Abstract
The little fire ant (LFA), Wasmannia auropunctata, is a serious invasive pest first reported on Hawaii Island in 1999, and has since spread and established itself across the island. LFA is considered one of the worst 100 invasive species and has significant ecological, agricultural, and public health impacts in invaded areas, which include much of the tropical New World. Although localized eradication efforts have proven successful, they are intensive and difficult to implement. Furthermore, LFA’s high invasive-ability resists these control efforts in areas where the species is established and can re-infest treated areas. This research set out to determine whether LFA queens have a suppressant effect on new queen production in nests, as a first step in identifying a potential queen pheromone for LFA. A queen pheromone could offer a means to shutdown LFA reproductive capability, potentially by suppressing the production of new queens or inducing the execution of queens or queen-destined larvae. When queenless experimental nests and polygyne experimental nests were compared, six out of eight queenless nests successfully reared both new alate queens (2.25 queens/nest) and drones (3.63 drones/nest) to adulthood, whereas only three of eight polygyne nests reared sexual larvae that failed to develop to adulthood or even the pupal stage. These results suggest that dealate mature LFA queens suppress the production of new alate queens in LFA nests, and is the first evidence that LFA may utilize a queen pheromone.
Keywords: Little fire ant, Tramp ant species, Invasive species, Queen pheromone
Introduction
The little fire ant (LFA), Wasmannia auropunctata (Roger) (Formicidae: Myrmicinae), is a cosmopolitan tramp ant species with pantropical distribution and is considered one of the 100 worst invasive species by the International Union for the Conservation of Nature (Lowe et al. 2000). LFA occurs throughout the warmer parts of the New World from northern Argentina to southern Mexico and throughout the Caribbean. It has steadily spread into the Galapagos Islands, West Africa, Australia, Melanesia, Polynesia, Florida, and the western US, including several islands of Hawaii, Guam, and possibly California (Wetterer and Porter 2003). The high invasive potential of LFA is evidenced by the fact that a single queen transported from Florida was sufficient to allow the species to spread across the island of Hawaii (Mikheyev et al. 2009; Foucaud et al. 2010). The impacts of LFA on agriculture and public health are severe (Wetterer and Porter 2003; Motoki et al. 2013).
Queen signaling wherein a fecund queen advertises her presence is ubiquitous among eusocial organisms, having widespread documentation throughout the various species of Hymenoptera (Van Oystaeyen et al. 2014; Holman 2018), in termites (Matsuura et al. 2010; Funero et al. 2018), and even in the eusocial mammal, the naked mole-rat, Heterocephalus glaber Rüppell (Clarke and Faulkes 1997). This signaling can be accomplished via chemical signals (Van Oystaeyen et al. 2014; Holman 2018) and/or overt physical dominance, with the latter often at play in cases of smaller-nest size and less derived species, such as those in the formicid subfamily Ponerinae (Monnin et al. 1998; Liebig et al. 1999). While these signals may operate differently to meet the needs of a given species’ biology, a number of parallel mechanisms achieve the common purpose of repressing reproduction by intraspecific individuals. Specifically, queen signals have been shown to: inhibit ovarian development in subordinate queens (Fletcher and Blum 1981) or gamergates (= reproductive workers) (Holman et al. 2010b); inhibit rearing of larvae into gynes via execution by workers (Vargo and Fletcher 1986; Vargo and Passera 1991; Oliveira et al. 2020); induce worker execution of non-dominant queens or gamergates that lack these signals (Fletcher and Blum 1981; Edwards 1991; Smith et al. 2009; Holman et al. 2010a; Smith et al. 2012). Queen signals also promote worker attendance of dominant queens or gamergates (Hannonen et al. 2002; Smith et al. 2012; Oliveira et al. 2020), and are often honest signals of fecundity, health, and hierarchical status of queens or gamergates (Keller et al. 1993; Hannonen et al. 2002; Holman et al. 2010a, b). The honesty of these signals is essential for the sustainability of the eusocial system.
Chemical queen signaling (i.e. queen pheromones) has been indicated for a number of species, including the polygynous and unicolonial red imported fire ant (Fletcher and Blum 1981) and Argentine ant (Vargo and Passera 1991), ~ 40 other ant species, ~ 20 other hymenopteran species, and five species of termites (reviewed in Van Oystaeyen et al. 2014). Conclusive structural identification of the pheromones involved has been completed for eight species of hymenopteran (reviewed in Van Oystaeyen et al. 2014) and two species of termite (Matsuura et al. 2010; Funero et al. 2018).
Suppression of new queen production by mature queens is a common consequence of queen signaling (Vargo and Fletcher 1986; Vargo and Passera 1991; Oliveira et al. 2020), and to our knowledge, neither queen suppression nor queen signaling has been investigated in LFA. Manipulation of suppression of new queen production via queen pheromones could offer potential behavioral and physiological control options for this species, by incapacitating their reproductive output if exposed to the queen pheromone. Thus, the purpose of this study was to demonstrate if the presence of mature dealate LFA queens within an artificial nest suppresses the rearing of new alate queens from the brood as a first step in evidencing and identifying queen signaling in LFA.
Methods
Ants
Little fire ants were collected from two locations: Pacific Basin Agricultural Research Center (PBARC), Hilo HI, and a fallow macadamia nut (Macadamia spp.) orchard in Papaikou (19.787029, − 155.124443), just north of Hilo. Ants were manually collected from ‘ohi’a (Metrosideros polymorpha) log piles at the former site, and from epiphytic moss on macadamia nut trees at the latter one; ants were separated from the moss manually, or by slow-dripping water into a bucket and skimming the ants off the top of the water. Ants were maintained in 1.2 L plastic bins (40 × 26 × 12 cm), with queens introduced into shell vials (4 × 0.6 cm cylindrical vials without the choke-point of regular capped vials; 1-NWV-C, Thermo-Fisher Scientific) with the vial caps having a hole cut into them large enough for workers to easily enter and exit the vial. Due to the unicolonial and clonal nature of invasive LFA populations (i.e. ants from different ephemeral nests are non-aggressive and act as one large colony) (Le Breton et al. 2004; Mikheyev et al. 2009; Foucaud et al. 2010), these collections of ants were aggregated into ‘source colonies’ based on the day the ants were collected; we utilized three separate field collections for the experimental nests (Table 1). The aggregated ants from a given collection day were provided with a 3 cm2 dollop of peanut butter (roasted peanut butter, sugar, palm oil, salt; Skippy Natural Peanut Butter, Hormel Foods, Austin MN USA) weekly, approximately 50 freeze-killed adult oriental fly (Bactrocera dorsalis (Hendel)) twice per week, and 25% sucrose water (by volume) and regular water (both DI-grade) ad libitum.
Table 1.
Experimental nests of queenless (QL) and polygyne (P) LFA, with the source colonies of ants used to put the nests together, total number of observations of the nest before being culled, and the total number of observations of sexual larvae and pupae (these numbers do not represent total numbers of sexual larvae and pupae, just total times either were counted across the observation periods)
| Exp. nest | trt | Source colony | Number of exp. nest observations | Total # of observations of sexual larvae | Total # of observations of sexual pupae |
|---|---|---|---|---|---|
| 1 | QL | A | 22 | 17 | 0 |
| 2 | P | A | 22 | 11 | 0 |
| 3 | QL | A | 22 | 35 | 4 |
| 4 | P | A | 22 | 2 | 0 |
| 5 | QL | B | 22 | 145 | 49 |
| 6 | P | B | 22 | 0 | 0 |
| 7 | QL | B | 21 | 152 | 31 |
| 8 | P | B | 21 | 27 | 0 |
| 9 | QL | C | 16 | 10 | 14 |
| 10 | P | C | 16 | 0 | 0 |
| 11 | QL | C | 16 | 24 | 17 |
| 12 | P | C | 16 | 0 | 0 |
| 13 | QL | C | 16 | 15 | 16 |
| 14 | P | C | 16 | 0 | 0 |
| 15 | QL | C | 16 | 12 | 10 |
| 16 | P | C | 16 | 0 | 0 |
Queenless vs. polygyne nest bioassay
There were two treatments: queenless nests (QL) that contained brood + workers only, and polygyne nests (P) that contained 20 mature dealate queens + brood + workers. The number of queens was based on field observations by RMC of LFA aggregates containing up to 24 dealate queens. Each treatment was tested using experimental nest formicaria and replicated eight times.
Experimental nest formicaria (Fig. 1) consisted of small plastic containers (14 × 14 × 5 cm) with the interior sides coated with polytetrafluoroethylene (Fluon; BioQuip, Rancho Dominguez CA). Queens and/or brood were placed into shell vials with the vial caps having a hole cut into them large enough for workers to easily enter and exit the vial (‘nest vial’; 4 × 0.6 cm), and with 100 workers from the same source colony, and another 300 workers from the same source colony placed into the experimental nest formicaria. As much young brood (eggs through second instar larvae) as available (estimated 250–500 brood) was placed directly into the nest vials, with QL and P experimental nests put together on a given day having close to equal amounts of brood. Because it took time for the source colonies to have enough brood for the assembly of the experimental nests, the experimental nests were assembled on a rolling basis, always with an equal number of QL and P nests being assembled on a given day.
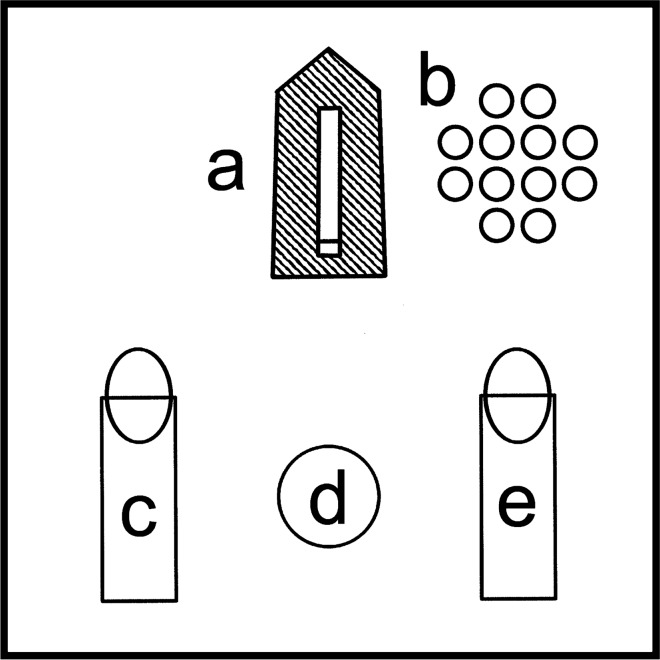
Fig. 1.
Experimental nest and formicarium schema. a Nest vial containing brood and queens, with workers able to enter and exit via the vial cap with hole in it at base of the vial, shaded area represents opaque black tent positioned over the nest vial; b placement of freeze-killed oriental fruit flies; c vial of 25% surcose water by volume with cotton wick at top; d 2 cm2 dollop of peanut butter; e vial of water with cotton wick at top
The experimental nest formicaria (Fig. 1) were fed, watered, and observed every 3–4 days: nests were provided with five female and five male freeze-killed adult oriental fruit flies, and a 2 cm2 dollop of peanut butter (same as above), and all old fly carcasses and peanut butter were removed; 25% sucrose water and regular water (both DI-grade) were provided ad libitum from 4 ml glass vials with cotton wicks. The shell vial brood nest was covered with an opaque piece of black construction paper to make it more appealing to the ants. All vials were sticky-tacked to the floor of the formicarium to reduce disruptions to the nests. The plastic containers had four 3 cm2 holes cut into the lid and covered with fine mesh to increase ventilation of the formicaria.
All source colonies and experimental nests were maintained in an environmental chamber set to 12:12 light:dark cycle, 82.5 ± 2.5% relative humidity, and 28 ± 0.5°C.
At each maintenance and observation session (every 3–4 days), total numbers of visually-identifiable sexual larvae, sexual pupae, and alate queens and drones were counted. The experimental nests were maintained and observed until all the brood from the QL nests were raised to maturity, and then all QL and P nests that were put together on the same day were culled at the same time; experimental nests went for 8–11 wks based on this criteria. We based culling of experimental nests off the QL nests because P nests had constant recruitment of new brood due to the live queens continuing to produce brood.
Data for the numbers of alate queens and drones reared to adulthood were analyzed in R 3.6.0 (R Core Team 2019). The data were heterogenous for variance based on Bartlett’s Test and thus analyzed via Kruskal Wallis Test (α = 0.05).
Results
The presence of mature dealate LFA queens significantly suppressed the production of new alate queens in experimental nests, with queenless nests producing significantly greater numbers of alate queens (P = 0.004) and drones (P = 0.01) than polygyne nests. A total of 18 alate queens were reared to adulthood in six of the eight queenless nests (mean ± SE: 2.25 ± 0.56 alate queens per nests), and no alate queens were reared in the polygyne nests (Table 2). A total of 29 drones were reared to adulthood in five of the eight queenless nests (mean ± SE: 3.63 ± 2.13 drones per nests), and no drones were reared in polygyne nests (Table 2).
Table 2.
Number of alate queens and drones (mean ± SE) reared from brood in queenless and polygyne LFA experimental nest formicaria
| Queenless nests | Polygyne nests | p value | |
|---|---|---|---|
| Alate queens | 18 (2.25 ± 0.56) | 0 (0 ± 0) | 0.004 |
| Drones | 29 (3.63 ± 2.13) | 0 (0 ± 0) | 0.01 |
While the polygyne nests did not rear any sexual larvae to the pupal or adult stage, three of the eight polygyne nests did begin to rear sexual larvae, about three to six weeks following the first appearance of sexual larvae in queenless nests, but none of these larvae made it to the pupal stage, as no sexual pupae were ever observed in polygyne nests. The sexual larvae were easily visually-identifiable once they had grown larger than the worker larvae; at full size, the sexual larvae are about 3–4 mm in length compared to worker larvae that are about 1 mm long. It took 20–30 days from when a QL experimental nest first had observable sexual larvae to when it had alate queens or drones; this does not include the developmental time of when the sexual larvae were indistinguishable by size from worker larvae.
The failed sexual larvae in polygyne nests, and occasionally in queenless nests (data not available), were found removed from the nest vial in the formicarium, neglected and typically shriveled and darkened brown or black, as opposed to the more translucent white turgidity of presumably healthy sexual larvae that remained in the nest vial and were reared to the pupal stage in queenless nests.
Discussion
The results of this study show that the presence of mature dealate queens suppresses new queen production in LFA nests. While this result was expected based on the many examples of queen signaling in other ant and eusocial species (Van Oystaeyen et al. 2014; Holman 2018), this is the first evidence of this phenomenon in this species.
At each observation session for the experimental nests, we would count the total number of sexual larvae in each experimental nest, but due to the difficulty in tracking individual sexual larvae from observation session to observation session, we were unable to quantify the actual number of sexual larvae produced in experimental nests over the course of the experiment. However, our records of total instances of sexual larvae observed at each twice-weekly maintenance and observational session indicate a large difference in the number of sexual larvae produced between the two experimental groups: 410 sexual larvae were observed in queenless nests, and only 40 observed in polygyne nests (Table 1). While these numbers represent observed instances from observational period to observational period, and thus many of these sexual larvae were likely counted multiple times and the actual number of sexual larvae produced would be much lower, the large difference between the two treatment groups does indicate that queenless nests were producing much greater numbers of sexual larvae.
The exact mechanisms underlying the suppression of sexual larval or pupal emergence are still unclear in LFA, although studies from other insect systems suggest that it is likely chemically mediated (reviewed in Van Oystaeyen et al. 2014). Due to the difficulty in tracking individual larvae and to what extent new recruitment of sexual larvae was occurring from unsexualized larvae (or larvae too small to be identified as sexual), we were unable to provide insight into the exact mechanisms of suppression of new queen production in LFA with the reported experiment. Our results suggest that there are multiple stages at which suppression can occur: firstly, during the early-to-mid larval stage, when we observed less sexual larvae in polygyne nests than queenless nests (only three out of eight polgyne nests produced visually-identifiable sexual larvae versus six out of eight queenless nests; also, 40 observed instances of visually-identifiable sexual larvae in polygyne nests versus 410 in queenless nests, a 10 × difference); and secondly, during the late larval stage, when we observed sexual larvae in polygyne nests culled and removed from the nest vial before they could reach the pupal stage. Another open question is when sexualization occurs in larvae, or if sexual potency is determined by genetics when the egg is laid.
Chemical analyses of the cuticular hydrocarbon (CHC) extracts of mature queens, immature alate queens, workers, and brood did reveal a straight-chain diene compound specific to mature queens, but due to issues working during the COVID-19 pandemic, we were unable to successfully test these extracts or this compound for biological activity. Future efforts should focus on testing the biological activity of CHC extracts of LFA.
This study is the first to demonstrate suppression of new queen production by LFA mature queens and is the first step in demonstrating and identifying a potential queen pheromone for this species. Future studies need to confirm that the queen signal is transmitted chemically by testing mature queen versus worker cuticular extracts, and isolating, identifying, and testing the candidate queen pheromone compound(s) for LFA.
Acknowledgements
We thank Michelle Montgomery of the Hawaii Ant Lab for consultation regarding little fire ant rearing and biology, and Lori Carvalho, Thomas Fezza, and Dominick Skabeikis of USDA-PBARC for logistical support. We also thank the anonymous reviewers of the article for their thoughtful and constructive reviews and comments. We gratefully acknowledge the financial support for this research provided by the USDA-AFRI-NIFA Postdoctoral Fellowship Program to RMC (Award No. 2019-67012-29629, Ascension No. 1019294). Opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA. USDA is an equal opportunity provider and employer.
Data availability
Apologies, we do not have a repository available for the generated data.
Declarations
Conflict of interest
Funding for this research was provided by a USDA-AFRI-NIFA Postdoctoral Fellowship Program to RMC (Award No. 2019-67012-29629, Ascension No. 1019294). The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole–rat, Heterocephalus glaber. Proc Roy Soc B. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP. Caste regulation in the pharaoh’s ant Monomorium pharaonis: recognition and cannibalism of sexual brood by workers. Physiol Entomol. 1991;16:263–271. doi: 10.1111/j.1365-3032.1991.tb00565.x. [DOI] [Google Scholar]
- Fletcher DJ, Blum MS. Pheromonal control of dealation and oogenesis in virgin queen fire ants. Science. 1981;212:73–75. doi: 10.1126/science.212.4490.73. [DOI] [PubMed] [Google Scholar]
- Foucaud J, Estoup A, Loiseau A, Rey O, Orivel J. Thelytokous parthenogenesis, male clonality and genetic caste determination in the little fire ant: new evidence and insights from the lab. Heredity. 2010;105:205–212. doi: 10.1038/hdy.2009.169. [DOI] [PubMed] [Google Scholar]
- Funero CF, Böröczky K, Vargo EL, Schal C. Identification of a queen and king recognition pheromone in the subterranean termite Reticulitermes flavipes. Proc Natl Acad Sci USA. 2018;115:3888–3893. doi: 10.1073/pnas.1721419115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannonen M, Sledge MF, Turillazzi S, Sundström L. Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim Behav. 2002;64:477–485. doi: 10.1006/anbe.2002.4001. [DOI] [Google Scholar]
- Holman L, Dreier S, d'Ettorre P. Selfish strategies and honest signalling: reproductive conflicts in ant queen associations. Proc Roy Soc B. 2010;277:2007–2015. doi: 10.1098/rspb.2009.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman L, Jørgensen CG, Nielsen J, d'Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc Roy Soc B. 2010;277:3793–3800. doi: 10.1098/rspb.2010.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman L. Queen pheromones and reproductive division of labor: a meta-analysis. Behav Ecol. 2018;29:1199–1209. doi: 10.1093/beheco/ary023. [DOI] [Google Scholar]
- Keller L, Nonacs P. The role of queen pheromones in social insects: queen control or queen signal? Anim Behav. 1993;45:787–794. doi: 10.1006/anbe.1993.1092. [DOI] [Google Scholar]
- Le Breton J, Delabie JHC, Chazeau J, Dejean A, Jourdan H. Experimental evidence of large-scale unicoloniality in the tramp ant Wasmannia auropunctata (Roger) J Insect Behav. 2004;17:263–271. doi: 10.1023/B:JOIR.0000028575.28700.71. [DOI] [Google Scholar]
- Liebig J, Peeters C, Hölldobler Worker policing limits the number of reproductives in a ponerine ant. Proc R Soc London. 1999;266:1865–1870. doi: 10.1098/rspb.1999.0858. [DOI] [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world's worst invasive alien species: a selection from the global invasive species database. Auckland: Invasive Species Specialist Group; 2000. [Google Scholar]
- Matsuura K, Himuro C, Yokoi T, Yamamoto Y, Vargo EL, Keller L. Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA. 2010;107:12963–12968. doi: 10.1073/pnas.1004675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheyev AS, Bresson S, Conant P. Single-queen introductions characterize regional and local invasions by the facultatively clonal little fire ant Wasmannia auropunctata. Mol Ecol. 2009;18:2937–2944. doi: 10.1111/j.1365-294X.2009.04213.x. [DOI] [PubMed] [Google Scholar]
- Monnin T, Maloose C, Peeters C. Solid-phase microextraction and cuticular hydrocarbon differences related to the reproductive activity in queenless ant Dinoponera quadriceps. J Chem Ecol. 1998;24:473–490. doi: 10.1023/A:1022360718870. [DOI] [Google Scholar]
- Motoki M, Lee DJ, Vanderwoude C, Nakamoto ST, Leung P. A bioeconomic model of Little Fire Ant Wasmannia auropunctata in Hawaii. Hawaii: University of Hawaii at Manoa; 2013. [Google Scholar]
- Oliveira RC, Warson J, Sillam-Dussès D, Herrera-Malaver B, Verstrepen K, Millar JG, Wenseleers T. Identification of a queen pheromone mediating the rearing of adult sexuals in the pharaoh ant Monomorium pharaonis. Biol Lett. 2020;16:20200348. doi: 10.1098/rsbl.2020.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019) R: A language and environment for statistical computing. R Foun- dation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Smith AA, Hölldober B, Liebig J. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol. 2009;19:78–81. doi: 10.1016/j.cub.2008.11.059. [DOI] [PubMed] [Google Scholar]
- Smith AA, Millar JG, Hanks LM, Suarez AV. Experimental evidence that workers recognize reproductives through cuticular hydrocarbons in the ant Odontomachus brunneus. Behav Ecol Sociobiol. 2012;66:1267–1276. doi: 10.1007/s00265-012-1380-x. [DOI] [Google Scholar]
- Smith AA, Liebig J. The evolution of cuticular fertility signals in eusocial insects. Curr Opin Insect Sci. 2017;22:79–84. doi: 10.1016/j.cois.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS, Romero C, Oi CA, d'Ettorre P, Khalesi M, Billen J, Wäckers F, Millar JG. Conserved class of queen pheromones stops social insect workers from reproducing. Science. 2014;343:287–290. doi: 10.1126/science.1244899. [DOI] [PubMed] [Google Scholar]
- Vargo EL, Fletcher DJ. Evidence of pheromonal queen control over the production of male and female sexuals in the fire ant, Solenopsis invicta. J Comp Physiol A. 1986;159:741–749. doi: 10.1007/BF00603727. [DOI] [Google Scholar]
- Vargo EL, Passera L. Pheromonal and behavioral queen control over the production of gynes in the Argentine ant Iridomyrmex humilis (Mayr) Behav Ecol Sociobiol. 1991;28:161–169. doi: 10.1007/BF00172167. [DOI] [Google Scholar]
- Wetterer JK, Porter SD. The little fire ant, Wasmannia auropunctata, distribution, impact, and control. Sociobiology. 2003;41:1–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Apologies, we do not have a repository available for the generated data.