Abstract
Defined as congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical, differences or disorders of sex development (DSDs) comprise many discrete diagnoses ranging from those associated with few phenotypic differences between affected and unaffected individuals to those where questions arise regarding gender of rearing, gonadal tumor risk, genital surgery, and fertility. Controversies exist in numerous areas including how DSDs are conceptualized, how to refer to the set of conditions and those affected by them, and aspects of clinical management that extend from social media to legislative bodies, courts of law, medicine, clinical practice, and scholarly research in psychology and sociology. In addition to these aspects, this review covers biological and social influences on psychosocial development and adjustment, the psychosocial and psychosexual adaptation of people born with DSDs, and roles for clinical psychologists in the clinical management of DSDs.
Keywords: disorders of sex development, differences of sex development, DSD, intersex, psychological adaptation
WHY A REVIEW OF DSDs?
Medical conditions—or categories of them, as in the case of differences or disorders of sex development (DSDs)1—are rarely the focus of topic reviews in the Annual Review of Clinical Psychology. Yet print, broadcast, and social media have raised public awareness of people identifying as intersex. What is the relationship between intersex and the medical conditions falling under the umbrella term DSD? What are the biological origins of DSDs? And how are DSDs specifically relevant to clinical psychology?
Nonspecialist health care providers and the public are more likely to see and hear the terms intersex or LGBTI than DSD. Similarly, it is likely that people have heard of women Olympians with an XY karyotype who have been disqualified from competition because of “naturally elevated” testosterone (Hirschberg 2019). Additional attention to DSDs/intersex comes from a growing number of US states introducing legislation to ban all genital or gonadal surgery in infants or young children born with intersex traits unless medically urgent (Gardner & Sandberg 2018). There are also reports by international governmental and nongovernmental organizations challenging medical standards of care for people born with medical conditions that fall under the DSD umbrella; some of these standards have been equated to a form of torture (Hum. Rights Watch 2017, Méndez & UN Hum. Rights Counc. 2013). Finally, disputes regarding medical care received by individuals born with atypical sex anatomies have played out in courts of law (Ghorayshi 2017). Western societies demonstrate increasing acceptance of men and women who cross conventional gender and sexual expression boundaries. Yet, when it comes to biological sex, societies lack frameworks—legal or otherwise—to accommodate anatomies that challenge the male-female binary (Ainsworth 2015).
A broad consensus exists among medical societies, patients, and patient advocates that clinical psychologists (and allied behavioral health specialists) should serve as fully integrated members of comprehensive interprofessional teams delivering care to this population (Cools et al. 2018, Lee et al. 2016). The dearth of educational content that can guide otherwise-qualified clinical psychologists in the assessment and care of people with DSDs and their families is justification enough for this topic review. Our goal is to provide readers a primer to the biology and psychosocial aspects of these conditions; we hope this review will spur interest among clinical psychologists to engage in informed discussion about DSDs and become involved in the clinical care of those affected by variations of somatic sex development.
WHAT ARE DSDs?
The term disorders of sex development was introduced at a 2005 consensus conference attended by an interprofessional group of international experts and patient advocates. The conference summary (hereafter referred to as the Consensus Statement) incorporated all variations in sex development under the umbrella of DSDs, defined as “congenital conditions in which development of chromosomal, gonadal, or anatomic sex is atypical” (Lee et al. 2006, e488).
Variations in genetic or hormonal determinants of somatic sex development can result in a newborn with characteristics that do not fit neatly into either the male or female category. It is frequently, but mistakenly, assumed that the term DSD necessarily implies ambiguity in external genital appearance. This is not a definitional requirement. DSDs can present with a wide range of genital phenotypes depending on the specific condition and its expression (Chan et al. 2020). Most children born with DSDs are identified soon after birth because of visible genital differences, health concerns [e.g., a positive newborn screening test for congenital adrenal hyperplasia (CAH)], or discordance between prenatal genetic testing, diagnostic ultrasound, and the newborn’s apparent sex. However, some DSDs may not be detected until later when an inguinal hernia is identified, a girl fails to ever menstruate (i.e., primary amenorrhea), or an adult experiences infertility. A commonly cited estimate of DSD incidence is 1:4,500 live births, but estimates as high as 1% are used when conditions considered borderline for inclusion are counted (Arboleda et al. 2014).
Typical and Atypical Sex Development
Somatic sex development is divided into three major components: chromosomal sex (the complement of X and Y chromosomes), gonadal sex (testes and/or ovaries), and anatomic sex (male and/or female internal and external genitalia). Sex development involves two sequential processes, each controlled by different mechanisms (Chan et al. 2020). Five weeks after conception, human embryos are identical and have the potential to form either male or female anatomy (i.e., we all start out the same).
Sex determination begins at 6 weeks: Sex chromosomes (XX or XY) guide bipotential gonads to develop into testes or ovaries. The SRY (sex-determining region Y) gene on the Y chromosome triggers a cascade of genetic signaling events resulting in development of the testes. Development of ovaries was once believed to be the default process [i.e., an ovary develops in the absence of a Y chromosome (and SRY)]; current understanding shows ovarian development is also an active process requiring activation of genes that promote ovarian, and interfere with testicular, development (Figure 1). A major consequence of deviation in typical sex determination includes development of atypical sex anatomy. A heightened likelihood of gonadal cancer (Hersmus et al. 2017), insufficient production of sex hormones for a spontaneous puberty or maintenance of secondary sex characteristics (Ahmed et al. 2021), and infertility (Finlayson et al. 2017) can be accompanying features.
Figure 1.
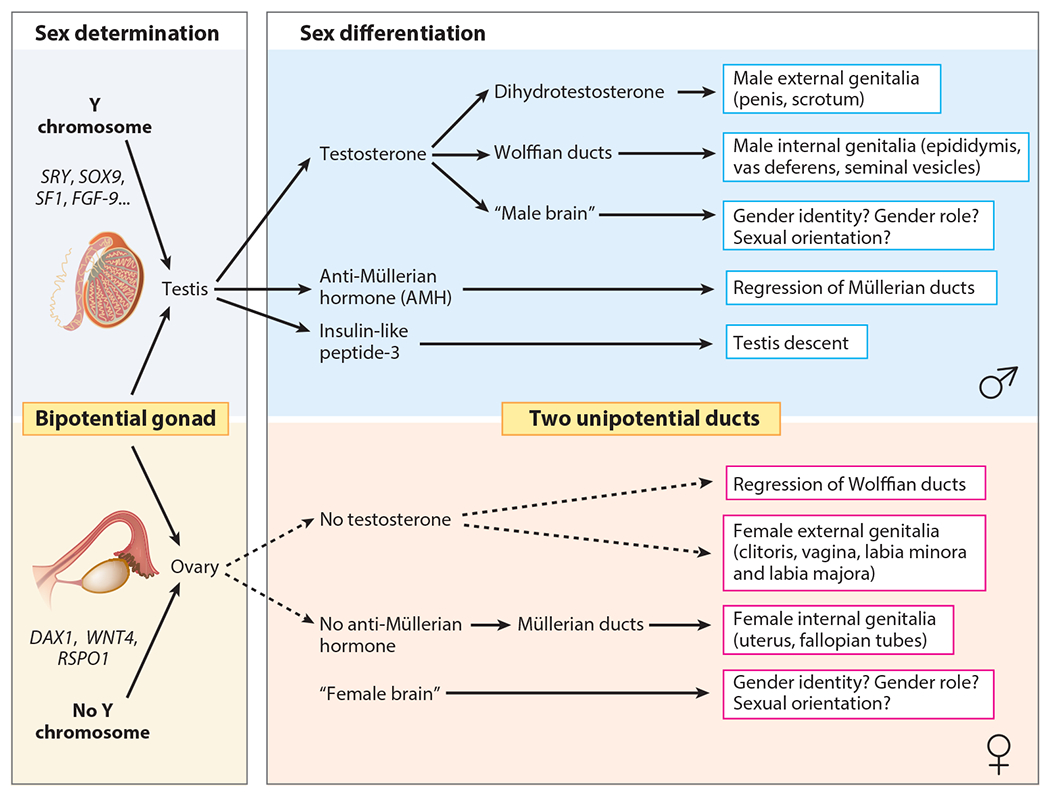
Simplified overview of sex determination and sex differentiation in humans. Figure provided by Eric Vilain, MD, PhD.
The second stage of sex development—sex differentiation—involves growth of the internal genital ducts and external genitalia. Testicular production of the peptide anti-Müllerian hormone (AMH) suppresses Müllerian structure development, the primordia of fallopian tubes, the uterus, and the upper two-thirds of the vagina. In the absence of AMH—as in the case when an ovary develops from the bipotential gonad—female-typical internal structures will differentiate under the control of multiple genes. Testes also produce testosterone, which is responsible for differentiating the Wolffian structures into the epididymides, vasa deferentia, and seminal vesicles (Figure 2).
Figure 2.
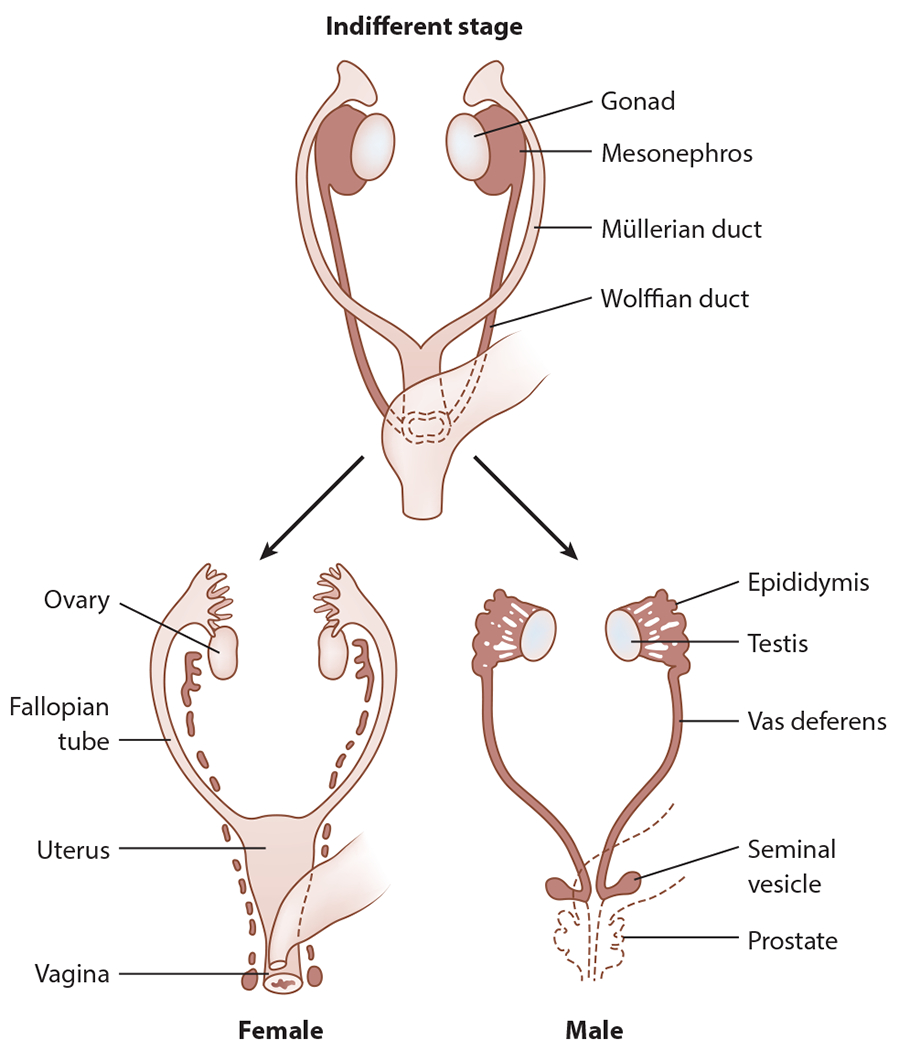
Differentiation of the female and male genital reproductive structures from the primordial Wolffian and Müllerian structures. Figure adapted with permission from Chan et al. (2020).
In contrast to the dual ducts of the internal genitalia—the Wolffian and Müllerian ducts—the external genitalia differentiate from a single anlage. Typically, testicular testosterone is converted into dihydrotestosterone, which is responsible for developing the typical appearance of the penis and scrotum. In the absence of testosterone (or the presence of a defect in the action of androgens at the end organ), the genital tubercle develops into the clitoris, urethral folds form the labia minora, and labioscrotal swellings form the labia majora (Figure 3).
Figure 3.
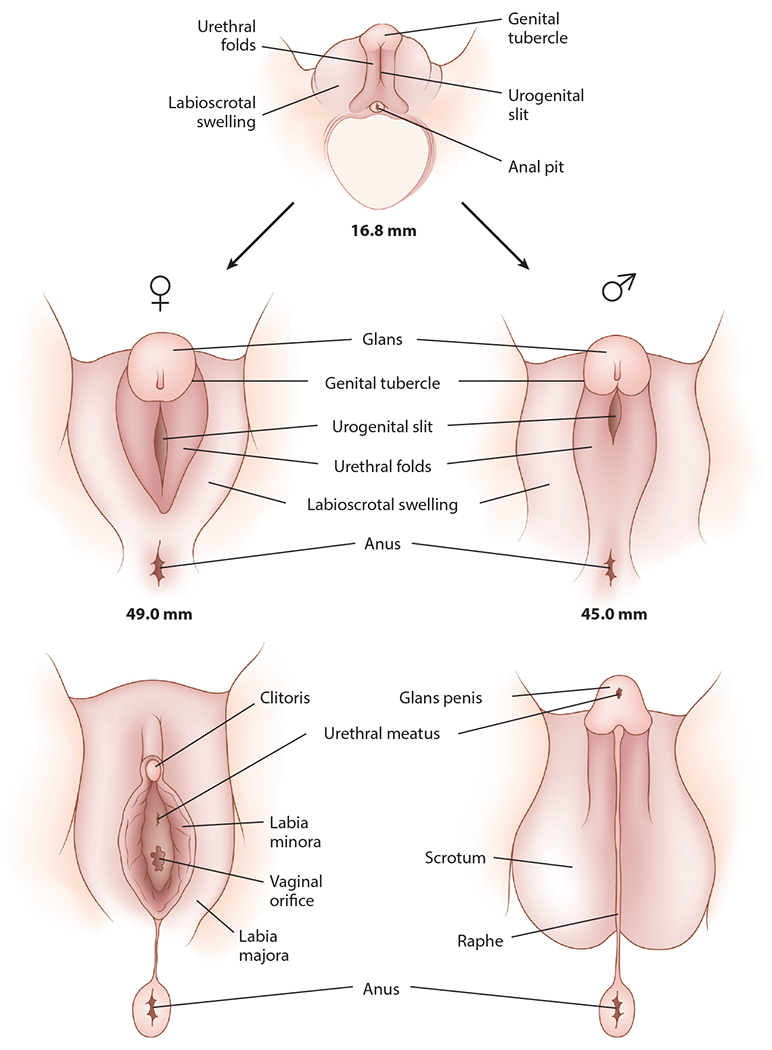
Differentiation of the male and female external genitalia. Figure adapted with permission from Chan et al. (2020).
Sex development culminates with puberty—the emergence of secondary sexual characteristics, a growth spurt, and fertility. At the time of puberty in females, estrogen synthesis by the ovaries stimulates breast and uterine development; over time, hypothalamic–pituitary–gonadal axis activity results in regular menstrual cycles. In males, testicular growth is often the first sign of puberty, typically beginning approximately 6 months after the average age at which breast development begins in girls. Fertility potential for males precedes the growth spurt, development of a mature body habitus, and adult concentrations of testosterone (Styne 2020). DSDs can result in a delayed, incomplete, or entirely absent puberty. Particular DSDs can cause a contrasexual puberty (i.e., physical feminization of children reared as boys through the production of excessive estrogens or virilization of females resulting from the production of excess androgen levels in girls).
Classification of DSDs
DSDs are classified according to karyotype, and when the genetic basis of the disorder is known, clinically descriptive terms combined with the molecular basis of the disorder are added—for instance, “46,XY complete gonadal dysgenesis with SF1 variant” or “46,XX testicular DSD, SRY+.” The Consensus Statement (Lee et al. 2006) classifies DSDs as follows:
46,XY DSD: including disorders of testis development (e.g., partial or complete gonadal dysgenesis, or the presence of both ovarian and testicular tissue, i.e., ovotesticular DSD) and defects in androgen synthesis (e.g., 5α-reductase 2 deficiency) and action (e.g., partial or complete androgen insensitivity syndrome)
46,XX DSD: including disorders of ovary development (e.g., gonadal dysgenesis) or androgen excess (e.g., CAH)
Sex chromosome DSD: represented by variations in the standard sex chromosome complement [e.g., Klinefelter syndrome and variants (47,XXY), Turner syndrome and variants (45,X), sex chromosome mosaicism and variants (45,X/46,XY), chimerism (46,XX/46,XY)]
For additional examples, we refer readers to Chan et al. (2020).
Reactions to DSD Classification and Terminology
Prior to the 2005 consensus conference, the term intersex was commonly used to refer to variations in somatic sex development. Intersex conditions were categorized based on gonadal histology: male pseudohermaphroditism (characterized by atypical external genitalia in the presence of testes), female pseudohermaphroditism (atypical external genitalia in the presence of ovaries), and true hermaphroditism (having both testicular and ovarian tissue regardless of external characteristics). Recognizing major genetic discoveries, and acknowledging that the older terminology was vague, confusing, and experienced as stigmatizing, consensus conference participants recommended change toward the term disorders of sex development. Surveys, scientific meeting proceedings, and clinical literature following publication of the Consensus Statement (Lee et al. 2006) suggested broad acceptance of the new terminology and associated classification scheme by medical and research communities (Davies et al. 2011, Pasterski et al. 2010) and intersex advocacy leadership (Dreger et al. 2005). However, some people born with DSDs and their advocates rejected DSD terminology, proposing alternatives—including differences of sex development, divergence of sex development, and, again, intersex—because of concerns that the word disorder fosters stigma and contributes to some health care providers’ insistence on surgical “normalization” procedures (Davis 2013). Clinicians, meanwhile, may gravitate to the word disorder over difference (and alternatives) over concerns that speaking of somatic sex atypicality as if it always involves benign variation may lead parents, patients, and health care providers to ignore serious health risks that accompany specific DSDs if left unattended, such as absent or arrested puberty (Chan et al. 2020).
Issues of identity further complicate terminology choices. Some people with DSDs adopt intersex as an identity rather than as a term for a medical condition. Others prefer not to be defined by their biology or want to distance themselves (or their children) from words that may imply atypical gender or sexuality. Although those born with DSDs may identify with lesbian, gay, bisexual, and transgender (LGBT) or other gender-nonconforming communities, many view themselves as cisgender (i.e., accepting the boy/man or girl/woman category in which they were reared). While making it convenient to identify societal subgroups that face what may be perceived as comparable forms of discrimination, the increasingly common addition of the letter I (for “intersex”) to LGBT fosters perceptions that these groups are homogeneous and that people subsumed by the acronym have parallel (or even identical) health care needs and concerns. Such unqualified groupings do not reflect clinical reality (Hollenbach et al. 2014, Mazur et al. 2016).
Distinctions Between DSDs and Transsexualism/Transgenderism
Important distinctions between transsexualism/transgenderism and DSDs can become blurred in both popular and scientific literature (see Table 1). People who identify as transsexual or transgender are characteristically born with typical male or female sex chromosomes, gonads, and anatomy but experience a mismatch between their somatic sex and their experienced gender identity. In contrast, a DSD is a consequence of atypical development of sex chromosomes, gonads, or sex anatomy. Although individuals born with DSDs may share experiences with transgender individuals (e.g., gender dysphoria incidence is higher in individuals with DSDs than in the general population), DSDs expose affected persons to saliently different life events. In other words, these are distinct populations; one should not simply apply lessons learned from research and clinical experience with the transgender population to the DSD population (or vice versa).
Table 1.
Comparison of DSDs and transsexualism/transgenderism
| Feature | DSDs | Transsexualism/transgenderism |
|---|---|---|
| Somatic sex characteristics | Atypical/discordant | Typical/concordant |
| Gender of rearing | Occasionally delayed (or reassigned) | Unquestioned and immediate |
| Diagnostic evaluation and medical/surgical care | ||
| a. Involvement of multiple health care providers | Considered a chronic health condition; some DSDs require life-sustaining hormone replacement from birth (e.g., 21-hydroxylase deficiency, CAH) | Variable and dependent on individual decisions regarding gender-affirming medical/surgical interventions |
| b. Surgical decision making and procedures | Feminizing or masculinizing surgery; gonadectomy Timing: at any point after ascertainment of DSD |
Feminizing or masculinizing surgery; gonadectomy Timing: age at majority (with exceptions) |
| c. Chronic medication(s) | Glucocorticoids (e.g., for CAH); hormone replacement therapy (for treatment of hypogonadism); both lifelong | Puberty-blocking hormones; feminizing or masculinizing surgeries; gonadectomy Timing: puberty blockers, peripubertally; otherwise, age of majority (with exceptions) |
| d. Puberty | Spontaneous or absent; isosexual or contrasexual | Spontaneous, but associated with dysphoria |
| Gender dysphoria | Higher than in general population | Always present |
Abbreviations: CAH, congenital adrenal hyperplasia; DSD, difference or disorder of sex development.
From the moment of detection, DSDs trigger a series of events not experienced by individuals without DSDs and consign them and their caregivers to monitoring by health care providers. Immediate concerns focus on determining if the child’s DSD is attributable to classic CAH, a potentially life-threatening condition that requires medication in the newborn period and throughout life (Speiser et al. 2018). If CAH is ruled out and the child’s sex remains unclear, attention turns toward diagnosis to inform the gender-of-rearing decision. Gender reassignment may involve reannouncing the child’s gender to family and friends. Surgical reconstruction considerations may also arise early in life (Mouriquand et al. 2016). In specific 46,XY DSD, children reared as girls will likely develop contrasexual secondary sex characteristics at the time of puberty unless endogenous hormone secretion is eliminated by removal of the testes or by administering pubertal hormone blockers (Lee et al. 2016, Mouriquand et al. 2016). An additional concern not shared with transgender populations is elevated gonadal cancer risk (Hersmus et al. 2017). The context within which children with DSDs grow and develop is much different from that of children born without such a condition. Accordingly, failure to differentiate between individuals with and without a clearly identifiable DSD may hamper research and optimal clinical care.
BIOLOGICAL INFLUENCES: HORMONES, GENES, AND PUBERTAL TIMING
Natural Experiments
The study of biological influences on human development makes use of “natural experiments,” wherein exposure to atypical hormone levels occurs for reasons other than experimental manipulations (e.g., O’Reilly et al. 2010, Sandberg et al. 2003). Because the pathogenesis of a DSD involves departure from typical sex determination or differentiation pathways, those with DSDs have been studied as natural experiments to better understand downstream effects on psychological development and adjustment—in particular, gendered behavior (Berenbaum & Meyer-Bahlburg 2015). Gender role refers to behaviors and attitudes that differ in frequency or level between males and females in a specific culture and period of time. Influenced by genetics, fetal and neonatal testosterone exposure, socialization by parents, peers, teachers, and others, and self-socialization based on cognitive developmental processes associated with gender, the gendering process begins at the moment of conception and continues through the life span (Hines 2020). The most extensively studied DSD is 46,XX classic CAH, a genetic condition that results in exposure to high levels of androgens beginning early in gestation (Claahsen-van der Grinten et al. 2021).
Influence of Sex Hormones
Sex hormones (e.g., androgens and estrogens) influence behavior via two routes: organizational and activational effects (Arnold & McCarthy 2016). Organizational effects occurring early in development cause permanent changes to brain structure and physiology that produce long-lasting changes to behavior. Activational effects produce temporary changes to the brain, affecting behavior only when sex hormones are present (Berenbaum & Meyer-Bahlburg 2015).
A substantial experimental research literature in nonhuman animals demonstrates that sex hormones present during early development produce long-lasting effects on behaviors that exhibit sex differences—for instance, learning and memory, aggression, play, and sexual behavior (Berenbaum & Beltz 2011). Early programming influences of sex hormones occur during species-specific sensitive periods of prenatal or neonatal life; sex hormones do not have the same effect if exposure occurs before or after this period. There is strong evidence human behavior is also influenced by hormones present during prenatal development, particularly androgens. Sex hormones continue to organize the brain well beyond the prenatal and early neonatal periods, with later periods of brain organization building upon and refining neural circuits established during earlier development (Berenbaum & Beltz 2011). Pubertal hormones may have immediate and temporary effects on the brain and behavior and/or reorganize a brain system (as they do during prenatal development), in which case hormones would be required to initiate or modulate the behavior but not to maintain it (Berenbaum et al. 2015). Behaviors likely to be organized by sex hormones during prenatal and pubertal development include psychological changes in adolescence (e.g., increased risk-taking in early adolescence, sex differences in internalizing symptoms with greater anxiety and self-esteem problems in girls than in boys), sex-typed activity interests (e.g., occupational choice), gender identity, sexual orientation, cognitive abilities, and behavior problems (for reviews, see Berenbaum & Meyer-Bahlburg 2015, Berenbaum et al. 2015). Depending on the particular DSD, there may be variations in these expected effects; for instance, rates of gender change appear to be higher in individuals with 46,XY DSD reared as girls who go on to experience a contrasexual puberty (e.g., 5α-reductase type 2 deficiency and HSD17B3 deficiency) (Cohen-Kettenis 2005), whereas in 46,XY complete androgen insensitivity syndrome, increases in testosterone associated with puberty do not result in typical androgen-related behavior changes as the body is insensitive to the effects of androgens (Mazur 2005).
Beyond Hormonal Organization and Activation: Direct Genetic Influences and Sex Chromosome Effects
In rodent studies, sex differences have been identified in the brain prior to gonadal differentiation—that is, before the developing fetus can be identified as male or female other than by the sex chromosomes (Dewing et al. 2003). This finding suggests evidence for a direct effect of genetics on the brain and, potentially, on behavior.
Mice are the preferred species for experimental studies in which the genetic and hormonal influences on behaviors that exhibit sex-related variability are separated. These studies have shown direct influences of sex chromosomes on a wide range of behaviors, including aggression, parenting behavior, juvenile social interaction, learning and cognition, and partner preference (Cox et al. 2014). Investigations such as these were spurred by development of a line of mice in which genetic and gonadal sex were separated by moving the Sry gene (which codes for testis development) to an autosome and deleting it from the Y chromosome. This led to XY mice with ovaries and XX mice with testes, which allows investigators to independently evaluate gonadal and sex chromosome effects by comparing, for example, XX females with testes to XX females with ovaries (McCarthy 2017).
Pubertal Timing
Puberty is a complex series of processes involving growth and development resulting in reproductive maturity and adult anatomy and physiology. The timing, tempo, and order of development may vary across individuals, and significant deviations from one’s peer group serve as a risk factor for maladaptation (Berenbaum et al. 2015). In a DSD, typical progression of all or some aspects of pubertal development may be early, delayed, or absent; differences between one’s development and that of one’s peers can be particularly stressful and result in inadequately resolved interpersonal dilemmas—for instance, lies about repeated doctor visits, menstruation, and medications—which appropriate support and counseling can help address (Howe 2021).
SOCIAL INFLUENCES ON PSYCHOSOCIAL DEVELOPMENT AND ADJUSTMENT
Parenting a Child with a DSD
Parents are tasked with promoting children’s physical, emotional, social, and intellectual development. The “normal rules” of parenting apply in DSDs (e.g., parenting styles/capacities and their relationships to long-term outcomes in children). Added to this, when parents learn their child has a DSD, they can feel upset and overwhelmed (Delozier et al. 2019). Despite this experience, parents are integral members of their children’s health care team.
Parenting styles, practices, and capacities.
Characterized in terms of parental responsiveness and demandingness, parenting styles reflect the emotional climate in which children are raised (Baumrind 2005). There is no reason to believe that overarching styles are fundamentally different for parents of children with DSDs; however, parents of chronically ill children are at increased risk for maladaptive parenting practices that are, in turn, associated with children’s poor behavioral and social outcomes (Wolfe-Christensen et al. 2014). A growing area of research examines relationships between child DSD characteristics, parental adaptation, and clinical management decisions (e.g., Kirk et al. 2011, Suorsa et al. 2015). The psychosocial challenges DSD patients share with children affected by other congenital body anomalies or chronic diseases (Kirk et al. 2011, Meyer-Bahlburg 2008) warrant a review of parenting in chronic illness, supplemented by issues and challenges specific to DSDs.
Parenting in the context of pediatric chronic illness.
Research generally shows few differences on measures of general adaptation or psychopathology between groups within the chronic illness population (Wisniewski & Sandberg 2015), suggesting that challenges associated with chronic illness generally outweigh those associated with specific conditions or management approaches. Added parental responsibilities of providing care for children with chronic illnesses affect family functioning (including finances and family relationships), contribute to parental anxiety, stress, and depression, and affect parental quality of life (Wisniewski 2017). Parents are at risk for perceiving their children as more vulnerable and overprotecting them (Cousino & Hazen 2013, Fedele et al. 2010). Compared with fathers, mothers report greater impact of their child’s illness on their own adjustment, presumably since mothers typically shoulder more burden of care (Rolston et al. 2015, Wisniewski 2017). Together, these issues affect parenting capacities—parents’ behaviors toward, and beliefs about, their child—which are predictive of children’s poorer emotional, behavioral, and social adjustment (Colletti et al. 2008, Holmbeck et al. 2002).
Adaptation to stress is expressed as resiliency—a process wherein positive adaptation is achieved despite significant adversity. As applied to trajectories of parental adjustment in pediatric chronic illness, research demonstrates a consistent tendency toward initial moderate levels of distress that diminish over several months to a year following disease identification or diagnosis (Dolgin et al. 2007, Perez et al. 2021).
DSD-specific challenges.
Challenges germane (though not exclusive) to DSDs include accessing balanced and authoritative information, stress associated with the birth of a child for whom gender of rearing is not obvious, anticipated stigma, and evaluating what constitute the best interests of the child in the process of clinical decision making.
Information that is lacking, conflicted, and anxiety-provoking.
Parents are often unaware DSDs exist until they receive their child’s diagnosis (Crissman et al. 2011). Unfamiliarity with DSDs, lack of clear information, and cognitive confusion contribute to stress and feelings of isolation (Pasterski et al. 2014). Conflicting clinical management strategies contribute to and complicate information-collecting and promote parental distress (Magritte 2012). The interplay of information and anxiety also poses a challenge: Having information can cause anxiety and confusion; seeking information can counter anxiety (Chan et al. 2019).
Stress.
Birth of a child with a DSD and uncertainties about the child’s gender and psychosexual development are believed to be extraordinarily stressful (Chase 2003, Crissman et al. 2011), with conditions exerting sizeable strain on families (e.g., Fedele et al. 2010). Cultural understandings about biological sex can contribute to parental distress, confusion, uncertainty, and trauma as the birth of a child with sexual ambiguity may violate deeply held world views and elicit feelings of shame and guilt (Chase 2003). Posttraumatic stress symptoms rise to levels consistent with post-traumatic stress disorder in 18–31% of parents (Pasterski et al. 2014, Wolfe-Christensen et al. 2017). Parents also report that inadequate information or information overload, medical jargon and dismissive comments from providers, strong negative emotions (e.g., fear, guilt, uncertainty, shock, disbelief), and feeling overwhelmed (Boyse et al. 2014, Sanders et al. 2008) all result in difficulty processing their child’s diagnosis. Specific to the newborn period, concerns include chaotic delivery room environments, medication side effects, and sleep deprivation contributing to difficulty processing information (Chan et al. 2019); complications associated with pregnancy, delivery, and prematurity; and maternal emotional vulnerability (Duguid et al. 2007).
Stigma.
Having a child born with atypical genitalia is often perceived as problematic and brings about changes in parental roles, responsibilities, goals, and social status (Sanders et al. 2008). Parents worry sharing information about their child’s condition will lead to rumors, gossip, and teasing, resulting in isolation and withdrawal from usual support systems (Crissman et al. 2011, Duguid et al. 2007, Wisniewski 2017). Decisions in favor of early genital surgery can stem from perceived or anticipated stigma, as parents feel pressure to resolve this psychosocial problem through surgical intervention. Downstream effects of secrecy include shame experienced by the child and/or withholding of information about the condition from the child (Crissman et al. 2011, Sanders et al. 2012). A child’s right to privacy must be balanced against risks associated with secrecy. Failure to achieve balance contributes to parental guilt and family isolation.
Ethics.
A major issue in the clinical management of DSDs involves elective genital or gonadal surgery. In 2018, the American Academy of Pediatrics reaffirmed its endorsement of “patient- and family-centered care”—core principles include shared decision making (SDM) involving the family and child (AAP Comm. Hosp. Care & Inst. Patient Fam.-Cent. Care 2012). Legal and ethical questions about such policies’ applicability to genital surgery are raised by others on the basis of children’s bodily autonomy and “right to an open future” (Feinberg 1980, p. 124; Greenberg 2017; Hum. Rights Watch 2017). Less often considered are risks or outcomes associated with performing surgery later in life. One argument posits that deferring decisions until the age of consent closes an important window of opportunity for the child. For a thorough review, we refer readers to Wiesemann et al. (2010).
Legislative and Legal Factors
Legislative changes and seminal legal cases have the potential to affect clinical management strategies and one’s day-to-day lived experience (ISNA 2008). In the United States, legislative change efforts have been perhaps most visible within the California Senate. Following the 2018 Senate Concurrent Resolution 110, advocates—including InterACT, a nonprofit with a mission statement “[to use] innovative legal and other strategies, to advocate for the human rights of children born with intersex traits” (https://interactadvocates.org/about-us/mission-history/)—supported legislation (SB-225: the Intersex Bodily Autonomy, Dignity and Choice Act) to prohibit “sex organ modification procedures on an individual born with variations in their physical sex characteristics who is under 12 years of age unless the procedure is a surgery required to address an immediate risk of physical harm” (Calif. Legis. 2021). After three consecutive years of work to pass this bill, it was withdrawn in early 2022; subsequently, an unrelated bill assumed the numeric identifier SB-225. Advocacy organization sponsors expressed disappointment and reaffirmed their intent to continue with efforts in this area (Wiener 2022). Prior to these legislative efforts, activists who equated genital surgery to torture worked with the United Nations (UN) High Commissioner for Human Rights and the UN Special Rapporteur on Torture and Other Cruel, Inhuman or Degrading Treatment or Punishment to “repeal any law allowing intrusive and irreversible treatments, including forced genital-normalizing surgery” (Méndez & UN Hum. Rights Counc. 2013, p. 23).
These efforts are not widely supported by patient and family support organizations or by health care professionals. The CARES (Congenital Adrenal Hyperplasia Research Education and Support) Foundation, a nonprofit that “leads in the effort to improve the lives of the Congenital Adrenal Hyperplasia community and seeks to advance quality health care through advocacy, education, research and support” (https://caresfoundation.org/our-mission/), sharply opposes blanket legislative bans. Instead, “CARES recognizes that any surgical decision, including timing, for some girls born with CAH is a deeply personal one to be made by the family in consultation with a multi-disciplinary team of CAH experts and evidence-based data” (https://caresfoundation.org/treatment-surgery/). When asked to “affirm that medically unnecessary surgeries in individuals born with differences of sex development are unethical and should be avoided until the patient can actively participate in decision-making” and to “oppose the assignment of gender binary sex to infants with differences in sex development through surgical intervention outside of the necessity of physical functioning for an infant and [recognize that] children should have meaningful input into any gender assignment surgery,” in 2019, the Council on Ethical and Judicial Affairs of the American Medical Association (AMA) declined to do either (AMA 2019, p. 1). The resulting white paper affirmed that health care decisions for children are made in the context of a three-way relationship among patient, parents (or guardians), and physician and that, as the persons best positioned to understand their child’s unique needs and interests, parents/guardians are ethically and legally expected to make health care decisions in their children’s best interests. The Pediatric Endocrine Society (PES) issued a 2020 position statement on genital surgery in individuals with DSDs that states that “there can be no single approach to individuals with DSD conditions. The PES opposes government bans on genital surgery for DSD because legislation cannot integrate the myriad of factors that determine the choices for any specific individual” (PES 2020, p. 2).
Efforts at curbing early surgery have also been made through the courts. There was a well-publicized legal case involving a young child with ovotesticular DSD in which the plaintiffs claimed there had been inadequate informed consent for the surgery. The adoptive parents of the child were represented in court by the Southern Poverty Law Center and received legal counsel from the intersex advocacy organization InterACT (Ghorayshi 2017, SPLC 2013). The case, ultimately, settled out of court.
In addition to legal challenges to genital surgery during childhood, decisions about legal sex exert large downstream effects. Advances in the genetics of sex development show there is no one biological parameter that clearly defines sex. Nevertheless, traditional options on legal documents are either “M” (male) or “F” (female) (ISNA 2008). Some countries have started to include a third category (Lee et al. 2014)—for instance, “X” for “Indeterminate/Intersex/Unspecified” in Australia (Aust. Gov. 2013) and “gender diverse” in New Zealand (NZ Gov. 2021). Not all countries or systems recognize a third sex; consequently, one may be required to declare themselves as either M or F elsewhere.
Society: The Person, Peers, and Providers
Personal experiences across the life span, including interactions with family, peers, and providers, serve as powerful social influences on adjustment. These include limited information (or exposure to misinformation) about the condition and its implications, inaccessibility of specialized services, religious and cultural factors, stigma and shame, and care experienced as nosocomial abuse and trauma.
Information and resources.
Information deficits extend from birth through childhood (Crissman et al. 2011, Magritte 2012) and into adulthood (Karkazis 2008, MacKenzie et al. 2009). These can include a lack of education and inaccessible medical care—particularly when financial concerns, such as the high cost of medication or travel from afar to reach specialty care providers, constitute primary burdens (Lee et al. 2014). Limited understanding of DSD conditions in the general population contributes to distress in affected adults and can be associated with a feeling of being different, further contributing to secrecy and shame (Alderson et al. 2004, MacKenzie et al. 2009). Participation in resource, support, or advocacy groups can be empowering as well as painful and emotionally taxing as an individual learns more about their condition; referral to and engagement with groups are generally low (Alderson et al. 2004, Baratz et al. 2014).
Religion and culture.
Religion and culture have been shown to influence clinical management decisions and quality of life for those affected by DSDs. The marriage imperative varies greatly by culture and religion (Warne & Raza 2008). Plausible deniability of suspected or known infertility and/or heritable conditions may affect decisions regarding the extent to which full assessment and diagnosis of a suspected DSD are made. Further, some cultures in the Middle and Far East and elsewhere favor male over female offspring, and these views have been reported to influence gender assignment in DSDs (Lee et al. 2014).
Stigma, secrecy, and shame or feeling different.
Stigma, isolation, and shame are not limited to parents (Alderson et al. 2004). Because anatomic differences in DSDs are not directly observable by others, parents may believe that secrecy can avert stigma, and, in the past, caregivers may have received backing from health care providers in withholding information about the child’s DSD—not only from those outside the immediate family but also from the child—by concealing some particularly sensitive details (e.g., karyotype, gonadal status, fertility potential) (Brinkmann et al. 2007, Lossie & Green 2015). Thus, a foundation for these experiences, set in early childhood, may continue throughout adult life.
Nosocomial trauma.
Medical management standards in DSDs include promoting positive psychological adaptation, achieving full sexual function through surgical habilitation, and preserving gonadal function and fertility if possible. Health care services for those with DSDs and their families are designed to prevent or minimize maladaptive responses to the diagnosis and effects of the condition (Alderson et al. 2004). These goals, however, have not always been fulfilled.
An interview with those affected by androgen insensitivity syndrome showed the most distressing aspects of medical interventions were related to psychological sequelae of physical treatments; for example, few felt prepared for the discomfort of a demonstration of vaginal dilators, others reported the rationale for the treatment itself was not always discussed, and most mentioned secrecy within their medical management. Secrecy included a range of limited information-giving, omission of information, and explicit directions to other professionals to keep the diagnosis from patients (Alderson et al. 2004). Further, reports exist of nosocomial abuse (Chase 2003, Howe 2021, Money & Lamacz 1987) and trauma generated by the health care system itself.
Given the rarity of many DSDs, genital examinations have historically been used as training opportunities for medical students, residents, and other medical colleagues. The Consensus Statement noted that “repeated examination of the genitalia, including medical photography, may be experienced as deeply shaming” (Lee et al. 2006, p. e493). Despite this admonition, a 2017 survey of 22 US medical centers providing care for patients with DSDs found that less than half of the sites reported setting a maximum number of providers/trainees to be present during genital exams. Though the majority (71%) of sites reported that they “never” perform genital exams on awake patients primarily for education, the 29% that did report this practice “at least some of the time” remains an area of concern (Rolston et al. 2017). Most recently, Howe (2021) conjectured that reluctance and failure among clinicians to bring up subjects that patients may find pertinent may cause some patients to read into the omission that there is an underlying reason the clinician did not bring such subjects up. Patients may fear that the reason is that there is something wrong with them and conclude that they should feel shame.
PSYCHOSOCIAL AND PSYCHOSEXUAL ADAPTATION OF PEOPLE BORN WITH DSDs
The term psychosocial is used broadly to refer to psychological/psychosocial/behavioral variables exclusive of gender and sexuality. Psychosexual encompasses gender identity (i.e., identifying oneself as either girl/woman, boy/man, or nonbinary gender), gender role (i.e., behaviors and attitudes that differ in frequency or level between males and females in a particular culture and time), sexual function, and sexual orientation [i.e., sexual arousal to individuals of the same sex (homosexual), opposite sex (heterosexual), or both sexes (bisexual), or a lack of sexual attraction to others of any gender (asexual)]. Not covered by these broad categories are neuropsychological aspects of specific DSDs, particularly the extensively studied neurocognitive profiles of Klinefelter and Turner syndromes (Gravholt et al. 2017, van Rijn 2019). Because of space constraints, features of these sex chromosome DSDs are not covered here except to note that the neurocognitive profiles associated with these particular conditions predispose individuals to significant psychosocial difficulties (Gravholt et al. 2017, van Rijn 2019, van Rijn & Swaab 2020, Wolstencroft & Skuse 2019).
Methodological Considerations
Interpretation of clinical research findings is conditioned by the quality of the studies being summarized. Investigations in this space range from case reports to investigations employing quantitative, qualitative, or mixed methods that have been conducted in large samples. Participants have been identified and recruited using a variety of strategies: medical chart review (e.g., Hanauer et al. 2014), DSD health care specialists’ referral (e.g., endocrinologists, gynecologists) (e.g., Schweizer et al. 2009), requests to patient/family peer support organizations representing specific or combined DSDs (e.g., Lampalzer et al. 2020), advertising on social media platforms (e.g., Schlomer et al. 2014), and combinations thereof (e.g., Schönbucher et al. 2012). Representative sampling is hampered because most DSDs are individually rare conditions and historically associated with shame and stigma. It is therefore difficult to ascertain how representative any given sample is of the population of patients—with particular concern that participants differ in outcomes from those who cannot be located or elect not to participate (Erens et al. 2014).
Methods adopted to assess psychosocial and psychosexual function are notable for their diversity and absence of a core set of measures. Studies of psychological endpoints have been conducted internationally and in diverse cultural settings (e.g., Banu & Chowdhury 2020, Ediati et al. 2015, Jürgensen et al. 2013, Khorashad et al. 2018, Warne et al. 2005). Most of the studies are cross-sectional; many combine varying developmental stages and examine a variety of DSDs in the aggregate (e.g., de Neve-Enthoven et al. 2016, Johannsen et al. 2006, Schützmann et al. 2009). Finally, many studies use reference values for standardized measures as an alternative to an internal comparison/control group, limiting the opportunity to statistically control for potentially confounding demographic background variables (e.g., Brinkmann et al. 2007, de Neve-Enthoven et al. 2016, Schweizer et al. 2017).
Until the 2006 publication of the Consensus Statement, research elucidating the influence of atypical sex hormone exposure during steroid-sensitive periods of brain development represented a major focus (e.g., Berenbaum et al. 2000, Collaer & Hines 1995, Hines 2004, Hines et al. 2004). DSDs (in particular, 46,XX CAH) were (and continue to be) studied as natural experiments for testing hormonal hypotheses related to the influence of early prenatal sex hormone exposure on behaviors exhibiting sex-related variability (Stout et al. 2010). In more recent years, studies of psychological adaptation in people affected by DSDs have examined a broader range of outcomes and moderating variables that is similar to that seen in health psychology more generally (e.g., Callens et al. 2021, Perez et al. 2021).
Generalized Observations
Notwithstanding the heterogeneity of research on psychosocial and psychosexual adaptation in DSDs, several themes emerge.
Stigma.
Across specific DSDs, reluctance to share details about the condition is common. It begins with parents withholding information from extended family and close friends (Crissman et al. 2011, Lee et al. 2016, Sanders et al. 2012) because of perceived and/or anticipated stigma (Rolston et al. 2015). Experiences of shame and stigma are commonly reported (e.g., Meyer-Bahlburg et al. 2018, Meyer-Bahlburg et al. 2017). Ironically, the very efforts employed in the hope of avoiding stigmatization (i.e., secrecy) may actually engender internalized stigma (MacKenzie et al. 2009, Meyer-Bahlburg et al. 2017). According to former patients and advocates, stigma, shame, and secrecy are more strongly predictive of psychological outcomes than the objective severity of the DSD condition or questions about gender (Chase 2003). The degree to which the experience of shame and secrecy potentially mediates the relationship between the medical condition and psychosocial/psychosexual outcomes remains to be investigated.
Increased psychological and social problems.
Whereas some studies of the psychosocial adaptation of people with DSDs have reported outcomes indistinguishable from those in comparison groups (e.g., Messina et al. 2020), others have reported elevated emotional distress and impairment (e.g., de Vries et al. 2019, Godfrey 2021, Hansen-Moore et al. 2021, Ortqvist et al. 2019). Psychiatric and social morbidities are clearly higher among those with the sex chromosome DSD Klinefelter or Turner syndrome—conditions in which there are demonstrable effects on brain development and function beyond what might be attributable to the early influences of sex hormone exposure (Davenport et al. 2020, Gravholt et al. 2018). In the case of 46,XY DSD and 46,XX DSD, it is difficult to synthesize the findings into general statements because of the heterogeneity of samples, methods, and findings. Nevertheless, recent reviews examining psychosocial morbidities and large cohort studies of those in either DSD category, reared as males or females, suggest that anticipatory guidance and surveillance of psychosocial adaptation across developmental stages are prudent because of heightened risk factors and reports suggesting dysfunction (Falhammar et al. 2018, Godfrey 2021, Rapp et al. 2018).
Experiences and preferences regarding reconstructive surgery.
One of the most contentious areas of DSD care involves urogenital surgery and, if performed, when and who decides. There are increasing activist calls for a ban on any surgeries performed prior to individuals being able to meaningfully decide for themselves (Hum. Rights Watch 2017). These demands are based in part on reports of individuals who have experienced significant complications from surgical procedures performed in early childhood (Crouch et al. 2008, Long & Canning 2016, Long et al. 2017). These negative outcomes notwithstanding, multiple other studies indicate that the majority of those who have undergone surgery are largely satisfied with outcomes and believe that surgery in early life is preferable to deferring until the patient can make their own decision (Bennecke et al. 2021, Binet et al. 2016, Fagerholm et al. 2011, Nordenskjold et al. 2008, Rapp et al. 2021).
Psychosexual milestones and sexual function.
Sexuality in adolescence is a normative and essential part of human development that provides opportunities for companionship, exploration, and intimacy. Factors disrupting the emergence of psychosexual milestones (e.g., holding hands, kissing, sexual petting) (O’Sullivan et al. 2007) can exert lasting effects on the expression of an individual’s sexuality (Haydon et al. 2014). DSDs have been shown to be associated with delayed (Avellan 1976, Meyer-Bahlburg 2014) and arrested (Gravholt et al. 2017, Skakkebaek et al. 2018) patterns of sexual relationships.
Dissatisfaction with sexual function.
Many studies have reported sexual discomfort and dysfunction accompanying DSDs (Köhler et al. 2012, Kreukels et al. 2019). Insofar as early genital surgery has been a feature of standards of care in DSDs, participants in long-term follow-up studies have generally received surgery. Those reports suggest that sexual dysfunction is common, and complications of surgery are believed to be one of or the major contributing factor (Crouch et al. 2008, van de Grift et al. 2021). Notwithstanding suggestions of a causal relationship between early genital surgery and adult sexual function, sexual dissatisfaction and dysfunction are common in the general population (Bancroft et al. 2003, Dunn et al. 2000, McCool-Myers et al. 2018). Furthermore, it would be counterfactual to assume that outcomes in this domain would be better had surgery not been performed in infancy or early childhood.
Sexual orientation.
Most individuals with 46,XY DSD, reared as girls, report a heterosexual orientation irrespective of the extent of prenatal androgen exposure as indexed by the degree of genital masculinization (Wisniewski et al. 2019). Similarly, individuals with 46,XY DSD reared as boys have reported almost exclusively heterosexual orientation (Bouvattier et al. 2006, Callens et al. 2016). The same pattern holds true for women with 46,XX classic CAH, although the proportion of study participants reporting a nonheterosexual orientation is higher than in the general population (Gondim et al. 2018).
Gender dysphoria.
Unlike atypical gender-role behavior or a nonheterosexual orientation, which are not targets of clinical care, there are good reasons for wanting to avoid rearing in a gender that increases the likelihood that the person will experience gender dysphoria. In DSDs, the most common finding is that gender identity follows the gender of rearing regardless of sex chromosome complement or prenatal androgen exposure (e.g., Callens et al. 2016). But there are exceptions. In individuals with 46,XX DSD caused by classic CAH and reared female, estimates of self-initiated gender change are approximately 5% (Babu & Shah 2021). The recommendation of rearing as girls individuals with 46,XY DSD caused by complete androgen insensitivity syndrome (Babu & Shah 2021, Mazur 2005) or complete 46,XY gonadal dysgenesis (Jürgensen et al. 2013, Kreukels et al. 2018) is strongly supported by studies showing gender identity stability across the life span. Self-initiated gender change in individuals with 46,XY partial androgen insensitivity syndrome depends on the gender of rearing: 12% and 25% in those reared as girls and boys, respectively (Babu & Shah 2021). Nevertheless, a trend exists for individuals with this diagnosis to be reared as males, even after accounting for associations between external genital appearance and gender of rearing (Kolesinska et al. 2014). Two additional 46,XY conditions caused by defects in androgen biosynthesis are associated with the highest incidence of self-initiated gender change from female to male: In 5α-reductase 2 deficiency and 17β-hydroxysteroid dehydrogenase deficiency type 3, external genital appearance at birth commonly leads to female gender rearing. If the testes remain in place, a contrasexual puberty occurs. Gender reassignment, typically occurring in adolescence or young adulthood, has been reported as high as 56–63% in individuals with 5α-reductase 2 deficiency and in 39–64% in individuals with 17β-hydroxysteroid dehydrogenase deficiency who were reared as girls (Cohen-Kettenis 2005). The incidence of gender change may be far lower if the testes of these girls are prophylactically removed (Chuang et al. 2013, Maimoun et al. 2011).
CLINICAL MANAGEMENT OF DSDs
Model of Care
There are several models of care pertinent to the clinical management of DSDs—namely, those pertaining to how health care specialists work together and with patients and families. In DSDs, interdisciplinary patient- and family-centered care are recommended.
Interdisciplinary care.
Given the complexity seen in DSDs, multiple specialists are routinely involved in their clinical management. Specialties recommended by the Consensus Statement include pediatric subspecialists in endocrinology, surgery or urology (or both), psychology/psychiatry, gynecology, genetics, neonatology, and, if available, social work, nursing, and medical ethics; the core composition of any one team will vary depending on DSD condition, local resources, developmental context, and location (Lee et al. 2006, 2016). Small variations to this list are found in other sources (Kyriakou et al. 2016). Several models are available for adoption, the most common of which include multidisciplinary and interdisciplinary approaches reflecting the varying degree of collaboration and professional autonomy (Lee et al. 2016). Optimal team care is labeled multidisciplinary by the Consensus Statement and others; however, descriptions of team composition and roles better match an interdisciplinary model. In multidisciplinary care, professionals from different disciplines work independently from discipline-specific perspectives with the goal of eventually combining efforts to address a common problem. Interdisciplinary care involves working jointly from discipline-specific perspectives; providers on an interdisciplinary team meet regularly to discuss and collaboratively set treatment goals and carry out treatment. Adoption of this model is projected to improve health outcomes through enhanced communication and coordination.
Patient- and family-centered care.
Patient- and family-centered care is grounded in collaboration among patients, families, physicians, and other professionals in clinical care, education, and research. As endorsed (and reaffirmed in 2018) by the American Academy of Pediatrics (AAP Comm. Hosp. Care & Inst. Patient Fam.-Cent. Care 2012), patient- and family-centered care is based on the understanding that the family is the child’s primary source of strength and support, that perspectives and information provided by families, children, and young adults are essential components of high-quality clinical decision making, and that patients and family are integral partners in the health care team.
Given these principles, patient- and family-centered care is recommended in the management of DSDs (Lee et al. 2006, Sandberg & Mazur 2014). As applied to DSDs, patient-centered care includes the following:
Provide medical and surgical care when real and present threats exist to patient physical well-being
Recognize that what is typical/acceptable for one individual may not be for another; providers should not force patients into a social norm (e.g., for phallic size or gender-typical behaviors)
Minimize potential for patients and families to feel ashamed, stigmatized, or overly fixated on genital appearance; avoid the use of stigmatizing terminology (e.g., ambiguous, mutation) and medical photography in the awake state; avoid a “parade of white coats” and limit genital exams, as medically appropriate; and promote openness with others
Ask oneself, when elective surgical or hormonal treatments are considered, whether they are needed for the child’s benefit versus to allay parental distress; behavioral health professionals can help assess this
Respect parents by addressing their concerns and distress empathetically, honestly, and directly and helping them obtain behavioral health care
Directly address children’s psychosocial distress through psychosocial interventions and peer support
Speak the truth to the family and the child, answering questions promptly and honestly—including the patient’s medical history and about areas of clinical uncertainty (Consort. Manag. Disord. Sex Dev. 2006)
While patient- and family-centeredness is regarded as a core principle of DSD care, particularly regarding pediatric practice, it is incumbent upon clinicians to appreciate potentially competing interests between the patient and the family. Using phrases such as “delaying treatment” (Consort. Manag. Disord. Sex Dev. 2006) or preserving a “child’s right to an open future” (Feinberg 1980), some advocate for withholding treatment, other than what is necessary to preserve life, until a child can meaningfully participate in decision making (Feder & Dreger 2016). Adopting a contrasting viewpoint, the AMA’s Counsel on Ethical and Judicial Affairs noted that “choosing not to have a treatment or procedure performed also forecloses a future choice” (AMA 2019, p. 4; emphasis in original) and concluded that because families provide a child’s usual, often only, source of support and care, the family’s needs and interests can also be relevant to treatment decisions.
Noting dissatisfaction with the direction and pace of desired changes to models of care offered by medicine, intersex advocates frame their push for changes in the language of human rights (Hum. Rights Watch 2017, Méndez & UN Hum. Rights Counc. 2013) and make use of the social model of disability (Crocetti et al. 2021). Applied to DSDs, advocates argue clinical practice is underpinned by incorrect assumptions that intersex characteristics are disabling and require intervention. Further, they offer that intersex constitutes a difference or an aspect of human diversity (versus a disorder) and that care options are affected by the frame through which intersex is viewed. Noting neglect of lived realities of impairment associated with DSDs and the lack of guidelines for incorporating social models of intersex into care, Crocetti et al. (2021) concluded that while a social model of intersex health is central to surfacing the cultural underpinnings of medical overreach, it is not, in itself, sufficient in addressing intersex health. Attempts to balance perspectives of the child’s right to bodily autonomy and right to an open future with parents’ decision-making authority regarding what they perceive as their child’s future best interests are present in the literature (e.g., Krishna et al. 2021).
Quality improvement.
Ideally, the state of health care should be such that if two patients with the same condition and presentation sought care at different medical centers, both would have the same clinical assessment and management services available. This, however, is not likely to occur. In a study of clinical service delivery in the United States, differences between medical centers were found in which diagnoses/phenotypes were considered to constitute a DSD, availability of specialist providers, how informed consent was conducted and documented, continuing education activities for providers, and research participation (Rolston et al. 2017). Internationally, differences were seen in formal national network participation; organization and collaboration of specialist providers; participation in registries, audits, and quality improvement exercises; professional development in DSD care; selection and availability of endocrine and cytogenetic diagnostic and molecular genetic tests; and diagnostic pathways preferred by clinicians in the investigation of 46,XY DSD (Kyriakou et al. 2016). To advance quality of care provided to patients and their families, there should be at least a baseline standard of universal comprehensive services (Kavanaugh et al. 2021).
Pressing Medical Needs Versus Elective Procedures
Depending on the specific DSD condition and its effects on the body, those with DSDs may require urgent medical or surgical care. Others may require lifelong hormone replacement and monitoring for gonadal cancer or other risks to physical health. A major area of contention in clinical management revolves around elective and values-sensitive genital and gonadal surgery.
Surgical terminology.
Gonadal and genital surgery are controversial elements of clinical management—particularly in the pediatric population. Surgical interventions are generally classified as either urgent or elective. Urgent surgeries are performed to avoid life-threatening circumstances or prevent permanent disability. In a DSD, urgent surgery may be needed to create unobstructed outlets for urine or stool. Elective surgeries include those that address nonurgent issues. Cosmetic surgeries, a subset of elective surgery, are designed to enhance appearance without changing function. In DSDs associated with atypical reproductive anatomy and/or genitalia, most surgical interventions are elective, but they would not be considered exclusively cosmetic because altered function is an additional objective (Gardner & Sandberg 2018).
Physical health.
Although arguments have been made that intersex/DSD conditions should not be viewed as disorders but rather as variations on an aspect of human diversity (Crocetti et al. 2021), research uncovers differences in perceived and objective health statuses of those with DSDs that compare negatively to those of nonaffected persons. In a cross-sectional observational study involving 1,040 participants with DSDs recruited from 14 European tertiary centers, 84.3% reported the presence of a medical condition (other than a DSD) compared with 24.6% of controls. Conditions identified in the study as being higher in the DSD cohort than in the general population included cardiovascular and metabolic disorders, osteoporosis and fractures despite the young age of the cohort, cancer risk, psychiatric disorders, and suicide attempt rates; additional conditions were specific to Turner syndrome, Klinefelter syndrome, and CAH (Falhammar et al. 2018).
Psychological Assessment and Intervention
Despite assumed benefits and a significant proportion of parents indicating a high need for psychological support, a clear mismatch exists between these factors and the availability of behavioral health services and their integration within the model of care (Sandberg & Mazur 2014). A recent US-based survey of DSD clinical services showed behavioral health was “always” involved in patient care in the majority (67%) of centers and involved on a referral/consult-only basis for the rest (Rolston et al. 2017) (also see Kavanaugh et al. 2021), while an international survey showed a minority of centers (41%) included a behavioral health provider (Kyriakou et al. 2016). Implementation difficulties center on administrative burden within the health care system and limitations on resources needed to administer and score measures, expertise required to interpret and communicate results, costs to purchasing proprietary measures, and patients’ and families’ willingness/ability to complete multiple measures (Ernst et al. 2018a, Sandberg et al. 2017). Further, limited availability of providers with specialized training to implement interventions constitutes another barrier that needs to be addressed at a systems level (Gardner & Sandberg 2018). A concerning effect of the lack of psychological services was brought to international attention by several reports that documented ongoing breeches of national standards and guidelines of care regarding patients with DSDs. Specifically, Great Ormond Street Hospital for Children in the United Kingdom was reported as having performed genital surgery without first providing psychological support to families, not discussing all cases at DSD team meetings, and having a flawed informed consent process. This prompted investigation by the National Health Service (Ernst et al. 2018b) and should stand as encouragement to follow recommendations for interdisciplinary care with integrated behavioral health services and as a warning against uncoordinated and less than comprehensive care.
Evaluation.
Historically, categorical (disease-specific) approaches favoring research on the process of psychosexual differentiation have dominated the field. This has left major gaps in our understanding of other factors that likely contribute to adaptation in those with DSDs. In contrast, a noncategorical approach considers the unique aspects of the child’s medical condition while also considering the total life experience of the child, family, and broader social context. As such, it has become a recommended approach to clinical assessment and management (Claahsen-van der Grinten et al. 2021, Sandberg & Mazur 2014, Sandberg et al. 2017). Nevertheless, research with adults using a wide range of methods demonstrates that specific difficulties exist, often despite medical/surgical interventions; these include negative body image, functional sexual difficulties, complications surrounding “normalizing” surgery, fear of devaluation, social isolation, barriers to communication with significant others, and feeling not entitled to relationships (Lee et al. 2016). These all require ongoing attention.
Intervention.
Proper assessment informs the urgency and direction of treatment. The Pediatric Psychosocial Preventative Health Model provides a path forward by calling for screening of all members of the population, universal delivery of interventions promoting resiliency and preventing deterioration in adaptation, and provision of specific interventions that match the level of need of families for whom risk factors are identified (Ernst et al. 2018a, Kazak et al. 2015). In practice, this means that all patients seen for assessment or management of DSDs should be screened and receive interventions carefully targeted to match their needs—which may range from providing nondirective psychoeducational counseling about predictable issues arising throughout the course of development to immediate intervention to address significant psychosocial crises (Ernst et al. 2018a, Sandberg et al. 2017). In a recent study of psychosocial risk, one-third of families seeking services for the assessment or management of their child’s DSD demonstrated some level of psychosocial risk: 27.9% targeted risk and 6.1% clinical level of risk (Ernst et al. 2018a). Targeted risk reflects acute distress and some psychosocial risk; clinical risk reflects ongoing, escalating, or high-intensity distress with multiple risk factors present (Kazak et al. 2015).
The importance of a behavioral health component in the management of DSDs stems from recognizing that clinical decisions are frequently driven by considerations of promoting positive psychological adaptation and well-being rather than by exclusively addressing a specific medical health need (Sandberg et al. 2017). Although evidence-based psychosocial interventions specific to DSDs have not been developed, cognitive behavioral and problem-solving psychosocial interventions have demonstrable efficacy in improving the psychosocial functioning of patients and families in other conditions (Ernst et al. 2018b, Gardner & Sandberg 2018). Examples of this can be found in group work drawing on cognitive and narrative approaches improving self-evaluation of women with Turner syndrome, cognitive behavioral therapy reducing specific stresses, and psychological interventions improving well-being for women with Mayer–Rokitansky–Küster-Hauser syndrome (Lee et al. 2016).
Advocates and professionals alike recommend psychosocial interventions designed to promote open and developmentally appropriate information-sharing with the child, continuing patient education throughout the lifetime, interventions to address shame, and active decisional support (Lee et al. 2016, Sandberg & Mazur 2014). Specific recommendations for clinicians concerning setting the stage for assessment, outreach teaching, careful use of language and demeanor, tackling shame and reducing stigma, helping parents think about elective surgery, and truth telling within the context of a treatment protocol can be found in the Clinical Guidelines for the Management of Disorders of Sex Development in Childhood (Consort. Manag. Disord. Sex Dev. 2006).
Decision Making in DSDs
Providers seek to deliver holistic care that includes anticipating and managing predictable challenges associated with DSDs and their care—including facilitation of decision making about gender of rearing, timing of surgery, and sex hormone replacement (Lee et al. 2016). Examples of other decisions concern the balance between secrecy and sharing details about the condition and its implications—including who should be told, when the information should be given, who should deliver it, how best to do it, and whether various medical/surgical interventions are warranted at all (Sandberg et al. 2017).
In addition to not recognizing the need for decisions to be made or one’s role in such decisions (Crissman et al. 2011), barriers to active parent or patient participation in decision making include uncertainty in the medical community regarding best practices in the diagnosis, medical/surgical management, and strategies for optimal psychosocial management; the experience of DSDs as rare and stigmatizing, resulting in neglecting support and resources that can come from extended family, friends, peer support organizations, and trusted physicians in the medical home; lack of rigorous methods for assessing patient (and, in pediatrics, family) values and preferences relevant to decision making; and emotional distress and fear of stigma-related social isolation interfering with cognitive processes necessary to balance the risks and benefits of treatment options. Under high-stress circumstances and limited information, people struggle to absorb unfamiliar and highly complex concepts (Sandberg & Mazur 2014). Decisional regret is an easily understood consequence of such circumstances (Siminoff & Sandberg 2015).
The objective of SDM is to help patients (or, in pediatrics, parents) make informed, preference-based clinical management choices among several relevant options (Siminoff & Sandberg 2015). SDM comprises three essential elements: (a) explicit acknowledgment that a decision is required; (b) review and understanding of the best available evidence concerning the harms and benefits of each option; and (c) a process that integrates the patient’s/family’s values and preferences together with the provider’s guidance (Gardner & Sandberg 2018). SDM does not imply that providers and patients/parents must have equal responsibility for the final decision or that decisions are based entirely on patient/parental preference; rather, it requires involvement of providers and patients/parents, with bidirectional information exchange, mutual deliberation on treatment options, and agreement on treatment plans. The US federal government defined informed consent as “a process, not just a form” (OHRP 1993), highlighting the importance of thorough and meaningful informed consent. Integrated into ongoing clinical management, the SDM process can help form the basis of truly informed consent.
CONCLUSION: KEY POINTS AND ROLES FOR CLINICAL PSYCHOLOGY
DSDs are congenital conditions arising from atypicality in the sex determination and/or differentiation processes. Controversy exists in numerous areas including how DSDs are conceptualized, how to refer to the set of conditions and those affected by them, and aspects of clinical management. Historically, research focused on psychosexual development and the effects of sex hormones on behavior. Increased attention to issues pertaining to aspects of psychosocial adaptation is occurring. Future research should focus on multisite, registry-based strategies that allow for long-term multi-informant prospective follow-up.
DSDs share several aspects with other conditions, including congenital conditions (generally) and chronic illnesses, as well as issues pertaining to LGBT communities; however, the issues are not one and the same and require careful thought regarding the application of lessons learned in these areas to DSDs. Issues commonly affecting the DSD community include information about specific conditions and/or aspects of care that are lacking, conflicted, or anxiety-provoking; parental stress; stigma, shame, and secrecy; legal and ethical questions in pediatric care surrounding consent, assent, child rights, and parental responsibilities, largely in regard to surgical procedures; influence of religion and culture; and nosocomial trauma. Psychosocial and psychosexual adaptation in DSDs can differ from unaffected persons regarding experienced stigma and shame, psychological and social problems, experiences and preferences regarding genital surgery, psychosexual milestones and sexual function, sexual orientation, and gender dysphoria.
Recommended clinical management models include multispecialty interdisciplinary team care that, in pediatrics, carefully attends to the best interests of children within the context of their families. Roles and considerations for clinical psychologists within multispecialty team care include the following: integrate with other specialists caring for the patient/family; in clinical assessment and intervention, balance a noncategorical approach, which assumes that patients and families are influenced by a similar set of factors that affect adaptation in general, with condition-specific knowledge (e.g., needs for medication or regular screening for tumor risk) gleaned from the full range of specialty team providers; educate patients, families, and health care provider colleagues about the implications of medical conditions for quality of life and strategies to address quality of life concerns; within SDM, promote patient self-efficacy (in pediatrics, based on developmentally qualified understanding of one’s condition)—for instance, choosing how the person wants the genital exam to be performed; and promote psychologically informed research—both in its conduct and uptake to inform practice.
SUMMARY POINTS.
Differences or disorders of sex development (DSDs) are congenital conditions arising from atypicality in the sex determination and/or differentiation processes.
DSDs share some aspects with other conditions; however, the issues are not one and the same and require careful thought regarding the application of lessons learned in these areas to DSDs.
Controversy exists in numerous areas ranging from terminology to legal and ethical questions surrounding parental rights and responsibilities and the role of parental permission and child assent to genital and gonadal surgery.
Increased attention to issues pertaining to aspects of psychosocial adaptation is occurring.
Broad consensus exists regarding the desirability and importance of integrated behavioral health care in DSDs; this goal is rarely achieved in practice.
FUTURE DIRECTIONS.
Future research should focus on multisite, registry-based strategies that allow for long-term multi-informant prospective follow-up.
Recommended clinical management models include interdisciplinary team care that is patient-centered (and, in pediatrics, family-centered).
Increased integration of clinical psychology (and allied behavioral health) in interdisciplinary team care is needed and can be accomplished.
ACKNOWLEDGMENTS
Funded in part by grants from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R01 HD093450 and R01 HD086583).
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Footnotes
The acronym DSD is used in this review to refer exclusively to a category of medical conditions that share common features; in this context, DSD carries no implications for the identity of the person, some of whom prefer the term intersex. In general, we adopt the principle of person-first language, which refers to the person first and the potentially associated disability second.
The Annual Review of Clinical Psychology is online at clinpsy.annualreviews.org
LITERATURE CITED
- AAP (Am. Acad. Pediatr.) Comm. Hosp. Care, Inst. Patient Fam.-Cent. Care. 2012. Patient- and family-centered care and the pediatrician’s role. Pediatrics 129:394–404 [DOI] [PubMed] [Google Scholar]
- Ahmed SF, Achermann J, Alderson J, Crouch NS, Elford S, et al. 2021. Society for Endocrinology UK guidance on the initial evaluation of a suspected difference or disorder of sex development (revised 2021). Clin. Endocrinol 95(6):818–40 [DOI] [PubMed] [Google Scholar]
- Ainsworth C 2015. Sex redefined. Nature 518:288–91 [DOI] [PubMed] [Google Scholar]
- Alderson J, Madill A, Balen A. 2004. Fear of devaluation: understanding the experience of intersexed women with androgen insensitivity syndrome. Br. J. Health Psychol 9:81–100 [DOI] [PubMed] [Google Scholar]
- AMA (Am. Med. Assoc.). 2019. Report of the Council on Ethical and Judicial Affairs. CEJA Rep. 3-I-18, AMA, Chicago. https://www.ama-assn.org/system/files/2019-12/i18-ceja-report-3.pdf [Google Scholar]
- Arboleda VA, Sandberg DE, Vilain E. 2014. DSDs: genetics, underlying pathologies and psychosexual differentiation. Nat. Rev. Endocrinol 10:603–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, McCarthy MM. 2016. Sexual differentiation of the brain and behavior: a primer. In Neuroscience in the 21st Century, ed. Pfaff DW, Volkow ND, pp. 2139–68. New York: Springer [Google Scholar]
- Aust. Gov. 2013. Australian Government Guidelines on the Recognition of Sex and Gender. Canberra: Aust. Gov. https://www.ag.gov.au/sites/default/files/2020-03/AustralianGovernmentGuidelinesontheRecognitionofSexandGender.pdf [Google Scholar]
- Avellan L 1976. The development of puberty, the sexual debut and sexual function in hypospadiacs. Scand. J. Plast. Reconstr. Surg 10:29–44 [DOI] [PubMed] [Google Scholar]
- Babu R, Shah U. 2021. Gender identity disorder (GID) in adolescents and adults with differences of sex development (DSD): a systematic review and meta-analysis. J. Pediatr. Urol 17:39–47 [DOI] [PubMed] [Google Scholar]
- Bancroft J, Loftus J, Long JS. 2003. Distress about sex: a national survey of women in heterosexual relationships. Arch. Sex. Behav 32:193–208 [DOI] [PubMed] [Google Scholar]
- Banu T, Chowdhury TK. 2020. Cultural differences in the developing world. In Disorders|Differences of Sex Development, ed. Hutson JM, Grover SR, O’Connell MA, Bouty A, Hanna CA, pp. 295–304. Singapore: Springer [Google Scholar]
- Baratz AB, Sharp MK, Sandberg DE. 2014. Disorders of sex development peer support. In Endocrine Development, Vol. 27: Understanding Differences and Disorders of Sex Development (DSD), ed. Hiort O, Ahmed SF, pp. 99–112. Basel, Switz.: Karger; [DOI] [PubMed] [Google Scholar]
- Baumrind D 2005. Patterns of parental authority and adolescent autonomy. New Dir. Child Adolesc. Dev 2005:61–69 [DOI] [PubMed] [Google Scholar]
- Bennecke E, Bernstein S, Lee P, van de Grift TC, Nordenskjöld A, et al. 2021. Early genital surgery in disorders/differences of sex development: patients’ perspectives. Arch. Sex. Behav 50:913–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. 2011. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front. Neuroendocrinol 32:183–200 [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM, Corley R. 2015. The importance of puberty for adolescent development: conceptualization and measurement. In Advances in Child Development and Behavior, ed. Benson JB, pp. 53–92. Waltham, MA: Academic; [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Duck SC, Bryk K. 2000. Behavioral effects of prenatal versus postnatal androgen excess in children with 21-hydroxylase-deficient congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab 85:727–33 [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Meyer-Bahlburg HF. 2015. Gender development and sexuality in disorders of sex development. Horm. Metab. Res 47:361–66 [DOI] [PubMed] [Google Scholar]
- Binet A, Lardy H, Geslin D, Francois-Fiquet C, Poli-Merol ML. 2016. Should we question early feminizing genitoplasty for patients with congenital adrenal hyperplasia and XX karyotype? J. Pediatr. Surg 51:465–68 [DOI] [PubMed] [Google Scholar]
- Bouvattier C, Mignot B, Lefevre H, Morel Y, Bougneres P. 2006. Impaired sexual activity in male adults with partial androgen insensitivity. J. Clin. Endocrinol. Metab 91:3310–15 [DOI] [PubMed] [Google Scholar]
- Boyse KL, Gardner M, Marvicsin DJ, Sandberg DE. 2014. “It was an overwhelming thing”: parents’ needs after infant diagnosis with congenital adrenal hyperplasia. J. Pediatr. Nurs 29:436–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L, Schuetzmann K, Richter-Appelt H. 2007. Gender assignment and medical history of individuals with different forms of intersexuality: evaluation of medical records and the patients’ perspective. J. Sex. Med 4:964–80 [DOI] [PubMed] [Google Scholar]
- Calif. Legis. 2021. SB-225 Medical procedures: individuals born with variations in their physical sex characteristics. Calif. State Senate Bill 225, Mar. 2. https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202120220SB225 [Google Scholar]
- Callens N, Kreukels BPC, van de Grift TC. 2021.Young voices: sexual health and transition care needs in adolescents with intersex/differences of sex development—a pilot study. J. Pediatr. Adolesc. Gynecol 34:176–89.e2 [DOI] [PubMed] [Google Scholar]
- Callens N, Van Kuyk M, van Kuppenveld JH, Drop SLS, Cohen-Kettenis PT, Dessens AB. 2016. Recalled and current gender role behavior, gender identity and sexual orientation in adults with disorders/differences of sex development. Horm. Behav 86:8–20 [DOI] [PubMed] [Google Scholar]
- Chan KH, Panoch J, Carroll A, Wiehe S, Downs S, et al. 2019. Parental perspectives on decision-making about hypospadias surgery. J. Pediatr. Urol 15:449.e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y-M, Hannema SE, Achermann JC, Hughes IA. 2020. Disorders of sex development. In Williams Textbook of Endocrinology, ed. Melmed S, Auchus RJ, Goldfine AB, Koenig RJ, Rosen CJ, pp. 867–936.e14. Philadelphia: Elsevier [Google Scholar]
- Chase C 2003. What is the agenda of the intersex patient advocacy movement? Endocrinologist 13:240–42 [Google Scholar]
- Chuang J, Vallerie A, Breech L, Saal HM, Alam S, et al. 2013. Complexities of gender assignment in 17β-hydroxysteroid dehydrogenase type 3 deficiency: Is there a role for early orchiectomy? Int. J. Pediatr. Endocrinol 2013:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, et al. 2021. Congenital adrenal hyperplasia—current insights in pathophysiology, diagnostics, and management. Endocr. Rev 10.1210/endrev/bnab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kettenis PT. 2005. Gender change in 46,XY persons with 5α-reductase-2 deficiency and 17β-hydroxysteroid dehydrogenase-3 deficiency. Arch. Sex. Behav 34:399–410 [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. 1995. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol. Bull 118:55–107 [DOI] [PubMed] [Google Scholar]
- Colletti CJ, Wolfe-Christensen C, Carpentier MY, Page MC, McNall-Knapp RY, et al. 2008. The relationship of parental overprotection, perceived vulnerability, and parenting stress to behavioral, emotional, and social adjustment in children with cancer. Pediatr. Blood Cancer 51:269–74 [DOI] [PubMed] [Google Scholar]
- Consort. Manag. Disord. Sex Dev. 2006. Clinical Guidelines for the Management of Disorders of Sex Development in Childhood. Whitehouse Station, NJ: Accord Alliance. http://www.accordalliance.org/dsd-guidelines/ [Google Scholar]
- Cools M, Nordenstrom A, Robeva R, Hall J, Westerveld P, et al. 2018. Caring for individuals with a difference of sex development (DSD): a consensus statement. Nat. Rev. Endocrinol 14:415–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousino MK, Hazen RA. 2013. Parenting stress among caregivers of children with chronic illness: a systematic review. J. Pediatr. Psychol 38:809–28 [DOI] [PubMed] [Google Scholar]
- Cox KH, Bonthuis PJ, Rissman EF. 2014. Mouse model systems to study sex chromosome genes and behavior: relevance to humans. Front. Neuroendocrinol 35:405–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman HP, Warner L, Gardner M, Carr M, Schast A, et al. 2011. Children with disorders of sex development: a qualitative study of early parental experience. Int. J. Pediatr. Endocrinol 2011:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocetti D, Monro S, Vecchietti V, Yeadon-Lee T. 2021.Towards an agency-based model of intersex, variations of sex characteristics (VSC) and DSD/dsd health. Cult. Health Sex 23:500–15 [DOI] [PubMed] [Google Scholar]
- Crouch NS, Liao LM, Woodhouse CR, Conway GS, Creighton SM. 2008. Sexual function and genital sensitivity following feminizing genitoplasty for congenital adrenal hyperplasia. J. Urol 179:634–38 [DOI] [PubMed] [Google Scholar]
- Davenport ML, Cornea E, Xia K, Crowley JJ, Halvorsen MW, et al. 2020. Altered brain structure in infants with Turner syndrome. Cereb. Cortex 30:587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JH, Knight EJ, Savage A, Brown J, Malone PS. 2011. Evaluation of terminology used to describe disorders of sex development. J. Pediatr. Urol 7:412–15 [DOI] [PubMed] [Google Scholar]
- Davis G 2013. The power in a name: diagnostic terminology and diverse experiences. Psychol. Sex 5:15–27 [Google Scholar]
- de Neve-Enthoven NG, Callens N, van Kuyk M, van Kuppenveld JH, Drop SL, et al. 2016. Psychosocial well-being in Dutch adults with disorders of sex development. J. Psychosom. Res 83:57–64 [DOI] [PubMed] [Google Scholar]
- de Vries ALC, Roehle R,Marshall L, Frisen L, van de Grift TC, et al. 2019. Mental health of a large group of adults with disorders of sex development in six European countries. Psychosom. Med 81:629–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delozier AM, Gamwell KL, Sharkey C, Bakula DM, Perez MN, et al. 2019. Uncertainty and posttraumatic stress: differences between mothers and fathers of infants with disorders of sex development. Arch. Sex. Behav 48:1617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. 2003. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Mol. Brain Res 118:82–90 [DOI] [PubMed] [Google Scholar]
- Dolgin MJ, Phipps S, Fairclough DL, Sahler OJ, Askins M, et al. 2007. Trajectories of adjustment in mothers of children with newly diagnosed cancer: a natural history investigation. J. Pediatr. Psychol 32:771–82 [DOI] [PubMed] [Google Scholar]
- Dreger AD, Chase C, Sousa A, Gruppuso PA, Frader J. 2005. Changing the nomenclature/taxonomy for intersex: a scientific and clinical rationale. J. Pediatr. Endocrinol. Metab 18:729–33 [DOI] [PubMed] [Google Scholar]
- Duguid A, Morrison S, Robertson A, Chalmers J, Youngson G, et al. 2007. The psychological impact of genital anomalies on the parents of affected children. Acta Paediatr. 96:348–52 [DOI] [PubMed] [Google Scholar]
- Dunn KM, Croft PR, Hackett GI. 2000. Satisfaction in the sex life of a general population sample. J. Sex Marital Ther 26:141–51 [DOI] [PubMed] [Google Scholar]
- Ediati A, Faradz SMH, Juniarto AZ, van der Ende J, Drop SLS, Dessens AB. 2015. Emotional and behavioral problems in late-identified Indonesian patients with disorders of sex development. J. Psychosom. Res 79:76–84 [DOI] [PubMed] [Google Scholar]
- Erens B, Burkill S, Couper MP, Conrad F, Clifton S, et al. 2014. Nonprobability web surveys to measure sexual behaviors and attitudes in the general population: a comparison with a probability sample interview survey. J. Med. Internet Res 16:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MM, Gardner M, Mara CA, Delot EC, Fechner PY, et al. 2018a. Psychosocial screening in disorders/differences of sex development: psychometric evaluation of the Psychosocial Assessment Tool. Horm. Res. Paediatr 90:368–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MM, Liao L-M, Baratz AB, Sandberg DE. 2018b. Disorders of sex development/intersex: gaps in psychosocial care for children. Pediatrics 142(2):e20174045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm R, Santtila P, Miettinen PJ, Mattila A, Rintala R, Taskinen S. 2011. Sexual function and attitudes toward surgery after feminizing genitoplasty. J. Urol 185:1900–4 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Claahsen-van der Grinten H, Reisch N, Slowikowska-Hilczer J, Nordenstrom A, et al. 2018. Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr. Connect 7:466–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele D, Kirk K, Wolfe-Christensen C, Phillips T, Mazur T, et al. 2010. Primary caregivers of children affected by disorders of sex development: mental health and caregiver characteristics in the context of genital ambiguity and genitoplasty. Int. J. Pediatr. Endocrinol 2010:690674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder EK, Dreger A. 2016. Still ignoring human rights in intersex care. J. Pediatr. Urol 12:436–37 [DOI] [PubMed] [Google Scholar]
- Feinberg J 1980. The child’s right to an open future. In Whose Child? Children’s Rights, Parental Authority, and State Power, ed. Aiken W, LaFollette H, pp. 124–53. Totowa, NJ: Rowman and Littlefield [Google Scholar]
- Finlayson C, Fritsch MK, Johnson EK, Rosoklija I, Gosiengfiao Y, et al. 2017. Presence of germ cells in disorders of sex development: implications for fertility potential and preservation. J. Urol 197:937–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, Sandberg DE. 2018. Navigating surgical decision making in disorders of sex development (DSD). Front. Pediatr 6:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorayshi A 2017.A landmark lawsuit about an intersex baby’s genital surgery just settled for $440,000. Buzz-Feed News, July 26. https://www.buzzfeed.com/azeenghorayshi/intersex-surgery-lawsuit-settles?utm_term=.esZyYNva9O#.waJdG581ng [Google Scholar]
- Godfrey LM. 2021. Mental health outcomes among individuals with 46,XY disorders of sex development: a systematic review. J. Health Psychol 26:40–59 [DOI] [PubMed] [Google Scholar]
- Gondim R, Teles F, Barroso U Jr. 2018. Sexual orientation of 46, XX patients with congenital adrenal hyperplasia: a descriptive review. J. Pediatr. Urol 14:486–93 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, et al. 2017. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur. J. Endocrinol 177:G1–70 [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebaek A. 2018. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr. Rev 39:389–423 [DOI] [PubMed] [Google Scholar]
- Greenberg JA. 2017. Legal, ethical, and human rights considerations for physicians treating children with atypical or ambiguous genitalia. Semin. Perinatol 41:252–55 [DOI] [PubMed] [Google Scholar]
- Hanauer DA, Gardner M, Sandberg DE. 2014. Unbiased identification of patients with disorders of sex development. PLOS ONE 9:e108702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Moore JA, Kapa HM, Litteral JL, Nahata L, Indyk JA, et al. 2021. Psychosocial functioning among children with and without differences of sex development. J. Pediatr. Psychol 46:69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon AA, Cheng MM, Herring AH, McRee A-L, Halpern CT. 2014. Prevalence and predictors of sexual inexperience in adulthood. Arch. Sex. Behav 43:221–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersmus R, van Bever Y, Wolffenbuttel KP, Biermann K, Cools M, Looijenga LH. 2017. The biology of germ cell tumors in disorders of sex development. Clin. Genet 91:292–301 [DOI] [PubMed] [Google Scholar]
- Hines M 2004. Psychosexual development in individuals who have female pseudohermaphroditism. Child Adolesc. Psychiatr. Clin. N. Am 13:641–56 [DOI] [PubMed] [Google Scholar]
- Hines M 2020. Human gender development. Neurosci. Biobehav. Rev 118:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Brook C, Conway GS. 2004. Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH). J. Sex. Res 41:75–81 [DOI] [PubMed] [Google Scholar]
- Hirschberg AL. 2019. Hyperandrogenism in female athletes. J. Clin. Endocrinol. Metab 104:503–5 [DOI] [PubMed] [Google Scholar]
- Hollenbach AD, Eckstrand KL, Dreger A, eds. 2014. Implementing Curricular and Institutional Climate Changes to Improve Health Care for Individuals Who Are LGBT, Gender Nonconforming, or Born with DSD: A Resource for Medical Educators. Washington, DC: Assoc. Am. Med. Coll. https://store.aamc.org/downloadable/download/sample/sample_id/129 [Google Scholar]
- Holmbeck GN, Johnson SZ, Wills KE, McKernon W, Rose B, et al. 2002. Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: the mediational role of behavioral autonomy. J. Consult. Clin. Psychol 70:96–110 [DOI] [PubMed] [Google Scholar]
- Howe EG. 2021. People with differences of sexual development: Can we do better? J. Clin. Ethics 32:3–12 [PubMed] [Google Scholar]
- Hum. Rights Watch. 2017. “I Want to Be Like Nature Made Me”: Medically Unnecessary Surgeries on Intersex Children in the US. New York: Hum. Rights Watch. https://www.hrw.org/sites/default/files/report_pdf/lgbtintersex0717_web_0.pdf [Google Scholar]
- ISNA (Intersex Soc. N. Am.). 2008. Law. Intersex Society of North America. https://isna.org/legal/
- Johannsen TH, Ripa CP, Mortensen EL, Main KM. 2006. Quality of life in 70 women with disorders of sex development. Eur. J. Endocrinol 155:877–85 [DOI] [PubMed] [Google Scholar]
- Jürgensen M, Kleinemeier E, Lux A, Steensma TD, Cohen-Kettenis PT, et al. 2013. Psychosexual development in adolescents and adults with disorders of sex development—results from the German Clinical Evaluation Study. J. Sex. Med 10:2703–14 [DOI] [PubMed] [Google Scholar]
- Karkazis K 2008. Fixing Sex: Intersex,Medical Authority, and Lived Experience. Durham, NC: Duke Univ. Press [Google Scholar]
- Kavanaugh GL, Mohnach L, Youngblom J, Kellison JG, Sandberg DE, Accord Alliance. 2021. “Good practices” in pediatric clinical care for disorders/differences of sex development. Endocrine 73:723–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak AE, Schneider S, Didonato S, Pai ALH.2015. Family psychosocial risk screening guided by the Pediatric Psychosocial Preventative Health Model (PPPHM) using the Psychosocial Assessment Tool (PAT). Acta Oncol. 54:574–80 [DOI] [PubMed] [Google Scholar]
- Khorashad BS, Aghili Z, Kreukels BPC, Reid AG, Roshan GM, et al. 2018. Mental health and disorders of sex development/intersex conditions in Iranian culture: congenital adrenal hyperplasia, 5-α reductase deficiency-type 2, and complete androgen insensitivity syndrome. Arch. Sex. Behav 47:931–42 [DOI] [PubMed] [Google Scholar]
- Kirk KD, Fedele DA, Wolfe-Christensen C, Phillips TM, Mazur T, et al. 2011. Parenting characteristics of female caregivers of children affected by chronic endocrine conditions: a comparison between disorders of sex development and type 1 diabetes mellitus. J. Pediatr. Nurs 26:e29–36 [DOI] [PubMed] [Google Scholar]
- Köhler B, Kleinemeier E, Lux A, Hiort O, Gruters A, et al. 2012. Satisfaction with genital surgery and sexual life of adults with XY disorders of sex development: results from the German clinical evaluation study. J. Clin. Endocrinol. Metab 97:577–88 [DOI] [PubMed] [Google Scholar]
- Kolesinska Z, Ahmed SF, Niedziela M, Bryce J, Molinska-Glura M, et al. 2014. Changes over time in sex assignment for disorders of sex development. Pediatrics 134:e710–5 [DOI] [PubMed] [Google Scholar]
- Kreukels BPC, Cohen-Kettenis PT, Roehle R, van de Grift TC, Slowikowska-Hilczer J, et al. 2019. Sexuality in adults with differences/disorders of sex development (DSD): findings from the dsd-LIFE Study. J. Sex Marital Ther 45:688–705 [DOI] [PubMed] [Google Scholar]
- Kreukels BPC, Kohler B, Nordenstrom A, Roehle R, Thyen U, et al. 2018. Gender dysphoria and gender change in disorders of sex development/intersex conditions: results from the dsd-LIFE Study. J. Sex. Med 15:777–85 [DOI] [PubMed] [Google Scholar]
- Krishna KB, Kogan BA, Mazur T, Hoebeke P, Bogaert G, Lee PA. 2021. Individualized care for patients with intersex (differences of sex development): part 4/5. considering the ifs, whens, and whats regarding sexual-reproductive system surgery. J. Pediatr. Urol 17(3):338–45 [DOI] [PubMed] [Google Scholar]
- Kyriakou A, Dessens A, Bryce J, Iotova V, Juul A, et al. 2016. Current models of care for disorders of sex development—results from an international survey of specialist centres. Orphanet J. Rare Dis 11:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampalzer U, Briken P, Schweizer K. 2020. Dealing with uncertainty and lack of knowledge in diverse sex development: controversies on early surgery and questions of consent. Sex. Med 8:472–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, Houk CP, Ahmed SF, Hughes IA, Int. Consens. Conf. Intersex, Lawson Wilkins Pediatr. Endocr. Soc., Eur. Soc. Paediatr. Endocrinol. 2006. Consensus statement on management of intersex disorders. Pediatrics 118:e488–500 [DOI] [PubMed] [Google Scholar]
- Lee PA, Nordenstrom A, Houk CP, Ahmed SF, Auchus R, et al. 2016. Global disorders of sex development update since 2006: perceptions, approach and care. Horm. Res. Paediatr 85:158–80 [DOI] [PubMed] [Google Scholar]
- Lee PA, Wisniewski AB, Baskin L, Vogiatzi MG, Vilain E, et al. 2014. Advances in diagnosis and care of persons with DSD over the last decade. Int. J. Pediatr. Endocrinol 2014:19 [Google Scholar]
- Long CJ, Canning DA. 2016. Hypospadias: Are we as good as we think when we correct proximal hypospadias? J. Pediatr. Urol 12:196.e1–5 [DOI] [PubMed] [Google Scholar]
- Long CJ, Chu DI, Tenney RW, Morris AR, Weiss DA, et al. 2017. Intermediate-term followup of proximal hypospadias repair reveals high complication rate. J. Urol 197:852–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossie AC, Green J. 2015. Building trust: the history and ongoing relationships amongst DSD clinicians, researchers, and patient advocacy groups. Horm. Metab. Res 47:344–50 [DOI] [PubMed] [Google Scholar]
- MacKenzie D, Huntington A, Gilmour JA. 2009. The experiences of people with an intersex condition: a journey from silence to voice. J. Clin. Nurs 18:1775–83 [DOI] [PubMed] [Google Scholar]
- Magritte E 2012.Working together in placing the long term interests of the child at the heart of the DSD evaluation. J. Pediatr. Urol 8:571–75 [DOI] [PubMed] [Google Scholar]
- Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, et al. 2011. Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients. J. Clin. Endocrinol. Metab 96:296–307 [DOI] [PubMed] [Google Scholar]
- Mazur T. 2005. Gender dysphoria and gender change in androgen insensitivity ormicropenis. Arch. Sex. Behav 34:411–21 [DOI] [PubMed] [Google Scholar]
- Mazur T, Gardner M, Cook A, Sandberg DE. 2016. Disorders of sex development (DSD): definition, gender dysphoria, and differentiation from transsexualism. In Principles of Transgender Medicine and Surgery, ed. Ettner R, Monstrey S, Coleman E, pp. 222–48. New York: Taylor & Francis [Google Scholar]
- McCarthy MM. 2017. Overcoming the hegemony of hormones: Genes matter too. In Sex and the Developing Brain, ed. McCarthy MM, pp. 83–93. Williston, VT: Morgan & Claypool [Google Scholar]
- McCool-Myers M, Theurich M, Zuelke A, Knuettel H, Apfelbacher C. 2018. Predictors of female sexual dysfunction: a systematic review and qualitative analysis through gender inequality paradigms. BMC Women’s Health 18:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez JE, UN Hum. Rights Council. 2013. Report of the Special Rapporteur on torture and other cruel, inhuman or degrading treatment or punishment, Juan E. Méndez. Rep. A/HRC/22/53, United Nations, New York. http://www.ohchr.org/Documents/HRBodies/HRCouncil/RegularSession/Session22/A.HRC.22.53_English.pdf [Google Scholar]
- Messina V, Hirvikoski T, Karlsson L, Vissani S,Wallensteen L, et al. 2020. Good overall behavioural adjustment in children and adolescents with classic congenital adrenal hyperplasia. Endocrine 68:427–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL. 2008. Treatment guidelines for children with disorders of sex development. Neuropsychiatr. Enfance Adolesc 56:345–49 [Google Scholar]
- Meyer-Bahlburg HFL. 2014. Psychoendocrinology of congenital adrenal hyperplasia. In Genetic Steroid Disorders, ed. New MI, Lekarev O, Parsa A, O’Malley B, Hammer GD, pp. 285–300. London: Elsevier [Google Scholar]
- Meyer-Bahlburg HFL, Khuri J, Reyes-Portillo J, Ehrhardt AA, New MI. 2018. Stigma associated with classical congenital adrenal hyperplasia in women’s sexual lives. Arch. Sex. Behav 47:943–51 [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HFL, Reyes-Portillo JA, Khuri J, Ehrhardt AA, New MI. 2017. Syndrome-related stigma in the general social environment as reported by women with classical congenital adrenal hyperplasia. Arch. Sex. Behav 46:341–51 [DOI] [PubMed] [Google Scholar]
- Money J, Lamacz M. 1987. Genital examination and exposure experienced as nosocomial sexual abuse in childhood. J. Nerv. Ment. Dis 175:713–21 [DOI] [PubMed] [Google Scholar]
- Mouriquand PD, Gorduza DB, Gay CL, Meyer-Bahlburg HF, Baker L, et al. 2016. Surgery in disorders of sex development (DSD) with a gender issue: if (why), when, and how? J. Pediatr. Urol 12:139–49 [DOI] [PubMed] [Google Scholar]
- Nordenskjold A, Holmdahl G, Frisen L, Falhammar H, Filipsson H, et al. 2008. Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab 93:380–86 [DOI] [PubMed] [Google Scholar]
- NZ Gov. 2021. Change your gender in your passport. New Zealand Government. https://www.passports.govt.nz/change-your-name-or-gender/change-your-gender-in-your-passport [Google Scholar]
- OHRP (Off. Hum. Res. Prot.). 1993. Tips on informed consent. US Department of Health & Human Services. http://www.hhs.gov/ohrp/policy/ictips.html [Google Scholar]
- O’Reilly EJ, Mirzaei F, Forman MR, Ascherio A. 2010. Diethylstilbestrol exposure in utero and depression in women. Am. J. Epidemiol 171:876–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortqvist L, Engberg H, Strandqvist A, Nordenstrom A, Holmdahl G, et al. 2019. Psychiatric symptoms in men with hypospadias—preliminary results of a cross-sectional cohort study. Acta Paediatr. 108:1156–62 [DOI] [PubMed] [Google Scholar]
- O’Sullivan LF, Cheng MM, Harris KM, Brooks-Gunn J. 2007. I wanna hold your hand: the progression of social, romantic and sexual events in adolescent relationships. Perspect. Sex. Reprod. Health 39:100–7 [DOI] [PubMed] [Google Scholar]
- Pasterski V, Mastroyannopoulou K, Wright D, Zucker KJ, Hughes IA. 2014. Predictors of posttraumatic stress in parents of children diagnosed with a disorder of sex development. Arch. Sex. Behav 43:369–75 [DOI] [PubMed] [Google Scholar]
- Pasterski V, Prentice P, Hughes IA. 2010. Consequences of the Chicago consensus on disorders of sex development (DSD): current practices in Europe. Arch. Dis. Child 95:618–23 [DOI] [PubMed] [Google Scholar]
- Perez MN, Clawson AH, Baudino MN, Austin PF, Baskin LS, et al. 2021. Distress trajectories for parents of children with DSD: a growth mixture model. J. Pediatr. Psychol 46(5):588–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PES (Pediatr. Endocr. Soc.). 2020. Position statement on genital surgery in individuals with differences of sex development (DSD)/intersex traits. Position Statement, PES, McLean, VA. https://mk0pesendoklgy8upp97.kinstacdn.com/wp-content/uploads/2020/10/44-DSD-paperPosition-Statement-DSD-SIG.pdf [Google Scholar]
- Rapp M, Duranteau L, van de Grift TC, Schober J, Hirschberg AL, et al. 2021. Self- and proxy-reported outcomes after surgery in people with disorders/differences of sex development (DSD) in Europe (dsd-LIFE). J. Pediatr. Urol 17:353–65 [DOI] [PubMed] [Google Scholar]
- Rapp M, Mueller-Godeffroy E, Lee P, Roehle R, Kreukels BPC, et al. 2018. Multicentre cross-sectional clinical evaluation study about quality of life in adults with disorders/differences of sex development (DSD) compared to country specific reference populations (dsd-LIFE). Health Qual. Life Outcomes 16:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston AM, Gardner M, van Leeuwen K, Mohnach L, Keegan C, et al. 2017. Disorders of sex development (DSD): clinical service delivery in the United States. Am. J. Med. Genet. C Semin. Med. Genet 175:268–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston AM, Gardner M, Vilain E, Sandberg DE. 2015. Parental reports of stigma associated with child’s disorder of sex development. Int. J. Endocrinol 2015:980121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg DE, Gardner M, Callens N, Mazur T, DSD-TRN Psychosoc. Workgr., et al. 2017. Interdisciplinary care in disorders/differences of sex development (DSD): the psychosocial component of the DSD-Translational Research Network. Am. J.Med. Genet. C Semin. Med. Genet 175:279–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg DE, Mazur T. 2014. A noncategorical approach to the psychosocial care of persons with DSD and their families. In Gender Dysphoria and Disorders of Sex Development, ed. Kreukels BPC, Steensma TD, de Vries ALC, pp. 93–114. New York: Springer [Google Scholar]
- Sandberg DE, Vena JE, Weiner J, Beehler GP, Swanson M, Meyer-Bahlburg HF. 2003. Hormonally active agents in the environment and children’s behavior: assessing effects on children’s gender-dimorphic outcomes. Epidemiology 14:148–54 [DOI] [PubMed] [Google Scholar]
- Sanders C, Carter B, Goodacre L. 2008. Parents’ narratives about their experiences of their child’s reconstructive genital surgeries for ambiguous genitalia. J. Clin. Nurs 17:3187–95 [DOI] [PubMed] [Google Scholar]
- Sanders C, Carter B, Goodacre L. 2012. Parents need to protect: influences, risks and tensions for parents of prepubertal children born with ambiguous genitalia. J. Clin. Nurs 21:3315–23 [DOI] [PubMed] [Google Scholar]
- Schlomer B, Breyer B, Copp H, Baskin L, DiSandro M. 2014. Do adult men with untreated hypospadias have adverse outcomes? A pilot study using a social media advertised survey. J. Pediatr. Urol 10:672–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbucher V, Schweizer K, Rustige L, Schützmann K, Brunner F, Richter-Appelt H. 2012. Sexual quality of life of individuals with 46,XY disorders of sex development. J. Sex. Med 9:3154–70 [DOI] [PubMed] [Google Scholar]
- Schützmann K, Brinkmann L, Schacht M, Richter-Appelt H. 2009. Psychological distress, self-harming behavior, and suicidal tendencies in adults with disorders of sex development. Arch. Sex. Behav 38:16–33 [DOI] [PubMed] [Google Scholar]
- Schweizer K, Brunner F, Gedrose B, Handford C, Richter-Appelt H. 2017. Coping with diverse sex development: treatment experiences and psychosocial support during childhood and adolescence and adult well-being. J. Pediatr. Psychol 42(5):504–19. 10.1093/jpepsy/jsw058 [DOI] [PubMed] [Google Scholar]
- Schweizer K, Brunner F, Schützmann K, Schönbucher V, Richter-Appelt H. 2009. Gender identity and coping in female 46, XY adults with androgen biosynthesis deficiency (intersexuality/DSD). J. Couns. Psychol 56:189–201 [Google Scholar]
- Siminoff LA, Sandberg DE. 2015. Promoting shared decision making in disorders of sex development (DSD): decision aids and support tools. Horm. Metab. Res 47:335–39 [DOI] [PubMed] [Google Scholar]
- Skakkebaek A, Moore PJ, Chang S, Fedder J, Gravholt CH. 2018. Quality of life in men with Klinefelter syndrome: the impact of genotype, health, socioeconomics, and sexual function. Genet. Med 20:214–22 [DOI] [PubMed] [Google Scholar]
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, et al. 2018. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab 103:4043–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPLC (South. Poverty Law Cent.). 2013. M.C. V. Aaronson. Southern Poverty Law Center. https://www.splcenter.org/seeking-justice/case-docket/mc-v-aaronson
- Stout SA, Litvak M, Robbins NM, Sandberg DE.2010. Congenital adrenal hyperplasia: classification of studies employing psychological endpoints. Int. J. Pediatr. Endocrinol 2010:191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne DM. 2020. Physiology and disorders of puberty. In Williams Textbook of Endocrinology, ed. Melmed S, Auchus R, Goldfine A, Koenig R, Rosen C, pp. 1023–164.e25. Philadelphia: Elsevier [Google Scholar]
- Suorsa KI, Mullins AJ, Tackett AP, Reyes KJ, Austin P, et al. 2015. Characterizing early psychosocial functioning of parents of children with moderate to severe genital ambiguity due to disorders of sex development. J. Urol 194:1737–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Grift TC, Rapp M, Holmdahl G, Duranteau L, Nordenskjold A, DSD-Life Group. 2021. Masculinizing surgery in disorders/differences of sex development: clinician- and participant-evaluated appearance and function. BJU Int. In press. 10.1111/bju.15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S 2019. A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47, XYY). Curr. Opin. Psychiatry 32:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Swaab H. 2020. Emotion regulation in adults with Klinefelter syndrome (47,XXY): neurocognitive underpinnings and associations with mental health problems. J. Clin. Psychol 76:228–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne G, Grover S, Hutson J, Sinclair A, Metcalfe S, et al. 2005. A long-term outcome study of intersex conditions. J. Pediatr. Endocrinol. Metab 18:555–67 [DOI] [PubMed] [Google Scholar]
- Warne GL, Raza J. 2008. Disorders of sex development (DSDs), their presentation and management in different cultures. Rev. Endocr. Metab. Disord 9:227–36 [DOI] [PubMed] [Google Scholar]
- Wiener S 2022. Senator Wiener will not seek to advance SB 225—intersex civil rights legislation—due to lack of votes in committee. News Release, Jan. 5. https://sd11.senate.ca.gov/news/20220105-senator-wiener-willnot-seek-advance-sb-225-%E2%80%94-intersex-civil-rights-legislation-%E2%80%94-due [Google Scholar]
- Wiesemann C, Ude-Koeller S, Sinnecker GH, Thyen U. 2010. Ethical principles and recommendations for the medical management of differences of sex development (DSD)/intersex in children and adolescents. Eur. J. Pediatr 169:671–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski AB. 2017. Psychosocial implications of disorders of sex development treatment for parents. Curr. Opin. Urol 27:11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski AB, Batista RL, Costa EMF, Finlayson C, Sircili MHP, et al. 2019. Management of 46,XY differences/disorders of sex development (DSD) throughout life. Endocr. Rev 40:1547–72 [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Sandberg DE. 2015. Parenting children with disorders of sex development (DSD): a developmental perspective beyond gender. Horm. Metab. Res 47:375–79 [DOI] [PubMed] [Google Scholar]
- Wolfe-Christensen C, Fedele DA, Kirk K, Mullins LL, Lakshmanan Y, Wisniewski A. 2014. Caregivers of children with a disorder of sex development: associations between parenting capacities and psychological distress. J. Pediatr. Urol 10:538–43 [DOI] [PubMed] [Google Scholar]
- Wolfe-Christensen C, Wisniewski AB, Mullins AJ, Reyes KJ,Austin P, et al. 2017. Changes in levels of parental distress after their child with atypical genitalia undergoes genitoplasty. J. Pediatr. Urol 13:32.e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstencroft J, Skuse D. 2019. Social skills and relationships in Turner syndrome. Curr. Opin. Psychiatry 32:85–91 [DOI] [PubMed] [Google Scholar]


