Key Points
Question
Is medical cannabis treatment associated with improvements in health-related quality of life?
Findings
In this case series of 3148 patients, significant improvements were reported on all 8 domains of the 36-Item Short Form Health Survey health-related quality of life assessment after commencing treatment with medical cannabis. Improvements were largely sustained over time.
Meaning
These findings suggest that medical cannabis treatment may be associated with improvements in health-related quality of life among patients with a range of health conditions.
This case series investigates whether patients using medical cannabis report improvements in health-related quality of life over time.
Abstract
Importance
The use of cannabis as a medicine is becoming increasingly prevalent. Given the diverse range of conditions being treated with medical cannabis, as well as the vast array of products and dose forms available, clinical evidence incorporating patient-reported outcomes may help determine safety and efficacy.
Objective
To assess whether patients using medical cannabis report improvements in health-related quality of life over time.
Design, Setting, and Participants
This retrospective case series study was conducted at a network of specialist medical clinics (Emerald Clinics) located across Australia. Participants were patients who received treatment for any indication at any point between December 2018 and May 2022. Patients were followed up every mean (SD) 44.6 (30.1) days. Data for up to 15 follow-ups were reported. Statistical analysis was conducted from August to September 2022.
Exposure
Medical cannabis. Product types and cannabinoid content varied over time in accordance with the treating physician’s clinical judgement.
Main Outcomes and Measures
The main outcome measure was health-related quality of life as assessed using the 36-Item Short Form Health Survey (SF-36) questionnaire.
Results
In this case series of 3148 patients, 1688 (53.6%) were female; 820 (30.2%) were employed; and the mean (SD) age was 55.9 (18.7) years at baseline before treatment. Chronic noncancer pain was the most common indication for treatment (68.6% [2160 of 3148]), followed by cancer pain (6.0% [190 of 3148]), insomnia (4.8% [152 of 3148]), and anxiety (4.2% [132 of 3148]). After commencing treatment with medical cannabis, patients reported significant improvements relative to baseline on all 8 domains of the SF-36, and these improvements were mostly sustained over time. After controlling for potential confounders in a regression model, treatment with medical cannabis was associated with an improvement of 6.60 (95% CI, 4.57-8.63) points to 18.31 (95% CI, 15.86-20.77) points in SF-36 scores, depending on the domain (all P < .001). Effect sizes (Cohen d) ranged from 0.21 to 0.72. A total of 2919 adverse events were reported, including 2 that were considered serious.
Conclusions and Relevance
In this case series study, patients using medical cannabis reported improvements in health-related quality of life, which were mostly sustained over time. Adverse events were rarely serious but common, highlighting the need for caution with prescribing medical cannabis.
Introduction
Medical cannabis was legalized in Australia in November 2016.Aside from Sativex and Epidiolex, all other cannabinoid products are considered unapproved therapeutic goods at the time of this writing. Physicians must obtain regulatory approval to prescribe via one of several special access pathways. These approvals have increased rapidly over the last 2 years and now total more than 332 000.1 Most approvals have been for chronic pain (55%), followed by anxiety (23%) and insomnia and/or sleep disorders (6%).2 Major reviews have generally concluded there is evidence for cannabinoid efficacy in the treatment of several conditions: pain in adults, chemotherapy-induced nausea and vomiting, and spasticity associated with multiple sclerosis.3,4,5 Moderate evidence exists for cannabinoid efficacy in treating secondary sleep disturbances, and there is limited, insufficient, or absent evidence for other conditions. Despite this, enrollment in medical cannabis programs increased 4.5-fold in the US between 2016 and 2020,6 and a recent survey conducted in the US and Canada found that 27% of all respondents (n = 27 169) had used cannabis for medical purposes at some point.7
The term medical cannabis encompasses a vast array of products (eg, dried flower, oils, edibles) containing multiple bioactive constituents including, but not limited to, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Because patients are using these products to manage such a wide range of health conditions—in addition to the paucity of evidence from randomized clinical trials—clinical evidence incorporating patient-reported outcomes is becoming increasingly recognized as a vital source of safety and efficacy data.8,9 Validated health-related quality of life measures can help provide important, global insights into associations between medical cannabis treatment and daily functioning, physical mobility, and mental health among patients with various and disparate conditions. Here, we examine changes in health-related quality of life over time in a cohort (n = 3148) of Australian patients receiving medical cannabis treatment between 2018 and 2022.
Methods
Study Design and Procedures
We conducted a retrospective case series analysis of patients prescribed medical cannabis through Emerald Clinics, a network of specialist medical clinics across Australia. After providing informed written consent, patients presenting to Emerald Clinics first undergo a comprehensive consultation with a physician, who reviews their medical history and determines suitability for cannabinoid treatment. In addition to meeting Australia’s regulatory requirements for access to unapproved products (physicians must provide a suitable clinical justification for the use of medical cannabis, including reasons why products included in the Australian Register of Therapeutic Goods are not suitable for treatment of the patient), patients are also required to have exhausted other treatment options for the clinical indication(s) they are presenting with. Moreover, site-specific contraindications for treatment include: (1) urine positive for carboxy-THC (THC-COOH), (2) pregnant and/or breastfeeding, (3) serious cardiac disease, or (4) serious mental health conditions, such as suicidal ideation or a history of psychosis. Patients are instructed to slowly increase their dose via a “start low, go slow” principle. The target dose is determined on a case-by-case basis and is subject to regular reviews by the prescribing physician to assess treatment efficacy and side effects, including any interactions with concomitant medication. Although no official prescribing guidelines exist in Australia, clinical judgement of appropriate dose and product type may be influenced by various factors such as health condition, age, concomitant medications, comorbidities, dose form, and the cost of treatment. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Collection
In accordance with Australia’s National Statement on Ethical Conduct in Human Research (2007) requirements for exemption from review, data collection commenced in December 2018 and remains ongoing. For this study, we included every observation available (as of May 5, 2022) comprising baseline and up to and including the first 15 follow-up consultations of each patient. We limited the number of follow-ups to 15 as patient numbers become much smaller thereafter (n <80). Besides providing detailed clinical and demographic information (such as age, gender, employment status, and any other medications currently being used), at each consultation patients were also asked to complete several validated questionnaires, including the 36-Item Short Form Health Survey (SF-36) which is the focus of this study. eTable 1 in Supplement 1 presents a consult-by-consult overview of data availability for each measure used in our analysis, but also the mean (SD) time elapsed between consultations. On average, patients attended a mean of (SD) 5.6 (4.9) consultations with a mean (SD) time between consultations of 44.6 (30.1) days.
Outcome
The primary outcome was change from baseline in patient scores on the SF-36,10,11 a widely used measure of health-related quality of life. The SF-36 includes 36 items which form 8 distinct scales, including: (1) limitations in physical activities due to health problems; (2) limitations in social activities due to physical or emotional problems; (3) limitations in usual role activities due to physical health problems; (4) bodily pain; (5) general mental health (psychological distress and well-being); (6) limitations in usual role activities due to emotional problems; (7) vitality (energy and fatigue); and (8) general health perceptions. Scores can range from 0 to 100, with higher values indicating better outcomes. A recent review considered a 10-point change to be the minimally clinically important difference.12 Finally, as an additional outcome we also report any reported adverse events.
Statistical Analyses
Our analysis followed a conventional ordinary least squares model. We first estimated a univariate regression using a binary treatment indicator for taking medical cannabis as the sole estimator for each of the 8 domain scores. We then moved to a more complete framework, estimating each score y for patient i at consult t with: yi,t = β1Treatmentt + β2Xi,t + β3Zi + εi,t (equation 1). The coefficient associated with β1 represents the effect of commencing with the treatment on a patient’s quality of life. Xi,t represents a set of control variables that could potentially influence yi,t. These include the number of medications a patient takes daily (at the time of consult), binary indicators for both 8 medication categories (simple analgesics, opioids, antidepressants, benzodiazepines, GABA analogues, antipsychotic medications, compound analgesics, and other pain medications) and 4 primary diagnosis categories (pain, psychiatric, neurological, or other), the number of other comorbidities reported, the patient’s age, gender, and employment status, and a nonlinear treatment trend (equal to the reciprocal of the number of follow-up consults since commencing treatment), as well as month- and year-fixed effects. Furthermore, Zi incorporates patient-fixed effects and εi,t corresponds to the usual error term. Note that throughout all estimations, 95% CIs were clustered at the patient level while statistical significance was tested at the 5% level (P = .05). We then reestimated the same regression analysis displayed in equation 1 for the separate treatment categories, focusing on whether a patient was using a balanced (40% to <60% CBD content), CBD-dominant (≥60% CBD content), or THC-dominant (≥60% THC content) treatment as the main regressors of interest. Effect sizes equivalent to Cohen d were calculated by dividing the associated treatment coefficients in our patient fixed-effects model by the SDs of the respective SF-36 scores at baseline. All analyses were performed in R 4.2.2 (R Project for Statistical Computing) using the lfe package from August to September 2022.
Results
Demographics and Patient Characteristics
Among the 3148 patients included in this data set, 1688 (53.6%) were female; 820 (30.2%) were employed; and the mean (SD) age was 55.9 (18.7) years at baseline before treatment. Table 1 summarizes the demographics and characteristics of the 3148 patients included in this study. Chronic non-cancer pain was the most common indication for treatment (68.6% [2160 of 3148]), followed by cancer pain (6.0% [190 of 3148]), insomnia (4.8% [152 of 3148]), and anxiety (4.2% [132 of 3148]). Number of comorbidities ranged from 0 to 36, with a mean (SD) of 5.2 (3.9). On average, patients were taking a mean (SD) of 6.58 (4.58) medications a day prior to commencing treatment. The most common medications included simple analgesics (54.1% [1703 of 3148]), opioid analgesics (48.4% [1523 of 3148]), antidepressants (44.5% [1401 of 3148]), benzodiazepines (34.4% [1084 of 3148]), and GABA analogues (22.0% [693 of 3148]). Except for the mental health measure (mean [SD]: 54.06 [22.27]), all mean (SD) pretreatment SF-36 scores were well below the halfway mark on the respective 0 to 100 scales: 40.22 (22.40) for general health; 29.85 (24.16) for bodily pain; 40.99 (30.49) for physical functioning; 14.02 (28.99) for role-physical; 28.37 (37.30) for role-emotional; 36.57 (26.84) for social functioning; and 30.19 (20.83) for vitality.
Table 1. Summary Statistics of All Variables at Baseline.
| Variable | No. (%) (N = 3148) |
|---|---|
| Basic patient characteristics | |
| Age, mean (SD) | 55.9 (18.7) |
| Sex | |
| Female | 1688 (53.6) |
| Male | 1460 (46.4) |
| Employed | 820 (30.2)a |
| Primary diagnosis | |
| Chronic noncancer pain | 2160 (68.6) |
| Cancer pain | 190 (6.0) |
| Insomnia | 152 (4.8) |
| Anxiety | 132 (4.2) |
| Posttraumatic stress disorder | 76 (2.4) |
| Autism | 60 (1.9) |
| Migraine/headache | 50 (1.6) |
| Spasticity | 44 (1.4) |
| Parkinson disease | 38 (1.2) |
| Epilepsy | 26 (0.8) |
| Otherb | 220 (7.1) |
| No. comorbidities, mean (SD) | 5.19 (3.86) |
| Concomitant medicationsc | |
| Simple analgesics | 1703 (54.1) |
| Opioid analgesics | 1523 (48.4) |
| Antidepressants | 1401 (44.5) |
| Benzodiazepines | 1084 (34.4) |
| GABA analogues | 693 (22.0) |
| Antipsychotics | 162 (5.1) |
| Compound analgesics | 137 (4.4) |
| Cannabinoidsd | 25 (0.8) |
| Other pain medications | 350 (11.1) |
| Total No. daily medications, mean (SD) | 6.58 (4.58) |
| Quality of life measures, mean (SD) (SF-36) | |
| General health | 40.22 (22.40) |
| Bodily pain | 29.85 (24.16) |
| Physical functioning | 40.99 (30.84) |
| Role-physical | 14.02 (28.99) |
| Mental health | 54.06 (22.27) |
| Role-emotional | 28.37 (37.30) |
| Social functioning | 36.57 (26.84) |
| Vitality | 30.19 (20.83) |
Abbreviations: GABA, gamma-aminobutyric acid; SF-36, 36-Item Short Form Health Survey.
Relative to the 2713 who disclosed their employment status.
Includes chemotherapy-induced nausea and vomiting, irritable bowel syndrome, depression, Alzheimer disease, inflammatory bowel disease, dementia, refractory nausea and vomiting, anorexia and wasting, alcohol use disorder, obsessive compulsive disorder, attention deficit/hyperactivity disorder, back pain, behavioral disorder, complex regional pain syndrome, essential tremor, hereditary spastic paraplegia, motor neuron disease, panic disorder and benzodiazepine dependence, tinnitus, Tourette syndrome, and vaginismus.
The number displayed for each medication category relates to the count of patients who take the respective medication daily.
Corresponds to use of cannabidiol at baseline.
Prescribing Patterns and Cannabinoid Dose
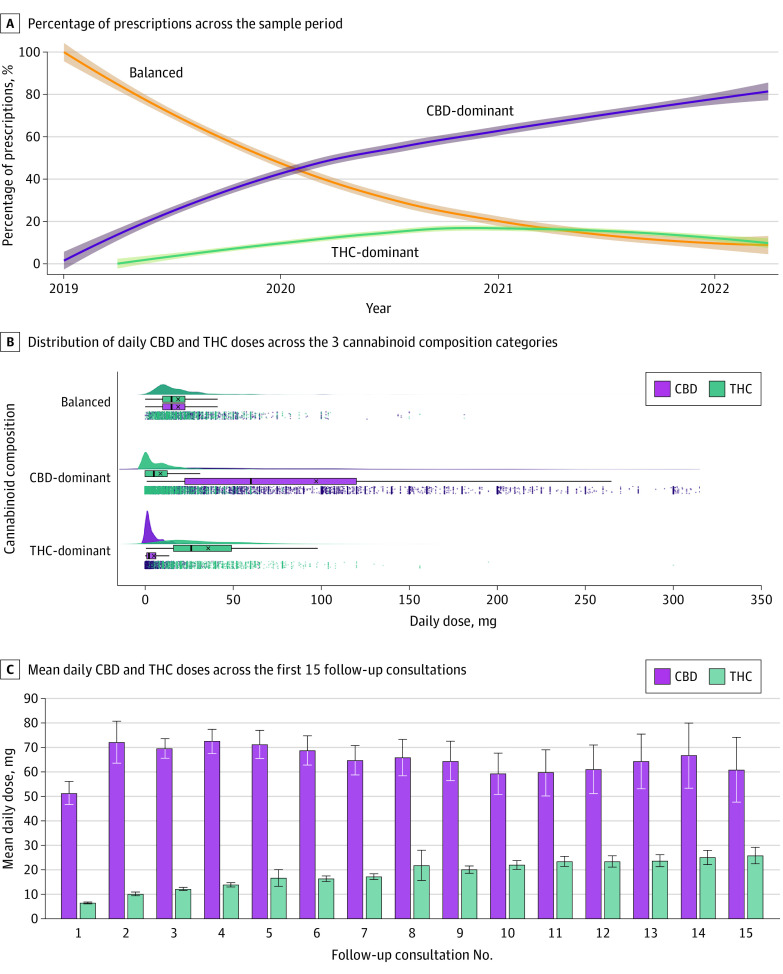
Figure 1A shows the percentage of prescriptions by cannabinoid category across the sample period. Prescriptions for CBD-dominant treatments increased consistently from February 2019, and accounted for approximately 80% of all monthly prescriptions (compared with 7.5% and 12.5% for balanced and THC-dominant categories, respectively) at the end of the data collection period. Most of these prescriptions were for orally administered products including oils (n = 14 779 [90.1%]) and capsules (n = 631 [3.8%]). There were only a small number of prescriptions for dried flower for inhalation either alone (n = 244 [1.5%]) or in combination with an oil (n = 168 [1.0%]). Figure 1B compares daily THC and/or CBD doses across categories. For balanced treatments, the mean (SD) CBD dose was 18.8 (19.2) mg and the mean (SD) THC dose was 18.8 (19.0) mg. For CBD-dominant treatments, the mean (SD) CBD dose was 97.1 (155.0) mg and the mean (SD) THC dose was 8.7 (12.2) mg. For THC-dominant treatments, the mean (SD) CBD dose was 5.0 (6.9) mg while the mean (SD) THC dose was 35.9 (71.6) mg. As Figure 1C illustrates, the mean (SD) daily CBD dose initially increased from 51.4 (128.4) mg at follow-up 1 (approximately 45 days after treatment initiation) to 72.2 (217.6) mg at follow-up 2 (approximately 90 days after treatment initiation), but then stayed relatively stable across subsequent consults. The mean (SD) daily THC dose, on the other hand, increased steadily over time from 6.5 (8.2) mg at follow-up 1 to 25.8 (23.6) mg at follow-up 15 (approximately 675 days after treatment initiation).
Figure 1. Treatment Characteristics Over Time.
A, The y-axis corresponds to the cannabinoid composition of medical cannabis prescriptions (balanced, CBD-dominant, THC-dominant). The x-axis represents time in years over the sample period (December 2018 to May 2022). The solid fitted lines are locally estimated scatterplot smoothing curves with bandwidths of 0.9 and 2-sided 95% CIs around the smooths. B, Raincloud plots for the daily dose amounts of CBD and THC (x-axis) across the 3 main cannabinoid composition categories (y-axis) are shown. Each dot in the panel corresponds to a single patient-consult dose recording (measured in mg), whereas the boxplot showcases the associated means (denoted by the x), medians (middle line of the box), first and third quartiles (left and right hinges), and 1.5 times the interquartile range left and right of the first and third quartiles, respectively (left and right whiskers), for both CBD and THC. Finally, the split-violin plot visualizes the distribution density of CBD/THC dosing behavior. C, The y-axis represents the daily dose of CBD and THC taken, while the x-axis denotes the number of consultations since commencing treatment. Error bars show 95% CI. CBD indicates cannabidiol; THC, delta-9-tetrahydrocannabinol.
SF-36 Domain Scores
Figure 2 and Figure 3 display mean scores for all SF-36 domains across 15 follow-up consults, with the red horizontal line showing the mean score at baseline as a pretreatment reference point. The gray line provides a comparison to the mean Australian score as reported in the 2015 wave of the Household, Income and Labour Dynamics in Australia survey.13 As can be seen in Figure 2, patients reported an increase relative to baseline on all 4 physical component domains, yet scores remain substantially lower than the mean Australian score. For physical functioning (Figure 2C), mean scores regressed toward baseline at follow-up 10, but did not decrease beyond this point. For all other physical domains, gains relative to baseline were maintained across all 15 follow-ups. For bodily pain (Figure 2B) and role-physical (Figure 2D), the change from baseline was statistically significant across all time points (P < .05). Figure 3 shows a similar if not greater (relative to physical component domains) improvement in mental health domain scores. We observed pronounced and statistically significant improvements on all 4 domains across all 15 follow-ups (P < .01). For both Figure 2 and Figure 3, wider 95% CIs at later time points (ie, longer treatment duration) reflect smaller patient numbers.
Figure 2. Mean 36-Item Short Form Health Survey (SF-36) Scores for General Health, Bodily Pain, Physical Functioning, and Role-Physical .
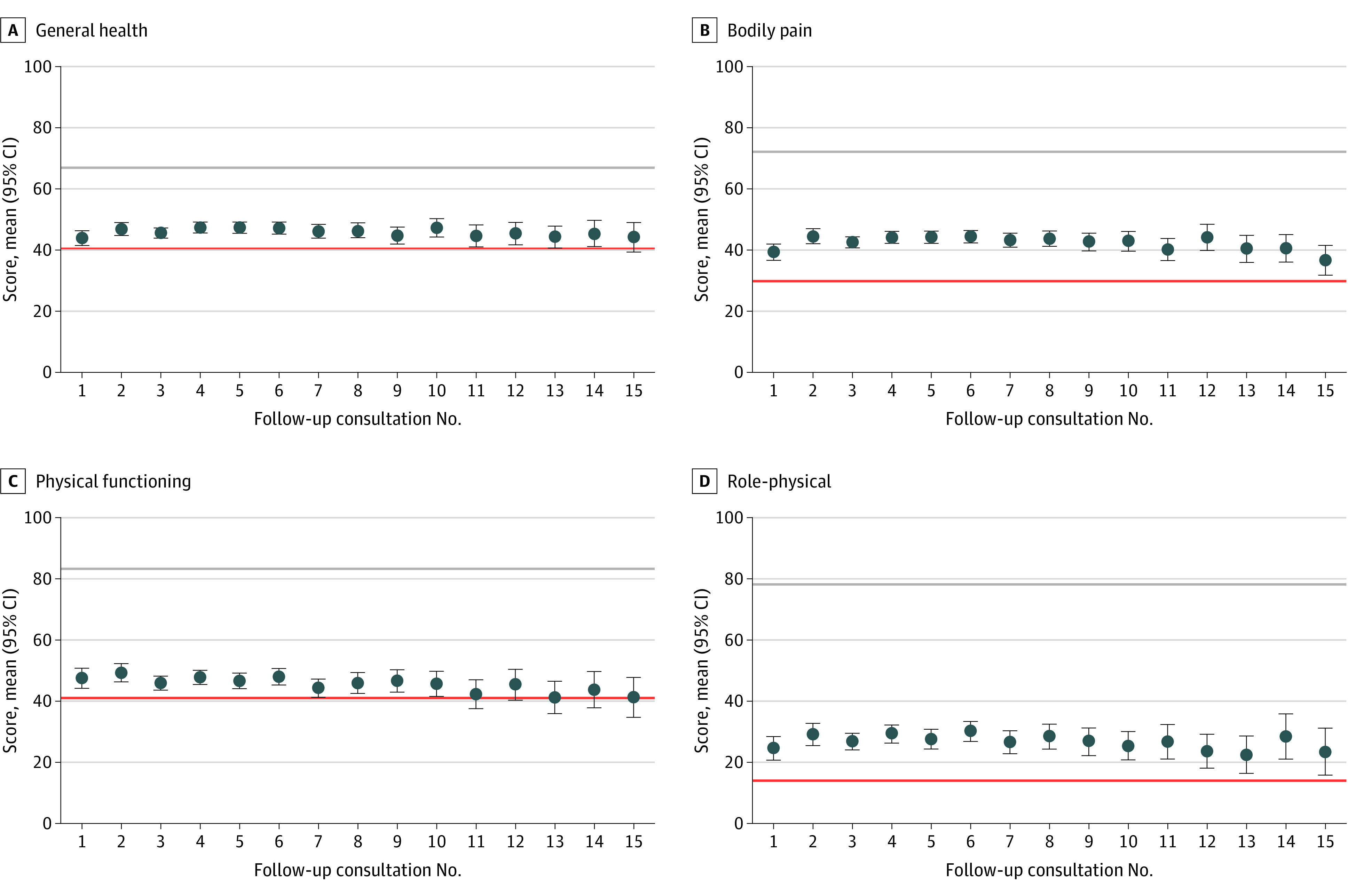
Mean scores on the y-axes correspond to the respective 0 to 100 subscales for general health (A), bodily pain (B), physical functioning (C), and role-physical (D) from the SF-36, respectively. The follow-up on the x-axes represents the number of consultations since commencing treatment. Mean levels of the 4 domain scores are computed for each follow-up consult. The red horizontal lines show the respective pretreatment means at baseline. The gray horizontal lines illustrate the associated means reported by individuals in the 2015 wave of the Household, Income and Labour Dynamics in Australia survey (see reference in text). Error bars show 95% CIs.
Figure 3. Mean 36-Item Short Form Health Survey (SF-36) Scores for Mental Health, Role-Emotional, Social Functioning, and Vitality Scales.
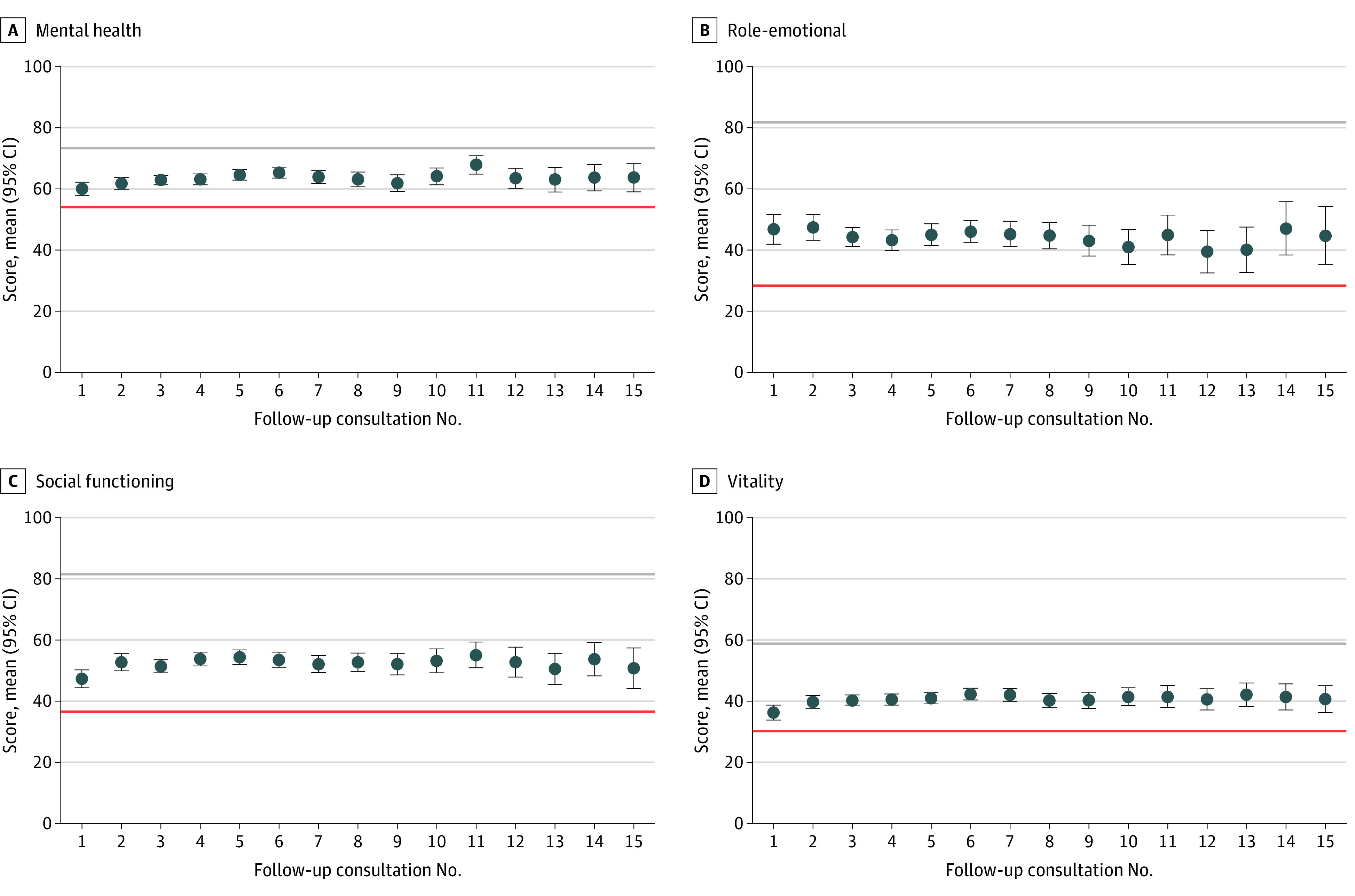
Mean scores on the y-axes correspond to the respective 0 to 100 subscales for mental health (A), role-emotional (B), social functioning (C), and vitality (D) from the SF-36, respectively. The follow-up on the x-axes represents the number of consultations since commencing treatment. Mean levels of the 4 domain scores are computed for each follow-up consult. The red horizontal lines show the respective pre-treatment means at baseline. The gray horizontal lines illustrate the associated mean reported by individuals in the 2015 wave of the Household, Income and Labour Dynamics in Australia survey (see reference in text). Error bars show 95% CIs.
Ordinary Least Squares Regression Results
Table 2 reports the ordinary least squares regression results for all 8 SF-36 domain scores. Here, we only display the primary coefficient of interest with the corresponding 95% CIs, R2 value, and effect size (Cohens d). The complete regression output can be found in eTables 2 to 9 in Supplement 1. Our complete regression model accounts for a relatively high proportion of variance (41% to 79%) in SF-36 domain scores. Overall (Table 2), treatment with medical cannabis was associated with improvements on all physical and mental health domain scores: general health (β = 8.42; 95% CI, 6.73-10.11; P < .001); bodily pain (β = 17.34; 95% CI, 15.41-19.27; P < .001); physical functioning (β = 6.60; 95% CI, 4.57-8.63; P < .001); role-physical (β = 16.81; 95% CI, 13.58-20.04, P < .001); mental health (β = 11.00; 95% CI, 9.32-12.68; P < .001); role-emotional (β = 14.19; 95% CI, 10.01-18.36; P < .001); social functioning (β = 18.31; 95% CI, 15.86-20.77; P < .001); and vitality (β = 12.91; 95% CI, 11.02-14.79; P < .001). Effect sizes were small-moderate in magnitude, ranging from 0.21 to 0.72. For all domains except for physical functioning and role-physical, balanced products were associated with marginally greater improvements than either CBD-dominant or THC-dominant products. CBD-dominant products were associated with largest improvements on the role-physical domain, while THC-dominant products were associated with largest improvements on the physical functioning domain.
Table 2. Main Results From OLS Regressions, Estimating the Treatment Effect of Taking Medical Cannabis on Quality-of-Life Outcomes (SF-36).
| Outcome | Univariate regression (1)a | Control variables (2)b | Patient-fixed effects (3)c | ES | |||
|---|---|---|---|---|---|---|---|
| β (95% CI) | R2 | β (95% CI) | R2 | β (95% CI) | R2 | ||
| Estimated change from baseline on the SF-36 associated with medical cannabis treatment | |||||||
| General health | 5.94 (4.77 to 7.10) | 0.02 | 6.22 (4.29 to 8.15) | 0.15 | 8.42 (6.73 to 10.11) | 0.73 | 0.38 |
| Bodily pain | 13.12 (11.89 to 14.36) | 0.07 | 15.55 (13.77 to 17.33) | 0.33 | 17.34 (15.41 to 19.27) | 0.68 | 0.72 |
| Physical functioning | 5.29 (3.76 to 6.82) | 0.01 | 6.24 (4.01 to 8.48) | 0.33 | 6.60 (4.57 to 8.63) | 0.79 | 0.21 |
| Role-physical | 13.37 (11.55 to 15.18) | 0.03 | 14.52 (11.65 to 17.39)d | 0.16 | 16.81 (13.58 to 20.04) | 0.49 | 0.58 |
| Mental health | 9.35 (8.26 to 10.44) | 0.04 | 9.87 (8.12 to 11.62)d | 0.18 | 11.00 (9.32 to 12.68) | 0.69 | 0.49 |
| Role-emotional | 16.17 (14.11 to 18.23) | 0.04 | 12.91 (9.53 to 16.29)d | 0.09 | 14.19 (10.01 to 18.36) | 0.41 | 0.38 |
| Social functioning | 15.97 (14.53 to 17.41) | 0.07 | 17.03 (14.72 to 19.35)d | 0.18 | 18.31 (15.86 to 20.77) | 0.61 | 0.68 |
| Vitality | 10.41 (9.26 to 11.55) | 0.05 | 11.85 (9.91 to 13.80) | 0.14 | 12.91 (11.02 to 14.79) | 0.63 | 0.62 |
| Estimated change from baseline on the SF-36 associated with medical cannabis treatment (THC/CBD balanced products only) | |||||||
| General health | 6.39 (3.86 to 8.93) | 0.02 | 7.41 (4.56 to 10.27) | 0.15 | 9.06 (7.18 to 10.93) | 0.73 | 0.41 |
| Bodily pain | 13.00 (10.57 to 15.42) | 0.07 | 15.92 (13.36 to 18.48) | 0.33 | 18.03 (15.86 to 20.21) | 0.68 | 0.75 |
| Physical functioning | 0.39 (−2.67 to 3.45) | 0.01 | 6.07 (2.92 to 9.23) | 0.33 | 6.81 (4.55 to 9.07) | 0.79 | 0.22 |
| Role-physical | 13.12 (9.58 to 16.66) | 0.03 | 15.61 (11.56 to 19.65) | 0.16 | 16.73 (13.30 to 20.16 | 0.49 | 0.58 |
| Mental health | 12.11 (10.08 to 14.13) | 0.05 | 11.44 (9.10 to 13.78) | 0.18 | 11.62 (9.66 to 13.58) | 0.69 | 0.52 |
| Role-emotional | 17.41 (13.55 to 21.27) | 0.04 | 15.43 (10.86 to 19.99) | 0.09 | 15.84 (11.17 to 20.51) | 0.41 | 0.43 |
| Social functioning | 18.35 (15.55 to 21.15) | 0.08 | 19.49 (16.29 to 22.69) | 0.18 | 20.19 (17.46 to 22.93) | 0.61 | 0.75 |
| Vitality | 12.55 (10.24 to 14.87) | 0.05 | 13.85 (11.15 to 16.54) | 0.14 | 13.66 (11.48 to 15.85) | 0.63 | 0.66 |
| Estimated change from baseline on the SF-36 associated with medical cannabis treatment (CBD-dominant products only) | |||||||
| General health | 6.10 (4.73 to 7.46) | 0.02 | 6.19 (4.03 to 8.35) | 0.15 | 8.67 (6.89 to 10.45) | 0.73 | 0.39 |
| Bodily pain | 13.89 (12.44 to 15.34) | 0.07 | 16.41 (14.36 to 18.46) | 0.33 | 17.65 (15.61 to 19.68) | 0.68 | 0.73 |
| Physical functioning | 7.18 (5.36 to 9.01) | 0.01 | 6.86 (4.43 to 9.29) | 0.33 | 6.68 (4.54 to 8.81) | 0.79 | 0.22 |
| Role-physical | 14.48 (12.33 to 16.64) | 0.04 | 15.35 (12.06 to 18.63) | 0.16 | 17.61 (14.13 to 21.09) | 0.49 | 0.61 |
| Mental health | 8.86 (7.56 to 10.15 | 0.04 | 9.48 (7.44 to 11.51) | 0.18 | 11.07 (9.31 to 12.83) | 0.69 | 0.50 |
| Role-emotional | 16.96 (14.53 to 19.39) | 0.04 | 12.81 (8.94 to 16.67) | 0.09 | 13.61 (9.21 to 18.00) | 0.41 | 0.37 |
| Social functioning | 16.33 (14.64 to 18.02) | 0.07 | 17.30 (14.65 to 19.95) | 0.18 | 18.08 (15.48 to 20.68) | 0.61 | 0.67 |
| Vitality | 10.00 (8.68 to 11.32 | 0.05 | 11.65 (9.50 to 13.81) | 0.14 | 13.08 (11.13 to 15.04 | 0.63 | 0.63 |
| Estimated change from baseline on the SF-36 associated with medical cannabis treatment (THC-dominant products only) | |||||||
| General health | 5.55 (2.88 to 8.22) | 0.02 | 5.61 (2.76 to 8.46) | 0.15 | 7.06 (4.83 to 9.29) | 0.73 | 0.32 |
| Bodily pain | 11.90 (9.09 to 14.71) | 0.07 | 14.76 (12.08 to 17.43) | 0.33 | 17.17 (14.74 to 19.60) | 0.68 | 0.71 |
| Physical functioning | 6.58 (2.43 to 10.73) | 0.01 | 6.19 (2.08 to 10.29) | 0.33 | 7.18 (4.67 to 9.69) | 0.79 | 0.23 |
| Role-physical | 11.28 (7.14 to 15.42) | 0.03 | 12.72 (8.37 to 17.06) | 0.16 | 16.29 (11.98 to 20.60) | 0.49 | 0.56 |
| Mental health | 9.16 (6.65 to 11.67) | 0.04 | 10.18 (7.58 to 12.79)d | 0.18 | 11.44 (9.17 to 13.71)d | 0.69 | 0.51 |
| Role-emotional | 13.12 (8.53 to 17.70 | 0.04 | 11.00 (5.89 to 16.10) | 0.09 | 14.12 (8.69 to 19.55) | 0.41 | 0.38 |
| Social functioning | 13.58 (10.28 to 16.88) | 0.07 | 14.77 (11.30 to 18.24) | 0.18 | 17.10 (13.93 to 20.27) | 0.61 | 0.64 |
| Vitality | 9.93 (7.36 to 12.50) | 0.05 | 10.58 (7.63 to 13.52) | 0.14 | 12.52 (10.17 to 14.87) | 0.63 | 0.60 |
Abbreviations: CBD, cannabidiol; ES, effect size (Cohen d); SF-36, 36-Item Short Form Health Survey; THC, delta-9-tetrahydrocannabinol.
Includes only the binary indicator for taking medical cannabis.
Introduces control variables for the number of medications patient takes every day, binary indicators for both 8 medication categories and 4 primary diagnosis categories, the number of other comorbidities, the patient’s age, gender and employment status, the reciprocal of the number of follow-up consults since commencing treatment, and month- and year-fixed effects.
Incorporates patient-fixed effects (in addition to the before-mentioned control variables).
95% CIs clustered at the patient level are displayed in parenthesis.
Adverse Events
A total of 2919 adverse events were reported over the sampling period (eTable 10 in Supplement 1). Most were either mild (n = 1905) or moderate (n = 922); 86 were severe. Two adverse events were considered serious, including 1 incidence of hallucination. In order of frequency, adverse events included sedation and/or sleepiness (13.1% of patients), dry mouth (11.4%), lethargy and/or tiredness (7.4%), dizziness (7.1%), difficulty concentrating (6.4%), nausea (6.3%), diarrhea and/or loose stools (4.9%), feeling high (4.7%), increased appetite (3.7%), headache (3.2%), anxiety and/or panic attack (2.7%), vivid dreams (1.7%), hallucination (1.4%), and impaired coordination (1.3%). The incidence of adverse events did not differ significantly across cannabinoid composition categories.
Discussion
In this retrospective case series, patients reported improvements on all 8 health-related quality of life domains assessed by the SF-36 after commencing treatment with medical cannabis. In our most complete regression model, observed treatment effects suggest improvements relative to baseline (pretreatment) ranging from 6.60 to 18.31 points. Even though the mean daily THC/CBD dose differed considerably across the balanced (18.8 mg THC; 18.8 mg CBD), CBD-dominant (8.7 mg THC; 97.1 mg CBD) and THC-dominant (35.9 mg THC; 5.0 mg CBD) treatment categories, estimated treatment effects were very similar. The mean daily THC dose increased consistently across the sample period from 6.5 mg at follow-up 1 to 25.8 mg at follow-up 15, consistent with a standard dose titration protocol. The mean CBD dose, on the other hand, stayed relatively stable across the sample period after reaching 72.2 mg at follow-up 2.
Commensurate with the Therapeutic Goods Administration data reflecting broader prescription patterns across Australia,2 chronic noncancer pain was by far the most common primary diagnosis in this sample population (n = 2160), followed by cancer pain (n = 190), insomnia (n = 152), and anxiety (n = 132). As might be expected given the high incidence of pain conditions, almost half of all patients were using simple and/or opioid analgesics at baseline. Patient-reported bodily pain and physical functioning scores at baseline were more than 40% below the Australian mean score, while patient-reported role-physical scores (limitations in usual role activities due to physical health problems) were more than 70% below the Australian mean. Patient-reported social functioning and role-emotional (limitations in usual role activities due to emotional problems) were also more than 40% below the Australian mean. Considering this, the estimated treatment effects reported here (ranging from 6.60 to 18.31 points) suggest substantial absolute gains across all functional domains, although it is important to contextualize the magnitude of these changes within the broader literature.
In a recent systematic review and meta-analysis of randomized clinical trials of medical cannabis for chronic pain (n = 32 trials with 5174 patients), oral medical cannabis was associated with a 4% increase in the proportion of patients experiencing an improvement of more than 10 points (the minimally clinically important difference) on the physical functioning scale of the SF-36 relative to placebo.12 No evidence was found for improvements on the role-emotional, role-physical, or social functioning scales; however, the median follow-up time was only 50 days (maximum: 154 days), and there was considerable variability in active drug type and route of administration. Here, clinically important improvements (>10 points) were observed for the role-emotional, role-physical, and social functioning scales, with associated effect sizes (0.38 to 0.68), suggesting considerable clinical gains over the long term.
Pritchett et al14 reported significant improvements on 5 SF-36 domains when comparing scores prior to commencing medical cannabis with posttreatment scores. In a sample of 2183 patients in Florida, large mean differences of 43.64, 35.15 and 26.55 points were noted for the social functioning, bodily pain, and physical functioning scales. However, pretreatment scores were retrospectively reported by patients, which limits their reliability, and only a single posttreatment measure was obtained. To better determine the long-term effects of medical cannabis treatment, Safakish et al15 examined changes on the SF-12 (a short-form version of the SF-36) over 12 months in 751 patients with chronic pain commencing medical cannabis treatment. While statistically significant improvements were seen on both the physical and mental health domains, these changes were notably smaller than those seen here. Nevertheless, patients did experience a clinically important reduction in pain severity of 2.09 points on the brief pain inventory.
Pain severity was also significantly reduced in 274 patients with chronic pain when assessed 6 months after treatment, as was pain interference and most social and emotional disability scores on the S-TOPS.16 An analysis of 190 patients with chronic pain in the UK Medical Cannabis Registry likewise revealed improvements on a range of scales (including the EQ-5D, Sleep Quality Scale, General Anxiety Disorder-7) at 1, 3, and 6 months relative to baseline.17 Changes in EQ-5D scores after 6 weeks of treatment were less consistent in a study involving 214 Canadian patients commencing medical cannabis treatment; improvements were seen for patients with anxiety and PTSD, but not for patients with arthritis and other rheumatic disorders or sleep disorders.18 Despite an improvement in quality of life among patients with anxiety, there were no significant changes in the anxiety subscale of the Depression, Anxiety and Stress Scale. These data suggest that treatment with medical cannabis may, in some circumstances, improve quality of life without reducing the severity of the underlying condition.
A recent study by Aviram et al19 provides some evidence to support this notion. In a sample of 429 patients who consumed medical cannabis via inflorescence inhalation and were followed up monthly over 6 months, there was no change over time in the least, average, and worst weekly pain intensities, or in pain frequency. There was, however, an increase in the proportion of patients reporting better quality of life on the EQ-5D and a decrease in the proportion reporting consumption of analgesic medications at subsequent time points. There was also a reduction in the mean (SD) morphine equivalent dose of opioid analgesics from 21 (91) mg at baseline to 5.2 (27) mg at 6 months, suggesting a possible opioid-sparing association with medical cannabis, consistent with several other recent studies.(20,21,22) These data are also supported by epidemiological evidence for reduced state-level opioid overdose mortality rates in US states with medical cannabis laws,23 although as Noori et al24 caution in a recent review,24 extant evidence from randomized and observational studies is of very low certainty.
Limitations
This study is limited by the use of a retrospective case series design without a control, which restricts what conclusions can be drawn around treatment efficacy, and limits generalizability to other clinical populations. Given the ongoing increase in medical cannabis prescribing, other clinics should strongly consider implementing a similarly rigorous clinical data collection protocol in order to monitor clinical safety and patient-reported outcomes associated with medical cannabis use. As most patients began treatment at some point during the sampling period, patient numbers at later consults (ie, reflecting longer treatment periods) are lower than patient numbers at earlier consults. As a result, mean SF-36 domain scores show considerably greater variability at later consults and should be interpreted with caution. We intend to conduct a follow-up study in the future with larger patient numbers and a longer follow-up period. Furthermore, patients were not required to complete the questionnaires described here, and so these data may be biased upwards if patients experiencing a positive effect of medical cannabis were more likely to respond. Finally, the clinical care model used by Emerald Clinics may have also contributed to perceived improvements in quality of life.
Conclusions
This study suggests a favorable association between medical cannabis treatment and quality of life among patients with a diverse range of conditions. However, clinical evidence for cannabinoid efficacy remains limited, and further high-quality trials are required. While we cannot exclude the possibility that adverse events may have been caused in whole or part by the disease state and concomitant medications, the relatively high incidence of adverse events still affirms the need for caution with THC prescribing and careful identification of patients with contraindications.
eTable 1. Data Availability on Quality of Life (SF-36) Measures by Follow-up
eTable 2. OLS Regression Results, Estimating General Health (Increasing From 0 to 100)
eTable 3. OLS Regression Results, Estimating Bodily Pain (Decreasing From 0 to 100)
eTable 4. OLS Regression Results, Estimating Physical Functioning (Increasing From 0 to 100)
eTable 5. OLS Regression Results, Estimating Role-Physical (Decreasing From 0 to 100)
eTable 6. OLS Regression Results, Estimating Mental Health (Increasing From 0 to 100)
eTable 7. OLS Regression Results, Estimating Role-Emotional (Decreasing From 0 to 100)
eTable 8. OLS Regression Results, Estimating Social Functioning (Increasing From 0 to 100)
eTable 9. OLS Regression Results, Estimating Vitality (Increasing From 0 to 100)
eTable 10. Reported Adverse Events Across Different Severity Levels
eFigure. Flow of Patients Through the Study of the Association of Medicinal Cannabis With Health-Related Quality of Life
Data Sharing Statement
References
- 1.MacPhail SL, Bedoya-Perez MA, Cohen R, Kotsirilos V, McGregor IS, Cairns EA. Medicinal cannabis prescribing in australia: an analysis of trends over the first five years. Front Pharmacol. 2022;13:885655. doi: 10.3389/fphar.2022.885655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Australian Government. Therapeutic Goods Administration . Medicinal cannabis Special Access Scheme Category B data. Accessed September 28, 2022. https://www.tga.gov.au/medicinal-cannabis-special-access-scheme-category-b-data
- 3.Bilbao A, Spanagel R. Medical cannabinoids: a pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 2022;20(1):259. doi: 10.1186/s12916-022-02459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456-2473. doi: 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 5.Abrams DI. The therapeutic effects of cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7-11. doi: 10.1016/j.ejim.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Boehnke KF, Dean O, Haffajee RL, Hosanagar A. U.S. trends in registration for medical cannabis and reasons for use from 2016 to 2020: an observational study. Ann Intern Med. 2022;175(7):945-951. doi: 10.7326/M22-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung J, Chan G, Stjepanović D, Chung JYC, Hall W, Hammond D. Prevalence and self-reported reasons of cannabis use for medical purposes in USA and Canada. Psychopharmacology (Berl). 2022;239(5):1509-1519. doi: 10.1007/s00213-021-06047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee R, Erridge S, Salazar O, et al. Real world evidence in medical cannabis research. Ther Innov Regul Sci. 2022;56(1):8-14. doi: 10.1007/s43441-021-00346-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlag AK, Zafar RR, Lynskey MT, Athanasiou-Fragkouli A, Phillips LD, Nutt DJ. The value of real world evidence: The case of medical cannabis. Front Psychiatry. 2022;13:1027159. doi: 10.3389/fpsyt.2022.1027159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 11.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350-357. doi: 10.3109/07853890109002089 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Hong PJ, May C, et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: a systematic review and meta-analysis of randomised clinical trials. BMJ. 2021;374(1034):n1034. doi: 10.1136/bmj.n1034 [DOI] [PubMed] [Google Scholar]
- 13.Watson N, Wooden M. The Household, Income and Labour Dynamics in Australia (HILDA) Survey. Jahrb Natl Okon Stat. 2021;241(1):131-141. doi: 10.1515/jbnst-2020-0029 [DOI] [Google Scholar]
- 14.Pritchett CE, Flynn H, Wang Y, Polston JE. Medical cannabis patients report improvements in health functioning and reductions in opiate use. Subst Use Misuse. 2022;57(13):1883-1892. doi: 10.1080/10826084.2022.2107673 [DOI] [PubMed] [Google Scholar]
- 15.Safakish R, Ko G, Salimpour V, et al. Medical Cannabis for the Management of Pain and Quality of Life in Chronic Pain Patients: A Prospective Observational Study. Pain Med. 2020;21(11):3073-3086. doi: 10.1093/pm/pnaa163 [DOI] [PubMed] [Google Scholar]
- 16.Haroutounian S, Ratz Y, Ginosar Y, et al. The Effect of Medicinal Cannabis on Pain and Quality-of-Life Outcomes in Chronic Pain: A Prospective Open-label Study. Clin J Pain. 2016;32(12):1036-1043. doi: 10.1097/AJP.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 17.Harris M, Erridge S, Ergisi M, et al. UK Medical Cannabis registry: an analysis of clinical outcomes of medicinal cannabis therapy for chronic pain conditions. Expert Rev Clin Pharmacol. 2022;15(4):473-485. doi: 10.1080/17512433.2022.2017771 [DOI] [PubMed] [Google Scholar]
- 18.Cahill SP, Lunn SE, Diaz P, Page JE. Evaluation of Patient Reported Safety and Efficacy of Cannabis From a Survey of Medical Cannabis Patients in Canada. Front Public Health. 2021;9:626853. doi: 10.3389/fpubh.2021.626853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviram J, Lewitus GM, Vysotski Y, et al. Prolonged medical cannabis treatment is associated with quality of life improvement and reduction of analgesic medication consumption in chronic pain patients. Front Pharmacol. 2021;12:613805. doi: 10.3389/fphar.2021.613805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capano A, Weaver R, Burkman E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med. 2020;132(1):56-61. doi: 10.1080/00325481.2019.1685298 [DOI] [PubMed] [Google Scholar]
- 21.Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: a preliminary cohort study. PLoS One. 2017;12(11):e0187795. doi: 10.1371/journal.pone.0187795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takakuwa KM, Hergenrather JY, Shofer FS, Schears RM. The impact of medical cannabis on intermittent and chronic opioid users with back pain: how cannabis diminished prescription opioid usage. Cannabis Cannabinoid Res. 2020;5(3):263-270. doi: 10.1089/can.2019.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014;174(10):1668-1673. doi: 10.1001/jamainternmed.2014.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noori A, Miroshnychenko A, Shergill Y, et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta-analysis of randomised and observational studies. BMJ Open. 2021;11(7):e047717. doi: 10.1136/bmjopen-2020-047717 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Data Availability on Quality of Life (SF-36) Measures by Follow-up
eTable 2. OLS Regression Results, Estimating General Health (Increasing From 0 to 100)
eTable 3. OLS Regression Results, Estimating Bodily Pain (Decreasing From 0 to 100)
eTable 4. OLS Regression Results, Estimating Physical Functioning (Increasing From 0 to 100)
eTable 5. OLS Regression Results, Estimating Role-Physical (Decreasing From 0 to 100)
eTable 6. OLS Regression Results, Estimating Mental Health (Increasing From 0 to 100)
eTable 7. OLS Regression Results, Estimating Role-Emotional (Decreasing From 0 to 100)
eTable 8. OLS Regression Results, Estimating Social Functioning (Increasing From 0 to 100)
eTable 9. OLS Regression Results, Estimating Vitality (Increasing From 0 to 100)
eTable 10. Reported Adverse Events Across Different Severity Levels
eFigure. Flow of Patients Through the Study of the Association of Medicinal Cannabis With Health-Related Quality of Life
Data Sharing Statement