ABSTRACT
Objective
Posttraumatic stress disorder (PTSD) and traumatic life events are often coupled to chronic pain, possibly linked by central sensitization. We wanted to assess the prevalence of traumatic events and PTSD in chronic pain patients of a German university hospital outpatient pain clinic. Moreover, we evaluated the extent of indicators and co-occurring traits of central sensitization in comorbid patients.
Methods
We retrospectively divided 914 chronic pain patients into four groups depending on their trauma severity: no trauma, accidental trauma, interpersonal trauma, and PTSD. We collected electronic pain drawings focusing on pain area and widespreadness, as well as information about pain intensity, sleep impairment, disability, stress, anxiety, depression, and somatization. Differences between groups were calculated using Kruskal-Wallis with post-hoc Mann-Whitney tests.
Results
Of 914 patients, 231 (25%) had no trauma, 210 (23%) had accidental traumas, 283 (31%) had interpersonal traumas, 99 (11%) had PTSD, and 91 (10%) could not be classified. We observed statistically significant differences between groups in pain area and widespreadness, as well as maximal pain, sleep impairment, disability, stress, anxiety, depression, and somatization. The severity of symptoms increased with trauma severity.
Conclusions
Traumatic life events and PTSD are frequent in chronic pain patients. The increased pain area and widespreadness, as well as the increased negative impact on co-occurring traits of sensory sensitivity (anxiety, depression, somatization), are compatible with central sensitization in comorbid patients. Therefore, a heightened awareness of the comorbidity between traumatic experiences and chronic pain is recommended.
Key words/Abbreviations: chronic pain; posttraumatic stress disorder; pain area; pain widespreadness; central sensitization; ICD-10 = International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; PDI = Pain Disability Index; PDS = Posttraumatic Diagnostic Scale; PHQ-D = Patient Health Questionnaire, German version; PTSD = posttraumatic stress disorder; VAS = visual analog scale; WPI = Widespread Pain Index
INTRODUCTION
Chronic pain, that is, pain lasting longer than 3 months, is a commonly encountered health issue that decreases quality of life and leads to a great burden on society (1). A meta-analysis showed that 9.7% of chronic pain patients had posttraumatic stress disorder (PTSD) (2). This prevalence was even more pronounced in four university hospital outpatient pain clinics, ranging from 21% to 29% (3–5). This elevated prevalence of PTSD compared with the general population (approximately 7%) (6) suggests that the two disorders often coexist and might exacerbate each other (4,7).
There is some uncertainty about the extent to which psychological trauma and PTSD may promote the development of chronic pain (8). However, early trauma is associated with an increased risk of developing chronic pain in adulthood (9–11), which suggests at least indirect causality. Further research indicates that chronic pain can develop from traumatic psychological events independently of affective factors and in a dose-response relation (11,12).
Early life stress can interact with genetic factors, especially in vulnerable phases of live, and, with the involvement of epigenetic mechanisms, create the foundation for a persistently disturbed responsiveness of the allostatic systems (13,14). One significant example of such a process is the epigenetic dysregulation of central glucocorticoid receptors, resulting in a disruption of stress processing (15). Both overactivation and underactivation of the hypothalamo-pituitary-adrenal axis can lead to an imbalance of other systems, especially the endocannabinoid (16) and the corticomesolimbic (17) systems. The dysfunction of the latter can be understood as a central neurobiological correlate of chronic pain (17,18), which can remain active even without sustained nociceptive input (17). Childhood trauma also seems be able to directly influence pain sensitivity through epigenetic changes in ion channels (19). This fits with the observation that early life stress is associated with the development of proinflammatory responsiveness throughout life, for example, via priming of microglia (20,21).
Although earlier proxy definitions of central pain sensitization exclusively used pain dimensions such as widespread pain, disproportionate pain intensity, and sensory amplification (22), today a multidimensional nature is assumed, including further factors such as generalized sensory sensitivity, heightened somatic awareness, cognitive disturbance, and sleep difficulties (23). Furthermore, a recent review on studies of central sensitization in chronic low back pain showed that these factors correlate with psychosocial metrics such as depression, anxiety, and somatization (24). We have to rely on this characterization of central sensitization because the direct proof of a “hyperexcitability of the central nervous system” (25) by means of direct electrophysiological recordings is not reasonable in humans.
From a neurobiological perspective, the emergence of central pain sensitization, that is, the hyperexcitability of the central nervous system, was discussed as a significant mechanism for the development of chronic pain in the context of traumatic life events (10,26). In a cross-sectional study on 202 patients with chronic pain, both traumatic events and PTSD symptoms were significantly associated with clinical indicators of central sensitization, such as pain extent, pain intensity, and polysomatic complaints measured by the Central Sensitization Inventory (27). Moreover, compared with controls, pain-free PTSD subjects also showed higher pain ratings and significantly increased temporal summation after intramuscular capsaicin stimulus, indicating acute sensitization (28).
The first objective of this study was to assess the prevalence of traumatic life events and PTSD in a large sample of chronic pain patients of a German university hospital outpatient clinic. Second, we wanted to evaluate the impact of comorbidity on proxy measures for central sensitization such as pain area and widespreadness, as well as co-occurring traits of sensory sensitivity (e.g., anxiety, depression, somatization) (29–31). We also analyzed associated symptoms such as pain intensity, sleep impairment, disability, and stress.
METHODS
This retrospective study took place at Hannover Medical School (Hannover, Germany), was approved by the ethics committee of Hannover Medical School, and was registered at ClinicalTrials.gov (NCT05190367). Between February 2019 and July 2020, 914 of 1047 patients (87%) who visited our outpatient pain department gave written consent to use their routinely collected data anonymously for research purposes. We cannot provide any information about the 133 who did not give their consent to use their data for research purposes. M.D. and M.K. were the attending physicians, and J.M. had access to all anonymized data. Our article follows the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement (32).
Participants were divided into four groups depending on their trauma severity: a) no trauma, b) accidental trauma (e.g., illness, accident, natural disaster), c) interpersonal trauma (e.g., assault, rape, war), and d) PTSD (diagnosed according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]). This subdivision followed previous reports that interpersonal traumas are more prone to develop into PTSD than accidental traumas (33). Patients fulfilling two or more categories were assigned to the highest group. Patients who could not be classified because of missing information on traumas were excluded from the analysis. Their measures can be found in Supplemental Digital Content, Table S1, http://links.lww.com/PSYMED/A907. All the remaining data were included in the statistical analyses.
Data were collected using the SymptomMapper application (34). Participants provided information about their traumas (first part of the Posttraumatic Diagnostic Scale [PDS] (35)), current pain intensity (visual analog scale [VAS] (36)), mean and maximal pain in the last 4 weeks (VAS), sleep impairment (VAS), acceptable pain (VAS), Pain Disability Index (PDI (37)), pain area (digital drawings), pain widespreadness (Widespread Pain Index [WPI] (38), derived from drawings), stress (Patient Health Questionnaire, German version [PHQ-D] (39)), anxiety (PHQ-D), depression (PHQ-D), and somatization symptoms (PHQ-D).
Differences between groups were calculated using Kruskal-Wallis tests (and χ2 test for sex), followed by post-hoc Mann-Whitney tests. In a supplementary analysis, we tested for sex differences in all parameters and groups using Mann-Whitney tests. We used the nonparametric equivalents to the analysis of variance and two-sample t tests because our data were not normally distributed (Figure S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A907). Moreover, we calculated the Pearson cross-correlation matrix of all measures. All analyses were Bonferroni corrected for multiple comparisons, and we only report corrected p values.
Data Availability
Raw data and necessary scripts for reproducing the results of this study are available at Zenodo (https://doi.org/10.5281/zenodo.7498710).
RESULTS
Of 914 patients, 231 (25%) had no trauma, 210 (23%) had accidental traumas, 283 (31%) had interpersonal traumas, 99 (11%) had PTSD, and 91 (10%) could not be classified. There were 623 women (68%), the average age was 54.0 (16.2) years, and the average body mass index was 26.4 (5.4) kg/m2.
We observed highly significant (p < .001) differences between groups in pain area, WPI, sleep impairment, PDI, stress, anxiety, depression, and somatization symptoms. Moreover, there were significant differences (p = .001) in maximal pain during the previous 4 weeks and acceptable pain (p = .010). Patients diagnosed with PTSD showed more severe symptoms than patients without trauma. Specifically, they showed larger pain area (13.2% [18.5%] versus 6.9% [12.6%]; p < .001; d = 0.43), WPI (7.3 [5.7] versus 4.1 [4.4]; p < .001; d = 0.67), maximal pain (80.6 [17.4] versus 70.3 [25.2]; p = .005; d = 0.45), acceptable pain (31.7 [21.4] versus 22.6 [19.4]; p < .001; d = 0.45), sleep impairment (70.6 [28.1] versus 45.6 [32.2]; p < .001; d = 0.81), PDI (42.3 [13.4] versus 31.3 [15.5]; p < .001; d = 0.74), stress (10.3 [4.4] versus 5.0 [3.4]; p < .001; d = 1.44), anxiety (12.1 [5.4] versus 6.3 [4.7]; p < .001; d = 1.17), depression (15.4 [5.5] versus 9.3 [5.5]; p < .001; d = 1.11), and somatization (16.8 [5.3] versus 10.4 [4.9]; p < .001; d = 1.28; Table 1 and Figure S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A907).
TABLE 1.
Group Differences
| Measure | Statistical Significance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No Trauma (n = 231) | Accidental Trauma (n = 210) | Interpersonal Trauma (n = 283) | PTSD (n = 99) | Kruskal-Wallis | Post-Hoc Mann-Whitney | ||||||
| 0 versus 1 | 0 versus 2 | 0 versus 3 | 1 versus 2 | 1 versus 3 | 2 versus 3 | ||||||
| Women | 159 (69%) | 135 (64%) | 195 (69%) | 72 (73%) | |||||||
| Age, y | 53.9 (18.0) | 55.9 (15.7) | 53.0 (16.0) | 50.7 (12.0) | |||||||
| Body mass index, kg/m2 | 26.1 (5.0) | 26.6 (5.8) | 26.4 (5.4) | 26.2 (5.7) | |||||||
| Pain area, % | 6.9 (12.6) | 7.3 (11.4) | 8.5 (12.8) | 13.2 (18.5) | *** | *** | ** | * | |||
| Widespread Pain Index (0–19) | 4.1 (4.4) | 4.4 (4.5) | 5.6 (5.0) | 7.3 (5.7) | *** | * | *** | *** | |||
| Current pain, VAS (0–100) | 48.3 (29.8) | 52.6 (27.9) | 52.9 (27.2) | 59.4 (24.5) | |||||||
| Mean pain, VAS (0–100) | 55.8 (24.7) | 59.3 (22.7) | 59.8 (21.6) | 65.0 (20.8) | |||||||
| Maximal pain, VAS (0–100) | 70.3 (25.2) | 75.6 (21.0) | 79.5 (18.5) | 80.6 (17.4) | ** | *** | ** | ||||
| Acceptable pain, VAS (0–100) | 22.6 (19.4) | 25.8 (19.6) | 25.4 (17.2) | 31.7 (21.4) | ** | *** | |||||
| Sleep impairment, VAS (0–100) | 45.6 (32.2) | 53.3 (31.6) | 52.2 (32.9) | 70.6 (28.1) | *** | *** | *** | *** | |||
| Pain Disability Index (0–70) | 31.3 (15.5) | 35.3 (15.7) | 38.0 (14.7) | 42.3 (13.4) | *** | * | *** | *** | *** | * | |
| Patient Health Questionnaire | |||||||||||
| 10-item stress scale (0–20) | 5.0 (3.4) | 6.1 (3.3) | 7.4 (4.1) | 10.3 (4.4) | *** | *** | *** | *** | ** | *** | *** |
| 7-item anxiety scale (0–21) | 6.3 (4.7) | 7.3 (4.7) | 8.3 (5.0) | 12.1 (5.4) | *** | *** | *** | *** | *** | ||
| 9-item depression scale (0–27) | 9.3 (5.5) | 10.4 (5.1) | 11.8 (5.3) | 15.4 (5.5) | *** | *** | *** | * | *** | *** | |
| 15-item somatic scale (0–30) | 10.4 (4.9) | 11.8 (4.9) | 13.3 (5.1) | 16.8 (5.3) | *** | * | *** | *** | ** | *** | *** |
PTSD = posttraumatic stress disorder; VAS = visual analog scale.
Data are expressed as mean (standard deviation) unless otherwise stated. (0) no trauma; (1) accidental trauma; (2) interpersonal trauma; (3) PTSD.
* p < .05.
** p < .01.
*** p < .001.
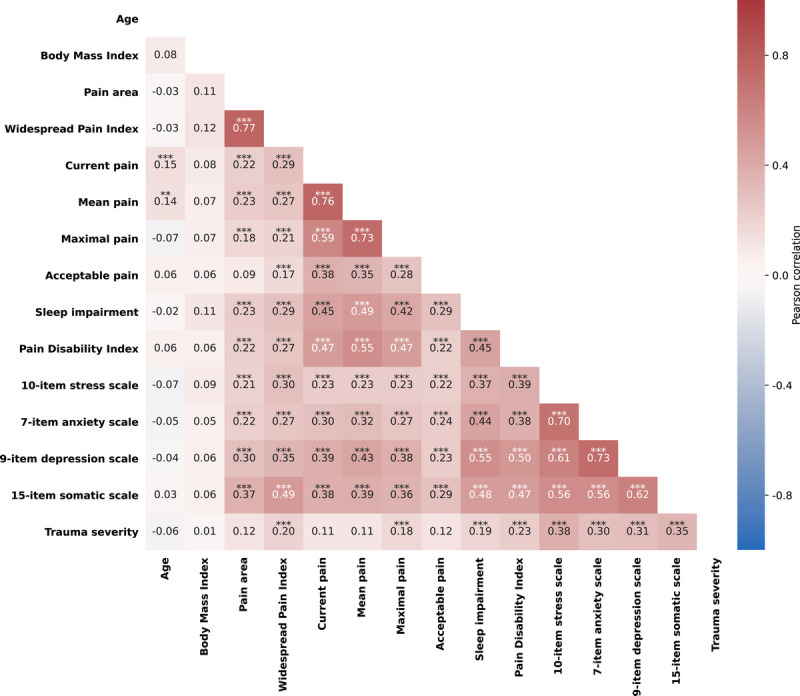
We also calculated the Pearson cross-correlation matrix of all measures. WPI, current pain, mean pain, maximal pain, acceptable pain, sleep impairment, PDI, stress, anxiety, depression, and somatization significantly correlated with each other. Age correlated with current pain (r = 0.15; p < .001) and mean pain (r = 0.14; p = .002). Furthermore, trauma severity correlated with WPI (r = 0.20; p < .001), maximal pain (r = 0.18; p < .001), sleep impairment (r = 0.19; p < .001), PDI (r = 0.23; p < .001), stress (r = 0.38; p < .001), anxiety (r = 0.30; p < .001), depression (r = 0.31; p < .001), and somatization (r = 0.35; p < .001; Figure 1).
FIGURE 1.
Cross-correlation matrix of all measures. **p < .01; ***p < .001. Color image is available only in the online version of the article.
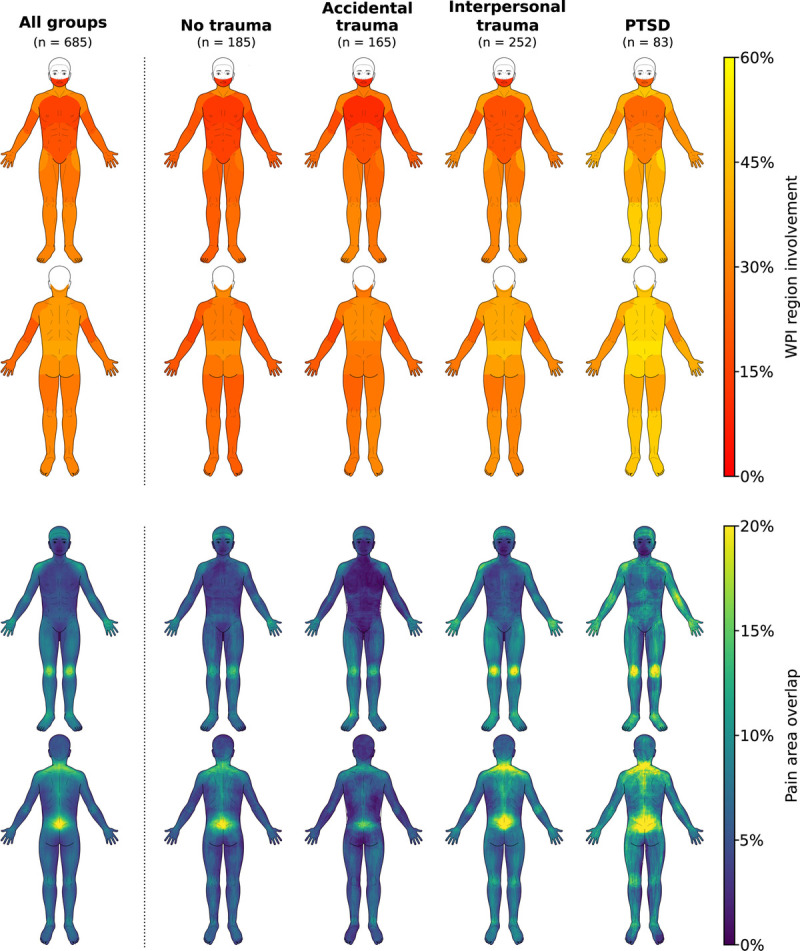
Average pain drawings (Figure 2) showed that pain was centered at the lumbar region, spinal cord, shoulders, knees, wrists, and temples. Patients with PTSD additionally reported pain across the limbs, in the back, and in the abdomen.
FIGURE 2.
WPI region involvement and pain area overlap. Note that the WPI does not include head regions. Moreover, not all patients made pain drawings. WPI = Widespread Pain Index. Color image is available only in the online version of the article.
We did not observe sex differences in any of the parameters (Figure S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A907).
DISCUSSION
We found a PTSD prevalence of 11% in our cohort of 914 patients with chronic pain. Moreover, further 54% reported traumatic life events. These results are in line with data from a previous meta-analysis (2). In contrast, pain units of four Scandinavian university clinics showed a prevalence rate of PTSD at least twice as high as our sample (3–5).
Moreover, we observed a positive correlation between trauma severity and pain widespreadness, maximal pain, sleep impairment, PDI, stress, anxiety, depression, and somatization (Figure 1). This correlation underpins the notion that trauma severity can be roughly subdivided into accidental and interpersonal traumas, being the latter closer in severity to PTSD (33).
The increased pain area and widespreadness, as well as the impact on outcomes such as pain intensity, sleep impairment, disability, and stress, are compatible with the concept of central sensitization in patients with PTSD as elaborated in Introduction. Both chronic pain and PTSD alter similar nuclei in the brainstem, hypothalamus, and amygdala (40). For this reason, it has been hypothesized by some authors that dysregulation of these regions, which would be exacerbated by the comorbidity, could lead to changes in neurons and glia and hence to central sensitization (41). Other authors found that changes in the amygdala could also lead to affective disorders (42), which could result in increased somatization and pain (43).
Because our sample included a relatively large number of female patients (68%), we conducted a supplementary analysis to figure out whether there were sex differences. We could not find any sex difference in the acquired parameters (Figure S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A907). Nonetheless, the percentage of women in our study was larger than in people with chronic pain in Germany, of which roughly 54% are female (44). One explanation for this discrepancy could be that women visit the pain outpatient department more often.
Limitations
The usage of routinely collected data might distort the results because of missing values or inconsistent data quality. We minimized these errors by acquiring all data electronically. Therefore, no questionnaires had missing values, and pain drawing instructions were standardized. However, some patients were not capable of completing the drawings. We minimized misclassification bias in trauma severity by using the first part of the PDS to reduce free text to a minimum. Moreover, PTSD was diagnosed by experienced physicians following ICD-10 criteria. Patients who could not be classified because of missing information (10%) were excluded from the analysis. Their measures can be found in Table S1, Supplemental Digital Content, http://links.lww.com/PSYMED/A907. We might hypothesize that these patients felt uncomfortable sharing such sensitive information, which would suggest an underreporting of traumatic events. In addition, the absence of the second to fourth parts of the PDS limits the quantification of trauma severity.
We had no information from specific measures for central sensitization such as quantitative sensory testing, functional magnetic resonance imaging, or blood markers (22). However, pain area, intensity, and widespreadness have been found to be significant proxies for central sensitization (31,45). In addition, patient-reported phenomena relating to psychological and mental impairments seem to be significant cues of central sensitization (see Introduction) (31,46). Given the results from this and previous studies, it may be sensible to screen chronic pain patients for central sensitization using specialized questionnaires like the Central Sensitization Inventory (47) and using quantitative sensory testing for confirmation.
Moreover, neither sociodemographic data nor other relevant factors such as wider social dimensions (e.g., partnership), somatic and mental comorbidities, and medication were part of the SymptomMapper database. Hence, they were not included in this analysis. Being potential confounders, these parameters should be investigated in further studies to elucidate their impact on chronic pain with comorbid PTSD.
A further limitation of our study is that it did not include people with traumas but without comorbid pain. Such a group could provide insightful information on the risks of developing or exacerbating chronic pain after a trauma. It might well be that people with traumas but without pain develop nonpainful symptoms such as fatigue or no symptoms at all. On the other hand, there is some evidence that pain-free PTSD patients are also affected by central sensitization mechanisms (28).
Clinical Implications
A previous study showed that the majority of a cohort of 83 patients with chronic pain and comorbid PTSD benefited from a 3-week group-based interdisciplinary pain rehabilitation program (48). However, no conclusion could be drawn regarding long-term effects, and a subset of patients experienced no change or even an increase in symptoms. Chronic pain may mask an existing psychological trauma that plays an important role in the way pain is experienced and coped with. On the other hand, chronic pain and the therapeutic interactions themselves can have a (re)traumatizing effect on patients (49). As long as a specific therapy for patients with chronic pain and a traumatic background has not been established, a holistic, person-centered and trauma-informed care approach seems to be elementary. This approach emphasizes the importance of active and compassionate listening from the first encounter (50). The frequent occurrence of PTSD as comorbidity and the severity of symptoms should make healthcare professionals working in pain management aware of and sensitive to their patients.
In conclusion, because of the correlation between severity of traumatic events, anxiety, stress, depression, and pain widespreadness, we believe that chronic pain patients should be screened for trauma and PTSD. A better understanding of the comorbidity of PTSD, chronic pain, and central sensitization may lead to better clinical care for these severely affected patients.
Supplementary Material
Acknowledgments
Source of Funding and Conflicts of Interest: We declare no conflicts of interest. F.B. was supported by the Horst Görtz Foundation. The founder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contribution: J.M.: study design, data acquisition, analysis, visualization, discussion of results, manuscript drafting and revision. L.R., F.B., T.A.N., and M.D.: data acquisition, discussion of results, manuscript revision. M.K.: study design, data acquisition, discussion of results, manuscript drafting and revision, supervision.
Footnotes
Article Editor: Harald Guendel
Supplemental Digital Content
Contributor Information
Linda Rudolph, Email: Rudolph.Linda@mh-hannover.de.
Florian Beissner, Email: Beissner.Florian@mh-hannover.de.
Till-Ansgar Neubert, Email: tillneubert@nbrt.de.
Martin Dusch, Email: Dusch.Martin@mh-hannover.de.
Matthias Karst, Email: Karst.Matthias@mh-hannover.de.
REFERENCES
- 1.Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;13:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siqveland J, Hussain A, Lindstrøm JC, Ruud T, Hauff E. Prevalence of posttraumatic stress disorder in persons with chronic pain: a meta-analysis. Front Psych 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen TE, Andersen PG, Vakkala MA, Elklit A. The traumatised chronic pain patient—Prevalence of posttraumatic stress disorder—PTSD and pain sensitisation in two Scandinavian samples referred for pain rehabilitation. Scand J Pain 2012;3:39–43. [DOI] [PubMed] [Google Scholar]
- 4.Åkerblom S, Perrin S, Rivano Fischer M, McCracken LM. The impact of PTSD on functioning in patients seeking treatment for chronic pain and validation of the posttraumatic diagnostic scale. Int J Behav Med 2017;24:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linnemørken LTB, Granan LP, Reme SE. Prevalence of posttraumatic stress symptoms and associated characteristics among patients with chronic pain conditions in a Norwegian university hospital outpatient pain clinic. Front Psychol 2020;11:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 7.Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev 2001;21:857–77. [DOI] [PubMed] [Google Scholar]
- 8.Gasperi M, Panizzon M, Goldberg J, Buchwald D, Afari N. Posttraumatic stress disorder and chronic pain conditions in men: a twin study. Psychosom Med 2021;83:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood?: a meta-analytic review of the literature. Clin J Pain 2005;21:398–405. [DOI] [PubMed] [Google Scholar]
- 10.Afari N Ahumada SM Wright LJ Mostoufi S Golnari G Reis V, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med 2014;76:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RC, Plener PL, Braehler E, Fegert JM, Huber-Lang M. Associations of adverse childhood experiences and bullying on physical pain in the general population of Germany. J Pain Res 2018;11:3099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atwoli L, Platt JM, Basu A, Williams DR, Stein DJ, Koenen KC. Associations between lifetime potentially traumatic events and chronic physical conditions in the South African Stress and Health Survey: a cross-sectional study. BMC Psychiatry 2016;16:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson S, Burns M, McEwen B, Borsook D. Stressful experiences in youth: “set-up” for diminished resilience to chronic pain. Brain Behav Immun Health 2020;5:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agorastos A, Pervanidou P, Chrousos GP, Baker DG. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psych 2019;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan PO Sasaki A D’Alessio AC Dymov S Labonté B Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009;12:342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 2016;41:80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vachon-Presseau E Centeno MV Ren W, et al. The emotional brain as a predictor and amplifier of chronic pain. J Dent Res 2016;95:605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmers I, Quaedflieg CWEM, Hsu C, Heathcote LC, Rovnaghi CR, Simons LE. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehav Rev 2019;107:641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach J Rhein M Gombert S, et al. Childhood traumatization is associated with differences in TRPA1 promoter methylation in female patients with multisomatoform disorder with pain as the leading bodily symptom. Clin Epigenetics 2019;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke NN, Fan CY, Trang T. Microglia in health and pain: impact of noxious early life events. Exp Physiol 2016;101:1003–21. [DOI] [PubMed] [Google Scholar]
- 21.Burke NN, Finn DP, McGuire BE, Roche M. Psychological stress in early life as a predisposing factor for the development of chronic pain: clinical and preclinical evidence and neurobiological mechanisms. J Neurosci Res 2017;95:1257–70. [DOI] [PubMed] [Google Scholar]
- 22.Gatchel RJ, Neblett R. Central sensitization: a brief overview. J Appl Biobehav Res 2018;23:e12138. [Google Scholar]
- 23.Schrepf A Williams DA Gallop R Naliboff B Basu N Kaplan C, et al. Sensory sensitivity and symptom severity represent unique dimensions of chronic pain: a MAPP research network study. Pain 2018;159:2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuttert I Timmerman H Petersen KK McPhee ME Arendt-Nielsen L Reneman MF, et al. The definition, assessment, and prevalence of (human assumed) central sensitisation in patients with chronic low back pain: a systematic review. J Clin Med 2021;10:5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.den Boer C Dries L Terluin B van der Wouden JC Blankenstein AH van Wilgen CP, et al. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res 2019;117:32–40. [DOI] [PubMed] [Google Scholar]
- 26.Janssen J, Abou-Assaly E, Rasic N, Noel M, Miller JV. Trauma and pain sensitization in youth with chronic pain. Pain Reports 2022;7:e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKernan LC, Johnson BN, Crofford LJ, Lumley MA, Bruehl S, Cheavens JS. Posttraumatic stress symptoms mediate the effects of trauma exposure on clinical indicators of central sensitization in patients with chronic pain. Clin J Pain 2019;35:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moeller-Bertram T, Strigo IA, Simmons AN, Schilling JM, Patel P, Baker DG. Evidence for acute central sensitization to prolonged experimental pain in posttraumatic stress disorder. Pain Med 2014;15:762–71. [DOI] [PubMed] [Google Scholar]
- 29.Clark J, Nijs J, Yeowell G, Goodwin PC. What are the predictors of altered central pain modulation in chronic musculoskeletal pain populations? A systematic review. Pain Physician 2017;20:487–500. [PubMed] [Google Scholar]
- 30.van Wilgen CP Vuijk PJ Kregel J, et al. Psychological distress and widespread pain contribute to the variance of the Central Sensitization Inventory: a cross-sectional study in patients with chronic pain. Pain Pract 2018;18:239–46. [DOI] [PubMed] [Google Scholar]
- 31.Nijs J George SZ Clauw DJ Fernández-de-las-Peñas C Kosek E Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol 2021;3:e383–92. [DOI] [PubMed] [Google Scholar]
- 32.Benchimol EI Smeeth L Guttmann A, et al. The REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forbes D Lockwood E Phelps A, et al. Trauma at the hands of another: distinguishing PTSD patterns following intimate and nonintimate interpersonal and noninterpersonal trauma in a nationally representative sample. J Clin Psychiatry 2013;75:147–53. [DOI] [PubMed] [Google Scholar]
- 34.Neubert TA, Dusch M, Karst M, Beissner F. Designing a tablet-based software app for mapping bodily symptoms: usability evaluation and reproducibility analysis. JMIR Mhealth Uhealth 2018;6:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foa EB McLean CP Zang Y, et al. Psychometric properties of the Posttraumatic Diagnostic Scale for DSM-5 (PDS-5). Psychol Assess 2016;28:1166–71. [DOI] [PubMed] [Google Scholar]
- 36.Dworkin RH Turk DC Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 37.Pollard CA. Preliminary validity study of the Pain Disability Index. Percept Mot Skills 1984;59:974. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F Clauw DJ Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 39.Löwe B, Spitzer RL, Zipfel S, Herzog W. Gesundheitsfragebogen für Patienten (PHQ-D) Manual und Testunterlagen. 2nd ed. Karlsruhe, Germany: Pfizer; 2002. [Google Scholar]
- 40.Scioli-Salter ER, Forman DE, Otis JD, Gregor K, Valovski I, Rasmusson AM. The shared neuroanatomy and neurobiology of comorbid chronic pain and PTSD: therapeutic implications. Clin J Pain 2015;31:363–74. [DOI] [PubMed] [Google Scholar]
- 41.Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci 2018;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray RJ, Brosch T, Sander D. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex 2014;60:10–33. [DOI] [PubMed] [Google Scholar]
- 43.Stonnington CM, Locke DEC, Hsu CH, Ritenbaugh C, Lane RD. Somatization is associated with deficits in affective theory of mind. J Psychosom Res 2013;74:479–85. [DOI] [PubMed] [Google Scholar]
- 44.Häuser W, Schmutzer G, Hinz A, Hilbert A, Brähler E. Prävalenz chronischer Schmerzen in Deutschland. Der Schmerz 2013;27:46–55. [DOI] [PubMed] [Google Scholar]
- 45.Arendt-Nielsen L Morlion B Perrot S Dahan A Dickenson A Kress HG, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–41. [DOI] [PubMed] [Google Scholar]
- 46.Schäfer AGM, Joos LJ, Roggemann K, Waldvogel-Röcker K, Pfingsten M, Petzke F. Pain experiences of patients with musculoskeletal pain + central sensitization: a comparative group Delphi study. PLoS One 2017;12:e0182207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer TG Neblett R Cohen H Howard KJ Choi YH Williams MJ, et al. The development and psychometric validation of the Central Sensitization Inventory. Pain Pract 2012;12:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilliam WP Schumann ME Craner JR, et al. Examining the effectiveness of pain rehabilitation on chronic pain and post-traumatic symptoms. J Behav Med 2020;43:956–67. [DOI] [PubMed] [Google Scholar]
- 49.Defontaine-Catteau M-C, Bioy A. Place du traumatisme psychique en clinique de la douleur. Douleur et Analgésie 2014;27:68–74. [Google Scholar]
- 50.Tidmarsh LV, Harrison R, Ravindran D, Matthews SL, Finlay KA. The influence of adverse childhood experiences in pain management: mechanisms, processes, and trauma-informed care. Frontier Pain Res 2022;3:923866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data and necessary scripts for reproducing the results of this study are available at Zenodo (https://doi.org/10.5281/zenodo.7498710).


