMed] [Google Scholar]
- 8.Bär P, Amelink GJ, Oldenburg B, Blankenstein M. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988;42:2677-2681. [DOI] [PubMed] [Google Scholar]
- 9.Drake SM, Evetovich T, Eschbach C, Webster M. A pilot study on the effect of oral contraceptives on electromyography and mechanomyography during isometric muscle actions. J Electromyogr Kinesiol. 2003;13:297-301. [DOI] [PubMed] [Google Scholar]
- 10.Ekenros L, Hirschberg AL, Heijne A, Fridén C. Oral contraceptives do not affect muscle strength and hop performance in active women. Clin J Sport Med. 2013;23:202-207. [DOI] [PubMed] [Google Scholar]
- 11.Elliott KJ, Cable NT, Reilly T. Does oral contraceptive use affect maximum force production in women? Br J Sports Med. 2005;39:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon D, Hughes F, Young K, et al. The effects of menstrual cycle phase on the development of peak torque under isokinetic conditions. Isokinet Exerc Sci. 2013;21:285-291. [Google Scholar]
- 13.Hackney AC, Kallman AL, Agˇgön E. Female sex hormones and the recovery from exercise: menstrual cycle phase affects responses. Biomed Human Kinet. 2019;11:87-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks KM, Onambélé-Pearson G, Winwood K, Morse CI. Oral contraceptive pill use and the susceptibility to markers of exercise-induced muscle damage. Eur J Appl Physiol. 2017;117:1393-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janse de Jonge X, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sport Exer. 2019;51:2610-2617. [DOI] [PubMed] [Google Scholar]
- 16.Joyce S, Sabapathy S, Bulmer AC, Minahan C. The effect of prior eccentric exercise on heavy-intensity cycling: the role of gender and oral contraceptives. Eur J Appl Physiol 2014;114:995-1003. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Tamadon A, Park HT, Kim H, Ku S-Y. The role of sex steroid hormones in the pathophysiology and treatment of sarcopenia. Osteoporos Sarcopenia. 2016;2:140-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komulainen J, Koskinen SOA, Kalliokoski R, Takala TES, Vihko V. Gender differences in skeletal muscle fibre damage after eccentrically biased downhill running in rats. Acta Physiol Scand. 1999;165:57-63. [DOI] [PubMed] [Google Scholar]
- 19.Konopka JA, Hsue LJ, Dragoo JL. Effect of oral contraceptives on soft tissue injury risk, soft tissue laxity, and muscle strength: a systematic review of the literature. Orthop J Sports Med. 2019;7:2325967119831061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackay K, González C, Zbinden-Foncea H, Peñailillo L. Effects of oral contraceptive use on female sexual salivary hormones and indirect markers of muscle damage following eccentric cycling in women. Eur J Appl Physiol. 2019;119:2733-2744. [DOI] [PubMed] [Google Scholar]
- 21.Minahan C, Joyce S, Bulmer AC, Cronin N, Sabapathy S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur J Appl Physiol. 2015;115:1493-1500. [DOI] [PubMed] [Google Scholar]
- 22.Morse CI, Spencer J, Hussain AW, Onambele GL. The effect of the oral contraceptive pill on the passive stiffness of the human gastrocnemius muscle in vivo. J Musculoskelet Neuronal Interactions. 2013;13:97-104. [PubMed] [Google Scholar]
- 23.Nicolay CW, Kenney JL, Lucki NC. Grip strength and endurance throughout the menstrual cycle in eumenorrheic and women using oral contraceptives. Int J Ind Ergon. 2008;38: 211-221. [Google Scholar]
- 24.Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010;40:207-227. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496:551-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechichi C, Dawson B, Goodman C. Athletic performance and the oral contraceptive. Int J Sports Physiol Perform. 2009;4:151-162. [DOI] [PubMed] [Google Scholar]
- 27.Sakamaki-Sunaga M, Min S, Kamemoto K, Okamoto T. Effects of menstrual phase-dependent resistance training frequency on muscular hypertrophy and strength. J Strength Cond Res. 2016;30:1727-1734. [DOI] [PubMed] [Google Scholar]
- 28.Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493:267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage KJ, Clarkson PM. Oral contraceptive use and exercise-induced muscle damage and recovery. Contraception. 2002;66: 67-71. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;12;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung E, Han A, Hinrichs T, Vorgerd M, Manchado C, Platen P. Effects of follicular versus luteal phase-based strength training in young women. SpringerPlus. 2014;3:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson B, Almarjawi A, Sculley D, Janse de Jonge X. The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med. 2020;50:171-185. [DOI] [PubMed] [Google Scholar]
- 33.Thompson HS, Hyatt JP, De Souza MJ, Clarkson PM. The effects of oral contraceptives on delayed onset muscle soreness following exercise. Contraception. 1997;56:59-65. [DOI] [PubMed] [Google Scholar]
- 34.Tiidus PM, Holden D, Bombardier E, Zajchowski S, Enns D, Belcastro A. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can J Physiol Pharmacol. 2001;79:400-406. [PubMed] [Google Scholar]
- 35.Wikström-Frisén L, Boraxbekk CJ, Henriksson-Larsen K. Effects on power, strength and lean body mass of menstrual/oral contraceptive cycle based resistance training. J Sports Med Phys Fitness. 2017;57:43-52. [DOI] [PubMed] [Google Scholar]