Abstract
A possible role for the γ subunit of immunoglobulin Fc receptors (FcR) in mucosal defenses against intestinal nematode parasites was studied using age-matched FcRγ-knockout (FcRγ−/−) and wild-type (FcRγ+/+) C57BL/6 mice. Mice were infected subcutaneously with 3,000 infective larvae of Strongyloides venezuelensis, and the degree of infection was monitored by daily fecal egg counts and adult worm recovery on days 8 and 13 postinfection. Mucosal mast cell (MMC) responses were assayed by in situ intestinal mast cell counts in stained histological sections of the jejunum and by measuring mouse mast cell protease 1 (MMCP-1) release in serum using sandwich enzyme-linked immunosorbent assay. FcRγ−/− mice had significantly higher egg counts (P < 0.01) and numbers of adult worms (P < 0.05) than FcRγ+/+ mice, but mastocytosis and serum MMCP-1 release were comparable. It was concluded that MMCP-1 release may be spontaneous, does not depend on mast cell degranulation via the FcRγ signaling system, and appears to play no role in the expulsion of S. venezuelensis. The delay in worm expulsion in the FcRγ−/− mice might be related to inability of the MMC to degranulate and release effector molecules other than MMCP-1, since FcRγ deletion abrogates mast cell degranulative responses.
Fc receptors (FcR) are hetero-oligomeric complexes present on most effector cells of the immune system and, upon cross-linking by their ligand (antigen-antibody complex), mediate phagocytosis, antibody-dependent cell-mediated cytotoxicity, activation of inflammatory cells, and many other effector responses (20). However, several of the FcR require for cell surface assemblage and signal transduction into the interior of the cell an additional chain, the homodimeric γ subunit (20). Targeted disruption of this subunit results in pleiotropic defects in cell functions, including the loss of immunoglobulin E (IgE)-mediated mast cell degranulation (27). This is because the high-affinity FcR for IgE (FcɛRI), which is also associated with host resistance to parasitic infections (12), requires the γ subunit to express receptor-mediated cellular functions (20). Intestinal mucosal mastocytosis is observed in certain helminth infections, and it was therefore speculated that mast cells were important in the expulsion of Strongyloides ratti in rodents (19). Subsequently, in a series of experiments in infected rodents, it was demonstrated that mucosal mast cells (MMC) induced by the mast cell growth/differentiation factor interleukin 3 (IL-3) were the effector cells in the immune expulsion of Strongyloides spp. (1, 3, 8, 17, 18). The exact mechanism of the mast cell-mediated parasite expulsion is still not clear, although it has been suggested that granular contents released by activated mast cells may be the ultimate effector molecules (7, 17). Since Fc γ subunit deletion results in loss of mast cell function, including degranulation and granular content release (27), the aim of this study was to determine whether the MMC-mediated parasite expulsion mechanism actually involves MMC degranulative responses through the FcɛRI γ subunit signaling system. To do this, we infected FcRγ−/− and FcRγ+/+ mice with S. venezuelensis and indexed their immune protectiveness by fecal egg counts, by degree of adult parasite burden, and by intestinal mast cell counts and assay of mouse mast cell protease 1 (MMCP-1) release in serum. We report that FcRγ subunit deletion has no effect on MMC and MMCP-1 responses but results in significant increase in fecal egg output, worm burden, and delay in adult worm expulsion during primary infection.
MATERIALS AND METHODS
Animals.
C57BL/6 FcRγ−/− mice were produced in our laboratory and verified as previously described (27); age-matched specific-pathogen-free FcRγ+/+ mice were purchased from Japan SLC (Shizuoka, Japan). All animals were males between 8 and 10 weeks of age at the start of the experiment. Feed and water were supplied ad libitum. Male Wistar rats used for the maintenance and recovery of S. venezuelensis for experimental infections were purchased from Kyudo Co. (Kumamoto, Japan).
Parasite and parasitological techniques.
The strain of S. venezuelensis used is currently maintained in our laboratory but was originally isolated from a wild brown rat in Okinawa Prefecture, Japan, and later established as a laboratory strain (21). Stage 3 larvae (L3) were obtained by the filter paper fecal culture method (21), washed several times in phosphate-buffered saline (PBS), counted, and adjusted with fresh PBS to 15,000 L3/ml. Each mouse was infected subcutaneously (s.c.) with 3,000 L3 in 0.2 ml of PBS. The degree of infection was assessed by the level of the daily fecal egg counts (eggs per gram of feces [EPG]) and number of adult worms recovered from sacrificed animals on the days specified. The methods for fecal egg counts and adult worm recovery were as described elsewhere (10, 21).
Histology.
For the enumeration of mast cells, a ∼1.5-cm piece of the jejunum was taken from a distance 6 cm distal to the pylorus from each mouse and fixed in Carnoy's fluid. The samples were dehydrated, cleared in d-limonene (HemoDe; Fisher Scientific, Springfield, Calif.), and embedded in paraffin wax. Sections (4 μm thick) were cut and stained overnight with alcian blue (pH 0.3) and safranin O (pH 0.1). The number of mast cells were counted in 50 villus crypt units (VCU) and expressed as mast cell numbers per 10 VCU (14).
Serum MMCP-1 ELISA.
Serum MMCP-1 concentration was assayed using a commercial MMCP-1 enzyme-linked immunosorbent assay (ELISA) kit (MS-RM 3; Moredun Scientific, Ltd., Edinburgh, United Kingdom). Slight modification of the manufacturer's ELISA protocol was used. Briefly, plates were coated with 50 μl of polyclonal sheep anti-MMCP-1 capture antibody diluted to 2 μg/ml with 0.1 M carbonate buffer, incubated overnight at 4°C, and washed eight times with PBS-Tween 20. Single (1/3,000) and log (0.1, 0.3, 1, 3, and 10 ng/ml) dilutions of the test serum and MMCP-1 standard, respectively, were made using PBS-Tween 20 containing 4% bovine serum albumin, and the washed plates were loaded with 50 μl of each as appropriate. They were incubated at 37°C for 1 h, washed as before, loaded with 50 μl of rabbit anti-MMCP-1–horseradish peroxidase conjugate diluted 1/600 with PBS-Tween 20-bovine serum albumin, and incubated at 37°C for 1 h. Plates were washed and incubated at 37°C with 3,3′,5,5′-tetramethylbenzidine (TMB; 50 μl/well; DAKO TMB One-Step Substrate System; DAKO, Carpinteria, Calif.) for 15 min. The reaction was stopped with a mixture of equal volumes of 1 N HCl and 3 N H2SO4 (50 μl/well) and read at 450 nm. The amounts of MMCP-1 (nanograms per milliliter) in the test sera were calculated from the standard curve.
Experimental design.
The experiment was designed to monitor daily fecal egg output until final expulsion, adult worm load at peak of establishment, and mastocytosis at the start and peak periods. In S. venezuelensis infection in mice, peak worm establishment occurs at about day 8 whereas mastocytosis starts and peaks at days 8 and 12 postinfection (p.i.), respectively (8). Ten each of the FcRγ−/− and FcRγ+/+ mice kept in groups of five per cage were used for the primary infection. In each mouse type, daily EPG was based on counts obtained from the first group of five mice, which were also the last to be sacrificed on day 13. The others were sacrificed on day 8. All animals were killed by anesthetic overdose using ether.
Statistics.
Differences between groups were analyzed by Student's t test for unpaired samples, and differences at P ≤ 0.05 were considered significant.
RESULTS
Fecal egg output.
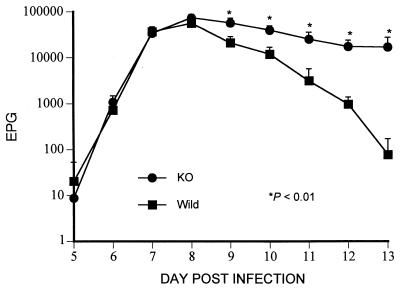
The daily EPG following the primary infection is presented in Fig. 1. EPG did not differ significantly (P > 0.05) between FcRγ−/− and FcRγ+/+ mice at the time of logarithmic rise in fecal egg count (days 5 to 8 p.i.), but during the time of logarithmic expulsion of adult worms from the intestine (days 9 to 13 p.i.), the daily EPG of FcRγ−/− mice were significantly higher than those of the FcRγ+/+ mice (P < 0.01). These results were reproduced in another experiment (data not presented) during which the FcRγ−/− mice continued to discharge eggs in the feces until treated with mebendazole on day 25 p.i.
FIG. 1.
Mean daily EPG ± standard deviation in FcRγ−/− (●) and FcRγ+/+ (■) mice following a primary infection by s.c. injection of 3,000 L3 of S. venezuelensis.
Adult worm burden.
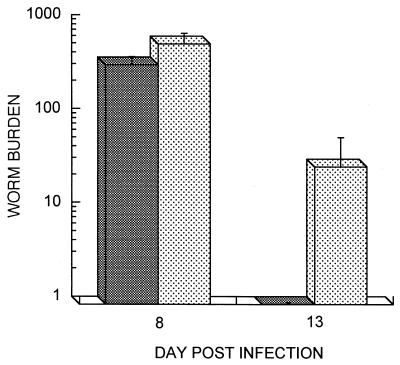
Adult worms were recovered from the small intestines of both groups on days 8 and 13 p.i. (Fig. 2). Significantly more adult worms were recovered from FcRγ−/− mice on day 8 p.i. (P < 0.05). Similarly, the number of adult worms recovered from the FcRγ−/− mice on day 13 p.i. was significantly higher (P < 0.05) than that in the FcRγ+/+ mice, among which only one adult worm was recovered from just one of the five mice in the group.
FIG. 2.
Mean numbers ± standard deviation of adult worms (worm burden) recovered from the small intestines of FcRγ−/− (▩) and FcRγ+/+ (░⃞) mice following a primary infection by s.c. injection of 3,000 L3 of S. venezuelensis.
Intestinal mastocytosis.
Examination of the stained jejunal sections showed that as expected, primary infection of FcRγ+/+ C57BL/6 mice with S. venezuelensis induced both hyperplasia and intraepithelial migration of mast cells. Expectedly, similar intense mastocytosis and intraepithelial migration of mast cells were also observed following primary infection of the FcRγ−/− mice with S. venezuelensis. In contrast, practically no mast cells were evident in stained jejunal sections of naive animals. In situ intestinal mast cell counts on days 8 and 13 p.i. in both groups of mice are presented in Table 1. The numbers of mast cells per 10 VCU were again expectedly similar in FcRγ−/− and FcRγ+/+ mice. While the numbers were low on day 8 p.i., similar significant increases in the FcRγ−/− and FcRγ+/+ mice (approximately five- and sixfold, respectively) were observed on day 13 p.i. (P < 0.001). On both days, however, the numbers of MMC were surprisingly higher in FcRγ−/− than FcRγ+/+ mice, although the difference was marginal and insignificant (P > 0.05).
TABLE 1.
Mean intestinal mast cell numbers in FcRγ−/− and FcRγ+/+ mice with primary S. venezuelensis infection
| Day p.i. | Mean no./10 VCU ± SD
|
P | |
|---|---|---|---|
| FcRγ−/− | FcRγ+/+ | ||
| 8 | 174 ± 45 | 130 ± 36 | 0.07 |
| 13 | 788 ± 74 | 786 ± 152 | 0.49 |
Serum MMCP-1 concentration.
The results of the ELISA for serum MMCP-1 concentration during the primary S. venezuelensis infection in the FcRγ−/− and FcRγ+/+ mice are presented in Table 2. As with mast cell numbers, the results were against our expectation, as comparable amounts of MMCP-1 were detected in the sera of both groups of mice on days 8 and 13 p.i.; as for the MMC number, there was marginally more MMCP-1 in FcRγ−/− than FcRγ+/+ mice, although the differences were again insignificant (P > 0.05).
TABLE 2.
Mean MMCP-1 concentrations in sera of FcRγ−/− and FcRγ+/+ mice with primary S. venezuelensis infection
| Day p.i. | Mean MMCP-1 ± SD (ng/ml)
|
P | |
|---|---|---|---|
| FcRγ−/− | FcRγ+/+ | ||
| 8 | 210 ± 230 | 200 ± 100 | 0.08 |
| 13 | 11,730 ± 3,009 | 9,528 ± 2,303 | 0.08 |
DISCUSSION
Mastocytosis is associated with the expulsion of Strongyloides spp. (8, 17). It was therefore surprising that worm expulsion was delayed in FcRγ−/− mice, although clearly FcRγ deletion did not interfere with mucosal mastocytosis following the primary S. venezuelensis infection. Thus, it is clear that mast cell hyperplasia per se is not solely responsible for worm expulsion and that the mast cell-mediated worm expulsion involves an effector mechanism which is affected by targeted disruption of the FcRγ subunit. The nature of the effector molecule in mast cell-mediated worm expulsion has been a matter for speculation. Ironically, a clue came from studies of hamsters infected with S. venezuelensis, in which the expulsion is associated with goblet cell hyperplasia and production of large quantities of mucins and not with mastocytosis (24, 26). It was shown that goblet cell mucins of four different species of hamsters were sulfated and that the degree of sulfation determined the rapidity of expulsion of adult S. venezuelensis from the hamsters (25). These studies suggest that it is possible for highly sulfated mucins to substitute for any mast cell-derived effector molecules in the expulsion of Strongyloides species from mice and rats. This possibility was confirmed in rats by reserpine treatments to induce sulfated intestinal goblet cell mucins, which showed that intraduodenally implanted S. venezuelensis adults were unable to establish in treated rats compared with the untreated controls (6). Preformed high-molecular-weight proteoglycans in mouse mast cell granules such as chondroitin and heparin are highly sulfated, and it was thus suggested that they might be the effector molecules in the prevention of the establishment and subsequent expulsion of adult Strongyloides (7). Furthermore, in treating mice with various carbohydrates including glycosaminoglycans of the type produced by mast cells, such as chondroitin sulfate A, chondroitin sulfate E, heparin, and dextran sulfate, it was demonstrated that these molecules actually mediate the expulsion of S. venezuelensis from mice and that this is achieved by their preventing the invasion of the intestinal mucosa by the adult parasite through the inhibition of binding of the adhesion molecules of the parasite to intestinal epithelial cells (11). Since Fc γ subunit deletion results in loss of mast cell function, including degranulation and granular contents release (27), it is possible that the defect in worm expulsion following primary S. venezuelensis infection in FcRγ−/− mice results from failure of the MMC to degranulate and release high-molecular-weight sulfated proteoglycans. This possibility is currently being investigated in our laboratory.
Systemic release of mast cell proteinases during primary and challenge nematode infections has been described in rodents and sheep and cited as evidence that MMC are active during nematode expulsion (4, 5, 13, 15, 22, 23, 29, 30). Our data on MMC and MMCP-1 release support the proposition that MMCP-1 is a correlate for mast cell activity. However, it was also suggested that the coincidence in rodents of the accumulation and secretion of the highly soluble β-chymases (MMC proteases) with the time of worm expulsion is strongly indicative of a major function for the proteases in the process (31). Similar levels of serum MMCP-1 release in FcRγ−/− and FcRγ+/+ mice in this study argue against this suggestion and indicate that MMCP-1 does not appear to be a reliable index of mast cell functionality in relation to MMC-mediated mucosal immunity against and expulsion of adult S. venezuelensis. It would therefore appear that other mast cell molecules, including preformed mediators such as proteoglycans, as discussed above, or newly synthesized mediators such as leukotrienes, which have been associated with the rapid expulsion of Trichinella spiralis from rats (16), may be more important than MMCP-1 as a measure of MMC function in the expulsion of S. venezuelensis. This possibility is also under investigation in our laboratory. Thus, in both FcRγ−/− and FcRγ+/+ mice, mast cells may have released MMCP-1 spontaneously as a result of other host- and/or parasite-derived molecules not requiring specific antibody-mediated degranulation of mast cells prior to mediator release. In fact, there is evidence that repeated treatment of normal mice twice daily for 5 days with 104 U of IL-3 resulted in mastocytosis and spontaneous release of MMCP-1 at concentrations up to 200 times higher than the concentration in control animals given only medium (2), and that murine IL-3 could induce the spontaneous release of histamine by mouse peritoneal mast cells (28). However, MMCP-1 may still have an indirect role in S. venezuelensis expulsion since ex vivo and in vivo studies showed that MMC proteinases permeabilize enterocyte tight junctions and promote the escape of MMC and the translocation of plasma-derived molecules into the gut lumen (9, 23).
Lack of FcR γ chain also appeared to have enhanced worm establishment. Following primary S. venezuelensis infection, roughly 50% of larvae reach patency whereas the rest either fail to attach as adults in the intestine and are immediately expelled or are trapped and killed by professional phagocytes at the tissue migratory stage (8, 21). It is therefore conceivable that more larvae would have survived the tissue migratory stage to reach and establish in the intestines in knockout mice since FcRγ deletion abrogates phagocytic activities (27). Larval worm recovery from and histology of the lungs at day 3 p.i. should clarify the kinetic status of migrating larvae following primary infection in these animals. However, it is possible that in our system, the fact that FcRγ−/− mice had significantly more adult worms recovered on day 8 p.i. and subsequently significantly higher EPG than the FcRγ+/+ mice may not be a reflection of an enhanced worm establishment but may reflect a more gradual and rapid expulsion of adult worms from FcRγ−/− and FcRγ+/+ mice, respectively, since at the period of logarithmic rise in EPG, when adult worms were still entering and attaching in the intestines (days 5 to 8 p.i.), there were no differences in the EPG of the two groups of mice.
Finally, FcRγ subunit deficiency resulted in significantly higher EPG, worm numbers, and delay in worm expulsion but no effect on mastocytosis and serum MMCP-1 release. Slightly higher numbers of MMC and serum MMCP-1 concentrations in FcRγ−/− than FcRγ+/+ mice suggest that the expulsion of adult Strongyloides does not depend on mastocytosis per se and that MMCP-1 release may be spontaneous and independent of degranulative responses upon IgE-parasite antigen cross-linking. Furthermore, since the expulsion of adult Strongyloides parasites is definitely associated with hyperplasia and intraepithelial migration of MMC (7), both of which were observed in our system, we speculate that the expulsion does not involve MMCP-1 and that the delay in expulsion in FcRγ−/− mice might be related to failure of the MMC to degranulate and release effector molecules other than MMCP-1, such as high-molecular-weight sulfated proteoglycans. This speculation could be clarified by secondary infection studies and by the measurement of mast cell-derived sulfated sugars released into the luminal contents of the small intestine during primary and challenge S. venezuelensis infections in these mice, both of which form the focus of our ongoing effort to elucidate the precise effector mechanisms involved in the immune-mediated expulsion of the parasite from infected rodents.
ACKNOWLEDGMENTS
D.N.O. is a JSPS postdoctoral fellow, and this work is supported by a grant-in-aid from MOMBUSHO, Japan.
We thank Eri Ono and Ayumi Tanaka for excellent technical assistance.
REFERENCES
- 1.Abe T, Nawa Y. Worm expulsion and mucosal mast cell response induced by repetitive IL-3 administration in Strongyloides ratti-infected nude mice. Immunology. 1988;63:181–185. [PMC free article] [PubMed] [Google Scholar]
- 2.Abe T, Sugaya H, Ishida W, Khan I, Tasdemir I, Yoshimura K. Intestinal protection against Strongyloides rattiand mastocytosis induced by administration of interleukin-3 in mice. Immunology. 1993;80:116–121. [PMC free article] [PubMed] [Google Scholar]
- 3.Abe T, Sugaya H, Yoshimura K, Nawa Y. Induction of the expulsion of Strongyloides ratti and retention of Nippostrongylus brasiliensisin athymic nude mice by repetitive administration of recombinant IL-3. Immunology. 1992;76:10–14. [PMC free article] [PubMed] [Google Scholar]
- 4.Huntley J F, Gibson S, Brown D, Smith W D, Jackson F, Miller H R P. Systemic release of a mast cell proteinase following nematode infections in sheep. Parasite Immunol. 1987;9:603–608. doi: 10.1111/j.1365-3024.1987.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 5.Huntley J F, Gooden C, Newlands G F J, Mackellar A, Lammas D A, Wakelin D, Tuohy M, Woodbury R G, Miller H R P. Distribution of intestinal mast cell proteinase in blood and tissue of normal and Trichinella-infected mice. Parasite Immunol. 1990;12:85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa N, Shi B B, Khan A I, Nawa Y. Reserpine-induced sulphomucin production by goblet cells in the jejunum of rats and its significance in the establishment of intestinal helminths. Parasite Immunol. 1995;17:581–586. doi: 10.1111/j.1365-3024.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishiwata K, Uchiyama F, Maruyama H, Kobayashi T, Kurokawa M, Nawa Y. Glycoconjugates and host-parasite relationship in the mucosal defense against intestinal nematodes. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 925–933. [Google Scholar]
- 8.Khan A I, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol. 1993;23:551–555. doi: 10.1016/0020-7519(93)90159-v. [DOI] [PubMed] [Google Scholar]
- 9.King S J, Miller H R P. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology. 1984;51:653–659. [PMC free article] [PubMed] [Google Scholar]
- 10.Korenaga M, Nawa Y, Mimori T, Tada I. Effects of preintestinal larval antigenic stimuli on the generation of intestinal immunity in Strongyloides rattiinfection. J Parasitol. 1983;69:78–82. [PubMed] [Google Scholar]
- 11.Maruyama H, Yabu Y, Yoshida A, Nawa Y, Ohta N. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J Immunol. 2000;164:3749–3754. doi: 10.4049/jimmunol.164.7.3749. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda H, Watanabe N, Kiso Y, Hirota S, Ushio H, Kannan Y, Azuma M M, Koyama H, Kitamura Y. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornisticks in mice. J Immunol. 1990;144:259–262. [PubMed] [Google Scholar]
- 13.Miller H R P. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996;54:331–336. doi: 10.1016/s0165-2427(96)05696-6. [DOI] [PubMed] [Google Scholar]
- 14.Miller H R P, Jarrett W F H. Immune reactions in mucous membranes. I. Intestinal mast cell responses during helminth expulsion in the rat. Immunology. 1971;20:277–288. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller H R P, Woodbury R G, Huntley J F, Newlands G F J. Systemic release of mucosal mast cell proteinase in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983;49:471–478. [PMC free article] [PubMed] [Google Scholar]
- 16.Moqbel R, Wakelin D, MacDonald A J, King S J, Grencis R K, Kay A B. Release of leukotrienes during rapid expulsion of Trichinella spiralisfrom immune rats. Immunology. 1987;60:425–430. [PMC free article] [PubMed] [Google Scholar]
- 17.Nawa Y, Ishikawa N, Tsuchiya K, Horii Y, Abe T, Khan A I, Shi B, Itoh H, Ide H, Uchiyama F. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol. 1994;16:333–338. doi: 10.1111/j.1365-3024.1994.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 18.Nawa Y, Kiyota M, Korenaga M, Kotani M. Defective protective capacity of W/Wv mice against Strongyloides rattiinfection and its reconstitution with bone marrow cells. Parasite Immunol. 1985;7:429–438. doi: 10.1111/j.1365-3024.1985.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 19.Nawa Y, Korenaga M. Mast and goblet cell responses in the small intestine of rats concurrently infected with Nippostrongylus brasiliensis and Strongyloides ratti. J Parasitol. 1983;69:1168–1170. [PubMed] [Google Scholar]
- 20.Ravetch J V. Fc receptors: rubor redox. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Toma H. Strongyloides venezuelensisinfections in mice. Int J Parasitol. 1990;20:57–62. doi: 10.1016/0020-7519(90)90173-k. [DOI] [PubMed] [Google Scholar]
- 22.Scudamore C L, McMillan L, Thornton E M, Wright S H, Newlands G F J, Miller H R P. Mast cell heterogeneity in the gastrointestinal tract: variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode infected and normal BALB/c mice. Am J Pathol. 1997;150:1661–1672. [PMC free article] [PubMed] [Google Scholar]
- 23.Scudamore C L, Thornton E M, McMillan L, Newlands G F J, Miller H R P. Release of mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995;182:1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi B B, Ishikawa N, Khan A I, Tsuchiya K, Horii Y, Nawa Y. Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus, with reference to the phenotype of intestinal mucosal mast cells. Parasite Immunol. 1994;16:545–551. doi: 10.1111/j.1365-3024.1994.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 25.Shi B B, Ishikawa N, Itoh H, Ide H, Tsuchiya K, Horii Y, Uchiyama F, Nawa Y. Goblet cell mucins of four genera of the subfamily Cricetinae with reference to the protective activity against Strongyloides venezuelensis. Parasite Immunol. 1994;16:553–559. doi: 10.1111/j.1365-3024.1994.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 26.Shi B B, Ishikawa N, Itoh H, Khan A I, Tsuchiya K, Horii Y, Nawa Y. Goblet cell hyperplasia induced by Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus. Int J Parasitol. 1995;25:399–402. doi: 10.1016/0020-7519(94)00100-3. [DOI] [PubMed] [Google Scholar]
- 27.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. FcR γ chain deletion results in pleiotropic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 28.Takaishi T, Morita Y, Hirai K, Yamaguchi M, Yokota T, Arai K, Ito K, Miyamoto T. Mouse IL-3 induces histamine release from mouse peritoneal mast cells. Int Arch Allergy Immunol. 1992;98:205–210. doi: 10.1159/000236186. [DOI] [PubMed] [Google Scholar]
- 29.Wastling J M, Knight P, Ure J, Wright S, Thornton E M, Scudamore C L, Mason J, Smith A, Miller H R P. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the β-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153:491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wastling J M, Scudamore C L, Thornton E M, Newlands G F J, Miller H R P. Constitutive expression of mouse mast cell protease-1 in normal BALB/c mice and its up-regulation during intestinal nematode infection. Immunology. 1997;90:308–313. doi: 10.1046/j.1365-2567.1997.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodbury R G, Miller H R P, Huntley J F, Newlands G F J, Palliser A C, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infection in rat. Nature. 1984;312:450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]