Abstract
In the present study the effect of ozone therapy on hydrofluoric acid (HFA) related eye burn was investigated in rats. A Total 20 healthy male Wistar albino rats (weighing 250 - 300 g with the age of 16 weeks) were used. They were divided into groups (experimental and control groups) of 10 rats being housed individually and fed ad libitum. The HFA (2.00%) burn was created in all animals. The ozonized (20.00 µg O3 mL-1) bi-distilled water was applied as a drop (10.00 µL each drop) every 8 hr for 7 days in the experimental group. At the same time, 0.90% NaCl was applied as drop (10.00 µL each drop) every 8 hr for 7 days in the control group. In the experimental group, intensive inflammation, angiogenesis, epithelial damage and stromal edema were detected in one animal. Epithelial vascularization and stromal edema were seen in four animals. In control group, only two animals’ corneal structures were normal. Inflammation, angiogenesis, epithelial damage, fibrosis, epithelial vascularization and stromal edema were detected in the rest. As a result of this study, it was observed that local usage of ozone therapy had a positive effect on the healing of corneal burns caused by HFA. It was concluded that more ozone-related studies should be done to enlighten the subject.
Key Words: Burn, Cornea, Hydrofluoric acid, Ozone, Rat
Introduction
Due to the industrial development associated changes in the environmental condition, contact risk to the chemical agent increases in human and animals. Acidic, alkaline, oxidizing and reducing agents are the most widely used ones in industry. Therefore, animal contact risk to these agents is as high as in humans. Chemical eye burns are also among increasing eye diseases. Ocular chemical burns are the most common cause of limbal stem cell failure and they are characterized by non-healing epithelial corneal defect, stromal inflammation, neovascularization and corneal opacification. In acute chemical ocular burn treatment, removal of chemical agent from the ocular surface and suppression of the inflammation are very important for the recovery process.1,2
Hydrofluoric acid (HFA) is a hazardous acidic substance and readily penetrates to the tissue. Generally, acidic substances cause coagulative necrosis in the corneal epithelium of the eye. On the other hand, HFA is more dangerous in eye burns due to higher penetration of dissociated fluoride anion content.3
Ozone is a medical gas used for clinical treatment in recent years, and proper concentration of ozone can improve oxygen supply in local tissues, inhibit inflammatory and stress responses and reduce angio-genesis.4-6 The use of ozone in eye diseases could be therapeutic due to its anti-inflammatory, bactericidal and tissue repairing properties.7
The aim of the study was to investigate the effect of ozone therapy on HFA related eye burn in rats, and whether corneal oxygen demand can be provided with ozone or not during the early period of chemical corneal damage healing.
Materials and Methods
Animal grouping. The study was conducted on 20 healthy male Wistar albino rats (weighing 250 - 300 g, with the age of 16 weeks); they were divided into two groups (experimental and control groups) of 10 rats being housed individually and fed ad libitum. The experimental protocol was approved by the Animal Ethical Committee of University of Erciyes, Kayseri, Türkiye (Approval Number: 19/187).
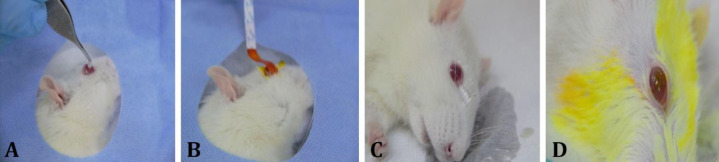
Experimental corneal acidic burn. After routine examination of the animals, using the modified experimental procedure of Altan and Oğurtan2 and Hatipoğlu et al.,8 under general and under anesthesia by 5.00 mg kg-1 xylazine (Bayer, Kiel, Germany) and 60.00 mg kg-1 ketamine (Richter Pharma AG, Wels, Germany), a 2.00% HFA (Merck, Burlington, USA) impregnated 0.10 mm diameter filter paper was placed on the right corneas surface for 10 sec (Fig. 1A). After 10 sec, the filter paper was removed and the eyes were washed with 0.90% NaCl solution for 2 min (Fig. 1C). Then, 1.00% fluorescein strip (ERC, Ankara, Türkiye) was contacted to the cornea to detect HFA burn (Fig. 1B) and all eyes were washed again with 0.90% NaCl solution for 1 min to clean fluorescein staining residue from them (Fig. 1D). Then, 4.80 mg kg-1 paracetamol (q24hr; GlaxoSmithKline, Istanbul, Türkiye) for 3 days and 10 mg kg-1 amoxicillin + clavulanic acid (q24hr; GlaxoSmithKline, Istanbul, Türkiye) for 5 days were given orally as analgesic and to avoid secondary infection risk in all animals in the groups, respectively.
Fig. 1.
Hydrofluoric acid-induced corneal burn in a rat. A) Placing the 0.10 mm diameter filter paper on the right cornea surface. B) Fluorescein staining. C) Corneal washing after filter paper removal. D) Cleaning of fluorescein staining residue and induced corneal burn
Ozonized water preparation and administration. The ozone generator (Evozone GmbH, Reutlingen, Germany) was adjusted to 80.00 µg mL-1 for ozone concentration and fed with pure oxygen with flow rate of 500 mL per min. The bi-distilled water (10.00 mL) was ozonized for 10 min at 20.00 ˚C and maximum ozone concentration was obtained. The ozonized (20.00 µg O3 mL) bi-distilled water was applied as drop (10.00 µL each drop) every 8 hr for 7 days to experimental group. At the same time 0.90% NaCl was applied as drop (10.00 µL each drop) every 8 hr for 7 days to the control group. The ozonization procedure of bi-distilled water was repeated before each application, for giving the same amount of ozone.
Clinical and histopathological evaluations. The experimentally induced corneal HFA burns formed opacity in limited areas was detected in both groups. In the experimental group, the opacity developed on the burned eyes started to reduce at the 3rd day and reduction in the opacity continued until the 7th day. In contrast, the formed opacity was found to continue until 7th day in the control group. In daily control of all groups, no secondary complications were seen in the other structures of the eyes. All animals were euthanized with 100 mg kg-1 ketamine and 10.00 mg kg-1 xylazine through intra-peritoneal injection at the end of 7th day. Following euthanasia, the eye globes were removed. After removal, formalin was injected into eye globes and corneal tissues were fixed in 10.00% buffered formalin during 72 hr for histopathological examination. To determine histo-pathological changes (inflammation, neovascularization, fibrosis, stromal edema, and epithelialization), Hematoxylin and Eosin staining was performed on the corneas.9 The obtained preparations were evaluated histopathologically with light microscope (BX51; Olympus, Tokyo, Japan). Histological changes were recorded for each sample separately. Each sample was evaluated in five different areas at 4×, 10× and 40× magnifications under the microscope. Considering the mentioned criteria, microscopical evaluation was performed according to the following scoring system: 0 = no damage, 1 = minimal damage, 2 = intermediate damage, 3 = severe damage.10-12
Statistical analysis. The Q-Q plot, histogram plot, and the Shapiro-Wilk test were used to check the normal distribution of data. To determine statistical differences of inflammation score between the experimental and control groups, Mann-Whitney U test was used. Data were presented as median (1st - 3rd quarters). The R Software (version 4.4.2; RStudio Inc., Boston, USA) was used for statistical analysis of data. Significance level was determined as p < 0.05.
Results
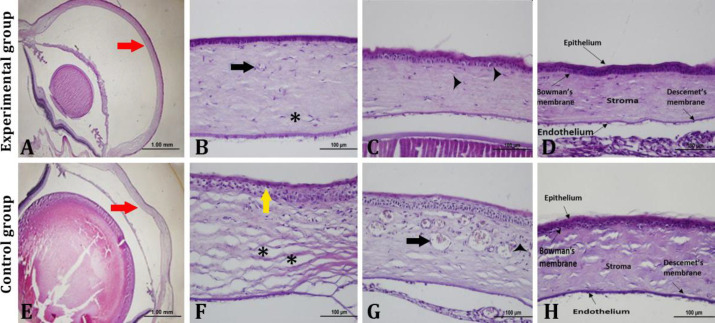
The healing and damage of the cornea in groups were demonstrated in Figure 2. In microscopic examination, the experimental group’s corneal structures were smoother and more normal than the controls (Figs. 2A and 2E). In the experimental group, vascularization and stromal edema were detected in only one animal (Fig. 2B). Epithelial damage and fibroblast were seen in four animals (Fig. 2C). In the remaining five animals, corneal structures were close normal healthy corneal structure unlike others (Fig. 2D). In the control group; corneal structure was normal only in two animals. Epithelial damage, epithelial vascularization and stromal edema (Fig. 2F) and inflammation and vascularization (Fig. 2G) were detected in the rest.
Fig. 2.
Histopathological evaluation of the experimental and control groups using Hematoxylin and Eosin staining. A) Red arrow indicates the cornea in the experimental group. B) Black arrow and asterisk indicate fibroblasts and stromal edema in cornea, respectively. C) Black arrowheads indicate vessels transection in cornea. D) Corneal layers are depicted. E) Red arrow indicates the cornea in the control group. F) Yellow arrow and asterisk indicate the epithelial vacuolization and stromal edema in cornea, respectively. G) Black arrow and black arrowheads indicate vessel and inflammatory cells, respectively. H) Corneal layers are depicted
At the same time, the corneal layers of the control group were obvious (Fig. 2H). The scoring results are given in Table 1.
Table 1.
Scoring results of the animals
| Animal No. | Control (n = 10) | Experimental (n = 10) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 1 | 1 |
| 3 | 2 | 0 |
| 4 | 1 | 0 |
| 5 | 0 | 0 |
| 6 | 2 | 1 |
| 7 | 1 | 1 |
| 8 | 1 | 0 |
| 9 | 3 | 1 |
| 10 | 1 | 3 |
| Total point | 12 | 7 |
| Scoring point | 1.00 (0.75 - 2.00) | 0.50 (0.00 - 1.00)* |
0 = no damage, 1 = minimal damage, 2 = intermediate damage, 3 = severe damage
* No statistically significant difference was observed between groups (p = 0.19).
Discussion
Nowadays, various new treatment methods have been investigated and used for the treatment of corneal damages. In recent years, ozone has been preferred intensely in human medicine because of super-activating oxygen feature, inhibiting inflammatory and stress factors, increasing oxygenation in local lesions and suppressing angiogenesis through oxidation properties.6,13 The corneal epithelial damage usually heals in a week or more depending on the severity of damage. If the healing processes are not well managed, it can cause corneal ulcer, and perforation and blindness.14,15 The researchers have focused on alkaline corneal burns and investigated the effects of different treatments methods and drugs on corneal healing.16-18 In the present study, acidic corneal burn and the effect of ozone therapy were investigated. The HFA is different than other acidic agents due to its chemical structure. The fluoride ion constituent in HFA causes extensive liquefactive necrosis in soft tissues as well as decalcification and corrosion in hard tissues. In HFA burns being not treated immediately, it diffuses and penetrates deep tissues and induces coagulation necrosis like alkaline agents. The coagulation necrosis is formed due to tissue hypoxia. The opacification of cornea caused by HFA has been attributed to the powerful penetration of the fluoride ion damaging trabecular corneal structure, and the release of prostaglandin.19 In the present study, opacification was detected after HFA burn in experimental and control groups. But, opacification started to decrease in experimental group after ozone therapy at the third day. This decrease showed that the atmospheric oxygen required for corneal healing could be supplied with ozone therapy. It was recorded that ozone reacts with biological membranes to produce anti-oxidant substances and enzymes contributing to corneal healing.7,13
Liu has investigated the ozone therapy effect on cell apoptosis and angiogenesis in retina tissue of rats and compared the apoptotic cell numbers between diabetic retinopathy model and control groups.4 It was found that the apoptotic cell numbers were lower in ozone therapy group than other groups, explaining the inhibitory effect of ozone on the Nogo receptors in diabetic retinopathy decreasing the nerve cells damage in retina. In several studies, anti-inflammatory effect of systemically used ozone has been reported. Uysal et al. used ozone in an acute necrotizing pancreatitis model in rats and evaluated its anti-inflammatory effect.20 They found that the inflammation was lower in ozone used group, and being attributed to the ozone anti-inflammatory effect. Furthermore, Yamanel et al. have induced a lung damage in sepsis model and used ozone therapy in rats.21 They have detected a decrease in serum pro-inflammatory cytokine levels and inflammation suppression in histopathological examination as a result of ozone therapy. In the present study, corneal opacification was determined after HFA burn in both groups. In the clinical evaluation, the opacification decreased in experimental group. The epithelial damage and stromal edema were not determinate in 50.00% of animals in the experimental group. The number of animals having inflammation, angiogenesis, epithelial damage and stromal edema was lower in experimental groups compared to control group. Furthermore, fibrosis was also seen in two animals from control group. However, there was no statistical significance between groups regarding the histopathological score evaluation. Although no statistical significance was found in the scoring results, the clinical results of this study showed that zone therapy may be effective for early acidic corneal burn healing in rats. The better corneal healings in experimental group support this outcome. These results may even suggest that early use of ozone in acidic burn can minimize possible corneal damage and contribute to the recovery process.
Hatipoğlu et al. have induced HFA burn in rabbits and emphasized that using of 500 mL saline solution twice a day for two weeks contributes to recovery.8 In another study it has been suggested that using 500 mL saline solution twice a day during one week period leads to better results in rabbits.22 The saline solution is known effective for early corneal healing in chemical eye burn and routinely proposed to use in humans.23,24 In our study, ozonized bi-distilled water being used as a drop (10.00 µL each drop) three times per day (8 hr interval) during one week period resulted in a better outcome than saline solution group (control group) in rats. The researchers have speculated that the first phase of healing in corneal injuries is the latent phase and the cells do not show a numerical change. The healing in chemical eye burns is linked to the type of chemical agent, its pH, duration of contact with the chemical agent and medical intervention time.1,23,25 Therefore, the researchers have suggested the cornea washing when the eye contacts with HFA to minimize the corneal damage.26,27 In the present study, the corneas were washed with saline solution for cleaning HFA and fluorescein staining in all animals, and the saline solution was continued to be used as a drop-in control group. At the same time, histopathological data of the present study also showed the comparison of ozone and saline solutions for the treatment of acidic corneal burns. As a result, although there was no statistical difference between the groups, it was concluded that ozone may be more effective in the treatment of acidic corneal burns regarding the numerical evaluation.
Spadea et al. have examined the effect of the ozonized oil on the cornea irritation during recovery period.7 Thus, they have suggested the usage of medically formalized ozonized oil to avoid corneal irritation during healing period of corneal damages. However, the formulation and preserving of ozonized oils can be more expensive, and desired recovery rate lower due to the physical and chemical properties of ozone. Especially, bi-distilled water was preferred in the ozonization in order not to cause a secondary irritation in cornea in the present study. In addition, bi-distilled water was ozonized before application to diffuse high quality ozone on corneal damage and accelerate the healing process. It is thought that this procedure is cheaper and has more positive effect in treatment of HFA corneal burns.
Varol has measured the level of ozone saturation in bi-distilled water.28 Furthermore, Varol et al. in another study, have emphasized that the half-life of ozone is very short at room temperature. Therefore, they have suggested that the application of ozonated water should be made immediately after ozonization.13 In the present study bi-distilled water was ozonized for 10 min and maximum ozone concentration obtained as reported by Varol.28 Considering the application time interval according to Varol,28 the average of 20.00 µg O3 mL-1 ozone was used per animal in experimental group. This situation revealed that the importance of considering the application time and the amount of ozone to be used in future studies.
As a result of this study, it was thought that locally used ozone therapy had a positive effect on the healing of HFA corneal burns. It was concluded that more ozone related studies should be done to enlighten the subject.
Conflict of interest
None.
Acknowledgments
This study was supported Scientific Research Projects Coordination Unit, Erciyes University, Kayseri, Türkiye (Project Number: TYL-2020-10056).
References
- 1.Müftüoğlu IK, Akova YA, Çetinkaya A. Clinical spectrum and treatment approaches in corneal burns [Turkish] Turk J Opthalmol. 2015;45(5):182–187. doi: 10.4274/tjo.99267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altan S, Oğurtan Z. Dimethyl sulfoxide but not indomethacin is efficient for healing in hydrofluoric acid eye burns. Burns. 2017;43(1):232–244. doi: 10.1016/j.burns.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Spöler F, Frentz M, Först M, et al. Analysis of hydrofluoric acid penetration and decontamination of the eye by means of time-resolved optical coherence tomography. Burns. 2008;34(4):549–555. doi: 10.1016/j.burns.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Liu X. Effect of ozone therapy on cell apoptosis and angiogenesis in retina tissue of diabetic retinopathy rats. J Hainan Med Univ. 2016;22(10):1–4. [Google Scholar]
- 5.Altınbilek T, Kaya E, Uyar M, et al. Ozone therapy for treatment of wounds [Turkish] Interg. Tıp Derg. 2014;2(2):44–48. [Google Scholar]
- 6.Kaya A, Sonmez M, Kar T, et al. Efficiency of ozone therapy in a rat model of experimental uveitis. Ocul Immunol Inflamm. 2017;25(5):695–700. doi: 10.3109/09273948.2016.1161057. [DOI] [PubMed] [Google Scholar]
- 7.Spadea L, Tonti E, Spaterna A, et al. Use of ozone-based eye drops: a series of cases in veterinary and human spontaneous ocular pathologies. Case Rep Ophthalmol. 2018;9(2):287–298. doi: 10.1159/000488846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatipoğlu F, Ogurtan Z, Sezer AD, et al. Effect of laminarin and chitosan gel formulations on the treatment of hydrofluoric acid induced corneal burns in the rabbits. Revue Méd Vét. 2008;159(4):207–214. [Google Scholar]
- 9.Lee CM, Jung WK, Na G, et al. Inhibitory effects of the platelet-activating factor receptor antagonists, CV-3988 and Ginkgolide B, on alkali burn-induced corneal neovascularization. Cutan Ocul Toxicol. 2015;34(1):53–60. doi: 10.3109/15569527.2014.903573. [DOI] [PubMed] [Google Scholar]
- 10.Sancak IG, Bozkurt MF. Healing effect of cornea conjunctival transposition (CCT) technique on corneal healing in New Zealand rabbits (Oryctolagus cuniculus) [Turkish] Ankara Üniv Vet Fak Derg. 2010;57(4):235–240. [Google Scholar]
- 11.Singh P, Tyagi M, Kumar Y, et al. Ocular chemical injuries and their management. Oman J Ophthalmol. 2013;6(2):83–86. doi: 10.4103/0974-620X.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghadam MH, Jafarinasab MR, Yousefi Z, et al. Aloe vera gel-derived eye drops for alkaline corneal injury in a rabbit model. J Ophthalmic Vis Res. 2020;15(1):7–15. doi: 10.18502/jovr.v15i1.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varol K, Koç AN, Çakır Bayram L, et al. Studies on the effectiveness of ozone therapy on the treatment of experimentally induced keratitis with Candida albicans in rabbits. Semin Ophthalmol. 2022;37(2):253–264. doi: 10.1080/08820538.2021.1995006. [DOI] [PubMed] [Google Scholar]
- 14.Ollivier FJ. Bacterial corneal diseases in dogs and cats. Clin Tech Small Anim Pract. 2003;18(3):193–198. doi: 10.1016/s1096-2867(03)90016-8. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo-Martin E, Gallego-Muñoz P, Mar S, et al. Dynamic changes of the extracellular matrix during corneal wound healing. Exp Eye Res. 2019;186:107704. doi: 10.1016/j.exer.2019.107704. [DOI] [PubMed] [Google Scholar]
- 16.Yi Q, Zou WJ. The wound healing effect of doxycycline after corneal alkali burn in rats. J Ophthamol. 2019;5168652 :10. doi: 10.1155/2019/5168652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EC, Kim TK, Park SH, et al. The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats. Acta Ophthalmolog. 2012;90(7):e540–e546. doi: 10.1111/j.1755-3768.2012.02496.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoffart L, Matonti F, Conrath J, et al. Inhibition of corneal neovascularization after alkali burn: comparison of the different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Exp Opthalmol. 2010;38(4):346–352. doi: 10.1111/j.1442-9071.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick JJ, Enion DS, Burd DA. Hydrofluoric acid burns: a review. Burns. 1995;21(7):483–493. doi: 10.1016/0305-4179(95)93254-h. [DOI] [PubMed] [Google Scholar]
- 20.Uysal B, Demirbag S, Poyrazoglu Y, et al. Medical ozone therapy decreases postoperative uterine adhesion formation in rats. Arch Gynecol Obstet. 2012;286(5):1201–1207. doi: 10.1007/s00404-012-2435-y. [DOI] [PubMed] [Google Scholar]
- 21.Yamanel L, Kaldirim U, Oztas Y, et al. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011;8(1):48–55. doi: 10.7150/ijms.8.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oğurtan Z, Hatipoğlu F, Ceylan C, et al. Treatment of corneal hydrofluoric acid burns in rabbits. Revue Méd Vét. 2002;153(4):269–74. [Google Scholar]
- 23.Barrientez B, Nicholas SE, Whelchel A, et al. Corneal injury: clinical and molecular aspects. Exp Eye Res. 2019;186:107709. doi: 10.1016/j.exer.2019.107709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol. 2020;65(1):1–11. doi: 10.1016/j.survophthal.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Sağsöz H, Topaloğlu U, Güney Saruhan B, et al. Distribution of CD8 and CD68 positive cells in acid corneal burns in rabbit [Turkish] Dicle Üniv Vet Fak Derg. 2018;11(1):22–28. [Google Scholar]
- 26.Schrage NF, Langefeld S, Zschocke J, et al. Eye burns: an emergency and continuing problem. Burns. 2000;26(8):689–699. doi: 10.1016/s0305-4179(00)00044-9. [DOI] [PubMed] [Google Scholar]
- 27.Gerard M, Merle H, Chiambaretta F, et al. An amphoteric rinse used in the emergency treatment of serious ocular burn. Burns. 2002;28(7):670–673. doi: 10.1016/s0305-4179(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 28.Varol K. Determination of maximum ozone concentrations and half-life of ozone in some drinking water and high quality pure water produced in new system devices. Fresenius Environ Bull. 2022;31(2):2285–2294. [Google Scholar]