Abstract
Background
COVID-19 vaccines are recommended for people with multiple sclerosis (pwMS). Adequate humoral responses are obtained in pwMS receiving disease-modifying therapies (DMTs) after vaccination, with the exception of those receiving B-cell-depleting therapies and non-selective S1P modulators. However, most of the reported studies on the immunity of COVID-19 vaccinations have included mRNA vaccines, and information on inactivated virus vaccine responses, long-term protectivity, and comparative studies with mRNA vaccines are very limited. Here, we aimed to investigate the association between humoral vaccine responses and COVID-19 infection outcomes following mRNA and inactivated virus vaccines in a large national cohort of pwMS receiving DMTs.
Methods
This is a cross-sectional and prospective multicenter study on COVID-19-vaccinated pwMS. Blood samples of pwMS with or without DMTs and healthy controls were collected after two doses of inactivated virus (Sinovac) or mRNA (Pfizer-BioNTech) vaccines. PwMS were sub-grouped according to the mode of action of the DMTs that they were receiving. SARS-CoV-2 IgG titers were evaluated by chemiluminescent microparticle immunoassay. A representative sample of this study cohort was followed up for a year. COVID-19 infection status and clinical outcomes were compared between the mRNA and inactivated virus groups as well as among pwMS subgroups.
Results
A total of 1484 pwMS (1387 treated, 97 untreated) and 185 healthy controls were included in the analyses (male/female: 544/1125). Of those, 852 (51.05%) received BioNTech, and 817 (48.95%) received Sinovac. mRNA and inactivated virus vaccines result in similar seropositivity; however, the BioNTech vaccination group had significantly higher antibody titers (7.175±10.074) compared with the Sinovac vaccination group (823±1.774) (p<0.001). PwMS under ocrelizumab, fingolimod, and cladribine treatments had lower humoral responses compared with the healthy controls in both vaccine types. After a mean of 327±16 days, 246/704 (34.9%) of pwMS who were contacted had COVID-19 infection, among whom 83% had asymptomatic or mild disease. There was no significant difference in infection rates of COVID-19 between participants vaccinated with BioNTech or Sinovac vaccines. Furthermore, regression analyses show that no association was found regarding age, sex, Expanded Disability Status Scale score (EDSS), the number of vaccination, DMT type, or humoral antibody responses with COVID-19 infection rate and disease severity, except BMI Body mass index (BMI).
Conclusion
mRNA and inactivated virus vaccines had similar seropositivity; however, mRNA vaccines appeared to be more effective in producing SARS-CoV-2 IgG antibodies. B-cell-depleting therapies fingolimod and cladribine were associated with attenuated antibody titer. mRNA and inactive virus vaccines had equal long-term protectivity against COVID-19 infection regardless of the antibody status.
Keywords: COVID-19, Humoral response, Inactivated virus vaccine, mRNA vaccine, Multiple sclerosis
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected officially 650 million confirmed cases and led to approximately 6.6 million deaths globally; the numbers are likely to be at least 2–3 times higher. The disease first emerged in December 2019, rapidly spread worldwide, and was declared as a global pandemic by the World Health Organization (WHO) in February 2020 (WHO coronavirus disease. https://www.who.int/director-general/speeches/detail/who-directorgeneral-s-opening-remarks-at-the-media-briefingon-covid-19–11-march-2020). The development of COVID-19 vaccines has been heralded as a milestone in the management of this global pandemic. Several vaccine subtypes with different modes of action have been developed, aiming to promote an immune response against COVID-19 infection. Major vaccine approaches were inactivated vaccines, recombinant protein-based vaccines, non-replicated viral vector vaccines, replicated viral vector vaccines, and nucleic acid vaccines (Negahdaripour et al., 2020). The Multiple Sclerosis International Federation and MS experts have recommended that all people with multiple sclerosis (pwMS) be vaccinated against SARS-CoV-2, given that the risks of serious illness due to COVID-19 greatly outweigh the potential risks of the vaccines (Toscano et al., 2021). The introduction of COVID-19 vaccines has initially raised clinical concerns among some MS healthcare providers in that these vaccines may cause MS relapses or even induce MS development (Gustavo C.R. et al., 2021). The exclusion of patients from the vaccine trials has also resulted in considerable vaccine hesitancy among pwMS. However, subsequent studies have confirmed that COVID-19 vaccination is safe for pwMS (A. Achiron et al., 2021a; Kelly et al., 2021; Brunn et al., 2022; Di Filippo et al., 2022) Concurrently with the vaccination program, a significant amount of studies leading to a better understanding of the immune responses associated with vaccines in pwMS have been published (Gombolay et al., 2022). Studies have revealed information on the humoral and later cellular immune responses achieved with these vaccines both in untreated and treated pwMS. The immune response to COVID-19 vaccines in untreated pwMS was found to be similar to that of healthy controls, and factors such as older age, comorbid conditions, and male sex were associated with reduced humoral response (Wu et al., 2022). Regarding the association between COVID-19 vaccine responses and MS treatments, many studies have revealed that a reduced humoral response was elicited with B-cell-depleting therapies and fingolimod, whereas a robust cellular response could be obtained with B-cell-depleting therapies but not with fingolimod (Gadani et al., 2021; Sormani et al., 2021; Apostolidis et al., 2021). Most of these studies have also shown that the degree of response was closely correlated with the timing of vaccination in B-cell-depleting therapy infusions, as well as other immunosuppressive therapies such as alemtuzumab and cladribine (Rico et al., 2021; Drulovic et al., 2021). Since most of the related research has involved mRNA vaccines, information on other types of vaccines, such as inactivated virus or adenovirus vector COVID-19 vaccines, remains limited (Etemadifar et al., 2020). Some of the inactivated virus vaccine-related studies had either limited sample size or some methodological issues, such as the timing of sampling, necessitating larger and more standardized studies to address the efficacy of inactivated virus versus mRNA vaccines (Ozakbas et al., 2022; Ghadiri et al., 2022). A few studies have investigated the association between humoral vaccine responses and COVID-19 infection rate or COVID-19 infection outcome after vaccination. Here, to better clarify the comparative efficacy of mRNA versus inactivated virus vaccines, we included detailed demographical and clinical variables that may influence immune responses in a large national cohort consisting of treated and untreated pwMS with all available DMTs in Turkey, as well as healthy controls. In this cross-sectional national multicenter study, we examined humoral responses elicited with mRNA and inactivated vaccines, their protective effects against COVID-19 infection, and, if infected, the severity of the disease.
2. Methods
2.1. Study population and setting
This cross-sectional study included data from 11 MS centers in Turkey. The study was initiated after the approval of the central ethics committee of Istanbul University-Cerrahpasa (10.09.2021-162329). Written informed consent was obtained from all participants. PwMS (N = 1484), who fulfilled the McDonald's 2017 diagnostic criteria, were between 18 and 65 years of age, and had received either two doses of inactivated (Sinovac) or mRNA (BioNTech) vaccines, were included. Treated pwMS (N = 1387) were sub-grouped according to the mode of action of the DMT that they were receiving. Untreated pwMS (N = 97) and healthy controls (N = 185) were also randomly selected. Detailed demographical and clinical variables, including sex, age, body mass index (BMI), type of MS onset, disease duration, clinical phenotype, total relapse number, disability level (Expanded Disability Status Scale [EDSS] score) at the time of the sampling, and the presence of any comorbidity, were noted.
2.2. Sampling and contact time
The blood sampling period was between April 1, 2021, and September 30, 2021. The sampling time was a minimum of 28 days (±7 days) and a maximum of 84 days (±7 days) after the second dose of the vaccination. The main inclusion criteria for the MS cohort were: no clinical attack in the past month, no previous COVID-19 infection (based on the patients’ statement and official COVID-19 infection registration records), no clinical attack after vaccination, and not receiving corticosteroid therapy for any reason after the vaccination. Treated pwMS had to be on a regular DMT for at least six months at the time of sampling and on no other medication affecting vaccination response. Patients treated with ocrelizumab had received at least two doses of treatment six months apart. In this group, DMTs were ocrelizumab, fingolimod, interferon beta (interferon beta-1a and interferon beta-1b), glatiramer acetate, dimethyl fumarate, teriflunomide, natalizumab, and cladribine. Untreated pwMS who have never been treated with a DMT or previously treated but not being on therapy in the past year were included. The healthy control group consisted of age- and sex-matched individuals.
Complete blood count–mainly white blood cell count and lymphocyte count–of the study population was obtained during the blood sampling time. The lymphocyte levels were graded as follows: normal: >1000 cells/mcL, grade I: 800–1000 cells/mcL, grade II: 500–800 cells/mcL, grade III: 200–500 cells/mcL, and grade IV: <200 cells/mcL.
After a mean of 327±16 days following the blood sampling, patients were contacted by phone and asked whether they were vaccinated with booster doses or not. They were also asked whether they had COVID-19 infection and the time of the infection. The severity of the infection was documented as follows: asymptomatic disease, mild symptoms without pneumonia, pneumonia or severe symptoms that required hospitalization or intensive care unit treatment, or death.
2.3. Antibody measurements
SARS-CoV-2 quantitative IgG test was performed using the chemiluminescent microparticle immunoassay method to detect SARS-CoV-2 IgG titers (ARCHITECT IgG II Quant test, Abbott, USA), demonstrating the quantity of neutralizing antibodies against the receptor-binding region of the spike protein S1 subunit of SARS-CoV-2. (WHO, Reference Panel for anti-SARS-CoV-2 antibody, https://www.who.int/publications/m/item/WHO-BS-2020.2403). The antibody results of the studied sera were evaluated as Arbitrary Unit/mL (AU/mL). The antibody concentrations obtained in AU/mL were multiplied by the correlation coefficient of 0.142 and converted to the Binding Antibody Unit (BAU/mL)–WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Accordingly, 50 AU/mL or 7.1 BAU/mL and above concentrations were considered positive. This test has been reported to be 100% compatible with the plaque reduction neutralization test (PRNT), and a concentration of 1050 AU/mL was associated with a 1:80 dilution of PRNT (Abbott-Sars-Cov-2-immunoassays, https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2)
2.4. Statistical analysis
Statistical analyses were performed using the SPSS software (v25.0, IBM Corp., Armonk, NY, USA). All antibody titer levels were transformed into log units and compared on a log10 scale. The Kolmogorov–Smirnov test was used to determine the normal distribution of the data. Mann–Whitney U test was performed for the comparison of antibody levels between the BioNTech and Sinovac groups. For multiple comparisons, the one-way analysis of variance (ANOVA) and post-hoc Tukey–Kramer tests were used to compare the parametric data, while the Kruskal–Wallis test was used for the non-parametric data. Pearson's or Spearman's correlation tests were performed, as applicable, to determine the correlations between antibody titer vs. the time between the second vaccine dose and sample collection date (TBVS), age, and BMI. The associations of the factors (age, sex, EDSS, BMI, vaccination status, and medication) with COVID-19 severity (asymptomatic/mild vs. moderate/severe/death) were assessed by univariate and multivariate (enter method) logistic methods. Nominal data were compared using Fisher's exact test. All tests were two-sided, with statistical significance set at p<0.05.
3. Results
In the initial phase of the study, a total of 1857 participants were enrolled. After the re-screening, 127 participants due to the sampling time, 43 participants due to incomplete clinical-demographic data, and 18 participants due to suspected prior COVID-19 infection were excluded from the study. Finally, data from 1669 participants (1387 treated pwMS, 97 untreated pwMS, and 185 healthy controls) were analyzed.
The average age of the participants was 39.64±10.65 years. Patients having progressive forms of MS were older than those having relapsing forms of MS (RRMS: 38.33±10.34, SPMS: 46.32±8.73, and PPMS: 47.36±10.14; p<0.001). There were 544 men (32.59%) and 1125 women (67.41%). The average BMI was 24.76±4.25. Among pwMS, 1263 (85.11%) had RRMS, 161 (10.85%) had SPMS, and 60 (4.04%) had PPMS. Detailed demographic data is presented in Table 1 . The mean disease duration was 9.32±10.65 years, and the mean EDSS was 2.14±1.92. Among the entire study population, 852 (51.05%) had received BioNTech and 817 (48.95%) had received Sinovac. TBVS (50.85±16.90) was not significantly different between the Sinovac and BioNTech groups. The subgroups according to the DMT type and vaccines are shown in Table 1. The majority of DMTs included ocrelizumab (N = 368), fingolimod (N = 332), and interferons (N = 193). There were 97 pwMS who have not received any DMT for at least a year and 185 healthy controls.
Table 1.
Demographic and clinical profiles of the study participants.
| Age | Sex |
BMIa | MS Type |
Disease Duration (in years) | Last EDDSe | Total Relapses | TBVSf | Vaccine Type (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Male | Female | Mean (SD) | RRMSb | SPMSc | PPMSd | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | BioNTech | Sinovac | |
| n (%) | n (%) | n (%) | n (%)n (%) | Median (IR) | (SD) | n (%) | n (%) | ||||||
| Total | 39.64 (10.65) | 544 (32.59) | 1125 (67.41) | 24.76 (4.25) | 1263 (85.11) | 161 (10.85) | 60 (4.04) | 9.32 (10.65) | 2.14 (1.92) 1.5 (1–3) |
3.30 (2.70) | 50.85 (16.90) | 852 (51.05) | 817 (48.95) |
| Healthy Controls | 40.27 (11.21) | 76 (41.08) | 109 (58.92) | 25.15 (4.23) | - | - | - | - | - | - | 47.84 (15.56) | 79 (42.70) | 106 (57.30) |
| pwMS w/o Tg | 39.48 (10.23) | 28 (28.87) | 69 (71.13) | 24.46 (3.92) | 79 (81.44) | 12 (12.37) | 6 (6.19) | 7.40 (7.10) | 1.41 (1.72) | 2.35 (2.10) | 53.67 (16.57) | 64 (65.98) | 33 (34.02) |
| pwMS DMTh | 39.57 (10.61) | 440 (31.72) | 947 (68.28) | 24.74 (4.28) | 1184 (85.36) | 149 (10.74) | 54 (3.89) | 9.44 (7.14) | 2.19 (1.92) | 3.38 (2.74) | 51.05 (17.05) | 709 (51.12) | 678 (48.88) |
| Ocrelizumab | 44.23 (10.03) | 132 (35.87) | 236 (64.13) | 25.21 (4.40) | 208 (56.22) | 113 (30.71) | 47 (12.77) | 12.45 (7.11) | 4.03 (1.97) | 4.31 (3.10) | 49.73 (15.72) | 194 (52.72) | 174 (47.28) |
| Fingolimod | 38.19 (10.23) | 94 (28.83) | 232 (71.17) | 24.65 (4.19) | 312 (95.71) | 13 (3.99) | 1 (0.31) | 9.89 (7.02) | 1.73 (1.50) | 3.78 (2.70) | 52.22 (17.72) | 162 (49.69) | 164 (50.31) |
| Interferons | 38.48 (10.82) | 59 (30.57) | 134 (69.43) | 24.20 (3.92) | 182 (94.30) | 8 (4.15) | 3 (1.55) | 8.10 (6.24) | 1.22 (1.19) | 2.38 (1.71) | 50.08 (18.80) | 88 (45.60) | 105 (54.40) |
| Glatiramer Acetate | 37.02 (10.07) | 26 (21.14) | 97 (78.86) | 23.92 (4.36) | 117 (95.12) | 6 (4.88) | 0 | 7.44 (7.31) | 1.18 (1.26) | 2.22 (1.85) | 51.29 (17.26) | 65 (52.85) | 58 (47.15) |
| Dimethyl fumarate | 36.47 (10.41) | 57 (40.43) | 84 (59.57) | 24.80 (4.44) | 136 (96.45) | 2 (1.42) | 3 (2.13) | 5.86 (5.72) | 1.47 (1.24) | 2.29 (1.50) | 49.77 (16.46) | 74 (52.48) | 67 (47.52) |
| Teriflunomide | 39.21 (10.35) | 45 (36.00) | 80 (64.00) | 24.75 (4.10) | 121 (97.80) | 4 (3.20) | 0 | 11.87 (8.35) | 1.26 (1.22) | 2.14 (2.00) | 52.82 (17.42) | 66 (52.80) | 59 (42.20) |
| Natalizumab | 37.95 (10.50) | 14 (20.90) | 53 (79.10) | 24.69 (4.37) | 65 (97.02) | 2 (2.99) | 0 | 7.40 (7.10) | 2.36 (1.64) | 5.17 (3.72) | 52.69 (17.60) | 37 (55.22) | 30 (44.78) |
| Cladribine | 36.17 (8.11) | 13 (29.55) | 31 (70.45) | 24.70 (4.37) | 43 (97.27) | 1 (2.27) | 0 | 8.56 (5.29) | 2.13 (1.61) | 3.89 (2.94) | 53.77 (13.42) | 23 (52.27) | 21 (47.73) |
Body mass index.
Relapsing-remitting multiple sclerosis.
Secondary-progressive multiple sclerosis.
Primary-progressive multiple sclerosis.
Expanded Disability Status Scale.
The time between the second vaccine dose and sample collection date.
People with MS without treatment.
People with MS treated with disease-modifying therapies.
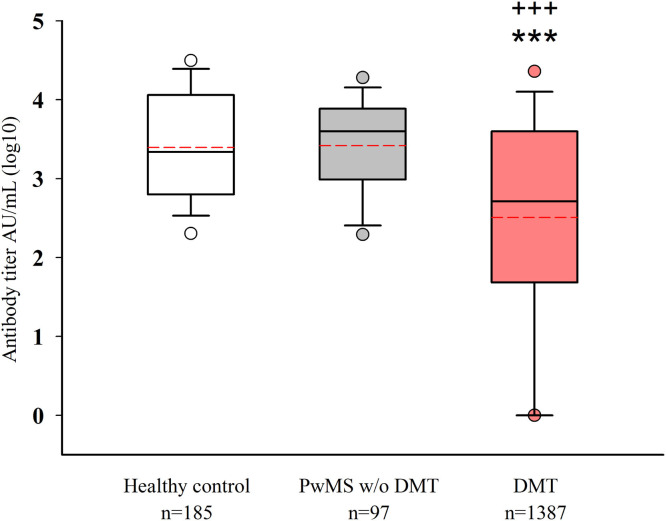
Patients who were under a DMT had a lower antibody response than healthy controls and untreated pwMS (both p<0.001). No significant difference was detected in antibody titers between healthy controls and untreated pwMS (Fig. 1 ). Any detectable level above the cut-off value (50 AU/ml) was considered a positive response for each vaccination (Fig. 2 ). This response was not significant for any of the subgroups between the two vaccine types.
Fig. 1.
A) Comparison of the antibody levels (log10) among healthy controls, untreated pwMS, and pwMS receiving DMTs. pwMS, people with multiple sclerosis; DMT, disease-modifying therapy; ***p<0.001: compared with healthy controls; +++p<0.001: compared with untreated pwMS.
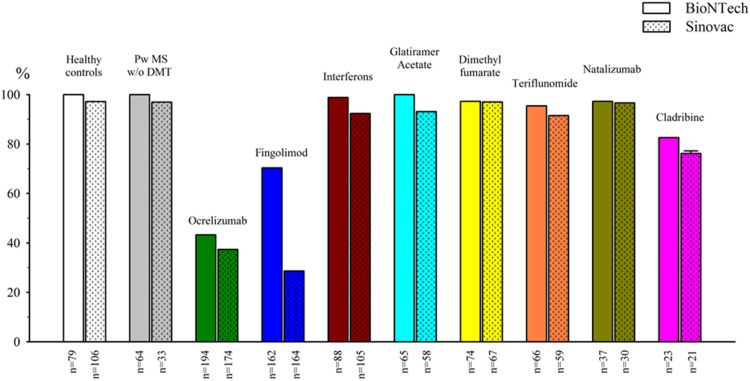
Fig. 2.
Percentage of seropositivity (antibody titer ≥ 50 AU/ml) among the groups.
The lowest seropositivity for both vaccine groups were for those receiving ocrelizumab and fingolimod. When the humoral response was evaluated based on antibody titers, the individuals who received BioNTech showed significantly higher antibody titers (7.175±10.074) compared with those who received Sinovac (823±1.774) (p<0.001). The mean antibody titers in the BioNTech and Sinovac groups were as follows, respectively: healthy controls: 15.817±11.134, 1.425±2.104 (p<0.001); untreated pwMS: 8.038±7.476, 1.394±2.527 (p<0.001); ocrelizumab group: 1.204±4.174, 374±1.126 (p<0.001); fingolimod group: 1.192±2.771, 142±410 (p<0.001); interferon group: 14.079±12.174, 1.547±2.846 (p<0.001); glatiramer acetate group: 11.382±10.677, 839±1.098 (p<0.001); dimethyl fumarate group: 10.494±10.031, 710±1.202 (p<0.001); teriflunomide group: 9.415±1.422, 1.544±2.386 (p<0.001); natalizumab group: 10.264±1.867, 1.021±1.408 (p<0.001); and cladribine group: 7.212±2.088, 320±379 (p<0.001).
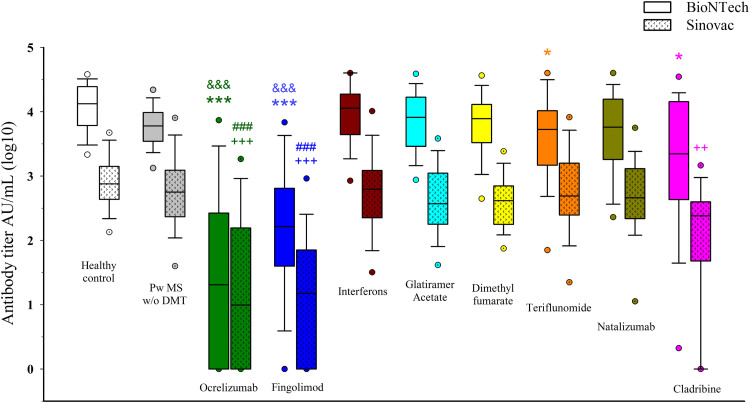
In a subgroup analysis of the BioNTech cohort, pwMS treated with ocrelizumab (p<0.001), fingolimod (p<0.001), teriflunomide (p<0.05), and cladribine (p<0.05) had lower antibody titers compared with the healthy controls. However, only the ocrelizumab and fingolimod groups had lower antibody titers compared with untreated pwMS (p<0.001; p<0.001, respectively). In the Sinovac cohort, pwMS treated with ocrelizumab (p<0.001), fingolimod (p<0.001), and cladribine (p<0.01) had lower antibody titers compared with the healthy controls. Again, only the ocrelizumab and fingolimod groups had lower antibody titers compared to untreated pwMS (p<0.001; p<0.001, respectively). (Fig. 3 ).
Fig. 3.
Comparison of the antibody levels (log10) among the groups. PwMS w/o DMT: People with MS without disease-modifying therapies. *p<0.05, ⁎⁎⁎p<0.001: compared with healthy controls (BioNTech) &&&p<0.001: compared with untreated pwMS (BioNTech) ++p<0.01, +++p<0.001: compared with healthy controls (Sinovac) ###p<0.001: compared with untreated pwMS (Sinovac).
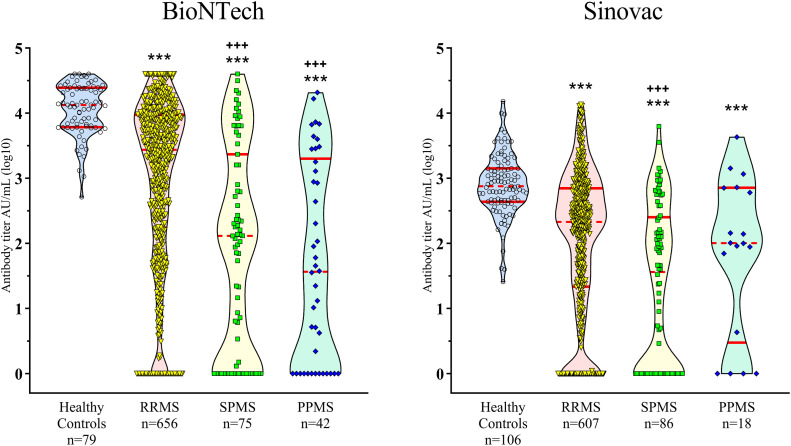
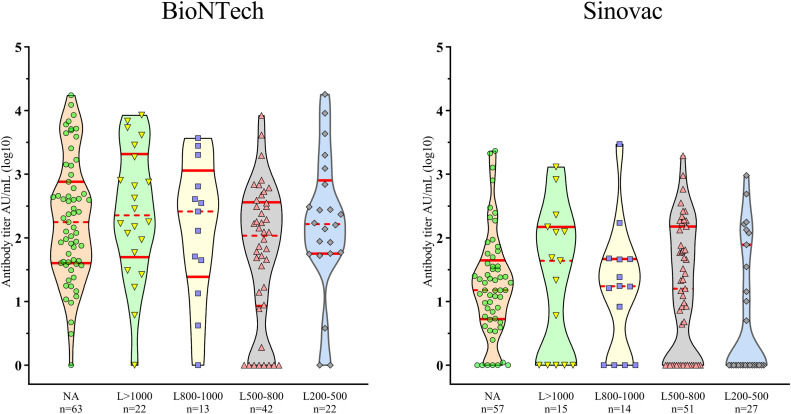
When the vaccination response was evaluated according to MS clinical phenotypes, all MS groups had lower antibody titers than the healthy controls in both BioNTech (p<0.001) and Sinovac (p<0.001) cohorts. SPMS or PPMS groups had lower antibody titers than the RRMS group in the BioNTech cohort (both p<0.001). In the Sinovac cohort, only the SPMS group had a lower antibody titer than the RRMS group (p<0.001) (Fig. 4 ). Low absolute lymphocyte count was observed only in the fingolimod-treated group, which did not significantly affect the humoral response of either vaccinatin cohort in this group (Fig. 5 ).
Fig. 4.
Comparison of the antibody levels (log10) among MS types. RRMS: relapsing-remitting multiple sclerosis, SPMS: secondary progressive multiple sclerosis, PPMS: primary progressive multiple sclerosis. ***p<0.001: compared with healthy controls; +++p<0.001: compared with the RRMS group.
Fig. 5.
The effect of lymphocyte distribution on antibody titers in patients receiving fingolimod treatment.
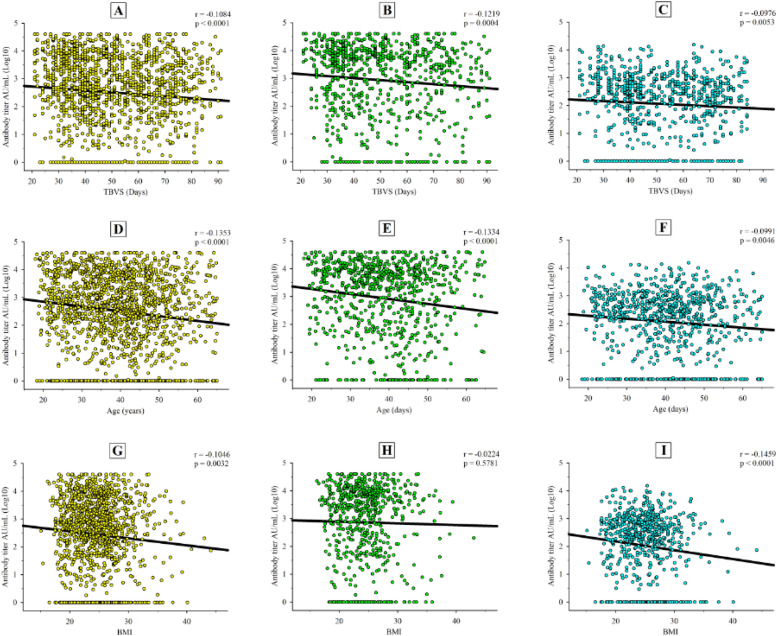
Negative correlations were detected between the TBVS and antibody titer in the entire cohort (r=−0.1084; p<0.0001) (Fig. 6 A to 6C) and in BioNTech (r=−0.1219; p = 0.0004) and Sinovac (r=−0.0976; p = 0.0053) vaccination groups. There was a significant correlation between age and antibody titer in the entire cohort (r=−0.1353; p<0.0001) (Fig. 6D to 6F), as well as in each vaccination group (BioNTech: r=−0.1334, p<0.0001; Sinovac: r=−0.0991; p = 0.0046). BMI was negatively correlated with the humoral response in the entire cohort (r=−0.1046, p = 0.0032) (Fig. 6G) and Sinovac group (r=−0.1459, p<0.0001) (Fig. 6I); however, there was no significant correlation in the BioNTech group (r=−0.0224, p = 0.5781) (Fig. 6).
Fig. 6.
Correlations between antibody levels (log10) and the time between the second vaccine dose and sample collection date (TVBS) in the A) entire population, B) BioNTech group, and C) Sinovac group. Partial correlations between antibody levels (log10) and age, after controlling for TVBS, in the D) entire population, E) BioNTech group, and F) Sinovac group. Partial correlations between antibody levels (log10) and body mass index (BMI), after controlling for TVBS, in the G) entire population, H) BioNTech group, and I) Sinovac group.
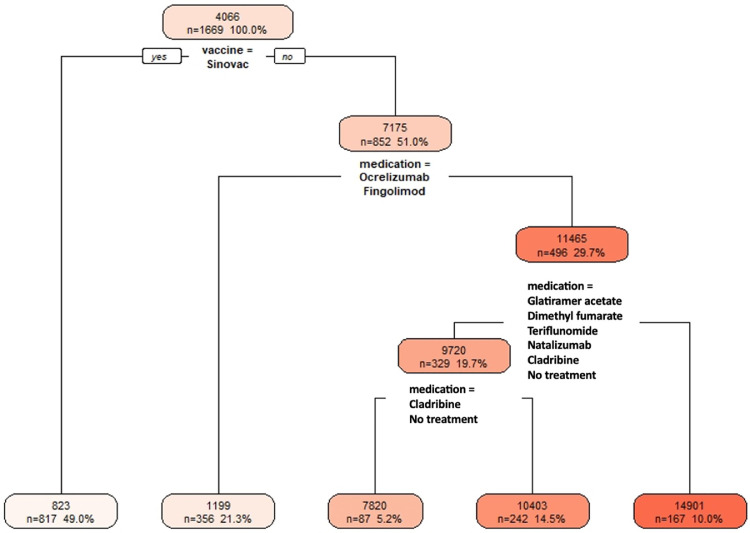
We also evaluated the COVID-19 antibody response in pwMS using a decision tree. We used Recursive Partitioning and Regression Trees (rpart) in the R Programming environment. Participants who received the Sinovac vaccine produced the lowest antibody response; those who received ocrelizumab or fingolimod treatments had also very low antibody responses (Fig. 7 ).
Fig. 7.
Decision tree shows on the top node that the average antibody count for the entire cohort is 4066. Those who received Sinovac–the bottom left leaf of the tree–consists of 817 participants, yielding the lowest average antibody count of 823. The second lowest antibody response group–the second leaf from the bottom left of the tree–consists of pwMS who received BioNTech and were on ocrelizumab or fingolimod, with 356 participants with an average of 1199 antibody count. The highest antibody group consists of healthy controls and pwMS on interferon beta who received BioNTech, with 167 participants with an average of 14,901 antibody count.
We contacted 704 patients by telephone; 246 (34.9%) of them were infected with COVID-19 between the sampling time and contact time. Detailed demographic data are presented in Table 2 . There was no correlation between age, sex, or BMI and COVID-19 infection among the patients. Among pwMS, 203 patients had only two doses of Sinovac or BioNTech vaccines; the rest of the patients had one or more booster doses. The time from the second vaccine to COVID-19 infection was 190 days, from the first booster dose to COVID-19 infection was 118 days, and from two or more booster doses to COVID-19 infection was 104 days. Between infected and non-infected patients, no significant differences were detected in the vaccine type, time from vaccination to COVID-19 infection, number of vaccination, or antibody titers (Table 2).
Table 2.
Demographic characteristics, vaccination status, and antibody titers in participants infected and non-infected with COVID-19.
| Total | Infected with COVID-19 | Non-infected | p-value | |
|---|---|---|---|---|
| Contacted patients (n, %) | 704 (100%) | 246 (34.9%) | 458 (65.1%) | - |
| Age (mean±SD) | 38.67±10.20 | 37.56±9.65 | 39.27±10.44 | 0.109 |
| Sex (male/female, n) | 221/483 | 68/178 | 153/305 | 0.137 |
| BMI (mean±SD) | 24.29±4.13 | 24.02±3.91 | 24.43±4.24 | 0.303 |
| Vaccination status | ||||
| 2 doses of BioNTech (n, %) | 134 (100%) | 58 (43.3%) | 76 (56.7%) | - |
| 2 doses of Sinovac (n, %) | 69 (100%) | 22 (31.9%) | 47 (68.1%) | 0.155 |
| 1 booster dose (n, %) | 270 (100%) | 93 (34.4%) | 177 (65.6%) | 0.311 |
| 2 or more booster doses (n, %) | 231 (100%) | 73 (31.6%) | 158 (68.4%) | 0.110 |
| Participants | ||||
| Healthy controls (n, %) | 21 (100%) | 7 (33.3%) | 14 (66.7%) | - |
| Untreated pwMS (n, %) | 41 (100%) | 14 (34.1%) | 27 (65.9%) | 0.949 |
| pwMS under DMTs | ||||
| Ocrelizumab (n, %) | 202 (100%) | 73 (36.1%) | 129 (63.9%) | 0.949 |
| Fingolimod (n, %) | 118 (100%) | 50 (42.4%) | 68 (57.6%) | 0.459 |
| Others (n, %) | 322 (100%) | 102 (31.7%) | 220 (68.3%) | 0.887 |
| Antibody titer (AU/mL) | ||||
| Healthy controls (mean±SD) | - | 7810±9253 | 6211±7621 | 0.927 |
| Untreated pwMS (mean±SD) | - | 5335±5127 | 4302±4553 | 0.714 |
| pwMS under DMTs | ||||
| Ocrelizumab (mean±SD) | - | 555±2149 | 1238±4650 | 0.075 |
| Fingolimod (mean±SD) | - | 1014±2989 | 703±1921 | 0.424 |
| Others (mean±SD) | - | 5751±8943 | 5551±8193 | 0.625 |
Among patients who had COVID-19 infection, 206 (83%) patients were asymptomatic or had mild infection symptoms without pneumonia, 37 had moderate symptoms, three patients were treated in the intensive care unit, and one patient under ocrelizumab treatment died. Only high BMI was a risk factor for severe COVID-19 infection responses (Table 3 ). There was no correlation between age, sex, EDSS, the number of vaccination, or DMT type and humoral antibody.
Table 3.
Univariate and multivariate analyses for COVID-19 severity.
| Covid-19 Severity (N=246) | Univariate Analysis (N=246) | Multivarite Analysis (N=246) | ||||
|---|---|---|---|---|---|---|
| Variable | Asymptomatic Mild (N=206) | Moderate Severe Death (N=40) | OR (95% C.I.) | P | OR (95% C.I.) | p |
| Age (mean±SD) | 37.35±9.64 | 38.64±9.75 | 1.014 (0.979–1.050) | 0.440 | 0.999 (0.957–1.043) | 0.958 |
| Sex (female/male) (n) | 147/59 | 31/9 | 1.382 (0.620–3.081) | 0.428 | 1.923 (0.825–4.484) | 0.130 |
| EDSS (mean±SD) | 2.19±1.80 | 2.54±1.86 | 1.107 (0.923–1.329) | 0.273 | 1.115 (0.841–1.480) | 0.449 |
| BMI (mean±SD) | 23.79±3.85 | 25.17±4.06 | 1.091 (1.001–1.189) | 0.047 | 1.106 (1.007–1.215) | 0.035 |
| Vaccination status (n, %) | ||||||
| 2 doses | 12 (15%) | 68 (85%) | Ref. | Ref. | ||
| 3 doses | 15 (16%) | 78 (84%) | 1.090 (0.477–2.489) | 0.838 | 1.237 (0.507–3.020) | 0.640 |
| 4–5 doses | 13 (18%) | 60 (82%) | 1.228 (0.521–2.896) | 0.639 | 1.450 (0.576–3.649) | 0.455 |
| Medication (n, %) | ||||||
| Healthy controls + untreated pwMS | 4 (19%) | 17 (81%) | Ref. | Ref. | ||
| Ocrelizumab | 13 (18%) | 60 (82%) | 0.921 (0.266–3.193) | 0.897 | 0.519 (0.106–2.544) | 0.419 |
| Fingolimod | 8 (16%) | 42 (84%) | 0.810 (0.215–3.048) | 0.907 | 0.570 (0.134–2.429) | 0.645 |
| Other drugs | 15 (15%) | 87 (85%) | 0.733 (0.216–2.480) | 0.976 | 0.620 (0.171–2.251) | 0.697 |
4. Discussion
The COVID-19 pandemic has caused significant mortality and morbidity globally, with an unprecedented challenge to public health. During this burden and uncertainty, rapidly developed vaccines have created hope and succeeded in controlling the spread and severe consequences of COVID-19. However, this situation has also raised the issue of vaccine immunogenicity, especially in people with autoimmune diseases and receiving drugs that may interfere with the efficacy of these vaccines.
It has been shown that mRNA vaccines are more effective than viral vector vaccines (Doroftei et al., 2021), but there is limited information about the effects of inactive vaccines in comparison with mRNA vaccines. Three studies from Chile, Jordan, and China examining the humoral response in the general population have shown that mRNA vaccines were superior to inactivated vaccines regarding seropositivity and SARS-Cov2 antibody titers (Mok et al. 2022; Alqassieh et al. 2021). In the study with the Chile population, seropositivity was 77.4% and 96.5% after two doses of Sinovac and mRNA vaccination, respectively(Saure et al., 2022). To our knowledge, our study is the largest to evaluate and compare the humoral responses of an mRNA vaccine (BioNTech) and an inactivated virus vaccine (Sinovac) in pwMS, as well as the outcomes of the infection in this population.
Our results are in accordance with recent studies indicating that MS itself does not affect the antibody response, but DMTs appear to be associated with attenuated humoral response (Ozakbas et al., 2022; Habek et al., 2022; Di Filippo et al., 2022; A. Achiron et al., 2021a; Sormani et al., 2021). The main result obtained in this study is the absence of a significant difference between the vaccine groups regarding seropositivity. Lower positivity rates were observed in patients who received ocrelizumab, fingolimod, and cladribine treatments in both vaccine groups. However, it should be noted that although values above certain thresholds were considered positive in the tests of different brands used, the sensitivity and specificity of these tests may be different.
Our study demonstrated that mRNA vaccines appear to be more effective in inducing an immune response compared with inactivated virus vaccines. Importantly, when SARS-CoV-2 IgG levels, which are used to measure the humoral antibody response, were examined, the mRNA vaccine was significantly superior to the inactivated virus vaccine in all treated and untreated pwMS and healthy control groups. Also, SARS-CoV-2 IgG levels were approximately 9 times higher in patients receiving BioNTech in all DMT groups compared with patients receiving Sinovac. The highest prevalence of antibody response was observed among those receiving immunomodulators (natalizumab, 97%; interferons and glatiramer acetate, 98%). In meta-analyses of humoral studies on COVID-19 vaccination responses in pwMS, antibodies were detected in 93% of healthy controls and 77% of pwMS, with >93% response in all DMT groups (interferon beta, glatiramer acetate, cladribine, natalizumab, dimethyl fumarate, alemtuzumab, and teriflunomide) except for sphingosine-1-phosphate modulators (72%) and anti-CD20 monoclonal antibodies (44%) (Etemadifar et al., 2022; Gombolay et al., 2022). Cladribine was reported not to affect vaccine immunity. However, in our cohort, the antibody titers were decreased in patients using cladribine compared with the healthy control group. These results differ from the majority of previous studies but are consistent with the study of Tortorella et al. (Tortorella et al., 2022; Tallantyre et al., 2022; A. Achiron et al., 2021b; Sormani et al., 2021). This inconsistency may be due to the differences in the comparisons–whether antibody titers or antibody response rates were evaluated. Teriflunomide was expected not to reduce the response to vaccines; however, surprisingly, lower antibody responses were found in patients treated with teriflunomide in the BioNTech vaccination group. Previously, however lower vaccination responses were found in the inactivated vaccine groups but not in theb mRNA groups (Ozakbas et al., 2022).
Pre-COVID vaccine studies in patients receiving ocrelizumab and fingolimod treatments have shown that the formation of antibody response is reduced compared with healthy controls. Regarding the effect of the DMTs on humoral response, attenuated humoral responses were detected in pwMS receiving ocrelizumab and fingolimod, irrespective of which vaccine was used. In the light of previous research, it is now well-established that fingolimod and B-cell-depleting therapies can impair post-vaccination antibody formation (Bar-Or et al., 2020; Kappos et al., 2015), and similar results have been observed in all vaccine subgroups.
Fingolimod is an antagonist of the sphingosine-1-phosphate receptor and prevents lymphocyte egression from secondary lymphoid tissues and marked peripheral blood lymphopenia. PwMS treated with fingolimod had lower humoral and a few countable cellular immune responses due to low lymphocyte counts (Achiron et al., 2022). However, despite these low T- and B-cell numbers and low antibody titers after vaccination, fingolimod use is not a risk factor for severe COVID-19 infection (Achtnichts et al., 2022; Turkoglu et al., 2022). Accordingly, we did not find any relationship between lymphocyte count and humoral responses in our study.
The results of our study confirmed the negative correlation between age and vaccine response; this seems to be consistent with most previous studies, although there are also conflicting results (Pitzalis et al., 2021; Tallantyre et al., 2022; Etemadifar et al., 2022; Sormani et al., 2021). This relationship seems legit, given the presence of immune senescence. Moreover, TVBS was negatively correlated with the vaccine response. This is in line with the results of previous studies arguing that it seems important how long after the last dose of the vaccine is administered, especially for treatments administered as intermittent infusions. In our study, BMI was negatively correlated with antibody titer. In a study, BMI was not found to be effective on the antibody response (Sormani et al., 2021); however, there is little data on this issue in the literature.
The relationship between phenotypes of MS and the humoral response to SARS-CoV-2 vaccines has remained speculative. In this study, patients with progressive forms of MS developed lower antibody titers. Such a relationship has not been observed in other studies (Ozakbas et al., 2022; Sormani et al., 2021; Etemadifar et al., 2022). This inconsistency may be due to the demographical factors of progressive MS groups and the drugs used in these patients. In our study, progressive MS patients were older compared with RRMS patients and used fingolimod and ocrelizumab treatments at higher rates, which affected antibody responses.
In our study, patients were followed prospectively. Within one year after blood sampling and determination of humoral response levels, patients’ COVID-19 status and the severity of COVID-19 infection were questioned via contacting them by telephone. The type of vaccine, humoral antibody level, number of vaccinations, age, sex, BMI, EDSS, and MS type were examined in relation to COVID-19 infection. Unexpectedly, there was no association between COVID-19 infection rate and age, sex, vaccine type, antibody level, the number of vaccinations, EDSS, and DMTs. The rates of COVID-19 infection were also similar between the Sinovac and BioNTech groups.
Considering the severity of the infection, 83% of the patients who had COVID-19 infection were asymptomatic or recovered with mild symptoms. The remaining 40 patients developed pneumonia or more severe symptoms. Only three patients had to be followed up in the intensive care unit, and only one patient on ocrelizumab died. In the multivariant analysis of COVID-19 infection severity, no association was observed between age, sex, EDSS, vaccine type, and DMT type. Only those with high BMI were associated with a higher risk of severe infection. Moreover, no association was found between susceptibility to the infection and infection severity, whether it was the mRNA vaccine, which induces a high humoral response, or the inactivated vaccine, which induces a low humoral response. A possible explanation for this might be that the rate of the Omicron variant of COVID-19 infection was dominant at the time of the follow-up period, which had a low risk of severe infection (Barouch 2022; Sormani et al., 2022).
There are several limitations to this study. One limitation is that COVID-19 infection before vaccination was determined based on the patients’ statements and not serological data. Since there was no antibody titer before the study, antibody change could not be detected. Moreover, these results may underestimate the effect of age and other covariates on the humoral response. Second, the antibody titers of the patients were not checked after the booster injections or anytime in the follow-up period. Lastly, some patients preferred different vaccines for booster injections during the follow-up period; this might have interfered with the COVID-19 infection severity. Although the findings should be interpreted with caution, this study has several strengths. This is the largest multicenter study evaluating vaccine immunogenicity, and this sample size allowed us to reach sufficient statistical power, producing more generalizable findings. Moreover, the long follow-up period allowed us to more reliably compare the inactivated virus and mRNA vaccines’ protection against COVID-19 infection.
Although much progress has been made in the issue of vaccine immunogenicity in MS, there is abundant room for further progress in determining the causal factors associated with post-vaccination antibody formation and protection against the infection or risk of severe infection.
5. Conclusion
In this study, we determined the effect of vaccination type and DMTs on the humoral response upon vaccination in pwMS. Overall, our findings strengthen the idea that MS itself does not affect the humoral response. In general, the mRNA vaccine appeared to be more effective in producing COVID-19 IgG antibodies. B-cell-depleting therapies and fingolimod were associated with an attenuated humoral response. Finally, both inactive virus and mRNA vaccines were equally protective against COVID-19 infection, regardless of the antibody status, and the severity of COVID-19 infection did not differ between the vaccine groups.
Funding
The author (s) have received no financial support for research, authorship, and/or publication of this article.
Melih Tütüncü: Conceptualization, Visualization, Resources, Investigation, Writing- original draft, Data curation, Formal analysis, Methodology
Serkan Demir: Data curation, Formal analysis, Methodology
Gökhan Arslan:: Formal analysis, Methodology
Öykü Dinç: Laboratory analysis
Sedat Şen: Data curation, Formal analysis, Methodology
Tuncay Gündüz: Data curation, Formal analysis, Methodology
Cihat Uzunköprü: Data curation, Formal analysis, Methodology
Haluk Gümüş: Data curation, Formal analysis, Methodology
Mesude Tütüncü: Data curation, Formal analysis, Methodology, Formal analysis
Rüveyda Akçin : Laboratory analysis
Serkan Özakbaş: Data curation, Formal analysis, Methodology
Mesrure Köseoğlu: Data curation, Formal analysis, Methodology
Sena Destan Bünül: Data curation, Formal analysis, Methodology
Ozan Gezen: Data curation,
Damla Çetinkaya Tezer: Data curation,
Cavid Baba: Data curation,
Pınar Acar Özen: Data curation,
Rabia Koç: Data curation,
Tuğrul Elverdi: Formal analysis, Methodology
Uğur Uygunoğlu: Data curation, Formal analysis, Methodology
Murat Kürtüncü: Data curation, Formal analysis, Methodology
Yeşim Beckmann: Data curation, Formal analysis, Methodology
İpek Güngöz Doğa: Data curation,
Ömer Faruk Turan: Data curation, Formal analysis, Methodology
Cavit Boz: Data curation, Formal analysis, Methodology
Murat Terzi: Data curation, Formal analysis, Methodology
Asli Tuncer: Data curation, Formal analysis, Methodology
Sabahattin Saip: Data curation, Formal analysis, Methodology
Rana Karabudak: Data curation, Formal analysis, Methodology
Bekir Kocazeybek; Data curation, Formal analysis, Methodology
Hüsnü Efendi; Data curation, Methodology
Uğur Bilge; Formal analysis, Methodology, supervision
Aksel Siva; Conceptualization, Visualization, Supervision, Resources, Investigation, Writing – review & editing, Data curation, Formal analysis, Methodology
Declaration of Competing Interest
M.Tutuncu, S. Demir, S. Sen, T. Gunduz, C Uzunköprü, H Gumus have received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and regis- tration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma.
Asli Tuncer has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma.
Serkan Ozakbas has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma.
H. Efendi has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim
Rana Karabudak has received honoraria for giving educational lectures, consultancy fees for participating advisory boards, and travel grants for attending scientific congresses or symposia from Roche, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Abdi Ibrahim Ilac, Deva and ARIS.
Aksel Siva has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Sanofi-Genzyme, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey and Abdi Ibrahim Ilac.
The rest of authors declare no conflict of interest with the study project.
References
- Abbott SARS-COV-2 IMMUNOASSAYS, https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2.
- Achiron A., Mathilda M., Sapir D.A., et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 Months Cross-Sectional Study. J. Neuroimmunol. 2021;361 doi: 10.1016/j.jneuroim.2021.577746. 2021 Dec 15Epub 2021 Oct 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. 2021 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Gurevich M., et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022;269(5):2286–2292. doi: 10.1007/s00415-022-11030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtnichts L., Ovchinnikov A., Jakopp B., et al. SARS-CoV-2 MRNA vaccination in people with multiple sclerosis treated with fingolimod: protective humoral immune responses may develop after the preferred third shot. Vaccines (Basel) 2022;10(2):341. doi: 10.3390/vaccines10020341. 2022 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqassieh R., Suleiman A., Abu-Halaweh S., et al. Pfizer-Biontech and sinopharm: a comparative study on post-vaccination antibody titers. Vaccines (Basel) 2021;9(11):1223. doi: 10.3390/vaccines9111223. 2021 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 MRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. 2021 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Calkwood J.C., Chognot C., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D.H. Covid-19 vaccines—immunity, variants, boosters. N. Engl. J. Med. 2022;387(11):1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn J.A., Galit L.D., Andrew R.R., et al. SARS-CoV-2 infection and vaccination outcomes in multiple sclerosis. Neurol. 2022;12(3):e14–e21. doi: 10.1212/CPJ.0000000000001164. 2022 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M., Cordioli C., Malucchi S., et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2022;93(4):448–450. doi: 10.1136/jnnp-2021-327200. 2022 Apr. [DOI] [PubMed] [Google Scholar]
- Doroftei B., Ciobica A., Ilie O.D., Maftei R., Ilea C. Mini-review discussing the reliability and efficiency of covid-19 vaccines. Diagnostics (Basel) 2021;11(4):579. doi: 10.3390/diagnostics11040579. 2021 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drulovic J., Ivanovic J., Martinovic V., et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. 2021 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Sedaghat N., Nouri H., et al. SARS-CoV-2 serology among people with multiple sclerosis on disease-modifying therapies after BBIBP-CorV (Sinopharm) inactivated virus vaccination : same story, different vaccine. Mult. Scler. Relat. Disord. 2020;57 doi: 10.1016/j.msard.2021.103417. 2022 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar M., Nouri H., Pitzalis M., et al. Multiple sclerosis disease-modifying therapies and COVID-19 vaccines: a practical review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2022;93(9):986–994. doi: 10.1136/jnnp-2022-329123. [DOI] [PubMed] [Google Scholar]
- Gadani P.S., Reyes-Mantilla M., Jank L., et al. Discordant humoral and t cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadiri F., Sahraian M.A., Azimi A., Moghadasi A.N. The study of COVID-19 infection following vaccination in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombolay G.Y., Monideep D., William T. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis. Ann. Clin. Transl. Neurol. 2022;9(8):1321–1331. doi: 10.1002/acn3.51628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavo C.R., Fernando G., Antonio T., Alexis P., et al. Acute Transverse Myelitis (ATM):clinical review of 43 patients with COVID-19-Associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653786. 2021 Apr 26; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habek M., Željko C., Savić M.A., et al. Humoral and cellular immunity in convaaaaalescent and vaccinated COVID-19 people with multiple sclerosis: effects of disease modifying therapies. Mult. Scler. Relat. Disord. 2022;59 doi: 10.1016/j.msard.2022.103682. 2022 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L., Mehling M., Arroyo R., et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015;84(9):872–879. doi: 10.1212/WNL.0000000000001302. [DOI] [PubMed] [Google Scholar]
- Kelly H., Brent S., Hesham A. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021;356 doi: 10.1016/j.jneuroim.2021.57759. 2021 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok C.K.P., Cohen C.A., Cheng S.M.S., et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2022;27(4):301–310. doi: 10.1111/resp.14191. 2022 Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahdaripour, M., Mojtaba S., Seyed M.I.M., et al., 2020. Int Immunopharmacol. 2021 Oct;99:108021. doi: 10.1016/j.intimp.2021.108021. [DOI] [PMC free article] [PubMed]
- Ozakbas S., Baba C., Dogan Y., et al. Comparison of SARS-CoV-2 antibody response after two doses of MRNA and inactivated vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult. Scler. Relat. Disord. 2022;58 doi: 10.1016/j.msard.2022.103486. 2022 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis M., Idda M.L., Lodde V., et al. Effect of different disease-modifying therapies on humoral response to BNT162b2 vaccine in sardinian multiple sclerosis patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.781843. 2021 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A., Ninove L., Maarouf A., et al. Determining the best window for BNT162b2 MRNA vaccination for SARS-CoV-2 in patients with multiple sclerosis receiving anti-CD20 therapy. Mult. Scler. J. Exp. Transl. Clin. 2021;7(4) doi: 10.1177/20552173211062142. 2021 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauré D., O'Ryan M., Torres J.P., et al. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect. Dis. 2022;22(1):56–63. doi: 10.1016/S1473-3099(21)00479-5. 2022 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., et al. Effect of SARS-CoV-2 MRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. 2021 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., et al. Breakthrough SARS-CoV-2 infections after COVID-19 MRNA vaccination in ms patients on disease modifying therapies during the delta and the omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. 2022 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., et al. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella C., Aiello A., Gasperini C., et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 MRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):E541–E554. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano S., Clara G.C., Francesco P. Multiple sclerosis, COVID-19 and vaccines: making the point. Neurol. Ther. 2021;10(2):627–649. doi: 10.1007/s40120-021-00288-7. 2021 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkoğlu R., Baliç N., Kızılay T., et al. Fingolimod impairs inactivated vaccine (CoronaVac)-induced antibody response to SARS-CoV-2 spike protein in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2022;58 doi: 10.1016/j.msard.2022.103524. 2022 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO coronavirus disease, https://www.who.int/director-general/speeches/detail/who-directorgeneral-s-opening-remarks-at-the-media-briefingon-covid-19–11-march-2020.
- World health organization, Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody, https://www.who.int/publications/m/item/WHO-BS-2020.2403.
- Wu X., Lu W., Lu S., Kefu T. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: a meta-analysis. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104102. 2022 Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]