Abstract
BACKGROUND:
We analyzed bleeding and thrombotic complications in COVID-19–associated ARDS requiring extracorporeal membrane oxygenation (ECMO).
METHODS:
This was a single-center observational study of adult subjects undergoing ECMO for COVID-19 (n = 67) or all other cause of ARDS (n = 60), excluding trauma patients.
RESULTS:
In the COVID-19 group, duration of invasive mechanical ventilation prior to ECMO was lower (2 [0–4] d vs 3 [1–6] d) and ECMO retrieval less frequent (71% vs 87%). No significant differences were found in Simplified Acute Physiology Score II, Acute Physiology and Chronic Health Evaluation II (APACHE II), or in the in-hospital survival predicted by the Respiratory ECMO Survival Prediction score. During the first 7 d of ECMO support, the COVID-19 group presented higher platelets and fibrinogen, lower activated partial thromboplastin time, but no differences in D-dimer. Thrombotic complications were similar between groups. Higher rates of severe bleeding, namely airway bleeding (37.3% vs 15.0%) and hemothorax (13.4% vs 3.3%), were found in COVID-19, with lower hemoglobin and higher red blood cell transfusions. COVID-19 ARDS was associated with longer ECMO duration (47 [17–80] d vs 19 [12–30] d) and absence of a statistically significant difference concerning in-hospital mortality.
CONCLUSIONS:
COVID-19–associated ARDS requiring ECMO presented high rates of severe bleeding complications and a protracted course. Further studies are needed to clarify the risks and benefits of ECMO in severe COVID-19–associated ARDS.
Keywords: ARDS, COVID-19, bleeding, thrombosis, extracorporeal membrane oxygenation
Introduction
COVID-19 can lead to severe ARDS. Extracorporeal membrane oxygenation (ECMO) may be considered in refractory respiratory failure when positive-pressure ventilation alone is insufficient to maintain adequate gas exchange or when adherence to lung-protective ventilation strategies and prone position results in unacceptable levels of hypoxemia and respiratory acidosis.1 From the beginning of the pandemic, ECMO has been used in refractory severe COVID-19–associated ARDS,2,3 with a recent systematic review describing a ∼7% use rate, with overall in-hospital mortality of 39%.4
However, ECMO is a resource-intensive and invasive technique with a procoagulant effect5 and major bleeding and thrombotic complications. This could be particularly relevant given that severe COVID-19 is an acute inflammatory disease and hypercoagulable state, with endothelial injury6–8 and increased circulating prothrombotic factors.9–11 This COVID-19–associated coagulopathy is characterized by a high rate of thrombotic events.12–15 Bleeding, despite being less frequent, still occurs in ∼30% of subjects,12,13,16 having a greater impact than thrombosis in the mortality of ECMO subjects.12,17 This finding is in line with pre-pandemic data on subjects receiving ECMO for ARDS of other causes.18
In this context, to better ascertain the potential benefits and risks associated with ECMO use in severe COVID-19, the aim of the present study was to compare the incidence of thrombotic and hemorrhagic complications between subjects with COVID-19 and non–COVID-19 subjects with severe ARDS requiring ECMO. Namely, we compared baseline characteristics, pre-ECMO ventilatory and gas exchange parameters, blood and coagulation, bleeding and thrombotic complications, as well as clinical outcome, between adult non-trauma subjects with COVID-19 or all other causes of ARDS.
QUICK LOOK.
Current Knowledge
COVID-19 is a hypercoagulable state with increased thrombotic and bleeding risk. Thrombotic complications are described as more frequent than bleeding events in patients with COVID-19 requiring extracorporeal membrane oxygenation (ECMO); however, the latter appears to have greater impact on mortality. Reported mortality in these patients varies from 18–68%.
What This Paper Contributes to Our Knowledge
Thrombotic complications were similar between groups. COVID-19 subjects presented more bleeding events, namely airway bleeding and hemothorax. These complications were associated with prolonged ECMO courses.
Methods
A retrospective cohort study of adult subjects without trauma and with severe respiratory failure treated with ECMO for > 7 d in Hospital S. João (Porto, Portugal) between January 2018–September 2021 was performed. We excluded patients with ECMO runs shorter than 7 d. For this period, we screened 171 potentially eligible patients. Of these, 16 were excluded due to polytrauma, 7 due to ECMO runs shorter than 7 d, and 21 because important data were missing, namely regarding hemostatic parameters. Our primary outcome was the incidence of bleeding and thrombotic complications under ECMO support. São João University Hospital is a 1,100-bed tertiary hospital. With a current case volume of ∼100 patients/y (∼50% respiratory ECMO; neonatal and pediatric ECMO representing < 10% of the total), our ECMO reference center is an Extracorporeal Life Support Organization member (center 227). Being the sole ECMO reference center in the north of Portugal, a region with approximately 4 million inhabitants, most of our respiratory patients are transported by our ECMO team from referring hospitals, with cannulation in loco and patient transfer to our center already under ECMO support. Specific ECMO data were collected and presented from a dedicated database.
Subjects undergoing ECMO for COVID-19 (COVID-19 group, n = 67) or all other cause of ARDS (non-COVID, n = 60) were compared. In the COVID-19 group, confirmation of SARS-CoV-2 infection on hospital admission was performed via nasopharyngeal swabs and tracheal aspirate (in mechanically ventilated subjects) with polymerase chain reaction assays. Airway bleeding severity was classified as mild/moderate or severe based on bronchoscopy reports. Bleeding was considered severe whenever a high volume of active bleeding was documented, large clots obstructing the main airways were present, and/or hemostatic interventions were required.
Study Population and Technique of Extracorporeal Support
Criteria and contraindications for ECMO in refractory acute respiratory failure, the technique of extracorporeal support, subjects' management on ECMO, including anticoagulation therapy, and weaning from extracorporeal support were described previously.19 No relevant alterations were made to this protocol since its publication. Regarding anticoagulation, unfractionated heparin was used for a target activated partial thromboplastin time (aPTT) of 1.5 times the normal.
Data Collection and Statistical Analysis
The Ethics Committee of the Hospital S. João approved the study and waived the requirement for subject consent. Variables are reported either as number of cases and percentage or median and interquartile ranges. Comparisons between groups (COVID-19 vs other etiologies) were performed using independent samples t test (normal distributed data) or Mann-Whitney U test (non-normal distributed data) for continuous variables, whereas the chi-square test or Fisher exact test was used for categorical variables, as appropriate. Results were considered statistically significant if P < .05. For statistical analysis, SPSS 28.0 (IBM, Armonk, New York) was used.
Results
Baseline Subject Characteristics
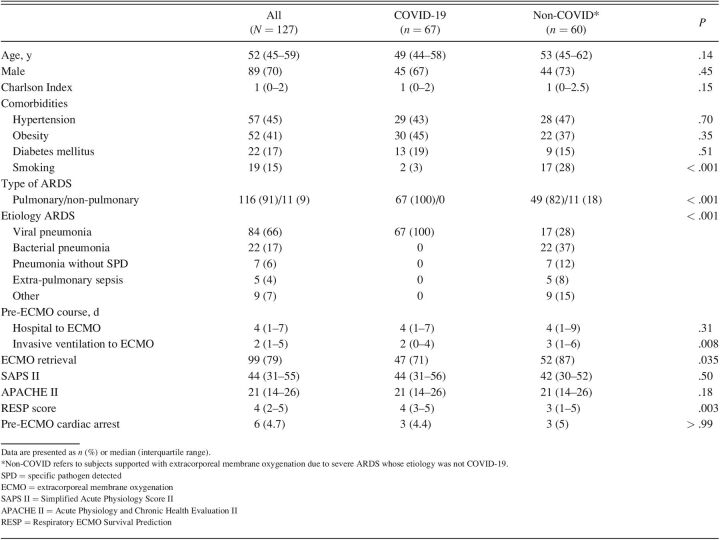
Our study included relatively young subjects, mostly male, with no significant comorbidities (Table 1). Smoking was less frequent in the COVID-19 group (3% vs 28%). Non-pulmonary ARDS was present in 18% of subjects in the non-COVID group.
Table 1.
Baseline Characteristics of Subjects Requiring Extracorporeal Membrane Oxygenation for Severe ARDS
Notably, subjects with COVID-19 had a shorter duration of invasive mechanical ventilation pre-ECMO. Most subjects were retrieved from referring hospitals, although this occurred less frequently in the COVID-19 group. No significant differences were found in Simplified Acute Physiology Score II and Acute Physiology and Chronic Health Evaluation II (APACHE II). In-hospital survival predicted by the Respiratory ECMO Survival Prediction (RESP) score (76%) was similar in both groups. Three subjects in each group suffered cardiac arrest before ECMO cannulation. The preferred cannulation strategy was femoro-jugular in both groups (93.9% in the COVID-19 group and 75.9% in the non-COVID cohort).
Ventilatory Parameters and Gas Exchange Before ECMO
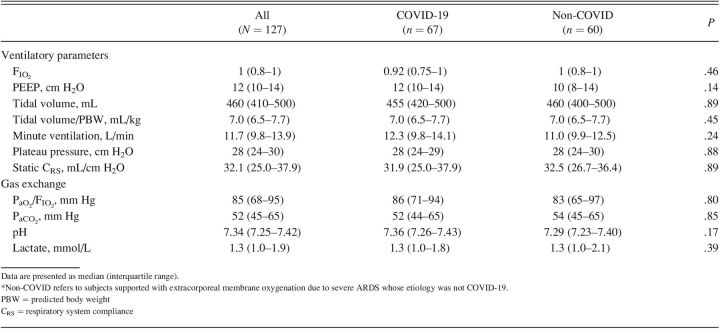
Regarding ventilatory parameters (Table 2), no significant differences were detected between groups in the level of FIO2, PEEP, tidal volume, minute ventilation, plateau pressure, or static respiratory system compliance before starting ECMO support. Likewise, similar pre-ECMO levels in gas exchange parameters such as PaO2/FIO2, PaCO2, pH, and blood lactate were observed in both groups.
Table 2.
Ventilatory Parameters and Gas Exchange Before Starting Extracorporeal Membrane Oxygenation Support
Blood and Coagulation Before and During ECMO
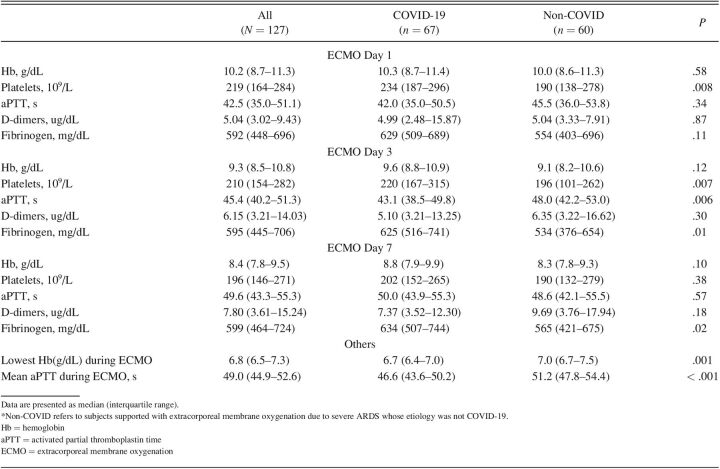
Hemoglobin, platelets, aPTT, D-dimers, and fibrinogen values on the first (day 1), third (day 3), and seventh day (day 7) of ECMO support are presented (Table 3).
Table 3.
Blood and Coagulation Before and During Extracorporeal Membrane Oxygenation
At day 1 and day 3, COVID-19 had higher platelet count, but at day 7 no significant differences were detected between groups. Inversely, aPTT raised at a similar extent in both groups during the first week of ECMO support as systemic anticoagulation ensued, except in day 3, where it was significantly lower in COVID-19 group. Except for day 1, fibrinogen was higher in COVID-19 group. No differences were observed between groups concerning hemoglobin and D-dimer levels. COVID-19 group had an inferior lowest hemoglobin and mean aPTT during the first week of ECMO support.
Regarding transfusion support, COVID-19 group had a significantly higher need for red blood cell (RBC) units when compared with non-COVID group (8 [2–16] vs 2 [1–6], P = .001). Other blood products, such as platelets, fresh frozen plasma, or fibrinogen, were rarely administered (< 25% of subjects) and did not differ between groups.
ECMO Complications and Clinical Outcome
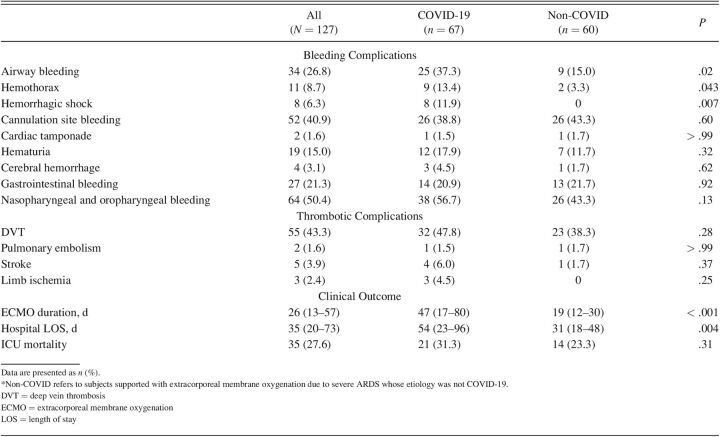
A significantly higher rate of severe bleeding was found in COVID-19 group (Table 4). Airway bleeding was significantly higher in the COVID-19 group, both the mild/moderate bleeding (18 [26.9%] vs 8 [13.3%], P = .03) and the severe cases (7 [10.4%] vs 1 [1.7%)], P = .03). Within the 7 subjects with COVID-19 who suffered severe airway bleeding, 4 died, whereas mild/moderate airway bleeding 8 of 18 subjects did not survive. Hemorrhagic shock was mainly associated with hemothorax (n = 4; ∼50% of cases); 2 cases were caused by lower-limb hematomas, 1 by retroperitoneal bleeding, and 1 due to nontraumatic lesion of an iliolumbar artery. Of note, 6 of the 9 subjects with COVID-19 with hemothorax died, whereas the 2 subjects with hemothorax in the non-COVID group survived. Ocular, nasal, and pharyngeal bleeding was common, whereas cardiac tamponade and intracerebral hemorrhage were rare.
Table 4.
Incidence of Post-Cannulation Complications
Regarding thrombotic complications, no differences were detected between groups. Deep vein thrombosis, mostly related to ECMO cannulation, was common in both groups. Stroke, limb ischemia, and pulmonary embolism were rare events. Acute kidney injury was a notably frequent complication in both COVID-19 and non-COVID groups (40.3% vs 46.6%, P = .47). The COVID-19 group required longer ECMO support and hospital stay.
Discussion
In our single-center experience, severe COVID-19–associated ARDS requiring ECMO presented high rates of severe bleeding complications and a protracted course, with a statistically significant difference concerning in-hospital mortality.
No relevant differences were found between groups in the severity scoring systems. Of note, although the RESP score significantly differed between groups (COVID-19: 4 [3–5] vs non-COVID-19: 3 [1–5]), both scores 3 and 4 are associated with similar in-hospital survival. Accordingly, no significant differences were found in the pre-ECMO ventilatory and gas exchange parameters between group. Subjects with COVID-19 had a shorter duration of invasive mechanical ventilation pre-ECMO when compared with non-COVID group. However, in contrast to pre-pandemic evidence, pre-ECMO invasive ventilation duration in COVID-19 does not seem to have impact on survival,20 although more studies are needed to confirm this finding.
In our study, severe COVID-19–associated ARDS supported with ECMO did not show increased thrombotic complications. ECMO support requires systemic anticoagulation, which could have contributed to decreased thrombotic complications in COVID-19 group. Pre-ECMO cardiac arrest, higher PaCO2 at ECMO initiation, and obesity, all recognized risk factors for thrombosis,18 were similar between groups and, therefore, are unlikely to have confounded the results. In our medical center, computed tomography pulmonary angiogram (CTPA) is not routinely performed in every patient in the first day of ECMO support, which could have underestimated incidence of pulmonary thromboembolism (PTE). The difference between incidentally detected PTE on CTPA and clinically suspected PTE in ICU-treated subjects with COVID-19 can be as high as 93–7%.21
Bleeding events occurred more frequently in COVID-19 group. Accordingly, statistically significant inferior mean lowest hemoglobin and higher need for RBC transfusion was detected in this group. This could not have been caused by higher systemic anticoagulation in this group given that the mean aPTT during ECMO support was lower. Moreover, ECMO-associated coagulopathy most likely did not account for the bleeding risk observed in COVID-19 group given the higher platelet count and fibrinogen observed in this group during the first week of ECMO support. Additionally, it's worth mentioning the absence of differences concerning D-dimer levels between cohorts, as D-dimers have popularly been attributed with prognostic significance; and although pre-cannulation D-dimer levels have been associated with an increased predicted disease severity and longer ECMO course,22 their value under ECMO must be carefully interpreted as it may reflect thrombus within the oxygenator or hemostatic perturbance rather than hypercoagulability.23 Furthermore, D-dimer has poor specificity, with increased levels seen in a variety of conditions, and fails to capture the dynamic interplay between platelets, endothelium, and coagulation cascade phenomena.24 Thus, interpretation of D-dimer levels is difficult and should be careful given the complexity of these subjects.
In our COVID-19 cohort, airway bleeding was more common and more severe. In subjects with severe airway bleeding and large blood clots causing significant airway obstruction, clots were removed by cycles of saline instillation and aspiration. In 2 cases, debridement with tissue-grasping forceps was required. In one case, cryoextraction and thrombectomy with Fogarty catheter were performed. When active bleeding was observed or emerged as a complication of clot extraction, cold saline, adrenaline, and tranexamic acid instillation were used to control the hemorrhage. Bleeding relapse was common, and a bronchoscopy to review hemostasis was often required.
Similarly, hemothorax was a frequent and severe complication with difficult management and high associated mortality. In 4 of 9 subjects with hemothorax, thoracic surgery was required. Two thoracotomies and 2 video-assisted thoracoscopic surgeries were performed. Surgical re-intervention was needed in 2 occasions due to relapsing bleeding, and in one case intrathoracic gauze packing was performed in the presence of a hemorrhagic suffusion that involved all thoracic cavity without visible bleeding point. A possible underlying etiology for these events may be the development of peripheral medium and small pulmonary artery branch aneurysms in COVID-19's highly inflammatory setting.25
Conclusions
In our single-center experience, severe COVID-19–associated ARDS requiring ECMO presented high rates of severe bleeding complications and a protracted course. This could not have been accounted for by differences in the severity of the acute respiratory failure or in ECMO-associated coagulopathy. Further studies are needed to further clarify the risks and benefits of ECMO in severe COVID-19–associated ARDS.
Footnotes
The authors have disclosed no conflicts of interest.
REFERENCES
- 1. Rajagopal K, Keller SP, Akkanti B, Bime C, Loyalka P, Cheema FH, et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO-A “Living Working Document.” ASAIO J 2020;66(6):588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1,591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. Extracorporeal membrane oxygenation for COVID-19–associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med 2021;204(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertini P, Guarracino F, Falcone M, Nardelli P, Landoni G, Nocci M, et al. ECMO in COVID-19 patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2022;36(8 Pt A):2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granja T, Hohenstein K, Schüssel P, Fischer C, Prüfer T, Schibilsky D, et al. Multimodal characterization of the coagulopathy associated with extracorporeal membrane oxygenation. Crit Care Med 2020;48(5):e400–e408. [DOI] [PubMed] [Google Scholar]
- 6. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol 2020;20(7):389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowenstein CJ, Solomon SD. Severe COVID-19 is a microvascular disease. Circulation 2020;142(17):1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 2020;41(32):3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18(7):1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ranucci M, Ballotta A, Di Dedda U, Baryshnikova E, Dei Poli M, Resta M, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18(7):1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID-19–associated hyperviscosity: a link between inflammation and thrombophilia? Lancet 2020;395(10239):1758–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arachchillage DJ, Rajakaruna I, Scott I, Gaspar M, Odho Z, Banya W, et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with venovenous extracorporeal membrane oxygenation in the United Kingdom: a multi-center observational study. Br J Haematol 2022;196(3):566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurihara C, Manerikar A, Gao CA, Watanabe S, Kandula V, Klonis A, et al. Outcomes after extracorporeal membrane oxygenation support in COVID-19 and non–COVID-19 patients. Artif Organs 2022;46(4):688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weir-McCall JR, Galea G, Mun Mak S, Joshi K, Agrawal B, Screaton N, et al. Vascular thrombosis in severe coronavirus disease 2019 requiring extracorporeal membrane oxygenation: a multi-center study. Crit Care Med 2022;50(4):624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doyle AJ, Hunt BJ, Sanderson B, Zhang J, Mak SM, Benedetti G, et al. A comparison of thrombosis and hemorrhage rates in patients with severe respiratory failure due to coronavirus disease 2019 and influenza requiring extracorporeal membrane oxygenation. Crit Care Med 2021;49(7):e663–e672. [DOI] [PubMed] [Google Scholar]
- 16. Autschbach T, Hatam N, Durak K, Grottke O, Dreher M, Nubbemeyer K, et al. Outcomes of extracorporeal membrane oxygenation for acute respiratory distress syndrome in COVID-19 patients: a propensity-matched analysis. J Clin Med 2021;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weatherill A, Laffan M, Gasper M, Bianchi P, Passariello M, Singh S, et al. Impact of thrombosis and bleeding in patients with severe COVID-19 versus other viral pneumonias in the context of extracorporeal membrane oxygenation. Semin Thromb Hemost 2022;48(1):118–123. [DOI] [PubMed] [Google Scholar]
- 18. Nunez JI, Gosling AF, O'Gara B, Kennedy KF, Rycus P, Abrams D, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 2022;48(2):213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roncon-Albuquerque R, Jr., Basílio C, Figueiredo P, Silva S, Mergulhão P, Alves C, et al. Portable miniaturized extracorporeal membrane oxygenation systems for H1N1-related severe acute respiratory distress syndrome: a case series. J Crit Care 2012;27(5):454–463. [DOI] [PubMed] [Google Scholar]
- 20. Hermann M, Laxar D, Krall C, Hafner C, Herzog O, Kimberger O, et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care 2022;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mirsadraee S, Gorog DA, Mahon CF, Rawal B, Semple TR, Nicol ED, et al. Prevalence of thrombotic complications in ICU-treated patients with coronavirus disease 2019 detected with systematic CT scanning. Crit Care Med 2021;49(5):804–815. [DOI] [PubMed] [Google Scholar]
- 22. Shakoor A, Chen S, Hyde J, Wu B, Kon Z, Piper G, et al. 335: D-dimer trend in COVID-19 patients requiring extracorporeal membrane oxygenation: a clinical dilemma. Crit Care Med 2022;50(1):154–154. [Google Scholar]
- 23. Chandel A, Patolia S, Looby M, Bade N, Khangoora V, King CS. Association of D-dimer and fibrinogen with hypercoagulability in COVID-19 requiring extracorporeal membrane oxygenation. J Intensive Care Med 2021;36(6):689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaudhary R, Garg J, Houghton DE, Murad MH, Kondur A, Chaudhary R, et al. Thrombo-inflammatory biomarkers in COVID-19: systematic review and meta-analysis of 17,052 patients. Mayo Clin Proc Innov Qual Outcomes 2021;5(2):388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Desnos C, Boussouar S, Hekimian G, Redheuil A, Combes A. Spontaneous hemothorax in 4 COVID-19 ARDS patients on VV-ECMO revealing pulmonary artery aneurysms. Crit Care 2020;24(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]