Abstract
OBJECTIVE
Some anti-cytokine treatments are being used to control the hyperinflammatory condition defined as cytokine storm that develops during COVID-19 infection. In this study, we aim to investigate the effects of anakinra, an IL-1 antagonist, on the clinical status and laboratory values of hospitalized patients with the COVID-19 infection. The aim of the study was to investigate the effects of anakinra, an IL-1 antagonist, on the clinical and laboratory results of hospitalized patients with COVID-19 infection.
METHODS
This study was planned as a retrospective study. The age, gender, and current comorbidities of a total of 66 patients who were treated with anakinra for COVID-19 infection from November 2020 to January 2021 were analyzed. The amount of oxygen demand (L/s), the type of oxygen supplementation, oxygen saturation, radiological findings, WBC count, lymphocyte count, neutrophil count, C-reactive protein, LDH, ferritin, fibrinogen, D-dimer levels were monitored before the treatment, and after the anakinra treatment, newly gathered results were compared. Patients’ hospitalization period, oxygen need, and their clinical status at discharge were evaluated. The effects of early anakinra treatment (9 days before and after the onset of symptoms) on the prognosis were evaluated. SPSS version 21.0 provided by IBM Company in the USA, Chicago, IL was used for statistical analysis and p<0.05 was considered significant.
RESULTS
Sixty-six patients were included in the study. There was no significant gender difference in the prognosis of the patients. There was a significant difference in the statistical deterioration in patients with comorbidities (p=0.004). Patients who started the anakinra treatment at an early stage developed less need for intensive care and low mortality ratios (p=0.019). There were significant improvements on the levels of WBC (p=0.045), neutrophils (p=0.016), lymphocyte (p=0.001), LDH (p=0.005), ferritin (p=0.02), and fibrinogen (p=0.01) after the administration of anakinra therapy.
CONCLUSION
We found that earlier and appropriate use of anakinra therapy in COVID-19 patients with the signs of macrophage activation syndrome reduces the need for oxygen support in patients and contributes to improvement in laboratory results and radiological findings, and most importantly reduces the need for intensive care.
Keywords: Anakinra, COVID-19, macrophage activation syndrome
Highlight key points
Early and appropriate use of anakinra treatment reduces the need for intensive care.
Cytokine storm that develops during COVID-19 infection can be controlled.
Anakinra treatment showed improvement in WBC, neutrophil, lymphocyte count, CRP, LDH, ferritin, and fibrinogen levels, which are considered prognostic markers.
Despite numerous interventions, there is no proven cure for COVID-19 yet.
Chloroquine, a widely used anti-malarial drug, has been reported as a potential broad-spectrum anti-viral drug. This effect was thought to be an immune modulation provided by the inhibition of membrane fusion and the binding receptor of the virus. Consequently, chloroquine is added to the guidelines by a group of experts [1].
Lopinavirin-Ritonavir is an antiviral agent that has been determined to have inhibitory activity in vitro against Sars-CoV. However, in studies conducted on COVID-19 patients, it has been shown that lopinavir-ritonavir treatment does not reduce mortality and does not accelerate clinical recovery [2].
Studies of Remdesivir in Sars-CoV-2 infection have shown that patients in the Remdesivir group have shorter recovery and hospital stay compared to the placebo group [3]. However, studies are continuing to reveal its effectiveness.
Favipiravir is an antiviral agent that is an RNA-dependent RNA polymerase inhibitor. In a study on 80 patients diagnosed with COVID-19 in China, a significant reduction in Sars-CoV-2 disease duration was found in patients treated with favipiravir [4].
Cytokines are at the heart of the pathophysiology of COVID-19. While some of these cytokine responses, such as IL-7, are beneficial for the healing process, especially cytokines such as IL-1, IL-6, and TNF-alpha are particularly damaging to the organism. Considering this information, several anti-cytokine therapies have been introduced in treating patients diagnosed with COVID-19. Benefits of anti-cytokine therapies such as IL-6 receptor inhibitors (tocilizumab and sarilumab) and IL-6 inhibitors (siltuximab, clazakizumab, and sirukumab), IL-1 receptor inhibitors (anakinra), and a monoclonal antibody targeting IL-1 (canakinumab) have been demonstrated [5].
Cytokine storm syndrome that develops in the course of COVID-19 infection is reviving research on this. As a result of these studies, it was determined that the rising interleukin level in patients with severe COVID-19 infection was associated with a poor prognosis, and then the research on anti-inflammatory and anti-cytokine therapies accelerated [6]. In light of the increasing evidences, anti-cytokine therapies are considered to be promising options. Numerous studies have been conducted specifically for anti-cytokine therapies’ inhibition of IL-6 and IL-1, and promising results have emerged [5]. In many clinics, more tocilizumab and less anakinra are used to treat COVID-19 patients, but insufficient information is available to guide patient selection, dosing, and monitoring of treatment response.
In this study, we aimed to evaluate the effects of anakinra treatment on the prognosis of patients hospitalized with COVID-19.
MATERIALS AND METHODS
This study was carried out retrospectively between November 2020 and January 2021 in patients.
Study Design and Population
Our study was designed as a retrospective study. Our study included 66 patients who were diagnosed with COVID-19 and admitted to pandemic services. Criteria for inclusion of patients in the study were: (1) to have been hospitalized in the pandemic service of our hospital (2) to have received anakinra treatment. Detailed history and physical examination findings of all patients were retrospectively obtained from the system. The results of biochemical blood tests (WBC, lymphocyte count, neutrophil count, CRP, LDH, D-dimer, ferritin, and fibrinogen) were examined. Pre-admission thoracic tomography involvements of the patients were categorized as mild, moderate, and severe, as well as bilateral and unilateral. The dose of corticosteroids received by the patients during their hospitalization and the total duration of treatment was determined as days. The duration of onset of the patient’s symptoms, the number of days after admission to take anakinra, oxygen saturations before and after anakinra treatment, and the time elapsed in between (days) were recorded. In this study, we recorded the duration of onset of the patient’s symptoms, the number of days between the admission and the start of anakinra treatment, and oxygen saturations before and after anakinra treatment. Patients were divided into three groups based on how they exited the clinic: Discharged to home, intensive care unit (ICU) referral, and exitus. In our study, the way the patients were discharged from the hospital as a result of anakinra treatment, L/min we aimed to investigate how the prognostic markers change in the need for oxygen support and laboratory values. At the same time, the prognosis of patients with early anti-cytokine therapy, we aimed to evaluate the effect on.
Statistical Method
In the statistical analysis, the data were expressed as mean±SD, the normal distribution was confirmed by the Kolmogorov–Smirnov test, one-way ANOVA (Tukey), Kruskal–Wallis test was used for differences between groups, and Pearson r coefficient was used for univariate correlations. SPSS 21.0 was used and the statistical significance was accepted as p<0.05. Power analysis was performed using G*Power (v3.1.9) program to determine the sample size. The power of the study is expressed as 1-β (β=II type error probability) and has 80% power. According to the effect size coefficients determined by Cohen (d=0.311); it was determined that at least 66 patients were required.
RESULTS
Sixty-six patients were included in the study. Twenty-one (31.8%) were female and 45 (68.2%) were male. There were 51 patients (77.3%) with comorbidity. The number of those who received treatment at home was 35 (53%), and the number of those who started treatment at the hospital for the 1st time was 31 (22.7%). The number of patients who died or had been referred to the was 21 (31.8%) and those who were discharged home were 45 (68.1%). 26 (39.3%) patients received high flow, while 28 (42.4%) patients received oxygen by mask, and 12 (18.1%) patients received nasal oxygen. The number of patients with bilateral severe lung involvement was 46 (69.7%), and the number of patients with moderate bilateral involvement was 20 (30.3%). The distribution of parameters according to gender, comorbidity, whether to receive home treatment, mode of exit, the form of oxygen support, and severity of lung involvement are given in Table 1.
Table 1.
Distribution and percentage of parameters
| n | % | |
|---|---|---|
| Gender | ||
| Female | 21 | 31.8 |
| Male | 45 | 68.2 |
| Comorbidity | ||
| Yes | 51 | 77.3 |
| No | 15 | 22.7 |
| Home treatment | ||
| Yes | 35 | 53 |
| No | 31 | 47 |
| Form of discharge | ||
| Exitus-ICU | 21 | 31.8 |
| Discharged | 45 | 68.1 |
| Oxygen support | ||
| High flow | 26 | 39.3 |
| Mask | 28 | 42.4 |
| Nasal | 12 | 18.1 |
| Lung involvement | ||
| Mid-dual | 20 | 30.3 |
| Heavy-dual | 46 | 69.7 |
ICU: Intensive care unit.
There were comorbidities in 20 (100%) patients with poor prognosis and p=0.004 was statistically significant. There was no significant difference between both sexes in the course of the disease (Table 2).
Table 2.
Comparison of parameters with the bad course (exitus-ICU) and good course (discharge)
| Bad situation | Good situation | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Gender | 0.714 | ||||
| Female | 7 | 35 | 14 | 30.4 | |
| Male | 13 | 65 | 32 | 69.6 | |
| Comorbidity | 0.004 | ||||
| Yes | 20 | 100 | 31 | 67.4 | |
| No | 0 | 0 | 15 | 32.6 | |
| Home treatment | 0.199 | ||||
| No | 7 | 35 | 24 | 52.2 | |
| Yes | 13 | 65 | 22 | 47.8 | |
| Oxygen support | 0.064 | ||||
| High flow | 9 | 50 | 15 | 33.3 | |
| Mask | 9 | 50 | 19 | 42.2 | |
| Nasal | 0 | 0 | 11 | 24.4 | |
| Application method | 0.727 | ||||
| Iv | 16 | 80 | 35 | 76.1 | |
| Sc | 4 | 20 | 11 | 23.9 | |
| Form of involvement | 0.074 | ||||
| Heavy | 17 | 85 | 29 | 63 | |
| Mid | 3 | 15 | 17 | 37 | |
ICU: Intensive care unit.
The mean age of the patients in the poor prognosis group was found to be significantly higher (p=0.045) than in the good prognosis group. It was observed that a statistically significant higher dose of anakinra treatment was needed in patients with a poor prognosis. Nonetheless, according to this analysis, the more days between the date of the first symptom and the date of the first anakinra administration, the worse the prognosis (Table 3).
Table 3.
Mean – standard deviation data and p values of the parameters
| Course condition | Mean±SD | p | |
|---|---|---|---|
| Age (years) | 0.045 | ||
| General | 62±16 | ||
| Bad | 67.9±10.8 | ||
| Good | 59.5±16.8 | ||
| Hospital stay (days) | 0.208 | ||
| General | 17±7 | ||
| Bad | 15±7.5 | ||
| Good | 17±6.3 | ||
| Administered dose (mg) | 0.000 | ||
| General | 332±134 | ||
| Bad | 425±125 | ||
| Good | 291±117 | ||
| Number of days implemented | 0.110 | ||
| General | 5±2 | ||
| Bad | 4.6±2.5 | ||
| Good | 5.6±2.2 | ||
| Temperature (°C) | 0.578 | ||
| General | 36.6±0.4 | ||
| Bad | 36.6±0.25 | ||
| Good | 36.6±0.43 | ||
| Steroid dose (mg) | 0.331 | ||
| General | 301±200 | ||
| Bad | 264±140 | ||
| Good | 316±220 | ||
| Number of days between anakinra treatment and first symptom date | 0.019 | ||
| General | 10.2±5.0 | ||
| Bad | 12.4±6.4 | ||
| Good | 9.3±3.9 |
SD: Standard deviation.
According to the correlation analysis, WBC and neutrophil (r=0.976, p=0.000), lymphocyte and WBC (r=0.439, p=0.000), neutrophil and lymphocyte (r=0.295, p=0.016), CRP and fibrinogen (r=0.455, p=0.000), a positive correlation was found between LDH and ferritin (r=0.480, p=0.000), and ferritin and D-dimer (r=0.497, p=0.000) (Table 4).
Table 4.
Correlation analysis of parameters before treatment
| WBC | Neutrophil | Lymphocyte | CRP | LDH | D-Dimer | Ferritin | Fibrinogen | |
|---|---|---|---|---|---|---|---|---|
| WBC | ||||||||
| r | 1.000 | 0.976** | 0.439** | 0.047 | 0.123 | 0.095 | -0.066 | 0.145 |
| p | 0.045 | 0.000 | 0.000 | 0.705 | 0.495 | 0.461 | 0.623 | 0.256 |
| Neutrophil | ||||||||
| r | 0.976** | 1.000 | 0.295* | 0.114 | 0.132 | 0.132 | -0.069 | 0.150 |
| p | 0.000 | – | 0.016 | 0.362 | 0.463 | 0.304 | 0.606 | 0.241 |
| Lymphocyte | ||||||||
| r | 0.439** | 0.295* | 1.000 | -0.086 | -0.149 | -0.065 | 0.103 | 0.093 |
| p | 0.000 | 0.016 | – | 0.494 | 0.409 | 0.615 | 0.443 | 0.470 |
| CRP | ||||||||
| r | 0.047 | 0.114 | -0.086 | 1.000 | 0.286 | 0.211 | 0.060 | 0.455** |
| p | 0.705 | 0.362 | 0.494 | – | 0.106 | 0.097 | 0.653 | 0.000 |
| LDH | ||||||||
| r | 0.095 | 0.132 | -0.065 | 0.211 | 0.497** | 1.000 | 0.480** | 0.045 |
| p | 0.461 | 0.304 | 0.615 | 0.097 | 0.003 | – | 0.000 | 0.733 |
| D-dimer | ||||||||
| r | 0.123 | 0.132 | -0.149 | 0.286 | 1.000 | 0.497** | 0.211 | 0.009 |
| p | 0.495 | 0.463 | 0.409 | 0.106 | – | 0.003 | 0.280 | 0.959 |
| Ferritin | ||||||||
| r | -0.066 | -0.069 | 0.103 | 0.060 | 0.211 | 0.480** | 1.000 | -0.007 |
| p | 0.623 | 0.606 | 0.443 | 0.653 | 0.280 | 0.000 | – | 0.956 |
| Fibrinogen | ||||||||
| r | 0.145 | 0.150 | 0.093 | 0.455** | 0.009 | 0.045 | -0.007 | 1.000 |
| p | 0.256 | 0.241 | 0.470 | 0.000 | 0.959 | 0.733 | 0.956 | – |
: p=0.016; **: p=0.000; WBC: White blood cell; CRP: C-reactive protein; LDH: Lactate dehydrogenase.
The graphic and numerical progress of the parameters in different groups after the treatment is given in Figures 1–6.
Figure 1.
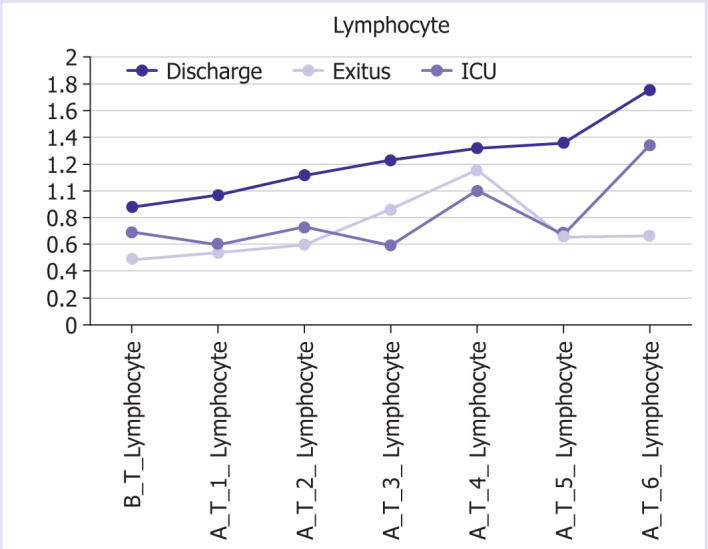
Graphical and numerical course of lymphocyte values in different groups after treatment.
Figure 6.
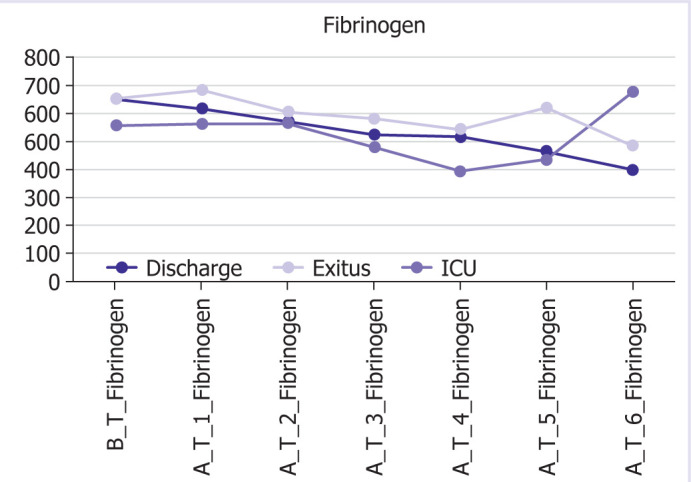
Graphical and numerical course of fibrinogen values in different groups after treatment.
Figure 2.
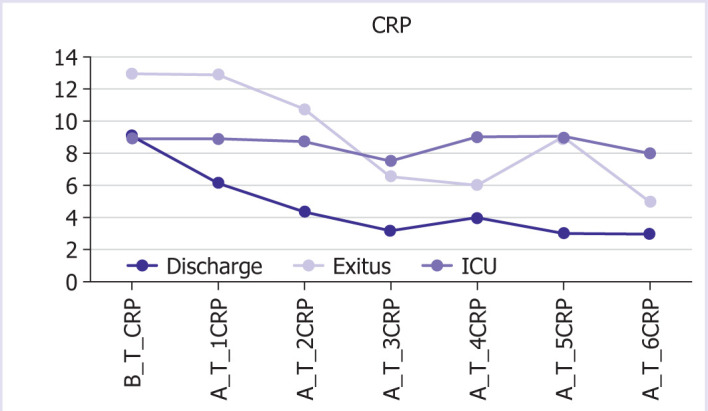
Graphical and numerical progression of CRP values in different groups after treatment.
Figure 3.
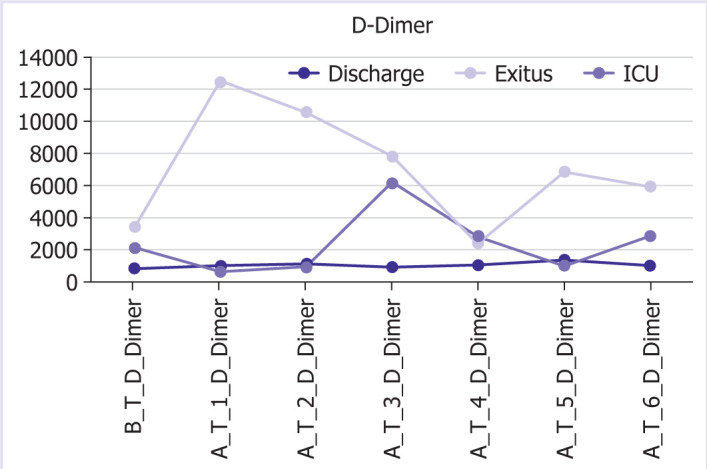
Graphical and numerical course of D-dimer values in different groups after treatment.
Figure 4.
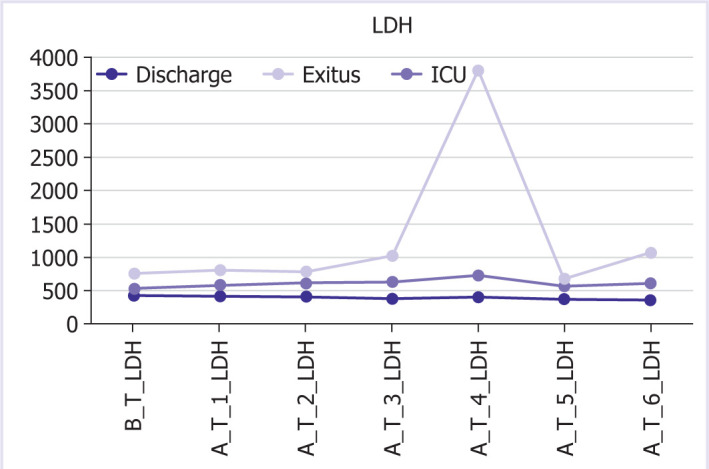
Graphical and numerical course of LDH values in different groups after treatment.
Figure 5.
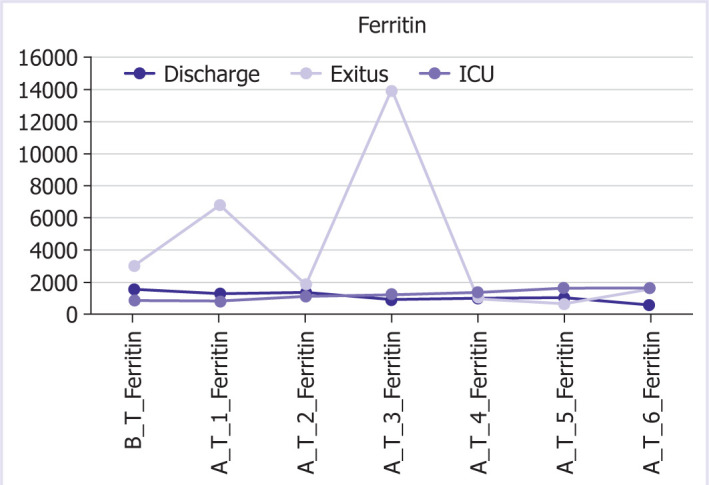
Graphical and numerical course of ferritin values in different groups after treatment.
DISCUSSION
In this study, we aimed to evaluate the results of anakinra treatment in COVID-19 patients with macrophage activation syndrome symptoms. We have determined that the use of anakinra treatment at an early and accurate stage reduces the need for oxygen, and it contributes to laboratory and radiological recovery, and most importantly, reduces the need for intensive care.
In our study, 65% of the patients with poor prognosis were men and 35% were women. In a study of 8916 people in China, it was seen that men have a high rate of disease severity. In the same study, comorbidities such as hypertension, diabetes, malignancy, cardiovascular disease, coronary artery disease, cerebrovascular disease, chronic kidney disease, and especially respiratory system diseases, were found to be significantly associated with the severity of the disease [7]. In our study, comorbidity was found in 100% of the patients with a poor prognosis, and comorbidity was found in 67.4% of the group with a good prognosis.
In the analysis of our study, the time between the first symptom date and the date of receiving anakinra treatment was 12.4 days in patients with a bad prognosis and it was 9.3 days in patients with a good prognosis. These data indicate that initiation of the treatment at the early stage in the course of the disease reduces mortality and morbidity. In a study conducted in France that compares with 22 patients with acute respiratory failure and systemic inflammation, 10 of them received standard care and 12 of them started early anakinra treatment. A comparison of these two groups revealed that early use of anakinra prevented the poor outcome [8]. Therefore, early prediction of the severity of the disease and the timely initiation of anti-cytokine treatments such as IL-1 antagonists, without delay, directly affect the prognosis of the disease. The data in our study also support this.
In the study of 5700 people conducted in New York in March–April 2020, one of the largest case series consisting of hospitalized COVID-19 patients; 14.02% of the patients were treated in the ICU, 12.2% underwent mechanical invasive ventilation, and 9.7% died [9]. In our study, 30.3% of the patients included in the study were either deceased or transferred to ICU, and 69.7% of them were discharged.
In a study examining the underlying diseases in hospitalized COVID-19 patients, it was determined that the most common comorbidity was hypertension [10].
In light of the current data regarding the COVID-19 pandemic, experts believe that patients with any comorbidities may be severely affected. Many studies have been published concerning this subject. In a review including 27 studies, one of them revealed, 57.7% of patients diagnosed with COVID-19 had at least one of the comorbidities classified as cardiovascular diseases, hypertension, diabetes, chronic kidney failure, chronic obstructive pulmonary disease, malignancy, and others. It was observed that 42.3 of them did not have any comorbidity. Comorbidity distribution cardiovascular diseases (10.7%), hypertension (33.1%), diabetes (21%), chronic obstructive pulmonary disease (9.1%), malignancy (3%), chronic kidney disease (4.3%), and other (18.8%). One or more comorbidities were associated with a 2.57-fold increased risk of death. As a result of this review, the relationship between comorbidity and COVID-19 death was evaluated and it was shown that there is a strong relationship between having one or more comorbidities and death [11]. In our study, it was observed that 100% of the patients with a poor prognosis had at least one comorbidity, on the other hand patients with a good prognosis and discharged from the clinic, 64.4% of them have comorbidities.
Severe hematological, biochemical, and inflammatory markers have been identified in COVID-19 patients. High WBC neutrophil count, low lymphocyte and platelet count, T, B, and NK cell count as hematological markers in COVID-19 patients, high ALT, AST, bilirubin, blood urea nitrogen, creatinine, creatine kinase (CK), LDH, myoglobin as biochemical markers, low albumin level, high prothrombin time as a clotting marker, and high D-dimer levels as an inflammatory marker, high ESR, CRP, ferritin, IL-1, IL-2, IL-6, IL-8, and IL-10 levels were detected [12].
In a single-center study in Wuhan, it was reported that severe cases had lower lymphocyte, monocyte, eosinophil and basophil counts, higher leukocyte counts, and neutrophil/lymphocyte ratio than mild cases [13]. In another study, lymphocyte count <500/mL was associated with death [14].
In our patient group, the mean leukocyte count before the treatment was 11100/mm3, the neutrophil count was 9800/mm3, and the lymphocyte count was 800/mm3 before the treatment in patients who were treated with anakinra. Total WBC and neutrophil counts of the patients were high throughout the treatment course, and lymphocyte counts improved with anakinra treatment. This shows similarity with other studies based on the severity of the disease.
When biochemical markers were examined, ALT, AST, creatinine, CK, LDH, and D-dimer levels were found to be significantly higher in patients with a poor prognosis than in those with a good prognosis in the study of Chen et al. [15] with 799 patients. In our study, D-dimer levels were higher in deceased patients compared to discharged patients from the 1st day.
RESULTS
In our study, we found that the application of anakinra treatment in patients with a diagnosis of COVID-19 who showed cytokine storm symptoms had a positive effect on both laboratory results and clinical recovery and reduced the need for intensive care.
We found improvement in WBC, neutrophil, lymphocyte count, CRP, LDH, ferritin, and fibrinogen levels, which are considered prognostic markers, after anakinra treatment.
As a result of our research, we found that gender did not play a role in the good or bad prognosis of the patients, but the presence of one or more comorbidities was directly related to the poor prognosis.
In the patient group included in the study, the mean age of the patients with a poor course (exitus-ICU) was higher than the mean age of the patients with a good course.
Another crucial point is that the time elapsed from the first symptom date to the date of receiving anakinra treatment was longer in patients with a poor prognosis than in patients with a good prognosis. We found that those who received early anakinra treatment had a better prognosis.
Anakinra is used to treat patients with COVID-19, but insufficient information is available to guide patient selection, dosing, and monitoring of treatment response. Our study is retrospective; supportive, controlled, prospective, and clinical studies are needed for these treatments.
Conclusion
We found that earlier and appropriate use of anakinra therapy in COVID-19 patients with the signs of macrophage activation syndrome reduces the need for oxygen support in patients and contributes to improvement in laboratory results and radiological findings, and most importantly reduces the need for intensive care.
Footnotes
Cite this article as: Siyer O, Aksakal B, Basat S. Evaluation of the effects of anakinra treatment on clinic and laboratory results in patients with COVID-19. North Clin Istanb 2023;10(2):189–196.
Ethics Committee Approval
The Umraniye Training and Research Hospital Clinical Research Ethics Committee granted approval for this study (date: 14.08.2020, number: 27042).
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
The authors declared that this study has received no financial support.
Authorship Contributions
Concept – SB; Design – OS; Supervision – BA; Fundings – OS; Materials – OS; Data collection and/or processing – OS; Analysis and/or interpretation – SB; Literature review – OS; Writing – OS; Critical review – BA.
References
- 1.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4539–47. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19-preliminary report. Reply. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 4.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–4. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, et al. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021;20:102763. doi: 10.1016/j.autrev.2021.102763. [DOI] [PubMed] [Google Scholar]
- 7.Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, et al. Emory COVID-19 Quality and Clinical Research Collaborative ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799–804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A. 2020;117:18951–3. doi: 10.1073/pnas.2009017117. Erratum Proc Natl Acad Sci U S A 2020, 117, 22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher CJ, Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, et al. IL-1RA Sepsis Syndrome Study Group Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 11.Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: a rapid review of current literature. Am J Infect Control. 2021;49:238–46. doi: 10.1016/j.ajic.2020.06.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–99. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and igg level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51–60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019 retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. Erratum BMJ 2020, 368:m1295. [DOI] [PMC free article] [PubMed] [Google Scholar]


