Abstract
Aim
H3G34 diffuse hemispheric glioma is a CNS tumor that is difficult to diagnose and treat and accompanied with poor prognosis. It is becoming clear that extra CNS metastasis may present in a subset of patients with H3G34 gliomas, further complicating diagnosis and treatment.
Materials & methods
We present a case of a 19-year-old female with a H3G34 mutant diffuse hemispheric glioma with osseous metastases. We then provide a literature review of the most recent understanding of H3G34 mutant malignancies.
Conclusion
Given the stress that patients with H3G34 can experience and the poor prognosis, it is imperative to expand our knowledge and ascertain accurate diagnostic methodologies and targeted therapeutic approaches.
Keywords: diffuse hemispheric glioma, H3G34-mutant, osseous metastases
Case presentation
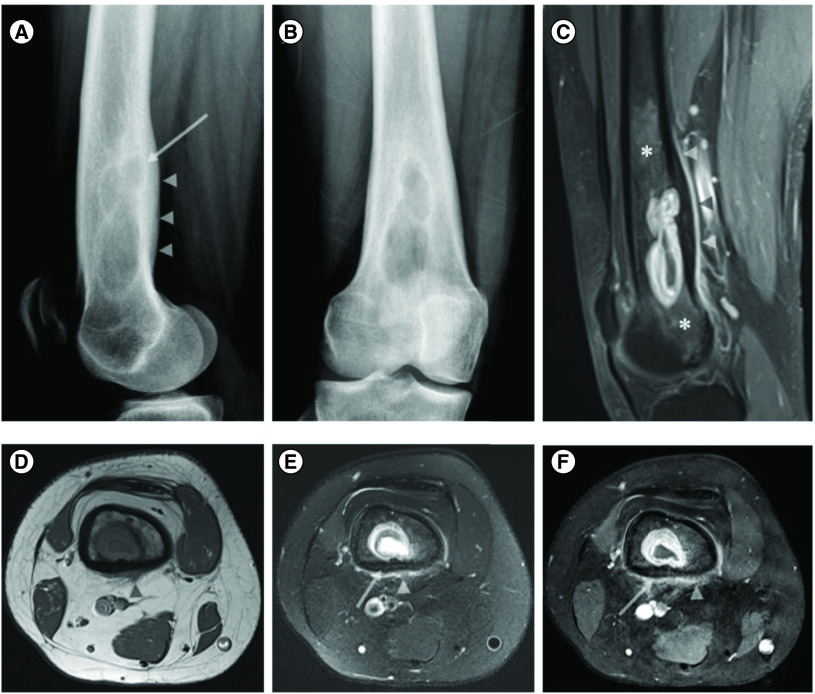
A 19-year-old female with no significant past medical history initially presented at an outside facility with involuntary jerking symptoms in her right knee/lower leg which progressed to right foot rhythmic episodic movements (<1 min duration) after approximately 1 year later. The patient had been prescribed levetiracetam (Keppra) but was not taking the medication when she presented at our institution 2 months later. Upon arrival to our center, the patient reported right lower extremity ‘jerking movements’. These focal seizures were accompanied with her right leg feeling ‘heavier’ and reports that Keppra was not alleviating the symptoms. X-ray followed by contrast enhanced MRI showed an ill-defined right distal femur expansile osteolytic lesion with central fluid signal, thick rim enhancement, endosteal scalloping, cortical invasion and smooth enhancing periosteal reaction/soft tissue extension associated with surrounding enhancing marrow signal abnormality worrisome for lesion extension (Figure 1). Differential considerations included primarily Brodie’s abscess, or neoplastic lesion such as Langerhans’ cell histiocytosis. In general, the differential considerations for a lytic bone lesion in the distal femur is summed up in the famous radiographic pneumonic ‘FEGNOMASHIC’ which includes fibrous dysplasia or fibrous cortical defect, enchondroma, eosinophilic granuloma, giant cell tumor (GCT) or geode, non ossifying fibroma (NOF), osteoblastoma, metastasis(es)/myeloma, aneurysmal bone cyst, simple (unicameral) bone cyst, hyperparathyroidism (brown tumor), infection (osteomyelitis) or infarction (bone infarction), chondroblastoma or chondromyxoid fibroma. We excluded fibrous dysplasia due to the lack of ground glass matrix and presence of periosteal reaction (PR), fibrous cortical defect due to the involvement of the underlying medullary cavity and presence of PR, enchondroma due to the absence of the ‘rings and arcs’ characteristic of a chondroid matrix, GCT due to the lack of extension to the subchondral bone, geode due to absence of degenerative changes, non ossifying fibroma due to the presence of PR, OB due to the absence of rim of reactive sclerosis, myeloma is unusual at this age, aneurysmal bone cyst due to the lack of characteristic fluid-fluid levels ls on MR, simple bone cyst due to the shape of the lesion and PR, brown tumor due to lack of clinical suspicion and imaging features associated with hyperparathyroidism, infarction due to lack of serpiginous peripheral low signal, chondroblastoma which are usually epiphyseal or apophyseal in location and finally chondromyxoid fibroma due to the presence of PR. Therefore, the main differential considerations were infection (Brodie’s abscess), a neoplastic lesion such as eosinophilic granuloma (Langerhans’ cell histiocytosis) or metastasis.
Figure 1. . X-ray of distal right femur and coronal postcontrast fat suppressed images.
X-ray of distal right femur in lateral (A) and AP (B) views showing an ill-defined expansile lytic lesion in the right distal femoral shaft with endosteal scalloping and posterior cortical sclerotic reaction and thickening (arrow and arrowhead in (A), respectively). Coronal postcontrast fat suppressed sagittal (C) and axial (F) T1 WI and axial T1 (D) and fat suppressed T2 images of the right distal femur showing a rim enhancing lesion with central fluid signal. There is cortical thickening and invasion evidenced by loss of the normal low signal in the cortical bone (arrow in (E & F)), associated with endosteal scalloping and smooth periosteal/soft tissue extension (arrow heads in (C–F)). Note the surrounding enhancing marrow signal abnormality worrisome for lesion extension more than red marrow islands (asterisks in (C)).
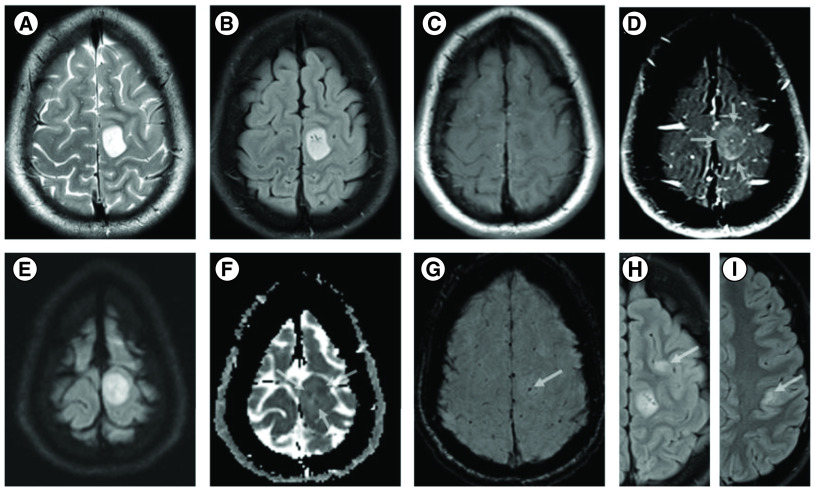
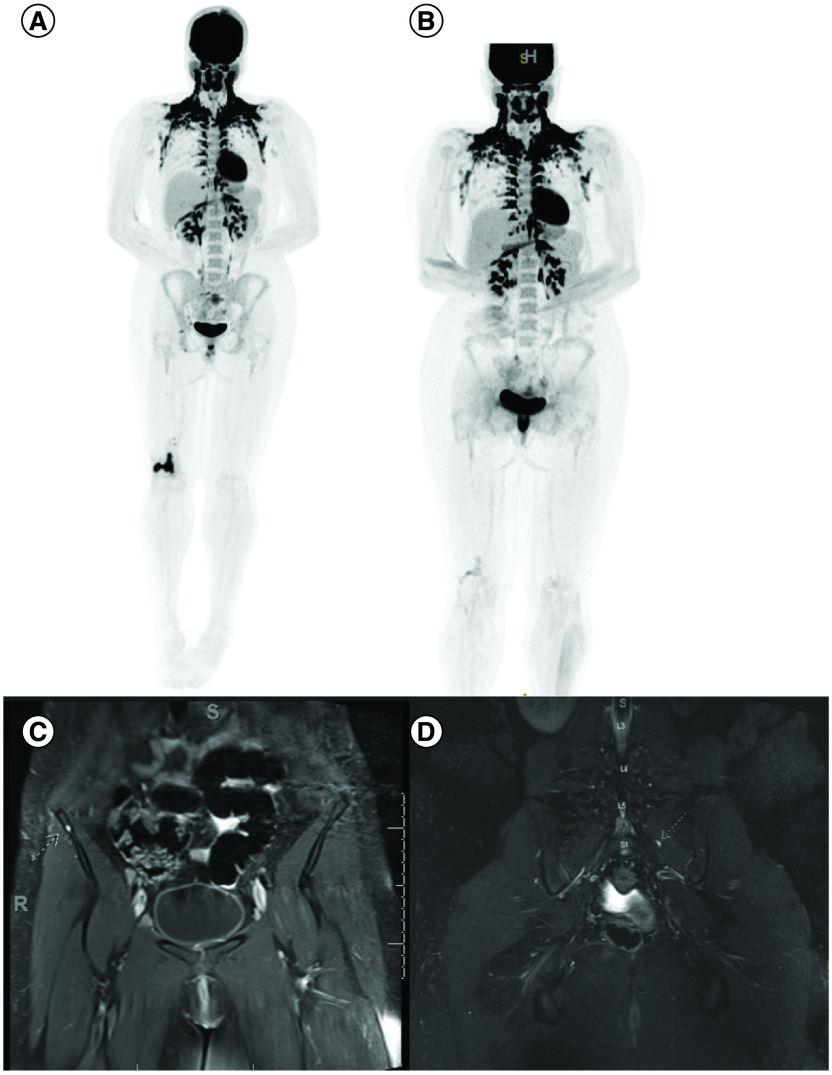
The patient’s ‘spells’ were captured with continuous EEG with intermittent 3 Hz amplitude polymorphic delta waves in the left centroparietal region. For seizure workup, contrast enhanced MRI of the brain exhibited multiple frontal and partial lobe T2 FLAIR hyperintense lesions with the largest lesion demonstrating contrast enhancement, internal foci of restricted diffusion and a focus of intratumoral hemorrhage (Figure 2) differential consideration was a low-grade glioma versus a demyelinating process. MRI of the full spine and CT chest, abdomen and pelvis were negative for malignancy. Basic CSF studies were not suggestive of infection or autoimmune etiology such as meningoencephalitis and oligoclonal bands were negative. Biopsy of the right femur lesion was nondiagnostic. A whole-body PET/CT showed intensely FDG-avid lytic lesion in the right distal femur, with FDG activity along the biopsy tract. Hypermetabolic lytic metastases to the right femoral head and mid-sacral body extending to S3 were also observed, along with suspected metastatic adenopathy in the right popliteal region, right groin, right external and common iliac chains and mesentery (Figure 3A, B, C & D).
Figure 2. . MRI images of brain lesion.
MRI shows a well circumscribed cortical based 2.1 × 1.6 cm mass in the left peri-Rolandic region demonstrating hyperintense T2/FLAIR SI (A & B), hypointense T1-WI (C) and thick subtle incomplete ring enhancement on the postcontrast fat suppressed T1 WI (arrows) (D). DWI and ADC maps show internal foci of restricted diffusion (arrows) (E & F) and T2*-SWI images shows a punctate focus of intratumoral hemorrhage (arrow) (G). Axial FLAIR images and different axial levels showing similar but nonenhancing cortical/subcortical lesions in the left frontal and parietal lobes (arrows) (H & I).
DWI: Diffusion weighted imaging.
Figure 3. . Images showing multiple extra CNS lesions.
Intensely FDG-avid lytic lesion of the right distal femur (A) as well as lytic metastases involving the right popliteal region, right groin, right external and common iliac chains. Improvement in sacrum and right femoral FDG uptake after treatment (B). Note, physiological brown fat activation in the neck. Coronal contrast enhanced fat suppressed T1 WI showing corresponding punctate enhancing metastatic nodules in the right iliac bone (C) and left S1 level (D).
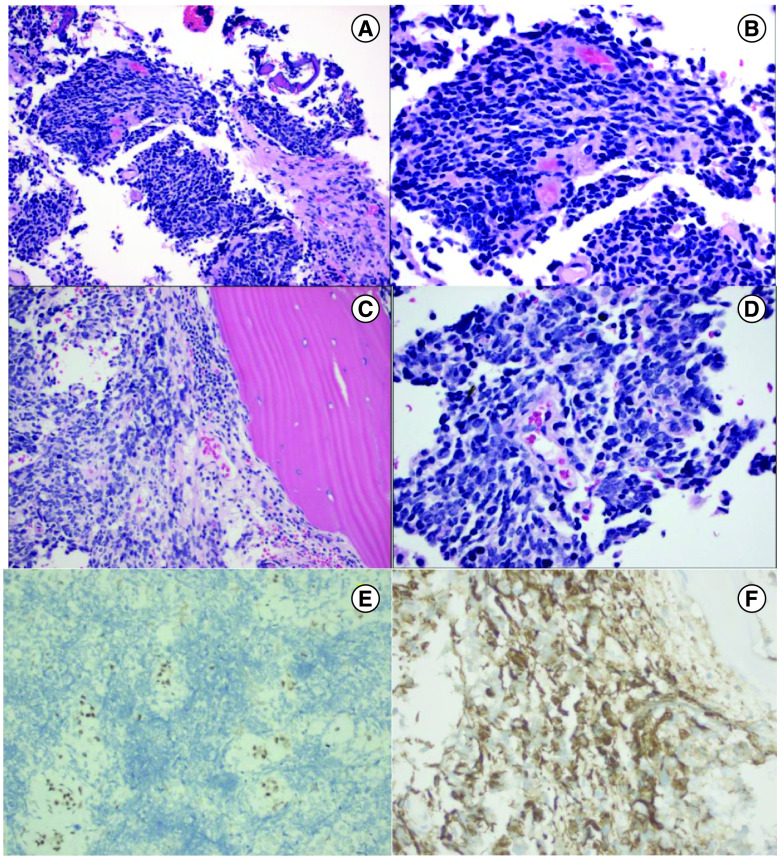
About 2 months later, the patient underwent a craniotomy with biopsy which showed histological features of malignant small round blue cell neoplasm. Intraoperatively, the lesion was noted to include prominent calvarial and subperiosteal components, raising the further possibility of a neoplasm arising in bone. The initial histological features were primarily of a malignant small round blue cell neoplasm (Figure 4A & B) with CD99 staining, leading to a preliminary impression of Ewing Sarcoma. Following the patient’s diagnosis, a bone biopsy of the distal right femur and a sacral bone lesion biopsy were conducted (Figure 4). The biopsy from the distal right femur found abundant granulation tissue with bone remodeling changes and fibrosis. The sacral bone biopsy reported malignant small blue cell neoplasm (Figure 4C & D), consistent with involvement by the patient’s known cranial neoplasm. A contrast enhanced pelvic MRI was performed around the same time of the biopsy results indicated sacral metastatic lesions extending to the margins of the S1–S3 neural foramina, punctate enhancing metastatic nodules throughout the pelvis and femur and enlarged bilateral external iliac and inguinal lymph nodes (Figure 3C & D).
Figure 4. . H&E histology images, and ATRX and GFAP images.
H&E 400X histology image from brain tissue showing small blue cell neoplasms composed of sheets of ribbons of cells with scant cytoplasm, hyperchromatic nuclei, and high nucleus/cytoplasm ratio (200× magnification in (A), 400× magnification in (B)). H&E image from bone biopsy showing small blue cell neoplasm involving bone, mitosis present (200× magnification in (C), 400× magnification in (D)). ATRX 200× image from brain tissue showing ATRX loss (200× magnification, (E)). GFAP 400X image from brain tissue showing GFAP positivity (400× magnification, (F)).
Based on these pathology findings, the patient was started on the PEDS AEWS1031 regimen for sarcoma. The first cycle included vincristine, cyclophosphamide, doxorubicin and mesna. The second cycle consisted of ifosfamide, etoposide and mesna. Concurrent chemoradiation with temozolomide (TMZ) chemotherapy was then started. During this time and following the absence of EWSR gene rearrangements by FISH, tissue was submitted for evaluation by UCSF500 next generation sequencing which resulted in the finding of ATRX, p53 and H3F3A gene alterations, suggesting the diagnosis of H3G34-mutant diffuse hemispheric glioma. Subsequent evaluation by methylation profiling at the NIH supported classification as H3G34-mutant diffuse hemispheric glioma, WHO grade 4 with a high confidence score. Retrospectively, the biopsy tissues were further evaluated by immunohistochemistry, showing typical findings including ATRX loss, GFAP positivity (Figure 4E & F) and staining for the H3G34-R/V (data not shown) specific antibody. Of note that there have been proposed updates to H3 gene and protein nomenclature according to sequence alterations provided by the human genome variation society [1]. In this report, we chose to continue using previously known classification for this entity. With chemoradiotherapy, the patient was seizure-free for more than 20 days. It was determined that the patient would continue with adjuvant TMZ therapy after radiation, continue systemic staging and re-evaluate at tumor board if she had progression.
Following the sixth cycle of TMZ, MRI brain imaging was stable. Apart from holocephalic headaches, the patient had no significant side effects to the medication and was seizure free. A second whole-body PET scan showed improvement and interval decrease in FDG uptake of the sacrum and right femoral head (Figure 4).
Discussion
High grade gliomas are malignant brain tumors that exhibit poor prognosis [2–4]. Mutations in the gene H3F3A, which encodes the replication independent histone 3 variant H3.3, are particularly prevalent in pediatric gliomas including diffuse intrinsic pontine glioma, non brainstem high grade glioma, as well as in giant cell tumor of bone (GCTB) [5–7]. H3.3 has been found to be vulnerable to somatic missense mutations primarily at three amino acids on the N-terminus tail, including lysine-27 (K27), glycine-34 (G34) and lysine-36 (K36) [5]. While there is growing literature about the mechanism of H3K27M tumors, knowledge about H3G34 mutations remains in its infancy. To date, mutations in H3G34 to arginine or valine (H3G34R/V) have largely been associated with brain tumors [8–10] and mutations in H3G34 to tyrosine or lysine (H3G34W/L) have been linked to GCTB [8,11,12]. However, it is notable that there are cases in which missense mutations such as H3G34V are implicated in both gliomas and GCTB [12,13]. Recent studies posit that G34R/V/D mutations result in steric change that block H3K36 demethylation and trimethylation in cis by impairing enzymes including SETD2, an H3K36-specific methyltransferase and MutSα, a mismatch repair protein, thereby dually promoting genetic instability and tumor growth [14]. In 2020, Jain et al. added to the literature demonstrating that in H3G34W mutations in vivo, the reduction of SETD2-dependent H3K36 methylation leads to a gain of aberrant polycomb repressive complex 2-mediated H3K27 di-/tri-methylation that promotes tumor development [15].
Co-occurring alterations have been observed in MGMT promotor methylation and mutations in ATRX, TP53, mucin genes (MUC), EGFR and platelet derived growth factor receptor alpha (PDGFRA) [16–18]. It has been proposed via in vivo murine models that H3G34R/V mutations arise from GSX2+-expressing interneuron progenitor cells and that the mutations delay neuronal differentiation and increase PDGFRA overexpression, ultimately linking PDGFRA aberration to gliomagenesis [19]. While some studies indicate that the presence of mutations in PDGRA or EGFR may confer prognosis better than others [16,17], further investigation is required.
Histologically, H3G34 diffuse hemispheric gliomas can display characteristics similar to glioblastoma with increased mitotic activity, microvascular proliferation, and palisading necrosis or resemble primitive neuroectodermal-like (PNET-like) small blue cell tumor morphology cytology of small cells with hyperchromatic nuclei and little cytoplasm [20]. Studies have shown H3G34R mutations in both glioblastoma and PNET-like tumor, as well as anaplastic astrocytoma, other non specified high-grade gliomas and more [21]. This heterogeneity can complicate diagnosis and treatment for patients [22].
Neuroimaging studies have confronted similar challenges. Among pediatric patients, H3G34 mutations have been most evident in the supratentorial regions of the brain [21], and a case report by Onishi et al. showed hyperintensity on diffusion weighted imaging, edema on T2WI/FLAIR and slight partial gadolinium enhancement [23]. The presence of restricted diffusion is unusual in classic glial tumors, and we postulate that it could be due to the high tumor cellularity which may point to the diagnosis in this patient population. While Kurokawa et al. noted significant differences in survival prognosis of patients with DHGs-G34m with ill-defined margins and those with well-delineated margins [21], the data remains sparse and inconsistent [24,25].
Kay et al. reported the utility of FDG PET/CT to illuminate extracranial metastases in the midthoracic spine and right shoulder after detecting vertebral masses on MRI, suggesting that this modality may assist metastatic detection [26]. Despite these tools, neuroimaging can be difficult to reconcile with clinical presentation. Jethanandani et al. reported a case of H3F3A G34R mutation glioma wherein the initial contrast and non contrast MRI demonstrated a faintly peripherally enhanced lesion in the right paramedian cortex but was thought to be unrelated to the patient’s presenting symptoms [10]. The patient was first diagnosed with Guillain–Barré Syndrome and the lesion progressed soon after and additional imaging including FDG PET/CT showed additional lesions in the ipsilateral iliac. Though the diagnosis of H3F3A G34R mutation with extraneural metastases ultimately surfaced, this report continues to demonstrate the difficulties underlying diagnosis [10].
As mentioned earlier, the heterogeneity of H3G34 mutations can make diagnosis and treatment complex. Most cases are treated with surgical resection or biopsy depending on the tumor location, radiation and chemotherapy [21,27–29].
Diffuse extraneural metastases of H3G34 mutated high-grade gliomas are rare and just beginning to be described in the literature [28,30,31]. In reports, patients have varying initial presentation, but experience multimodal treatment and overall, have poor outcome.
Conclusion
High grade gliomas are complex malignancies and recent literature is beginning to expose how histone mutations in high grade gliomas can also present as extra-CNS malignancies. These findings reiterate how challenging diagnosis can be and emphasize the necessity of accurate and timely diagnostic techniques. Additionally, H3G34 mutated tumors lean toward poor prognosis, but our case report indicates that H3G34 mutants may have a more variable outcome than what has been reported in the literature thus far.
Summary points.
H3G34 diffuse hemispheric glioma is a CNS tumor that may present with extra CNS metastasis.
Our study underscores the need for both careful diagnostic screening and special treatment protocol for this subset of patients.
Footnotes
Author contributions
All listed authors participated in the writing of the manuscript and have read and approved the final version.
Financial & competing interests disclosure
O Aboud is supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through grant #2K12CA138464-11. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The study protocol was approved by the Institutional Review Board of The University of California Davis.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Leske H, Dalgleish R, Lazar AJ, Reifenberger G, Cree IA. A common classification framework for histone sequence alterations in tumours: an expert consensus proposal. J. Pathol. 254(2), 109–120 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Crowell C, Mata-Mbemba D, Bennett J et al. Systematic review of diffuse hemispheric glioma, H3 G34-mutant: outcomes and associated clinical factors. Neurooncol. Adv. 4(1), vdac133 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Shao L, Li H et al. Histone H3.3 G34-mutant diffuse gliomas in adults. Am. J. Surg. Pathol. 46(2), 249–257 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Lim KY, Won JK, Park CK et al. H3 G34-mutant high-grade glioma. Brain Tumor Pathol. 38(1), 4–13 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Schwartzentruber J, Korshunov A, Liu XY et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384), 226–231 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Broniscer A, Mceachron TA et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44(3), 251–253 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behjati S, Tarpey PS, Presneau N et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 45(12), 1479–1482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe BR, Yadav RK, Henry RA et al. Surprising phenotypic diversity of cancer-associated mutations of Gly 34 in the histone H3 tail. Elife 10, e65369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimoto K, Hatae R, Sangatsuda Y et al. Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor. Pathol. 34(3), 103–112 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Jethanandani A, Gule-Monroe MK, Chen M, Johnson JM. Extraneural metastases from a high-grade glioma (HGG) with an H3F3A G34R mutation. Front. Oncol. 9, 373 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangatsuda Y, Miura F, Araki H et al. Base-resolution methylomes of gliomas bearing histone H3.3 mutations reveal a G34 mutant-specific signature shared with bone tumors. Sci. Rep. 10(1), 16162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto H, Iwasaki T, Yamada Y et al. Diagnostic utility of histone H3.3 G34W, G34R, and G34V mutant-specific antibodies for giant cell tumors of bone. Hum. Pathol. 73, 41–50 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Sweha SR, Chung C, Natarajan SK et al. Epigenetically defined therapeutic targeting in H3.3G34R/V high-grade gliomas. Sci. Transl. Med. 13(615), eabf7860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J, Huang Y, Mao G et al. Cancer-driving H3G34V/R/D mutations block H3K36 methylation and H3K36me3-MutSα interaction. Proc. Natl Acad. Sci. USA 115(38), 9598–9603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain SU, Khazaei S, Marchione DM et al. Histone H3.3 G34 mutations promote aberrant PRC2 activity and drive tumor progression. Proc. Natl Acad. Sci. USA 117(44), 27354–27364 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuong HG, Le HT, Dunn IF. The prognostic significance of further genotyping H3G34 diffuse hemispheric gliomas. Cancer 128(10), 1907–1912 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Duan H, Zhong S, Zeng J, Mou Y. High frequency of PDGFRA and MUC family gene mutations in diffuse hemispheric glioma, H3 G34-mutant: a glimmer of hope? J. Transl. Med. 20(1), 64 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picart T, Barritault M, Poncet D et al. Characteristics of diffuse hemispheric gliomas, H3 G34-mutant in adults. Neurooncol. Adv. 3(1), vdab061 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CCL, Deshmukh S, Jessa S et al. Histone H3.3G34-mutant interneuron progenitors Co-opt PDGFRA for gliomagenesis. Cell 183(6), 1617–1633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korshunov A, Capper D, Reuss D et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol. 131(1), 137–146 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kurokawa R, Baba A, Kurokawa M et al. Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J. Neuroimaging 32(1), 17–27 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Gessi M, Gielen GH, Hammes J et al. H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: possible diagnostic and therapeutic implications? J. Neurooncol. 112(1), 67–72 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Onishi S, Amatya VJ, Karlowee V et al. Radiological and immunostaining characteristics of H3.3 G34R-mutant glioma: a report of 3 cases and review of the literature. Pediatr. Neurosurg. 55(5), 319–325 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Weinberg DN, Allis CD, Lu C. Oncogenic mechanisms of histone H3 mutations. Cold Spring Harb. Perspect. Med. 7(1), a026443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vettermann FJ, Felsberg J, Reifenberger G et al. Characterization of diffuse gliomas with histone H3-G34 mutation by MRI and dynamic 18F-FET PET. Clin. Nucl. Med. 43(12), 895–898 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Kay MD, Pariury HE, Perry A, Winegar BA, Kuo PH. Extracranial metastases from glioblastoma with primitive neuronal components on FDG PET/CT. Clin. Nucl. Med. 45(3), e162–e164 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Wood MD, Neff T, Nickerson JP et al. Post-treatment hypermutation in a recurrent diffuse glioma with H3.3 p.G34 mutation. Neuropathol. Appl. Neurobiol. 47(3), 460–463 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Ray A, Manjila S, Hdeib AM et al. Extracranial metastasis of gliobastoma: three illustrative cases and current review of the molecular pathology and management strategies. Mol. Clin. Oncol. 3(3), 479–486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki S, Tomomasa R, Nobusawa S et al. Anaplastic pleomorphic xanthoastrocytoma associated with an H3G34 mutation: a case report with review of literature. Brain Tumor Pathol. 36(4), 169–173 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H. Extracranial metastases of high-grade glioma: the clinical characteristics and mechanism. World J. Surg. Oncol. 15(1), 181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohiuddin S, Maraka S, Usman Baig M et al. Case series of diffuse extraneural metastasis in H3F3A mutant high-grade gliomas: clinical, molecular phenotype and literature review. J. Clin. Neurosci. 89, 405–411 (2021). [DOI] [PubMed] [Google Scholar]