Abstract
Purpose
Prior data suggest driver-mutated NSCLC, especially EGFR and ALK tumors, poorly respond to immunotherapy. However, little research using real-world cohorts have been performed, nor is it clear whether PD-L1 and smoking history are predictive of outcomes in such tumors. This study assessed rwPFS in a large cohort with driver-mutated advanced NSCLC treated with single-agent PD-1/PDL-1 inhibitors.
Methods
Real-world data from 1746 patients were analyzed and rwPFS with immunotherapy was determined for EGFR, ALK, BRAF, and KRAS tumors. Kaplan–Meier curves characterized rwPFS and correlated with PD-L1 and smoking history. Comparisons were tested using log-rank.
Results
Median rwPFS and the percent progression-free at 12 months were greater among KRAS (3.3 months, 21.1%) and BRAF (3.6 months, 20.6%) as compared to EGFR (2.5 months, 8.1%) and ALK tumors (2.3 months, 11.2%). KRAS tumors with PD-L1 ≥ 1% had longer rwPFS than PD-L1 < 1% tumors (4.1 versus 3.2 months, p = 0.001). PD-L1 positivity did not predict rwPFS in EGFR, ALK, or BRAF tumors. However, a smoking history was associated with longer rwPFS in EGFR (2.6 versus 2.3 months, p = 0.048) and ALK tumors (3.0 versus 2.1 months, p = 0.049) as compared to no smoking history.
Conclusion
Real-world PFS with immunotherapy was greater in KRAS and BRAF as compared to EGFR and ALK tumors. PD-L1 positivity was predictive in KRAS and not associated with rwPFS in other mutation types. While median rwPFS was short for EGFR and ALK tumors, small subsets were progression-free at 12 months. Better characterizing these subsets that benefit, along with developing strategies to overcome immunotherapy resistance in EGFR/ALK tumors are needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04089-9.
Keywords: Immunotherapy, Driver-mutation, EGFR, ALK, NSCLC, Real-world cohort
Introduction
The emergence of checkpoint inhibitors has dramatically changed the treatment landscape of advanced non-small cell lung cancer (NSCLC). PD-1/PD-L1 inhibitors can be utilized as monotherapies in the second-line setting or be used as a first-line therapy, either alone (Borghaei et al. 2015; Reck et al. 2019a, b) or in combination with chemotherapy (Gandhi et al. 2018) or CTLA-4 inhibition (Hellmann et al. 2019). Such checkpoint-inhibitor based therapies can result in sustained durable responses and extended survival; however, only a fraction of patients benefit from such favorable outcomes.
Patients with tumors possessing certain oncogenic drivers, in particular aberrations in the epidermal growth factor receptor (EGFR) and the anaplastic lymphoma kinase (ALK) genes, generally appear to obtain little benefit from checkpoint inhibitor monotherapy in prior studies. Meta-analyses of EGFR subgroups from second-line registration trials showed no benefit of single-agent immunotherapy as compared to docetaxel (Lee et al. 2017, 2018). A small number of retrospective cohorts have similarly demonstrated poorer outcomes with single-agent immunotherapy in EGFR-mutated and ALK-rearranged tumors (Gainor et al. 2016; Hastings et al. 2019; Mazieres et al. 2019; Yamada et al. 2019). Targeted therapies with oral tyrosine kinase inhibitors can be highly effective in such driver-mutated tumors (Peters et al. 2017; Soria et al. 2018); however, eventual disease progression is inevitable and standard chemotherapeutic options provide modest and short-lived benefits. Given this, continued research on the utility of checkpoint inhibitors in patients with tumors possessing oncogenic mutations is needed. In addition, further study is needed examining the clinical and biological markers identifying patients with driver-mutated tumors who are more likely to derive benefit. Little research has been performed studying outcomes in driver-mutated tumors treated with immunotherapy utilizing large real-world patient cohorts. Moreover, it is still not clear whether biomarkers, like PD-L1 expression or smoking history, are predictive of outcomes in oncogene-addicted tumors. In particular, the value of PD-L1 in predicting immunotherapy outcomes in EGFR-mutated tumors has been controversial, with studies thus far demonstrating conflicting findings (Hastings et al. 2019; Lisberg et al. 2018; Masuda et al. 2021; Mazieres et al. 2019).
The purpose of this research was to assess real-world progression-free survival (rwPFS) in a large cohort of driver-mutated advanced NSCLC patients treated with single-agent checkpoint inhibitors and to evaluate tumor PD-L1 expression and patient smoking history as predictive markers of outcomes. This retrospective cohort analysis was performed using data gathered from a real-world electronic health record (EHR)-derived de-identified database and included advanced NSCLC patients with tumors possessing oncogenic mutations in EGFR, ALK, BRAF, and KRAS.
Methods
Study design
Advanced NSCLC (stage IIIB or IV) patients with driver-mutated tumors treated with single-agent immunotherapy between April 23, 2014 and February 28, 2019 were selected from the nationwide Flatiron Health EHR-derived de-identified database. This longitudinal database, comprising de-identified patient-level structured and unstructured data curated via technology-enabled abstraction, includes clinical and biomarker data from patients seen in approximately 280 community and academic cancer clinics across the U.S. (~ 800 sites of care) (Howlader et al. 2020; Ma et al. 2020). Biomarker data were abstracted from unstructured EHR biomarker testing or pathology reports. When these data sources were not available, information was abstracted from clinician notes. The majority of patients in the database originated from community oncology settings; relative community/academic proportions may vary depending on study cohort. Further details describing abstraction methods used by Flatiron Health to gather data for their nationwide database have been previously described (Khozin et al. 2019). Institutional Review Board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent. The original database provided to this study’s investigators included 53,591 advanced NSCLC patients, of which 10,033 patients had tumors with a documented mutation in EGFR, ALK, BRAF, or KRAS. Of these patients with a mutated tumor, 1815 were treated with either the PD-1/PD-L1 inhibitor pembrolizumab, nivolumab, or atezolizumab as monotherapy. Patients with incomplete historical treatment data (i.e. a gap of > 90 days between their advanced diagnosis date and start of structured data in the EHR) or patients missing data on key study variables were excluded. The final cohort used for this study’s analyses included 1746 patients.
Patient and clinical variables evaluated included sex, age, line of therapy of checkpoint inhibitor, tumor histology, history of smoking, and PD-L1 expression. Tumor histologies included non-squamous, squamous, and NSCLC NOS (not otherwise specified). Information on smoking history was available as a dichotomous variable (a history of smoking vs. no history of smoking). Level of PD-L1 expression was determined using data on the percentage of tumor cells positive-staining for PD-L1.
The primary outcome variable was rwPFS as defined as the time from single-agent checkpoint inhibitor initiation to date of real-world progression or death. Patients alive and without documented progression were censored at date of last follow-up. Dates of real-world progression events were ascertained using clinician documentation of growth or worsening of disease and abstracted from data obtained from the EHR by trained medical reviewers. This methodology used to determine real-world progression was evaluated with a validity framework and previously described (Griffith et al. 2019).
Statistical analyses
Standard descriptive analyses were performed for patient and clinical variables by tumor driver-mutation type and differences were tested using the Pearson’s chi-square test. In this study’s primary analyses, median rwPFS in months was determined for patients by each tumor mutation type (i.e. EGFR, ALK, BRAF, and KRAS) and differences in rwPFS were assessed within each tumor mutation type by tumor PD-L1 positivity (< 1% vs. ≥ 1%) and by a history of smoking (yes vs. no). Among patients with EGFR or KRAS tumors, subgroup sizes were sufficient to perform additional analyses assessing median rwPFS by a trichotomous PD-L1 expression variable comprised of the categories: < 1%, 1–49%, and ≥ 50%. Kaplan–Meier curves characterized rwPFS and comparisons were evaluated in the overall cohort and in tumor mutation subgroups using the log-rank test. Multivariable Cox regression was used to assess time to real-world progression by tumor mutation type, after adjusting for sex, age (< 65 years vs. ≥ 65 years), and line of therapy single agent checkpoint inhibitor given (first line vs. second line vs. ≥ third line). p values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, N.Y., USA).
Results
In this study’s cohort, 458 patients had EGFR, 65 had ALK, 146 had BRAF, and 1077 had KRAS-positive tumors (Table 1). Women were greater represented in the overall cohort, and especially among patients with EGFR-mutated disease. Mean age for the entire cohort was 68.7 years and ALK-positive tumors had a higher percentage of patients below the age of 65 years as compared to other tumor mutation types. Tumors were predominantly of non-squamous histology, and this did not differ by oncogene mutation type. A significantly higher percentage of patients with KRAS (38.3%) and BRAF (39.0%) tumors received a checkpoint inhibitor as a first-line therapy as compared to patients with EGFR (8.7%) and ALK positive (16.9%) disease. Approximately half of patients with EGFR (47.6%) or ALK tumors (55.4%) did not have a history of smoking. In contrast, most patients with KRAS (94.4%) and BRAF-mutated tumors (86.3%) had a smoking history. A subset of 792 patients had data available on percentage of tumor cells staining for PD-L1. Presence of PD-L1 positivity on tumor cells did not vary significantly by oncogene mutation type.
Table 1.
Characteristics of study cohort (N = 1746)
| Patient/clinical characteristic | Total (N = 1746) | EGFR (n = 458) | ALK (n = 65) | BRAF (n = 146) | KRAS (n = 1077) | p value |
|---|---|---|---|---|---|---|
| Sex, n (%) | ||||||
| Male | 730 (41.8) | 163 (35.6) | 33 (50.8) | 68 (46.6) | 466 (43.3) | 0.008 |
| Female | 1016 (58.2) | 295 (64.4) | 32 (49.2) | 78 (53.4) | 611 (56.7) | |
| Age, n (%) | ||||||
| < 65 years | 609 (34.9) | 161 (35.2) | 34 (52.3) | 46 (31.5) | 368 (34.2) | 0.022 |
| ≥ 65 years | 1137 (65.1) | 297 (64.8) | 31 (47.7) | 100 (68.5) | 709 (65.8) | |
| Histology, n (%) | ||||||
| Nonsquamous | 1584 (90.7) | 425 (92.8) | 58 (89.2) | 124 (84.9) | 977 (90.7) | 0.111 |
| Squamous | 115 (6.6) | 24 (5.2) | 6 (9.2) | 17 (11.6) | 68 (6.3) | |
| NSCLC NOS | 47 (2.7) | 9 (2.0) | 1 (1.5) | 5 (3.4) | 32 (3.0) | |
| Checkpoint inhibitor, n (%) | ||||||
| First line | 520 (29.8) | 40 (8.7) | 11 (16.9) | 57 (39.0) | 412 (38.3) | < 0.001 |
| Second line | 751 (43.0) | 161 (35.2) | 19 (29.2) | 73 (50.0) | 498 (46.2) | |
| ≥ Third line | 475 (27.2) | 257 (56.1) | 35 (53.8) | 16 (11.0) | 167 (15.5) | |
| Smoking history, n (%) | ||||||
| Yes | 1412 (80.9) | 240 (52.4) | 29 (44.6) | 126 (86.3) | 1017 (94.4) | < 0.001 |
| No | 334 (19.1) | 218 (47.6) | 36 (55.4) | 20 (13.7) | 60 (5.6) | |
| PD-L1 expression, n (%)* | ||||||
| ≥ 1% | 587 (74.1) | 116 (69.5) | 25 (80.6) | 62 (77.5) | 384 (74.7) | 0.370 |
| < 1% | 205 (25.9) | 51 (30.5) | 6 (19.4) | 18 (22.5) | 130 (25.3) | |
*A subset of 792 patients had available data on percentage of tumor cells staining for PD-L1
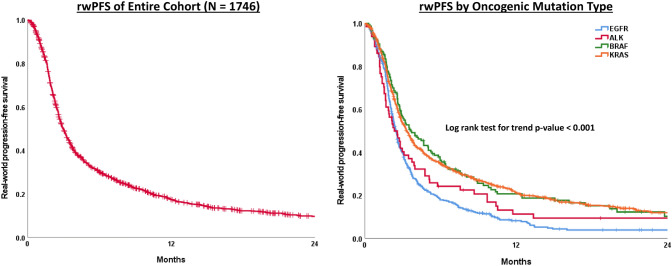
In the overall cohort, rwPFS with a single-agent PD1/PD-L1 inhibitor was 3.0 months (95% Confidence Interval 2.8–3.1)’ however, this varied significantly by mutation type (p < 0.001)(Fig. 1). Patients with KRAS-mutated tumors treated with a checkpoint inhibitor had a median rwPFS of 3.3 months and 21% were progression-free at 12 months. Similarly, patients with BRAF-mutated tumors had a median rwPFS of 3.6 months and 21% were progression-free at 12 months. Patients with EGFR-mutated tumors had significantly shorter rwPFS as compared to those with KRAS (p < 0.001) and BRAF-mutated tumors (p < 0.001) in pairwise comparisons. Patient with ALK-positive tumors had significantly shorter rwPFS as compared to patients with KRAS-mutated tumors (p = 0.025) and the difference in rwPFS between those with ALK versus BRAF-positive disease neared statistical significance (p = 0.06) in pairwise comparisons. Patients with EGFR-mutated tumors had a median rwPFS of 2.5 months and 8% were progression-free at 12 months, while for patients with ALK-positive disease median rwPFS was 2.3 months and 11% were progression-free at 12 months. After adjusting for sex, age, and what line of therapy checkpoint inhibitor was given, this study’s multivariable Cox regression model demonstrated superior outcomes with respect to rwPFS in patients with KRAS (HR 0.75, 95% CI 0.66–0.85) and BRAF tumors (HR 0.74, 95% CI 0.59–0.91) as compared to patients with EGFR tumors (reference group). No statistical difference was observed between patients with ALK and EGFR tumors (reference group) in this model (HR 0.89, 95% CI 0.67–1.17) (Table 2).
Fig. 1.
Kaplan–Meier curves of real-world progression free survival of entire cohort and by oncogenic mutation type
Table 2.
Real-world progression free survival (months) by oncogenic mutation type (N = 1746)
| # Events/total N | Median rwPFS (95% CI) | 6-month % PFS | 12-month % PFS | HR (95% CI) * | |
|---|---|---|---|---|---|
| Entire cohort | 1423/1746 | 3.0 (2.8–3.1) | 30.0 | 17.3 | – |
| Mutation type | |||||
| EGFR | 406/458 | 2.5 (2.3–2.6) | 17.8 | 8.1 | Reference |
| ALK | 58/65 | 2.3 (1.6–3.1) | 24.1 | 11.2 | 0.89 (0.67–1.17) |
| BRAF | 115/146 | 3.6 (2.6–4.7) | 36.2 | 20.6 | 0.74 (0.59–0.91) |
| KRAS | 844/1077 | 3.3 (3.0–3.6) | 34.7 | 21.1 | 0.75 (0.66–0.85) |
rwPFS Real-world progression free survival, CI confidence interval, HR hazard ratio
*Cox regression model adjusted for sex, age, and line of therapy checkpoint inhibitor given
In the subgroup of 792 patients with data available on tumor PD-L1 expression, rwPFS did not differ significantly by PD-L1 positivity (≥ 1% vs. < 1%) for EGFR, ALK, or BRAF-mutated tumors. Among patients with EGFR-mutated NSCLC, median rwPFS was 2.5 months for patients with PD-L1 positive tumors vs. 3.1 months for patients with PD-L1 negative tumors (p = 0.966). Among those with ALK (2.2 months vs. 1.6 months, p = 0.108) and BRAF-positive NSCLC (4.1 months vs. 3.1 months, p = 0.686) there were numerical trends indicating longer rwPFS in PD-L1 positive tumors, however none of these findings were statistically significant. Only in patients with KRAS-mutant disease did rwPFS significantly differ by PD-L1 positivity (4.1 vs. 3.2 months, p = 0.001) (Table 3, Fig. 2).
Table 3.
Real-world progression free survival (months) in each oncogenic mutation type by PD-L1 expression or smoking history
| Mutation | Median rwPFS (95% CI) | |||||
|---|---|---|---|---|---|---|
| PD-L1 expression* | Smoking history | |||||
| ≥ 1% | < 1% | p value | Yes | No | p value | |
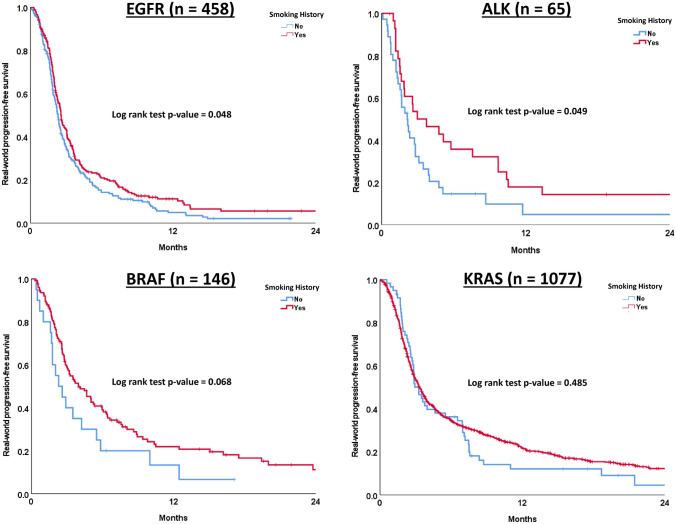
| EGFR | 2.5 (2.2–2.9) | 3.1 (2.3–3.9) | 0.966 | 2.6 (2.4–2.8) | 2.3 (2.1–2.5) | 0.048 |
| ALK | 2.2 (0.0–4.4) | 1.6 (0.0–3.3) | 0.108 | 3.0 (0.1–5.8) | 2.1 (1.3–2.9) | 0.049 |
| BRAF | 4.1 (1.4–6.9) | 3.1 (0.3–5.9) | 0.686 | 4.0 (2.5–5.4) | 2.3 (1.1–3.5) | 0.068 |
| KRAS | 4.1 (3.3–4.9) | 3.2 (2.6–3.7) | 0.001 | 3.3 (3.0–3.6) | 3.3 (2.6–4.0) | 0.485 |
rwPFS real-world progression free survival, CI confidence interval
*A subset of 792 patients had available data on percentage of tumor cells staining for PD-L1
Fig. 2.
Kaplan–Meier curves of real-world progression free survival in each oncogenic mutation type by PD-L1 positivity
Additional analyses using the tumor PD-L1 expression categories of < 1%, 1–49%, and ≥ 50% were performed in patients with EGFR and KRAS tumors (Supplemental Figure). Again, among patients with EGFR-mutant disease, median rwPFS did not significantly differ (p = 0.985) by the level of tumor PD-L1 expression: 3.1 months for PD-L1 < 1% tumors (n = 51) vs. 2.6 months for PD-L1 1–49% tumors (n = 60) vs. 2.4 months for PD-L1 ≥ 50% tumors (n = 56). Among patients with KRAS tumors, rwPFS varied significantly by the level of tumor PD-L1 expression (p < 0.001). The greatest median rwPFS was among patients with KRAS tumors in the highest PD-L1 expression category: 3.2 months for PD-L1 < 1% tumors (n = 130) vs. 3.1 months for PD-L1 1–49% tumors (n = 110) vs. 4.8 months for PD-L1 ≥ 50% tumors (n = 274).
A history of smoking as compared to no smoking history resulted in a statistically significant, albeit modest, improvement in rwPFS among patients with EGFR-mutated tumors (2.6 vs. 2.3 months, p value = 0.048) and ALK-rearranged tumors (3.0 vs. 2.1 months, p value = 0.049). Among patients with BRAF-mutated tumors, there was a trend towards longer rwPFS observed in those with a smoking history as compared to no smoking history; however, this was not statistically significant (4.0 vs. 2.3 months, p value = 0.068). No association between history of smoking and rwPFS was seen for patients with KRAS-mutated tumors (Table 3, Fig. 3).
Fig. 3.
Kaplan–Meier curves of real-world progression free survival in each oncogenic mutation type by smoking history
Discussion
In this analysis, of one of the largest real-world cohorts of patients with driver-mutated NSCLC to date, rwPFS with single-agent checkpoint inhibitors varied significantly between oncogenic mutation types. Patients with KRAS and BRAF-mutated tumors had longer rwPFS times, and a greater percentage were progression-free at 12 months as compared to patients with EGFR and ALK-positive tumors. It is important to acknowledge that a significantly greater percentage of patients with KRAS and BRAF-positive NSCLC received a checkpoint inhibitor as a first-line treatment when compared to patients with EGFR and ALK tumors. This treatment pattern is expected given the highly effective oral targeted therapy options available as first-line therapies for EGFR and ALK-positive disease. However, the superior outcomes with checkpoint inhibitors observed among those with KRAS and BRAF tumors were not solely a function of these patients receiving such therapies as a first-line treatment, as this study’s multivariable Cox regression model yielded findings congruent with the unadjusted results, even after accounting for what line of therapy a checkpoint inhibitor was given.
Previous research has demonstrated greater activity of PD1/PD-L1 inhibitors in KRAS-mutated tumors both in subgroup analyses of clinical trials (Borghaei et al. 2015; Lee et al. 2018) and in retrospective cohorts (Mazieres et al. 2019). No clinical trial data exist for BRAF-mutated NSCLC treated with immunotherapy; although several small retrospective studies similarly indicate favorable activity of checkpoint inhibitors in this tumor mutation type (Dudnik et al. 2018; Guisier et al. 2020; Mazieres et al. 2019; Rihawi et al. 2019). The results from our large real-world cohort of patients with KRAS and BRAF-mutant disease are consistent with the findings from these earlier smaller cohorts. However, it must be acknowledged that both KRAS and BRAF-positive NSCLC are each heterogenous categories and genetic variants within each mutation type or the presence of certain co-mutations have been noted in prior retrospective work that may impact tumor immunogenicity (Dong et al. 2017a, b; Skoulidis et al. 2019). For instance, among KRAS-mutant disease, tumors with a co-mutation in serine/threonine kinase 11/liver kinase B1 (STK11/LKB1) exhibit greater primary resistance to immune-based therapies, including in tumors that are PD-L1 positive (Skoulidis et al. 2018). Among BRAF-mutated tumors in the Mazieres et al. IMMUNOTARGET registry retrospective analyses (Mazieres et al. 2019), there was a numerical trend towards shorter PFS in the V600E subgroup as compared to other BRAF mutation types, however not statistically significant. Our study’s analysis did not include granular data on tumor mutation subtypes (e.g. specific BRAF mutation class or KRAS molecular subtype), nor was information available on co-occurring mutations, however future research that incorporates such data should be an area of continued work.
In addition, consistent with prior clinical studies (Bylicki et al. 2017; Gainor et al. 2016; Lee et al. 2017; Mazieres et al. 2019), patients with EGFR and ALK NSCLC in our analysis overall seemed to benefit little from single-agent immunotherapy and demonstrated relatively short PFS times. The lack of responses with checkpoint inhibitors seen in EGFR/ALK positive cases is thought to be due to such tumors possessing uninflamed tumor microenvironments characterized by low tumor-infiltrating lymphocyte (TIL) concentrations (Gainor et al. 2016), low tumor mutational burdens (Dong et al. 2017a, b), or perhaps TILs that are “inactive” (Toki et al. 2018). However, it is noteworthy that a small subset of patients with EGFR (8%) and ALK (11%) tumors were progression-free at 12 months in our study. The IMMUNOTARGET analyses similarly found a small subgroup of patients with EGFR and ALK positive disease (6.4% and 5.9%, respectively) in their cohort to be progression-free at one year with single-agent immunotherapy (Mazieres et al. 2019). Admittedly, these percentages are small, but given the limited therapeutic options available after targeted treatments in such driver-mutated tumors, further clinical and molecular characterization of these small EGFR and ALK subsets that may derive sustained benefit from checkpoint inhibitors is still of great interest. For example, some retrospective studies suggest that particular mutation subtypes of EGFR may be associated with immunotherapy response and have noted better outcomes in L858R tumors (Hastings et al. 2019), or tumors possessing exon 20 insertions and other uncommon EGFR mutations (Hastings et al. 2019; Lau et al. 2021; Yamada et al. 2019). Continued research on the influence of genetic variants in EGFR and ALK or the impact of co-occurring mutations on the responsiveness of such tumors to checkpoint inhibitors is of interest.
PD-L1 expression on tumor cells is the current standard to identify advanced NSCLC tumors more likely to respond to immunotherapy. However, in our analysis, PD-L1 positivity was significantly correlated to rwPFS only in KRAS tumors, with patients possessing KRAS tumors with PD-L1 ≥ 50% appearing to derive the greatest benefit. Tumor PD-L1 positivity otherwise was not correlated to rwPFS in EGFR, ALK, and BRAF tumors in our study.
The utility of PD-L1 in predicting immunotherapy outcomes in EGFR-mutated NSCLC, in particular, has been a point of controversy. Much of the prior literature examining this have been in small patient cohorts and have produced inconsistent findings. Results from a phase II trial by Lisberg et al. demonstrated poor outcomes with single-agent pembrolizumab in TKI naïve patients with PD-L1 positive, EGFR-mutated NSCLC; including among patients possessing tumors with PD-L1 ≥ 50% (Lisberg et al. 2018). Due to the lack of observed efficacy, this trial was stopped early after only 11 of 25 planned patients were treated. In addition, retrospective cohort studies by Hastings et al. and Yamada et al., in patients with EGFR disease treated with single-agent checkpoint inhibitors, did not find associations between PD-L1 expression and immunotherapy response (Hastings et al. 2019; Yamada et al. 2019). In contrast, there are also several studies supporting a relationship between higher PD-L1 expression and improved outcomes with immunotherapy in EGFR disease. For instance, the phase II ATLANTIC trial (Garassino et al. 2018) found a greater response rate of 12.2% for EGFR/ALK tumors with PD-L1 > 25% as compared to 3.6% for PD-L1 < 25% after treatment with the checkpoint inhibitor durvalumab, and the retrospective IMMUNOTARGET analyses found PD-L1 positivity (PD-L1 ≥ 1%) correlated to longer PFS in EGFR tumors treated with immunotherapy (Mazieres et al. 2019). A recent retrospective study by Masuda et al. examining a small cohort of EGFR-mutant NSCLC cases found that patients possessing tumors with PD-L1 expression ≥ 50% had longer median PFS with single-agent immunotherapy as compared to those with low expression (5.3 months versus 1.6 months) (Masuda et al. 2021). As noted, many of these prior studies were limited by their small cohort sizes and the number of study cases with available PD-L1 data. To our knowledge, our study includes the largest cohort to date of EGFR-mutant NSCLC cases with PD-L1 expression information (n = 167), and our findings clearly do not indicate any association between PD-L1 positivity and rwPFS. Moreover, dissimilar to the abovementioned findings from Masuda et al., even among patients in our study possessing EGFR tumors with PD-L1 ≥ 50%, median rwPFS was short and not different from patients possessing tumors with lower levels of PD-L1 expression.
Among patients with ALK-positive NSCLC in our cohort, there was a non-significant trend towards longer rwPFS in PD-L1 positive tumors. However, this analysis was in a small sample of patients (n = 31), thus limiting the ability to draw any firm conclusions.
While PD-L1 positivity on tumor cells did not significantly correlate with rwPFS in EGFR and ALK tumors in our analyses, a history of smoking was positively associated with rwPFS in patients with such mutated-tumors. Admittedly, the additional PFS benefits seen were modest, however further investigation of patient smoking history as a clinical predictor of response in such tumors should be considered.
These analyses have several limitations. First, this was a retrospective cohort study using EHR data; thus there may be missing data not captured in the database (e.g. any off-site care, documentation lapses). Second, biomarker data were abstracted from unstructured EHR biomarker testing or pathology reports, rather than determined via a centralized molecular assessment. Third, despite the large overall sample size, there were some small subgroups examined (e.g. PD-L1 subgroups among patients with ALK or BRAF tumors), limiting the power of detecting noteworthy differences in such analyses. Last, data on smoking history were only available as a binary variable (i.e. a history of smoking vs. no history of smoking), and no additional data on years of smoking, number of cigarettes smoked, or current versus former smoking were available. Thus, the history of smoking category was likely quite heterogenous with a wide range of smoking histories which may have diluted study associations.
The inferior outcomes seen in EGFR and ALK-mutated NSCLC treated with single-agent checkpoint inhibitors as compared to KRAS and BRAF-mutated tumors in this large cohort of patients are consistent with prior studies and reinforces the need for continued research on strategies that overcome immunotherapy resistance in EGFR/ALK disease. Promising data from subgroup analyses from the phase III clinical trial IMPower150 revealed superior PFS with the combination regimen that included bevacizumab, carboplatin, paclitaxel, and the checkpoint inhibitor atezolizumab as compared to bevacizumab, carboplatin, paclitaxel alone in patients with EGFR/ALK NSCLC (Reck et al. 2019a, b). However, prospective validation of such a combination regimen is needed and currently no checkpoint inhibitor with chemotherapy ± VEGF inhibitor combination regimens are FDA approved for patients with EGFR and ALK NSCLC. In addition, while our findings generally indicate that most patients with EGFR/ALK tumors derive little benefit from checkpoint inhibitor monotherapy, a small subset of patients appear to have durable PFS times. Better clinical and molecular characterization of this small subgroup that may obtain benefit from single-agent immunotherapy is of interest and should be an area of ongoing research.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Fig. Kaplan-Meier curves of real-world progression free survival in EGFR and KRAS tumors by PD-L1 expression categories (< 1%, 1 – 49%, ≥ 50%) (PPTX 82 kb)
Acknowledgements
This work was supported by the Comprehensive Cancer Center Program at Fox Chase (P30 CA006927) and by the TUFCCC/HC Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704(5) from the National Cancer Institute of National Institutes of Health (NCI/NIH).
Author contributions
JNB: conceptualization, investigation, formal analysis, writing—original draft preparation. JRB: investigation, writing—review and editing. EAH: formal analysis, writing—review and editing. EAR: writing—review and editing. MLC: writing—review and editing. JT: conceptualization, investigation, writing—review and editing.
Funding
This work was supported by the Comprehensive Cancer Center Program at Fox Chase (P30 CA006927) and by the TUFCCC/HC Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704(5) from the National Cancer Institute of National Institutes of Health (NCI/NIH).
Data availability
The data underlying this article were provided by Flatiron Health via a data sharing agreement.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Fox Chase Cancer Center (IRB # 18-9047).
Consent for publication
This manuscript contains no individual person’s data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylicki O, Paleiron N, Margery J, Guisier F, Vergnenegre A, Robinet G et al (2017) Targeting the PD-1/PD-L1 immune checkpoint in EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol 12(5):563–569. 10.1007/s11523-017-0510-9 [DOI] [PubMed] [Google Scholar]
- Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z et al (2017a) EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 6(11):e1356145. 10.1080/2162402X.2017.1356145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY et al (2017b) Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 23(12):3012–3024. 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- Dudnik E, Peled N, Nechushtan H, Wollner M, Onn A, Agbarya A et al (2018) BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol 13(8):1128–1137. 10.1016/j.jtho.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z et al (2016) EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 22(18):4585–4593. 10.1158/1078-0432.CCR-15-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H et al (2018) Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 19(4):521–536. 10.1016/S1470-2045(18)30144-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith SD, Miksad RA, Calkins G, You P, Lipitz NG, Bourla AB et al (2019) Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform 3:1–13. 10.1200/CCI.19.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisier F, Dubos-Arvis C, Vinas F, Doubre H, Ricordel C, Ropert S et al (2020) Efficacy and safety of Anti-PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01–2018. J Thorac Oncol 15(4):628–636. 10.1016/j.jtho.2019.12.129 [DOI] [PubMed] [Google Scholar]
- Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J et al (2019) EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 30(8):1311–1320. 10.1093/annonc/mdz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E et al (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381(21):2020–2031. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2020) SEER cancer statistics review, 1975–2017. National Cancer Institute, Bethesda [Google Scholar]
- Khozin S, Miksad RA, Adami J, Boyd M, Brown NR, Gossai A et al (2019) Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 125(22):4019–4032. 10.1002/cncr.32383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SCM, Fares AF, Le LW, Mackay KM, Soberano S, Chan SW et al (2021) Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin Lung Cancer 22(4):253–259. 10.1016/j.cllc.2020.12.015 [DOI] [PubMed] [Google Scholar]
- Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 12(2):403–407. 10.1016/j.jtho.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V et al (2018) Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 4(2):210–216. 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T et al (2018) A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol 13(8):1138–1145. 10.1016/j.jtho.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Long L, Moon S, Adamson BJS, Baxi SS (2020) Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPCR. medRxiv. 10.1101/2020.03.16.2003714333330879 [Google Scholar]
- Masuda K, Horinouchi H, Tanaka M, Higashiyama R, Shinno Y, Sato J et al (2021) Efficacy of anti-PD-1 antibodies in NSCLC patients with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin Oncol 147(1):245–251. 10.1007/s00432-020-03329-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L et al (2019) Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 30(8):1321–1328. 10.1093/annonc/mdz167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW et al (2017) Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377(9):829–838. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F et al (2019a) Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 7(5):387–401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A et al (2019b) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37(7):537–546. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- Rihawi K, Giannarelli D, Galetta D, Delmonte A, Giavarra M, Turci D et al (2019) BRAF mutant NSCLC and immune checkpoint inhibitors: results from a real-world experience. J Thorac Oncol 14(3):e57–e59. 10.1016/j.jtho.2018.11.036 [DOI] [PubMed] [Google Scholar]
- Skoulidis F, Heymach JV (2019) Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 19(9):495–509. 10.1038/s41568-019-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF et al (2018) STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8(7):822–835. 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- Toki MI, Mani N, Smithy JW, Liu Y, Altan M, Wasserman B et al (2018) Immune marker profiling and programmed death ligand 1 expression across NSCLC mutations. J Thorac Oncol 13(12):1884–1896. 10.1016/j.jtho.2018.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hirai S, Katayama Y, Yoshimura A, Shiotsu S, Watanabe S et al (2019) Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med 8(4):1521–1529. 10.1002/cam4.2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. Kaplan-Meier curves of real-world progression free survival in EGFR and KRAS tumors by PD-L1 expression categories (< 1%, 1 – 49%, ≥ 50%) (PPTX 82 kb)
Data Availability Statement
The data underlying this article were provided by Flatiron Health via a data sharing agreement.