To The Editor Sir,
Myotonic dystrophy (DM) is an autosomal dominant genetic disorder characterized by both muscle dystrophy and myotonia along with involvement of other systems such as the central nervous system (CNS), eye, heart, and endocrine system. There are two major forms of myotonic dystrophy – type 1 (DM1) and the milder form type 2 (DM2). Weakness in distal muscles is generally more prominent in DM1, whereas proximal muscle involvement is the main disability in DM2.[1] DM1 is caused by a (CTG) n micro-satellite repeat expansion in the untranslated 3′ region of the dystrophin myotonin protein kinase (DMPK) gene in chromosome 19q13.3, whereas DM2 is because of a (CCTG) n expansion in intron 1 of the nucleic acid-binding protein (CNBP) gene in chromosome 3q21.3. Here, we present a genetically proven case of DM1, with early proximal muscle involvement along with Parkinsonian features.
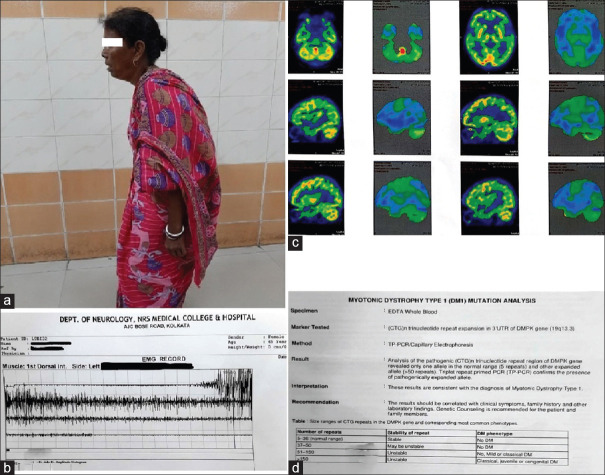
A 45-year-old normotensive, euglycemic lady presented with insidious onset, gradually progressive spastic quadriparesis for the past 5 years, initially involving lower limbs followed by upper limbs, with early involvement of the proximal muscles followed by distal muscles without any fasciculations or diurnal variation of weakness. The patient has stooped-forward posture, and she has been speaking in a low voice and walking in a stooping posture for the past 2 years [Figure 1a]. There was no history suggestive of any cranial nerve, sensory, or sphincter involvement.
Figure 1.
(a) Patient photograph showing stooped forward posture; (b) needle electromyography showed myotonic discharges on both proximal and distal muscles; (c) FDG-PET scan of brain showed hypometabolism in bilateral pallidum and lateral frontal and superior temporal gyrus; (d) genetic study confirmed myotonic dystrophy type 1 mutation
On examination, the patient had hypomimia with a decreased blink rate, alopecia, and wasting of temporalis muscle. The patient had hypophonic and monotonous speech. On motor examination, proximal muscles were found to be weak with a normal power in distal muscles in all four limbs. Handgrip myotonia was present along with percussion myotonia over thenar eminence and tongue. There was both axial and appendicular rigidity. Cogwheel rigidity was present around both wrist joints. Despite having normal power in small muscles of hand, she had bradykinesia on finger tapping. Gait examination showed stooped posture and bilaterally reduced arm swing [Video 1]. The patient has Parkinsonism, as evidenced by symmetrical bradykinesia, rigidity, and camptocormia, with the absence of rest tremor [Video 2]. She has frequent forward fall, two to three times per week. She has normal cognition, MMSE 23/23 (as the patient is illiterate, she cannot read, write, or calculate). Autonomic testing shows no abnormality in postural blood pressure change, heart rate variability with respiration, sweating abnormality, blood pressure changes with mental arithmetic, isometric hand grip, and cold emersion.
Her routine blood investigations revealed normal hemogram, blood glucose, creatine kinase, electrolytes, thyroid, liver and renal function tests, electrocardiogram (ECG), and chest roentgenogram. ECG showed sinus bradycardia. Needle electromyography showed myotonic discharges on both proximal and distal muscles [Figure 1b]. Magnetic resonance imaging of brain and spine were non-contributory. Muscle biopsy showed some atrophic muscle fibers with clumped and pyknotic nuclei. FDG-PET scan of brain showed hypometabolism in bilateral pallidum and lateral frontal and superior temporal gyrus [Figure 1c]. Genetic study confirmed myotonic dystrophy type 1 mutation [Figure 1d]. The whole exome sequencing data identified ~ 52,412 variants including insertion/deletion and single-nucleotide variants, which include six variants (three frameshift and three missense) in a heterozygous manner in VPS13C (p.Asn1964MetfsTer2 and p.His1963Pro), SYNJ1(p.Leu610SerfsTer22 and p.Lys648Met), and DNAJC6 (p.Val678AspfsTer5 and p.Val678Gly) genes [Table 1]. All these missense variants were predicted to be disease-causing using several bioinformatic tools, whereas all other frameshift changes lead to pre-mature termination codon mutations. The patient was tried with levodopa therapy, and there was minimal improvement.
Table 1.
In silico prediction of identified variants in SYNJ1, VPS13C, and DNAJ6 genes (predicted to be damaging are indicated by the values written in BOLD)
| Gene | Nucleotide variant | Amino Acid change | SIFT score | PolyPhen-2 score | Mutation Taser score | FATHMM score | Provean PRED | MetaSVM score | MetaLR score | Mutation Assessor score |
|---|---|---|---|---|---|---|---|---|---|---|
| SYNJI | c. 1827_1828insT | p.Leu610SerfsTer22 | NA | NA | NA | NA | NA | NA | NA | NA |
| c. 1943A >T | P.Lys648Met | 0.001 | 1.00 | 1.00 | 1.43 | -4.64 | -0.7712 | 0.1904 | 2.22 | |
| VPS13C | c. 5891delA | p.Asn1964MetfsTer26 | NA | NA | NA | NA | NA | NA | NA | NA |
| c. 5888A >C | P.His1963Pro | 0.021 | 0.891 | 0.999 | 0.91 | -3.12 | -0.7471 | 0.1979 | 2.76 | |
| DNAJC6 | c. 2032_2033insA | p.Val678AspfsTer5 | NA | NA | NA | NA | NA | NA | NA | NA |
| c. 2033T >G | P.Val678Gly | 0.21 | 0.359 | 1.00 | -3.17 | -0.83 | 0.1398 | 0.6157 | 2.08 |
DM1 affects the distal muscles of the extremities more than proximal muscles. However, in our case, the proximal muscles are affected more and distal limb muscles are spared.
Parkinsonian features are rare in myotonic dystrophy. Only few cases have been reported worldwide. Among the reported cases, most cases are because of DM2. In our case, Parkinsonian features were found in a DM1 patient, where features of Parkinsonism appeared three years after the onset of myopathic illness and showed minimal response to levodopa therapy. To the best of our knowledge, this is the first reported case from India of a genetically proven case of DM1 with Parkinsonian features and hypometabolism in bilateral pallidum in FDG-PET scan.
Clinically, the patient presented with stooped posture (camptocormia), which is a feature of Parkinsonism. However, severe trunk flexor myotonia may produce this stooped posture. In this clinical context, electromyography study was undertaken, which showed non-specific positive waves in rectus abdominis (Grade 1, Streib and Sun score). There are several other spine-related local and CNS and peripheral nervous system-related causes which can give rise to camptocormia. All those possible causes were tried to be ruled out by relevant spine and brain imaging and electro-physiological studies.
Among the previous reported patients with DM who developed Parkinsonism [Table 2],[2–8] an autopsy was performed on a patient reported by Okuma et al.,[2] which was clinically diagnosed to be DM1 and Parkinsonism, but was not genetically tested. The autopsy in the patient showed marked cell loss and Lewy bodies in the substantia nigra (SN) and diffuse myelin loss in the cerebral white matter. However, Lewy bodies in the SN have been reported in other patients with genetically proven or clinically diagnosed DM without Parkinsonism.
Table 2.
Descriptions of our patient along with similar case reports
| Similar case reports | Age | Gender | Type of Myotonic dystrophy | Salient features | Levodopa response |
|---|---|---|---|---|---|
| Okuma et al.[2] | 63 | F | One | Asymmetric tremor, small step with freezing, bradykinesia, postural instability, and hypomimia | Good |
| Hund et al.[3] | 59 | F | Two | Stooped posture, hypomimia, and low voice | Good |
| Chu et al.[4] | 65 | M | Two | Asymmetric tremor, rigidity, bradykinesia, hypomimia, stooped posture, and gait disturbance | Partial |
| Celik et al.[5] | 41 | F | Two | Bilateral resting tremor, rigidity, bradykinesia, and hypomimia | Good |
| Annic et al.[6] | 47 | F | Two | Bilateral tremor, bradykinesia, rigidity, and gait disturbance | Partial |
| Choi et al.[7] | 54 | F | One | Bilateral bradykinesia, rigidity, stooped posture, hypomimia, gait freezing, and postural instability | Minimal |
| Sansone et al.[8] | 72 | F | Two | Parkinsonism, postural instability, distal limb weakness in upper limbs, neck flexor, and trunk weakness | Minimal |
| Our case | 45 | F | One | Bilateral bradykinesia, rigidity, stooped posture, hypomimia, low voice, alopecia, quadriparesis (lower limb followed by upper limb, proximal followed by distal limb) | Minimal |
Sansone et al.[8] showed in their case of DM presenting with Parkinsonism hypometabolism in bilateral superior temporal gyrus and parietal operculum in FDG-PET. In our case, we have also found a similar finding in FDG-PET with bilateral pallidal hypometabolism. Chu et al.[4] categorized these patients as a different disease group, named ‘parkinsonism-myotonia myopathia complex’.
In conclusion, DM1 patients may present with proximal muscle weakness and Parkinsonism may be one of the presenting features in myotonic dystrophy patients. Parkinsonian clinical features including cogwheel rigidity, bilateral pallidal involvement in FDG-PET, and minimal levodopa responsiveness are suggestive of cerebral involvement. Further research is needed to understand the pathophysiology of Parkinsonism in myotonic dystrophy patients.
There were a few limitations in our case. One of the previous studies by Choi et al.[7] used DAT imaging in DM1 patients to establish pathology at the dopamine transporter system of basal ganglia structures of DM1 patients. As this imaging facility is not available in our institute, we could not establish pathology at the dopamine transport system, which is characteristic of many Parkinsonian disorders. One of the previous studies by Okuma et al.[2] involved autopsy studies of DM1 patients and found characteristic pathological findings of Parkinsonian disorders such as Lewy bodies in basal ganglia structures of DM1 patients. This is also lacking in our case as we could not establish any histopathological corroboration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.annalsofian.org
REFERENCES
- 1.Udd B, Krahe R. The myotonic dystrophies:Molecular, clinical, and therapeutic challenges. Lancet Neurol. 2012;11:891–905. doi: 10.1016/S1474-4422(12)70204-1. [DOI] [PubMed] [Google Scholar]
- 2.Okuma Y, Tanaka S, Nomura Y, Mori H, Yan H, Shirai T, et al. A63-year-old woman with muscle weakness, myotonia, and parkinsonism. No To Shinkei. 1996;48:287–97. [PubMed] [Google Scholar]
- 3.Hund E, Jansen O, Koch MC, Ricker K, Fogel W, Niedermaier N, et al. Proximal myotonic myopathy with MRI white matter abnormalities of the brain. Neurology. 1997;48:33–7. doi: 10.1212/wnl.48.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Cho J-W, Song E-C, Jeon BS. A patient with proximal myotonic myopathy and parkinsonism. Can J Neurol Sci. 2002;29:188–90. [PubMed] [Google Scholar]
- 5.Celik Y, Turgut N, Balci K, Kabayel L. Proximal myotonic dystrophy associated with parkinsonism. J Clin Neurosci. 2006;13:275–6. doi: 10.1016/j.jocn.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Annic A, Devos D, Destée A, Defebvre L, Lacour A, Hurtevent J, et al. Early dopasensitive parkinsonism related to myotonic dystrophy type 2. 2008 doi: 10.1002/mds.22239. doi:10.1002/mds. 22239. [DOI] [PubMed] [Google Scholar]
- 7.Choi J-H, Lee J-Y, Kim H-J, Jeon B. A patient with myotonic dystrophy type 1 presenting as parkinsonism. J Mov Disord. 2018;11:145–8. doi: 10.14802/jmd.18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansone V, Meola G, Perani D, Fazio F, Garibotto V, Cotelli M, et al. Glucose metabolism and dopamine PET correlates in a patient with myotonic dystrophy type 2 and parkinsonism. J Neurol Neurosurg Psychiatry. 2006;77:425–6. doi: 10.1136/jnnp.2005.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.