Abstract
Vibrio cholerae strain VB1 secretes a number of enzymes into the outside medium that utilize ATP as a substrate. Such enzymes are found in the outside medium during the mid-log phase of growth, when the optical density at 650 nm is about 0.4, and they demonstrate nucleoside diphosphate kinase (Ndk), 5′ nucleotidase, and adenylate kinase (Ak) activities. We report that the filtered growth medium of V. cholerae, as well as the flowthrough fraction of a green Sepharose column during fractionation of the growth medium, had very little cytotoxicity by itself towards macrophages and mast cells but exhibited significant cytotoxicity in the presence of exogenous ATP. Such fractions, harboring 5′ nucleotidase, Ndk, and presumably other ATP-utilizing enzymes, demonstrated enhanced macrophage and mast cell death; periodate-oxidized-ATP (oATP)-treated macrophage and mast cells or such cells exposed to 0.1 mM Mg2+, where surface-associated P2Z receptors could not be activated, were not susceptible to subsequent ATP addition. Microscopic visualization of mast cells clearly demonstrated cell morphological changes such as swelling, vacuolization, and nuclear fragmentation following treatment with ATP and the growth medium of V. cholerae; however, these effects were suppressed if the mast cells were pretreated with oATP. These results strongly imply that the secreted ATP-utilizing enzymes of V. cholerae modulate the external ATP levels of the macrophage and mast cells, leading to their accelerated death, presumably through activation of P2Z receptors. Thus, development of inhibitors for such enzymes may reduce the level of V. cholerae infection; alternatively, mutations in such genes may eliminate V. cholerae survival in the gut and contribute to a safer live vaccine.
Macrophages and thymocytes express various purinergic nucleotide receptors that, in the presence of ATP and other nucleotides, regulate immune development and microbial infections (3, 10). A major group of receptors, called the P2Z or P2X7 receptors, have been implicated in the killing of invading pathogens by modulating production of oxygen radicals and reactive nitrogen intermediates (17, 25); in the presence of millimolar concentrations of external ATP effluxed from the macrophages or other mammalian cells (1, 7, 9, 25), the surface-associated P2Z receptors of macrophages and other phagocytic or inflammatory cells are activated, thereby altering the permeability of the plasma membrane. Such membrane perturbation leads to formation of pores on the surface through which hydrophilic molecules with masses of up to 900 Da can leak out, causing phagocytic cell death (3, 6, 7). Thus, external adenine nucleotides, particularly ATP, are involved in the death of the phagocytic cells. Since macrophage cell death leads to the death of the engulfed pathogen (17, 25), activation of the purinergic receptors is an accepted part of the phagocytic process.
Vibrio cholerae, the causative organism of the intestinal disease cholera, manifests its virulence through production of a number of cytotoxic agents, the best known of which is the cholera toxin (4, 13). This organism, particularly serovars O1 and O139, is a mucosal pathogen, known for its ability to adhere to the intestinal epithelial cells through production of toxin-coregulated pili that help in the colonization process. Subsequently the cells secrete cholera toxin and other ancilliary toxins that produce severe diarrheal symptoms by interfering with the Gsα-regulated chloride channels (4, 13). The genes encoding cholera toxin (ctxAB) and other ancilliary toxins (ace and zot) are clustered together and represent the genome of lysogenic filamentous bacteriophage CTXφ (2, 5, 27), while the toxin-coregulated pilus accessory colonization factor genes, along with several other genes, comprise a pathogenicity island which is present in epidemic and pandemic strains of V. cholerae (14, 16). The characterization of a number of toxin genes has led to various attempts to develop vaccine strains free of such toxin production (4, 13, 15, 26). In spite of a great deal of effort, no ideal vaccine strain is currently available that does not produce weak diarrhea and other symptoms (8).
While many studies have been directed towards an improved understanding of how V. cholerae colonizes the intestinal epithelial cells, very little is known about how V. cholerae evades the host defense to survive in the gut. Since purinergic receptors such as P2Z receptors are involved in macrophage cell death, an intriguing possibility for how a pathogen evades host cell defense is that it might enhance the activation of P2Z-type receptors and thereby enhance phagocytic cell death through modulation of external adenine nucleotide levels. In this article, we show that V. cholerae VB1 secretes various ATP-transforming enzymes into the outside medium that enhance the ATP-induced activation of the macrophage and mast cell surface-associated P2Z receptors, thereby enhancing cell death. This mode of induction of phagocytic cell death is analogous to that reported for Pseudomonas aeruginosa (30) and Burkholderia cepacia (20).
MATERIALS AND METHODS
Bacterial strains and reagents.
V. cholerae strain VB1, harboring a deletion in the ctxAB operon but otherwise capable of secreting a number of other enzymes, such as protease, chitinase, etc. (23, 24), was the subject of our study. V. cholerae VB1 was inoculated in Luria broth (LB) medium containing thymine, 100 μg/ml, and was grown at 37°C in a shaker. For determination of ATP-utilizing activities, aliquots were withdrawn at each hour of growth from 0 to 8 or 10 h and centrifuged, supernatants were filtered through 0.22-μm-pore-size filters, and the filtrates were assayed for ATP-utilizing activities.
Enzymatic assay.
The aliquots were analyzed for 5′ nucleotidase or ATPase activity as follows. In a 10-μl assay volume, 5 μl of the filtered supernatant fraction was incubated with 0.15 μl [γ-32P]ATP, 10 mCi/ml, in TM (50 mM Tris-HCl, pH 7.5–10 mM MgCl2) buffer for 5 min. [32P]phosphate-labeled products were visualized after the products of the reaction were separated on a polyethyleneimine–thin-layer chromatography (PEI-TLC) plate in 0.75 M KH2PO4 and then autoradiographed (29). To assay for nucleoside diphosphate kinase (Ndk) activity, 5 μl of the sample was incubated with 0.15 μl of [γ-32P]ATP, 10 mCi/ml, and 100 μM (each) nucleoside diphosphates (NDPs) such as GDP, CDP, and UDP for 5 min. Terminal [32P]phosphate was transferred by Ndk to NDPs to produce the corresponding radioactive nucleoside triphosphates (NTPs) (GTP, CTP, and UTP), which were separated on a PEI-TLC plate in 0.75 M KH2PO4. To assay for adenylate kinase (Ak) activity, 5 μl of the sample was incubated with 0.15 μl of [γ-32P]ATP and 100 μM AMP for 5 min. [32P]ADP was formed in a reversible reaction ([γ-32P]ATP + AMP ⇋ ADP + [32P]ADP) and visualized by autoradiography.
Enzyme fractionation.
V. cholerae (4 liters) was grown for 4 to 6 h up to an optical density at 650 nm (OD650) of around 0.7 to 1.0. The cells were removed by centrifugation, and the supernatant was concentrated and changed for TM buffer with an Amicon YM10 membrane by ultrafiltration. The concentrated supernatant in TM buffer was loaded onto a variety of columns such as hydroxyapatite Bio-Gel HT Gel (Bio-Rad), ATP-agarose, Mono Q, and Q-Sepharose, as used earlier by us to separate secreted enzymes of P. aeruginosa (30). The various ATP-utilizing enzymes, as well as the cytotoxic activities, did not bind to any of these columns and were always found in the flowthrough fraction. Only with the green Sepharose column were we able to separate the Ak activity, which remained bound to the column. Two fractions were collected from the green Sepharose column, including an eluant, when the protein was eluted by a linear gradient of 0 to 3 M KCl in TM buffer, and active fractions that were collected at 3 M KCl and contained primarily Ak activity. The flowthrough fraction having the orthophosphate Pi-releasing activity from [γ-32P]ATP as well as [α-32P]GTP or [α-32P]CTP (5′ nucleotidase or phosphatase) was also obtained.
HPLC assay for ATP-related activities.
The high-performance liquid chromatography (HPLC) assay was performed using a strong anion-exchange Protein-Pak TMQ-8HR column (Waters) in gradient A:B (7 mM KH2PO4, pH 3.8–0.5 M KH2PO4, pH 4.5) with a linear gradient from 0% B to 100% B for 60 min and a flow rate of 0.5 ml/min. AMP, ADP, and ATP were eluted at retention times of 12, 35, and 55 min, respectively. Various column fractions were incubated with ATP, ADP, or AMP at final concentrations of 5 mM for 2 h at room temperature, and 20 μl of each reaction mixture was used for separation of the products by HPLC.
Animal and cell populations.
BALB/c mice were obtained from Jackson Laboratory (Bar Harbor, Maine) and maintained in the Biological Laboratory of the University of Illinois at Chicago. Mice were sex matched and used at 6 to 10 weeks of age.
Mast cells were purified from peritoneal exudate as described by Ishizawa et al. (11). Briefly, a 1-ml volume of the peritoneal cell suspension was layered on top of 2 ml of 35% Ficoll (Sigma Chemical Co., St. Louis, Mo.) in Tris-EDTA buffer, pH 7.6, containing 0.025 M Tris, 0.12 M NaCl, 0.005 M KCl, 0.01 M EDTA, and 0.3 g of human serum albumin per liter. The tubes were centrifuged at 500 × g for 30 min at room temperature. Mast cells were recovered from the Ficoll layer, washed, and resuspended in RPMI 1640 containing l-glutamine, buffered with 10 mM HEPES, and supplemented with 10% fetal bovine serum (FBS)–penicillin (100 U/ml)–streptomycin (100 μg/ml).
Mast cells from six to eight mice were pooled, and a direct count of the cell suspension with toluidine blue showed that 85 to 95% of the total cells were mast cells.
Macrophage cultures and cytotoxicity assays.
Macrophages were obtained by culturing J774 cells suspended at 0.5 × 106 cells/ml in RPMI 1640 medium containing l-glutamine buffered with 10 mM HEPES and supplemented with 10% FBS. Such macrophages were plated in 96-well plates (Becton Dickinson Labware, Lincoln Park, N.J.) at a final concentration of 105 cells/well in 200 μl of complete RPMI 1640 medium supplemented with 10% FBS and allowed to adhere to wells for 2 h at 37°C and 5% CO2 before gentle rinsing to remove nonadherent cells. Macrophages were then activated with 50 ng of lipopolysaccharide (LPS) per ml (Sigma) for 24 h as previously described (30). LPS-primed cells were washed and incubated for 2 h in the presence of 1 mM ATP (Sigma) with or without concentrated supernatant samples from V. cholerae strain VB1 or the eluant and flowthrough fractions from the green Sepharose column. For mast cells, a similar procedure was followed. At the end of each incubation, 50 μl of the supernatants was transferred to 96-well plates and lactate dehydrogenase (LDH) activity was determined using a CytoTox 96 assay kit (Promega, Madison, Wis.). Triplicate samples were tested for each data point. Prior to challenge with macrophages, the reaction of various fractions with nucleotides was allowed to proceed for 2 to 4 h. In experiments with the P2Z antagonist periodate-oxidized ATP (oATP; Sigma), macrophages were pretreated with 1 mM oATP for 2 h prior to cytotoxicity assay with ATP and the various supernatant or column chromatography fractions.
Microscopy.
LPS-stimulated macrophages or mast cells (106 cells/ml) were cultured in a chambered coverglass system (Nunc, Naperville, Ill.) with a volume of 1 ml/well. After 2 h with various treatments, cells were washed with warm medium and incubated at 37°C in 5% CO2. Phase-contrast pictures were taken with an inverted microscope (Nikon Diaphot 200) equipped with a 40× objective.
RESULTS
Elaboration of ATP-transforming enzymes by V. cholerae strain VB1.
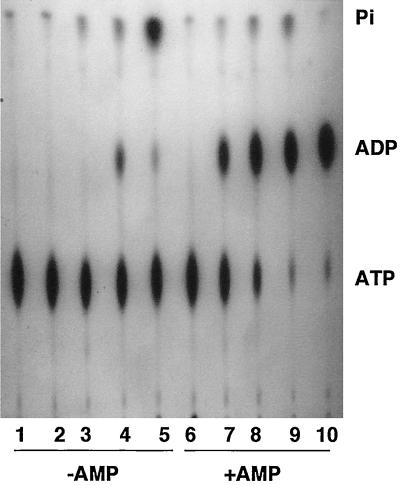
We have recently reported that an intracellular pathogen such as Mycobacterium bovis secretes Ndk and ATPase, which sequester and remove ATP from macrophage surface-associated P2Z (P2X7) receptors, thereby preventing macrophage cell death (29). Prevention of macrophage cell death is important for virulent mycobacteria such as M. bovis, since the pathogenic bacteria grow within the macrophages and if the infected macrophage dies, the engulfed bacteria die with it (17, 25). In contrast, a mucosal respiratory tract pathogen, the mucoid cystic fibrosis isolate P. aeruginosa, was shown to secrete a number of ATP-utilizing enzymes that contributed to enhanced killing of macrophages (30). We therefore became interested in knowing if a well-known intestinal mucosal pathogen such as V. cholerae, capable of colonizing the gut, would demonstrate the secretion of ATP-utilizing enzymes to the outside medium. A ctxAB deletion mutant of V. cholerae strain VB1 was grown in LB containing 100 μg of thymine per ml at 37°C; samples were taken at various times and filtered through 0.22-μm-pore size filters, and the filtrates were tested for the presence of ATP-transforming enzymes by incubating the samples with [γ-32P]ATP in either the absence or presence of 0.1 mM AMP. Addition of exogenous AMP allows detection of Ak, which transfers the terminal [32P]phosphate from [γ-32P]ATP to AMP, leading to the formation of [32P]ADP (30). The results in Fig. 1 demonstrate that in the absence of exogenous AMP, the supernatants allowed the formation of small amounts of ADP (lane 4) and the release of increasing amounts of Pi. In the presence of external 0.1 mM AMP, clear bands of ADP were detected even when the supernatants from 3-h-grown cultures (mid-log phase) were incubated with [γ-32P]ATP (lanes 7, 8, and 9). The release of 32Pi was more pronounced as the cells entered late log to early stationary phase (lanes 4, 5, 8, and 9).
FIG. 1.
Secretion of various ATP-transforming enzymes by V. cholerae strain VB1 during growth in LB. At various times during the growth phase (0, 3, 6, and 10 h, corresponding to OD650s of 0.005, 0.3, 0.9, and 1.2), aliquots were centrifuged and filtered through 0.22-μm-pore-size filters, and the cell-free supernatants were incubated with [γ-32P]ATP in the absence or presence of 0.1 mM AMP for 5 min. The formation of various radioactive products was followed by separation on PEI-TLC plates followed by autoradiography as previously described (29, 30). Lane 1, [γ-32P]ATP control; lanes 2, 3, 4, and 5, [γ-32P]ATP incubated with supernatants of cells grown for 0, 3, 6, and 10 h in the absence of AMP; lanes 6, 7, 8, and 9, the same as lanes 2, 3, 4, and 5 but included AMP; lane 10, purified Ak incubated with [γ-32P]ATP plus 0.1 mM AMP.
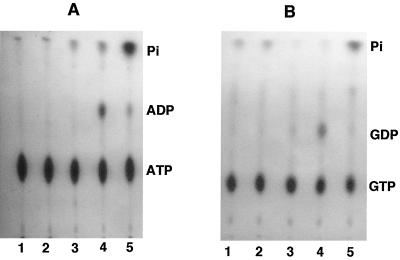
The detection of the release of 32Pi from [γ-32P]ATP by supernatant fractions from mid-log- to early-stationary-phase cultures raised the question of whether this release was due to an ATPase activity, as has been shown for M. bovis (29), or to a 5′ nucleotidase activity as demonstrated for P. aeruginosa (30). In the latter case, we demonstrated that the 5′-nucleotidase activity allows release of 32Pi even from α-32P-labeled NTPs such as GTP or CTP. We additionally demonstrated that the P. aeruginosa supernatant allowed the formation of [32P]ADP from [γ-32P]ATP alone by a combined action of 5′ nucleotidase (or phosphatase) and Ak, such that 5′ nucleotidase generated nonradioactive ADP, AMP, and adenosine from [γ-32P]ATP, and the resultant AMP was phosphorylated to [32P]ADP by terminal phosphotransfer from [γ-32P]ATP in presence of Ak (30). Indeed, the formation of [32P]ADP (lane 4) and its subsequent conversion to 32Pi (lane 5) strongly suggested that a 5′-nucleotidase activity becomes prominent later during the growth phase (6 h). We therefore tested the ability of V. cholerae supernatant to release 32Pi from [α-32P]GTP. The supernatant from 6-h-grown V. cholerae VB1 was capable of producing [32P]GDP from [α-32P]GTP with very little release of 32Pi (Fig. 2B, lane 4). The supernatant from a 10-h (early-stationary-phase) culture, however, demonstrated considerable release of 32Pi from [α-32P]GTP without accumulation of [32P]GDP (Fig. 2B, lane 5), demonstrating the presence of this enzyme in small amounts in the 6-h culture supernatant but in higher amounts in the supernatant of the 10-h culture. Similar accumulation of [32P]ADP by 6-h culture, but more pronounced release of 32Pi from [γ-32P]ATP by the 10-h culture of V. cholerae VB1, can be seen in Fig. 2A.
FIG. 2.
Secretion of 5′ nucleotidase by V. cholerae VB1 during growth in LB. Supernatant samples from various growth phases (0, 3, 6, and 10 h) were prepared as described in the legend to Fig. 1 and incubated with [γ-32P]ATP (A) or [α-32P]GTP (B). Lanes 1, [γ-32P]ATP and [α-32P]GTP controls; lanes 2 to 5, labeled nucleotide plus cell-free supernatants from cells grown for 0, 3, 6, and 10 h, respectively.
Fractionation of the supernatant from the late-log-phase-grown culture of V. cholerae.
The presence of 5′ nucleotidase and Ak in the supernatant of V. cholerae VB1 raised the question of the presence of additional ATP-utilizing enzymes in such supernatants. In order to identify individual enzymatic activities, we fractionated the growth medium (4 liters) of strain VB1 by column chromatography. The growth medium after 6 h of growth was centrifuged to remove cells and was concentrated by ultrafiltration with a YM10 Amicon membrane. The concentrated supernatant was loaded on hydroxyapatite, ATP-agarose, Mono Q, and Q-Sepharose columns, but, in each case, the enzymatic activities (as well as cytotoxicities, which will be described later) were found in the effluents, showing a lack of binding to these columns. Loading on a green Sepharose column and elution with a linear gradient of 0 to 3 M KCl, however, demonstrated the separation of the Ak activity from the other enzymatic activities.
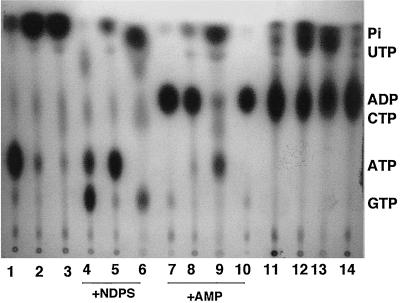
The results in Fig. 3 demonstrate that the V. cholerae VB1 supernatant, before fractionation, allowed release of 32Pi from [γ-32P]ATP (lane 2) and of both 32Pi and small amounts of GTP, CTP, and UTP in the presence of [γ-32P]ATP plus 0.1 mM concentrations of each NDP (GDP, CDP, and UDP), demonstrating the presence of traces of Ndk as well as 5′ nucleotidase or ATPase (lane 5). In the presence of [γ-32P]ATP plus 0.1 mM AMP, the supernatant fraction demonstrated the release of small amounts of 32Pi and formation of a major band of [32P]ADP, demonstrating the presence of 5′ nucleotidase (or ATPase) and Ak (Fig. 3, lane 8). In contrast, the green Sepharose column flowthrough (GSFT) fraction showed release of 32Pi from [γ-32P]ATP (Fig. 3, lane 3), while the 3.0 M KCl eluate had no such activity either with [γ-32P]ATP alone or in the presence of [γ-32P]ATP plus NDPs (data not shown). Thus, the 3.0 M KCl eluate fractions showed little 5′-nucleotidase or Ndk activity. When the GSFT fraction was incubated with [γ-32P]ATP + NDPs, release of 32Pi as well as formation of small amounts of GTP-CTP-UTP was detected (Fig. 3, lane 6), suggesting the presence of 5′ nucleotidase (ATPase) and Ndk activities in this fraction. In contrast, incubation of GSFT fraction in presence of [γ-32P]ATP + AMP demonstrated the release of 32Pi and traces of GTP-CTP-UTP but very little [32P]ADP (lane 9), suggesting that the Ak activity was removed from the GSFT fraction. Indeed, when fractions 25 to 32 of the KCl eluate, corresponding to about 3.0 M KCl, were incubated with [γ-32P]ATP plus 0.1 mM AMP, a major single band of [32P]ADP was observed (Fig. 3, lane 10), suggesting that the Ak activity was retained within the green Sepharose column and was subsequently eluted at 3.0 M KCl.
FIG. 3.
Presence of ATP-transforming enzymes in the flowthrough and eluted fractions from a green Sepharose column. The loading and elution conditions are described in Materials and Methods. Lanes: 1, [γ-32P]ATP control; 2, [γ-32P]ATP plus 10-fold-concentrated supernatant; 3, [γ-32P]ATP plus GSFT fraction; 4, [γ-32P]ATP plus 0.1 mM (each) NDPs (GDP, CDP, and UDP) plus purified Ndk; 5, [γ-32P]ATP plus NDPs plus supernatant; 6, [γ-32P]ATP plus NDPs plus GSFT; 7, [γ-32P]ATP plus 0.1 mM AMP plus purified Ak; 8, [γ-32P]ATP plus 0.1 mM AMP plus supernatant; 9, [γ-32P]ATP plus 0.1 mM AMP plus GSFT; 10, [γ-32P]ATP plus 0.1 mM AMP plus green Sepharose column eluate; 11, [α-32P]CTP control; 12, [α-32P]CTP plus supernatant; 13, [α-32P]CTP plus GSFT; 14, [α-32P]CTP plus green Sepharose column eluate. Protein (20 μg) from the eluted and flowthrough fractions was used in each assay.
To confirm that the 32Pi-releasing activity was not solely due to ATPase but a general 5′-nucleotidase action, we tested the supernatant, the GSFT, and the 3.0 M KCl eluate from the green Sepharose column in releasing 32Pi from [α-32P]CTP. The commercial [α-32P]CTP was slightly contaminated with 32Pi (Fig. 3, lane 11). Both the supernatant and the GSFT fractions caused release of considerable amounts of 32Pi from [α-32P]CTP (Fig. 3, lanes 12 and 13). Very little 32Pi, however, was released when the 3.0 M KCl eluate from the green Sepharose column was incubated with [α-32P]CTP (Fig. 3, lane 14), again demonstrating that this fraction had high Ak activity but very little 5′-nucleotidase activity.
HPLC for the detection of 5′ nucleotidase and adenylate kinase.
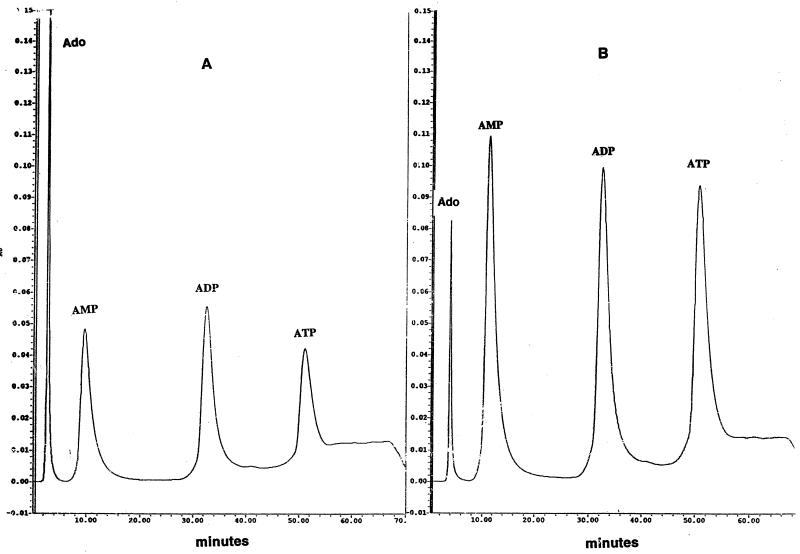
To characterize further the nature of the 5′ nucleotidase and Ak, we incubated the supernatant samples with ADP alone (Fig. 4A) and with ATP plus AMP (Fig. 4B). The nature of the products formed was then analyzed by HPLC. 5′ Nucleotidase (or phosphatase) dephosphorylates the 5′-terminal phosphates from nucleoside phosphates such as AMP, ADP, or ATP, while Ak catalyzes the reversible reaction AMP + ATP ⇋ 2 ADP. All nucleosides (such as adenosine) or nucleoside phosphates (such as AMP, ADP, or ATP) formed a single peak with appropriate retention times (data not shown). When, however, ADP was incubated with the supernatant, both AMP and ATP peaks were clearly visible as well as a peak of adenosine (Fig. 4A). While AMP and adenosine could result from the action of 5′ nucleotidase on ADP, the formation of a large peak of ATP indicated the presence of Ak in the supernatant. Similarly, the formation of a high level of ADP from ATP plus AMP demonstrated the presence of Ak (Fig. 4B). Incubation of the supernatant with ATP alone demonstrated the presence of a much smaller peak for ADP (data not shown), suggesting that the substantial peak for ADP seen in Fig. 4B is due to the presence of Ak. A substantial peak for adenosine is seen in both Fig. 4A and B.
FIG. 4.
Product characterization by HPLC during incubation of the supernatant fractions with ADP (A) or ATP plus AMP (B). For each experiment, 20 μg of protein from the supernatant fraction was used. Ado, adenosine.
Do ATP-transforming enzymes modulate virulence of V. cholerae?
An important question regarding the secretion of ATP-transforming enzymes by strain VB1 is that of their potential role in subverting host defense. Phagocytic cells such as macrophages and mast cells play a major role in defending the host epithelial cells from outside attack by various pathogens. It has recently been demonstrated that different mammalian cells, including macrophages, extrude ATP to the outside (1, 9). Macrophages efflux ATP on stimulation by bacterial LPSs or intact bacteria (6, 7). This external ATP then activates a number of receptors called purinergic receptors that, when activated by ATP or other nucleoside phosphates derived from it, allow various cellular functions to be performed. For example, it has been demonstrated that macrophages have surface-associated P2 purinergic receptors such as P2Y and P2Z. P2Z receptors, when activated by millimolar concentrations of external ATP, can trigger macrophage cell death by pore formation in the membrane through which intracellular metabolites with masses of up to 900 Da can leak out (3, 17). Thus, increasing concentrations of external ATP promote macrophage cell death. This can be seen from the results in Fig. 5A, where treatment of macrophages with 1 mM ATP leads to about 20% macrophage death in 2 h. Increasing concentrations of ATP or increasing periods of incubation led to higher rates of macrophage cell death. In order to assess the cytotoxic effect of V. cholerae supernatant, we kept the macrophage cell death at about 20% by adjusting the ATP concentration to 1 mM and the period of incubation to 2 h.
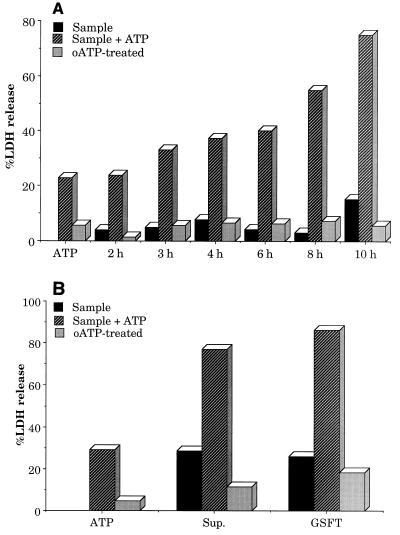
FIG. 5.
(A) Cytotoxicity towards macrophages exhibited by external ATP or the supernatant fraction of V. cholerae VB1 grown in LB at 37°C for various times. Macrophages were prepared, plated, and stimulated with LPS as described in Materials and Methods. For oATP treatment, macrophages were treated with 1.0 mM oATP for 2 h before being subjected to toxicity tests with supernatant samples in the presence or absence of exogenous ATP (1 mM). The measurement of LDH release is described in Materials and Methods as well as previously reported (29, 30). About 1.0 μg of protein was used in each experiment. (B) Macrophage cytotoxicity demonstrated by the supernatant or the GSFT fractions of V. cholerae VB1 cultures. The protocol of the experiments is very similar to that used for panel A. The supernatant (Sup.) from a 10-h-grown culture of VB1 was used for these experiments. About 1.0 μg of protein was used in each experiment.
Incubation of the macrophages with the supernatant fractions obtained at various stages of growth exhibited low levels of cytotoxicity. However, with increasing growth, as the ATP-utilizing enzymes were secreted into the medium, increasing macrophage cytotoxicities were observed in the presence of 1 mM ATP (Fig. 5A). Interestingly, such cytotoxicities were greatly reduced if the macrophages were pretreated with oATP, suggesting that the enhancement of macrophage cell death is mediated via activation of the macrophage surface-associated P2Z receptors, since activation of the P2Z receptors is known to be blocked on pretreatment of the macrophages with oATP (21). Heating of the supernatant fractions in a boiling-water bath for 5 min abolished both enzymatic activity and cytotoxicity, suggesting that the cytotoxicity might be due to the presence of the ATP-utilizing enzymes.
To evaluate whether Ak, which was removed during green Sepharose column chromatography, plays any major role in virulence, we determined the cytotoxicity of growth medium of 10-h-grown V. cholerae VB1 and the fractionated GSFT fraction lacking Ak activity (Fig. 5B). The GSFT fraction showed essentially as much cytotoxicity as the supernatant fraction, with lower cytotoxicity in the absence of or higher cytotoxicity in the presence of ATP. The ATP-induced enhancement of cytotoxicity in both cases was reduced when the macrophages were pretreated with oATP, suggesting that P2Z receptor activation was a major route to macrophage cell death. Very little macrophage cell-killing activity was demonstrated by the 3.0 M KCl eluate fraction, in either the absence or presence of ATP (data not shown), suggesting that separated Ak by itself had no significant activity for P2Z receptor activation.
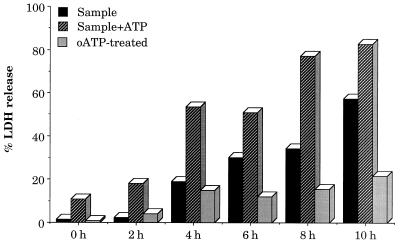
To determine if the cytotoxicity towards macrophages exhibited by the V. cholerae supernatant fractions may extend to other phagocytic cells, such as mast cells, we determined the effect of the growth medium supernatants of strain VB1, after different periods of growth, on mast cell death in the absence and presence of ATP. Very little cytotoxicity was observed from supernatants grown for 2 h, and the extent of mast cell death remained between 10 and 20% in the presence of ATP (Fig. 6), a value similar to that for 1.0 mM ATP without any supernatant. When supernatants were obtained from mid-log- to stationary-phase cultures, which demonstrated significant accumulation of ATP-utilizing enzymes, increasing cytotoxicities were observed in the presence of ATP and were inhibited when the mast cells were pretreated with oATP, suggesting that P2Z receptor activation might be the critical factor in triggering mast cell death, similar to that which happens with macrophages. P2Z receptor activation is also known to be blocked in the presence of Mg2+ (6, 7). When the cell death assays were done in the presence of 0.1 to 1.0 mM Mg2+, the cytotoxicity was reduced by 75 to 80%, again confirming that inhibitors of P2Z receptor activation inhibit the supernatant-mediated ATP-induced enhanced cytotoxicity towards phagocytic cells.
FIG. 6.
Cytotoxic effects on mast cells of ATP and the supernatant fraction of V. cholerae strain VB1 grown in LB at 37°C for various times. Mast cells were prepared, plated, and stimulated with LPS as described in Materials and Methods. Cells, either pretreated or not pretreated with oxidized ATP (oATP) for 2 h, were subjected to toxicity tests with supernatant samples in the presence or absence of exogenous ATP (1 mM). LDH assays were conducted as described in the legend to Fig. 5. Two micrograms of protein was used for each supernatant sample.
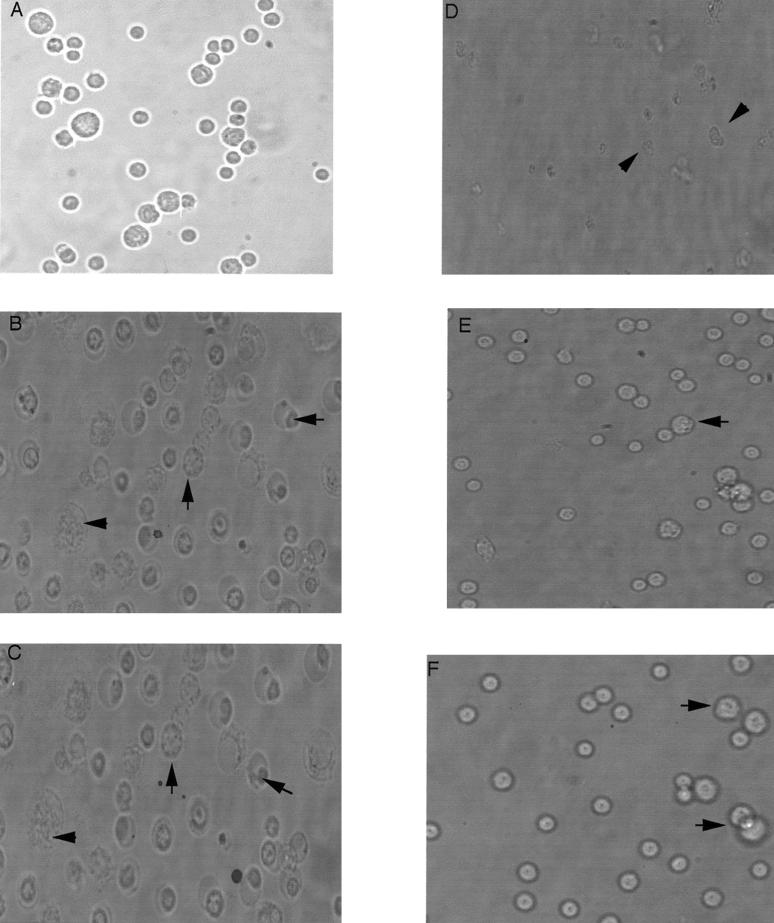
To gain further understanding of the nature of the cell death, we examined, by phase-contrast microscopy, the morphological features of the mast cells in the absence or presence of ATP with and without the supernatant samples obtained from 8-h-grown V. cholerae VB1. Normal mast cells have a round morphology (Fig. 7A). In the presence of 0.5 mM ATP, many cells underwent swelling and vacuolization (Fig. 7B), as demonstrated previously for macrophages undergoing P2Z receptor activation (30). Similar swelling and vacuolization were observed when the mast cells were treated with the 8-h samples from strain VB1 (Fig. 7C). Treatment of mast cells with a combination of 0.5 mM ATP and the 8-h supernatant sample led to dramatic changes in the morphology in which swelling, nuclear condensation, and fragmentation were common (Fig. 7D). Interestingly, pretreatment of the mast cells with oATP largely prevented changes in the morphology, induced either by ATP (Fig. 7E) or by the combination of ATP and the 8-h supernatant sample (Fig. 7F). Thus, oATP-induced irreversible blockage of P2Z receptor activation appeared to block mast cell death, likely due to cell swelling, membrane perturbation, and nuclear fragmentation induced by P2Z receptor activation.
FIG. 7.
Changes in morphology of LPS-primed mast cells after treatment with ATP and various supernatant fractions. Cells pretreated with 50 ng of LPS per ml were either treated with oATP or not treated and then incubated with ATP (0.5 mM), 8-h supernatants from V. cholerae strain VB1, or both. (A) Control, untreated cells; (B) cells treated with 0.5 mM ATP; (C) cells treated with supernatants from V. cholerae; (D) cells treated with supernatants from V. cholerae plus 0.5 mM ATP; (E) cells pretreated with oATP (1 mM) for 2 h before addition of 0.5 mM ATP; (F) cells pretreated with oATP (1 mM) for 2 h before addition of 0.5 mM ATP and supernatants from V. cholerae. Various abnormal morphological forms indicative of cell death are indicated by arrows. Two micrograms of supernatant proteins was used. Phase-contrast photomicrographs were taken with a 40× objective.
DISCUSSION
Phagocytic cells are important in the engulfment and removal of foreign pathogens from infected tissues. Successful V. cholerae colonization must therefore somehow involve elimination of such phagocytic cells. It has become increasingly clear that macrophages use purinergic receptors that, on activation in the presence of adenine nucleotides, will allow the macrophages to deal with the incoming pathogens, including allowing their own cell death (3, 17). Such receptors are also present on mast cells. An interesting feature of the purinergic receptors is that they have specific agonist profiles. For example, adenosine and AMP activate P1 receptors while ATP and ADP activate the P2 receptors. The ionic ATP4− form and benzoyl benzoyl ATP are much better agonists for P2Z receptor activation than ATP, which is a better agonist than ADP (3, 10). Thus, secretion of ATP-transforming enzymes by a pathogen may have enormous consequences for macrophage function and its survival, since macrophages are known to efflux ATP on LPS stimulation, and the secreted enzymes may act on such external ATP to either biotransform it, thereby preventing P2Z receptor activation and macrophage cell death, or convert it to a better agonist than ATP itself, thus accelerating macrophage cell death. Our finding that V. cholerae elaborates a number of such ATP-transforming enzymes, which may enhance phagocytic cell death, provides important insights into how a mucosal pathogen such as V. cholerae deals with phagocytic cells. It is important to point out that V. cholerae must exert its toxicity while living in the microaerophilic to anaerobic environment of the gut. It was thus of interest to us to determine if V. cholerae, grown under low oxygen tension, could secrete the ATP-utilizing enzymes. We demonstrated that when strain VB1 was grown in LB under stationary conditions, Ak activity was detectable in the cell-free growth medium as early as an OD650 of 0.1, while the Ndk and 5′-nucleotidase activities were detectable when the OD650 reached about 0.4. Thus, V. cholerae may contend with phagocytic cells while growing in the host gastrointestinal tract by secreting ATP-utilizing enzymes even at low cell densities.
Our observations raise a number of questions that remain unanswered. What's the nature of the secretion system? Using a type II secretion-deficient mutant VB12 that is unable to secrete cholera toxin, protease, and chitinase (24), we have shown that such a mutant is proficient in secreting the ATP-transforming enzymes (unpublished observations), suggesting that the type II secretion system is not involved in the secretion of ATP-utilizing enzymes. We have recently reported that in P. aeruginosa, Ndk is secreted by a type I mechanism which involves the presence of a secretion motif in the carboxy terminus (12). The secretion of Ak is detected when the OD650 is about 0.4, while 5′ nucleotidase become detectable at an OD650 of 0.7, long before cell lysis occurs and coincidental to cholera toxin secretion (23, 24). For M. bovis and P. aeruginosa, we have shown that secretion of ATP-utilizing enzymes is facilitated by the presence of eukaryotic proteins in the media such as those present in LB, and it occurs within 60 to 90 min of the addition of the proteins (29, 30). Although we have not specifically studied the effect of eukaryotic proteins on the secretion of ATP-utilizing enzymes by V. cholerae VB1, it is likely that such a process would be modulated in the same way as reported for M. bovis or P. aeruginosa. One of the limitations of the present studies is to show a direct effect of the secreted ATP-utilizing enzymes on P2Z receptor activation. We are in the process of obtaining mice that harbor knockout mutations in the P2Z receptor genes. Isolation of macrophages and mast cells from such knockout mutant mice and an examination of their susceptibility to ATP in the absence and presence of the V. cholerae supernatants will provide important clues regarding the role of V. cholerae secreted cytotoxic factors, presumably the ATP-utilizing enzymes, in P2Z receptor-mediated phagocytic cell death. Characterization of the individual enzymes and their genes in V. cholerae and introduction of knockout mutations in such genes will allow an evaluation of the role of the individual genes in V. cholerae pathogenesis. If indeed such enzymes play important roles in the survival of V. cholerae in the gut, then mutations in these genes, in addition to mutations in various toxin genes, may provide a better live-vaccine candidate than is presently available.
One of the intriguing observations in this report is the elaboration of so many ATP-transforming enzymes by V. cholerae. Mammalian cells are known to have such enzymes as Ecto-Ndk, Ecto-nucleotidase, Ecto-Ak, etc., on the outside of their membranes (18, 19, 22), which are believed to be important in the maintenance of a balanced level of various nucleotides in the outside of the cell (31). Secretion of such enzymes by the pathogens may thus be one way to subvert the cellular physiology of the host cells. Another interesting observation is the nature of the products formed from ATP that influence macrophage survival. Intracellular pathogens that are known to require live macrophages for their growth, such as M. bovis, Legionella pneumophila, or even the parasite Leishmania, have been shown to elaborate only Ndk and ATPase types of enzymes (29; O. Zaborina and K. P. Chang, unpublished observations) but not the 5′-nucleotidase/Ak type of enzymes. The latter have been observed to be elaborated only by mucosal pathogens such as mucoid P. aeruginosa (30), B. cepacia (20), and V. cholerae, which do not need live macrophages and are likely to kill macrophages for their survival. Since different ionic forms of ATP and adenine nucleotides have differential agonistic activities towards P2Z receptor activation (3, 10), it would be of interest to examine whether elaboration of 5′ nucleotidase and other enzymes by the mucosal pathogens may allow formation of modified adenine nucleotides that are potent agonists of the P2Z receptor activation and hence of macrophage cell death. The secretion of 5′ nucleotidase by V. cholerae VB1 cells that can generate adenosine, AMP, and ADP from ATP (Fig. 1 and 4) can modulate macrophage cell death through multiple mechanisms. Indeed, it has been reported that a continuous generation of adenosine within the human epidermoid carcinoma cells can lead to an intracellular nucleotide imbalance with pyrimidine starvation, triggering suicidal processes ending up in apoptosis of the cells (28). It would be interesting to see if the secreted enzymes from V. cholerae may allow the pathogen to evade the immune system through activation of multiple purinergic receptors, with P2Z receptors playing a primary role.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI 16790-20 from the National Institutes of Health.
REFERENCES
- 1.Al-AwQati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- 2.Davis B M, Kimsey H H, Chang W, Waldor M K. The Vibrio cholerae O139 Calcutta bacteriophage CTXφ is infectious and encodes a novel repressor. J Bacteriol. 1999;181:6779–6787. doi: 10.1128/jb.181.21.6779-6787.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Virgilio F. The P2Z purinoreceptor—an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 4.Faruque S M, Albert M J, Mekalanos J J. Epidemology, genetics and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque S M, Asadulghani, Alim A R M A, Albert M J, Islam K M N, Mekalanos J J. Induction of the lysogenic phage encoding cholera toxin in naturally occurring strains of toxigenic Vibrio cholerae O1 and O139. Infect Immun. 1998;66:3752–3757. doi: 10.1128/iai.66.8.3752-3757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari D, Chiozzi P, Falzoni S, Dalsusino M, Melchiorri L, Baricordi O R, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 7.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox J L. Advice, rules, diets to improve food, water safety. ASM News. 1998;64:439–440. [Google Scholar]
- 9.Guidotti G. ATP transport and ABC proteins. Chem Biol. 1996;3:703–706. doi: 10.1016/s1074-5521(96)90244-6. [DOI] [PubMed] [Google Scholar]
- 10.Harden T K, Boyer J L, Nicholas R A. P2-purinergic receptors: subtype-associated signaling and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa T, Konig W, Kurata M, Mauser L, Ishizaka K. Immunologic properties of mast cells from rats infected with Nippostrongylus brasiliensis. J Immunol. 1975;115:1078–1083. [PubMed] [Google Scholar]
- 12.Kamath S, Chen M L, Chakrabarty A M. Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion. J Bacteriol. 2000;182:3826–3831. doi: 10.1128/jb.182.13.3826-3831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaper J B, Morris J G, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaolis D K R, Johnson J A, Bailey E C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenner J R, Coster T S, Taylor D N, Trofa A, Barreraoro M, Hyman T, Adams J M, Beattie D T, Killeen K P, Spriggs D R, Mekalanos J J, Sadoff J C. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 16.Kovach M E, Shaffer M D, Peterson K M. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology. 1996;142:2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 17.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingan S, Kumararatne D S. ATP induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 18.Lazarowski E R, Homolya L, Boucher R C, Harden T K. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- 19.Lee K S, Schubert P, Reddington M, Kreutzberg G W. The distribution of adenosine A1 receptors and 5′-nucleotidase in hippocampal formation of several mammalian species. J Comp Neurol. 1986;246:427–434. doi: 10.1002/cne.902460402. [DOI] [PubMed] [Google Scholar]
- 20.Melnikov A, Zaborina O, Dhiman N, Prabhakar B S, Chakrabarty A M, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000;36:1481–1493. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 21.Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP—an irreversible inhibitor of the macrophage purinergic-P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- 22.Nagy A K, Shuster T A, Delgado-Escueta A V. Rat brain synaptosomal ATP:AMP-phosphotransferase activity. J Neurochem. 1989;53:1166–1172. doi: 10.1111/j.1471-4159.1989.tb07410.x. [DOI] [PubMed] [Google Scholar]
- 23.Overbye M L, Sandkvist M, Bagdasarian M. Specificity of the protein secretory apparatus: secretion of the heat-labile enterotoxin B subunit pentamers by different species of Gram (−) bacteria. Gene. 1995;152:41–45. doi: 10.1016/0378-1119(94)00691-k. [DOI] [PubMed] [Google Scholar]
- 24.Sandkvist M, Overbye M L, Hough L, Morales V, Bagdasarian M M, Koomey M, DiRita V, Bagdasarian M. General secretion pathway genes, eps, required for toxin secretion and for outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom B R. Purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- 26.Tacket C O, Kotloff K L, Losonsky G, Nataro J P, Michalski J, Kaper J B, Edelman R, Levine M M. Volunteer studies investigating the safety and efficacy of live oral E1 Tor Vibrio cholerae O1 vaccine strain CVD111. Am J Trop Med Hyg. 1997;56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 27.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 28.Wiendl H S, Schneider C, Ogilvie A. Nucleotide metabolizing ectoenzymes are upregulated in A431 cells periodically treated with cytostatic ATP leading to partial resistance without preventing apoptosis. Biochim Biophys Acta. 1998;1404:282–298. doi: 10.1016/s0167-4889(98)00040-8. [DOI] [PubMed] [Google Scholar]
- 29.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty A M. Secretion of ATP utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;30:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 30.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, El-Idrissi M E, Prabhakar B S, Chakrabarty A M. P2Z independent and P2Z receptor mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]