Abstract
Lipopolysaccharide (LPS) is an amphipathic macromolecule that is highly aggregated in aqueous preparations. LPS-binding protein (LBP) catalyzes the transfer of single LPS molecules, segregated from an LPS aggregate, to high-density lipoproteins (HDL), which results in the neutralization of LPS. When fluorescein isothiocyanate-labeled LPS (FITC-LPS) is used, this transfer of LPS monomers to HDL can be measured as an increase in fluorescence due to dequenching of FITC-LPS. Recently, serum amyloid P component (SAP) was shown to neutralize LPS in vitro, although only in the presence of low concentrations of LBP. In this study, we show that SAP prevented HDL-mediated dequenching of FITC-LPS, even in the presence of high concentrations of LBP. Human bactericidal/permeability-increasing protein (BPI), a very potent LPS-binding and -neutralizing protein, also prevented HDL-mediated dequenching of FITC-LPS. Furthermore, SAP inhibited HDL-mediated neutralization of both rough and smooth LPS in a chemiluminescence assay quantifying the LPS-induced priming of neutrophils in human blood. SAP bound both isolated HDL and HDL in serum. Using HDL-coated magnetic beads prebound with SAP, we demonstrated that HDL-bound SAP prevented the binding of LPS to HDL. We suggest that SAP, by preventing LPS binding to HDL, plays a regulatory role, balancing the amount of LPS that, via HDL, is directed to the adrenal glands.
Lipopolysaccharide (LPS) is the major component of the outer membrane of gram-negative bacteria. When bacteria are exposed to blood or plasma, LPS is released from the bacterial surface as either membrane fragments, membrane blebs, or mixed vesicles of bacterial phospholipid and LPS. To date, several studies have shown that plasma proteins play an important role in mediating cell responses to LPS. Two of these plasma proteins, LPS-binding protein (LBP) and soluble CD14 (sCD14), both phospholipid transfer proteins, have been demonstrated to play a role in enhancing responses to LPS. LBP can catalytically transfer an LPS monomer to CD14 expressed on monocytes, macrophages, and neutrophils, initiating the activation of these cells (14, 35). LBP can also enhance the transfer of LPS monomers to sCD14 (12), enabling LPS activation of CD14-negative cells, such as epithelial, endothelial, and smooth muscle cells (8, 30). Recently, strong evidence was provided that Toll-like receptor 4 plays a role in LPS-induced signal transduction via CD14 or sCD14 (15, 29). LBP facilitates the binding of LPS not only to CD14 or sCD14 but also to high-density lipoproteins (HDL) either by direct transfer or by a two-step reaction in which LPS is transferred first to sCD14 and then to HDL (41). Besides binding to HDL, LPS can interact with other lipoproteins present in plasma, for example, low-density lipoproteins, very low-density lipoproteins, and chylomicrons (7, 13, 38). Several studies have shown that the interaction of LPS with lipoproteins reduce its biological activity in vitro and in vivo. For example, HDL-bound LPS is less potent in the induction of cytokine release by monocytes or macrophages than free LPS (2, 7), while lipoproteins at physiological concentration reduce the LPS-induced oxidative burst in human neutrophils (39). Moreover, transgenic mice expressing high levels of plasma HDL levels are protected against LPS challenge (16).
Recently, our group identified a novel LPS-binding protein, serum amyloid P component (SAP) (4). SAP is a decameric serum glycoprotein composed of identical 25.5-kDa subunits noncovalently associated in two pentameric rings interacting face to face. Together with C-reactive protein (CRP), SAP belongs to the pentraxin protein family. SAP and CRP have a 51% amino acid homology; however, unlike CRP, SAP is not an acute-phase reactant in humans. It is constitutively present in serum at 30 to 50 μg/ml (6). Although its exact physiologic function is not known, it is believed to play a role in the binding and clearance of host- or pathogen-derived cellular debris at sites of inflammation (9). SAP binds to LPS with high affinity and, in the presence of low concentrations of LBP, inhibits LPS-induced responses in phagocytes (3, 4). Using gel filtration of serum preincubated with fluorescein isothiocyanate-labeled LPS (FITC-LPS), we showed that SAP binds to aggregated LPS in serum. In the same report, we showed that HDL is one of the major components in serum that can dequench FITC-LPS (5).
The present study investigated whether the interaction of SAP with aggregated LPS influences the transfer of LPS monomers to HDL and thereby affects the LPS-neutralizing ability of HDL. Because human bactericidal/permeability-increasing protein (BPI) is another well-known LPS-binding and -neutralizing protein, its effect on HDL-mediated dequenching of FITC-LPS was also studied.
MATERIALS AND METHODS
Reagents.
LPSs from Salmonella enterica serovar Minnesota strain R595 (ReLPS) and Escherichia coli O111:B4 were obtained from Sigma (St. Louis, Mo.). FITC-LPS was prepared as described by Troelstra et al. (36) with a molar labeling efficiency of 1:1. Recombinant human LBP (rLBP) was a generous gift from Henri S. Lichenstein (Amgen, Boulder, Colo.). Human native BPI was purified from neutrophils using E. coli J5 as the affinity matrix, as described by Mannion et al. (19). rBPI21 was a gift from XOMA Corporation (Berkeley, Calif.).
Serum.
Human serum was obtained from healthy donors and stored until use at −70°C. For SAP depletion, the serum was incubated for two 1-h periods with DNA-cellulose (Sigma) under constant agitation on ice. It was then collected, filtered (0.2-μm Spin-X tubes; Costar, Cambridge, Mass.), and stored at −70°C. This procedure reduced the SAP concentration in serum to about 1% of its original concentration, as determined by a SAP-specific enzyme-linked immunosorbent assay (ELISA) (4).
Isolation of SAP from serum.
To isolate SAP, fresh serum was applied to a column containing DNA-cellulose. The column was washed with Hanks' balanced salt solution (HBSS; Gibco BRL, Life Technologies, Breda, The Netherlands), and SAP was eluted with an EDTA buffer (140 mM NaCl, 0.01 M Tris-HCl, 10 mM EDTA [pH 8.0]). The eluate was then applied to a gel filtration column (Hiload 26/60, Superdex 200; Pharmacia, Uppsala, Sweden). The fractions containing SAP were concentrated in an Amicon filter system (10-kDa cutoff) and dialyzed against phosphate-buffered saline (PBS). SAP isolation was checked with sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie brilliant blue staining. SAP concentration was determined by ELISA as described by de Haas et al. (4).
Isolation of HDL.
HDL was isolated as described by Redgrave et al. (31). Briefly, EDTA plasma was applied on a gradient of potassium bromide and ultracentrifuged at 166,000 × g for 22 h at 4°C. The lipid fraction with a density between 1.063 and 1.210 was used as the HDL-containing fraction. The remaining fractions were pooled and referred to as HDL-depleted plasma. The fractions were then dialyzed against PBS, filtered (0.2-μm-pore-size filter), and kept at 4°C until use. The concentration of HDL was expressed as the equivalent concentration of cholesterol in micrograms per milliliter as determined by a cholesterol assay (Sigma).
Fluorometric dequenching assay for FITC-LPS.
To study the effect of SAP on the dequenching of FITC-LPS in serum, FITC-LPS (0.5 μg/ml) in HBSS–0.2% human serum albumin (HSA; Central Laboratory Blood Transfusion, Amsterdam, The Netherlands) was incubated with 2% normal serum, 2% SAP-depleted (SAP−) serum, and 2% SAP− serum replenished with SAP (0.1 to 3 μg/ml) in a 96-well flat-bottom microtiter plate (Greiner, Solingen, Germany) at 37°C under continuous agitation. In some experiments, normal serum was preincubated with rabbit anti-human SAP immunoglobulin G (IgG; 30 μg/ml; DAKO, Glostrup, Denmark) for 30 min at 37°C before FITC-LPS was added. Fluorescence was measured at set time periods in a Cytofluor II fluorescence multiwell plate reader (Perspective Biosystems, Framingham, Mass.) with excitation and emission wavelengths of 485 and 530 nm, respectively. To investigate the effect of SAP on HDL-induced dequenching, FITC-LPS (0.5 μg/ml) was incubated with HDL (30 μg/ml), rLBP (1 μg/ml), and increasing concentrations of SAP (0 to 10 μg/ml). In some experiments, native BPI or rBPI21 (0 to 3 μg/ml) was tested with the same concentrations of FITC-LPS and HDL. Results are presented as mean ± standard error of the mean (SEM).
LPS-induced priming of human blood.
To investigate the effect of SAP on the HDL-induced neutralization of LPS, LPS priming of whole blood was measured for an enhanced oxidative burst. Briefly, either ReLPS or O111:B4 LPS (1 ng/ml) was preincubated with or without HDL (30 μg/ml) with increasing concentrations of SAP (0 to 10 μg/ml) for 90 min at 37°C under constant agitation, in a total volume of 20 μl. All samples were diluted in HBSS–2% HSA. After preincubation, 80 μl of human heparinized blood, drawn from healthy volunteers, was added, the mixture was incubated for 30 min at 37°C under constant agitation, and then 900 μl of PBS–0.05% glucose was added. A total of 100 μl of this final mixture was used to measure the chemiluminescence response in a luminometer (Autolumat LB 953; Berthold GmbH & Co., Wildbad, Germany) after automated injection of N-formylmethionylleucyl phenylalanine (fMLP; final concentration of 1 μM) and HBSS containing 180 μM luminol (Sigma), as described elsewhere (4).
Determination of SAP binding to HDL.
To investigate whether SAP interacts with HDL, 96-well microtiter plates (Nunc Maxisorp; Nalge Nunc International, Kamstrup, Denmark) were coated overnight at 4°C with isolated HDL (3 μg/ml) and blocked for 1 h at 37°C with HBSS–0.05% Tween–4% bovine serum albumin (BSA). A concentration range of SAP (0 to 0.3 μg/ml) was diluted in HBSS–Tween–1% BSA and incubated for 1 h at 37°C; after a 1-h incubation with a biotinylated anti-human SAP monoclonal antibody (MAb; 1 μg/ml; Monosan; Sanbio, Uden, The Netherlands), peroxidase-labeled streptavidin (Southern Biotechnology Associates, Inc., Birmingham, Ala.) was added. After 1 h, the substrate composed of tetramethylbenzidine (Sigma) and H2O2 in 0.1 M acetate buffer was allowed to be converted for 10 min. To stop the enzymatic reaction, 2 N H2SO4 was added. The optical density at 450 nm (OD450) was then determined using a microtiter plate reader (Bio-Rad Laboratories, Hercules, Calif.). The plate was washed five times with HBSS–0.05% Tween between incubations. To check the amount of HDL coating, rabbit anti-human ApoAI (0.5 μg of IgG/ml; Calbiochem-Novabiochem, La Jolla, Calif.) was incubated for 1 h after blocking, with a subsequent 1-h incubation with peroxidase-labeled goat anti-rabbit IgG (1:5,000; Southern Biotechnology Associates). Two procedures were used to test whether SAP is associated with HDL in human serum. In the first procedure, anti-human ApoAI MAb 3F10 (0.33 μg/ml; ICN Biomedicals, Inc., Aurora, Ohio) was coated onto a microtiter plate, with biotinylated anti-human SAP MAb as the detection antibody. In the second procedure, anti-human SAP MAb 5.4A (1 μg/ml; Monosan; Sanbio) was coated onto a microtiter plate, with a rabbit anti-human ApoAI (0.5 μg/ml) as the detection antibody. Different dilutions of human serum (0 to 10%) were used as samples. The binding of SAP to ApoAI was also tested. To do this, purified ApoAI (1 μg/ml; Calbiochem) was coated overnight in PBS. After blocking, different concentrations of SAP (0 to 1 μg/ml) were added and SAP binding was detected as described above.
Binding of FITC-LPS to HDL-coated beads.
We developed a method to study the effect of SAP on the LPS binding to HDL in order to discriminate between the effect of SAP bound to HDL and SAP present in the fluid phase. Briefly, magnetic beads (4 × 108/ml; tosyl-activated Dyna M-450 beads; Dynal AS, Oslo, Norway) were coated with 500 μg of isolated HDL per ml for 4 h at 37°C under continuous agitation. The beads were then washed twice with HBSS–0.2% HSA and blocked with 1% HSA for 30 min at 37°C. HDL coating was checked on a FACScan (Becton Dickinson, Mountain View, Calif.) after incubation of the HDL-coated beads (106) with anti-human ApoAI MAb 3F10 (10 μg/ml), followed by incubation with FITC-labeled F(ab′)2 goat anti-mouse Ig (DAKO). To examine the interaction of LPS with HDL, the HDL-coated beads (106) were incubated with FITC-LPS (50 ng/ml) in the absence or presence of LBP (1 μg/ml) for 60 min at 37°C. To investigate the effect of SAP on the HDL-LPS interaction, different concentrations of SAP were also added to the HDL-coated beads. In some experiments, the HDL-coated beads were preincubated with increasing concentrations of SAP (0 to 100 μg/ml) for 30 min at 37°C. Then, the beads were washed four times before FITC-LPS and LBP were added for another 60-min incubation at 37°C. Finally, the interaction of FITC-LPS with the HDL-coated beads was analyzed on a FACScan. The interaction of SAP with the HDL-coated beads was checked by incubating them first with anti-human SAP MAb (clone 5; Sigma) and then with FITC-labeled F(ab′)2 goat anti-mouse Ig. Analysis was then conducted on the FACScan.
RESULTS
SAP inhibits the serum-induced dequenching of FITC-LPS.
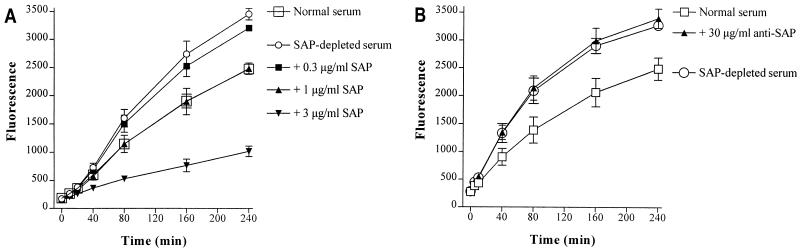
In an earlier study using gel filtration of FITC-LPS, we showed that SAP in whole serum interacts with aggregated LPS (5). In the present study, we were interested in the consequence of this interaction of SAP with LPS in serum. To investigate this, we determined the dequenching capacity of 2% SAP− serum kinetically in a fluorometer and compared it to 2% normal serum obtained from the same donor. Figure 1A shows that the dequenching of FITC-LPS is greater in SAP− serum than in normal serum. Repletion of SAP− serum with 1 μg of SAP per ml, corresponding to the concentration of SAP in 2% normal serum, restored the dequenching capacity to that of normal 2% serum. The addition of 3 μg of SAP per ml (three times the SAP concentration in 2% normal serum) decreased the dequenching capacity of the SAP− serum even more. When normal serum was preincubated with a polyclonal anti-human SAP antibody before the addition of FITC-LPS, the fluorescence signal increased again to the level of SAP− serum (Fig. 1B). This indicates that SAP inhibits the dequenching of FITC-LPS in serum.
FIG. 1.
SAP prevents the serum-induced dequenching of FITC-LPS. (A) Normal serum (2%) or SAP-depleted (SAP−) serum (2%), obtained from the same donor, replenished with increasing amounts of SAP was incubated with FITC-LPS (0.5 μg/ml) at 37°C under continuous agitation. (B) The contribution of SAP in the dequenching of FITC-LPS was also tested by preincubating normal serum with rabbit anti-human SAP antibodies (30 μg/ml) before the addition of FITC-LPS. Fluorescence, as a measure of serum-induced dequenching of FITC-LPS, was measured at set time periods on a fluorometer. Data represent the mean fluorescence ± SEM of three separate experiments performed in triplicate.
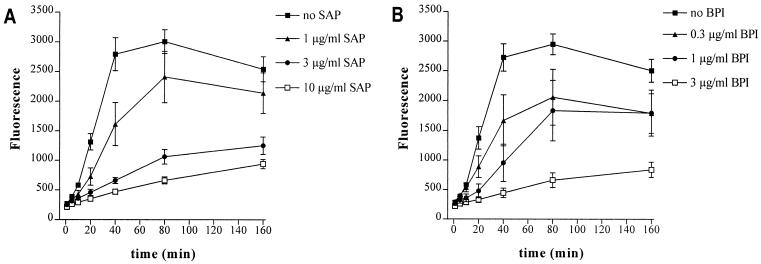
SAP inhibits the HDL-induced dequenching of FITC-LPS.
HDL is considered the major component of serum that is able to bind and neutralize LPS. Incubation of 2% HDL-depleted plasma with FITC-LPS resulted in a fluorescent signal 50% lower than that of 2% whole plasma, indicating that half of the dequenching capacity of serum or plasma is caused by HDL (data not shown). To provide extra evidence that indeed most of the dequenching capacity of plasma or serum is induced by HDL, we compared the dequenching capacity of isolated HDL with that of 2% serum. Figure 2 shows the effect of 30 μg of isolated HDL per ml, which is on average three times the concentration of HDL present in 2% normal serum (33). It is known from the literature that LBP catalyzes the transfer of LPS to HDL. Indeed, when isolated HDL was incubated with FITC-LPS in the presence of increasing concentrations of LBP (0.1 to 1 μg/ml), an increased fluorescence was observed. Incubation of FITC-LPS only in the presence of LBP did not result in a change in fluorescence (data not shown). For subsequent experiments with isolated HDL, 1 μg of LBP per ml was used. A comparison of Fig. 1 (effect of 2% serum) and Fig. 2 (effect of isolated HDL) shows that the increase in fluorescence induced by HDL at 40 min is about four- to fivefold higher than that of 2% serum. This result suggests that HDL is indeed the major component in serum able to dequench FITC-LPS. Therefore, we also examined the effect of SAP on the HDL-induced dequenching of FITC-LPS. Figure 2A shows that SAP clearly inhibited the dequenching of FITC-LPS by HDL, indicating the prevention of the transfer of LPS to HDL and thereby inhibiting the neutralization of LPS by HDL. Incubation of FITC-LPS only in the presence of LBP and SAP did not result in a change in fluorescence (data not shown). The effect of BPI, another well-known LPS-binding and -neutralizing protein, was also investigated. As shown in Fig. 2B, human native BPI also clearly inhibited the HDL-induced dequenching of FITC-LPS. The same was found for recombinant human BPI21 (data not shown).
FIG. 2.
SAP and BPI prevent the binding of FITC-LPS to HDL. HDL (30 μg/ml) was incubated with FITC-LPS (0.5 μg/ml) and LBP (1 μg/ml) in the presence of increasing amounts of SAP (A) or native BPI (B). Fluorescence, as a measure of HDL-induced dequenching of FITC-LPS, was measured at set time periods on a fluorometer. Data represent the mean fluorescence ± SEM of three separate experiments performed in duplicate.
SAP inhibits the HDL-induced neutralization of LPS in human blood.
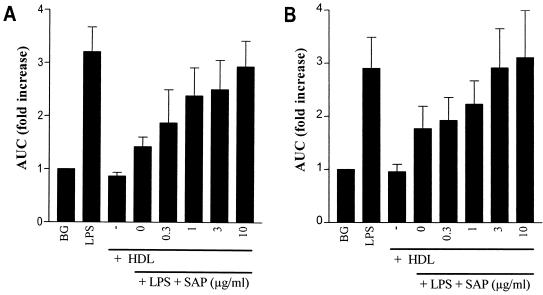
A chemiluminescence assay was used to examine whether SAP could also inhibit the HDL-induced neutralization of unlabeled LPS. Briefly, either ReLPS or O111:B4 LPS was preincubated with HDL in the presence of increasing amounts of SAP for 90 min before human whole blood was added. The fMLP-induced chemiluminescence response was then measured after an additional 30 min of incubation. Preincubating LPS only with HDL abrogated the priming activity of ReLPS (Fig. 3A) and O111:B4 LPS (Fig. 3B) by about 70 and 50%, respectively. When the LPS-HDL mixture was incubated in the presence of increasing concentrations of SAP, however, the priming activity of LPS was completely restored. Preincubation of LPS with only SAP did not affect the priming activity of ReLPS or O111:B4 LPS. These results indicate that SAP, regardless of whether unlabeled rough or smooth LPS is used, inhibits the capacity of HDL to neutralize LPS.
FIG. 3.
SAP prevents the HDL-mediated neutralization of LPS. HDL (30 μg/ml) was preincubated with ReLPS (A) and O111:B4 LPS (B) (1 ng/ml) in the presence of increasing amounts of SAP (0 to 10 μg/ml) for 90 min at 37°C at a volume of 20 μl. Then, 80 μl of undiluted heparinized human blood was added, and the mixture was incubated for 30 min at 37°C. The chemiluminescence response was measured for 10 min after automated injection of fMLP and luminol to 10-fold-diluted blood samples in a luminometer. The background (BG) represents the chemiluminescence response in the absence of LPS and HDL. Data represent the fold increase of the 10-min integral (area under the curve [AUC]) ± SEM of four separate experiments compared to the background.
SAP binds to HDL via ApoAI.
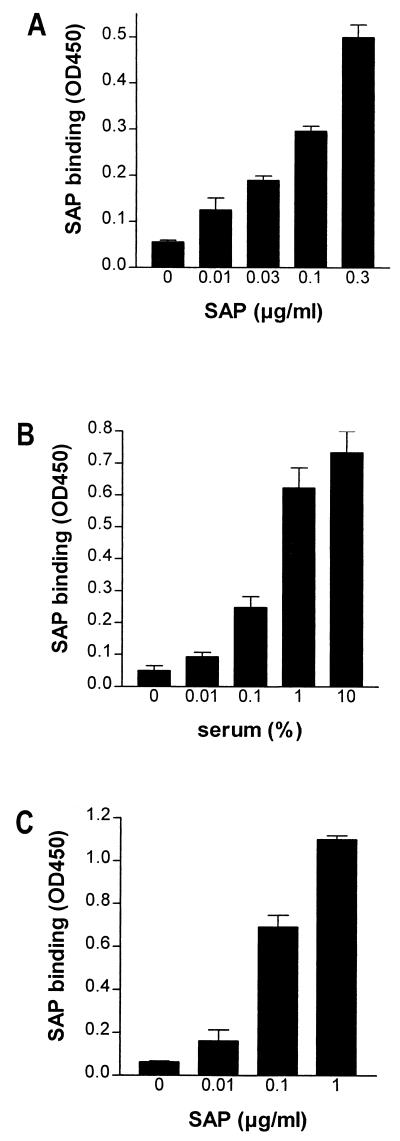
In an earlier study, we demonstrated that SAP was not able to inhibit the LPS-induced activation of neutrophils in the presence of high concentrations of LBP. Since the interaction of LPS with HDL is also LBP mediated, no inhibition by SAP was expected when high concentrations of LBP were available. However, the present experiments clearly show that SAP inhibited the interaction of LPS with HDL even in the presence of high concentrations of LBP. Therefore, it seems that the binding of SAP to LPS cannot solely explain this phenomenon. The binding of SAP to isolated HDL has already been discussed in the literature (17). Figure 4A demonstrates this binding of SAP to isolated HDL. To check whether SAP is also associated with HDL in serum, we performed a capture ELISA with anti-human SAP which was used to coat a microtiter plate and anti-human ApoAI as a detection antibody. As shown in Fig. 4B, there was a clear interaction between HDL and SAP in serum. Furthermore, when anti-human ApoAI which was used to coat a microtiter plate that was then incubated with serum and detected with anti-human SAP, an interaction between HDL and SAP also was demonstrated (data not shown). Since LBP and cationic protein 18-derived peptide LL-37 can interact with HDL via ApoAI (20, 40, 42), we tested whether SAP could also bind to HDL via ApoAI. Our results show that SAP was able to bind to ApoAI coating on a microtiter plate (Fig. 4C). This suggests that SAP associates with HDL via ApoAI. Thus, the binding of SAP to HDL may be the mechanism by which SAP prevents LPS from binding to HDL.
FIG. 4.
SAP binds to HDL and ApoAI. The binding of SAP was tested with isolated HDL (A), HDL in serum (B), and purified ApoAI (C). (A) For the binding of SAP to isolated HDL, HDL (3 μg/ml) was used to coat a microtiter plate overnight. After washing, increasing concentrations of SAP were tested for binding, as detected by a biotinylated anti-human SAP MAb and subsequent peroxidase-labeled streptavidin. (B) The binding of SAP to HDL in serum was tested by incubating serum in an anti-human SAP MAb-coated microtiter plate, followed by the detection of captured HDL with a polyclonal anti-human ApoAI antibody and a peroxidase-labeled goat anti-rabbit IgG. (C) The binding of SAP to ApoAI was tested by incubating increasing concentrations of SAP in an ApoAI-coated microtiter plate and detecting SAP binding as described above. Data represent the mean OD450 ± SEM of two (A and C) and three (B) separate experiments.
Binding of SAP to HDL prevents subsequent binding of LPS.
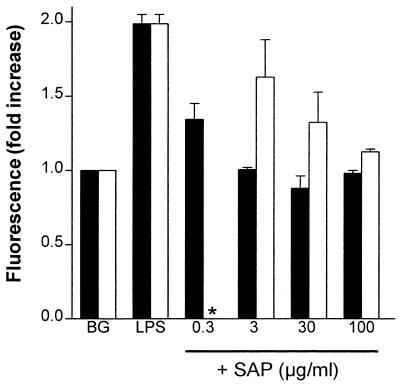
Next, we investigated whether SAP binding to HDL could prevent the binding of LPS to HDL. HDL-coated magnetic beads were used for this investigation. Incubation of the HDL-coated beads with an anti-human ApoAI MAb clearly showed that HDL was coated to the beads (uncoated beads gave a mean fluorescence of 8, while HDL-coated beads gave a mean fluorescence of 119). Figure 5 shows a clear LBP-dependent binding of FITC-LPS to the HDL-coated beads. This interaction was inhibited when SAP was added along with FITC-LPS and LBP to the beads. Even when the HDL-coated beads were preincubated with SAP and then washed to remove unbound SAP, the FITC-LPS binding to the HDL-coated beads was still inhibited, although higher concentrations of SAP were needed (Fig. 5). The association of SAP with HDL-coated beads was confirmed with an anti-SAP MAb staining (data not shown). These results suggest that HDL-bound SAP can prevent the association of LPS with HDL.
FIG. 5.
HDL-bound SAP prevents the binding of LPS to HDL. The effect of SAP on the binding of FITC-LPS to HDL-coated beads was studied to discriminate between HDL-bound SAP and fluid-phase SAP. The binding of FITC-LPS to HDL-coated beads preincubated with SAP (open bars) was compared to the binding of FITC-LPS to HDL-coated beads with no preincubation (solid bars). Briefly, the HDL-coated beads that were preincubated with SAP were washed to remove any unbound SAP. Then, the SAP-preincubated HDL-coated beads were incubated with FITC-LPS (50 ng/ml) and LBP (1 μg/ml) for 60 min at 37°C (∗, preincubation of HDL-coated beads with SAP [0.3 μg/ml] was not determined), while the non-SAP-preincubated HDL-coated beads were incubated with increasing concentrations of SAP, together with FITC-LPS and LBP, for 60 min at 37°C. The background (BG) represents the fluorescence of HDL-coated beads in the presence of LBP (1 μg/ml) only. LPS represents the fluorescence of the HDL-coated beads incubated with FITC-LPS and LBP. Data represent the fold increase of the fluorescence ± SEM of two separate experiments compared to the background.
DISCUSSION
Recently, we demonstrated that SAP binds with high affinity to LPS. However, it is not able to neutralize the LPS-induced activation of phagocytes in the presence of serum or high concentrations of LBP (3–5). Gel filtration of serum, preincubated with FITC-LPS, demonstrated that HDL is able to dequench FITC-LPS, giving rise to an increase in fluorescence, while SAP is not. These results indicate that HDL binds separate, monomeric LPS molecules, while SAP interacts with aggregated LPS. There is some debate in the literature as to whether the monomeric or aggregated state of LPS is more biologically active (32). Whatever the case may be, the binding of LPS to HDL is known to neutralize LPS (2, 27, 39, 42). Additionally, this report demonstrates that HDL neutralizes the LPS-induced priming of neutrophils in human blood. Since we and others could clearly show dequenching of FITC-LPS or BODIPY-LPS by HDL (5, 11, 43), we will use the terms “dequenching” and “neutralization” as synonyms as far as HDL is concerned.
In this study, the same dequenching properties of FITC-LPS were used to study the consequence of SAP binding to aggregated LPS on the monomerizing capacity of HDL. A fluorometer was used to measure the fluorescence of FITC-LPS. First, the dequenching capacity of serum was assessed; approximately 50% was attributed to HDL. This finding is consistent with those of Wurfel et al. (42), who demonstrated that the depletion of HDL from serum never resulted in more than a 66% reduction in the LPS-neutralizing capacity of the serum. The remaining LPS-neutralizing activity of the serum is most likely caused by other serum lipoproteins, such as low-density lipoproteins, very low-density lipoproteins, and chylomicrons, since they are also described as playing a role in the binding and neutralization of LPS (13, 26). SAP clearly inhibited the serum- and the HDL-induced dequenching of FITC-LPS. Furthermore, SAP inhibited the HDL-induced neutralization of both unlabeled rough ReLPS and smooth O111:B4 LPS. This strongly implies that the effects of SAP on FITC-LPS can be extrapolated to the effects of SAP on unlabeled LPS. SAP also inhibited the HDL-induced neutralization of smooth LPS, which is more relevant for an infection with a gram-negative bacterium than rough LPS. The reader might be concerned about the differences in the concentrations used for FITC-LPS and unlabeled LPS; unfortunately, the dequenching of FITC-LPS could not be evaluated using lower concentrations of FITC-LPS due to the limited sensitivity of the fluorometric assay. Like SAP, BPI inhibited the HDL-induced neutralization of LPS. The efficacy of BPI in the treatment of sepsis caused by a gram-negative organism has already been tested in humans. Although treatment with BPI showed promising results in a phase I/II trial of severe meningococcemia (10), investigators should take into account not only the direct neutralization of LPS but also the intervention of the natural occurring HDL-mediated LPS neutralization.
In an earlier report, we demonstrated that SAP could not inhibit the binding of LPS to either CD14 or sCD14 in the presence of high concentrations of LBP. It was hypothesized, therefore, that SAP competed with LBP in binding LPS (4). Thus, although SAP cannot inhibit the LBP-dependent route of LPS binding to CD14 or sCD14, it may inhibit the LBP-dependent route of LPS binding to HDL. Besides binding to LPS, the present report shows the binding of SAP to isolated HDL, as was also reported by Li et al. (17), and HDL in serum. Therefore, this SAP-HDL interaction might explain the different effects of SAP on LPS binding to CD14 or sCD14 versus HDL. This is not the first observation that an LPS-binding protein binds to HDL. LBP (20, 28, 42) and cationic protein 18-derived human antibacterial/cytotoxic peptide LL-37 (40) were also found associated with HDL via binding to ApoAI. Since SAP also bound purified ApoAI, it might also bind HDL via ApoAI. Using HDL-coated beads preincubated with SAP, it was demonstrated that HDL-bound SAP, and not LPS-bound SAP, prevented the binding of LPS to HDL. However, it is difficult to exclude the possibility that prebound SAP detaches to some degree from the HDL-coated beads and thereby still exerts some of its action in fluid phase by binding to LPS. We propose that SAP prevents the binding of LPS to HDL via competition with LBP. Wurfel et al. (42) showed that all LBP activity in serum can be captured via an anti-ApoAI column, suggesting that all LBP molecules are associated with HDL. They calculated that fewer than 1 in 100 HDL particles bear an LBP molecule and proposed that only this small subfraction of HDL is active in the binding and neutralization of LPS. If all HDL particles were able to bind LPS, the capacity of HDL to bind LPS would be 10- to 10,000-fold higher than the LPS concentrations reported in studies of septic patients (25, 34, 38). Since the infusion of exogenous lipoproteins still showed additional LPS-neutralizing effects (16), the hypothesis that only LBP-bound HDL particles can bind LPS might be true. Then, SAP and LBP may compete for the same binding site on HDL. This might be the mechanism by which SAP prevents LPS binding to HDL. Alternatively, SAP binding to an HDL-LBP complex could prevent the catalytic, LPS-monomerizing function of LBP. We have not yet been able to confirm either of these hypotheses.
We have shown that SAP inhibits the HDL-induced binding and neutralization of LPS. This does not seem to be very beneficial for the host. Besides SAP, antibodies to LPS have also been described to inhibit LPS binding to HDL, which results in an increased uptake of the injected LPS by the liver and spleen (24). Unlike free LPS, which is removed rapidly from the plasma, LPS bound to HDL has a prolonged half-life in plasma, as it is taken up slowly by tissues that utilize HDL cholesterol for purposes such as the synthesis of steroid hormones. Thus, free LPS is rapidly taken up by the liver and spleen, while the uptake of HDL-bound LPS is much slower, with a shift mainly toward the adrenal glands (18, 21, 23, 24). Data on the clearance of preformed HDL-LPS complexes in rabbits have even shown a preferential uptake of LPS in the adrenal glands that exceeded all other tissues, including liver and spleen, at least threefold (22, 37). The functional integrity of the adrenal cortex is described as an important factor in host survival during shock and disseminated intravascular coagulation arising from sepsis caused by a gram-negative organism. The adrenal glands produce the glucocorticoids that mediate the systemic stress response to infection. As suggested by some researchers (22, 23), the accumulation of LPS in the adrenal glands could provoke adrenal cortical insufficiency or hemorrhage in some patients. Others, however, have reported that LPS administration evokes the expression of macrophage migration inhibitory factor (MIF) in several organs, including the adrenal glands (1). MIF has been shown to counterregulate the inhibitory effect of glucocorticoids on inflammatory cytokine production. Controlling the amount of LPS that is transported to the adrenals by HDL could, therefore, regulate the expression of MIF and thereby the production of glucocorticoid. We suggest that SAP, by preventing LPS binding to HDL, plays a regulatory role, balancing the amount of LPS that, via HDL, is directed to the adrenal glands.
REFERENCES
- 1.Bacher M, Meinhardt A, Lan H Y, Mu W, Metz C N, Chesney J A, Calandra T, Gemsa D, Donnelly T, Atkins R C, Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997;150:235–246. [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon J M, Fitting C, Haeffner-Cavaillon N, Kirsch S J, Warren H S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Haas C J C, Haas P-J, Van Kessel K P M, Van Strijp J A G. Affinities of different proteins and peptides for lipopolysaccharide (LPS) as determined by biosensor technology. Biochem Biophys Res Commun. 1998;252:492–496. doi: 10.1006/bbrc.1998.9675. [DOI] [PubMed] [Google Scholar]
- 4.de Haas C J C, Van der Tol M E, Van Kessel K P M, Verhoef J, Van Strijp J A G. A synthetic lipopolysaccharide (LPS)-binding peptide based on amino acids 27–39 of serum amyloid P component inhibits LPS-induced responses in human blood. J Immunol. 1998;161:3607–3615. [PubMed] [Google Scholar]
- 5.de Haas, C. J. C., H. J. van Leeuwen, J. Verhoef, K. P. M. Van Kessel, and J. A. G. Van Strijp. Analysis of lipopolysaccharide (LPS)-binding characteristics of serum components using gel filtration of FITC-labeled LPS. J. Immunol. Methods, in press. [DOI] [PubMed]
- 6.Emsley J, White H E, O'Hara B P, Oliva G, Srinivasan N, Tickle I J, Blundell T L, Pepys M B, Wood S P. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 7.Flegel W A, Baumstark M W, Weinstock C, Berg A, Northoff H. Prevention of endotoxin-induced monokine release by human low- and high-density lipoproteins and by apolipoprotein A-I. Infect Immun. 1993;61:5140–5146. doi: 10.1128/iai.61.12.5140-5146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewurz H, Zhang X H, Lint T F. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 10.Giroir B P, Quint P A, Barton P, Kirsch E A, Kitchen L, Goldstein B, Nelson B J, Wedel N J, Carroll S F, Scannon P J. Preliminary evaluation of recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in children with severe meningococcal sepsis. Lancet. 1997;350:1439–1443. doi: 10.1016/s0140-6736(97)06468-4. [DOI] [PubMed] [Google Scholar]
- 11.Hailman E, Albers J J, Wolfbauer G, Tu A Y, Wright S D. Neutralization and transfer of lipopolysaccharide by phospholipid transfer protein. J Biol Chem. 1996;271:12172–12178. doi: 10.1074/jbc.271.21.12172. [DOI] [PubMed] [Google Scholar]
- 12.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris H W, Grünfeld C, Feingold K R, Rapp J H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Investig. 1990;86:696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch R J, Tobias P S, Glauser M P, Baumgartner J D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 15.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting Edge: Toll-like receptor 4 (TRL4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 16.Levine D M, Parker T S, Donnelly T M, Walsh A, Rubin A L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X A, Yutani C, Shimokado K. Serum amyloid P component associates with high density lipoprotein as well as very low density lipoprotein but not with low density lipoprotein. Biochem Biophys Res Commun. 1998;244:249–252. doi: 10.1006/bbrc.1998.8248. [DOI] [PubMed] [Google Scholar]
- 18.Maier R V, Mathison J C, Ulevitch R J. Interactions of bacterial lipopolysaccharides with tissue macrophages and plasma lipoproteins. Prog Clin Biol Res. 1981;62:133–155. [PubMed] [Google Scholar]
- 19.Mannion B A, Kalatzis E S, Weiss J, Elsbach P. Preferential binding of the cytoplasmic granule-derived bactericidal/permeability-increasing protein to target bacteria. J Immunol. 1989;142:2807–2812. [PubMed] [Google Scholar]
- 20.Massamiri T, Tobias P S, Curtiss L K. Structural determinants for the interaction of lipopolysaccharide binding protein with purified high density lipoproteins: role of apolipoprotein A-I. J Lipid Res. 1997;38:516–525. [PubMed] [Google Scholar]
- 21.Mathison J C, Ulevitch R J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979;123:2133–2143. [PubMed] [Google Scholar]
- 22.Mathison J C, Ulevitch R J. Uptake and subcellular localization of bacterial lipopolysaccharide in the adrenal gland. Am J Pathol. 1985;120:79–86. [PMC free article] [PubMed] [Google Scholar]
- 23.Munford R S, Andersen J M, Dietschy J M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Investig. 1981;68:1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munford R S, Dietschy J M. Effects of specific antibodies, hormones, and lipoproteins on bacterial lipopolysaccharides injected into the rat. J Infect Dis. 1985;152:177–184. doi: 10.1093/infdis/152.1.177. [DOI] [PubMed] [Google Scholar]
- 25.Munford R S, Hall C L, Dietschy J M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981;34:835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea M G, Demacker P N M, Kullberg B J, Boerman O C, Verschueren I, Stalenhoef A F H, Van der Meer J W M. Low-density lipoprotein receptor-deficient mice are protected against lethal endotoxemia and severe Gram-negative infections. J Clin Investig. 1996;97:1366–1372. doi: 10.1172/JCI118556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajkrt D, Doran J E, Koster F, Lerch P G, Arnet B, Van der Poll T, ten Cate J W, Van Deventer S J H. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. J Exp Med. 1996;184:1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park C T, Wright S D. Plasma lipopolysaccharide-binding protein is found associated with a particle containing apolipoprotein A-I, phospholipid, and factor H-related proteins. J Biol Chem. 1996;271:18054–18060. doi: 10.1074/jbc.271.30.18054. [DOI] [PubMed] [Google Scholar]
- 29.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 30.Pugin J, Schurer Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redgrave T G, Roberts D C K, West C E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- 32.Takayama K, Din Z Z, Mukerjee P, Coombe D R, Kirkland T N. Physicochemical properties of the lipopolysaccharide unit that activates B lymphocytes. J Biol Chem. 1990;265:14023–14029. [PubMed] [Google Scholar]
- 33.Tall A R, Small D M. Plasma high-density lipoproteins. N Engl J Med. 2000;299:1232–1236. doi: 10.1056/NEJM197811302992207. [DOI] [PubMed] [Google Scholar]
- 34.Tobias P S, McAdam K P, Soldau K, Ulevitch R J. Control of lipopolysaccharide-high-density lipoprotein interactions by an acute-phase reactant in human serum. Infect Immun. 1985;50:73–76. doi: 10.1128/iai.50.1.73-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobias P S, Soldau K, Kline L, Lee J D, Kato K, Martin T P, Ulevitch R J. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J Immunol. 1993;150:3011–3021. [PubMed] [Google Scholar]
- 36.Troelstra A, Antal-Szalmas P, de Graaf-Miltenburg L A M, Weersink A J L, Verhoef J, Van Kessel K P M, Van Strijp J A G. Saturable CD14-dependent binding of fluorescein-labeled lipopolysaccharide to human monocytes. Infect Immun. 1997;65:2272–2277. doi: 10.1128/iai.65.6.2272-2277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulevitch R J, Johnston A R, Weinstein D B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Investig. 1979;64:1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Lenten B J, Fogelman A M, Haberland M E, Edwards P A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1986;83:2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vosbeck K, Tobias P, Mueller H, Allen R A, Arfors K-E, Ulevitch R J, Sklar L A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J Leukoc Biol. 1990;47:97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Agerberth B, Lothgren A, Almstedt A, Johansson J. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J Biol Chem. 1998;273:33115–33118. doi: 10.1074/jbc.273.50.33115. [DOI] [PubMed] [Google Scholar]
- 41.Wurfel M M, Hailman E, Wright S D. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wurfel M M, Kunitake S T, Lichenstein H, Kane J P, Wright S D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J Exp Med. 1994;180:1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu B, Hailman E, Wright S D. Lipopolysaccharide binding protein and soluble CD14 catalyze exchange of phospholipids. J Clin Investig. 1997;99:315–324. doi: 10.1172/JCI119160. [DOI] [PMC free article] [PubMed] [Google Scholar]