Abstract
Background:
The presence of fatigue has been shown to modify running biomechanics. Throughout a run individuals become more fatigued, and the effectiveness of the musculoskeletal protective mechanism can diminish. Older adults are at an elevated risk for sustaining an overuse running related injury. This can be partially explained by changes in the musculoskeletal system and load attenuation.
Research question:
The purpose was to compare post-fatigue running mechanics between older and younger runners.
Methods:
Thirty runners (15 young, 15 older) between the ages of 18–65 participated in this study. All participants ran at least 15 miles/week. Running kinematics were captured using a 10-camera motion capture system while participants ran over a 10-m runway with force platforms collecting kinetic data under two conditions: C1: rested state at a controlled pace of 3.5 m/s ( ± 5%); C2: post-exertional protocol where pace was not controlled, rather it was monitored based on heartrate and RPE representative of somewhat-hard to hard intensity exercise. Prior to C2, participants underwent an exertional protocol that consisted of a maximal exercise test to induce fatigue and a required cool-down. A 2 (state of fatigue) × 2 (age) MANOVA was run to test for the effects of fatigue and age and their interactions.
Results:
No state of fatigue x age interaction was observed. A main effect of age for peak knee extension moment (Y > O; p = 0.01), maximum knee power (Y > O; p = 0.04), maximum hip power (O >Y; p = 0.04), and peak vertical ground reaction force (Y > O; p = 0.007). Regardless of age, participants exhibited decreased knee ROM (p = 0.007) and greater hip extension moment (p < 0.001) in C2 compared to C1.
Conclusion:
While different in knee and hip mechanics overall, the subtle differences observed demonstrate that older runners exhibit comparable gait adaptions post-fatigue to younger volume-matched runners.
Keywords: Gait, Aging, Kinematics, Kinetics, Running
1. Introduction
Running is a popular form of exercise with over 47 million people participating in the activity nationwide [1]. With the American population of people over the age 40 increasing by 42% over the past 20 years, the mean age of the recreational runner has increased as well [1]. Its popularity among aging adults can be attributed to two significant factors: (1) running is easily accessible and, in general, reasonably inexpensive; and (2) running can improve or maintain aspects of physical fitness and overall health that contribute to one’s quality of life [2–5]. While running provides many health benefits, it is also associated with a high rate of overuse injuries [6]. Older runners are at a greater risk of developing a running-related overuse injury [7,8] and exhibit a different injury profile than young runners, having a higher prevalence of lower extremity soft-tissue injuries [7,9]. The increased rate of injury may be explained in part by the changes in musculoskeletal function [10], joint mobility [11], and altered gait biomechanics [12] that occur with aging. The repetitive nature of running increases load impact throughout a run, and the accumulation of these loads can lead to overuse injuries [6]. The musculoskeletal system is partly responsible for attenuating loads; however, individuals become more fatigued throughout a run, and the effectiveness of the protective mechanism can diminish [13]. The presence of fatigue can lead to problems in a runner’s ability to generate muscular contractions, control joint motion, and ultimately one’s ability to maintain desired gait patterns [13–15].
Previous studies investigating the effect of maximum fatigue, induced by a running protocol, have reported modifications to gait mechanics including increases in ankle and knee flexion during the stance phase of gait [16,17] and changes in vertical ground reaction forces [17]. However, runners do not often run to exhaustive fatigue. The limited number of studies investigating mechanics in an exerted state (i.e., not maximal/exhaustive fatigue) report subtle yet significant changes in lower extremity kinematics. These include increases in rearfoot eversion, tibial and knee internal rotation, and knee flexion during the stance phase of gait [16,18]. The observed changes in joint range of motion may alter the shock attenuating capabilities of the lower extremity [19] resulting in an increased risk of overuse injury.
To the authors’ knowledge, no literature exists that has examined the effect of fatigue on running gait mechanics within an older population. Due to the reported biomechanical modifications observed within a younger population, there is a possibility older runners would demonstrate similar if not more pronounced movement alterations, increasing their already high risk of injury [7]. Previous studies have observed changes in lower extremity kinematics and impact forces within a younger population when a level of fatigue is present [16,18]. Additionally, previous literature largely supports age-related changes in gait mechanics [20–26]. Therefore, the purpose of this study was to investigate how running with a level of exertion, following a treadmill-based fatiguing protocol, influences the lower extremity mechanics of older and younger runners. We hypothesized that running in an exerted stated would expose different gait adaptations exhibited by older runners compared to young runners. Specifically, we expected to see increased utilization of more proximal joints (i.e. the hip) during the stance phase of gait by older runners post-fatiguing protocol, compared to young runners.
2. Methods
2.1. Participants
For this study we recruited healthy individuals between the ages of 18–35 and 45–65, who reported regularly running at least 15 miles/week. Data from the literature were used to estimate sample size for a minimum statistical power of 80% with an alpha level of 0.05 (GPower 3.1 Software). Dependent variables utilized in the power analysis included sagittal plane hip, knee, ankle joint kinetics [20,27,28]. The projected sample size to obtain a moderate effect size was approximately 10–14 participants per group for this between group comparisons. Therefore, thirty runners between the ages of 18–65 participated in this study. This allowed us to create two groups for comparison. Younger, consisting of individuals between 18 and 35, and older, consisting of individuals between the ages of 45–65 (Table 1). All participants reported running at least 15 miles/week, reported never having a lower extremity surgery that may affect their gait, and were free of lower extremity injury in the six months preceding and at the time of testing. Additionally, no participants reported wearing any medical device(s) (i.e., orthotics, ankle/knee braces) on the lower extremity that may affect their gait. Based on their completion of physical activity readiness [29] and health history questionnaires, all participants were considered low risk for participating in physical activity and were cleared to participate in a graded exercise test (GXT) according to the American College of Sports Medicine [2]. The University Institutional Review Board approved this study’s protocols, and all participants provided written informed consent before participation.
Table 1.
Participant demographics.
| Young | Older | |
|---|---|---|
|
| ||
| Sex | 10 F, 5 M | 6 F, 9 M |
| Age* | 26.07(7.11) | 51.93 (5.98) |
| Mass (kg)* | 62.18 (9.05) | 74.26 (14.43) |
| Height (m) | 1.71 (0.13) | 1.75 (0.14) |
| BMI (kg/m2) * | 21.17 (2.36) | 23.95 (2.03) |
| PBF (%) | 18.69 (8.71) | 22.90 (5.34) |
| Runs/week | 5.13 (1.3) | 4.60 (1.06) |
| Miles/week | 29.93 (11.77) | 31.67 (11.75) |
| Years running* | 8.47 (5.92) | 17.40 (12.94) |
| Self-reported pace (min-mile−1) * | 7.59 (0.50) | 9.09 (0.50) |
Mean (standard deviation); m: meters, kg: kilogram; BMI: body mass index; PBF: percent body fat; m/s: meters per second.
= significant difference between young and older runners (α = 0.05).
2.2. Experimental setup and protocol
The lab space consists of a 10-m runway with three embedded force platforms (OPT464508, AMTI, Watertown, MA) surrounded by a ten-camera three-dimensional motion capture system (Vantage 5, MX T40-S, Vicon Inc., Oxford, UK). Two photoelectric timing gates placed 4 m apart on either side of the force platform quantified running velocity. Before moving forward with data collection, participants completed questionnaires to provide information on training history (Table 1). Anthropometric data, including height, weight, and body fat percentage (inBody 770, Cerritos, CA) [30] were recorded. Participants were provided with neutral laboratory shoes (Brooks Ghost) and instructed to wear tight fit clothing. Retroreflective markers were placed on the pelvis and bilaterally on the thigh, shank, and foot [31].
A timeline overview of the study protocol can be found in Fig. 1. Participants were asked to warm up by running a short course, set up in our lab prior to data collection, where they gradually worked up to their preferred running velocity. While no kinematic or kinetic data was collected during the warmup, preferred velocity was recorded via timing gates. Following warm-up participants performed the rested state movement assessment (C1). Participants were instructed to run the short course mapped out in our laboratory allowing a dynamic start and avoiding acceleration and deceleration between trials. The course guided them through the capture area where they ran down the 10-m runway at a controlled pace of 3.5 m.s−1 ± 5% while kinematic and kinetic data were recorded at 200hz and 1000hz, respectively. A successful trial was defined as the right foot landing entirely on the force platform with no signs of targeting or alterations in gait. To prevent targeting, participants were not informed of the force platforms’ location and their starting position or short course was adjusted by a research team member to ensure a natural stride.
Fig. 1.
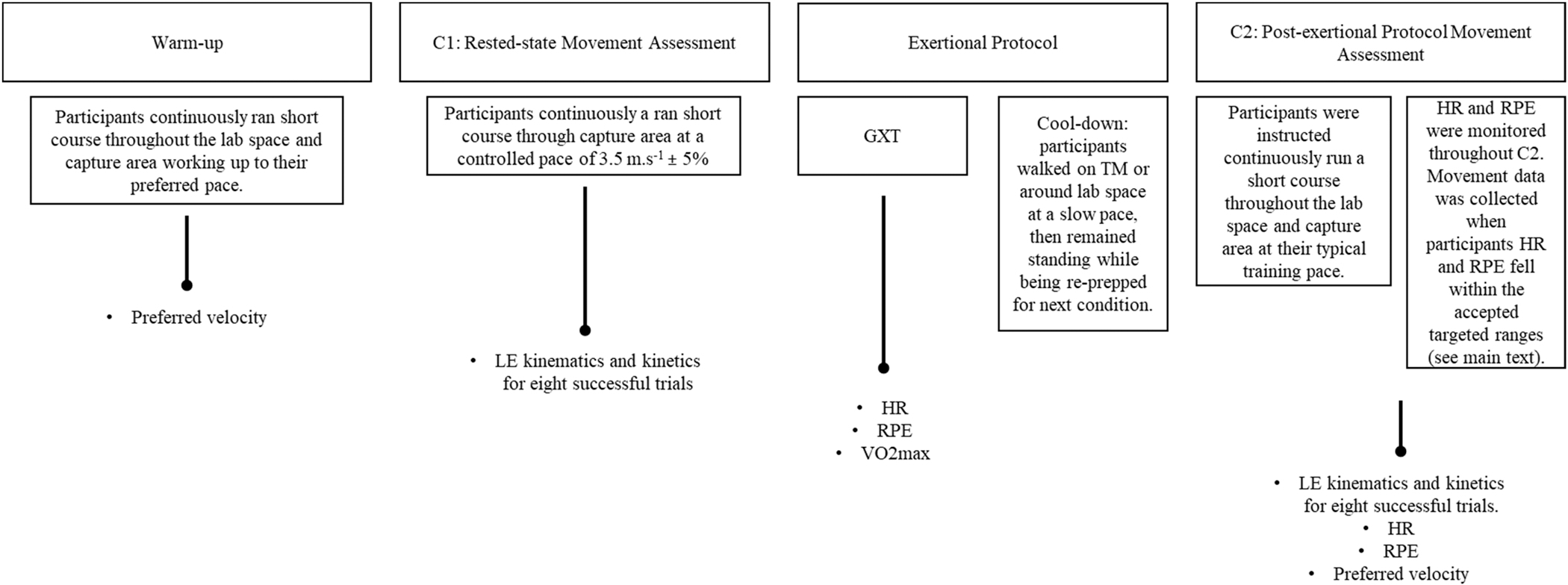
Timeline overview of study protocol and variables collected from each stage. LE: lower extremity; GXT: graded exercise test; HR: heart rate; RPE: rating of perceived exertion.
Following C1 all participants underwent an exertional protocol consisting of a graded exercise test (GXT) and 5-minute cool down. Prior to the GXT, thigh and shank tracking clusters, were removed. The exertional protocol began with a modified Astrand treadmill to elicit a state of maximal fatigue in which the incline of the treadmill was increased 2.5% every 2 min, while the treadmill’s speed remained constant throughout the testing [32]. The speed of the treadmill was blindly self-selected by participants to best represent their typical training pace. Heart rate (HR) (Polar H10 Heartrate Sensor) and rating of perceived exertion (RPE) were collected throughout the test. Participants ran until they reached volitional exhaustion, ending the test by pressing the stop button on the treadmill. Authors chose a maximal fatiguing GXT to determine maximal oxygen consumption (VO2max) (Parvo Medics,TrueOne) and to record subjective measures of fatigue over the course of an exhaustive run which would be later used during the post-exertional protocol movement assessment (C2). No kinematic or kinetic data were collected during the GXT.
Immediately following the GXT, participants were instructed to walk on the treadmill or around the lab at a slow pace to cool-down before moving forward with the next gait assessment. Following the cool-down, reflective markers that were removed prior to or that may have fallen off during the GXT were then replaced. Additionally all markers that remained attached during the GXT were double checked for accuracy in case marker movement occurred. A new static calibration was collected, and participants began C2. The amount of time between the end of the GXT and the beginning of C2 trials was around 10 min. This included the 5-minute active cooldown and an additional 5 min of quiet standing during marker placement and collection of a static trial. Participants were given similar instructions to C1 for C2, once again running through the mapped-out course within the lab space guiding them through the capture area and over the embedded force platforms. Participants ran continuously to avoid any acceleration or deceleration between trials. During C2, however, running velocity was not set to a controlled pace. Instead, participants were instructed to run at a pace that was like their typical training pace while we continued to monitor RPE and HR to ensure all participants were running in an exerted (i.e., not maximally exhausted) state. To do so, data recorded during the GXT was used to determine RPE and HR ranges for each participant that would be targeted during C2. Both RPE and HR are reliable and valid measurements of exercise intensity [33–35]. Previous studies investigating the effect of running in an exerted state on lower extremity mechanics used an RPE of 17 as a determinate of a level of fatigue similar to a prolonged training run [18]. Therefore, targeted RPE and HR for C2 were between 13 and 15 and less than the HR reported at an RPE of 17 during the GXT, respectively, for each individual participant. Movement data was collected when both of these conditions were met.
Participant’s HR was monitored and collected throughout the entire duration of C2 and RPE was collected at the beginning, end, and every 5th lap during C2. Because participants were instructed to run at a typical training pace, they were not aware we were monitoring their velocity based on their RPE or HR. If a participants HR rose above their respective target, and/or their reported RPE was < 13 or > 15, they were given feedback to “slow down” or “speed up”, respectively. Participants continued to run until 8 successful trials, defined similarly to C1 with the addition of RPE and HR targets, were collected.
2.3. Data analysis
Eight successful trials from each participant were used for analysis. Marker trajectories and ground reaction forces were exported to Visual 3D (C-Motion, Inc., Rockville MD) where they were filtered using a 4th order, zero lag, low-pass Butterworth filter with a cut off frequency of 12 Hz [36] and 50 Hz [37], respectively. Stance phase was defined using filtered ground reaction forces based on when forces rose above and fell below a 20 N threshold. Static trials were used to define anatomical coordinate systems for the rearfoot, shank, and thigh with coordinate systems defined based on recommendations of the International Society of Biomechanics [38]. Joint angles were calculated at the knee and ankle as rotations of the distal segment relative to the proximal segment using an XYZ Cardan rotation sequence corresponding to flexion/extension, ab/adduction, and axial rotation. Joint moments were calculated using a Newton-Euler inverse dynamics approach [39], with moments being expressed as internal joint moments.
Sagittal plane joint angles, moments, and powers were calculated at the ankle knee and hip during the stance phase of gait and exported to a custom Matlab (Mathworks, Natick, MA) program where additional variables of interest were calculated and averaged for eight trials for each participant. These included angles at initial contact, peak joint angles, moments, power, and peak ground reaction forces. All variables of interest were calculated for both C1 and C2 conditions. Kinetic variables were normalized to body mass (moments, power) and body weight (ground reaction forces). Additionally, heartrate, RPE, and velocity were averaged across the same 8 successful C2 trials used for kinematic and kinetic analyses.
2.4. Statistical analysis
Gait mechanics were compared pre- and post a fatiguing run with focus on sagittal plane variables, as this is the plane in which literature reports a distal-to-proximal shift in contribution to gait with age. A 2 (pre and post exertional protocol) × 2 (age) MANOVA was run to test for the effects of fatigue and age and their interactions on gait metrics. This included lower extremity kinematics, kinetics, and running velocity. Between group differences for additional GXT and C2 variables (Vo2 max, HR, preferred velocity, RPE) were determined using independent t-tests. An alpha level of p < 0.05 was used to indicate statistical significance. In the event of a significant omnibus F-test, post-hoc pairwise comparisons were conducted using an LSD correction to determine where differences occurred. Cohen’s d effect size calculations were also used to assess group differences in lower extremity mechanics. All statistical tests were performed using Statistical Package for the Social Sciences (SPSS, IBM Corp, Armonk, NY), version 25.
3. Results
Descriptive statistic for groups can be found in Table 1. Groups were statistically similar in weekly mileage as well as number of runs per week, percent body fat, and height. However, older (O) and younger (Y) self-reported pace (min⸳mile−1) was significantly different (O: 9.09 ± 0.5, Y: 7.59 ± 0.5, p = 0.02), as well as their preferred velocity (m/s) (Table 2) recorded during warm up, (O: 2.91 ± 0.3, Y: 3.25 ± 0.41, p = 0.02).
Table 2.
Additional variables.
| Young | Older | |
|---|---|---|
|
| ||
| Preferred warm-up velocity (m/s) * | 3.25 (0.41) | 2.91 (0.3) |
| Average velocity during C1 | 3.45 (0.05) | 3.46 (0.53) |
| During GXT | ||
| HR at 17 RPE | 170 (8) | 169 (9) |
| Max HR* | 182 (9) | 174 (10) |
| VO2 Max (ml/kg/min) * | 55.79 (9.30) | 45.93 (8.07) |
| During C2 trials | ||
| Preferred velocity (m/s) * | 3.66 (0.37) | 3.33(0.39) |
| Average HR* | 169 (7) | 161 (9) |
| %HR Max | 93.09 (14.92) | 93.06 (15.07) |
| Average RPE | 14 (0.76) | 15 (0.51) |
Warm-up velocity, average controlled velocity during C1; GXT results and C2 variables. HR: heart rate; %HR Max: percent of max heartrate; RPE: rate of perceived exertion.
= significant difference between young and older runners (α = 0.05).
Additional data collected during warm up, C1, the GXT and C2 can be found in Table 2. Young runners had higher Vo2 max scores compared to older runners (p = 0.004). No statistically significant differences in velocity between C1 and C2 (p 0.901) or between age group (p = 0.149) were found. However, during C2 specifically, older runners ran at a slower preferred velocity (p = 0.014) and had a lower average HR (p = 0.03) compared to the young runners. Still, older and young runners ran at similar percent of max heartrate (p = 0.93) and reported similar RPE values throughout C2 (p = 0.561).
Mean values for kinematic and kinetic variables during both C1 and C2 are listed in Table 3. No significant fatigue x age interactions were observed; however, moderate ES were calculated for certain kinematic and kinetic variables when comparing older and younger individuals in each condition (Table 3; * denotes moderate ES). Variables where main effects of either age or fatigue were observed are shown in Table 4. There was a main effect of age for peak knee extension moment (Y > O; p = 0.01), maximum knee power (Y > O; p = 0.04), maximum hip power (O >Y; p = 0.04), and peak vertical ground reaction force (Y > O; p = 0.007). Main effect of fatigue was also found for both knee ROM and peak hip extension moment Regardless of age, participants exhibited decreased knee ROM (p = 0.007) and greater hip extension moment (p < 0.001) during C2 compared to C1.
Table 3.
Mean (standard deviation) of sagittal plane lower extremity kinematics and kinetics during C1 and C2
| C1 |
C2 |
|||
|---|---|---|---|---|
| Young | Older | Young | Older | |
|
| ||||
| Kinematics | ||||
| (°) | ||||
| Ankle IC | 2.14 (4.74) | 2.75 (4.57) | 2.19 (4.41) | 2.36 (4.73) |
| Ankle Peak | 21.38 (1.70) | 21.78 (1.92) | 21.58 (1.75) | 21.65 (1.79) |
| Ankle ROM | 19.24 (4.56) | 19.03 (3.81) | 19.40 (4.10) | 19.29 (3.57) |
| Knee IC | −17.70 (2.32) | −18.43 (3.28) | −18.14 (2.31) | −18.73 (2.93) |
| Knee Peak | −41.86 (2.19) | −41.27 (2.97) | −41.59 (2.34) | −41.24 (3.34) |
| Knee ROM | 24.16 (2.78)* | 22.84 (2.62) | 23.45 (2.67) | 22.52 (2.57) |
| Knee IC | 46.16 (4.53) | 44.31 (6.86) | 46.24 (3.46) | 45.17 (6.86) |
| Knee ROM | 44.86 (5.66) | 44.41 (3.96) | 45.77 (5.83) | 44.54 (4.19) |
| Kinetics | ||||
| Peak vGRF (N/BW) | 2.52 (0.16)* | 2.38 (0.21) | 2.52 (0.17)* | 2.37 (0.23) |
| Peak PF moment (Nm/kg) | −2.72 (0.31) | −2.64 (0.35) | −2.73 (0.33) | −2.59 (0.38) |
| Peak KE moment (Nm/kg) | 2.43 (0.34)* | 2.20(0.22) | 2.40 (0.35)* | 2.20 (0.34) |
| Peak HE moment (Nm/kg) | −2.49 (0.44) | −2.54 (0.40) | −2.67 (0.58) | −2.63(0.50) |
| Max ankle power (W/kg) | 14.05 (2.6) | 13.67 (2.14) | 14.10 (3.36) | 13.06 (2.80) |
| Max knee power (W/kg) | 5.35 (1.02)* | 4.93 (0.72) | 5.42 (1.22)* | 4.78 (1.05) |
| Max hip power (W/kg) | 3.84 (1.22)* | 4.98 (2.38) | 4.14 (1.00)* | 5.43 (3.56) |
| Positive ankle work (J/kg) | 0.17 (0.03) | 0.16 (0.02) | 0.17 (0.03) | 0.15 (0.03) |
| Positive knee work (J/kg) | 0.07(0.02) | 0.07 (0.02) | 0.07 (0.02) | 0.07 (0.02) |
| Positive hip work (J/kg) | 0.06 (0.03) | 0.08 (0.03) | 0.07 (0.02) | 0.09 (0.04) |
IC: initial contact; ROM: range of motion; vGRF: vertical ground reaction force; PF: plantarflexion; KE: knee extension; HE: hip extension; (−) negative kinematic value represents a clockwise rotation
denotes moderate effect size (0.4–0.7) between older and young runners for respective condition.
Table 4.
Mean (standard deviation) of kinematic and kinetic variables where main effects of age or fatigue were found; α = 0.05.
| Main effect of age | Young | Older | p |
| Peak internal KE moment (Nm/kg) | 2.41(0.33) | 2.19 (0.28) | 0.01 |
| Max knee power (W/kg) | 5.38 (1.10) | 4.85 (0.89) | 0.04 |
| Max hip power (W/kg) | 3.98 (1.11) | 5.20 (2.98) | 0.04 |
| Peak vGRF (BW) | 2.45 (0.21) | 2.38 (0.22) | 0.007 |
| Main effect of fatigue | C1 | C2 | p |
| Knee ROM(°) | 23.49 (2.73) | 22.98 (2.67) | 0.007 |
| Peak HE moment (Nm/kg) | −2.51 (0.417) | −2.65 (0.417) | < 0.001 |
4. Discussion
The purpose of this study was to investigate how fatigue influences running mechanics in older runners. By comparing post fatigue running mechanics in older runners to volume matched younger runners, we hoped to expose differences in adaptation with exertion that may have implication for the higher rates of running related injuries in older runners. While not looked at concurrently, previous research has suggested that both the physiological effects of aging [20,22,40,41] and the presence of fatigue [16,18,42] can result in modifications to running biomechanics. Contrary to our hypothesis we did not observe any fatigue x age interactions which indicates that older and younger runners had comparable responses to the exertion experienced during C2.
The presence of fatigue has resulted in observed alterations in running kinematics and kinetics [16–18,43]. Regardless of age, participants in our study exhibited decreased knee ROM during C2 compared to C1 (C1: 23.49 ± 2.73, C2: 22.98 ± 2.61; p = 0.007). Previous studies have reported changes in knee flexion/extension during the stance phase when exposed to a level of fatigue [16,42]. With progressing fatigue, Mizrahi et al. reported a significant decrease in knee flexion following foot strike [42]. However, Derrick et al. report increase maximum knee flexion between the start and end of an exhaustive run [16]. Changes in knee joint mechanics are associated with altered shock absorbing capabilities [19]. While not analyzed in this study, it is possible that the decreased knee ROM observed during C2 could result in increased knee joint stiffness, affecting the attenuation of generated forces during stance and increasing the risk of injury [44]. While we did find significance, it is important to note that the difference in knee flexion ROM between conditions was approximately 0.5 degrees. However, when considering the knee, a joint that has ~135 degrees full range of motion, a difference of this magnitude may not be great enough to be clinically important. Although a main effect for age did not exist, when evaluating knee ROM regardless of condition the difference between groups is 1.13 degrees (O: 22.68 ± 0.67, Y: 23.81 ± 0.66; p = 0.249) which trends towards a more clinically meaningful difference. Though, a possible contribution to this difference may be due to the controlled velocity during C1 or C2 as gait velocity has been shown to influence knee kinematics [45]. When running at matched speeds, authors report older adults exhibit decreased knee ROM during stance phase [20,27,46]. While velocity was controlled during C1 on an absolute level (target of 3.5 m/s), during C2, we controlled RPE and HR and gave instructions to “speed up” or “slow down” based on these metrics. Therefore, it is possible that our results are based upon matching RPE rather than controlled velocity.
A distal-to-proximal shift in joint power has been reported in older adults during running in that older adults generate greater power at the hip and less power at the ankle compared to younger adults [22,46,47]. While we did not observe differences as distal as the ankle, we did observe differences at both the knee and hip between age groups. Overall, older adults generated less knee power and greater hip power compared to younger runners (Table 4). Our findings partially align with the established distal-to-proximal compensation strategy; however, we did not see a significant decrease in contribution from the ankle within our older population. Despite the lack of statistical significance, our findings suggest that young runners generated slight increases in power at each lower extremity post fatiguing protocol. In contrast, older adults only exhibited greater power generation at the hip joint demonstrating the need to maintain gait patterns using more proximal joints.
Previous studies that have investigated a similar level of fatigue to our study (i.e., not maximal, or exhaustive) and reported changes in mechanics had participants run on a treadmill for data collection [16, 18]. In these studies, while speed was self-selected, the treadmill speed was held constant for the duration of the data collection [16,18]. However, in our study, gait metrics for C1 and C2 were collected as participants ran overground and, for C2, they were instructed to run at a pace like their typical training pace. Older and younger runners ran at different preferred velocities during C2 (Table 2). Younger runners preferred velocity in C2 (3.66 ± 0.37 m/s) was similar to the controlled velocity during C1 (3.5 m/s), where older runners preferred velocity in C2 (3.33 ± 0.39 m/s) was less than the controlled velocity in C1 (3.5 m/s ± 5%). Because participants did not have to maintain a constant speed, like running on a treadmill, it is possible that the older runners adjusted their running velocity based on their perceived exertion during C2 to avoid compromising their ability to maintain running form. During C1, older adults exhibited subtle decreases in both KE moment and knee power, with an increase in hip power, representative of the distal-to-proximal aging shift. Since these changes occurred during a rested-state, it is possible that this made older runners more sensitive to exertion. Therefore, the slower velocity and reduce ROM during C2 is an adaptation to running with exertion.
To investigate how fatigue influences running mechanics in older runners, a protocol to elicit maximal volitional exhaustion was used. The maximal exhausting GXT was chosen by the authors to collect additional variables about participants’ level of fitness. Additionally, the authors saw this as another way to induce a level of fatigue other than having participants run a prolonged amount of time (i.e., ≥40 min) at a self-selected pace on the treadmill or throughout the lab space to induce fatigue. It should be noted that kinematic and kinetic data were not recorded in this exhaustive state. However, by monitoring HR and RPE throughout the GXT we were able to identify participant specific relationships between these variables that were representative of various levels of exertion, including somewhat hard-hard intensity exercise, which would later be used to help facilitate the post-fatigue running state during C2. Following the GXT participants were allowed a cool down while preparations for C2 were taking place. This cool down was an active cool down in which participants were instructed to walk on the treadmill or around the lab space at a slow pace for 5 min. An active cool down has been reported as a more suitable mode of HR recovery versus passive, as HR recovers more slowly in a sitting or supine position compared to standing [48]. Previous literature analyzing heart-rate recovery (HRR) report normal HRR as a reduction ≥ 12 bmp per minute for three minutes following exercise completion [49]. Following maximal exercise, however, authors report an average reduction of 79.9 bpm after three minutes of recovery in elite active adults [50]. While we did not record HR in the time between the completion of the GXT and beginning of C2 to calculate HRR, because the cool-down and preparation for C2 took a total of 10 min, we believe there was adequate time for the participants HR to recover from the maximal exercise testing but remain in some state of perceived exertion. Additionally, movement data was not recorded during C2 until both HR and RPE were within the targeted ranges. Because participants were given cues to “speed up” or “slow down” based on RPE and HR, those with too high of HRs were told to slow down. If a participants HR was still too high (i.e., not within their specific target range for C2) when they began running, they were instructed to slow down. This, by default, built in additional cool down time for those whose HR had not yet lowered enough to be within the subject specific ranges. Data was not collected until both the RPE and HR were within the targeted ranges.
During C2 it was important to collect additional metrics to ensure participants were running at the targeted level of fatigue, and not exhaustive fatigue. Exercise intensity was measured reliably and accurately with both RPE and HR [33–35]. An RPE of 17 has been previously used as a criterion of fatigue like that experienced at the end of a prolonged run [18,51]. In the present study, the target RPE for C2 was between 13 and 15 to mimic a level of fatigue experienced during a typical training run. This range is associated with somewhat hard to hard intensity in which participants should feel tired but can continue and is the typical target range when doing exercise [34]. By maintaining an RPE within this range, and a HR below that of which was reported at a 17 RPE during the GXT, we believe our participants were running in a state of moderate intensity exercise for the duration of C2. Additionally, based on the similar average RPE for older and young runners during C2 (Table 2), we are confident all participants were running in a comparable perceived state that was difficult yet attainable.
For moderate to vigorous intensity cardiorespiratory exercise, ACSM suggests a target heart rate between 64- < 96% of maximum heart rate [34]. During C2, both groups were running at 93% of their respective HR max. While high, this is still below the suggested endpoint for this level intensity of exercise. However, according the ACSM guidelines [34], these values do not necessarily match the RPEs during C2 and are more in line with an RPE > 16 representing a state of exertion that may be greater than a typical training run. After exercise, energy is used to return the body back to its resting state. Calories burned post-exercise, or exercise after-burn, is dependent on the intensity of exercise and results in oxygen consumption above resting levels which helps the body restore itself to this pre-exercise state [53]. Due to the high-intensity nature of a GXT, it is possible that participants HRs remained more elevated than normal as a result of exercise after-burn. Still, based on previous literature regarding HRR [49,50] and the RPEs reported throughout C2, we believe participants adequately recovered following the GXT and were not experiencing maximal fatigue during this condition. An example of the HR and RPE data collected throughout C2 can be found in Fig. 2A–B. While this figure shows data for only two individual participants, the trends of the data are representative of each of our groups. When considering data for all participants in each group, average HR recorded during C2 was significantly less than each group’s respective max HR recording during GXT, and between their C2 HR and age-predicted max HR. However, HR recorded during C2, and the HR reported at a 17 RPE during the GXT were similar for both (Table 5).
Fig. 2.
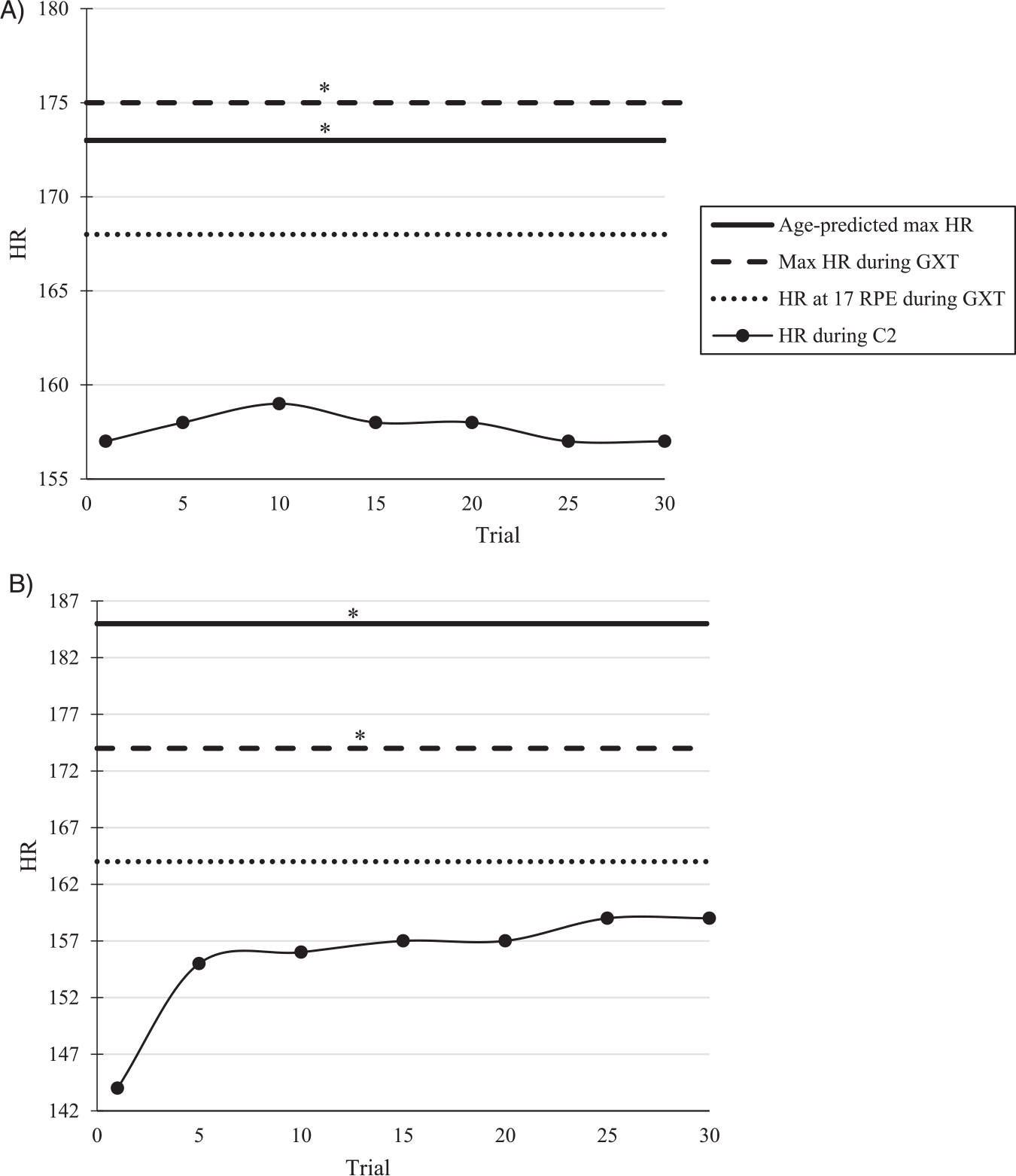
A-B. Heart rate (HR) data during C2 for two individual participants. (A) Older Participant (B) Younger participant. *Indicates significant difference from HR during C2 (α = 0.05).
Table 5.
Group averages (standard deviation) for heartrate data collected throughout the study.
| AP Max HR | Max HR during GXT | HR at 17 RPE during GXT | HR during C2 | |
|---|---|---|---|---|
|
| ||||
| Older | 161.69 (9.27) * | 174.07 (9.15) * | 169.62 (10.38) | 161.69 (9.27) |
| Younger | 193.93 (7.11) * | 182.5 (9.45) * | 168.94 (8.10) | 169.78 (7.69) |
AP: age-predicted; HR: heart rate; GXT: graded exercise test; RPE; rating of perceived exertion
denotes significant difference between respective group and their HR during C2 (α = 0.05).
As reported earlier, Vo2 max was significantly different between older and younger runners in our study. This is not surprising as it is reported that after the third decade of life, Vo2 max progressively decreases with age at a rate of around 10% per decade [54]. However, we found that older runners in our study were within the 95th percentile for their age range when compared to age-related Vo2 max norms [34]. The younger runners in our study fell within the 95th to 99th percentile for their age range. To evaluate fatigability, it is necessary to combine perceived exertion levels with physical activity performance, assuming that the activity is known or standardized [55]. Despite the reported age-related decline in Vo2 max, both older and younger subgroups fell within the same percentages for their respective age ranges. In this sense, the older runners have maintained their fitness as they age and suggests the relative perceived fatigability of older runners is comparable to younger counterparts.
The findings of our study provide insights into post-fatigue running mechanics, but we should also acknowledge the limitations of our study design. It was the authors’ intent to target a level of fatigue associated with that at the end of a typical training run. According to HR and RPE, neither group of runners was at maximum fatigue levels, however, their physical condition at the time of testing may not be comparable with that of a typical training run. This may be due to the fact that they had just previously completed a GXT where they ran to maximal exhaustion. As mentioned earlier, the maximal exhausting GXT was selected as a means to induce fatigue other than having participants run for a long period of time. According to our results, participants were running at a somewhat hard-to-hard intensity rather than the moderate intensity we associated with a typical run. It is possible that some participants experienced exertion similar to what they would usually experience during a run, however, it is also possible that some participants experienced exertion greater than normal. Therefore, we cannot conclude that the mechanical adaptations observed in this study are what one would experience during a typical run. Nonetheless, the results from our study do shed further light on the effect of fatigue on running mechanics, especially in an older population of runners. Additionally, some may view the differing pre- and post-fatigue running velocities a limitation. Establishing an acceptable velocity range is a common practice in over-ground running studies when collecting at a controlled velocity and, typically, this range is at minimum ± 5% [56–58]. It should be noted that while velocity during C2 was different between groups, both groups ran within 5% of the controlled C1 velocity. With that being said, while we did not have a controlled C2 velocity, participants did naturally run at velocities that would be deemed acceptable if we had controlled C2 velocity similarly to C1 (i.e., 3.5 m/s ± 5%). However, we do realize that trial to trial speeds may vary within the range. This may result in one group averaging a velocity on the lower end of the range, and the other averaging closer to the target or higher end. As reported earlier, we ran statistical tests to ensure that not only our older and young runners were running at a similar velocity during C1, but that there were no differences between C1 and C2 velocities. Both age and fatigue has been shown to affect gait velocity. Authors chose to control velocity during C1 to allow interstudy comparison as this is a pace commonly used when investigating the mechanics of regular runners. However, due to the known effects of both age [22] and fatigue [59] on gait velocity, authors chose not to control velocity during C2. In doing so we were able to observe differences that may be due in part to either of these two factors.
5. Conclusion
The findings from this study provide information regarding the effect of fatigue on running mechanics within an older population and to the authors’ knowledge, this is the first study to do so. Rather than using a combination of stressors such as increased, or controlled velocity and fatigue to elicit changes in mechanics, we targeted a familiar perceived level of exertion following a maximal exertion protocol to magnify subtle differences individuals experience throughout their run. While the exertion level experienced during C2 may not have been “typical” for participants, observing runners during a hard-intensity run may be more applicable than during a state of maximal fatigue. In our study, younger runners produced greater peak power at the knee regardless of exertion level, but the older runners generated greater power at the hip. In conjunction with the greater hip power output, older runners exhibited lower peak internal KE moments during stance. Based on effect size, older runners had slightly reduced knee ROM which was associated with a lower peak vGRF. Our findings suggest and support the idea that older individuals increase the utilization of hip mechanics and lessen their reliance on knee mechanics during running.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit-sectors.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Running U, 2019 National Runner Survey Results Released by Running USA, 2019. [Google Scholar]
- [2].M. American College of Sports, W.J. Chodzko-Zajko, D.N. Proctor, M.A. Fiatarone Singh, C.T. Minson, C.R. Nigg, et al., American College of Sports Medicine position stand. Exercise and physical activity for older adults, Med. Sci. Sports Exerc. 41 (7) (2009) 1510–1530. 〈https://www.ncbi.nlm.nih.gov/pubmed/19516148〉. [DOI] [PubMed] [Google Scholar]
- [3].Latham N, Anderson C, Bennett D, Stretton C, Progressive resistance strength training for physical disability in older people, Cochrane Database Syst. Rev. (2) (2003), CD002759. 〈https://www.ncbi.nlm.nih.gov/pubmed/12804434〉. [DOI] [PubMed] [Google Scholar]
- [4].Manini T, Marko M, VanArnam T, Cook S, Fernhall B, Burke J, et al. , Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life, J. Gerontol. A Biol. Sci. Med. Sci. 62 (6) (2007) 616–623. 〈https://www.ncbi.nlm.nih.gov/pubmed/17595417〉. [DOI] [PubMed] [Google Scholar]
- [5].Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. , Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study, J. Gerontol. A Biol. Sci. Med. Sci. 61 (11) (2006) 1157–1165. 〈https://www.ncbi.nlm.nih.gov/pubmed/17167156〉. [DOI] [PubMed] [Google Scholar]
- [6].Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD, A retrospective case-control analysis of 2002 running injuries, Br. J. Sports Med. 36 (2) (2002) 95–101. 〈https://www.ncbi.nlm.nih.gov/pubmed/11916889〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKean KA, Manson NA, Stanish WD, Musculoskeletal injury in the masters runners, Clin. J. Sport Med. 16 (2) (2006) 149–154. 〈https://www.ncbi.nlm.nih.gov/pubmed/16603885〉. [DOI] [PubMed] [Google Scholar]
- [8].Nielsen RO, Buist I, Parner ET, Nohr EA, Sorensen H, Lind M, et al. , Predictors of running-related injuries among 930 novice runners: a 1-year prospective follow-up study, Orthop. J. Sports Med. 1 (1) (2013), 2325967113487316. 〈https://www.ncbi.nlm.nih.gov/pubmed/26535228〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Knobloch K, Yoon U, Vogt PM, Acute and overuse injuries correlated to hours of training in master running athletes, Foot Ankle Int. 29 (7) (2008) 671–676. 〈https://www.ncbi.nlm.nih.gov/pubmed/18785416〉. [DOI] [PubMed] [Google Scholar]
- [10].Faulkner JA, Larkin LM, Claflin DR, Brooks SV, Age-related changes in the structure and function of skeletal muscles, Clin. Exp. Pharmacol. Physiol. 34 (11) (2007) 1091–1096. 〈https://www.ncbi.nlm.nih.gov/pubmed/17880359〉. [DOI] [PubMed] [Google Scholar]
- [11].Nonaka H, Mita K, Watakabe M, Akataki K, Suzuki N, Okuwa T, et al. , Age-related changes in the interactive mobility of the hip and knee joints: a geometrical analysis, Gait Posture 15 (3) (2002) 236–243. 〈https://www.ncbi.nlm.nih.gov/pubmed/11983498〉. [DOI] [PubMed] [Google Scholar]
- [12].Fukuchi RK, Stefanyshyn DJ, Stirling L, Duarte M, Ferber R, Flexibility, muscle strength and running biomechanical adaptations in older runners, Clin. Biomech. 29 (3) (2014) 304–310. 〈https://www.ncbi.nlm.nih.gov/pubmed/24380685〉. [DOI] [PubMed] [Google Scholar]
- [13].Radin EL, Role of muscles in protecting athletes from injury, Acta Med. Scand. Suppl. 711 (711 S) (1986) 143–147. 〈https://www.ncbi.nlm.nih.gov/pubmed/3465203〉. [DOI] [PubMed] [Google Scholar]
- [14].Christina KA, White SC, Gilchrist LA, Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running, Hum. Mov. Sci. 20 (3) (2001) 257–276. 〈https://www.ncbi.nlm.nih.gov/pubmed/11517672〉. [DOI] [PubMed] [Google Scholar]
- [15].Komi PV, Stretch-shortening cycle: a powerful model to study normal and fatigued muscle, 2000, pp. 1197–1206. [DOI] [PubMed] [Google Scholar]
- [16].Derrick TR, Dereu D, McLean SP, Impacts and kinematic adjustments during an exhaustive run, Med. Sci. Sports Exerc. 34 (6) (2002) 998–1002. 〈https://www.ncbi.nlm.nih.gov/pubmed/12048328〉. [DOI] [PubMed] [Google Scholar]
- [17].Bazuelo-Ruiz B, Dura-Gil JV, Palomares N, Medina E, Llana-Belloch S, Effect of fatigue and gender on kinematics and ground reaction forces variables in recreational runners, PeerJ 6 (2018), e4489. 〈https://www.ncbi.nlm.nih.gov/pubmed/29576960〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dierks TA, Davis IS, Hamill J, The effects of running in an exerted state on lower extremity kinematics and joint timing, J. Biomech. 43 (15) (2010) 2993–2998. 〈https://www.ncbi.nlm.nih.gov/pubmed/20663506〉. [DOI] [PubMed] [Google Scholar]
- [19].Derrick TR, The effects of knee contact angle on impact forces and accelerations, Med. Sci. Sports Exerc. 36 (5) (2004) 832–837. 〈https://www.ncbi.nlm.nih.gov/pubmed/15126718〉. [DOI] [PubMed] [Google Scholar]
- [20].Bus SA, Ground reaction forces and kinematics in distance running in older-aged men, Med. Sci. Sports Exerc. 35 (7) (2003) 1167–1175. 〈https://www.ncbi.nlm.nih.gov/pubmed/12840638〉. [DOI] [PubMed] [Google Scholar]
- [21].DeVita P, Hortobagyi T, Age causes a redistribution of joint torques and powers during gait, J. Appl. Physiol. 88 (5) (2000) 1804–1811. 〈https://www.ncbi.nlm.nih.gov/pubmed/10797145〉. [DOI] [PubMed] [Google Scholar]
- [22].Devita P, Fellin RE, Seay JF, Ip E, Stavro N, Messier SP, The relationships between age and running biomechanics, Med. Sci. Sports Exerc. 48 (1) (2016) 98–106. 〈https://www.ncbi.nlm.nih.gov/pubmed/26258853〉. [DOI] [PubMed] [Google Scholar]
- [23].Graf A, Judge JO, Ounpuu S, Thelen DG, The effect of walking speed on lower-extremity joint powers among elderly adults who exhibit low physical performance, Arch. Phys. Med. Rehabil. 86 (11) (2005) 2177–2183. 〈https://www.ncbi.nlm.nih.gov/pubmed/16271567〉. [DOI] [PubMed] [Google Scholar]
- [24].Judge JO, Davis RB 3rd, Ounpuu S, Step length reductions in advanced age: the role of ankle and hip kinetics, J. Gerontol. A Biol. Sci. Med. Sci. 51 (6) (1996) M303–M312. 〈https://www.ncbi.nlm.nih.gov/pubmed/8914503〉. [DOI] [PubMed] [Google Scholar]
- [25].Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ, Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments, Arch. Phys. Med. Rehabil. 79 (3) (1998) 317–322. 〈https://www.ncbi.nlm.nih.gov/pubmed/9523785〉. [DOI] [PubMed] [Google Scholar]
- [26].Winter DA, Patla AE, Frank JS, Walt SE, Biomechanical walking pattern changes in the fit and healthy elderly, Phys. Ther. 70 (6) (1990) 340–347. 〈https://www.ncbi.nlm.nih.gov/pubmed/2345777〉. [DOI] [PubMed] [Google Scholar]
- [27].Fukuchi RK, Duarte M, Comparison of three-dimensional lower extremity running kinematics of young adult and elderly runners, J. Sports Sci. 26 (13) (2008) 1447–1454. 〈https://www.ncbi.nlm.nih.gov/pubmed/18923957〉. [DOI] [PubMed] [Google Scholar]
- [28].Savelberg HHCM, Verdijk LB, Willems PJB, Meijer K, The robustness of age-related gait adaptations: can running counterbalance the consequences of ageing? Gait Posture 25 (2) (2007) 259–266. 〈https://www.ncbi.nlm.nih.gov/pubmed/16701997〉. [DOI] [PubMed] [Google Scholar]
- [29].Warburton DE, Jamnik V, Bredin SS, Shephard RJ, Gledhill N, The 2018 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+): 2018 PAR-Q+, Health Fit. J. Can. 11 (1) (2018) 31–34. [Google Scholar]
- [30].Antonio J, Kenyon M, Ellerbroek A, Carson C, Tyler-Palmer D, Burgess V, et al. , Body composition assessment: a comparison of the bod Pod, InBody 770, and DXA, J. Exerc. Nutr. 2 (2) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Manal K, McClay I, Stanhope S, Richards J, Galinat B, Comparison of surface mounted markers and attachment methods in estimating tibial rotations during walking: an in vivo study, Gait Posture 11 (1) (2000) 38–45. 〈https://www.ncbi.nlm.nih.gov/pubmed/10664484〉. [DOI] [PubMed] [Google Scholar]
- [32].Kang J, Chaloupka EC, Mastrangelo MA, Biren GB, Robertson RJ, Physiological comparisons among three maximal treadmill exercise protocols in trained and untrained individuals, Eur. J. Appl. Physiol. 84 (4) (2001) 291–295. [DOI] [PubMed] [Google Scholar]
- [33].Eston RG, Williams JG, Reliability of ratings of perceived effort regulation of exercise intensity, Br. J. Sports Med. 22 (4) (1988) 153–155. 〈https://www.ncbi.nlm.nih.gov/pubmed/3228684〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Co.S A. Medicine, ACSM’s Guidelines for Exercise Testing and Prescription, Lippincott Williams & Wilkins, 2013. [Google Scholar]
- [35].Skinner JS, Hutsler R, Bergsteinova V, Buskirk ER, The validity and reliability of a rating scale of perceived exertion, Med. Sci. Sports 5 (2) (1973) 94–96. 〈https://www.ncbi.nlm.nih.gov/pubmed/4721013〉. [PubMed] [Google Scholar]
- [36].Heiderscheit BC, Chumanov ES, Michalski MP, Wille CM, Ryan MB, Effects of step rate manipulation on joint mechanics during running, Med. Sci. Sports Exerc. 43 (2) (2011) 296–302. 〈https://www.ncbi.nlm.nih.gov/pubmed/20581720〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cavanagh PR, Lafortune MA, Ground reaction forces in distance running, J. Biomech. 13 (5) (1980) 397–406. 〈https://www.ncbi.nlm.nih.gov/pubmed/7400169〉. [DOI] [PubMed] [Google Scholar]
- [38].Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, et al. , ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion - part I: ankle, hip, and spine, J. Biomech. 35 (4) (2002) 543–548. [DOI] [PubMed] [Google Scholar]
- [39].Robertson DGE, Caldwell GE, Hamill J, Kamen G, Whittlesey S, Research methods in biomechanics, Human kinetics, 2013. [Google Scholar]
- [40].Kline PW, Williams DS 3rd, Effects of normal aging on lower extremity loading and coordination during running in males and females, Int. J. Sports Phys. Ther. 10 (6) (2015) 901–909. 〈https://www.ncbi.nlm.nih.gov/pubmed/26618069〉. [PMC free article] [PubMed] [Google Scholar]
- [41].Paquette MR, Devita P, Williams DSB 3rd, Biomechanical implications of training volume and intensity in aging runners, Med. Sci. Sports Exerc. 50 (3) (2018) 510–515. 〈https://www.ncbi.nlm.nih.gov/pubmed/29016393〉. [DOI] [PubMed] [Google Scholar]
- [42].Mizrahi J, Verbitsky O, Isakov E, Daily D, Effect of fatigue on leg kinematics and impact acceleration in long distance running, Hum. Mov. Sci. 19 (2) (2000) 139–151, https://www.google.com/search?q=Mizrahi+J%2C+Verbitsky+O%2C+Isakov+E%2C+Daily+D.+Effect+of+fatigue+on+leg+kinematics+and+impact+acceleration+in+long+distance+running.+Hum+Mov+Sci.+2000%3B19(2)%3A139-51.&rlz=1C1CHBF_enUS800US800&oq=Mizrahi+J%2C+Verbitsky+O. [Google Scholar]
- [43].Clansey AC, Hanlon M, Wallace ES, Lake MJ, Effects of fatigue on running mechanics associated with tibial stress fracture risk, Med. Sci. Sports Exerc. 44 (10) (2012) 1917–1923. 〈https://www.ncbi.nlm.nih.gov/pubmed/22525776〉. [DOI] [PubMed] [Google Scholar]
- [44].Williams DS 3rd, Davis IM, Scholz JP, Hamill J, Buchanan TS, High-arched runners exhibit increased leg stiffness compared to low-arched runners, Gait Posture 19 (3) (2004) 263–269. 〈https://www.ncbi.nlm.nih.gov/pubmed/15125915〉. [DOI] [PubMed] [Google Scholar]
- [45].Boyer KA, Johnson RT, Banks JJ, Jewell C, Hafer JF, Systematic review and meta-analysis of gait mechanics in young and older adults, Exp. Gerontol. 95 (2017) 63–70. 〈https://www.ncbi.nlm.nih.gov/pubmed/28499954〉. [DOI] [PubMed] [Google Scholar]
- [46].Karamanidis K, Arampatzis A, Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity, J. Exp. Biol. 208 (Pt 20) (2005) 3907–3923. 〈https://www.ncbi.nlm.nih.gov/pubmed/16215218〉. [DOI] [PubMed] [Google Scholar]
- [47].Kulmala JP, Korhonen MT, Kuitunen S, Suominen H, Heinonen A, Mikkola A, et al. , Which muscles compromise human locomotor performance with age? J. R. Soc. Interface 11 (100) (2014), 20140858. 〈https://www.ncbi.nlm.nih.gov/pubmed/25209406〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Buchheit M, Al Haddad H, Laursen PB, Ahmaidi S, Effect of body posture on postexercise parasympathetic reactivation in men, Exp. Physiol. 94 (7) (2009) 795–804, 10.1113/expphysiol.2009.048041. [DOI] [PubMed] [Google Scholar]
- [49].Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS, Heart-rate recovery immediately after exercise as a predictor of mortality, N. Engl. J. Med. 341 (18) (1999) 1351–1357, 10.1056/nejm199910283411804. [DOI] [PubMed] [Google Scholar]
- [50].Suzic Lazic J, Dekleva M, Soldatovic I, Leischik R, Suzic S, Radovanovic D, et al. , Heart rate recovery in elite athletes: the impact of age and exercise capacity, Clin. Physiol. Funct. Imaging 37 (2) (2017) 117–123, 10.1111/cpf.12271. [DOI] [PubMed] [Google Scholar]
- [51].Koblbauer IF, Van Schooten KS, Verhagen EA, Van Dieën JH, Kinematic changes during running-induced fatigue and relations with core endurance in novice runners, J. Sci. Med. Sport 17 (4) (2014) 419–424, 10.1016/j.jsams.2013.05.013. [DOI] [PubMed] [Google Scholar]
- [53].Mann TN, Webster C, Lamberts RP, Lambert MI, Effect of exercise intensity on post-exercise oxygen consumption and heart rate recovery, Eur. J. Appl. Physiol. 114 (9) (2014) 1809–1820, 10.1007/s00421-014-2907-9. [DOI] [PubMed] [Google Scholar]
- [54].Pimentel AE, Gentile CL, Hirofumi T, Seals DR, Gates PE, Greater rate of decline in maximal aerobic capacity with age in endurance-trained than in sedentary men, J. Appl. Physiol. 94 (2003) 2406–2413. [DOI] [PubMed] [Google Scholar]
- [55].Eldadah BA, Fatigue and fatigability in older adults, PM&R 2 (5) (2010) 406–413, 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- [56].Hamill J, Russell EM, Gruber AH, Miller R, Impact characteristics in shod and barefoot running, Footwear Sci. 3 (1) (2011) 33–40. [Google Scholar]
- [57].Paquette MR, Zhang S, Baumgartner LD, Acute effects of barefoot, minimal shoes and running shoes on lower limb mechanics in rear and forefoot strike runners, Footwear Sci. 5 (1) (2013) 9–18. [Google Scholar]
- [58].Ferber R, Davis IM, Williams DS 3rd, Gender differences in lower extremity mechanics during running, Clin. Biomech. 18 (4) (2003) 350–357. 〈https://www.ncbi.nlm.nih.gov/pubmed/12689785〉. [DOI] [PubMed] [Google Scholar]
- [59].Granacher U, Wolf I, Wehrle A, Bridenbaugh S, Kressig RW, Effects of muscle fatigue on gait characteristics under single and dual-task conditions in young and older adults, J. Neuroeng. Rehabil. 7 (1) (2010) 56. 〈https://www.ncbi.nlm.nih.gov/pubmed/21062458〉. [DOI] [PMC free article] [PubMed] [Google Scholar]


