Abstract
Objective:
Rates of e-cigarette use among adolescents and young adults (AYAs) remain high despite several federal policy changes intended to limit their availability and appeal. The current study examined how restricting flavors would affect current AYA users’ intentions to discontinue vaping, as a function of their current flavor preference.
Method:
In a national cross-sectional survey, AYA e-cigarette users (N = 1,414) completed measures of e-cigarette use, device type, e-liquid flavor (tobacco, menthol, cool mint, fruit ice, fruit/sweet), and intent to discontinue e-cigarette use in response to hypothetical federal product standards (i.e., tobacco and menthol or tobacco-only e-liquid). Logistic regression was used to model the association of preferred flavor with odds of discontinuing e-cigarette use (vs. continuing), for menthol and tobacco hypothetical product standards.
Results:
Overall, 38.8% of the sample reported intent to discontinue using their e-cigarette if tobacco and menthol-flavored e-liquid were the only options available, whereas 70.8% would discontinue under a tobacco-only product standard. AYAs preferring fruit/sweet flavor were most sensitive to either restricted scenario, with odds of discontinuing use ranging from adjusted odds ratio (aOR) = 2.22 to aOR = 2.38 under a tobacco and menthol product standard and aOR = 1.33 to aOR = 2.59 under a tobacco-only product standard, compared with other flavor preferences. In addition, AYAs using cooling flavors (e.g., fruit ice) reported higher odds of discontinuing use under a tobacco-only product standard, compared with menthol flavor users, indicating an important distinction between these groups.
Conclusions:
Results indicate potential for flavor restrictions to reduce use of e-cigarettes among AYAs and suggest that a tobacco flavor product standard may result in the greatest discontinuation of use.
Use of e-cigarettes among youth in the United States has increased substantially over time, despite considerable declines in combustible tobacco use (Tam, 2021; Tam & Brouwer, 2021). Point prevalence estimates indicate that between 20% and 30% of adolescents are current (past-30-day) e-cigarette users, prompting several federal agencies to call for increased resources to be allocated for prevention and treatment efforts (Tam, 2021; Wang et al., 2020). Estimates reported for 2021 show some encouraging signs of decrease in past-30-day e-cigarette use among adolescents, although it has been posited that this may be because of restrictions caused by the COVID-19 pandemic (Choi & Abraham 2021).
Recent literature has highlighted that product flavor is commonly cited as an important component in product selection, with adolescents and young adults (AYAs) primarily endorsing preference for sweet e-liquid flavor (e.g., fruit), compared with preferences reported by older adult e-cigarette users (Cullen et al., 2019; Huh et al., 2023; Leventhal et al., 2019a; Shang et al., 2018). Furthermore, use of mint and menthol e-liquids has recently increased among youth populations (Diaz et al., 2021; Leventhal et al., 2019b; Shang et al., 2018). Examining methods to decrease the prevalence of e-cigarette use among AYAs, including restrictions on product flavors, is an important method to consider in order to limit continued growth in prevalence rates and potentially reverse trends in nicotine vaping within this age group.
Some insight can be gained from flavor restrictions placed on combustible cigarettes and single e-cigarette manufacturers. In late 2019, JUUL suspended the sale of fruit flavors and mint. In February of the following year, the U.S. (Food and Drug Administration 2020) issued a policy to enforce compliance for sales of unauthorized e-cigarettes if they were flavored, closed cartridge-based electronic nicotine delivery systems products. Indeed, sales of pod- or cartridge-based e-cigarettes decreased; however, sales of disposable e-cigarettes, not included in the flavor restriction policy, increased (Wang et al., 2020). Importantly, these policies and standard changes excluded menthol-flavored products, and, subsequently, sales of menthol-flavored pods/cartridges increased by 155% from January 2019 to March 2020 and accounted for 60% of all pods/cartridge sales in March 2020 (Wang et al., 2021). More recently, on April 29, 2021, the Food and Drug Administration announced a commitment to ban menthol-combustible cigarettes and all flavored cigars in a step to reduce harm to youth. Although the press release noted that the Food and Drug Administration will remain focused on its regulatory oversight of e-cigarettes, no specific commitment was made to prohibit the sale of menthol flavors in noncombustible products at the time of release. The extent to which these results might extend to AYA e-cigarette use is unknown.
It is logical to expect that establishing a product standard that only allows tobacco- and menthol-flavored e-liquid may affect consumer preferences. However, evidence addressing this possibility is limited. How different flavor restrictions (e.g., removal of all flavors except tobacco or tobacco and menthol) may affect use intention by flavor preference has not yet been examined among AYA populations. Recent literature has documented emerging changes in preferred flavors used by AYAs, including growth in users of new fruit “ice” flavors (Leventhal et al., 2023). Given the likelihood of continued changes in the proportion of AYAs preferring various e-liquid flavors, it may prove valuable to understand how federal restrictions on e-liquid flavor would affect the intention to discontinue vaping, as a function of individual flavor preference.
Method
We used a national convenience sample of AYA e-cigarette users ages 14–21 years to capture data for participants during a period where e-cigarette initiation and use are high (Barrington-Trimis et al., 2020). Data for this study were collected in August 2020 using Lucid (Cint Group Company, New York, NY) online survey management company. Recruitment, data collection, and compensation were handled by the survey management company. No personal identifiable information was collected as part of this study. Per the Children's Online Privacy Protection Rule (2013), no children below age 13 were able to access the Qualtrics platform or participate in the study; participant age was verified by Lucid survey management. The study was approved by the institutional review board (IRB) at a midwestern academic medical center. The IRB-approved consent was presented to participants with an accompanying study description. Participants were informed that “by clicking the agree button below confirms that I agree with the above information and that I agree to take part in this study.”
Participants
Quota sampling was used to identify youth and young adults who met each of the following inclusion criteria: (a) between ages 14 and 21 at the time of enrollment and (b) self-reported current e-cigarette use. Data were restricted in the present analyses to current e-cigarette users only (N = 1,411), specifically users who reported use at least 1 day over the 30 days preceding survey completion.
Measures
Demographics. Participants provided information (categories detailed in Table 1) on participant and family characteristics, including age, race, gender identification, and socioeconomic status.
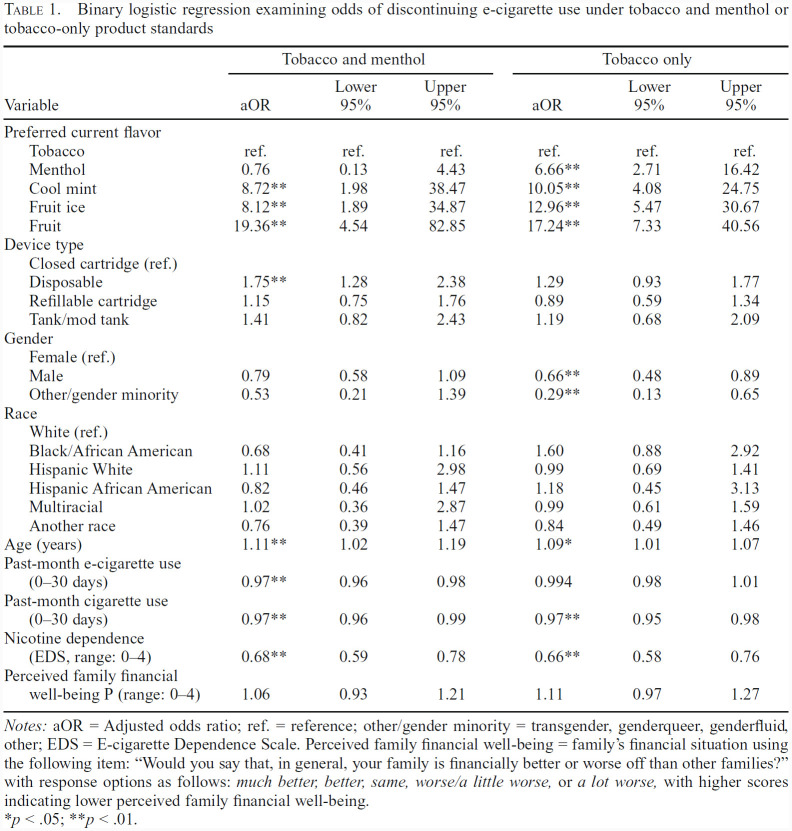
Table 1.
Binary logistic regression examining odds of discontinuing e-cigarette use under tobacco and menthol or tobacco-only product standards
| Variable | Tobacco and menthol | Tobacco only | ||||
|---|---|---|---|---|---|---|
| aOR | Lower 95% | Upper 95% | aOR | Lower 95% | Upper 95% | |
| Preferred current flavor | ||||||
| Tobacco | ref. | ref. | ref. | ref. | ref. | ref. |
| Menthol | 0.76 | 0.13 | 4.43 | 6.66** | 2.71 | 16.42 |
| Cool mint | 8.72** | 1.98 | 38.47 | 10.05** | 4.08 | 24.75 |
| Fruit ice | 8.12** | 1.89 | 34.87 | 12.96** | 5.47 | 30.67 |
| Fruit | 19.36** | 4.54 | 82.85 | 17.24** | 7.33 | 40.56 |
| Device type | ||||||
| Closed cartridge (ref.) Disposable | 1.75** | 1.28 | 2.38 | 1.29 | 0.93 | 1.77 |
| Refillable cartridge | 1.15 | 0.75 | 1.76 | 0.89 | 0.59 | 1.34 |
| Tank/mod tank | 1.41 | 0.82 | 2.43 | 1.19 | 0.68 | 2.09 |
| Gender | ||||||
| Female (ref.) Male | 0.79 | 0.58 | 1.09 | 0.66** | 0.48 | 0.89 |
| Other/gender minority | 0.53 | 0.21 | 1.39 | 0.29** | 0.13 | 0.65 |
| Race | ||||||
| White (ref.) Black/African American | 0.68 | 0.41 | 1.16 | 1.60 | 0.88 | 2.92 |
| Hispanic White | 1.11 | 0.56 | 2.98 | 0.99 | 0.69 | 1.41 |
| Hispanic African American | 0.82 | 0.46 | 1.47 | 1.18 | 0.45 | 3.13 |
| Multiracial | 1.02 | 0.36 | 2.87 | 0.99 | 0.61 | 1.59 |
| Another race | 0.76 | 0.39 | 1.47 | 0.84 | 0.49 | 1.46 |
| Age (years) | 1.11** | 1.02 | 1.19 | 1.09** | 1.01 | 1.07 |
| Past-month e-cigarette use (0-30 days) | 0.97** | 0.96 | 0.98 | 0.994 | 0.98 | 1.01 |
| Past-month cigarette use (0-30 days) | 0.97** | 0.96 | 0.99 | 0.97** | 0.95 | 0.98 |
| Nicotine dependence (EDS, range: 0-4) Perceived family financial | 0.68** | 0.59 | 0.78 | 0.66** | 0.58 | 0.76 |
| well-being P (range: 0-4) | 1.06 | 0.93 | 1.21 | 1.11 | 0.97 | 1.27 |
Notes: aOR = Adjusted odds ratio; ref. = reference; other/gender minority = transgender, genderqueer, genderfluid, other; EDS = E-cigarette Dependence Scale. Perceived family financial well-being = family's financial situation using the following item: “Would you say that, in general, your family is financially better or worse off than other families?” with response options as follows: much better, better, same, worse/a little worse, or a lot worse, with higher scores indicating lower perceived family financial well-being.
p < .05;
p < .01.
E-cigarette device preference. Participants were asked which type of e-cigarette was their main device (i.e., “Which e-cigarette type do you currently use most often?”). Response options were disposable e-cigarette (e.g., Puff Bar, Cali Bar, SEA Pod, Eon ST!K), cartridge-based closed e-cigarette (e.g., JUUL, Vuse, myblu, EON Pods), cartridge-based refillable e-cigarette (e.g., SMOK Novo, Caliburn KOKO, Suorin, Mi-Pod), and e-cigarette tank/mod tank (e.g., Horizon Falcon 2, Aspire Nautilus).
Past-30-day use covariates: E-cigarette and cigarette use. Frequency was assessed in the 30 days preceding study completion: “In the past 30 days, how many total days have you used this product” (range response options were 0 days–30 days).
E-cigarette flavor preference. Participants were also asked to indicate their primary flavor preference (i.e., “What flavor [type of e-cigarette device] do you typically use?”). Response options were fruit (sweet/nonmenthol; e.g., Pineapple Lemonade, Blue Raspberry), Fruit with menthol (e.g., Lycée Ice, Juicy Grape Ice, Banana Ice), tobacco, traditional menthol, and cool mint. Response options did not include an option for selecting “other”; thus, participants who chose to respond to the flavor question had to select one of the five general categories provided.
Nicotine dependence. E-cigarette nicotine dependence was assessed using the validated four-item E-cigarette Dependence Scale (EDS; Morean et al., 2018). Response options were provided in a 5-point Likert scale ranging from 0 (never) to 4 (always), and the mean total was used in analyses (Cronbach's α = .861).
Hypothetical product standard. Yes/no responses to a hypothetical product standard were assessed using the following questions: (1) “Would you use your [type of e-cigarette device] if it were only available in tobacco and menthol flavors?” and (2) “Would you use your [type of e-cigarette device] if it were only available in tobacco flavor?” This allowed for the formation of possible tobacco product standards (tobacco and menthol or tobacco only).
Analytic strategy
Binary logistic regression models generated odds ratios for a “yes” (vs. “no”) response to discontinuing e-cigarette use given a hypothetical flavor product standard for tobacco and menthol and tobacco only (presented in Table 1). The main referent for each analysis presented in Table 1 was tobacco flavor; other comparisons are listed in the text only. Comparisons were then made between each flavor preference group (e.g., odds of discontinuing use for fruit vs. cool mint preference groups). Covariates were selected a priori based on the extant e-cigarette literature and included age, race/ ethnicity, gender identification, past-30-day e-cigarette and cigarette use frequency (days: 0–30) respectively, device type, nicotine dependence, and perceived family financial well-being (all categories listed in Table 1). To determine if the number of individuals discontinuing e-cigarette use under the tobacco and menthol versus tobacco-only flavor product standards was significantly different, we used a McNemar's test with continuity correction. In addition, we conducted a sensitivity analysis to evaluate the potential for individuals age 21 to confound reporting given that they have legal access to products, whereas younger individuals did not. Analyses that included only individuals ages 14–20 revealed no differences in significance or direction as those reported in this article.
Results
Participants/sample
Respondents (N = 1,414) were majority female (79.6%) and White (61.9%) and had a mean age of 18.9 years (SD = 1.6 years). The average age at first e-cigarette use was 16.15 (SD = 2.04). The average self-reported days of e-cigarette use within the past month was 15.75 days (SD = 11.16). Fruit (n = 641; 45.3%) and fruit ice (n = 431; 30.5%) were endorsed as the most preferred flavors in the current sample, followed by cool mint (n = 151; 10.7%), menthol (n = 144; 10.2%), and tobacco (n = 47; 3.3%). Disposable devices were the most common device type used (n = 662; 47.0%). Among those endorsing past-month combustible cigarette use (n = 394, 27.8%), the average reported consumption was 12.6 days (SD = 11.1).
Primary analyses
Tobacco and menthol versus tobacco-only product standard. Overall, significantly more AYAs (70.8%) indicated that they would discontinue use of their e-cigarette if tobacco-flavored e-liquid was the only option available, compared with 38.8% discontinuing e-cigarette use under the tobacco and menthol flavor product standard, χ2(1) = 450.0, p < .001. In addition, when examining flavor preference groups in isolation, significantly more AYAs indicated discontinuing use under the tobacco-only product standard, compared with the tobacco and menthol standard within the fruit preference group (78.5% vs. 54.0%), χ2(1) = 155.01, p < .001; fruit ice (73.1% vs. 34.6%), χ2(1) = 164.01, p < .001; and cool mint (66.9% vs. 31.8%), χ2(1) = 51.02, p < .001, respectively.
Tobacco and menthol flavor product standard. Those who preferred fruit, fruit ice, and cool mint demonstrated significantly higher odds of discontinuing vaping if their preferred flavor was no longer available (Table 1). AYAs who preferred fruit flavors had the greatest odds of discontinuing e-cigarette use in response to a hypothetical tobacco and menthol product standard. Fruit preference was associated with higher odds of discontinuing e-cigarette use, compared with menthol (adjusted odds ratio [aOR] = 29.31, 95% CI [10.50, 81.89]), fruit ice (aOR = 2.38, 95% CI [1.81, 3.13]), and cool mint (aOR = 2.22, 95% CI [1.50, 3.35]). In addition, those preferring fruit ice (aOR = 11.96, 95% CI [4.23, 33.79]) and cool mint (aOR = 12.30, 95% CI [4.23, 35.74]) reported a higher odds of discontinuing use, compared with menthol users (data not presented in tables). No significant differences were observed between cool mint and fruit ice preference groups.
Tobacco flavor product standard. AYAs endorsing any other flavor preference than tobacco had significantly higher odds of discontinuing e-cigarette use, compared with those retaining their flavor preference under the hypothetical tobacco product standard (Table 1). Similar to the results of the tobacco and menthol product standard, AYAs preferring fruit flavor were most sensitive to the tobacco-only hypothetical product standard. This group had higher odds of discontinuing use, compared with those preferring menthol (aOR = 2.59, 95% CI [1.76, 4.10]) or cool mint (aOR = 1.71, 95% CI [1.12, 2.61]), and a trend toward significantly higher odds, compared with those preferring fruit ice (aOR = 1.33, 95% CI [0.98, 1.8], p = .06; data presented in text only). In addition, fruit ice users were significantly more likely to discontinue use of their e-cigarette under a tobacco-only standard, compared with menthol users. No significant differences were observed between cool mint and fruit ice or cool mint and menthol preference groups, respectively.
Discussion
Findings suggest that an e-cigarette product standard limiting the availability of e-liquid flavors to only tobacco and menthol is associated with increased intent to discontinue e-cigarette use among AYAs. Furthermore, significantly more AYAs reported intention to discontinue use under a tobacco-only standard in comparison to a tobacco and menthol standard. AYA e-cigarette users reported odds ranging from aOR = 6.66 to 29.31 of discontinuing vaping if their preferred flavors were unavailable in comparison to those retaining their preferred flavor under the product standards examined. This indicates that the effect size of e-liquid flavor preference on intentions to discontinue e-cigarette use is very large. In line with expectations, odds of hypothetically discontinuing e-cigarette use under both flavor product standards decreased with increasing nicotine dependence levels. In addition, significant but modest relationships were observed between discontinuing use and age (years), as well as past-month cigarette use under both flavor product standards.
More granular analysis between groups revealed that the greatest odds of discontinuing use were observed among those endorsing a preference for fruit flavors. Importantly, the fruit preference group was the most common, comprising nearly half of the current sample. Significantly more individuals in this group reported that they would discontinue use of their e-cigarette under a tobacco-only product standard, compared with a tobacco and menthol product standard allowing menthol products to be used. This has potential implications for policy considerations and demonstrates that a menthol ban may affect users beyond those specifically preferring menthol and other cooling flavors (e.g., fruit ice and cool mint). It is also important to highlight that fruit ice users represent the second largest group in the current study. Fruit ice (e.g., lychee ice, watermelon lime freeze) encompasses an emerging group of e-liquid flavors that combine fruit with the cooling sensation of menthol.
In this study, users of e-cigarettes with fruit ice flavor were more likely to discontinue use under a tobacco product flavor standard, compared with those using menthol-flavored e-cigarettes, which represents an important difference between these groups that are sometimes categorized together. Taken together, the current results demonstrate that a tobacco-only product standard may increase intent to discontinue vaping for all groups other than tobacco flavor users, with the largest effects observed for those using fruit and nonmenthol cooling flavors.
Limitations/future directions
The use of a convenience sample that was majority White and majority female limits the generalizability of our results, despite covariation for both variables. Future studies may benefit from an examination of hypothetical decisions to switch to combustible cigarettes or to attempt quitting nicotine use altogether in the context of flavor restrictions (Kasza et al., 2021; Smith et al., 2021). Likewise, it is possible that some participants may have interpreted the hypothetical product standard being applied to only their brand, rather than across all e-cigarette manufacturers. Thus, responses indicating intent to discontinue use may also include individuals who would switch to a different product if it were available. Flavor preference categories included five choices rather than an open response option; thus, participants who used flavors that did not fit well within the broad categories described may potentially be misclassified. In addition, future studies may benefit from including more detailed analysis of past combustible cigarette use, including use of menthol cigarettes. Given the nationwide survey method used, it is possible that some participants were located in areas that had differing bans in place at the time of survey completion. Thus, some participants’ responses could have been affected by these bans.
Conclusions
In summary, in this sample of AYA current e-cigarette users, flavor preference was differentially associated with discontinuing use under hypothetical product scenarios. Compared with a tobacco and menthol product standard, a tobacco-only standard resulted in substantial increases in intentions to discontinue e-cigarette use across all comparison groups. This included significant increases in reported intention to discontinue use if menthol flavor were unavailable that extended beyond those who prefer menthol or cooling flavors. We also demonstrated that menthol bans may have more of an impact on fruit ice users than on menthol users themselves. Future research that examines the effect of flavor preference on subsequent vaping behavior within experimental designs will provide valuable insight into the relationships we observed in this study. If the current self-reported intentions extend to behavior in the natural environment, significant resources and effective vaping cessation programs for youth should accompany any policies restricting flavors.
Footnotes
Research reported in this publication was supported by the National Cancer Institute and the U.S. Food and Drug Administration (FDA) Center for Tobacco Products under Award Number U54CA180905; Award Number K01HL148907 (to Alayna P. Tackett) from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH); and Award Number 092-016-0002 (to Alayna P. Tackett) from the Oklahoma Tobacco Settlement Endowment Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
References
- Barrington-Trimis J. L., Braymiller J. L., Unger J. B., McConnell R., Stokes A., Leventhal A. M., Goodwin R. D. Trends in the age of cigarette smoking initiation among young adults in the US from 2002 to 2018. JAMA Network Open. 2020;3:e2019022. doi: 10.1001/jamanetworkopen.2020.19022. doi:10.1001/jamanetworkopen.2020.19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children's Online Privacy Protection Rule (“COPPA”), 16 Part C.F.R. 312; Children's Online Privacy Protection Act of 1998, 15 U.S.C. 6501–6505, 78 Fed. Reg. 3972 et seq. (January 17, 2013). Retrieved from https://www.ftc.gov/system/files/2012-31341.pdf
- Choi B. M., Abraham I. The decline in e-cigarette use among youth in the United States—An encouraging trend but an ongoing public health challenge. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.12464. [DOI] [PubMed] [Google Scholar]
- Cullen K. A., Gentzke A. S., Sawdey M. D., Chang J. T., Anic G. M., Wang T. W., King B. A. E-cigarette use among youth in the United States, 2019. JAMA. 2019;322:2095–2103. doi: 10.1001/jama.2019.18387. doi:10.1001/jama.2019.18387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. C., Donovan E. M., Schillo B. A., Vallone D. Menthol e-cigarette sales rise following 2020 FDA guidance. Tobacco Control. 2021;30:700–703. doi: 10.1136/tobaccocontrol-2020-056053. doi:10.1136/tobaccocontrol-2020-056053. [DOI] [PubMed] [Google Scholar]
- Huh J., Yu S., Galimov A., Meza L., Galstyan E., Medel D., Sussman S. Hypothetical flavour ban and intention to vape among vape shop customers: The role of flavour reference and e-cigarette dependence. Tobacco Control. 2023;32:110–113. doi: 10.1136/tobaccocontrol-2020-056321. doi:10.1136/tobaccocontrol-2020-056321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza K. A., Edwards K. C., Gravely S., Coleman B., Kimmel H., Everard C., Hyland A. Adults’ e-cigarette flavor use and cigarette quit attempts: Population assessment of tobacco and health study findings. American Journal of Preventive Medicine. 2021;60:300–302. doi: 10.1016/j.amepre.2020.06.017. doi:10.1016/j.amepre.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A., Dai H., Barrington-Trimis J., Sussman S. ‘Ice’ flavoured e-cigarette use among young adults. Tobacco Control. 2023;32:114–117. doi: 10.1136/tobaccocontrol-2020-056416. doi:10.1136/tobaccocontrol-2020-056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. M., Goldenson N. I., Barrington-Trimis J. L., Pang R. D., Kirkpatrick M. G. Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug and Alcohol Dependence. 2019a;203:99–106. doi: 10.1016/j.drugalcdep.2019.05.020. doi:10.1016/j.drugalcdep.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A. M., Miech R., Barrington-Trimis J., Johnston L. D., O’Malley P. M., Patrick M. E. Flavors of e-cigarettes used by youths in the United States. JAMA. 2019b;322:2132–2134. doi: 10.1001/jama.2019.17968. doi:10.1001/jama.2019.17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean M. E., Krishnan-Sarin S., O’Malley S. S. Assessing nicotine dependence in adolescent e-cigarette users: The 4-item Patient-Reported Outcomes Measurement Information System (PROMIS) Nicotine Dependence Item Bank for electronic cigarettes. Drug and Alcohol Dependence. 2018;188:60–63. doi: 10.1016/j.drugalcdep.2018.03.029. doi:10.1016/j.drugalcdep.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C., Huang J., Chaloupka F. J., Emery S. L.2018The impact of flavour, device type and warning messages on youth preferences for electronic nicotine delivery systems: Evidence from an online discrete choice experiment Tobacco Control 27e152–e159doi:10.1136/tobaccocontrol-2017-053754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. T., Nahhas G. J., Carpenter M. J., Squeglia L. M., Diaz V. A., Leventhal A. M., Dahne J. Intention to quit vaping among United States adolescents. JAMA Pediatrics. 2021;175:97–99. doi: 10.1001/jamapediatrics.2020.2348. doi:10.1001/jamapediatrics.2020.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. E-cigarette, combustible, and smokeless tobacco product use combinations among youth in the United States, 2014–2019. Addictive Behaviors. 2021;112:106636. doi: 10.1016/j.addbeh.2020.106636. doi:10.1016/j.addbeh.2020.106636. [DOI] [PubMed] [Google Scholar]
- Tam J., Brouwer A. F. Comparison of e-cigarette use prevalence and frequency by smoking status among youth in the United States, 2014–19. Addiction. 2021;116:2486–2497. doi: 10.1111/add.15439. doi:10.1111/add.15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food U.S. and Drug Administration. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint [News release] 2020. Jan 2, Retrieved from https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-basede-cigarettes-appeal-children.
- Wang T. W., Gentzke A. S., Neff L. J., Glidden E. V., Jamal A., Park-Lee E., Hacker K. A. Disposable E-cigarette use among US youth—An emerging public health challenge [Letter to the Editor] The New England Journal of Medicine. 2021;384:1573–1576. doi: 10.1056/NEJMc2033943. doi:10.1056/NEJMc2033943. [DOI] [PubMed] [Google Scholar]
- Wang T. W., Neff L. J., Park-Lee E., Ren C., Cullen K. A., King B. A. E-cigarette use among middle and high school students—United States, 2020. Morbidity and Mortality Weekly Report. 2020;69:1310–1312. doi: 10.15585/mmwr.mm6937e1. doi:10.15585/mmwr.mm6937e1. [DOI] [PMC free article] [PubMed] [Google Scholar]