Abstract
Objective
Attention, working memory and executive processing have been reported to be consistently impaired in Neuro-Long coronavirus disease (COVID). On the hypothesis of abnormal cortical excitability, we investigated the functional state of inhibitory and excitatory cortical regulatory circuits by single “paired-pulse” transcranial magnetic stimulation (ppTMS) and Short-latency Afferent Inhibition (SAI).
Methods
We compared clinical and neurophysiological data of 18 Long COVID patients complaining of persistent cognitive impairment with 16 Healthy control (HC) subjects. Cognitive status was evaluated by means of the Montreal Cognitive Assessment (MoCA) and a neuropsychological evaluation of the executive function domain; fatigue was scored by the Fatigue Severity Scale (FSS). Resting motor threshold (RMT), the amplitude of the motor evoked potential (MEP), Short Intra-cortical Inhibition (SICI), Intra-cortical Facilitation (ICF), Long-interval Intracortical Inhibition (LICI) and Short-afferent inhibition (SAI) were investigated over the motor (M1) cortex.
Results
MoCA corrected scores were significantly different between the two groups (p = 0.023). The majority of the patients’ performed sub-optimally in the neuropsychological assessment of the executive functions. The majority (77.80%) of the patients reported high levels of perceived fatigue in the FSS. RMT, MEPs, SICI and SAI were not significantly different between the two groups. On the other hand, Long COVID patients showed a reduced amount of inhibition in LICI (p = 0.003) and a significant reduction in ICF (p < 0.001).
Conclusions
Neuro-Long COVID patients performing sub-optimally in the executive functions showed a reduction of LICI related to GABAb inhibition and a reduction of ICF related to glutamatergic regulation. No alteration in cholinergic circuits was found.
Significance
These findings can help to better understand the neurophysiological characteristics of Neuro-Long COVID, and in particular, motor cortex regulation in people with “brain fog”.
Keywords: Long COVID, TMS, LICI, ICF, SAI, SICI
1. Introduction
As new coronavirus disease (COVID19) variants keep scientists worried, more and more evidence suggest the existence of a post-infectious state known as Long COVID, or, more recently, Post-Acute Sequelae of COVID-19 (PASC, Groff et al., 2021). This clinical entity is defined by symptoms persisting more than four to twelve weeks after recovery from COVID-19 infection (Callard and Perego, 2021, Huang et al., 2021, Nalbandian et al., 2021). Shortness of breath, fatigue, hair loss, autonomic dysfunction, neuromuscular disorders, headache, and attention deficits have been frequently described (Manganotti et al., 2021, Buoite Stella et al., 2022, Cares-Marambio et al., 2021, Lopez-Leon et al., 2021, Michelutti et al., 2022). Other psychiatric and cognitive disorders have also been reported, with descriptions of low mood and “brain fog”, i.e., minor memory impairments and deficits in focusing (Kingstone et al., 2020, Maury et al., 2021, Michelutti et al., 2022). A proper clinical definition of a Long COVID Syndrome has not met experts’ consensus yet. However, this cluster of symptoms has been recently referred to as “Neuro-Long-COVID” (Ferrarese et al., 2020, Helbok et al., 2020). The degree of the impairment has been described to follow a gradient correlated to the severity of the acute phase of the infection (Blomberg et al., 2021). Nevertheless, the chance of developing Neuro-Long COVID has been demonstrated not to be strictly linked to the course of the acute phase of the infection (Carod-Artal, 2021, Su et al., 2022).
The cognitive deficits involve mainly executive functions such as working memory, attention, parallel processing, planning and problem-solving. The cognitive deficits have been described both in the acute stage of the COVID-19 infection, with severe involvement of the central nervous system (CNS) (Versace et al., 2021), and in Neuro-Long COVID, with differences in the presentation according to sex (Becker et al., 2021, Hosp et al., 2021, Michelutti et al., 2022). The cognitive deficits reported are typical of syndromes characterized by functional or structural impairment of the frontal and prefrontal lobes (Henri-Bhargava et al., 2018, Jones and Graff-Radford, 2021, Ajčević et al., 2023). These are crucial hubs for working memory, inhibition, cognitive flexibility, planning and problem-solving (Henri-Bhargava et al., 2018, Jones and Graff-Radford, 2021).
“Paired-Pulse” Transcranial Magnetic Stimulation (ppTMS) is a research tool used to investigate and measure the cortical excitation and inhibition in neurological and neuropsychiatric disorders including neurodegenerative disorders such as Mild Cognitive Impairment due to Alzheimer’s Disease (Benussi et al., 2021) and frontotemporal dementia (Benussi et al., 2018, Benussi et al., 2017b, Benussi et al., 2016, Benussi et al., 2017a, Benussi et al., 2017b, Benussi et al., 2016, Padovani et al., 2018). Short-latency Afferent Inhibition (SAI) is another TMS parameter that has been largely described to be sensitive to alterations caused by neurodegenerative diseases (Benussi et al., 2021, di Lazzaro et al., 2008, di Lazzaro et al., 2007, di Lazzaro et al., 2005, di Lazzaro et al., 2002, Padovani et al., 2018, Tokimura et al., 2000). On the basis of a possible alteration of the “cortical neurophysiology”, both SAI and ppTMS have been investigated in patients with neuro-long COVID. These parameters were found to be abnormal both after severe acute COVID-19 infection with neurological complications (Versace et al., 2021) and in Neuro-Long COVID (Ortelli et al., 2022) patients.
The aim of this study was to investigate cortical excitability in sensorimotor areas by SAI and ppTMS in a group of Long COVID outpatients complaining of cognitive impairment who did not require hospitalization in their acute infection phase.
2. Methods
2.1. Participants
Participants who were referred to the Neuro-Long COVID ambulatory service of the University Hospital of Trieste from January, the 1st, 2021 to April, the 1st, 2022 were screened for the presence of self-reported cognitive impairment in the post-acute COVID-19 period (diagnosis and recovery confirmed by positive and negative SARS-CoV-2 nasopharyngeal swab, respectively). The Montreal Cognitive Assessment was administered as a cognitive screening test (Nasreddine et al., 2005). Subsequently, a neuropsychological assessment was performed (Michelutti et al., 2022).
All the patients underwent a Magnetic Resonance Imaging (MRI) scan. Inclusion criteria for the study were: the persistence of self-reported cognitive impairment at least after 12 weeks from acute COVID-19 symptoms manifestation; age > 18 years and <75 years, positivity of a nasopharyngeal swab, full recovery from COVID-19 at the moment of the assessment (i.e., the absence of any acute COVID-19 symptom). Exclusion criteria for the study were: a history of COVID-19-related respiratory insufficiency or hospitalization; clinical and/or radiologic evidence of acute phase COVID-19 related pneumonia; the presence of severe psychiatric diseases that required management by a psychiatrist in the past; the presence of cardiologic, endocrine and neurologic major comorbidities, i.e. dementia or Mild Cognitive Impairment (MCI) due to other documented neurological diseases; current treatment with benzodiazepines, steroids, antidepressants and other drugs altering the brain cortex excitability (Robol et al., 2004, Ziemann et al., 2015), the presence of cortical atrophy and/or Fazekas score > 1 (Fazekas et al., 1987) on MRI. No restriction was considered in respect to the time between the onset of the symptoms and the time of the evaluation. All procedures were performed according to the declaration of Helsinki and the study was approved by the regional ethics board (CEUR FVG number 007-2021). All the subjects gave verbal consent for the procedures described.
The total number of patients enrolled was 20. Two patients were discarded due to the finding of previously not-diagnosed psychiatric comorbidities. Eighteen patients (50 ± 11 years, 100% right-handers; 12/18 females) underwent the neurophysiological assessment of cortical excitability and regulation. Sixteen healthy controls (HC) were recruited for comparison (50 ± 71 years, 100% right-handers; 10/16 females). All of them were recruited between hospital personnel undergoing weekly SARS-CoV-2 screening nasopharyngeal swabs (resulted negative). The recruited subjects underwent all the TMS protocols of this study.
2.2. Clinical assessment
Demographic characteristics, presence of neurological, psychiatric, cardiovascular, respiratory, metabolic, neoplastic, endocrine comorbidities and both acute and chronic (lasting for more than 12 weeks) COVID-19 symptoms were collected. We collected the presence of acute upper respiratory symptoms, fever, dyspnoea, headache, myalgia or joint pain, hyposmia or anosmia, palpitations, diarrhea or gastrointestinal tract symptoms and fatigue in the COVID-19 acute phase. Additional acute phase data were collected for the requirement of ventilation for respiratory failure (Michelutti et al., 2022). The presence of Long COVID was extensively studied, screening for symptoms lasting for more than 12 weeks after the infection onset: the patients were investigated for the presence of persistent fatigue, respiratory symptoms, palpitations, gastrointestinal tract symptoms, myalgia or joint pain, tinnitus, vertigo, visual disturbances, persistent fever. The patients were especially investigated for the presence of persisting neurological symptoms, such as paraesthesia, hyposmia or anosmia/ageusia, cognitive deficits, mood disturbances, headache, weakness of the arm/leg and insomnia (Michelutti et al., 2022).
2.3. Cognitive assessment
Cognitive impairment was screened by means of the Montreal Cognitive Assessment – MoCA test. The cut-off used was that suggested by Aiello (Aiello and Depaoli, 2022, Aiello et al., 2022), corrected for age and scholarity. A neuropsychological assessment by trained neuropsychologists was performed on all the patients. It was made of a series of psychometrically validated tests investigating attention and executive functions domain. The Fatigue Severity Scale (FSS) investigated the burden of fatigue on the daily activities of the subjects (Krupp et al., 1989): the subjects had to give a rating from 1 to 7 for every item, with 1 representing full disagreement and 7 representing full agreement. The pre-established cut-off was >4.67 (Krupp et al., 1989).
2.4. Neuropsychological evaluation of attention and executive functions
The Trail Making Test (Siciliano et al., 2019) is a neuropsychological evaluation tool consisting of two parts: part A of the test requires joining a series of numbers arranged in various spatial positions on a sheet of paper as quickly as possible; part B requires quickly joining numbers and letters variously arranged on a sheet of paper alternating between the two categories of stimuli. This test is designed to assess visual-spatial search ability, psycho-motor speed and set-shifting skills, i.e. the ability to alternate attention between two different categories of visual stimuli and to perform two cognitive tasks simultaneously. The Symbol Digit Modalities Test (SDMT) (Nocentini et al., 2006) requires quickly associating symbols with numbers, and measures the speed of processing visual information, oculomotor coordination, sustained attention and learning new visual information. The Paced Auditory Serial Addition Task (Saetti et al., 2021) consists in a task in which numbers are presented orally at a rate of 3 seconds and the person must quickly perform serial additions, adding each number to the previous one. The test measures the speed of processing auditory-verbal information, sustained attention in auditory mode, divided attention and verbal working memory. The Stroop test (Brugnolo et al., 2016) assesses attentional control and inhibition, i.e., the ability to inhibit interfering or irrelevant information in order to select the information that is relevant to the task goals. Finally, the graphical fluency tests (Cattelani et al., 2011) assess mental productivity and creativity, the ability to monitor one's own behaviour and to inhibit actions that have already been implemented but are no longer appropriate.
2.5. Neurophysiological evaluation
2.5.1. Transcranial magnetic stimulation (TMS) protocol
The subjects were instructed to sit in a quiet room in a resting position with eyes open. Stimuli were delivered with a stimulating figure-of-eight coil by using a MagPro® magnetic stimulator (MagVenture Inc., Alpharetta, GA, USA) delivering monophasic pulses. This was connected to an electromyography device (Synergy®, Natus®, Middleton, WI, USA). The electromyography signals were recorded with a bandpass of 10 to 1000 Hz. Ten stimuli were delivered for each ISI and protocol in a pseudo-randomized sequence. For all the protocols, the amplitude of the conditioned responses was expressed as a percentage of the corresponding mean unconditioned response.
Motor Evoked Potentials (MEPs) were recorded from the First Dorsal Interosseus (FDI) muscle of the dominant side with Ag/AgCl surface electrodes attached in a belly-tendon montage. We used a 7 cm figure-of-eight coil, tangentially oriented over the optimum scalp position to elicit MEPs in contralateral FDI, with the induced current flowing in a posterior-anterior direction (Rossini et al., 2015). Intensities were expressed as a percentage of maximum stimulator output (% MSO). The coil, whose position was continuously monitored during the entire experiment, was placed over the optimal site for eliciting MEPs in the contralateral FDI muscle. The optimal scalp position was determined by moving the coil around the area corresponding to the left M1 (approximately between C3 and P3) in 0.5 cm steps. Then, the optimal scalp position where the stimulation constantly produced the largest MEPs was marked in a tight-fitting plastic swimming cap. For each assessment peak-to-peak amplitude was measured and averaged offline for each participant. No substantial difference in the latencies of the MEPs of all the subjects was recorded.
Resting motor threshold (RMT) was defined as the lowest TMS intensity (expressed in percentage of the maximum stimulator output) that evoked motor potentials (MEPs) of at least 50 µV peak-to-peak amplitude in five of ten successive trials (Rossini et al., 2015). Paired-pulse TMS protocols were used to investigate inhibitory and excitatory intracortical networks; the chosen stimulation intensity and interstimulus interval (ISI) underlie different regulatory circuits (Kujirai et al., 1993). Short-Interval Intracortical Inhibition (SICI) at 3 ms and 5 ms ISI (henceforth SICI 2 and SICI 5) and Intracortical Facilitation (ICF) at ISI 10 and 15 ms (henceforth ICF 10 and ICF 15) were evaluated (Kujirai et al., 1993, Rossini et al., 2015). SICI reflects GABAA receptor-mediated fast inhibitory post-synaptic potentials (IPSPs) in corticospinal neurons; Intracortical Facilitation (ICF) reflects glutamatergic signaling (Ziemann et al., 2015). Stimulation intensities were 70% RMT for the conditioning stimulus and 130% RMT for the test stimulus. Long-interval Intracortical Inhibition (LICI) at ISI 100 ms (henceforth LICI 100) was evaluated; the stimulation intensity was of 130% RMT for both conditioning and test stimulus. LICI depends on slow IPSPs mediated through GABAB receptors (Kujirai et al., 1993, Ziemann et al., 2015).
2.5.2. Short afferent inhibition (SAI)
Short-latency Afferent Inhibition (SAI) was assessed to evaluate sensory afferents-mediated M1 inhibition. SAI is caused by the excitatory effect of cholinergic thalamocortical projections onto the inhibitory GABAergic cortical network (Tokimura et al., 2000, Valls-Solé et al., 1992). The conditioning stimulus was delivered to the ulnar nerve at the wrist (at an intensity just above the motor threshold for evoking a visible twitch in FDI) and preceded the TMS by an ISI of N20 −4 +0, 4, 8, ms (hence SAI 16, 20, 24, 28) relative to the latency of the N20 component of the ulnar nerve somatosensory evoked potentials (Alle et al., 2009, di Lazzaro et al., 2007). The intensity of the TMS test pulse over M1 was adjusted to elicit stable MEPs of more than 1 mV peak-to-peak amplitude in the relaxed FDI.
2.6. Statistics and data analyses
All statistical analyses were performed with SPSS version 23 (IBM). This is the primary analysis of these data. Data are reported as the medians, (25th—75th percentile) or counts and proportions (%) as appropriate. Two-tailed testing was performed. Mann-Whitney U test was used to assess differences between people with Long COVID and HC. To account for differences between groups in SAI, SICI and ICF considering the different ISI applied, the independent and interactive effect of health status (2 levels between subjects: Long COVID vs. healthy controls) and ISI (4 levels repeated measures for SAI: 16, 20, 24, 28 ms; 2 levels repeated measures for SICI: 3 ms and 5 ms; 2 levels repeated measures for ICF: 10 ms, 15 ms) was performed with a two-way mixed ANOVA. These analyses established the generalized effect of Long COVID MEPs over the different paired pulse protocols (different ISI), and their interaction. In the event of a statistically significant main group effect, a Bonferroni’s correction for multiple testing was performed for each ISI. Greenhouse–Geisser correction was applied in case of lack of sphericity. Normality testing using the Shapiro–Wilk test was performed for all datasets. Significance was set for p < 0.05.
2.7. Data availability
The authors confirm that the presented data of this study are saved at the Clinical Unit of Neurology, Trieste University Hospital ASUGI, Italy. They are available upon reasonable request and according to the local institutional and ethics regulation.
3. Results
The demographic and clinical features of our patients both for acute and Long COVID stage of the infection are shown in Table 1 . The average time from the diagnosis of COVID-19 infection to the evaluation was 165 ± 45 days. The time to access our outpatient service was always shorter than one year from SARS-COV2 nasopharyngeal swab positivity.
Table 1.
Demographic and clinical features of our Long COVID patients (n = 18). Data presented as mean ± standard deviation and frequencies.
| Characteristics | (n = 18) |
|---|---|
| Demographic Features | |
| Age [y] | 55 ± 10.90 |
| Right-handedness [n (%)] | 18 (100.0%) |
| Sex: female [n (%)] | 12 (66.7%) |
| Acute COVID19 symptoms | |
| Acute Upper Respiratory Symptoms [n (%)] | 9 (50.0%) |
| Acute Dyspnea [n (%)] | 0 (0.0%) |
| Acute Fever [n (%)] | 12 (66.7%) |
| Acute Headache [n (%)] | 11 (61.1%) |
| Acute Myalgia/Joint Pain [n (%)] | 7 (38.9%) |
| Acute Hyposmia/Anosmia [n (%)] | 7 (38.9%) |
| Acute Palpitations [n (%)] | 2 (11.1%) |
| Acute Gastrointestinal Tract Symptoms [n (%)] | 4 (22.2%) |
| Acute Fatigue [n (%)] | 10 (55.5%) |
| Acute Respiratory Failure [n (%)] | 0 (0.0%) |
| Ventilation [n (%)] | 0 (0.0%) |
| Administration of Heparine [n (%)] | 0 (0.0%) |
| Administration of Steroids [n (%)] | 0 (0.0%) |
| Administration of Antibiotics [n (%)] | 0 (0.0%) |
| Long COVID symptoms | |
| Persistent Fatigue [n (%)] | 17 (94.4%) |
| Persistent Respiratory Symptoms [n (%)] | 5 (27.8%) |
| Persistent Tinnitus [n (%)] | 0 (0.0%) |
| Persistent Vertigo [n (%)] | 1 (5.6%) |
| Persistent Headache [n (%)] | 3 (16.7%) |
| Persistent Visual Disturbances [n (%)] | 1 (5.6%) |
| Persistent Fever [n (%)] | 0 (0.0%) |
| Persistent Paresthesia [n (%)] | 0 (0.0%) |
| Persistent Myalgia/Joint Pain [n (%)] | 6 (33.3%) |
| Persistent Hyposmia [n (%)] | 4 (22.2%) |
| Persistent Palpitations [n (%)] | 1 (5.6%) |
| Persistent Gastrointestinal Tract Symptoms [n (%)] | 0 (0.0%) |
| Persistent Insomnia [n (%)] | 3 (16.7%) |
| Persistent Focal Weakness [n (%)] | 0 (0.0%) |
| Persistent Mood Disturbances [n (%)] | 2 (11.1%) |
3.1. Cognitive assessment
The results of the cognitive assessment are shown in Table 2 . All the patients self-reported being cognitively impaired at the time of the examination (18/18, 100%). Regarding the MoCA none of the patients totalized a score lower than the cut-off for pathological impairment, according to the normative data. The patients’ median MoCA corrected score was significantly lower than the healthy controls’ median score (p = 0.023). All the patients underwent a neuropsychological assessment of attentive and executive functions. The prevalence of the patients performing sub-optimally (Equivalent Score, ES < 3.00, 2.00 and 1.00) in at least one of the items of the assessment are shown in Table 2. All the patients were administered the Fatigue Severity Scale: 14 patients totalized a score higher than the cut-off for the scale.
Table 2.
Neuropsychological assessment in long COVID (n = 18) and healthy controls (n = 16). Data are presented as median (25th-75th percentile), and frequencies.
| Long COVID (n = 18) | HC (n = 16) | Significance | |
|---|---|---|---|
| MoCA assessment | |||
| MoCA (raw score) | 26 (25–28) | 30 (28.75–30) | <0.001 |
| MoCA (corrected score) | 24.74 (23.13–25.05) | 26.25 (25.83–26.74) | 0.023 |
| Neuropsychological assessment of executive functions in Long COVID patients | |||
| ES < 1.00 (%) | 6 (33.33%) | – | – |
| ES < 2.00 (%) | 9 (50.00%) | – | – |
| ES < 3.00 (%) | 14 (77.77%) | – | – |
Notes: Montreal Cognitive Assessment: MoCA (Nasreddine et al., 2005); Healthy Controls: HC; Equivalent Score: ES. Correction of the raw MoCA scores as indicated by Aiello et al. (Aiello et al., 2022). ES < 1.00: at least one test resulted < 1.00 in the neuropsychological assessment of executive functions. The ES was not assessed in HC. ES < 2.00: at least one test resulted < 2.00 in the neuropsychological assessment of executive functions. ES < 3.00: at least one test resulted < 3.00 in the neuropsychological assessment of executive functions. Significance for the median score comparison between the two groups was calculated with a Mann-Whitney U test. Bold values for p < 0.05.
3.2. Neurophysiological findings
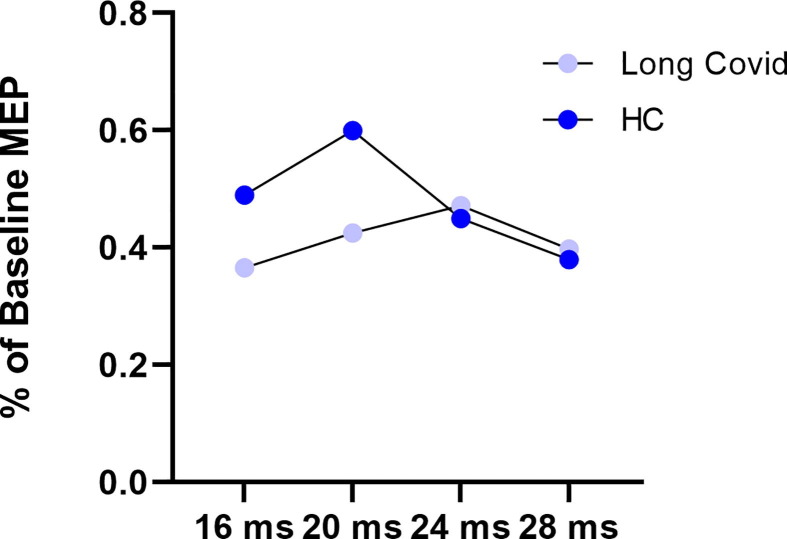
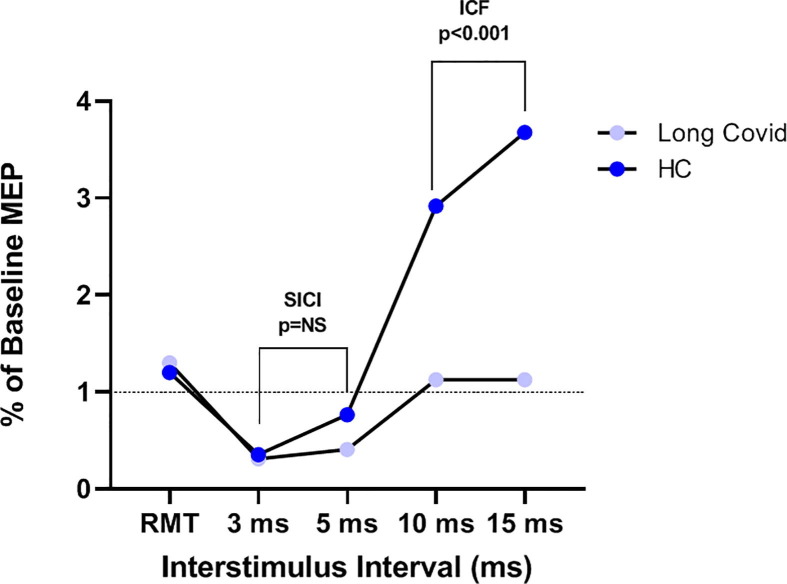
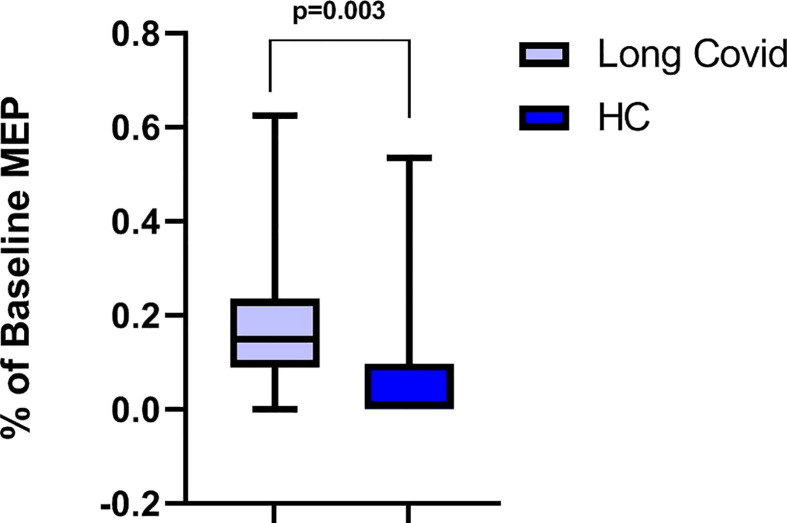
The results of the neurophysiological assessment are shown in Table 3 . We did not find any significant difference in the resting motor threshold (RMT, p = 0.410) and mean evoked motor potentials (MEP, p = 0.980) recorded at 130% intensity between the two groups. Regarding SAI no significant ISI effect (F3,96 = 2.352, p = 0.077, pη2 0.068), group effect (F1,32 = 0.764, p = 0.389, pη2 0.023), nor ISI × group effect (F3,96 = 2.113, p = 0.104; pη2 0.062) was found (Fig. 1 ). Regarding SICI a significant ISI effect (F1,32 = 7.299, p = 0.011, pη2 0.186) was found, in contrast to the non-significant group effect (F1,32 = 2.054, p = 0.161, pη2 0.060) and ISI × group effect (F1,32 = 2.913, p = 0.098; pη2 0.083) was found. Regarding ICF no significant ISI effect (F1,32 = 3.795, p = 0.060, pη2 0.106), whereas a significant ISI × group effect (F1,32 = 4.290, p = 0.046; pη2 0118) and a significant group effect were found (F1,32 = 20.215, p < 0.001, pη2 0.387) (Fig. 2 ). Post-hoc analysis with Sidak’s correction showed that a lower MEP amplitude was found in the long-COVID group compared to the HC (−2.160, 95 CI: −3.139 - −1.181, p < 0.001). Finally, LICI at 100 ms ISI was found to be significantly reduced in the Long-Covid group compared to HC (p = 0.003) (Fig. 3 ).
Table 3.
TMS assessment in long COVID (n = 18) and healthy controls (n = 16). Data are presented as median (25th-75th percentile).
| Long COVID n = 18 |
HC n = 16 |
Significance | |
|---|---|---|---|
| Baseline parameters | |||
| RMT (% SO) | 60 (55–70) | 65 (60–74) | 0.410 |
| Baseline MEP (mv) | 1.30 (0.83–1.57) | 1.20 (0.80–1.60) | 0.980 |
| Latency (ms) | 22.40 (20.90–23.90) | 24.00 (23.83–24.28) | 0.575 |
| Paired Pulse parameters (% of Baseline MEP) | |||
| SAI | 0.389 | ||
| 16 ms | 37 (22–42) | 40 (22–78) | |
| 20 ms | 38 (34–50) | 56 (38–64) | |
| 24 ms | 42 (36–55) | 41 (35–53) | |
| 28 ms | 40 (21–55) | 36 (27–42) | |
| SICI | 0.161 | ||
| 3 ms | 14 (11 –25) | 17 (0–62) | |
| 5 ms | 33 (19–63) | 74 (22–95) | |
| ICF | <0.001 | ||
| 10 ms | 100 (67–121) | 242 (159–420) | |
| 15 ms | 107 (86–111) | 317 (159–540) | |
| LICI 100 ms | 15 (11–23) | 1 (0–9) | 0.003 |
Notes: Healthy Controls: HC; MEP: motor evoked potential; rMT: resting motor threshold (% of stimulator output, SO); Short Afferent Inhibition: SAI; Short Interval intracortical Inhibition: SICI; Intracortical Facilitation: ICF; Long Interval intracortical Inhibition: LICI. Significance for group effect in the mixed factors ANOVA; in case of significant effect, post-hoc Sidak’s correction for the single ISI testing. Bold values for p < 0.05.
Fig. 1.
Short afferent inhibition (SAI) at 16, 20, 24 and 28 ms interstimulus intervals, in Long COVID (n = 18, violet) and healthy controls (HC, n = 16, blue). Motor evoked potentials (MEP) expressed as the percentage of baseline MEP (i.e., the MEP measured without any previous conditioning stimulus). Mixed-way ANOVA was used. No significant ISI effect (F3,96 = 2.352, p = 0.077, pη2 0.068), group effect (F1,32 = 0.764, p = 0.389, pη2 0.023), nor ISI × group effect (F3,96 = 2.113, p = 0.104; pη2 0.062) was found.
Fig. 2.
Resting motor threshold (RMT), short interval intracortical inhibition (SICI) at 3 and 5 ms interstimulus intervals, and intracortical facilitation (ICF) at 10 and 15 ms interstimulus intervals, in Long COVID (n = 18, violet) and healthy controls (HC, n = 21, blue). Motor evoked potentials (MEP) expressed as the percentage of baseline MEP (i.e., the MEP measured without any previous conditioning stimulus). Mixed-way ANOVA was used. Regarding SICI no significant ISI effect (F1,32 = 7.299, p = 0.011, pη2 0.186), group effect (F1,32 = 2.054, p = 0.161, pη2 0.060), nor ISI × group effect (F1,32 = 2.913, p = 0.098; pη2 0.083) was found. Regarding ICF no significant ISI effect (F1,32 = 3.795, p = 0.060, pη2 0.106), whereas a significant ISI × group effect (F1,32 = 4.290, p = 0.046; pη2 0118) and a significant group effect were found (F1,32 = 20.215, p < 0.001, pη2 0.387).
Fig. 3.
Long interval intracortical inhibition (LICI) at 100 ms interstimulus interval in Long COVID (n = 18, violet) and healthy controls (HC, n = 21, blue). Motor evoked potentials (MEP) expressed as the percentage of baseline MEP (i.e., the MEP measured without any previous conditioning stimulus). The difference was tested with the Mann-Whitney U test (p = 0.003).
4. Discussion
The main outcomes from this study suggest that LICI and ICF can be lower in people with Neuro-Long COVID with subjectively reported cognitive impairment than in healthy controls, whereas SICI and SAI are not significantly different. The finding of LICI dysregulation with suboptimal executive performances is partially in line with previous works held on patients after a severe COVID-19 infection complicated with neurological symptoms (Versace et al., 2021) and in patients with Long COVID (Ortelli et al., 2022) affected by a dysexecutive syndrome and an alteration in the perception of fatigue (Ortelli et al., 2022). Both our study and the study from Ortelli et al. (2022) did not find an alteration of GABAa circuits. Differently from them, we did not find any alteration in SAI; furthermore, we did find a reduction in ICF. The neurobiochemical circuits investigated by the TMS paired-pulse protocols (LICI, SICI, ICF) are regulated by GABAb, GABAa (Kujirai et al., 1993, Ziemann et al., 1996) and glutamate (Ziemann et al., 2015, Ziemann et al., 1996), respectively. SAI is regulated by a thalamocortical cholinergic circuit (Tokimura et al., 2000, Valls-Solé et al., 1992). These TMS parameters have been often considered as early biomarkers for neurodegenerative diseases. Indeed, SICI (Benussi et al., 2016, Benussi et al., 2018, Benussi et al., 2021), and ICF (Benussi et al., 2016, Benussi et al., 2017a, Benussi et al., 2017b, Benussi et al., 2018, Benussi et al., 2021) have been correlated with frontotemporal neurodegeneration. SICI is also reduced in central nervous system disorders inducing chronic fatigue (Liepert et al., 2005, McDonald et al., 2010, Vucic et al., 2011). In particular, reductions in LICI have been described in patients affected by frontotemporal dementia (FTD, Benussi et al., 2020a, Benussi et al., 2020b), and could be related to working memory in healthy middle-aged adults (Redondo-Camós et al., 2022). In addition, a trend for decreased LICI, although not significant, has also been observed in Mild Cognitive Impairment (MCI) due to FTD (Benussi et al., 2021) and in pre-symptomatic genetic FTD (Benussi et al., 2016). Abnormal LICI has also been considered a possible biomarker in different neuropsychiatric disorders such as mood disorder, schizophrenia, epilepsy, and amyotrophic lateral sclerosis (Fatih et al., 2021).
GABAa and GABAb receptors are both involved in the spontaneous fluctuation of the persistent activity, i.e., a sustained change in action potential discharge that long outlasts a stimulus (Major and Tank, 2004) in cortical networks (Mann et al., 2009). GABAa and GABAb receptor-mediated inhibition would have distinct roles in respectively balancing and terminating “Up” and “Down” states of activity (Mann et al., 2009). Disruption of GABAb signaling in the prefrontal cortex has been linked to the impairment of many executive functions such as working memory (Redondo-Camós et al., 2022, Bañuelos et al., 2014, Luo et al., 2016, Major and Tank, 2004, Mederos et al., 2021) and goal-directed behavior (Mederos et al., 2021). In our study, the pp-TMS findings could highlight possible alterations of GABAb-mediated inhibition in Long COVID patients. Such alterations could underlie an inefficient regulation of persistent activity states, as GABA represents one of the main inhibitory neurotransmitters in the brain. The decrease in its levels has been observed in patients with cognitive deficits (Porges et al., 2017, Sumner et al., 2010) and behavioral disinhibition in FTD (Murley et al., 2020). A rationale could be suggested considering persistently disinhibited up states of activity due to non-effective GABAb signaling.
In our study we also found an abnormal ICF pattern in Long COVID patients. This has not been shown in previous studies (Ortelli et al., 2022, Versace et al., 2021). ICF is related to glutamatergic excitatory signaling (Ziemann et al., 2015, 1996), and it has been reported to be impaired in dementia (Benussi et al., 2020a, Benussi et al., 2020b, Benussi et al., 2017a, Benussi et al., 2017b) and MCI (Benussi et al., 2021) due to frontotemporal degeneration. Our observation could be linked to the hypothesis of a neuroinflammation-induced increase in glutamate levels, which could be considered among the putative mechanisms of cognitive deficits in Long-COVID (Mohamed et al., 2022).
We did not find any significant difference between the two groups in all the ISIs (16, 20, 24, 28 ms) of SAI. SAI is largely accepted as a neurophysiological biomarker of severe chronic neurodegenerative disorders such as Alzheimer’s Disease (AD) di Lazzaro et al., 2002, di Lazzaro et al., 2004, di Lazzaro et al., 2005, di Lazzaro et al., 2008, Koch et al., 2016, Martorana et al., 2012, Nardone et al., 2006, Nardone et al., 2008, Noh et al., 2015) and Parkinson’s Disease (PD)/Dementia with Lewy Body (LBD) (Benussi et al., 2018, di Lazzaro Vincenzo et al., 2007, Mohamed et al., 2022, Marra et al., 2012). Conversely, normal SAI has been found in syndromes with a dysexecutive profile of cognitive deficits such as those that belong to the spectrum of frontal neurodegeneration (Benussi et al., 2021, Benussi et al., 2018, Benussi et al., 2017a, Benussi et al., 2017b, Benussi et al., 2016), whereas no correlation was demonstrated between frontal executive functioning and SAI in normal subjects and in patients with MCI (Young-Bernier et al., 2014).
Our findings show alterations in the TMS-elicited output of the motor cortex in subjects reporting persisting cognitive impairment months after COVID-19 infection. However, our assessment of motor descending pathways by means of single-pulse TMS showed no differences between the two groups of subjects. This indicates the integrity of motor signaling in our subjects. The finding of abnormal GABAb and glutammatergic (investigated by LICI and ICF respectively), as opposed to normal cholinergic (investigated by SAI) regulation of the excitability of M1 likely reflects a more widespread alteration in the regulatory crosstalk between a distributed network of regions that includes frontal and pre-frontal cortical hubs (Balzekas et al., 2018, Du et al., 2019). This taps into previous stages of motor processing such as planning and executive programming. The strength of the connections between motor, pre-motor and pre-frontal cortices during cognitive executive processing has been extensively supported by structural and functional connectivity data (Higashihara et al., 2021;96., Leisman et al., 2016, Mendoza and Merchant, 2014). For this reason, alterations in the regulation of the M1 output measured by ppTMS could be interpreted as a surrogate marker of activity happening elsewhere in the brain. In fact, alterations of ppTMS parameters in neurodegenerative and psychiatric diseases reflect alterations of cognitive rather than motor functions (Benussi et al., 2020a, Benussi et al., 2020b, Benussi et al., 2018, Benussi et al., 2017a, Benussi et al., 2017b, Benussi et al., 2016, Fatih et al., 2021). Given the correlation between LICI and executive functions (Redondo-Camós et al., 2022), whose heavy reliance on frontal processing has been extensively demonstrated (Peters, 2006, Sanger et al., 2001), one could suspect that the Long COVID dysexecutive deficits that have been reported in our sample of patients could be partially explained by pathogenetic processes involving the frontal areas of the brain. Unfortunately, to obtain a measurable TMS output from regions different than the motor cortex is technically demanding. Future studies including a TMS-EEG in their design would be useful to better localize the dysfunctions in the processes that underlie motor cortical regulation in Long COVID.
A frontal epicentre for the deficits in executive functions found in COVID-infected patients has also been suggested by frontoparietal hypometabolism revealed by PET imaging in a study conducted on patients in an early post-infectious time frame (Hosp et al., 2021) and in Long COVID patients complaining of persistent fatigue (Guedj et al., 2021). A recent study has also indicated structural damage to frontal lobe-located, olfactory network-adjacent areas in Long COVID patients (Douaud et al., 2022). These areas are also thought to be involved in executive processing (Arnold et al., 2020, Jones and Graff-Radford, 2021). Preferential involvement of frontal lobes and frontal-adjacent hubs of executive processing has been putatively explained by means of trans-synaptic direct viral transfer (Baig et al., 2020), or immune-mediated damage due to micro-glial reactive activation (Matschke et al., 2022, Poloni et al., 2021) and leakage of pro-inflammatory cytokines through the ematoencephalic barrier (Krasemann et al., 2022) caused by the proximity of the olfactory bulb, where a high amount of the virus-targeted ACE2 receptor are expressed (Douaud et al., 2022). Alterations in regulatory circuits in the brain might reflect a mild, often transitory, encephalopathy caused (directly or indirectly) by SARS-CoV2 (Buoite Stella et al., 2022, Liotta et al., 2020, Manganotti et al., 2021, Michelutti et al., 2022).
TMS could be a useful and interesting tool to test intracortical excitability in Long COVID patients complaining of cognitive impairment. We have described a neurophysiological phenotype corresponding to sub-optimal executive processing in Long COVID patients. Our findings attribute this to the sole glutamate and GABAb-dependent circuits’ dysfunction. We aimed to exclude the involvement of cholinergic circuits through an extensive investigation of SAI at multiple ISIs. These findings contribute to provide a more detailed and circumscribed picture of the cognitive Long COVID syndrome. Interesting implications for GABAb-dependent physiopathological mechanisms and therapeutic applications are warranted. Findings from the present study should be cautiously considered and could represent a preliminary investigation due to the limited sample size of both Long COVID and healthy controls; nevertheless, the Neuro-Long COVID participants who volunteered in this study were selected among the people who present to our Neuro-Long COVID ambulatory service and are characterized by symptoms persisting several months after the infection and therefore could be considered as chronically affected by this condition. In addition, to provide a fast and easily implementable protocol in this category of patients, the protocol used in this study applied 10 trials for each condition, and this might have affected the findings, recommending protocols considering a larger number of trials. Future studies on larger samples could not only confirm current literature, including the present findings, but could also assess if associations are present between the neurophysiological alterations and symptoms’ severity.
5. Conclusions
Neuro-Long COVID patients with self-reported persistent cognitive deficits and suboptimal executive functions could present a reduction of LICI and of ICF, related to GABAb inhibitory and glutamatergic facilitatory circuits, respectively. Conversely, GABAa and cholinergic-dependent regulation seems not to be significantly impaired. TMS might be useful in individuating pre-clinical cognitive impairment in Long COVID. Future studies could provide therapeutic suggestions targeted at the specific neurotransmitters involved in such condition.
6. Author contributions
P. Manganotti, MD PhD: conception and design of the study, interpretation of the data, revision of the manuscript, responsibility for the integrity of the work as a whole.
M. Michelutti, MD: writing of the manuscript, acquirement of the neurophysiological data, interpretation of the data.
G. Furlanis, MD: screening of the patients and inclusion to the study; theoretical input.
M Deodato, PT PhD: methodology and statistical analysis, revision of the manuscript.
A. Buoite Stella, PhD: methodology and statistical analysis, revision of the manuscript.
7. Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Figures are original and not previously published.
8. Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
9. Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Aiello E.N., Depaoli E.G. Norms and standardizations in neuropsychology via equivalent scores: software solutions and practical guides. Neurol Sci. 2022;43:961–966. doi: 10.1007/S10072-021-05374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello E.N., Gramegna C., Esposito A., Gazzaniga V., Zago S., Difonzo T., et al. The Montreal Cognitive Assessment (MoCA): updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin Exp Res. 2022;34:375–382. doi: 10.1007/S40520-021-01943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajčević M., Iscra K., Furlanis G., Michelutti M., Miladinović A., Buoite Stella A., et al. Cerebral hypoperfusion in post-COVID-19 cognitively impaired subjects revealed by arterial spin labeling MRI. Sci Rep. 2023;13(1):5808. doi: 10.1038/s41598-023-32275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H., Heidegger T., Kriváneková L., Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol. 2009;587:5163–5176. doi: 10.1113/JPHYSIOL.2009.179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T.C., You Y., Ding M., Zuo X.N., de Araujo I., Li W. Functional Connectome Analyses Reveal the Human Olfactory Network Organization. ENeuro. 2020;7:1–14. doi: 10.1523/ENEURO.0551-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/ACSCHEMNEURO.0C00122. [DOI] [PubMed] [Google Scholar]
- Balzekas I., Lewis C.P., Shekunov J., Port J.D., Worrell G.A., Joon Jo H., et al. A pilot study of GABA B correlates with resting-state functional connectivity in five depressed female adolescents. Psychiatry Res Neuroimaging. 2018:279. doi: 10.1016/j.pscychresns.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos C., Sofia Beas B., McQuail J.A., Gilbert R.J., Frazier C.J., Setlow B., et al. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.H., Lin J.J., Doernberg M., Stone K., Navis A., Festa J.R., et al. Assessment of cognitive function in patients after COVID-19 infection. JAMA Netw Open. 2021:4. doi: 10.1001/JAMANETWORKOPEN.2021.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A., Cosseddu M., Filareto I., Dell’Era V., Archetti S., Sofia Cotelli M., et al. Impaired long-term potentiation-like cortical plasticity in presymptomatic genetic frontotemporal dementia. Ann Neurol. 2016;80:472–476. doi: 10.1002/ANA.24731. [DOI] [PubMed] [Google Scholar]
- Benussi A., di Lorenzo F., Dell’Era V., Cosseddu M., Alberici A., Caratozzolo S., et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017:89. doi: 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- Benussi A., Dell’Era V., Cantoni V., Ferrari C., Caratozzolo S., Rozzini L., et al. Discrimination of atypical parkinsonisms with transcranial magnetic stimulation. Brain Stimul. 2018;11:366–373. doi: 10.1016/J.BRS.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Benussi A., Dell’Era V., Cosseddu M., Cantoni V., Cotelli M.S., Cotelli M., et al. Transcranial stimulation in frontotemporal dementia: A randomized, double-blind, sham-controlled trial. Alzheimers Dement (N Y) 2020;6:e12033. doi: 10.1002/trc2.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi A., Grassi M., Palluzzi F., Cantoni V., Cotelli M.S., Premi E., et al. Classification accuracy of TMS for the diagnosis of mild cognitive impairment. Brain Stimul. 2021;14:241–249. doi: 10.1016/J.BRS.2021.01.004. [DOI] [PubMed] [Google Scholar]
- Benussi A., Grassi M., Palluzzi F., Koch G., di Lazzaro V., Nardone R., et al. Classification accuracy of transcranial magnetic stimulation for the diagnosis of neurodegenerative dementias. Ann Neurol. 2020;87:394–404. doi: 10.1002/ANA.25677. [DOI] [PubMed] [Google Scholar]
- Benussi A., di Lorenzo F., Dell’Era V., Cosseddu M., Alberici A., Caratozzolo S., et al. Transcranial magnetic stimulation distinguishes Alzheimer disease from frontotemporal dementia. Neurology. 2017;89:665–672. doi: 10.1212/WNL.0000000000004232. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Mohn K.G.I., Brokstad K.A., Zhou F., Linchausen D.W., Hansen B.A., et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/S41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnolo A., de Carli F., Accardo J., Amore M., Bosia L.E., Bruzzaniti C., et al. An updated Italian normative dataset for the Stroop color word test (SCWT) Neurol Sci. 2016;37:365–372. doi: 10.1007/S10072-015-2428-2. [DOI] [PubMed] [Google Scholar]
- Buoite Stella A., Furlanis G., Frezza N.A., Valentinotti R., Ajcevic M., Manganotti P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol. 2022;269:587–596. doi: 10.1007/S00415-021-10735-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callard F., Perego E. How and why patients made Long Covid. Soc Sci Med. 2021:268. doi: 10.1016/J.SOCSCIMED.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cares-Marambio K., Montenegro-Jiménez Y., Torres-Castro R., Vera-Uribe R., Torralba Y., Alsina-Restoy X., et al. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chron Respir Dis. 2021;18 doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal F.J. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72:384–396. doi: 10.33588/RN.7211.2021230. [DOI] [PubMed] [Google Scholar]
- Cattelani R., Dal Sasso F., Corsini D., Posteraro L. The Modified Five-Point Test: normative data for a sample of Italian healthy adults aged 16–60. Neurol Sci. 2011;32:595–601. doi: 10.1007/S10072-011-0489-4. [DOI] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., Lange F., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. MedRxiv. 2022 doi: 10.1101/2021.06.11.21258690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Choa F., Chiappelli J., Wisner K.M., Wittenberg G., Adhikari B., et al. Aberrant Middle Prefrontal-Motor Cortex Connectivity Mediates Motor Inhibitory Biomarker in Schizophrenia. Biol Psychiatry. 2019:85. doi: 10.1016/j.biopsych.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatih P., Kucuker M.U., vande Voort J.L., Doruk Camsari D., Farzan F., Croarkin P.E. A systematic review of long-interval intracortical inhibition as a biomarker in neuropsychiatric disorders. Front Psychiatry. 2021:12. doi: 10.3389/FPSYT.2021.678088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/AJR.149.2.351. [DOI] [PubMed] [Google Scholar]
- Ferrarese C., Silani V., Priori A., Galimberti S., Agostoni E., Monaco S., et al. An Italian multicenter retrospective-prospective observational study on neurological manifestations of COVID-19 (NEUROCOVID) Neurol Sci. 2020;41:1355–1359. doi: 10.1007/S10072-020-04450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021:4. doi: 10.1001/JAMANETWORKOPEN.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H., et al. 18 F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823–2833. doi: 10.1007/S00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbok R., Chou S.H.Y., Beghi E., Mainali S., Frontera J., Robertson C., et al. NeuroCOVID: it’s time to join forces globally. Lancet Neurol. 2020;19:805–806. doi: 10.1016/S1474-4422(20)30322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri-Bhargava A., Stuss D.T., Freedman M. Clinical assessment of prefrontal lobe functions. Continuum (Minneap Minn) 2018;24:704–726. doi: 10.1212/CON.0000000000000609. [DOI] [PubMed] [Google Scholar]
- Higashihara M., Pavey N., van den Bos M., Menon P., Kiernan M.C., Vucic S. Association of cortical hyperexcitability and cognitive impairment in patients with amyotrophic lateral sclerosis. Neurology. 2021;96. doi: 10.1212/WNL.0000000000011798. [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Dressing A., Blazhenets G., Bormann T., Rau A., Schwabenland M., et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263–1276. doi: 10.1093/BRAIN/AWAB009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Yeming W., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Graff-Radford J. Executive dysfunction and the prefrontal cortex. Continuum (Minneap Minn) 2021;27:1586–1601. doi: 10.1212/CON.0000000000001009. [DOI] [PubMed] [Google Scholar]
- Kingstone T., Taylor A.K., O’Donnell C.A., Atherton H., Blane D.N., Chew-Graham C.A. Finding the “right” GP: a qualitative study of the experiences of people with long-COVID. BJGP Open. 2020;4:1–12. doi: 10.3399/BJGPOPEN20X101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., di Lorenzo F., del Olmo M.F., Bonní S., Ponzo V., Caltagirone C., et al. Reversal of LTP-like cortical plasticity in Alzheimer’s disease patients with Tau-related faster clinical progression. J Alzheimers Dis. 2016;50:605–616. doi: 10.3233/JAD-150813. [DOI] [PubMed] [Google Scholar]
- Krasemann S., Haferkamp U., Pfefferle S., Woo M.S., Heinrich F., Schweizer M., et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports. 2022;17:307–320. doi: 10.1016/J.STEMCR.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp L.B., Larocca N.G., Muir Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/ARCHNEUR.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Kujirai T., Caramia M.D., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A., et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/JPHYSIOL.1993.SP019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Lazzaro V., Oliviero A., Pilato F., Saturno E., Dileone M., Marra C., et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi: 10.1136/JNNP.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Lazzaro V., Oliviero A., Pilato F., Saturno E., Dileone M., Marra C., et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi: 10.1136/JNNP.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Lazzaro V., Oliviero A., Tonali P.A., Marra C., Daniele A., Profice P., et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/WNL.59.3.392. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V., Pilato F., Dileone M., Profice P., Marra C., Ranieri F., et al. In vivo functional evaluation of central cholinergic circuits in vascular dementia. Clin Neurophysiol. 2008;119:2494–2500. doi: 10.1016/J.CLINPH.2008.08.010. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V., Pilato F., Dileone M., Profice P., Ranieri F., Ricci V., et al. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–2214. doi: 10.1016/J.CLINPH.2007.07.005. [DOI] [PubMed] [Google Scholar]
- di Lazzaro Vincenzo, Pilato F., Dileone M., Saturno E., Profice P., Marra C., et al. Functional evaluation of cerebral cortex in dementia with Lewy bodies. Neuroimage. 2007;37:422–429. doi: 10.1016/J.NEUROIMAGE.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Leisman G., Moustafa A.A., Shafir T. Thinking, walking, talking: integratory motor and cognitive brain function. Front Public Health. 2016:4. doi: 10.3389/fpubh.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J., Mingers D., Heesen C., Bäumer T., Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler. 2005;11:316–321. doi: 10.1191/1352458505MS1163OA. [DOI] [PubMed] [Google Scholar]
- Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7:2221–2230. doi: 10.1002/ACN3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., et al. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. MedRxiv. 2021 doi: 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Chen C., Lu Y., Fu T.L., Lu Q., Xu X., et al. Baclofen ameliorates spatial working memory impairments induced by chronic cerebral hypoperfusion via up-regulation of HCN2 expression in the PFC in rats. Behav Brain Res. 2016;308:6–13. doi: 10.1016/J.BBR.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Major G., Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/J.CONB.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Manganotti P., Bellavita G., D’Acunto L., Tommasini V., Fabris M., Sartori A., et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: A case series. J Med Virol. 2021;93:766–774. doi: 10.1002/JMV.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E.O., Kohl M.M., Paulsen O. Distinct roles of GABAA and GABAB receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra C., Quaranta D., Profice P., Pilato F., Capone F., Iodice F., et al. Central cholinergic dysfunction measured “in vivo” correlates with different behavioral disorders in Alzheimer’s disease and dementia with Lewy body. Brain Stimul. 2012;5:533–538. doi: 10.1016/J.BRS.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Martorana A., Esposito Z., di Lorenzo F., Giacobbe V., Sancesario G.M., Bucchi G., et al. Cerebrospinal fluid levels of Aβ42 relationship with cholinergic cortical activity in Alzheimer’s disease patients. J Neural Transm (Vienna) 2012;119:771–778. doi: 10.1007/S00702-012-0780-4. [DOI] [PubMed] [Google Scholar]
- Matschke J., Lahann H., Krasemann S., Altmeppen H., Pfefferle S., Galliciotti G., et al. Young COVID-19 Patients Show a Higher Degree of Microglial Activation When Compared to Controls. Front Neurol. 2022;13:908081. doi: 10.3389/FNEUR.2022.908081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A., Lyoubi A., Peiffer-Smadja N., de Broucker T., Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev Neurol (Paris) 2021;177:51–64. doi: 10.1016/J.NEUROL.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C., Newton J., Lai H.M., Baker S.N., Jones D.E. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. J Hepatol. 2010;53:1095–1100. doi: 10.1016/J.JHEP.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Mederos S., Sánchez-Puelles C., Esparza J., Valero M., Ponomarenko A., Perea G. GABAergic signaling to astrocytes in the prefrontal cortex sustains goal-directed behaviors. Nat Neurosci. 2021;24:82–92. doi: 10.1038/S41593-020-00752-X. [DOI] [PubMed] [Google Scholar]
- Mendoza G., Merchant H. Motor system evolution and the emergence of high cognitive functions. Prog Neurobiol. 2014;122 doi: 10.1016/j.pneurobio.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Michelutti, Giovanni Furlanis, Alex Buoite Stella, Giulia Bellavita, Niccolò Frezza, Giovanna Torresin, et al. Sex-dependent characteristics of Neuro-Long-Covid: Data from a dedicated neurology ambulatory service. J Neurol Sci 2022;441:120355. 10.1016/j.jns.2022.120355. [DOI] [PMC free article] [PubMed]
- Mohamed M.S., Johansson A., Jonsson J., Schiöth H.B. Dissecting the Molecular Mechanisms Surrounding Post-COVID-19 Syndrome and Neurological Features. Int J Mol Sci. 2022;23. doi: 10.3390/IJMS23084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murley A.G., Rouse M.A., Simon Jones P., Ye R., Hezemans F.H., O’Callaghan C., et al. GABA and glutamate deficits from frontotemporal lobar degeneration are associated with disinhibition. Brain. 2020;143:3449–3462. doi: 10.1093/BRAIN/AWAA305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/S41591-021-01283-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R., Bergmann J., Kronbichler M., Kunz A., Klein S., Caleri F., et al. Abnormal short latency afferent inhibition in early Alzheimer’s disease: a transcranial magnetic demonstration. J Neural Transm (Vienna) 2008;115:1557–1562. doi: 10.1007/S00702-008-0129-1. [DOI] [PubMed] [Google Scholar]
- Nardone R., Bratti A., Tezzon F. Motor cortex inhibitory circuits in dementia with Lewy bodies and in Alzheimer’s disease. J Neural Transm (Vienna) 2006;113:1679–1684. doi: 10.1007/S00702-006-0551-1. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/J.1532-5415.2005.53221.X. [DOI] [PubMed] [Google Scholar]
- Nocentini U., Giordano A., di Vincenzo S., Panella M., Pasqualetti P. The Symbol Digit Modalities Test - Oral version: Italian normative data. Funct Neurol. 2006;21:93–96. [PubMed] [Google Scholar]
- Noh N.A., Fuggetta G., Manganotti P. Theta-burst transcranial magnetic stimulation alters the functional topography of the cortical motor network. Malays J Med Sci. 2015;22:35–43. [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., Maestri R., Dezi S., Spampinato D., et al. Altered motor cortex physiology and dysexecutive syndrome in patients with fatigue and cognitive difficulties after mild COVID-19. Eur J Neurol. 2022;29:1652–1662. doi: 10.1111/ENE.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani A., Benussi A., Cantoni V., Dell’Era V., Cotelli M.S., Caratozzolo S., et al. Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease with Transcranial Magnetic Stimulation. J Alzheimers Dis. 2018;65:221–230. doi: 10.3233/JAD-180293. [DOI] [PubMed] [Google Scholar]
- Peters R. Ageing and the brain. Postgrad Med J. 2006;82 doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni T.E., Medici V., Moretti M., Visonà S.D., Cirrincione A., Carlos A.F., et al. COVID-19-related neuropathology and microglial activation in elderly with and without dementia. Brain Pathol. 2021;31:12997. doi: 10.1111/BPA.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges E.C., Woods A.J., Edden R.A.E., Puts N.A.J., Harris A.D., Chen H., et al. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:38–44. doi: 10.1016/J.BPSC.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo-Camós M., Cattaneo G., Alviarez-Schulze V., Delgado-Gallén S., España-Irla G., Solana-Sanchez J., et al. Long-interval intracortical inhibition in primary motor cortex related to working memory in middle-aged adults. Front Psychol. 2022;13 doi: 10.3389/fpsyg.2022.998062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robol E., Fiaschi A., Manganotti P. Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci. 2004;221:41–46. doi: 10.1016/J.JNS.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., di Iorio R., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/J.CLINPH.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetti M.C., Difonzo T., Sirtori M.A., Negri L., Zago S., Rassiga C. The Paced Auditory Serial Addition Task (PASAT): normative data for the Italian population. Neuropsychol Trends. 2021:65–82. doi: 10.7358/NEUR-2021-029-SAET. [DOI] [Google Scholar]
- Sanger T.D., Garg R.R., Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530 doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano M., Chiorri C., Battini V., Sant’Elia V., Altieri M., Trojano L., et al. Regression-based normative data and equivalent scores for Trail Making Test (TMT): an updated Italian normative study. Neurol Sci. 2019;40:469–477. doi: 10.1007/S10072-018-3673-Y. [DOI] [PubMed] [Google Scholar]
- Su Y., Yuan D., Chen D.G., Ng R.H., Wang K., Choi J., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895.e20. doi: 10.1016/J.CELL.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P., Edden R.A.E., Bompas A., Evans C.J., Singh K.D. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/NN.2559. [DOI] [PubMed] [Google Scholar]
- Tokimura H., di Lazzaro V., Tokimura Y., Oliviero A., Profice P., Insola A., et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/J.1469-7793.2000.T01-1-00503.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J., Pascual-Leone A., Wassermann E.M., Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-G. [DOI] [PubMed] [Google Scholar]
- Versace V., Sebastianelli L., Ferrazzoli D., Romanello R., Ortelli P., Saltuari L., et al. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin Neurophysiol. 2021;132:1138–1143. doi: 10.1016/J.CLINPH.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Cheah B.C., Kiernan M.C. Maladaptation of cortical circuits underlies fatigue and weakness in ALS. Amyotroph Lateral Scler. 2011;12:414–420. doi: 10.3109/17482968.2011.597403. [DOI] [PubMed] [Google Scholar]
- Young-Bernier M., Tanguay A.N., Davidson P.S.R., Tremblay F. Short-latency afferent inhibition is a poor predictor of individual susceptibility to rTMS-induced plasticity in the motor cortex of young and older adults. Front Aging Neurosci. 2014;6 doi: 10.3389/FNAGI.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. doi: 10.1016/J.CLINPH.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Rothwell J.C., Ridding M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/JPHYSIOL.1996.SP021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the presented data of this study are saved at the Clinical Unit of Neurology, Trieste University Hospital ASUGI, Italy. They are available upon reasonable request and according to the local institutional and ethics regulation.
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.