Abstract
Objective:
Treatment refractory depression is a devastating condition with significant morbidity, mortality, and societal cost. At least 15% of major depressive disorder cases remain refractory to currently available treatments. We identified a young adult with treatment refractory depression and multiple suicide attempts with an associated severe deficiency of cerebrospinal fluid (CSF) tetrahydrobiopterin, a critical cofactor for monoamine neurotransmitter synthesis. Treatment with the tetrahydrobiopterin analogue sapropterin led to dramatic and long lasting remission of depression. This sentinel case led us to hypothesize that the incidence of metabolic abnormalities contributing to treatment refractory depression is under-recognized.
Methods:
We conducted a case-controlled, targeted, metabolomic evaluation of 33 adolescent and young adult patients with well-characterized history of depression refractory to at least three maximum-dose, adequate-duration medication treatments, and 16 healthy controls. Plasma, urine, and CSF metabolic profiling were performed by coupled gas chromatography/mass spectrometry, and high performance liquid chromatography, electrospray ionization, tandem mass spectrometry (LC-ESI-MS/MS).
Results:
CSF metabolite abnormalities were identified in 21 of 33 treatment refractory depression participants. Cerebral folate deficiency (n=12) was most common, with normal serum folate and low 5-methyltetrahydrofolate (5MTHF) in CSF. All patients with cerebral folate deficiency, including one patient with low 5MTHF and low tetrahydrobiopterin intermediates in CSF, showed improvement in depression symptom inventories after treatment with folinic acid and sapropterin, respectively. No healthy controls had a metabolite abnormality.
Conclusions:
Examination of metabolic disorders in treatment refractory depression identified an unexpectedly large proportion of patients with potentially treatable abnormalities. The etiology of these abnormalities remains to be determined.
Introduction
Major depressive disorder is the second-leading cause of disability world-wide and affects an estimated 350 million people.1 Although strides have been made in the treatment of depression, an estimated 15% of depressed individuals do not respond despite adequate pharmacotherapy, psychotherapy, and even electroconvulsive therapy.2 Current predictors of treatment response for depression, such as severity, chronicity, or exposure to adverse life events, are non-specific and without clear therapeutic implications in treatment refractory depression. The clinical management of treatment refractory depression has not advanced significantly in the past two decades, and the suicide rate in the US has been increasing.3 Recent emphasis on treatment refractory depression has focused on neuromodulatory treatments, such as deep brain stimulation, which, while promising, require neurosurgical intervention. We propose a novel diagnostic and therapeutic approach to treatment refractory depression based on a targeted analysis of metabolites in blood, urine, and CSF.
Metabolomics is the study of patterns of metabolic intermediates to characterize dysfunctional metabolic pathways potentially related to symptomatic presentation. This approach has previously contributed to our understanding of the pathophysiology of depression. For example, over 3 decades ago, decreased 5-hydroxyindoleacetic acid (5-HIAA) in CSF of depressed patients was shown to convey a markedly increased risk for suicide.4 This work helped to spur the development of selective serotonin reuptake inhibitors (SSRIs), which are now widely used for the treatment of depression.5–6 In addition, neuropsychiatric symptoms are common manifestations of many inborn errors of metabolism (IEMs). Adolescent and adult neuropsychiatric symptoms, possibly caused by metabolic abnormalities, are also a common comorbidity in both primary7 and secondary forms of mitochondrial dysfunction.8
We previously reported a 19 year old, male patient with unremitting treatment refractory depression and repeated suicide attempts with deficient tetrahydrobiopterin intermediates in CSF who was responsive to replacement with the tetrahydrobiopterin analogue sapropterin.9 CSF testing of five additional adolescent patients with treatment refractory depression revealed that three had low CSF 5-methyltetrahydrofolate (5-MTHF) and normal blood folate levels, indicating cerebral folate deficiency.10 None of the conventional clinical or research diagnostic or therapeutic approaches would have identified the source of these patients’ difficulties, nor would they have suggested the subsequent successful treatment strategies.
We hypothesize that the incidence of metabolic abnormalities contributing to treatment refractory depression will be significantly greater than in healthy controls Broader identification of such metabolic disorders could transform psychiatric practice, particularly in severe depression, when standard treatments are ineffective. We describe the systematic evaluation of 33 patients with treatment-refractory depression for primary and secondary disorders of central nervous system (CNS) metabolism. Targeted studies of CSF and peripheral samples (blood and urine) were utilized to identify patients with abnormalities in the monoamine neurotransmitter synthesis pathway, as well other metabolomic abnormalities leading to apparent isolated CNS dysfunction with depression.
Method
Sample Characteristics:
Participants aged 14 to 40 years with depression unresponsive to known treatments (at least three maximum dose medication trials for at least 6 weeks each), were recruited by advertisement through the Clinical and Translational Science Institute Registry at University of Pittsburgh or by clinical referral. All adult participants provided informed consent; adolescent participants provided informed assent for involvement in the study, with parents providing informed consent. Participants were compared with young adult healthy controls with no personal or first degree relative history of psychiatric disorder or suicidal behavior. Adolescent healthy controls were not included because lumbar puncture conferred greater than minimal risk. This study was approved by the Institutional Review Board of the University of Pittsburgh.
Healthy controls (n=22) and treatment refractory depression participants (n=38) were enrolled. One healthy control participant was subsequently excluded due to discovery of substance use disorder and suicidal behavior in one of the participant’s parents. One healthy control was excluded due to subsequent discovery of self-administration of large quantities of B vitamins. One treatment refractory depression participant was excluded due to diagnosis of Asperger’s Syndrome. Four treatment refractory depression participants and four healthy control participants were excluded because they did not complete the study due to refusal of lumbar puncture.
Participants were assessed with a structured psychiatric interview including the Family Interview for Genetics Studies11 at the time of referral to characterize depression course, comorbidity, family history, and history of trauma, psychosis, and anxiety. Treatment refractory depression status was confirmed with the Antidepressant Treatment History Questionnaire.12 The Beck Depression Inventory (BDI) 13 and 14 were collected for all participants and the Child Depression Rating Scale-Revised15 was collected from clinical assessment in adolescents. Patients remained on current medications and remained in current treatment during the course of the study.
Study Procedures:
The research staff and principal investigator a) completed a structured psychiatric interview, b) obtained self-report symptom questionnaires, and c) arranged for study laboratory testing. Assessment consisted of a psychiatric interview, review of records, and administered self-reports at intake (characterized depression course (Beck Depression Inventory (BDI)), suicidal ideation and behavior (Suicidal Ideation Questionnaire, Columbia Suicide History Form,16 and Beck Suicide Ideation Scale 17), comorbidity (anxiety, psychosis, substance use, attention disorders) (DSM 5 Cross Cutting Symptom Inventory) 18, family history (Family Interview for Genetic Studies and 3 generation pedigree)11. A neurologic examination was completed by the principal investigator (L.P.). Analysis of blood and CSF began immediately after collection.
Plasma and urine testing were performed by the Clinical Biochemical Genetics and Clinical Chemistry Laboratories of University of Pittsburgh Medical Center (UPMC) per their standard protocols including gas chromatography-mass spectrometry (GC-MS) of urine, and liquid chromatography-tandem mass spectrometry (LC-MS/MS) and high pressure liquid chromatography-MS/MS (HPLC-MS/MS) profiling of blood to examine known groups of metabolites contributing to depression. A lumbar puncture for CSF collection was performed under fluoroscopic guidance by Neuroradiology at UPMC, and samples were analyzed as for blood and urine by the Clinical Biochemical Genetics and Clinical Chemistry Laboratories of UPMC and Medical Neurogenetics, Inc. (Atlanta, GA). If a specific abnormality was suspected on the basis of the initial testing, the patient was referred to a biochemical geneticist for additional confirmatory testing. Upon receipt of results of testing, a follow-up appointment was scheduled for every affected participant to review results and provide additional referrals if needed. Participants with treatment refractory depression returned for a second appointment to review results. At this appointment, BDI, Suicidal Ideation Questionnaire, and DSM 5 Symptom Inventory were repeated. Participants for whom a novel treatment was available were re-contacted at least 6 weeks after start of treatment to determine outcome with repeated BDI, Suicidal Ideation Questionnaire, and DSM 5 Symptom Inventory.
Analyses were completed in SPSS v.21. We employed independent t-tests to examine the differences between treatment refractory depression participants and healthy controls in depression severity (BDI), suicidal ideation (Suicidal Ideation Questionnaire), and age. A Chi-Square goodness of fit test was calculated to compare the distribution of male to female participants in each group. Due to the small number of subjects who were assessed before and after intervention sample size (n=10), Wilcoxon signed-ranks tests were performed between pre and post-treatment BDI and Suicidal Ideation Questionnaire scores among the cerebral folate deficient participants.
Results
Demographics and Clinical Assessment:
The treatment refractory depression and healthy control groups were similar with respect to age and sex, and ethnicity (Table 1).
Table 1:
Demographics
| Healthy Controls (n=16) | Depressed (n=33) | |
|---|---|---|
| Average Age | 26.12 (SD=6.11) | 26.21 (SD=7.63) |
|
| ||
| Sex: | ||
| Number of Males | 7 | 8 |
| Number of Females | 9 | 25 |
|
| ||
| Race: | ||
| African American | 2 | 1 |
| Asian | 4 | 0 |
| Caucasian | 9 | 30 |
| Multiple Races | 1 | 2 |
|
| ||
| Ethnicity: | ||
| Hispanic | 0 | 2 |
| Not Hispanic | 16 | 31 |
Characterization of Depression (Table 2):
Table 2:
Characterization of Depression
| Cerebral Folate Deficiency-Depressed Participants | *Number of Depressed Episodes | *Age at Onset of Depression (years) | *Level of Impairment: 1=Impaired, 2=Incapacitated | *Duration of Longest Episode (weeks) | *Number of Suicide Attempts | Comorbid Disorder | History of Bipolar Disorder yes/no | Family History of MDD yes/no | Family History of BP yes/no | Number of Medication Trials | ECT yes/no |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 15 | 2 | 600 | 1 | PTSD and Anxiety | no | yes | yes | 6 | no |
| 2 | 1 | 9 | 2 | 468 | 1 | Anxiety | no | yes | no | >10 | yes |
| 3 | 2 | 12 | 2 | 468 | 5 | PTSD, Anxiety, and ADHD | no | yes | no | 8 | no |
| 4 | 1 | 26 | 2 | 208 | 4 | PTSD | no | no | yes | 4 | yes |
| 5 | >100 | 9 | 2 | 50 | 0 | PTSD, ADHD, and Substance Use Disorder | yes | yes | yes | 9 | no |
| 6 | 2 | 16 | 2 | 312 | 0 | Substance Use Disorder | no | yes | no | 4 | no |
| 7 | 2 | 12 | 2 | 72 | 0 | Anxiety | no | no | no | 4 | no |
| 8 | 2 | 13 | 2 | 156 | 5 | PTSD | no | yes | no | >10 | no |
| 9 | 1 | 20 | 2 | 28 | 1 | none | no | no | no | 3 | no |
| 10 | 1 | 4 | 2 | 1300 | 3 | PTSD and Anxiety | no | yes | no | 9 | no |
| 11 | 1 | 11 | 2 | 362 | 0 | PTSD | no | yes | yes | 3 | yes |
| 12 | 90 | 14 | 2 | 12 | 0 | Substance Use Disorder | no | no | yes | 3 | no |
| Other Metabolic Disorders-Depressed Participants | * Number of Depressed Episodes | * Age at Onset of Depression (years) | * Level of Impairment: 1=Impaired, 2=Incapacitated | * Duration of Longest Episode (weeks) | * Number of Suicide Attempts | Comorbid Disorder | History of Bipolar Disorder yes/no | Family History of MDD yes/no | Family History of BP yes/no | Number of Medication Trials | ECT yes/no |
| 13 | 1 | 5 | 2 | 1092 | 0 | OCD and PTSD | no | yes | no | 4 | no |
| 14 | 2 | 5 | 2 | 468 | 0 | Anxiety | no | yes | no | 5 | no |
| 15 | 1 | 12 | 2 | 1352 | 10 | PTSD and Psychosis NOS | yes | yes | yes | >10 | no |
| 16 | 2 | 13 | 2 | 364 | 0 | Anxiety | no | yes | no | 6 | yes |
| 17 | >100 | 21 | 2 | 104 | 1 | Anxiety | yes | no | no | >10 | no |
| 18 | 1 | 17 | 2 | 1092 | 0 | PTSD | yes | yes | yes | >10 | no |
| 19 | 1 | 13 | 2 | 364 | 1 | Anxiety and ADHD | no | yes | no | 9 | no |
| 20 | 2 | 11 | 2 | 208 | 0 | Anxiety and OCD | no | no | no | 4 | no |
| 21 | 15 | 8 | 2 | 12 | 2 | Anxiety | no | yes | yes | 10 | no |
| No Metabolic Disorder-Depressed Participants | * Number of Depressed Episodes | * Age at Onset of Depression (years) | * Level of Impairment: 1=Impaired, 2=Incapacitated | * Duration of Longest Episode (weeks) | * Number of Suicide Attempts | Comorbid Disorder | History of Bipolar Disorder yes/no | Family History of MDD yes/no | Family History of BP yes/no | Number of Medication Trials | ECT yes/no |
| 22 | 6 | 9 | 2 | 468 | 1 | PTSD and Anxiety | no | yes | no | 6 | no |
| 23 | 1 | 12 | 2 | 884 | 2 | none | no | yes | yes | 8 | no |
| 24 | 5 | 9 | 2 | 728 | 0 | none | no | yes | no | 7 | no |
| 25 | 1 | 23 | 2 | 572 | 0 | Anxiety | no | no | no | 10 | no |
| 26 | 2 | 6 | 2 | 364 | 0 | none | no | yes | no | 8 | no |
| 27 | 1 | 10 | 2 | 416 | 2 | Anxiety | no | yes | no | 6 | no |
| 28 | 1 | 13 | 2 | 364 | 4 | PTSD and Psychosis | no | yes | no | >10 | yes |
| 29 | 1 | 14 | 1 | 1042 | 0 | PTSD, Anxiety, and Substance Use Disorder | no | yes | yes | 7 | no |
| 30 | 1 | 7 | 2 | 780 | 0 | none | no | yes | no | 4 | no |
| 31 | 4 | 13 | 2 | 36 | 1 | Anxiety | no | yes | no | 4 | no |
| 32 | 1 | 12 | 2 | 208 | 1 | Anxiety | no | no | no | 5 | no |
| 33 | 5 | 16 | 2 | 24 | 0 | none | no | yes | no | >10 | no |
PTSD=Post Traumatic Stress Disorder, ADHD=Attention Deficit Hyperactivity Disorder, OCD= Obsessive Compulsive Disorder
Assessed from Family Interview for Genetic Studies (FIGS) reports.
Treatment refractory depression participants reported longstanding, unremitting or recurrent depression with an average of 5.13 episodes (SD= 15.98), with average longest duration of episode without symptom remission, having a mean of 453.88 weeks (SD 386.51 weeks). Mean age of onset of depressive symptoms in treatment refractory depression participants was 12.42 years old (SD 5.05). The BDI was much higher in the treatment refractory depression participants than in the healthy controls (29.2 (SD=8.4) vs. 0.6 (SD=1.2), ([t(47)=13.60], p<0.001). The treatment refractory depression group also showed much higher Suicidal Ideation Questionnaire scores than did the healthy controls (36.39 (SD=21.40) vs. 1.18 (SD=1.08), ([t(47)=6.57], p<0.001). Seventeen treatment refractory depression (52%) participants reported a history of at least one suicide attempt.
Of the 33 participants with treatment refractory depression, there was a positive family history for at least one first degree relative of any disorder (88%), depression (82%), and suicide attempts (24%) on the Family Interview for Genetic Studies. Healthy controls had no personal or first degree relative history of psychiatric disorder or suicidal behavior (by definition). On neurologic exam neither treatment refractory depression participants nor healthy controls had abnormalities with the exception of one adolescent treatment refractory depression participant with fine, benign hand tremor.
Metabolic Targeted Clinical Studies:
We identified evidence of metabolite abnormalities in 21 of 33 participants with treatment refractory depression (Table 3,4). CFD [CSF 5-methyltetrahydrofolate (5-MTHF) deficiency], a metabolic abnormality in which serum folate is normal, but CSF 5-MTHF is low (<40 nmol/L), was the most common metabolic abnormality identified, present in 12 participants (36%). One participant with CFD was also found to have low tetrahydrobiopterin metabolites in CSF, as seen in our previously published case who responded to treatment with sapropterin.9 Nine additional participants had identified abnormalities. Five of these participants had an acylcarnitine profile abnormality. One participant was found to have low guanidinoacetate and creatine/creatinine ratio consistent with a creatine synthesis deficit. One patient was found to have Fabry’s Disease. One patient had a 20p12.1 microdeletion and another had a 15q13.3 microduplication on microarray analysis.
Table 3:
Depressed Participants with Cerebral Folate Deficiency: Findings and Treatment Results
| Cerebral Folate Deficiency-Depressed Participants | Metabolic Findings | CSF 5-MTHF Levels nmol/L | Serum folate Levels ng/mL | Psychiatric Medications at Initial Visit | Initial SIQ | Initial BDI | Psychiatric Medications at Post-Treatment Visit | Post-Treatment SIQ | Post-Treatment BDI | Time lapse between initial and post treatment self-reports (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Low CSF 5MTHF | 40 | 6.1 | none | 13 | 22 | Folinic acid | 8 | 15 | 7 |
| 2 | Low CSF 5MTHF | 39 | >20.0 | Citalopram, Risperidone, Amitriptyline and Desyrel | 36 | 25 | Folinic acid, Amitriptyline, Desyrel, 5 HTP, Carbidopa | 17 | 14 | 36 |
| 3 | Low CSF 5MTHF | 37 | >20.0 | Desyrel | 61 | 29 | Folinic Acid, and Desyrel | 45 | 29 | 8 |
| 4 | Low CSF 5MTHF | 39 | 12.2 | Zolpidem and Vilazodone | 70 | 37 | Folinic Acid, Zolpidem, and Vilazodone | 58 | 36 | 11 |
| 5 | Low CSF 5MTHF | 36 | >20.0 | Lioresal, Gabapentin, Tramadol | 23 | 33 | Folinic Acid, Lioresal, Gabapentin, Tramadol, Lithium Carbonate, Dextroamphetamine | 18 | 17 | 20 |
| 6 | Low CSF 5MTHF | 15 | >20.0 | Fluoxetine | 14 | 24 | Folinic Acid and Fluoxetine | 13 | 13 | 15 |
| 7 | Low CSF 5MTHF | 14 | 18.3 | Sertraline, Carbidopa, and Lamotrigine | 41 | 28 | Folinic Acid, Sertraline, Carbidopa, and Lamotrigine | 20 | 16 | 7 |
| 8 | Low CSF 5MTHF | 35 | 14.4 | Sertraline | 67 | 43 | Folinic Acid and Sertraline | 15 | 0 | 80 |
| 9 | Low CSF 5MTHF | 37 | >20.0 | Bupropion | 20 | 20 | lost to follow up | lost | lost | |
| 10 | Low CSF 5MTHF | 40 | 17.6 | Lamotrigine, Escitalopram, Clonazepam, Desyrel | 53 | 31 | non-compliant with Folinic Acid | non-compliant | non-compliant | |
| 11 | Low CSF 5MTHF, and low tetrahydrobiopterin | 31 | >20.0 | Sertraline, Escitalopram, Lisdexamfetamine, and Hydroxyzine | 47 | 40 | Folinic Acid, 5HTP, Carbidopa, Sapropterin, Lisdexamfetamine, Hydroxyzine | 33 | 35 | 52 |
| 12 | Low CSF 5MTHF and abnormal acylcarnitine profile | 36 | >20.0 | none | 8 | 25 | Folinic acid | 4 | 21 | 10 |
CSF = Cerebrospinal Fluid, 5-MTHF = 5-Methyltetrahydrofolate, SIQ = Suicide Ideation Questionnaire, BDI = Beck Depression Inventory
Table 4:
Depressed Participants with a Metabolic Disorder and with No Metabolic Disorder: Findings
| Other Metabolic Disorders-Depressed Participants | Metabolic Findings | CSF 5-MTHF Levels nmol/L | Serum folate Levels ng/mL | Psychiatric Medications at Initial Visit | Initial SIQ | Initial BDI |
|---|---|---|---|---|---|---|
| 13 | Abnormal acylcarnitine profile | 68 | 17.6 | Citalopram, and Melatonin | 53 | 37 |
| 14 | Low creatine levels in urine/guan rat | 55 | 13.4 | Aripiprazole, Diazepam, Methylphenidate, Lamotrigine, and Bupropion | 15 | 38 |
| 15 | Abnormal acylcarnitine profile | 60 | >20.0 | Lamotrigine, Clonazepam, and Fluoxetine | 33 | 35 |
| 16 | Abnormal acylcarnitine profile | 109 | 19.3 | Levothyroxine Sodium, Lorazepam, Sertraline, Lurasidone, and Hydroxyzine | 36 | 29 |
| 17 | Fabry disease | 77 | 14.5 | Lithium Carbonate, and Desyrel | 15 | 19 |
| 18 | Abnormal acylcarnitine profile | 92 | 10.2 | Gabapentin, Lithium Carbonate, Lamotrigine, Clonazepam, and Olanzapine | 32 | 32 |
| 19 | Abnormal acylcarnitine profile and low creatine levels in urine/guan ratio | 59 | >20.0 | Nortriptyline and Sertraline | 72 | 32 |
| 20 | 20p12.1 microdeletion on chromosome analysis | 67 | >20.0 | Venlafaxine and Fluvoxamine | 22 | 24 |
| 21 | 15q13.3 microduplication on chromosome analysis | 45 | 16.6 | Desvenlafaxine, Divalproex Sodium, and Aripiprazole | 17 | 26 |
| No Metabolic Disorder-Depressed Participants | Other Findings | CSF 5-MTHF Levels nmol/L | Serum folate Levels ng/mL | Psychiatric Medications at Initial Visit | Initial SIQ | Initial BDI |
| 22 | 60 | 17.5 | Imipramine, Venlafaxine, and Quetiapine Fumarate | 37 | 41 | |
| 23 | 46 | >20.0 | Sertraline, and Quetiapine Fumarate | 59 | 33 | |
| 24 | 54 | >20.0 | Amitryptiline, Citalopram, Lovasal, and Levothyroxine Sodium | 12 | 12 | |
| 25 | 58 | 9.8 | Bupropion, Vilazodone, Temazepam, and Clonazepam | 11 | 19 | |
| 26 | 49 | 10.3 | Lamotrigine and Melatonin | 22 | 25 | |
| 27 | 52 | 17.6 | none | 41 | 22 | |
| 28 | 59 | >20.0 | Clozapine | 88 | 49 | |
| 29 | 53 | >20.0 | Venlafaxine | 11 | 17 | |
| 30 | 59 | 18.6 | Clonazepam | 55 | 39 | |
| 31 | 51 | >20.0 | none | 20 | 22 | |
| 32 | 78 | >20.0 | Fluoxetine, Lamotrigine, and Mirtazapine | 48 | 24 | |
| 33 | 48 | >20.0 | Lisdexamphetamine, Desyrel, and Levothyroxine Sodium | 49 | 30 |
CSF = Cerebrospinal Fluid, 5-MTHF = 5-Methyltetrahydrofolate, SIQ = Suicide Ideation Questionnaire, BDI = Beck Depression Inventory
Treatment:
Of the 12 patients with low 5MTHF in CSF, all were provided treatment with folinic acid 1–2 mg/kg for at least six weeks (range 6–79 weeks). Ten of the 12 patients treated with folinic acid along with their pre-evaluation treatment regimen showed reduction in symptom inventories at follow-up. One was lost to follow-up, and one was non-compliant with the folinic acid treatment. Eight participants with CFD who completed follow-up did not have a co-morbid biochemical findings. Of these 8 patients, 4 had a 50% reduction in BDI scores. Five of these 8 patients also met threshold scores for suicidal ideation on the Suicidal Ideation Questionnaire at intake, and three of these five had reduction in suicidal ideation scores to below threshold after treatment. One patient with CFD was also treated for low CSF tetrahydrobiopterin with sapropterin 20 mg/kg. FOLR1 gene sequencing was completed for eight of these patients, and no sequence abnormalities were identified. In patients with CFD, average BDI score decreased from 30.6 (SD 7.29) to 11.04 (SD 11.03) (Wilcoxon signed-ranks test Z=2.0, p<0.022. Average Suicidal Ideation Questionnaire score decreased from 38 (SD 23.13) to 23.1 (SD 17.09), although the change was not statistically significant on the Wilcoxon Sign Ranks test, Z=1.24, p<0.107. (Table 3, Figure 1).
Figure 1.
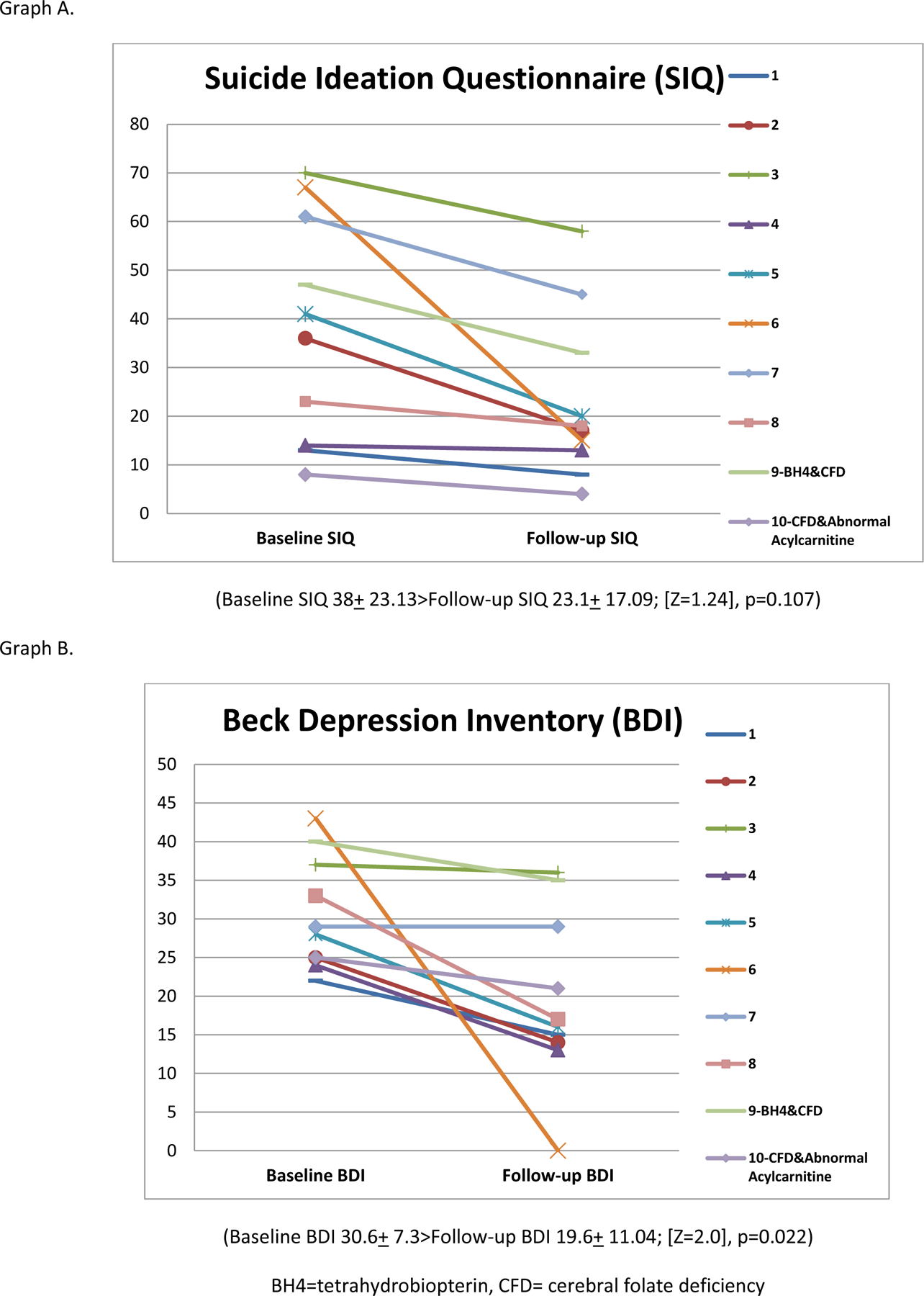
Symptom inventory outcomes in patients with cerebral folate deficiency before and after treatment with folinic acid.
Conclusions
Our CNS-specific metabolomic survey is the first to systematically evaluate abnormalities of neurotransmitter, vitamin, pterin, and energy metabolism in peripheral and CSF samples from patients with isolated psychiatric symptoms in the absence of primary neurologic symptoms. Testing for CNS-specific metabolic abnormalities included 5-methyltetrahydrofolate, tetrahydrobiopterin, neopterin, pyridoxal-5-phosphate, 5-hydroxyindoleacetic acid, homovanillic acid, and amino-adipic semialdehyde. It was spurred by our initial identification of a young man with deficient tetrahydrobiopterin and response to treatment with sapropterin, followed by discovery of CFD in 3 of 5 additional patients.9 In this case-control study of individuals with treatment refractory depression, 21 had metabolite abnormalities. Results were determined based on established clinical norms for all tests reported.
Twelve patients had CFD, including ten with reductions in BDI and Suicidal Ideation Questionnaire scores following treatment with folinic acid, one who was non-compliant with folinic acid treatment, and one who was lost to follow-up. Half of the eight patients who completed follow-up and did not have a co-morbid identified metabolic abnormality had a 50% reduction in BDI score. In all cases patients had normal serum metabolites associated with folate pathways, and therefore would have eluded conventional diagnostic approaches without a more comprehensive evaluation.
It should be noted that the abnormalities identified may either be due to a primary genetic disorder in a metabolic pathway or a secondary abnormality of indirect etiology (e.g. acquired injuries to the CNS from infections, autoimmune disease, asphyxia, or epilepsy) typically with other obvious systemic manifestations.19 The abnormalities identified could also be sequelae of unremitting depression. CFD is a CNS specific syndrome characterized by low 5-MTHF in CSF and normal folate in plasma.20–21 It can be caused primarily by mutations in the cerebral folate receptor (FOLR1), but can be secondarily reduced in any metabolic defect that increases use of methyl groups, or by changes in transport across the blood brain barrier. FOLR1 gene sequencing was normal in the first 8 patients with identified CFD. Secondary CFD is also seen in disorders of the mitochondrial respiratory chain such as Alpers syndrome,22 Kearnes-Sayre syndrome,23 and others. 24 Folate is involved in nearly 100 metabolic reactions,25 including the purine synthetic pathway, contributing to impaired tetrahydrobiopterin, serotonin, norepinephrine, and dopamine synthesis, and thus polygenic effects due to the accumulation of mutations in multiple genes may play a role in this population.26–27
The findings in this patient population are different than reports of empirical treatment with L-methylfolate. 28–31 Adjunctive treatment with L-methylfolate may improve depressive symptoms because of its role as a cofactor in monoamine metabolism.32 This is different from our findings in cerebral folate deficiency.19 Also, L-methylfolate is most helpful in those with the MTHFR polymorphism, which was negative in the 8 patients with CFD for whom we have completed gene testing. We continue to work to understand the biologic mechanism of our biochemical findings. It is clear that treatment with L-methylfolate is not sufficient in these individuals, because of a deficit earlier in the folate metabolic pathway that produces bioactive metabolites. Furthermore, the administration of L-methylfolate will falsely correct the CSF test to identify these individuals. While empirical treatment with folinic acid may seem appealing, this again is problematic with our current understanding. After folinic acid administration, definitive testing cannot be completed, and the full effects of treatment with folinic acid in an individual with CFD occur over years. This time frame is due to neuronal turnover and growth in the setting of availability of active folate metabolites needed for appropriate white matter structure. We cannot determine if the metabolite abnormalities are primary or secondary. However, our patients had no other overt clinical disease and thus secondary deficiencies related to other systemic conditions are unlikely. Applications of next generation sequencing technologies (i.e., whole exome or genome sequencing) on a focused, homogeneous patient population with treatment refractory depression will provide further insight into genetic components of the metabolic findings in our patients. After further evaluation and characterization of a larger population of patients, animal models of biochemical abnormalities will be an important next step.
Though the majority of patients in this study were recruited by advertisement through a research registry, 4 participants were clinically referred, and the possibility of an ascertainment bias cannot be excluded. This was a relatively small sample of mostly Caucasian patients, and the treatment component of the study was an open trial. Continued treatment as usual resulted in medication changes during the course of the study, meaning that in 3 cases of CFD, there were concomitant medication changes in addition to folinic acid supplementation. Preliminary experience with treatment has been promising; however, long term outcomes remain to be determined.
CSF testing is required to determine the presence of cerebral folate deficiency and subsequent need for treatment. Review of the CSF metabolic profile directs the diagnosis, and avoids missing other metabolic disorders that may be present. 5-L-methyltetrahydrofolate is the treatment specific to methylenetetrahydrofolate deficiency33, but in the majority of cases, folinic acid is preferable because of its superior stability and lower costs when compared to 5-L-methyltetrahydrofolate. Dihydrofolate reductase converts folic acid to dihydrofolate and then to tetrahydrofolate (Figure 1). Folinic acid (5-formyl-tetrahydrofolate) is converted to 5-,10- methenyltetrahydrofolate, then 5-,10- methylenetetrahydrofolate, and, eventually, to 5-methyltetrahydrofolate. Folinic acid, therefore, allows the availability of active metabolites in the folate pathway in individuals with cerebral folate deficiency that is not caused by methylenetetrahydrofolate deficiency. Furthermore, folinic acid has therapeutic effect in the setting of folate receptor autoantibodies, though there is controversy surrounding the assessment of these autoantibodies. 34
Folinic acid should be given by prescription. Doses of 1–2 mg/kg may be administered with prescription strength folinic acid. Treatment with either of these folate forms will preclude testing after administration because they correct deficient CSF 5MTHF levels. A clinical response should be expected in patients with treatment refractory depression and low CSF 5MTHF. Experience with more severe neurologic disorder associated with CFD35 indicates that the response may take several months but the full effect of treatment may take 1–3 years. This may be related to neuronal growth and turnover in an environment where folate metabolites are newly available. Folic acid is not recommended because of the potentially low activity of dihydrofolate reductase in cerebral folate deficiency, and because of its tight binding to folate receptor alpha that results in reduced cerebral transport of 5-methyltetrahydrofolate. Folinic acid treatment in our patients did have some side effects. It is important to note that vitamin B12 acts as a cofactor in this pathway. Blood folate and B12 levels should be evaluated in all patients with treatment refractory depression. The best option for patients with treatment refractory depression for whom metabolic disorder is suspected is consultation with a biochemical geneticist.
Our findings indicate a need for further evaluation of the role of neurometabolic abnormalities in treatment refractory depression. The remarkably high incidence of actionable abnormalities, and some evidence of symptom improvement with treatment strongly support the need for larger studies. Historically, a diagnosis of a metabolic disorder has been considered in the context of a family history of a known disorder, or symptoms that are exacerbated by significant physiologic stresses (e.g. fever, fasting, surgeries), especially with multi-system involvement,36 neither of which were present in our patient population. Our findings, if replicated suggest that, neurometabolic disorders may contribute to treatment-refractory psychiatric disorder even without other systemic illness. An important future question is whether early Identification and treatment of an underlying metabolic abnormality early in the course of psychiatric illness could prevent long-term emotional and cognitive complications. If these findings are replicated, they suggest that the identification of new inborn errors of metabolism or secondary disorders of metabolism contributing to psychiatric illness may allow repurposing of currently approved orphan drugs.
Supplementary Material
Figure 2.
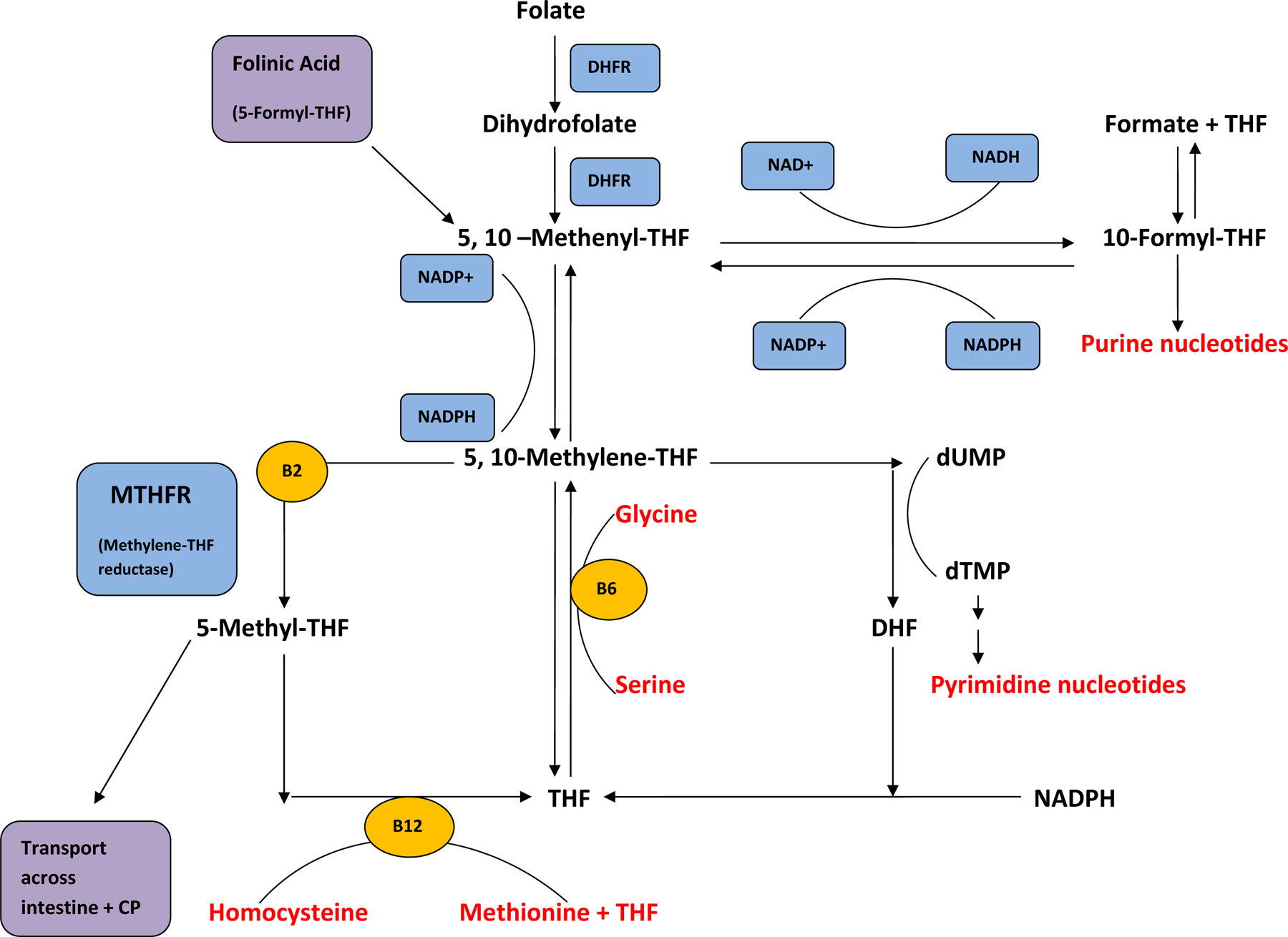
Summary of folate pathway. Folinic acid (5-formyl-THF) enters the pathway without the action of DHFR and contributes active metabolites. 5-Methyl-THF is a distal metabolite.
DHFR= dihydrofolate reductase, THF= tetrahydrofolate, DHF= dihydrofolate, dUMP= deoxy-uridine phosphate, dTMP= deoxy-thymidine phosphate, CP= choroid plexus, SAM= S-adenosylmethionine, NADP= nicotinamide adenine dinucleotide phosphate.
Acknowledgements
We thank the participants who made this research possible. We thank Katherine Wisner, M.D. and Vishwajit Nimgaonkar, M.D., Ph.D. for their review of this manuscript. All work was carried out within the Department of Psychiatry of the University of Pittsburgh. This pilot research was supported by the Brain and Behavior Research Foundation NARSAD Young Investigator Award, and The Clinical and Translational Research Institute of University of Pittsburgh NIH UL1 RR024153 and UL1 TR000005. Ongoing research is supported by the American Foundation for Suicide Prevention Standard Research Award, The Children’s Hospital of Pittsburgh Foundation, and Departmental funding supported by David Brent, M.D., Jerry Vockley, M.D., Ph.D, and David Lewis, M.D.
References
- 1.Souery D, Papkostas GI, Trivedi MH: Treatment resistant depression. J Clin Psychiatry 2006; 67(6): 16–22 [PubMed] [Google Scholar]
- 2.Anonymous: The burden of depression. Nature 2014; 515 (7526):163. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention: Center for Disease Control and Prevention Data and Statistical Fatal Injury Report, 2013
- 4.Asberg M, Traskman L, Thoren P: 5-HIAA in the cerebrospinal fluid: a biochemical suicide predictor? Arch Gen Psych 1976; 33(10):1193–7 [DOI] [PubMed] [Google Scholar]
- 5.Asberg M & Forslund K: Neurobiological aspects of suicidal behaviour. Int Rev Psychiatr 2000; 12(1): 62–74 [Google Scholar]
- 6.Axelrod J: Biogenic amines and their impact in psychiatry. Semin Psychiatry 1972; 4(3): 199– 210 [PubMed] [Google Scholar]
- 7.Anglin RE, Garside SL, Tarnopolsky MA, Mazurek MF, Rosebush PI: The psychiatric manifestations of mitochondrial disorders: a case and review of the literature. J Clin Psychiatry 2012; 73(4): 506–12 [DOI] [PubMed] [Google Scholar]
- 8.Morris G & Berk M: The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Medicine, 2015; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan L, McKain BW, Madan-Khetarpal S, Mcguire M, Diler RS, Perel JM, Vockley J, Brent DA: GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: relief of treatment-refractory depression and suicidal behaviour. BMJ Case Reports, 2011; (doi: 10.1136/bcr.03.2011.3927) [DOI] [PMC free article] [PubMed]
- 10.Hyland K, Shoffner J, Heales SJ: Cerebral folate deficiency. J Inherit Metab Dis 2010; 33(5):563–70 [DOI] [PubMed] [Google Scholar]
- 11.NIMH Genetics Initiative: Family Interview for Genetic Studies (FIGS) Rockville, MD, National Institute of Mental Health, 1992 [Google Scholar]
- 12.Fava M: Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 2003; 53(8): 649–59 [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961;4: 561–71 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds WM: Suicidal Ideation Questionnaire: Professional Manual, 1987. Odessa, FL: Psychological Assessment Resources [Google Scholar]
- 15.Poznanski EO & Mokros HB: Children’s depression rating scale, revised (CDRS-R) Los Angeles: Western Psychological Services, 1996 [Google Scholar]
- 16.Posner K, Oquendo MA, Gould M, Stanley B, Davies M: Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry; 2007; 164:1035–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck AT, Schuyler D, Herman I: Development of suicidal intent scales. In: Beck AT, Resnick HLP, Lettieri DJ, eds. The Prediction of Suicide Bowie, MD: Charles Press, 1974; 45–56 [Google Scholar]
- 18.American Psychiatric Association: DSM 5 Cross Cutting Symptom Inventory. In Diagnostic and Statistical Manual of Mental Disorders, 2013. (5th ed.) [Google Scholar]
- 19.Serrano M, Perez-Duenas B, Montoya J, Ormazabal A, Artuch R: Genetic causes of cerebral folate deficiency: clinical, biochemical, and therapeutic aspects. Drug Discov Today 2012; 17 (23–24):1299–1306 [DOI] [PubMed] [Google Scholar]
- 20.Pan L & Vockley J: Neuropsychiatric Symptoms in Inborn Errors of Metabolism: Incorporation of Genomic and Metabolomic Analysis into Therapeutics and Prevention. Curr Genet Med Rep 2013; 1(1): 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedel F: Cerebral Folate Deficiency. Presentation at Behavioural and Psychiatric Aspects of Inborn Errors of Metabolism, 2013, Orphan Europe Academy, Paris, France [Google Scholar]
- 22.Cohen BH, Chinnery PF, Copeland WC: POLG-related disorders. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews ® [Internet]. Seattle (WA): University of Washington, Seattle, 2010 [PubMed] [Google Scholar]
- 23.Quijada-Fraile P, O’Callaghan M, Martín-Hernández E, Montero R, Garcia-Cazorla À, de Aragón AM, Muchart J, Málaga I, Pardo R, García-Gonzalez P, Jou C, Montoya J, Emperador S, Ruiz-Pesini E, Arenas J, Martin MA, Ormazabal A, Pineda M, García-Silva MT, Artuch R: Follow-up of folinic acid supplementation for patients with cerebral folate deficiency and Kearns-Sayre Syndrome. Orphanet J Rare Dis 2014; 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Cazorla A, Quadros EV, Nascimento A, Garcia-Silva MT, Briones P, Montoya J, Ormazábal A, Artuch R, Sequeira JM, Blau N, Arenas J, Pineda M, Ramaekers VT : Mitochondrial diseases associated with cerebral folate deficiency. Neurology 2008; 70(16):1360–2 [DOI] [PubMed] [Google Scholar]
- 25.Bottiglieri T, Hyland K, Laundy M, Godfrey P, Carney MW, Toone BK, Reynolds EH: Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med-London 1992; 22: 871. [DOI] [PubMed] [Google Scholar]
- 26.Vockley J, Rinaldo P, Bennett MJ, Matern D Vladutiu GD : Synergisitic heterozygosity: disease resulting from multiple partial defects in one or more metabolic pathways. Mol Genet Metab 2000; 71 (1–2):10–8 [DOI] [PubMed] [Google Scholar]
- 27.Trefz F, Maillot F, Motzfeldt K, Schwarz M: Adult phenylketonuria outcome and management. Mol Genet Metab 2011; 104: S26–S30 [DOI] [PubMed] [Google Scholar]
- 28.Fava M & Mischoulon D: Folate in depression: efficacy, safety, differences in formulations, and clinical issues. The Journal of Clinical Psychiatry 2009; 70(5): 12–7 [DOI] [PubMed] [Google Scholar]
- 29.Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M: L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. The American Journal of Psychiatry 2012; 169(12), 1267–74 [DOI] [PubMed] [Google Scholar]
- 30.Jain R & Jackson WC: Beyond the resistance: how novel neurobiological understandings of depression may lead to advanced treatment strategies. The Journal of Clinical Psychiatry 2012; 73(11): e30. [DOI] [PubMed] [Google Scholar]
- 31.Papakostas GI, Shelton RC, Zajecka JM, Bottiglieri T, Roffman J, Cassiello C, Stahl SM, Fava M: Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. The Journal of clinical psychiatry, 2014; 75(8): 855–63 [DOI] [PubMed] [Google Scholar]
- 32.Ramaekers VT: Cerebral folate deficiency. Developmental Medicine and Child Neurology 2004; 46:843–51 [DOI] [PubMed] [Google Scholar]
- 33.Knowles L, Morris AA, Walter JH: Treatment with Mefolinate (5-Methyltetrahydrofolate), but Not Folic Acid or Folinic Acid, Leads to Measurable 5-Methyltetrahydrofolate in Cerebrospinal Fluid in Methylenetetrahydrofolate Reductase Deficiency. JIMD Rep 2016; Feb 23. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 34.Ramaekers VT, Thöny B, Sequeira JM, Ansseau M, Philippe P, Boemer F, Bours V, Quadros EV: Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol Genet Metab 2014. Dec;113(4):307–14 [DOI] [PubMed] [Google Scholar]
- 35.Molero-Luis M, Serrano M, O’Callaghan MM, Sierra C, Pérez-Dueñas B, García-Cazorla A, Artuch R. Clinical, etiological and therapeutic aspects of cerebral folate deficiency. Expert Rev Neurother 2015;15(7):793–802 [DOI] [PubMed] [Google Scholar]
- 36.Walterfang W, Bonnot O, Mocellin R: The neuropsychiatry of inborn errors of metabolism. J Inherit Metab Dis 2013; 36: 687–702 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


