Abstract
Aim
To explore the relationship between the serum sodium level on admission and all‐cause mortality in HF patients.
Design
A single‐center retrospective cohort study.
Methods
Patients hospitalized with HF at the Heart Failure Center, Fuwai Hospital, from November 2008 to November 2018 were enrolled.
Results
A total of 3649 patients were included, and the mean sodium level was 137.19 ± 4.36 mmol/L, with a range from 115.6 to 160.9 mmol/L. During a median follow‐up of 1101 days, mortality occurred in 1413 (38.7%) hospital survivors. After adjustment for age, sex, and other potential confounders, patients with sodium levels <135 mmol/L (hazard ratio [HR]: 1.67; 95% confidence interval [CI]: 1.29–2.16) and 135–137 mmol/L (HR: 1.34; 95% CI: 1.01–1.78) had an increased risk of all‐cause mortality compared to those with sodium levels of 139–141 mmol/L.
Keywords: heart failure, risk factor for mortality, serum sodium
1. INTRODUCTION
Heart failure (HF) is a clinical syndrome in which the structure and/or function of the heart is abnormal due to multiple causes. Data from the China Hypertension Survey (CHS) show that 1.3% of the Chinese adult population aged ≥35 years had HF. It has been emphasized that nurses play an important role in assessing, monitoring, and providing patient care as well as improving outcomes in HF (Bjornsdottir et al., 2021; Ordóñez‐Piedra et al., 2021; Sezgin et al., 2017; Yang et al., 2020; Zhang et al., 2020). However, more advanced and intensive care means greater burdens on nurses and other care‐givers. In consequence, it is important to screen for the HF patients who need more advanced care exactly for improving both the efficiency of nursing care and the prognosis of HF. Abnormal admission sodium levels are the most common electrolyte disturbances in HF patients (Hao et al., 2019). Hyponatremia, defined as serum sodium lower than 135 mmol/L, can be caused by an increase in arginine vasopressin (AVP), the use of loop diuretics, the control of sodium salt intake, and other factors that can induce overactivation of the neurohormonal system (Adrogué, 2017; Farmakis et al., 2009). The results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIMIZE‐HF) registry showed that 19.7% of HF patients had serum sodium values below 135 mmol/L (Gheorghiade et al., 2007). The China Heart Failure Registry Study (China‐HF) showed that the incidence of hyponatremia in hospitalized HF patients in China is 14.8% (Zhang et al., 2017). Research has shown that hyponatremia at admission is an independent predictor of increased mortality in patients with HF (Breen et al., 2020; Gheorghiade et al., 2007; Hiki et al., 2018; Klein et al., 2005). Hypernatremia refers to a serum sodium level higher than 145 mmol/L and is often accompanied by an increase in blood osmotic pressure. The main reasons include impaired fluid intake, excessive fluid loss, and neurological deficits (Adrogué & Madias, 2000). Hypernatremia is less common in HF patients, but previous research has shown that it is associated with poor prognosis (Shorr et al., 2011; Vicent et al., 2020). Recent research in America showed that hypernatremia on admission to the cardiac intensive care unit is associated with increased short‐term and long‐term mortality (Breen et al., 2020). Research on predictors of adverse events such as death and readmission in HF patients will help in making corresponding clinical decisions for people with HF, reduce patient mortality, and improve prognosis. Whether a narrower range of sodium values impacts outcomes in HF warrants further investigation. As a result, this study was designed to assess the effect of admission serum sodium levels on all‐cause post‐discharge mortality in hospitalized patients with HF to further provide a reliable method for accessing patients in nursing practice.
2. METHODS
2.1. Study population
The study population consisted of patients admitted to the HF care unit (HFCU) of Fuwai Hospital from November 2008 to November 2018 who were diagnosed with heart failure during hospital admission.The diagnostic criteria for HF refer to the “Chinese HF Diagnosis and Treatment Guideline” (Chinese Society of Cardiology & The Editorial Board of Chinese Journal of Cardiology, 2014). The diagnosis of each patient was confirmed by two cardiologists. For patients who had been hospitalized several times, the first in‐hospital data were used. Patients who died during hospitalization were excluded from the study.
Abnormal serum sodium levels were defined as serum sodium levels lower than 135 mmol/L (hyponatremia) or higher than 145 mmol/L (hypernatremia). The study population was divided into seven groups based on the serum sodium level at admission: Na < 135 mmol/L, 135 ≤ Na < 137 mmol/L, 137 ≤ Na < 139 mmol/L, 139 ≤ Na < 141 mmol/L (reference interval), 141 ≤ Na < 143 mmol/L, 143 ≤ Na ≤ 145 mmol/L and Na > 145 mmol/L (Table 1).
TABLE 1.
Predefined serum sodium interval.
| Sodium | Serum sodium concentration |
|---|---|
| Interval 1 (hyponatremia) | <135 mmol/L |
| Interval 2 (lower level of normal serum sodium levels) | 135–137 mmol/L |
| Interval 3 | 137–139 mmol/L |
| Interval 4 (reference interval) | 139–141 mmol/L |
| Interval 5 | 141–143 mmol/L |
| Interval 6 | 143–145 mmol/L |
| Interval 7 (hypernatremia) | >145 mmol/L |
2.2. Baseline study variable measurements
Comorbidities (hypertension and diabetes mellitus) were diagnosed according to the World Health Organization (WHO) International Classification of Disease (ICD). Blood samples for laboratory tests were collected via the cubital vein at 6 a.m. on the morning after patients were admitted to the HFCU to achieve an overnight fasting state. Serum sodium levels were measured using the indirect ion‐selective electrode method in the clinical laboratory of Fuwai Hospital. Left ventricular ejection fraction (LVEF) was measured by transthoracic echocardiogram according to the modified Simpson method. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation from baseline serum creatinine levels (Ma et al., 2006).
2.3. Outcomes and follow‐up
The outcome for the analysis was all‐cause mortality. Outcome data were collected through routine follow‐up, either by outpatient visits or phone calls. After discharge, patients were routinely followed up by outpatient visits or phone calls at 1, 6, and 12 months and then annually if they survived more than 1 year.
2.4. Statistical analysis
Measurement variables are expressed as the mean values ± standard deviation (SD) or median (first quartile [Q1], third quartile [Q3]) according to distribution. Categorical variables are presented as numbers (percentages). Measurement variables were compared using one‐way ANOVA or the Kruskal–Wallis H (K) test. Categorical variables were compared using the chi‐square test. The Kaplan–Meier method was used to estimate all‐cause mortality in the hospital survivors stratified by baseline serum sodium levels. Cox proportional hazards regression models were used to examine the association between all‐cause death and the seven categorical baseline serum sodium levels. Multivariable linear regression analyses were applied after adjusting covariates as follows: model 1 was adjusted for age and sex; model 2 was adjusted for age, sex, body mass index (BMI), hypertension, diabetes mellitus, smoking history, drinking history, alanine aminotransferase (ALT), aspartate transaminase (AST), eGFR, abnormal serum potassium level (serum potassium <3.5 or >5.5 mmol/L), N‐terminal pro‐brain natriuretic peptide (NT‐proBNP), New York Heart Association (NYHA) functional class IV, LVEF, and pharmacotherapy. Restricted cubic spline (RCS) multivariable Cox regression analysis was used to show the relationship between serum sodium as continuous variables and mortality. All data management and analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.0.2 (The R Foundation, Vienna, Austria).
3. RESULTS
3.1. Baseline characteristics
A total of 3649 HF patients were ultimately included in the analysis of this study (Figure S1) and the baseline characteristics of individuals included and individuals lost to follow‐up are shown in Table S1. The mean age of the patients was 56.9 ± 15.83 years, and 71.2% were male (2599/3649). HF patients with NYHA functional class IV symptoms accounted for 24.1% (878/3649) of the study population. The LVEF of the patients was 41.04 ± 14.67%. At admission, the mean serum sodium level was 137.19 ± 4.36 mmol/L, and the range was 115.6 to 160.9 mmol/L. The distribution of baseline serum sodium values of the study population is shown in Figure 1. Patients with normal serum sodium (135 mmol/L ≤ serum sodium ≤ 145 mmol/L) accounted for 74.2% (2709/3649) of the study population, while patients with abnormal serum sodium levels accounted for 25.8% (940/3649). Regarding abnormal serum sodium levels, patients with hyponatremia (serum sodium <135 mmol/L) accounted for 24.3% (887/3649) of the study population, and patients with hypernatremia (serum sodium >145 mmol/L) accounted for 1.5% (53/3649). Of the patients included in this analysis, 20.3% (741/3649) had sodium levels within the reference range of 139–141 mmol/L. The baseline characteristics of the included HF patients grouped by admission serum sodium level are listed in Table 2.
FIGURE 1.
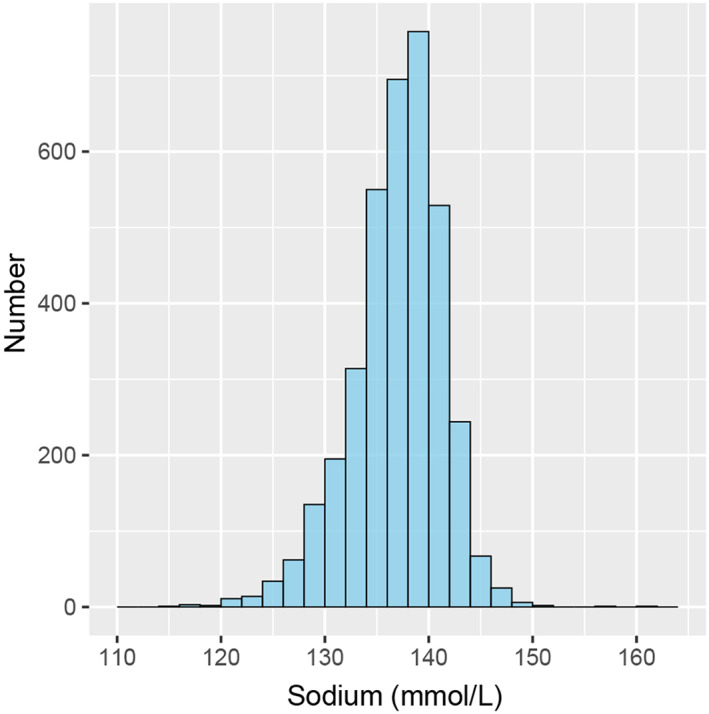
Distribution of baseline serum sodium values of the study population.
TABLE 2.
Baseline characteristics.
| Total, n = 3649 | Na < 135 mmol/L, n = 887 | 135 ≤ Na < 137 mmol/L, n = 607 | 137 ≤ Na < 139 mmol/L, n = 730 | 139 ≤ Na < 141 mmol/L, n = 741 | 141 ≤ Na < 143 mmol/L, n = 453 | 143 ≤ Na≤145 mmol/L, n = 178 | Na > 145 mmol/L, n = 53 | p‐Value | |
|---|---|---|---|---|---|---|---|---|---|
| Male sex, n (%) | 2599 (71.2%) | 610 (68.8%) | 422 (69.5%) | 520 (71.2%) | 543 (73.3%) | 339 (74.8%) | 124 (69.7%) | 41 (77.4%) | 0.171 |
| Age (years) | 56.9 ± 15.8 | 57.5 ± 16.1 | 57.2 ± 16.2 | 56.6 ± 15.9 | 55.9 ± 15.4 | 56.4 ± 15.7 | 58.6 ± 15.2 | 60.5 ± 14.9 | 0.136 |
| BMI (kg/m2) | 24.62 ± 4.30 | 23.40 ± 4.12 | 24.03 ± 4.31 | 24.74 ± 3.86 | 25.27 ± 4.40 | 25.54 ± 4.32 | 25.97 ± 4.67 | 26.72 ± 4.25 | <0.001 |
| Hypertension, n (%) | 1858 (50.9%) | 529 (59.6%) | 340 (56.0%) | 346 (47.4%) | 355 (47.9%) | 193 (42.6%) | 70 (39.3%) | 25 (47.2%) | <0.001 |
| Diabetes mellitus, n (%) | 2587 (70.9%) | 625 (70.5%) | 416 (68.5%) | 512 (70.1%) | 536 (72.3%) | 329 (72.6%) | 136 (76.4%) | 33 (62.3%) | 0.249 |
| Smoking, n (%) | 811 (47.2%) | 207 (48.7%) | 146 (49.0%) | 158 (44.9%) | 143 (43.1%) | 97 (46.9%) | 42 (55.3%) | 18 (62.1%) | 0.213 |
| Drinking, n (%) | 1050 (61.0%) | 275 (64.4%) | 178 (59.7%) | 220 (62.3%) | 194 (58.4%) | 119 (57.5%) | 45 (59.2%) | 19 (65.5%) | 0.559 |
| NYHA IV, n (%) | 2771 (75.9%) | 555 (62.6%) | 466 (76.8%) | 580 (79.5%) | 605 (81.6%) | 384 (84.8%) | 144 (80.9%) | 37 (69.8%) | <0.001 |
| ALT (IU/L) | 23.0 [14.0, 38.0] | 22.5 [15.0, 41.0] | 22.0 [14.0, 36.0] | 22.0 [14.0, 37.0] | 22.0 [15.0, 37.0] | 22.5 [14.0, 35.3] | 23.5 [14.0, 36.8] | 27.0 [17.0, 45.0] | 0.164 |
| AST (IU/L) | 24.0 [18.0, 33.0] | 26.0 [20.0, 38.0] | 24.0 [18.5, 32.0] | 23.0 [18.0, 33.0] | 23.0 [18.0, 32.0] | 22.0 [17.0, 28.0] | 23.0 [18.0, 29.0] | 29.0 [21.0, 37.0] | <0.001 |
| Serum Na (mmol/L) | 137.19 ± 4.36 | 131.29 ± 3.11 | 135.80 ± 0.58 | 137.78 ± 0.59 | 139.78 ± 0.57 | 141.70 ± 0.58 | 143.72 ± 0.65 | 147.20 ± 2.81 | <0.001 |
| Serum K (mmol/L) | 4.01 ± 0.50 | 4.05 ± 0.55 | 4.01 ± 0.49 | 3.98 ± 0.49 | 3.99 ± 0.44 | 3.99 ± 0.45 | 4.01 ± 0.57 | 4.08 ± 0.44 | 0.044 |
| Abnormal serum K, n (%) | 3176 (87.0%) | 755 (85.1%) | 530 (87.3%) | 628 (86.0%) | 662 (89.3%) | 407 (89.8%) | 146 (82.0%) | 48 (90.6%) | 0.022 |
| NT‐proBNP (pg/mL) | 1899.0 [802.0, 4396.0] | 2637.5 [1148.5, 5843.0] | 1935.0 [909.0, 4289.0] | 1872.5 [756.5, 4083.8] | 1614.0 [670.0, 3504.0] | 1539.0 [728.0, 3991.0] | 1606.0 [600.0, 5508.0] | 2260.5 [861.3, 4449.0] | <0.001 |
| eGFR (mL/min/1.73m2) | 75.28 ± 28.84 | 72.47 ± 31.01 | 74.94 ± 29.14 | 76.17 ± 29.08 | 78.08 ± 28.29 | 77.12 ± 25.79 | 73.91 ± 23.61 | 63.66 ± 26.43 | <0.001 |
| LVEF % | 41.04 ± 14.67 | 39.34 ± 15.15 | 40.59 ± 14.49 | 41.81 ± 14.46 | 41.24 ± 14.31 | 43.05 ± 14.73 | 41.54 ± 14.19 | 41.83 ± 14.92 | 0.001 |
| Pharmacotherapy | |||||||||
| Digoxin, n (%) | 1755 (49.5%) | 447 (53.3%) | 299 (50.9%) | 344 (47.7%) | 355 (48.7%) | 205 (45.8%) | 78 (44.8%) | 27 (52.9%) | 0.1 |
| ACEI/ARB, n (%) | 2096 (59.1%) | 408 (48.6%) | 324 (55.2%) | 454 (63.0%) | 484 (66.4%) | 285 (63.6%) | 116 (66.7%) | 25 (49.0%) | <0.001 |
| Beta‐blocker, n (%) | 3086 (87.0%) | 698 (83.2%) | 488 (83.1%) | 633 (87.8%) | 670 (91.9%) | 395 (88.2%) | 157 (90.2%) | 45 (88.2%) | <0.001 |
| MRA, n (%) | 2441 (68.8%) | 573 (68.3%) | 425 (72.4%) | 498 (69.1%) | 505 (69.3%) | 290 (64.7%) | 118 (67.8%) | 32 (62.7%) | 0.227 |
| Loop diuretic, n (%) | 2795 (76.6%) | 691 (77.9%) | 467 (76.9%) | 560 (76.7%) | 572 (77.2%) | 338 (74.6%) | 134 (75.3%) | 33 (623%) | 0.219 |
| Thiazide, n (%) | 113 (3.2%) | 21 (25%) | 22 (3.8%) | 22 (3.1%) | 22 (3.1%) | 17 (3.9%) | 7 (40%) | 2 (3.9%) | 0.771 |
| VRAs, n (%) | 70 (2.0%) | 18 (2.1%) | 9 (1.6%) | 17 (24%) | 12 (1.7%) | 11 (2.6%) | 3 (1.7%) | 0 (0.0%) | 0.774 |
Note: Data presented are mean ± SD, median [Q1, Q3], or N (%).
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate transaminase; AVP, arginine vasopressin; BMI, body mass index; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
3.2. Abnormal serum sodium levels and all‐cause mortality
During a median follow‐up of 1101 days, all‐cause mortality occurred in 1413 (38.7%) hospital survivors. In Kaplan–Meier analysis, hospital survivors with abnormal admission serum sodium levels had lower survival than those with normal admission serum sodium levels (p < 0.01 by log‐rank) (Figure S2). In unadjusted Cox analysis, abnormal serum sodium levels were associated with an increased risk of all‐cause mortality (hazard ratio [HR]: 1.82, 95% confidence interval [CI]: 1.64–2.03; p < 0.01) compared with normal serum sodium levels. After adjusting for age and sex, the hazard ratio was 1.78 (CI: 1.60–1.99; p < 0.01), and after adjusting for age, sex, and other confounders, the hazard ratio of abnormal serum sodium levels was 1.58 (CI: 1.32–1.89; p < 0.01) (Table 3).
TABLE 3.
Hazard ratios for mortality associated with abnormal serum sodium level in patients with heart failure.
| Crude | Model‐1 | Model‐2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| Abnormal serum sodium level | 1.82 (1.64, 2.03) | <0.001 | 1.78 (1.6, 1.99) | <0.001 | 1.58 (1.32, 1.89) | <0.001 |
Note: The risk of all‐cause mortality in patients with abnormal sodium level (<135 mmol/L or > 145 mmol/L) was examined with normal sodium level (135–145 mmol/L) as reference. The model 1 is adjusted for age and sex. The model 2 is adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, eGFR, abnormal serum potassium level, NT‐proBNP, NYHA Functional Class IV, LVEF, and pharmacotherapy.
To confirm that the findings were robust to potential confounders, stratified analyses were conducted by subgroups defined by covariates that had been demonstrated to have a major role in affecting the outcome of heart failure, including sex, age, BMI, hypertension, diabetes mellitus, eGFR, NYHA functional class, and LVEF. All of these analyses were adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, eGFR, abnormal serum potassium level, NT‐proBNP, NYHA functional class IV, LVEF, and pharmacotherapy except for the covariate, which was stratified. The associations of normal and abnormal serum sodium with all‐cause mortality were present in all examined subgroups and revealed a highly consistent pattern: the risk for all‐cause mortality increased with abnormal serum sodium regardless of the subgroup (all p for interaction >0.05) (Figure 2). The Kaplan–Meier survival curves of the subgroups are shown in Figure S3.
FIGURE 2.
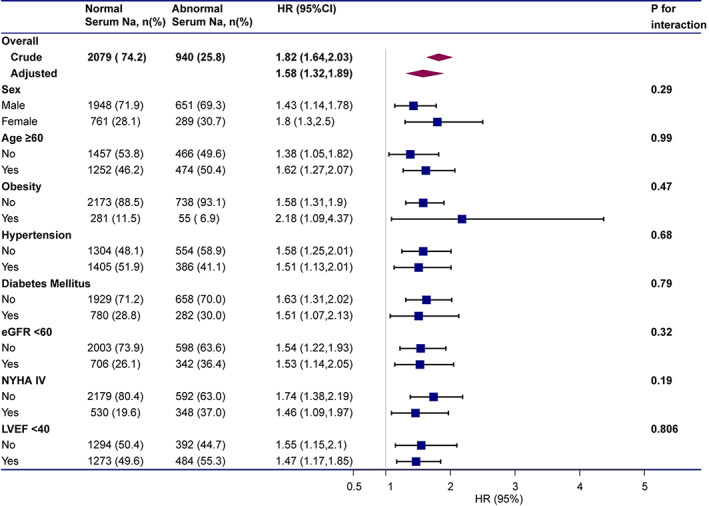
Subgroup analyses of hazard ratios (95% confidence intervals) associated with mortality associated with abnormal serum sodium levels. The model adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, eGFR, abnormal serum potassium level, NT‐proBNP, NYHA functional class IV, LVEF, and pharmacotherapy except for the covariate, which was stratified.
3.3. Association between baseline sodium level and all‐cause mortality—RCS
In unadjusted RCS analyses, there was a U‐shaped relationship between serum sodium level and mortality risk. The lowest hazard ratio for mortality was 140.5 mmol/L sodium, with an increase in risk with both higher and lower sodium levels (Figure 3a). In the spline analyses adjusted for age and sex, the lowest hazard ratio for mortality was 140.5 mmol/L sodium (Figure 3b), while when adjusted for age, sex, and other potential confounders, the lowest hazard ratio was 140.2 mmol/L sodium (Figure 3c).
FIGURE 3.
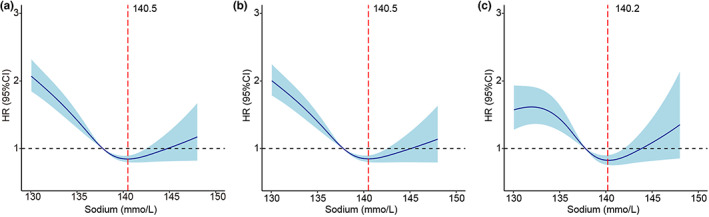
Restricted cubic spline curve showing the adjusted hazard ratios for all‐cause mortality as a function of baseline serum sodium level. (a) Unadjusted: 140.5 mmol/L was associated with the lowest risk of mortality. (b) Adjusted for age and sex: 140.5 mmol/L was associated with the lowest risk of mortality. (c) Adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, eGFR, abnormal serum potassium level, NT‐proBNP, NYHA functional class IV, LVEF and pharmacotherapy: 140.2 mmol/L was associated with the lowest risk of mortality.
3.4. Association between baseline sodium level and all‐cause mortality—Survival rates
Based on the results of RCS analyses, patients were further stratified into seven groups according to serum sodium levels, and patients with serum sodium within the 139–141 mmol/L range were taken as the reference. Kaplan–Meier analysis demonstrated that patients with serum sodium <135 mmol/L had the lowest survival rate. Compared with patients with serum sodium within the 139–141 mmol/L range, both patients with serum sodium <135 mmol/L and those with serum sodium between 135 and 147 mmol/L had a lower survival rate (Figure 4). However, no significant difference in the survival rate was noted between patients with serum sodium >145 mmol/L and patients with serum sodium between 139 and 141 mmol/L (Figure 4).
FIGURE 4.
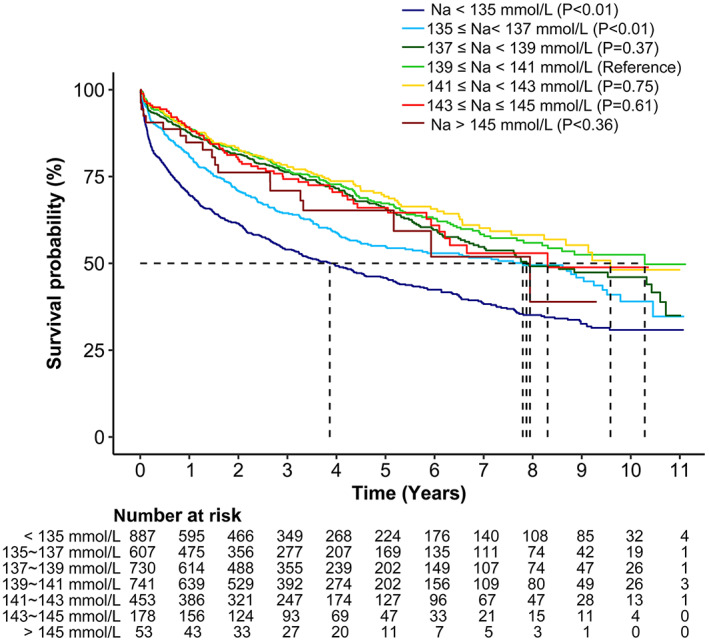
Kaplan–Meier chart of probability of survival among the different serum sodium levels. Maximal follow‐up survival of seven groups: Na < 135 mmol/L (hyponatremia), 135 ≤ Na < 137 mmol/L, 137 ≤ Na < 139 mmol/L, 139 ≤ Na < 141 mmol/L (reference interval), 141 ≤ Na < 143 mmol/L, 143 ≤ Na ≤145 mmol/L, and Na > 145 mmol/L (hypernatremia).
3.5. Association between baseline sodium level and all‐cause mortality—Cox regression
The results of the Cox proportional hazards regression analysis of all‐cause death and serum sodium level with 139–141 mmol/L as the reference are shown in Table 4. Unadjusted Cox analysis showed that patients with serum sodium <135 mmol/L (HR: 2.12, 95% CI: 1.81–2.48; p < 0.01) had a significantly higher risk of all‐cause mortality than those with a serum sodium level of 139–141 mmol/L. In the multivariable analysis adjusted for age, sex, and other potential confounders, the risk of mortality remained increased in patients with serum sodium <135 mmol/L (HR: 1.67, 95% CI: 1.29–1.16; p < 0.01). Moreover, patients with levels at the lower end of the normal serum sodium range (Na 135–137 mmol/L) were also at higher risk of all‐cause mortality than those with values between 139 and 141 mmol/L after adjusting for age, sex, and other potential confounders (HR:1.46, 95% CI: 1.22–1.75; p < 0.01). However, the differences in the risk of all‐cause mortality between patients with hypernatremia and with serum sodium between 139 and 141 mmol/L were not found to be statistically significant.
TABLE 4.
Hazard ratios for mortality associated with serum sodium levels in patients with heart failure.
| Crude | Model‐1 | Model‐2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| Na < 135 mmol/L | 2.12 (1.81, 2.48) | <0.001 | 2.05 (1.75, 2.41) | <0.001 | 1.67 (1.29, 2.16) | <0.001 |
| 135 ≤ Na < 137 mmol/L | 1.49 (1.24, 1.78) | <0.001 | 1.46 (1.22, 1.75) | <0.001 | 1.34 (1.01, 1.78) | 0.042 |
| 137 ≤ Na < 139 mmol/L | 1.11 (0.93, 1.34) | 0.243 | 1.11 (0.92, 1.33) | 0.266 | 0.94 (0.71, 1.25) | 0.676 |
| 139 ≤ Na < 141 mmol/L | 1 (Reference) | – | 1 (Reference) | – | 1 (Reference) | – |
| 141 ≤ Na < 143 mmol/L | 0.96 (0.78, 1.19) | 0.725 | 0.95 (0.77, 1.18) | 0.666 | 0.96 (0.68, 1.35) | 0.827 |
| 143 ≤ Na≤145 mmol/L | 1.09 (0.82, 1.46) | 0.543 | 1.06 (0.8, 1.42) | 0.682 | 0.93 (0.58, 1.47) | 0.745 |
| Na > 145 mmol/L | 1.32 (0.83, 2.11) | 0.245 | 1.28 (0.8, 2.05) | 0.298 | 1.6 (0.85, 3.03) | 0.146 |
Note: The reference interval is represented by the sodium interval 139–141 mmol/L. The model 1 is adjusted for age and sex. The model 2 is adjusted for age, sex, BMI, hypertension, diabetes mellitus, smoking history, drinking history, ALT, AST, eGFR, abnormal serum potassium level, NT‐proBNP, NYHA Functional Class IV, LVEF, and pharmacotherapy.
4. DISCUSSION
4.1. HF in China
The China Hypertension Survey (2012–2015) shows that there was an increase in the prevalence of HF in China, and among Chinese adults aged ≥35 years, the weighted prevalence of HF was 1.3% (Hao et al., 2019). With the increasing longevity of the Chinese population, HF has become one of the most prominent public health problems in China due to its high mortality, readmission rate, and medical expenses. In this study, the mean age of the patients was 56.9 ± 15.83 years, 71.2% were male (2599/3649), and 24.1% were in NYHA functional class IV (878/3649). The China Patient‐centered Evaluative Assessment of Cardiac Events Retrospective Study of Heart Failure (China PEACE 5r‐HF study) shows that the average age of Chinese patients hospitalized with HF was 73 [65, 80] years, 48.9% were women, and 30.9% were in NYHA functional class IV (Yu et al., 2019). China‐HF research shows that compared with HF patients in developed countries such as Europe and the United States, patients with HF in China are characterized by a low age of onset, poor risk factor control, and poor compliance (Zhang et al., 2017). Research on predictors of adverse events such as death and readmission in HF patients will help in making corresponding clinical decisions for people with HF, reducing patient mortality, and improving prognosis.
4.2. Mechanism of abnormal serum sodium levels in HF patients
Abnormal serum sodium is one of the most common types of electrolyte disorders in patients with HF. Furthermore, abnormal AVP regulation is one of the most common causes of hyponatremia in HF patients (Anderson et al., 1985). In the early stages of HF, affected by factors such as reduced blood pressure and blood volume, the baroreceptors located at the left ventricle, carotid sinus, aortic arch, and renal arteriole received less stimulation, leading to sympathetic nerve excitement, renin‐angiotensin‐aldosterone system (RAAS) activation, and the release of AVP from the posterior pituitary. As a result, an excess of aldosterone leads to sodium retention and extracellular fluid increase, causing peripheral edema but not hyponatremia. However, in advanced stages of HF, insufficient perfusion causes renal insufficiency, and the increase in AVP levels in the body leads to increased renal reabsorption of water and levels of angiotensin and aldosterone. This results in reduced sodium and water delivery to the renal collecting tubule. Coupled with resistance to the effects of natriuretic peptides, the use of potent diuretics, and strict salt limitation, hyponatremia is induced or exacerbated in patients (Fonarow et al., 2007; Mullens et al., 2017; Schrier & Abraham, 1999; Urso et al., 2015). Hypernatremia in patients with HF is related to the use of diuretics. Sufficient volume balance cannot be maintained in the patients because free water is removed by diuretics. At the same time, multiple organ dysfunction in patients with advanced HF, damage to the thirst center caused by age‐dependent degradation of the brainstem osmotic receptors and improper volume management during hospitalization can also cause hypernatremia (Adrogué & Madias, 2000; Sterns, 2015).
4.3. Prevalence of abnormal serum sodium levels in HF patients
Epidemiological studies report that the prevalence of hyponatremia is between 11% and 27% in heart failure patients (Bavishi et al., 2014; Cuffe et al., 2002; Gheorghiade et al., 2007; Hiki et al., 2018; Rusinaru et al., 2012; Zhang et al., 2017). In this study, the prevalence of hyponatremia was 24.3%. Although both studies focused on Chinese heart failure patients, the prevalence in the current study was higher than that in China‐HF research (13.1%). The reason may be that the patients in our HF care unit tend to have more complex conditions. Hypernatremia is much less common than hyponatremia in HF patients. Research has shown that the prevalence of hypernatremia ranges from 3.3% to 10.2% in HF patients (Breen et al., 2020; Polcwiartek et al., 2018). The prevalence of hypernatremia in this research was 1.5%, which is lower than that in other research. This may be caused by the exclusion of patients who died during hospitalization, as hypernatremia has been reported to contribute to a higher risk of in‐hospital mortality (Breen et al., 2020).
4.4. Abnormal serum sodium levels and risk of morality
The results of previous research have shown that hyponatremia is an independent predictor of poor prognosis in hospitalized HF patients and is related to a prolonged hospital stay, increased long‐term mortality after discharge, and high readmission rates (Bavishi et al., 2014; Gheorghiade et al., 2007; Klein et al., 2005). There are relatively few reports about the prognosis of HF patients with hypernatremia. However, a few studies have shown that hypernatremia can also lead to increased mortality in hospitalized patients with HF (Breen et al., 2020; Deubner et al., 2012; Gheorghiade et al., 2007; Kovesdy et al., 2012).
In this study, abnormal serum sodium levels were associated with a significantly greater risk of all‐cause mortality than normal serum sodium levels in multivariable adjusted models. The associations remained consistent in subgroups of patients stratified by sex, age, BMI, hypertension, diabetes mellitus, eGFR, NYHA functional class, and LVEF. Furthermore, RCS analyses showed that there was a U‐shaped relationship between serum sodium levels and mortality risk. The lowest hazard ratio for mortality was 140.5 mmol/L sodium in unadjusted analysis, 140.5 mmol/L sodium after adjusting for age and sex, and 140.2 mmol/L sodium after adjusting for age, sex, and other potential confounders. Our data were in line with other publications focusing on serum sodium levels in heart failure patients (Deubner et al., 2012; Kovesdy et al., 2012). In addition, patients with serum sodium <135 mmol/L and with serum sodium between 135 and 137 mmol/L had a significantly higher risk of all‐cause mortality than those with Na between 139 and 141 mmol/L. This result suggested a narrower optimum serum level for heart failure patients.
However, hypernatremia was not found to be a predictor of mortality in this research, possibly because of the small sample size. Nevertheless, recent research showed that although less common in heart failure patients, hypernatremia was associated with in‐hospital morality (Breen et al., 2020), whereas patients who died during the hospital stay were excluded in this study. Hypernatremia may be a marker of worse renal failure, altered mental status, preexisting neurologic dysfunction or aggravation of other conditions, which increases the risk of in‐hospital death or short‐term death. On the other hand, whether hypernatremia is an independent risk factor for long‐term mortality and whether correction of hypernatremia could reduce the risk of long‐term mortality need to be further verified.
Serum sodium ions are one of the most abundant cations in extracellular fluid. Serum sodium ions are important for maintaining extracellular fluid volume, regulating acid–base balance, maintaining normal osmotic pressure, and regulating maintenance of the cell membrane potential and other physiological functions of cells. When the serum sodium concentration is lower than the intracellular sodium concentration, extracellular water enters the cell by osmotic pressure gradient effects, resulting in cell swelling (Reynolds et al., 2006). In contrast, intracellular water is pushed into the extracellular space during hypernatremia, causing cellular dehydration (Adrogué & Madias, 2000). However, the mechanisms underlying the association between serum sodium concentration and long‐term mortality remain unclear. As serum sodium concentration is related to renal insufficiency, an imbalance in circulating blood volume and circulating AVP levels (Khan et al., 2016; Packer et al., 1987; Palmer, 2015), it is possible that abnormal serum sodium concentration may be a marker of underlying disease severity. At the same time, whether treatment focusing on correcting the sodium level would be beneficial remains to be further investigated.
4.5. Nursing care and HF
Previous research show that nursing care plays an important role in the management of HF. A systematic review focusing on effectiveness of nursing interventions showed that advanced practice nurse interventions imply a reduction in the number of hospital readmissions and an improvement in the quality of life of HF patients (Ordóñez‐Piedra et al., 2021). A randomized controlled study showed that nursing care conducted for the HF patients may improve the life quality (Zhang et al., 2020). More importantly, outpatient nursing support in HF patients has gradually been valued recently (Kitagawa et al., 2022; Østergaard et al., 2021; Taniguchi et al., 2021). Meanwhile, stratifying high‐risk patients is of great importance as caring HF patients has been proved associated with healthcare burden (Jackson et al., 2018). This study illustrated that both HF patients with hyponatremia and lower end of the normal serum sodium range (135–137 mmol/L) at admission had an increased long‐term risk of all‐cause death. This result indicates that not only patients with hyponatremia but also with lower end of the normal serum sodium range need more intensive and advanced nursing care in hospital and even need strengthened nursing support after discharge.
4.6. Limitations
This study certainly has some limitations. First, the study is a single‐center study, which may affect the extrapolation of the results. Second, this study has some limitations common to all retrospective cohort studies, such as unmeasured residual confounding factors that could partially or completely explain the observed findings. Third, there were 863 individuals lost to follow‐up, which may cause bias. Fourth, data on serum chloride were not always available in this study, so the risk interaction between serum sodium and chloride was not investigated. Fifth, serum sodium fluctuations were not obtained in our patient population, and we were unable to investigate the association between the change in serum sodium concentration and mortality. Sixth, cause‐specific mortality was not available in our study, and therefore, only all‐cause mortality was taken into consideration. Seventh, we set all‐cause mortality as the endpoint. However, the influence of serum sodium on hospitalization time and HF rehospitalization may also be of clinical significance.
In summary, serum sodium abnormalities (including hyponatremia and hypernatremia) are common types of electrolyte abnormalities in hospitalized HF patients. Serum sodium abnormalities at admission independently predict all‐cause mortality in hospitalized HF patients. In addition, levels in the lower end of the normal serum sodium range (135–137 mmol/L) at admission were associated with an increased long‐term risk of all‐cause mortality.
5. CONCLUSION
Serum sodium levels below 135 mmol/L and in the lower end of the normal serum sodium range (135–137 mmol/L) at admission were associated with an increased long‐term risk of all‐cause death in hospitalized HF patients.
5.1. Implications of the findings for nursing research and practice
It is suggested that HF patients with hyponatremia and lower normal serum sodium levels at admission are at higher risk of all‐cause death and need to be closely monitored and receive more intensive levels of care.
AUTHOR CONTRIBUTION
Conception and design of study: Lang Zhao, Yuhui Zhang, and Jian Zhang; acquisition of data: Xiaofeng Zhuang, Mei Zhai, Yunhong Wang, Yan Huang, Qiong Zhou, Pengchao Tian, Lin Liang, Boping Huang, Liyan Huang, and Jiayu Feng; analysis and interpretation of data: Lang Zhao and Xuemei Zhao; drafting the manuscript: Lang Zhao; Revising the manuscript critically for important intellectual content: Lang Zhao, Yuhui Zhang, and Jian Zhang.
FUNDING INFORMATION
This work was supported by the Key Projects in the National Science and Technology Pillar Program of the 13th Five‐Year Plan Period (grant number 2017YFC1308300), Beijing, People's Republic of China; the Key Projects in the National Science and Technology Pillar Program of the 12th Five‐Year Plan Period (grant number 2011BAI11B08), Beijing, People's Republic of China; and CAMS Innovation Fund for Medical Science (grant number 2020‐I2M‐1‐002).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
This study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (REDACTED). All included subjects provided written informed consent during hospitalization for the use of clinical data for the purpose of scientific research.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
We are grateful to the members of the Department of Cardiology, Cardiovascular Institute of Fuwai Hospital, for their help in recruiting patients. We thank all members who contributed to the study.
Zhao, L. , Zhao, X. , Zhuang, X. , Zhai, M. , Wang, Y. , Huang, Y. , Zhou, Q. , Tian, P. , Liang, L. , Huang, B. , Huang, L. , Feng, J. , Zhang, Y. , & Zhang, J. (2023). Hyponatremia and lower normal serum sodium levels are associated with an increased risk of all‐cause death in heart failure patients. Nursing Open, 10, 3799–3809. 10.1002/nop2.1638
Yuhui Zhang and Jian Zhang contributed equally to this work.
Clinical Trial Registration: URL: ClinicalTrials.gov; Unique Identifier: NCT02664818.
Contributor Information
Yuhui Zhang, Email: yuhuizhangjoy@163.com.
Jian Zhang, Email: fwzhangjian62@126.com.
DATA AVAILABILITY STATEMENT
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Adrogué, H. J. (2017). Hyponatremia in heart failure. Methodist DeBakey Cardiovascular Journal, 13(1), 40. 10.14797/mdcj-13-1-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrogué, H. J. , & Madias, N. E. (2000). Hypernatremia. The New England Journal of Medicine, 342(20), 1493–1499. 10.1056/nejm200005183422006 [DOI] [PubMed] [Google Scholar]
- Anderson, R. J. , Chung, H. M. , Kluge, R. , & Schrier, R. W. (1985). Hyponatremia: A prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Annals of Internal Medicine, 102(2), 164–168. 10.7326/0003-4819-102-2-164 [DOI] [PubMed] [Google Scholar]
- Bavishi, C. , Ather, S. , Bambhroliya, A. , Jneid, H. , Virani, S. S. , Bozkurt, B. , & Deswal, A. (2014). Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. The American Journal of Cardiology, 113(11), 1834–1838. 10.1016/j.amjcard.2014.03.017 [DOI] [PubMed] [Google Scholar]
- Bjornsdottir, K. , Ketilsdottir, A. , Gudnadottir, M. , Kristinsdottir, I. V. , & Ingadottir, B. (2021). Integration of nursing services provided to patients with heart failure living at home: A longitudinal ethnographic study. Journal of Clinical Nursing, 30(7–8), 1120–1131. 10.1111/jocn.15658 [DOI] [PubMed] [Google Scholar]
- Breen, T. , Brueske, B. , Sidhu, M. S. , Murphree, D. H. , Kashani, K. B. , Barsness, G. W. , & Jentzer, J. C. (2020). Abnormal serum sodium is associated with increased mortality among unselected cardiac intensive care unit patients. Journal of the American Heart Association, 9(2), e014140. 10.1161/jaha.119.014140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Society of Cardiology, & The Editorial Board of Chinese Journal of Cardiology . (2014). Chinese guidelines for the diagnosis and treatment of heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi, 42(2), 98–122. [PubMed] [Google Scholar]
- Cuffe, M. S. , Califf, R. M. , Adams, K. F., Jr. , Benza, R. , Bourge, R. , Colucci, W. S. , Massie, B. M. , O'Connor, C. M. , Pina, I. , Quigg, R. , Silver, M. A. , Gheorghiade, M. , & Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) Investigators . (2002). Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. Jama, 287(12), 1541–1547. 10.1001/jama.287.12.1541 [DOI] [PubMed] [Google Scholar]
- Deubner, N. , Berliner, D. , Frey, A. , Güder, G. , Brenner, S. , Fenske, W. , Allolio, B. , Ertl, G. , Angermann, C. E. , & Störk, S. (2012). Dysnatraemia in heart failure. European Journal of Heart Failure, 14(10), 1147–1154. 10.1093/eurjhf/hfs115 [DOI] [PubMed] [Google Scholar]
- Farmakis, D. , Filippatos, G. , Parissis, J. , Kremastinos, D. T. , & Gheorghiade, M. (2009). Hyponatremia in heart failure. Heart Failure Reviews, 14(2), 59–63. 10.1007/s10741-008-9109-7 [DOI] [PubMed] [Google Scholar]
- Fonarow, G. C. , Heywood, J. T. , Heidenreich, P. A. , Lopatin, M. , & Yancy, C. W. (2007). Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: Findings from acute decompensated heart failure National Registry (ADHERE). American Heart Journal, 153(6), 1021–1028. 10.1016/j.ahj.2007.03.012 [DOI] [PubMed] [Google Scholar]
- Gheorghiade, M. , Abraham, W. T. , Albert, N. M. , Gattis Stough, W. , Greenberg, B. H. , O'Connor, C. M. , She, L. , Yancy, C. W. , Young, J. , Fonarow, G. C. , & OPTIMIZE‐HF Investigators and Coordinators . (2007). Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE‐HF registry. European Heart Journal, 28(8), 980–988. 10.1093/eurheartj/ehl542 [DOI] [PubMed] [Google Scholar]
- Hao, G. , Wang, X. , Chen, Z. , Zhang, L. , Zhang, Y. , Wei, B. , Zheng, C. , Kang, Y. , Jiang, L. , Zhu, Z. , Zhang, J. , Wang, Z. , Gao, R. , & China Hypertension Survey Investigators . (2019). Prevalence of heart failure and left ventricular dysfunction in China: The Chinahypertension survey, 2012–2015. European Journal of Heart Failure, 21(11), 1329–1337. 10.1002/ejhf.1629 [DOI] [PubMed] [Google Scholar]
- Hiki, M. , Kasai, T. , Yatsu, S. , Murata, A. , Matsumoto, H. , Kato, T. , Suda, S. , Miyazaki, T. , Takagi, A. , & Daida, H. (2018). Relationship between serum sodium level within the low‐Normal range on admission and long‐term clinical outcomes in patients with acute decompensated heart failure. International Heart Journal, 59(5), 1052–1058. 10.1536/ihj.17-524 [DOI] [PubMed] [Google Scholar]
- Jackson, J. D. , Cotton, S. E. , Bruce Wirta, S. , Proenca, C. C. , Zhang, M. , Lahoz, R. , & Calado, F. J. (2018). Burden of heart failure on caregivers in China: Results from a cross‐sectional survey. Drug Design, Development and Therapy, 12, 1669–1678. 10.2147/dddt.S148970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, S. , Floris, M. , Pani, A. , & Rosner, M. H. (2016). Sodium and volume disorders in advanced chronic kidney disease. Advances in Chronic Kidney Disease, 23(4), 240–246. 10.1053/j.ackd.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Kitagawa, T. , Hidaka, T. , Watanabe, N. , Naka, M. , Yamaguchi, M. , Kanai, K. , Isobe, M. , Kihara, Y. , Nakano, Y. , & REAL‐HF Investigators . (2022). Current conditions and significance of outpatient cardiac rehabilitation and home nursing‐care services in heart failure patients with mid‐range or preserved ejection fraction: Post‐hoc analysis of the REAL‐HF registry. Heart and Vessels, 37(5), 745–754. 10.1007/s00380-021-01965-1 [DOI] [PubMed] [Google Scholar]
- Klein, L. , O'Connor, C. M. , Leimberger, J. D. , Gattis‐Stough, W. , Piña, I. L. , Felker, G. M. , Adams, K. F., Jr. , Califf, R. M. , Gheorghiade, M. , & OPTIME‐CHF Investigators . (2005). Lower serum sodium is associated with increased short‐term mortality in hospitalized patients with worsening heart failure: Results from the outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (OPTIME‐CHF) study. Circulation, 111(19), 2454–2460. 10.1161/01.Cir.0000165065.82609.3d [DOI] [PubMed] [Google Scholar]
- Kovesdy, C. P. , Lott, E. H. , Lu, J. L. , Malakauskas, S. M. , Ma, J. Z. , Molnar, M. Z. , & Kalantar‐Zadeh, K. (2012). Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation, 125(5), 677–684. 10.1161/circulationaha.111.065391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. C. , Zuo, L. , Chen, J. H. , Luo, Q. , Yu, X. Q. , Li, Y. , Xu, J. S. , Huang, S. M. , Wang, L. N. , Huang, W. , Wang, M. , Xu, G. B. , & Wang, H. Y. (2006). Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol, 17(10), 2937–2944. 10.1681/asn.2006040368 [DOI] [PubMed] [Google Scholar]
- Mullens, W. , Verbrugge, F. H. , Nijst, P. , & Tang, W. H. W. (2017). Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. European Heart Journal, 38(24), 1872–1882. 10.1093/eurheartj/ehx035 [DOI] [PubMed] [Google Scholar]
- Ordóñez‐Piedra, J. , Ponce‐Blandón, J. A. , Robles‐Romero, J. M. , Gómez‐Salgado, J. , Jiménez‐Picón, N. , & Romero‐Martín, M. (2021). Effectiveness of the advanced practice nursing interventions in the patient with heart failure: A systematic review. Nursing Open, 8(4), 1879–1891. 10.1002/nop2.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard, B. , Mahrer‐Imhof, R. , Shamali, M. , Nørgaard, B. , Jeune, B. , Pedersen, K. S. , & Lauridsen, J. (2021). Effect of family nursing therapeutic conversations on patients with heart failure and their family members: Secondary outcomes of a randomised multicentre trial. Journal of Clinical Nursing, 30(5–6), 742–756. 10.1111/jocn.15603 [DOI] [PubMed] [Google Scholar]
- Packer, M. , Lee, W. H. , Kessler, P. D. , Medina, N. , Yushak, M. , & Gottlieb, S. S. (1987). Identification of hyponatremia as a risk factor for the development of functional renal insufficiency during converting enzyme inhibition in severe chronic heart failure. Journal of the American College of Cardiology, 10(4), 837–844. 10.1016/s0735-1097(87)80278-4 [DOI] [PubMed] [Google Scholar]
- Palmer, B. F. (2015). Vasopressin receptor antagonists. Current Hypertension Reports, 17(1), 510–519. 10.1007/s11906-014-0510-4 [DOI] [PubMed] [Google Scholar]
- Polcwiartek, C. , Hansen, S. M. , Kragholm, K. , Krogager, M. L. , Aldahl, M. , Køber, L. , Torp‐Pedersen, C. , Jensen, S. E. , & Søgaard, P. (2018). Prognostic role of serum sodium levels across different serum potassium levels in heart failure patients: A Danish register‐based cohort study. International Journal of Cardiology, 272, 244–249. 10.1016/j.ijcard.2018.08.045 [DOI] [PubMed] [Google Scholar]
- Reynolds, R. M. , Padfield, P. L. , & Seckl, J. R. (2006). Disorders of sodium balance. BMJ, 332(7543), 702–705. 10.1136/bmj.332.7543.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinaru, D. , Tribouilloy, C. , Berry, C. , Richards, A. M. , Whalley, G. A. , Earle, N. , Poppe, K. K. , Guazzi, M. , Macin, S. M. , Komajda, M. , Doughty, R. N. , & MAGGIC Investigators . (2012). Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction: An individual patient data meta‐analysis(†): Meta‐analysis global Group in Chronic heart failure (MAGGIC). European Journal of Heart Failure, 14(10), 1139–1146. 10.1093/eurjhf/hfs099 [DOI] [PubMed] [Google Scholar]
- Schrier, R. W. , & Abraham, W. T. (1999). Hormones and hemodynamics in heart failure. The New England Journal of Medicine, 341(8), 577–585. 10.1056/nejm199908193410806 [DOI] [PubMed] [Google Scholar]
- Sezgin, D. , Mert, H. , Özpelit, E. , & Akdeniz, B. (2017). The effect on patient outcomes of a nursing care and follow‐up program for patients with heart failure: A randomized controlled trial. International Journal of Nursing Studies, 70, 17–26. 10.1016/j.ijnurstu.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Shorr, A. F. , Tabak, Y. P. , Johannes, R. S. , Gupta, V. , Saltzberg, M. T. , & Costanzo, M. R. (2011). Burden of sodium abnormalities in patients hospitalized for heart failure. Congestive Heart Failure, 17(1), 1–7. 10.1111/j.1751-7133.2010.00206.x [DOI] [PubMed] [Google Scholar]
- Sterns, R. H. (2015). Disorders of plasma sodium‐‐causes, consequences, and correction. The New England Journal of Medicine, 372(1), 55–65. 10.1056/NEJMra1404489 [DOI] [PubMed] [Google Scholar]
- Taniguchi, C. , Seto, N. , & Shimizu, Y. (2021). Outpatient nursing support for self‐monitoring in patients with chronic heart failure. PLoS One, 16(7), e0254019. 10.1371/journal.pone.0254019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso, C. , Brucculeri, S. , & Caimi, G. (2015). Acid‐base and electrolyte abnormalities in heart failure: Pathophysiology and implications. Heart Failure Reviews, 20(4), 493–503. 10.1007/s10741-015-9482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent, L. , Alvarez‐Garcia, J. , Gonzalez‐Juanatey, J. R. , Rivera, M. , Segovia, J. , Worner, F. , Bover, R. , Pascual‐Figal, D. , Vázquez, R. , Cinca, J. , Fernandez‐Aviles, F. , & Martinez‐Sellés, M. (2020). Prognostic impact of hyponatremia and hypernatremia at admission and discharge in heart failure patients with preserved, mid‐range, and reduced ejection fraction. Internal Medicine Journal, 51, 930–938. 10.1111/imj.14836 [DOI] [PubMed] [Google Scholar]
- Yang, X. M. , Li, Q. M. , & Gao, Q. N. (2020). Effect of high‐quality nursing intervention on anxiety and depression in patients with chronic heart failure companied malnutrition: A protocol for systematic review and meta‐analysis. Medicine (Baltimore), 99(22), e20261. 10.1097/md.0000000000020261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Gupta, A. , Wu, C. , Masoudi, F. A. , Du, X. , Zhang, J. , Krumholz, H. M. , Li, J. , & China PEACE Collaborative Group . (2019). Characteristics, management, and outcomes of patients hospitalized for heart failure in China: The China PEACE retrospective heart failure study. Journal of the American Heart Association, 8(17), e012884. 10.1161/jaha.119.012884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang, J. , Butler, J. , Yang, X. , Xie, P. , Guo, D. , Wei, T. , Yu, J. , Wu, Z. , Gao, Y. , Han, X. , Zhang, X. , Wen, S. , Anker, S. D. , Filippatos, G. , Fonarow, G. C. , Gan, T. , Zhang, R. , & China‐HF Investigators . (2017). Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: Results from the Chinaheart failure (China‐HF) registry. Journal of Cardiac Failure, 23(12), 868–875. 10.1016/j.cardfail.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Bai, J. , & Huang, Y. (2020). The efficacy of a nursing care and follow‐up program for patients with heart failure: Study protocol for a randomized controlled trial. Medicine (Baltimore), 99(49), e23380. 10.1097/md.0000000000023380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


