Abstract
Purposes
Despite reports of a declining incidence over the last decade, Clostridioides difficile infection (CDI) is still considered the most important healthcare-associated causes of diarrhea worldwide. In Germany, several measures have been taken to observe, report, and influence this development. This report aims to analyze the development of hospital coding for CDI in Germany over the last decade and to use it to estimate the public health burden caused by CDI.
Methods
Reports from the Institute for Hospital Remuneration Systems, German Federal Statistical Office (DESTATIS), the Robert-Koch-Institute (RKI), Saxonian authorities and hospital quality reports during 2010–2021 were examined for CDI coding and assessed in a structured expert consultation. Analysis was performed using 2019 versions of Microsoft Excel® and Microsoft Access®.
Results
Peaks of 32,203 cases with a primary diagnosis (PD) of CDI and 78,648 cases with a secondary diagnosis (SD) of CDI were observed in 2015. The number of cases had decreased to 15,412 PD cases (− 52.1%) and 40,188 SD cases (− 48.9%) by 2021. These results were paralleled by a similar decline in notifiable severe cases. However, average duration of hospitalization of the cases remained constant during this period.
Conclusions
Hospital coding of CDI and notification to authorities has approximately halved from 2015 to 2021. Potential influential factors include hospital hygiene campaigns, implementation of antibiotic stewardship programs, social distancing due to the COVID-19 pandemic, and a decrease in more pathogenic subtypes of bacteria. Further research is necessary to validate the multiple possible drivers for this development.
Keywords: Epidemiology, Clostridioides difficile, Infection, Incidence, Germany
Introduction
Clostridioides difficile (C. diff.) is a gram-positive spore-forming anaerobic bacterium that can be isolated from environmental sources and human feces [1, 2]. Colonization with C. diff. is not typically harmful, as other bacteria in the digestive system regulate the growth and toxin production of C. diff. through regulation of intraluminal intestinal bile acids [3, 4]. When commensal microbiota are disturbed through antibiotic exposure or other factors, C. diff. can grow in its vegetative state and produce toxins that damage the intestinal epithelium [5–7]. Risk factors for C. diff. infection (CDI) include hospitalization, advanced age, impaired immunocompetence, and any event that disturbs the balance of the intestinal microbiota [8–10]. The nature and severity of CDI vary considerably, ranging from self-limiting diarrhea to complications like an ileus, toxic megacolon, perforation, septic shock, and death [2, 11].
Until recent years, many reports about the growing incidence of CDI and its impact on the healthcare system were published [12–17]. CDI was repeatedly found to be the leading cause of healthcare-associated infections, accounting for most cases of hospital-acquired diarrhea in the United States [18–21]. CDI was also recognized as an emerging issue in German hospitals [22]. It is associated with a significant burden of morbidity and mortality; for instance, it has been reported to cause up to 10% of all recorded nosocomial infections [23, 24]. CDI treatment causes prolonged hospital stays, increases the need for antibiotics, transfusions, and transfer to intensive care units, also recurrence is a large problem and episodes of recurrence are associated with significant mortality in the frail and eldely population [25–27]. The Robert-Koch-Institute (RKI), the German government’s central scientific institution responsible for the surveillance of infectious diseases, included severe CDI in the chapter “Diseases with current importance” in its Epidemiological Yearbook 2014 [28]. A German National Reference Center (NRC) for C. diff. was established in 2017 in Homburg/Saar. The emergence of highly virulent strains, such as ribotype (RT)-027, was suspected to exacerbate the clinical problem, worsen the outcome, and increase the burden of illness in general [29–32].
This report aims to examine the development of hospital coding for CDI in Germany over the last decade and to estimate the development of the associated public health burden. Furthermore, patient demographics and clinical outcomes were analyzed as far as the data were available. In addition, the incidence of severe CDI in Germany as reported to the Robert-Koch Institute, which most likely reflects clinical reality, were related to the data obtained from hospital coding.
Methods
Collection of hospital data
Publicly available data from the Institute for the Hospital Remuneration System (www.g-drg.de), the Federal Statistical Office (DESTATIS; www.destatis.de), and hospital quality reports [33] covering the period 2010–2021 were examined. We searched for coding of primary diagnosis (PD) and secondary diagnosis (SD) of CDI, as well as coding for recurrent CDI. In addition, the chronological course of reports of serious illnesses related to CDI at the RKI during 2010–2020 and the Annual Report 2010–2020 of the National Institute for Health and Veterinary Investigation in Saxony were analyzed. Analysis was performed using Microsoft Excel® and Microsoft Access® (both version 2019).
In the hospital sector, the diagnosis of CDI can be coded as PD or SD. According to the German coding system, a PD is coded, if CDI is considered to be the main reason for the hospitalization. A SD is coded for patients hospitalized for reasons other than CDI, but if CDI occurred during the hospital stay or as a preexisting condition. In cases in which patients were referred from one hospital to another, they are usually registered as new cases. Thus, the sum of hospital cases with PD and SD will overestimate the total number of affected patients. In the German hospital reimbursement system (G-DRG) each case is denoted with a PCCL (Patient Clinical Complexity Level), which is an integer value between 0 and 6 calculated according to an annually adapted mathematical formula. It denotes the overall patient-related severity in medical-economic classification systems [34].
Consultation of experts
The qualitative results presented in this analysis were based on a structured narrative review through group consultation with clinical experts from Germany. The team consisted of experts in clinical gastroenterology and/or infectiology.
Results
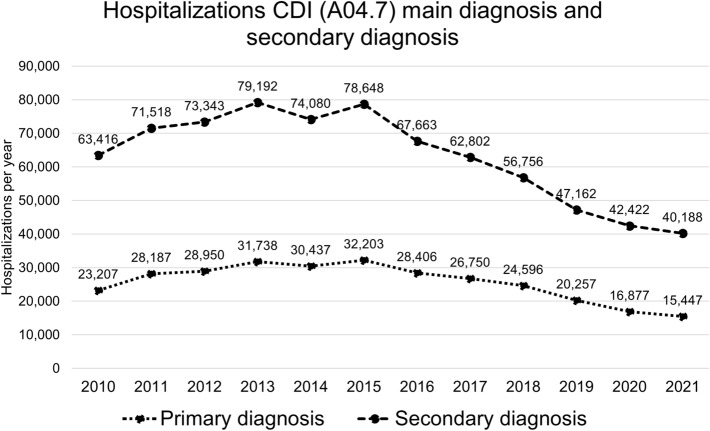
In Germany, coding of hospital cases of primary (PD) and secondary (SD) diagnoses of CDIs increased steadily from 2010 until a peak in 2015 followed by a decline to until 2021. In 2010, there were 23,207 PD cases and 63,416 SD cases. In 2015, numbers had risen to 32,203 (+ 38.8%) and 78,648 (+ 24.0%), respectively. After 2015, a downward trend saw case numbers decrease to 15,412 PD cases (− 52.1% from 2015) and 40,188 SD cases (− 48.9% from 2015) in 2021 (Fig. 1). In tandem, the CDI incidence dropped from 6.8 in 2015 to 4.3 in 2021 per 10,000 hospital-patient-days.
Fig. 1.
Development of coded CDI cases in German hospitals from 2010 to 2021 for primary and secondary diagnosis
The sex distribution hardly shifted; in 2010, 61.0% of PD-coded patients (14,150) and 53.0% of those with SD (33,604) were female. By 2021 the proportion of females had reduced to 59.1% (− 1.9%) of PD-coded patients (9122) and 51.0% (− 2.0%) of those with SD (19,940). CDI cases occurred more frequently in older patients than in younger patients. In 2020, patients between 70 and 95 years of age comprised 72.7% of cases with PD and 66.9% of cases with SD. The average age of CDI patients decreased over time. In 2015, the average ages were 75.1 years for PD and 75.9 years for SD of CDI. In 2021, these average ages had decreased to 71.8 years and 72.6 years, for PD and SD respectively (Fig. 2). Notably, the average age of all hospital cases in Germany rose from 55.2 in 2015 to 56.2 in 2021. The average patient clinical complexity level (PCCL) was different between the types of diagnosis in 2021: 1.6 for PD cases and 3.3 for SD cases. The differences in the PCCL compared to those in previous years were minimal.
Fig. 2.
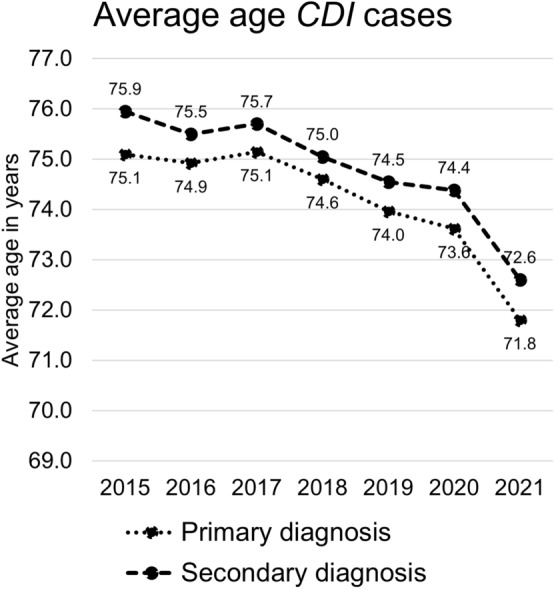
Development of the average age of coded CDI patient cases in German hospitals from 2015 to 2021 for primary and secondary diagnosis
The average length of hospitalization in each case remained constant over time. For PD, the average length of hospital stay was 10.6 days in 2015 and 9.9 days (− 6.1%) in 2021. For SD, hospital durations were 26.5 days in 2015 and 26.0 days (− 1.9%) in 2021. The overall length of stay in German hospitals was 7.4 days in 2015 and 7.2 days (− 2.7%) in 2021. Consequently, the hospital days with PD or SD of CDI were reduced by 47.5% from 2015 to 2021: from 2,424,511 days to 1,271,950 days owing to a reduction in cases, but not to a change in length of stay. Overall, 62.7% of PD and 67.1% of SD cases were classified at discharge as cases for supervised care and were assigned an official care classification (if not already existing).
In 2021, the in-hospital mortality rates of patients with SD and PD were 13.4% (5383 of 40,118) and 5.7% (886 of 15,412), respectively. The official German statistics based on death certificates reported 2666 deaths with CDI as the main cause of death in 2015. A reduction to 950 deaths (− 64.4%) was seen in 2020 [35]. Overall, 7.9% of the patients with SD and 3.1% with PD were transferred to another hospital.
CDI, as a coded hospital case, had five subcategories (Table 1). The case distributions of those with PD and SD were similar. Enterocolitis without megacolon or other organ complications accounted for 77.3% of PD cases and 74.3% of SD cases. Among cases with organ complications, 4.7% and 3.4% of the patients had PD and SD, respectively. Megacolon was coded in 1.3% of patients with PD and 1.0% of patients with SD. CDI as unspecified was labeled in 16.6% of PD cases and 21.9% of SD cases.
Table 1.
Enterocolitis caused by Clostridioides difficile according to the German modified (GM) International Classification of Diseases, Tenth Revisions (ICD-10)
| ICD-10GM-2022 | Description |
|---|---|
| A04.7 | Enterocolitis caused by Clostridioides difficile |
| • Including food poisoning caused by Clostridioides difficile | |
| • Including pseudomembranous colitis | |
| • If a recurrent infection with Clostridioides difficile is indicated, an additional key number (U69.40!) must be used | |
| A04.70 | Enterocolitis caused by Clostridioides difficile without megacolon, without other organ complications |
| A04.71 | Enterocolitis caused by Clostridioides difficile without megacolon, with other organ complications |
| A04.72 | Enterocolitis caused by Clostridioides difficile with megacolon, without other organ complications |
| A04.73 | Enterocolitis caused by Clostridioides difficile with megacolon, with other organ complications |
| A04.79 | Enterocolitis caused by Clostridioides difficile, unspecified |
In 2022, an internal medicine intensive care unit was present in 1250 German hospitals [33]. Overall, 1237 hospitals between 2015 and 2020 had at least one case of CDI as PD (data for 2021 not available). A total of 288 hospitals accounted for 50% of all cases. University hospitals treated 5.7% of all patients with PD. Of these patients, 89.4% were treated in an internal medicine department before discharge, followed by 5.7% who were treated in surgical departments.
In Germany, reporting of severe courses of CDI to local authorities is mandatory, and data are merged at federal level (RKI). At the time of the manuscript preparation, complete data were available until 2020, with 1595 severe cases reported. Overall, 96.5% of severe cases were hospitalized (n = 1502), and 19.2% of patients with survival data available died (n = 296). The median patient age was 79 years. In mid-2016, the RKI changed the definition for severe CDI. Specifically, in May 2016 the criterion ‘Inpatient readmission due to recurrent C. difficile infection’ was replaced by ‘Inpatient admission due to an outpatient acquired C. difficile disease’. This new definition was fully implemented in 2019 and complicated data comparability [36]. Under the former categorization, the number of reported cases increased from 470 to 2151 (+ 358%) between 2010 and 2015. The number of reported severe cases decreased from 2827 in 2018 to 1145 in 2021 (− 59.8%).
Saxony is the only federal state in Germany where reporting of any level of CDI is mandatory [37], with 4.9% of Germans living there in 2021 [38]. The highest number of CDI cases was reported in 2011 with 5837 notifications. By 2021, this number steadily decreased to 3017 (− 48.3%). In addition, the Saxonian authorities distinguished severe from non-severe cases by applying federal guidance. In 2015, the year before the change in definition of severe cases, 1.5% of all reported cases were classified as severe. In 2018, after full implementation of the definition, 4.2% of all cases were classified as severe.
Discussion
Until recent years, CDI was identified as an increasing concern to health care systems, based on its rising incidence in in- and outpatient settings worldwide. This development has led to growing concerns about CDI-associated mortality and the CDI-related economic burden on health care systems [12–17, 29, 39–41]. In the last few years, a change in this trend has been reported for hospital-acquired CDI in the United States of America [42]. In line with these findings, German hospital claims data demonstrated an approximately 50% decrease of CDI cases from 2015 to 2021. These findings are supported by similar trends in the statistics of severe cases reported to the responsible federal authority (RKI), which comprises mainly hospitalized cases. Furthermore, the data from Saxony, where reporting of any CDI in mandatory show a comparable result. Also, the official German statistics based on death certificates, where CDI is documented as a direct cause of death, confirmed the proportion of the decrease. These reductions can also be expressed in incidence per 10,000 patient-days. In Germany, the median values were 7.0 in 2007 [43] and 8.2 in 2011 [41]. In our analyses, we found a decrease in the CDI incidence per 10,000 patient-days from 6.8 in 2015 to 4.1 in 2021 in the hospitalization data. While all the reporting systems used for our analyses have their specific limitations, the fact that a broad range of data sources delivered similar results, suggests their general reliability.
Different reasons for this epidemiological trend reversal have been discussed. In the United States, the impact of the COVID-19 pandemic has been reported as a potential protective factor [44–46]. The overall number of hospitalizations declined from 2016 with 146.4 million hospital days and 20.1 million cases to 123.5 million hospital days (-15.7%) and 17.2 million cases (-14.6%), respectively in 2021 [47]. Less time spent in the hospital may protect against nosocomial infections. Social distancing and avoidance of hospitalization have also been observed in Germany. However, the emergence of COVID-19 protection measures does not fully explain the changing epidemiology, as the trend reversal was already underway in 2015.
The following factors are likely to play a role as well. In the last decade, the national public health institute of Germany (RKI) has run several campaigns to increase hospital hygiene. In 2011, the recommendations of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) and the Commission on Anti-Infectives, Resistance, and Therapy (ART) at RKI became compulsory for German hospitals and other health care facilities. Hospitals were obliged to create conditions in accordance with the KRINKO recommendations on organizational and human resource requirements for the prevention of nosocomial infections by 2019. A special financial program was established to promote hospital hygiene, with a budget of approximately 672 million Euros, which ended in 2022 [48]. A systematic evaluation of the program was not intended. Thus, the effects on the reduction in CDI are not quantifiable. In addition, the pathways for acquisition of C. diff. have not been fully explained to date [49].
As outlined above, antibiotic use is the main driver of CDI [5–7]. The ambulatory prescription of antibiotics peaked in Germany in 2013. Ever since, there has been a steady decrease of annual prescriptions. This may be partly to several concerted national activities aimed at the reduction of antibiotic consumption, such as the German antibiotic resistance strategy (Deutsche Antibiotika-Resistenzstrategie [DART]) implemented in 2015 and its follow-up project called DART 2020. After a reduction in the use of fluoroquinolones had shown to be a highly effective measure in reducing the burden of CDI [50], it is has been advised to restrict the use of fluoroquinolones in Germany [51].
Finally, the German state started in 2013 the funding of the systematic training of infectious disease specialists [52].
Changes in the prevalence of different CDI strains in Germany may also have contributed to incidence dynamics. The German NRC examined isolates obtained from samples sent between 2014 and 2019 for diagnostic reasons and those from a Tertiary Care University Center, and confirmed RT027 as the most prevalent strain; however, they also found that since 2016, there was a consistent decrease in RT027 cases [53]. It remains unclear whether this decrease in cases of the RT027 strain is a driver for the decrease in overall cases, or whether the mentioned measurements led to an overall decrease in cases, as well as in the RT027 strain. Another aspect that may have impacted incidence estimates is the quality of diagnostic measures. While in the last decade, it was assumed that the true incidence rate of CDI was being underestimated owing to a lack of awareness among physicians in combination with suboptimal laboratory diagnostics [39]. With the rise of PCR-based diagnostics, an overestimation of CDI cases may have followed [54]. Furthermore, the aggregated hospital data did not deliver detailed information on C. diff. diagnostics. Overall, any bias associated with the level of physicians’ awareness of CDI and the choice of adequate testing algorithms cannot be corrected for.
Readmissions of patients with CDI are common [55–57], and the rate of recurrence is estimated to be approximately 15–35% of all CDI cases. Patients who experience recurrent episodes are at risk of second and subsequent recurrences [58]. Hospital data did not allow for differentiation of readmission. In Germany, any readmission to the same hospital within 30 days after discharge was counted as one case. Historically, the readmission rate was reported to be 3.9% for CDI [41].
Conclusions
The number of hospital billing cases with a PD or SD of CDI was reduced to half between 2015 and 2021. This trend was confirmed by the cases resulting from mandatory reporting of severe CDI at the federal level, by reposting of any case of CDI in one German federal state, and finally by cases based on death certificates. Despite this quantitative development, the severity of the average case as reflected by length of stay, complexity and mortality remained unchanged indicating that the impact on the health care system is still high.
These data do not identify the reasons for this trend. There may be several possible explanations. German authorities campaigned to reduce the use of antibiotics with proven success. Additionally, a major hospital hygiene program was implemented. Social distancing and reduction in hospitalizations may have had an impact, as well. Finally, a reduction in the number of RT027 strains might be a cause for this decrease as well, it is difficult to judge whether this was an affected or rather a natural trend though. Overall, further research is necessary to determine the specific contributions of all possible factors.
Acknowledgements
We thank Dr. Nike Müller-Grage of Ferring Arzneimittel GmbH for providing support for this project. We also thank Marjan Arvand and Carsten Telschow for their input.
Author contributions
All authors were involved in the conception and design of the analysis. Similarly, all authors were involved in data collection. MJGTV and AS contributed to the analysis and writing of the manuscript. All authors approved the content for publication.
Funding
This work was supported by Ferring Arzneimittel GmbH. MV, AS, and LvM also received funding from Ferring for another independent project on hospitalization data on fecal microbiota transfer.
Declarations
Conflict of interest
MJGTV received research Grants from 3M, Astellas Pharma, Biontech, DaVolterra, Evonik, Gilead Sciences, Glycom, Immunic, MaaT Pharma, Merck/MSD, Organobalance, Seres Therapeutics, Takeda Pharmaceutical, as well as speaker fees or consulting fees from Alb Fils Kliniken GmbH, Arderypharm, Astellas Pharma, Basilea, Bio-Mérieux, DaVolterra, Farmak International Holding GmbH, Ferring, Gilead Sciences, Immunic AG, MaaT Pharma, Merck/MSD, Pfizer, Roche, Organobalance, SocraTec R&D GmbH, Tillots Pharma GmbH. CFM has given talks at Ferring-sponsored symposia. AS has been lecturing for AbbVie, Bristol Myers Squibb, Celltrion, CLS Behring, De Prom, Falk Foundation, Ferring, Janssen, Kompetenznetz Darmerkrankungen, MedUpdate, MSD, Recordati-Pharma, sobi rare strength, Takeda and consulting activities for AbbVie, Amgen, Bristol Myers Squibb, Consal, Galapagos, Gilead, Janssen, Lilly, MSD, Repha GmbH, Roche, Pfizer, Pharmacosmos GmbH, Takeda, Tillots Pharma GmbH. SS has received personal fees from Abbvie, Arena, BMS, Biogen, Celltrion, Celgene, Falk, Ferring, Fresenius Kabi, IMAB, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Sandoz, Takeda and Theravance. HJE received speaker fees or consulting fees from Astellas Pharma, Falk Foundation, Ferring Arzneimittel, MSD, Tillots Pharma, viiv Health care. JO and LvM stated no conflicts of interest.
Ethics statement
The risks for the participants were considered minimal, so no requirement of ethical approval was deemed necessary. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Contributor Information
Maria Johanna Gobertina Tetuanui Vehreschild, Email: Maria.Vehreschild@kgu.de.
Stefan Schreiber, Email: s.schreiber@mucosa.de.
Lutz von Müller, Email: lutz.mueller@christophorus-kliniken.de.
Hans-Jörg Epple, Email: hans-joerg.epple@charite.de.
Thomas Weinke, Email: th.weinke@gmail.com.
Carolin Manthey, Email: manthey@gim-witten.de.
Jun Oh, Email: j.oh@uke.de.
Steffen Wahler, Email: steffen.wahler@stbernward.de.
Andreas Stallmach, Email: Andreas.Stallmach@med.uni-jena.de.
References
- 1.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–1548. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 2.Smits WK, Lyras D, Lacy DB, et al. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138:1–11. doi: 10.1111/j.1365-2567.2012.03616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ofosu A. Clostridium difficile infection: a review of current and emerging therapies. Ann Gastroenterol. 2016;29:147–154. doi: 10.20524/aog.2016.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2013;69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 6.Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. 2016;14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzales-Luna AJ, Carlson TJ. Follow your gut: microbiome-based approaches in the developmental pipeline for the prevention and adjunctive treatment of Clostridioides difficile infection (CDI) Curr Infect Dis Rep. 2020;22:22. doi: 10.1007/s11908-020-00729-8. [DOI] [Google Scholar]
- 8.Khanna S, Gupta A, Baddour LM, et al. Epidemiology, outcomes, and predictors of mortality in hospitalized adults with Clostridium difficile infection. Intern Emerg Med. 2016;11:657–665. doi: 10.1007/s11739-015-1366-6. [DOI] [PubMed] [Google Scholar]
- 9.Vehreschild MJGT, Weitershagen D, Biehl LM, et al. Clostridium difficile infection in patients with acute myelogenous leukemia and in patients undergoing allogeneic stem cell transplantation: epidemiology and risk factor analysis. Biol Blood Marrow Transplant. 2014;20:823–828. doi: 10.1016/j.bbmt.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Lessa FC, Mu Y, Winston LG, et al. Determinants of Clostridium difficile infection incidence across diverse United States geographic locations. Open Forum Infect Dis. 2014 doi: 10.1093/ofid/ofu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett JG, Moon N, Chang TW, et al. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782. doi: 10.1093/cid/cix1085. [DOI] [PubMed] [Google Scholar]
- 12.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 13.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–549. doi: 10.1128/cmr.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvand M, Moser V, Schwehn C, et al. High prevalence of Clostridium difficile colonization among nursing home residents in Hesse, Germany. PLoS ONE. 2012;7:e30183. doi: 10.1371/journal.pone.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12:2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 16.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 17.Goudarzi M, Seyedjavadi SS, Goudarzi H, et al. Clostridium difficile infection: epidemiology, pathogenesis, risk factors, and therapeutic options. Scientifica Cairo. 2014;2014:916826. doi: 10.1155/2014/916826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerding DN, Lessa FC. The epidemiology of Clostridium difficile infection inside and outside health care institutions. Infect Dis Clin North Am. 2015;29:37–50. doi: 10.1016/j.idc.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGlone SM, Bailey RR, Zimmer SM, et al. The economic burden of Clostridium difficile. Clin Microbiol Infect. 2012;18:282–289. doi: 10.1111/j.1469-0691.2011.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burckhardt F, Friedrich A, Beier D, et al. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis. 2008;14:691–692. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen (NRZ) Deutsche nationale Punkt-Prävalenzerhebung zu nosokomialen Infektionen und Antibiotika-Anwendung 2016, Abschlussbericht. Nationales Referenzzentrum für Surveillance von nosokomialen Infektionen (NRZ); 2017.
- 24.Ott E, Saathoff S, Graf K, et al. The prevalence of nosocomial and community acquired infections in a university hospital: an observational study. Dtsch Arztebl Int. 2013;110:533–540. doi: 10.3238/arztebl.2013.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimann SM, Vehreschild JJ, Cornely OA, et al. Economic burden of Clostridium difficile associated diarrhoea: a cost-of-illness study from a German tertiary care hospital. Infection. 2015;43:707–714. doi: 10.1007/s15010-015-0810-x. [DOI] [PubMed] [Google Scholar]
- 26.Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect. 2012;18:5–12. doi: 10.1111/1469-0691.12064. [DOI] [PubMed] [Google Scholar]
- 27.Prechter F, Katzer K, Bauer M, et al. Sleeping with the enemy: Clostridium difficile infection in the intensive care unit. Crit Care. 2017;21:260. doi: 10.1186/s13054-017-1819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten für 2016. Infektionsepidemiologisches Jahrbuch meldepflichtiger Krankheiten. Berlin: Robert Koch-Institut (RKI); 2017.
- 29.Arvand M, Vollandt D, Bettge-Weller G, Harmanus C, Kuijper EJ. Increased incidence of Clostridium difficile PCR ribotype 027 in Hesse, Germany, 2011 to 2013. Euro Surveill. 2014;19(10):20732. doi: 10.2807/1560-7917.ES2014.19.10.20732. [DOI] [PubMed] [Google Scholar]
- 30.Marujo V, Arvand M. The largely unnoticed spread of Clostridioides difficile PCR ribotype 027 in Germany after 2010. Infect Prev Pract. 2020;2:100102. doi: 10.1016/j.infpip.2020.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steglich M, Nitsche A, von Müller L, et al. Tracing the spread of Clostridium difficile Ribotype 027 in Germany based on bacterial genome sequences. PLoS ONE. 2015;10:e0139811. doi: 10.1371/journal.pone.0139811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58:1394–1400. doi: 10.1093/cid/ciu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joint Federal Committee (G-BA). Qualitätsberichte der Krankenhäuser, gemäß § 137 Abs. 3 Satz 1 Nr. 4 SGB V. Joint Federal Committee (G-BA). 2022.
- 34.Gesetz zur wirtschaftlichen Sicherung der Krankenhäuser und zur Regelung der Krankenhauspflegesätze. Einführung eines pauschalierenden Entgeltsystems nach § 17b KHG. 2000.
- 35.Gesundheitsberichterstattung des Bundes (GBE). Mortalität und Todesursachen. Mortalität und Todesursachen. 2022.
- 36.Robert Koch-Institut (RKI) Infektionsepidemiologisches Jahrbuch meldepflichtiger Erkrankungen für 2019. Berlin: Robert Koch-Institut (RKI); 2020.
- 37.Merbecks S-S. Neue bundesweit geltende Meldepflichten: Bedeutung für Sachsen. Ärzteblatt Sachsen 2016. 235–6.
- 38.Statistisches Bundesamt (DESTATIS) 12411-0010: Bundesländer, Stichtag. Statistisches Bundesamt (DESTATIS). Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller). Statistisches Bundesamt (DESTATIS), Wiesbaden, 2022. www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Publikationen/Downloads-Krankenhaeuser/operationen-prozeduren-5231401217014.html.
- 39.Davies KA, Longshaw CM, Davis GL, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14:1208–1219. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 40.Hall AJ, Curns AT, McDonald LC, et al. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 41.Grube RF, Heinlein W, Scheffer H, et al. Economic burden of Clostridium difficile enterocolitis in German hospitals based on routine DRG data. Z Gastroenterol. 2015;53:391–397. doi: 10.1056/NEJMoa1910215. [DOI] [PubMed] [Google Scholar]
- 42.Guh AY, Mu Y, Winston LG, et al. Trends in U.S. Burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382:1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gastmeier P, Weitzel-Kage D, Behnke M, et al. Surveillance of Clostridium difficile-associated diarrhoea with the German nosocomial infection surveillance system KISS (CDAD-KISS) Int J Antimicrob Agents. 2009;33(Suppl 1):S19–23. doi: 10.1016/s0924-8579(09)70011-1. [DOI] [PubMed] [Google Scholar]
- 44.Weiner-Lastinger LM, Pattabiraman V, Konnor RY, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network. Infect Control Hosp Epidemiol. 2022;43:12–25. doi: 10.1017/ice.2021.362. [DOI] [PubMed] [Google Scholar]
- 45.Baker MA, Sands KE, Huang SS, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections. Clin Infect Dis. 2022;74:1748–1754. doi: 10.1093/cid/ciab688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans ME, Simbartl LA, Kralovic SM, et al. Lack of correlation between standardized antimicrobial administration ratios (SAARs) and healthcare-facility-onset Clostridioides difficile infection rates in Veterans Affairs medical facilitie. Infect Control Hosp Epidemiol. 2022 doi: 10.1017/ice.2022.93:1-24. [DOI] [PubMed] [Google Scholar]
- 47.Gesundheitsberichterstattung des Bundes (GBE). Hospitalisierungsdaten. Gesundheitsberichterstattung des Bundes (GBE) Berlin, 2022.
- 48.GKV-Spitzenverband. Bericht des GKV-Spitzenverbandes zum Hygienesonderprogramm in den Förderjahren 2013 bis 2021. 30.06.2022 ed. Berlin: GKV-Spitzenverband; 2022.
- 49.Crobach MJT, Vernon JJ, Loo VG, et al. Understanding Clostridium difficile colonization. Clin Microbiol Rev. 2018;31:e00021–e117. doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis. 2017;17:411–421. doi: 10.1016/s1473-3099(16)30514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helmut Schröder AZ, Katja Niepraschk-von-Dollen, Carsten Telschow, Jonas Lohmüller. Risikoreiche Verordnungen von Fluorchinolon-Antibiotika in Deutschland (23.05.2019). Im Internet: www.wido.de/fileadmin/Dateien/Dokumente/Forschung_Projekte/Arzneimittel/wido_arz_fluorchinolone_0519.pdf.
- 52.Stoliaroff-Pépin AAM, Mielke M. Zur Diskussion. Hygienefach personal—wann ist der Bedarf gedeckt? Epidemiologisches Bull. 2018 doi: 10.17886/EpiBull-2018-054. [DOI] [Google Scholar]
- 53.Abdrabou AMM, Ul Habib Bajwa Z, Halfmann A, et al. Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany, 2014–2019. Int J Med Microbiol. 2021;311:151507. doi: 10.1016/j.ijmm.2021.151507. [DOI] [PubMed] [Google Scholar]
- 54.Liu C, Lan K, Krantz EM, et al. Improving appropriate diagnosis of Clostridioides difficile infection through an enteric pathogen order set with computerized clinical decision support: an interrupted time series analysis. Open Forum Infect Dis. 2020;7:ofaa366. doi: 10.1093/ofid/ofaa366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drwiega EN, Danziger LH, Johnson S, et al. 766. Readmissions of hospitalized patients with Clostridioides difficile infection (CDI) for recurrent CDI is common. Open Forum Infect Dis. 2021;8:S480. doi: 10.1093/ofid/ofab466.963. [DOI] [Google Scholar]
- 56.Murphy CR, Avery TR, Dubberke ER, et al. Frequent hospital readmissions for Clostridium difficile infection and the impact on estimates of hospital-associated C. difficile burden. Infect Control Hosp Epidemiol. 2012;33:20–28. doi: 10.1086/663209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma S, Weissman S, Walradt T, et al. Readmission, healthcare consumption, and mortality in Clostridioides difficile infection hospitalizations: a nationwide cohort study. Int J Colorectal Dis. 2021;36:2629–2635. doi: 10.1007/s00384-021-04001-w. [DOI] [PubMed] [Google Scholar]
- 58.Singh T, Bedi P, Bumrah K, et al. Updates in treatment of recurrent Clostridium difficile infection. J Clin Med Res. 2019;11:465–471. doi: 10.14740/jocmr3854. [DOI] [PMC free article] [PubMed] [Google Scholar]