Abstract
BACKGROUND:
In refractory respiratory failure, extracorporeal membrane oxygenation (ECMO) is a rescue therapy to prevent ventilator-induced lung injury. Optimal ventilator parameters during ECMO remain unknown. Our objective was to describe the association between mortality and ventilator parameters during ECMO for neonatal and pediatric respiratory failure.
METHODS:
We performed a secondary analysis of the Bleeding and Thrombosis on ECMO dataset. Ventilator parameters included breathing frequency, tidal volume, peak inspiratory pressure, PEEP, dynamic driving pressure, pressure support, mean airway pressure, and FIO2. Parameters were evaluated before cannulation, on the calendar day of ECMO initiation (ECMO day 1), and the day before ECMO separation.
RESULTS:
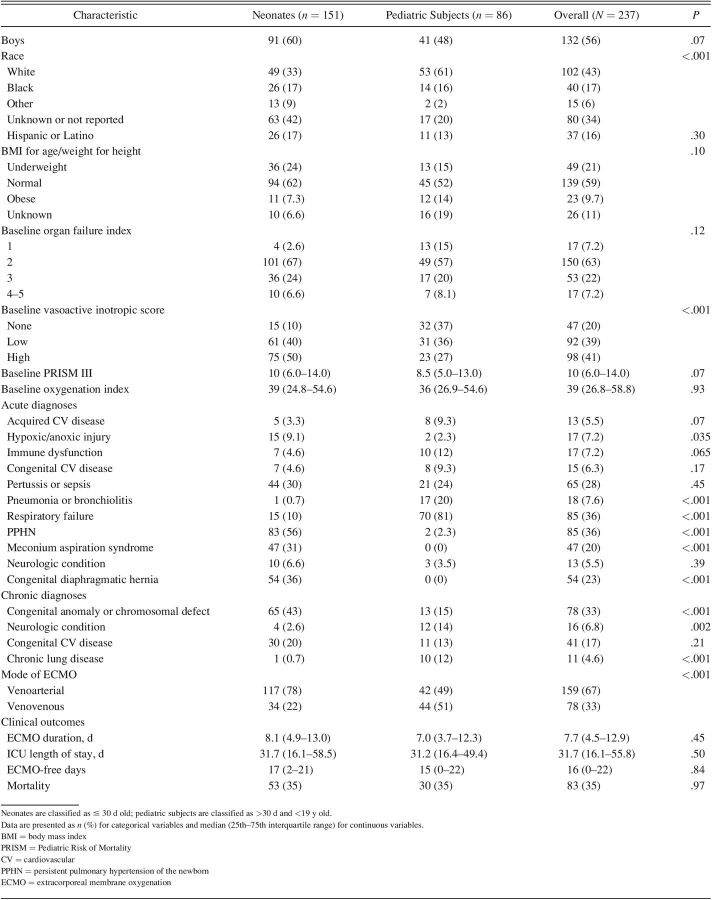
Of 237 included subjects analyzed, 64% were neonates, of whom 36% had a congenital diaphragmatic hernia. Of all the subjects, 67% were supported on venoarterial ECMO. Overall in-hospital mortality was 35% (n = 83). The median (interquartile range) PEEP on ECMO day 1 was 8 (5.0–10.0) cm H2O for neonates and 10 (8.0–10.0) cm H2O for pediatric subjects. By multivariable analysis, higher PEEP on ECMO day 1 in neonates was associated with lower odds of in-hospital mortality (odds ratio 0.77, 95% CI 0.62–0.92; P = .01), with a further amplified effect in neonates with congenital diaphragmatic hernia (odds ratio 0.59, 95% CI 0.41–0.86; P = .005). No ventilator type or parameter was associated with mortality in pediatric subjects.
CONCLUSIONS:
Avoiding low PEEP on ECMO day 1 for neonates on ECMO may be beneficial, particularly those with a congenital diaphragmatic hernia. No additional ventilator parameters were associated with mortality in either neonatal or pediatric subjects. PEEP is a modifiable parameter that may improve neonatal survival during ECMO and requires further investigation.
Keywords: extracorporeal membrane oxygenation, neonate, pediatric, respiratory failure, mortality, ventilatory-induced lung injury, mechanical ventilation, PEEP, hernias, diaphragmatic, congenital
Introduction
In refractory respiratory failure, extracorporeal membrane oxygenation (ECMO) is a rescue therapy to prevent ventilatory-induced lung injury, which includes alveolar overdistention (volutrauma), alveolar collapse and reopening (atelectrauma), and inflammatory response (biotrauma).1–3 However, the optimal lung-protective ventilation strategy during neonatal and pediatric ECMO remains unknown. Current guidelines for ventilating neonatal and pediatric patients on ECMO are based on expert opinion and recommend limiting peak inspiratory pressure (PIP), breathing frequency, and , while providing titratable ranges of PEEP (neonates: 5–10 cm H2O; pediatric patients: 5–15 cm H2O).4,5 Despite these guidelines, an international survey demonstrated wide practice variation between ventilator management concepts of “lung rest” and “lung recruitment” during neonatal and pediatric ECMO,6 which highlights persistent clinical uncertainty. Furthermore, it is difficult for clinicians to assess the degree of lung recovery while ECMO support is being used, which may delay optimal timing to liberate from ECMO. The ability for clinicians to determine optimal ventilator parameters for children on ECMO is currently precluded by conflicting research results, studies with small sample sizes, wide practice variation, and an inability to risk adjust for illness severity.6–13
Accordingly, the multi-center Bleeding and Thrombosis on ECMO (BATE) dataset contains prospectively collected clinical and ventilator data of children supported on ECMO. Our primary aim was to explore the associations between in-hospital mortality and ventilator parameters in subjects < 19 y old supported on ECMO for respiratory failure. The secondary aim was to evaluate ventilator parameters against ECMO-free days to capture the morbidity of prolonged ECMO duration.
QUICK LOOK.
Current Knowledge
In the setting of respiratory failure, extracorporeal membrane oxygenation (ECMO) is a rescue therapy to limit exposure of ventilator-induced lung injury. Ventilatory practices for children on ECMO are sparsely reported. The associations of various ventilator parameters on mortality in neonates and pediatric patients with refractory respiratory failure on ECMO remain unknown.
What This Paper Contributes to Our Knowledge
On initiation of ECMO for respiratory failure, the only ventilator parameter associated with a reduced odds of in-hospital mortality was higher levels of PEEP in neonates, particularly those with congenital diaphragmatic hernia. Outside of the neonatal population, no ventilator parameters were associated with outcomes in the remaining pediatric subjects.
Methods
Design and Setting
We performed a secondary analysis of the BATE dataset, originally conducted to describe the bleeding and thrombotic complications of neonates and pediatric subjects on ECMO at 8 pediatric institutions within the Eunice Kennedy Shriver National Institute of Child Health and Human Development's Collaborative Pediatric Critical Care Research Network between December 2012 and September 2014.14 The study was approved with a waiver of informed consent by the institutional review boards at each of the participating hospitals and the data coordination center at the University of Utah.
Study Population
The BATE study included neonatal and pediatric subjects supported on venoarterial (VA) and venovenous (VV) ECMO. We included subjects with a primary respiratory indication for ECMO. Subjects were excluded if ECMO was indicated for cardiac etiology or extracorporeal cardiopulmonary resuscitation. Neonates were classified as ≤ 30 d of age and pediatric subjects included those > 30 d and < 19 years of age. Prematurity was defined as neonates < 37 weeks gestation at birth. Acute and chronic diagnostic categories were previously described.14 The mode of ECMO was categorized as VV or VA, in which VA was defined as anytime an arterial cannula was in place, regardless of location. When multiple episodes or modes of ECMO occurred for a subject, only the first run was included. The vasoactive inotropic score15,16 and Pediatric Risk of Mortality III17 scores were calculated from baseline data before cannulation.
Ventilator Parameters
Ventilator parameters were analyzed at 3 time points: those most recently recorded before ECMO cannulation, on the calendar day of ECMO initiation (ECMO day 1), and those collected at 7:00 am on the calendar day before ECMO separation. Parameters included ventilation type, breathing frequency, tidal volume, PIP, PEEP, dynamic driving pressure (calculated as PIP minus PEEP), pressure support, mean airway pressure (), and FIO2. To assess lung strain, dynamic driving pressure provides a surrogate for traditional driving pressure (calculated as plateau pressure minus PEEP) given the clinical propensity to ventilate children on flow-decelerating modalities in which plateau pressures are not collected.18,19 Evaluating dynamic driving pressure also has practical implications, which align with organizational guidelines that address PIP rather than plateau pressure.4,5
Outcomes
The primary outcome was in-hospital mortality, including death after withdrawal of life-sustaining measures. To assess the effect of ventilator parameters on lung recovery and the timing of decannulation, ECMO-free days were included as a secondary outcome. ECMO-free days were defined as the number of days within the first 28 d after cannulation that subjects were both alive and no longer on ECMO.
Statistical Analysis
Demographic, clinical, and ventilatory parameters were summarized by using counts and percentages for categorical variables, and median (interquartile ranges [IQR]) were used for continuous variables. Differences in neonate and pediatric cohort characteristics were evaluated with the Fisher exact test for categorical (nominal) variables and with the Wilcoxon rank-sum test for continuous and ordinal variables. Associations with in-hospital mortality and ECMO-free days were evaluated separately in neonatal and pediatric cohorts. Univariable regression models were created to evaluate the association of outcomes with subject characteristics and ventilator parameters before ECMO and on the first day of ECMO. Logistic models were used for in-hospital mortality, and ordinary linear regression was used for ECMO-free days. The percentage of subjects with missing values for each parameter was also reported.
To identify independent predictors of in-hospital mortality and ECMO-free days, multivariable models were created. PEEP and dynamic driving pressure were presumed to have clinical importance and were forced into each model. Forcing PEEP and dynamic driving pressure restricted the analysis to those on conventional modes of ventilation and excluded those on high-frequency oscillatory ventilation (HFOV) in which PEEP, PIP, and, thus, dynamic driving pressure are not available. Although was available for all the subjects, this metric is dependent on PIP and PEEP in conventional ventilation. Moreover, the above method would inform the recommended practices by organizational guidelines that focus on the titration of PEEP rather than .4,5
Additional variables were considered for inclusion if they were missing for < 10% of the respective cohort and were at least moderately associated (P < .10) with in-hospital mortality or ECMO-free days in univariable analysis. The final multivariable models for each age group were developed by using bi-directional stepwise selection with a threshold of P <.10 to enter and P < .05 to stay in the final models. PEEP and congenital diaphragmatic hernia (CDH) were both independently predictive of in-hospital mortality among neonates. Hypothesizing that the association of PEEP and survival may differ in neonates with CDH compared with neonates without CDH, the interaction between PEEP and CDH was tested post hoc and found to be significant (P = .005). The final multivariable model of neonatal in-hospital mortality presented includes the interaction between PEEP and CDH. All P values were calculated based on 2-sided alternative hypotheses and were considered significant if < .05. Analyses were conducted by using SAS v.9.4 (SAS Institute, Cary, North Carolina).
Results
Cohort Characteristics
Of the initial 514 subjects enrolled in the original BATE study, 277 were excluded whose indication for ECMO was cardiac (n = 207) or extracorporeal cardiopulmonary resuscitation (n = 70). The remaining 237 subjects (151 neonates and 86 pediatric subjects) were eligible for analysis. Subject characteristics are summarized by age in Table 1. Of neonates, median (IQR) age was 2.0 (1.0–3.9) d, 21% (n = 31) were preterm, and 36% (n = 54) had a diagnosis of CDH. Of the pediatric subjects, the median (IQR) age was 3.2 (0.7–13.6) years, with the most frequent diagnosis listed as respiratory failure (81% [n = 70]). Seventy-eight percent of neonates (n = 117) and 48% of pediatric subjects (n = 42) were supported on VA ECMO. The median (IQR) oxygenation index was 38.7 (24.8–54.6) for neonates and 35.7 (26.9–54.6) for pediatric subjects. In-hospital mortality was 35% for both neonatal (n = 53) and pediatric (n = 30). The median (IQR) ECMO-free days was 15 (0–22) d for neonates and 17 (2–21) d for pediatric subjects. The median (IQR) duration of ECMO was 8.1 (4.9–13.0) d in neonates and 7.0 (3.7–12.3) d in pediatric subjects. The median (IQR) ICU length of stay was 31.7 (16.1–55.5) d in neonates and 31.2 (16.4–49.4) d in pediatric subjects.
Table 1.
Clinical Characteristics and Outcomes
Description of Ventilator Parameters on ECMO
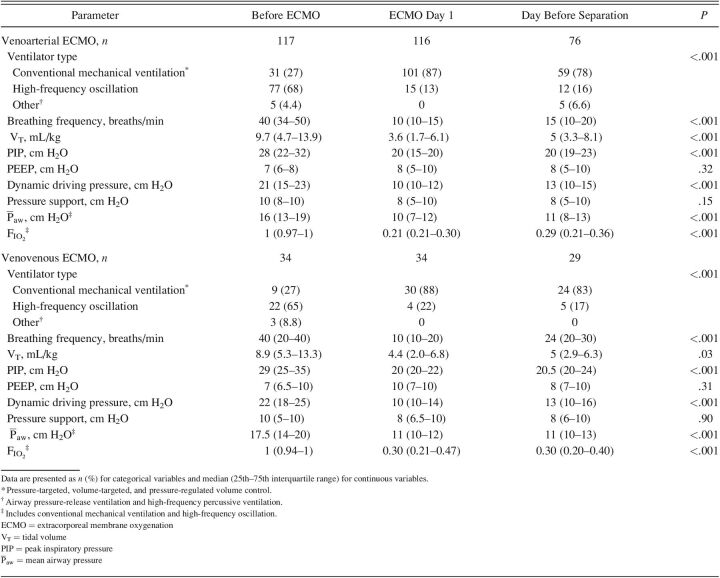
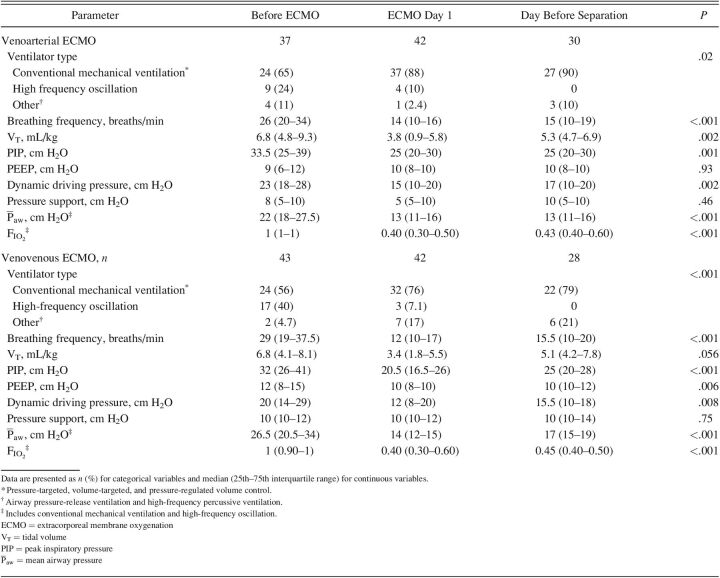
The majority of subjects underwent conventional mechanical ventilation during ECMO day 1, with the majority on pressure-targeted ventilation modalities (neonates, n = 125 [83%]; pediatric, n = 65 [77%]). In neonates, the median (IQR) PEEP on ECMO day 1 was 8 (5.0–10.0) cm H2O for subjects on VA ECMO and 10 (7.0–10.0) cm H2O for subjects on VV ECMO. In pediatric subjects, the median (IQR) PEEP on ECMO day 1 was 10 (8–10) cm H2O for both subjects on VA and those on VV ECMO. Full descriptions of ventilator parameters stratified across time points for neonatal and pediatric subjects are listed in Tables 2 and 3.
Table 2.
Ventilator Parameters Stratified by 3 Time Points in Neonates for Venoarterial and Venovenous ECMO
Table 3.
Ventilator Parameters Stratified by 3 Time Points in Pediatric Subjects for Venoarterial and Venovenous ECMO
Univariable Analysis
In neonates, ventilator parameters with lower odds of in-hospital mortality included higher before ECMO and higher , PIP, and dynamic driving pressure on ECMO day 1 (Supplementary Table 1 [see the supplementary materials at http://www.rcjournal.com]). Although ventilation type was not associated with mortality, neonates on HFOV before ECMO was associated with fewer ECMO-free days. Odds of mortality were greater for neonates with CDH, a higher Pediatric Risk of Mortality III17 score, and VA ECMO support. These clinical characteristics were also associated with fewer ECMO-free days, in addition to diagnoses of congenital anomalies and chromosomal defects. In pediatric subjects, no ventilator parameters either before or during ECMO, including breathing frequency, tidal volume, PIP, PEEP, dynamic driving pressure, pressure support, , and were associated with in-hospital mortality or ECMO-free days (Supplementary Table 2 [see the supplementary materials at http://www.rcjournal.com]). Furthermore, the mode of ECMO was not associated with mortality in pediatric subjects.
Multivariable Analysis
Neonates.
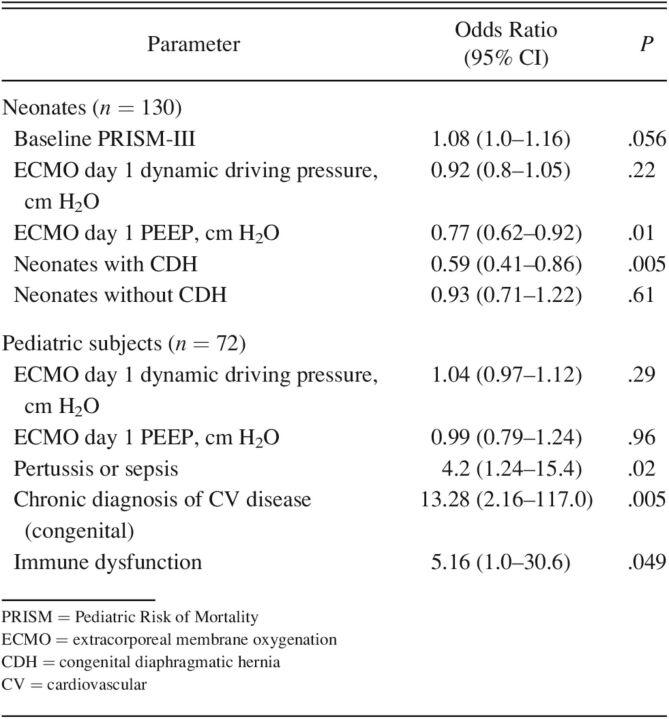
After excluding 21 neonates for missing data, 130 subjects were available for analysis. After adjusting for confounding variables, the only ventilator parameter independently associated with in-hospital mortality was higher PEEP on ECMO day 1 (odds ratio [OR] 0.77, 95% CI 0.62-0.95; P = .01) (Table 4). Odds of mortality was higher in the subjects with a concurrent CDH diagnosis (OR 8.8, 95% CI 3.71–22.67; P < .001) and higher Pediatric Risk of Mortality III17 score (OR 1.08, 95% CI 1.00–1.17; P = .056). The relationship between ECMO day 1 PEEP and mortality after accounting for the interaction between CDH and ECMO day 1 PEEP was further amplified in neonates with CDH (OR 0.59, 95% CI 0.41–0.86; P = .005). No ventilator parameters were independently associated with ECMO-free days.
Table 4.
Multivariable Models of In-Hospital Mortality
Pediatric Subjects.
After excluding 14 subjects for missing data, 72 subjects underwent multivariable analysis (Table 4). There was no association with in-hospital mortality or ECMO-free days and ECMO day 1 PEEP or dynamic driving pressure. Higher odds of in-hospital mortality was associated with acute diagnoses of immune dysfunction (OR 5.16, 95% CI 1.01–30.60; P = .049), congenital cardiovascular disease (OR 13.28, 95% CI 2.16–117.01; P = .005), and pertussis or sepsis (OR 4.20, 95% CI 1.24–15.40; P = .02).
Discussion
Optimal ventilation strategies during neonatal or pediatric ECMO for respiratory failure are not clearly established. By using the BATE dataset, we analyzed 237 subjects (age < 19 y) across 8 academic centers who received ECMO (VA or VV) for respiratory failure. We explored associations between in-hospital mortality and ECMO-free days with ventilator parameters before cannulation and on ECMO day 1. The neonates experienced lower odds of death with higher PEEP on ECMO day 1 with a stronger association of those with CDH. In the pediatric subjects, there were no independent associations between the outcomes among any observed ventilator parameters. Our findings represent a robust multi-center effort to delineate associated outcomes with ventilator parameters for both neonates and pediatric subjects on VA and VV ECMO. Given that PEEP during ECMO is largely modifiable, our findings have potential to improve outcomes.
Our neonatal results are similar to those found in adults with ARDS, in which higher PEEP when applied early during ECMO improves survival.20,21 The association between lower mortality with higher PEEP must be contextualized against variations of PEEP delivered, VA ECMO rates, subject inclusion, and mortality rates from previous studies in children. The only randomized trial that compared ventilator practices during neonatal ECMO was published in 1992, which compared “low PEEP” (3 to 5 cm H2O) with “high PEEP” (12 to 14 cm H2O) and found a shorter duration of ECMO in the high-PEEP group.11 The specified PEEP ranges in the trial extend beyond the IQR of our cohort, and, although all deaths in the trial occurred in the “low-PEEP” group, no significant association with mortality was detected (P = .08). This was likely due to a small sample size (n = 74) and an exceptionally low incidence of overall mortality (5%) not replicated in other studies.
Furthermore, only neonates supported on VA ECMO were assessed in this early trial, and, although VA ECMO remains the dominant modality, rates of VV ECMO for neonatal respiratory failure are increasing.22,23 This is particularly notable because there is a greater role for PEEP on alveolar gas exchange with pulmonary blood flow during VV ECMO. When evaluating subjects on both VA and VV ECMO, Alapati et al24 retrospectively analyzed Extracorporeal Life Support Organization (ELSO: Ann Arbor, MI) data between 2008 and 2013 (N = 3,040) and reported similar findings of shorter ECMO duration with higher levels of PEEP. Our mortality was greater at 35% compared with 21% in the study by Alapati et al,24 which may reflect differences between our inclusion of the subjects with CDH (known to have higher mortality rates), who were excluded from both previous studies. Unlike these reports, PEEP did not confer increased ECMO-free days in our cohort. This may also be related to the considerable proportion of subjects with CDH in our neonatal cohort.
Patients with CDH are often excluded from studies given their higher mortality related to lung hypoplasia with abnormal vascularity and pulmonary hypertension.25–29 This leads to a considerable paucity in the ECMO literature. Consistent with previous epidemiologic studies, 36% of our neonatal cohort was diagnosed with CDH.25,28 Mortality rates for those with CDH who received ECMO ranged between 48 and 61%27,28 and was 61% (33 of 54) in our cohort. Acknowledging that a proportion of our cohort may influence overall mortality while postulating that hypoplastic, noncompliant lungs seen in CDH may require higher distending pressures to maintain alveolar expansion, we explored a post hoc interaction between a diagnosis of CDH with PEEP on ECMO day 1. Our results support this hypothesis. Furthermore, suboptimal end-expiratory lung volumes at an already reduced functional reserve capacity increase pulmonary vascular resistance, exacerbating restriction of pulmonary blood flow through a pathologic pulmonary vasculature.30 Guidelines for patients with CDH in the absence of ECMO recommend minimal PEEP (2–5 cm H2O).31–33 We caution clinicians in maintaining these settings after ECMO cannulation. In a subanalysis of neonates with CDH in our study, each subject supported with PEEP < 6 cm H2O (n = 12) died. Our findings support Extracorporeal Life Support Organization guidelines5,34 on ventilation strategies in neonates with respiratory failure and those with CDH of advising PEEP delivery between 5 and 10 cm H2O during ECMO.
Consistent with previous reports of children on ECMO, higher PEEP in our subgroup of pediatric subjects did not confer any protection against mortality.7,8 Unlike older children, infants have a less rigid, underdeveloped chest wall with less outward elastic support, reducing functional reserve capacity and increasing closing capacity, which thereby results in a greater tendency toward atelectasis.35 PEEP may preferentially benefit neonates by increasing this gap between functional reserve capacity and closing capacity thus promoting alveolar expansion and reducing alveolar collapse and atelectrauma. There also was a larger range of PEEP in neonates, particularly in those on VA ECMO, compared with pediatric subjects in whom greater differences in lung physiology and the etiology of respiratory failure exist between 30 d and 19 years of life. We did not detect an association between higher and mortality as reported by Friedman in two separate multi-center cohorts of subjects ages 14 d to 18 years.7,8 Notably, these reports excluded VA ECMO and patients with CDH. Compared with VV ECMO, oxygen delivery during VA ECMO is less reliant on pulmonary blood flow, which makes less contributory. For this reason, our inclusion of pediatric subjects on VA ECMO may have lowered exposure of harmful and diluted an appreciable effect on outcomes.
Reduced mortality during neonatal ECMO for respiratory failure with increased PEEP is limited by the inherent maximum benefit of applied PEEP. Our analysis is constrained by the observed ventilation parameters of the cohort. It is an important distinction to note that levels of PEEP in neonates were not necessarily elevated after ECMO cannulation but rather maintained. Certainly, excessive amounts of PEEP is likely to result in harmful effects, therefore, interpretation of our findings should focus on the avoidance of low PEEP. Previous investigations have shown that using a lower PEEP relative to than recommended by the ARDS Network model36,37 had higher mortality. The highest upper quartile of PEEP before ECMO in our cohort was 10 cm H2O, far lower than those recommended by ARDS Network for the degree of administered . Although this concept supports our findings, this relationship has not been evaluated on ECMO and the identification of the nadir of in-hospital mortality against increasing levels of PEEP is an important topic for future studies.
To evaluate the current Extracorporeal Life Support Organization recommendations,4,5 PEEP and dynamic driving pressure were forced into the multivariable models. This not only excluded subjects on HFOV but may have limited the observed effect on . Univariate analysis in neonates demonstrated reduced odds of mortality with higher both before and after ECMO cannulation (Supplementary Table 1 [see the supplementary materials at http://www.rcjournal.com]). No independent relationships were captured between and mortality or ECMO-free days in multivariable models, possibly due to the intrinsic correlation between PEEP and during conventional ventilation or from exclusion of HFOV cases. As a shared metric across conventional and non-conventional types of ventilation, it is tempting to examine outcomes with rather than PEEP. However, applications of differ across ventilation types, which makes it challenging to target across modes. Compared with HFOV, in which is the primary distending pressure, during conventional ventilation, is also dependent on PIP, a variable associated with worse outcomes at higher pressures.38–42 Although a benefit of avoiding low during ECMO may exist, similar to PEEP in our study, this relationship may be complicated by the deleterious outcomes noted when higher is provided via HFOV to neonates with CDH before ECMO43 or when higher affects the ability to provide flow and support to patients on ECMO.20
Despite evidence of increased mortality with higher driving pressure (plateau pressure minus PEEP) seen in adults on ECMO,44–46 we did not find an independent association between dynamic driving pressure and outcomes in neonates or pediatric subjects. Our findings resemble previous investigations in children with respiratory failure while on ECMO8 as well as those supported without.19,47 This discrepancy between adult and pediatric subjects remains uncertain with theories, including lower absolute dynamic driving pressure applied8 or resiliency in the pediatric lung with greater elasticity against strain.47 Assessment of regional lung mechanics, especially in acute respiratory failure, can be challenging, and intrinsic patient factors, such as chest wall compliance, affect dynamic driving pressure, which makes the complex relationship among dynamic driving pressure, PEEP, and interrelated and warrants further investigation for risk stratification, select patient populations, and/or additional recommendations for subjects supported on non-conventional types of mechanical ventilation during peri-cannulation periods.
Limitations of this study are those inherent to observational design and causality cannot be inferred. Our findings are only generalizable to patients ventilated within the parameters studied, specifically applied PEEP from aforementioned ranges. Candidate variables were limited to those available in the BATE dataset. The analysis was limited to 2 time points, and any ventilator adjustments that occurred outside those time points were not evaluated. No protocol existed for ECMO candidacy or titration of ventilator settings. Although the mode of ECMO was included in the multivariable model, our sample size limited the ability to separately analyze subjects on VA and VV ECMO. The smaller sample size also limited the ability to assess PEEP as categorical ranges, which may provide more practical results. Our analysis did not risk adjust for contemporary pre-ECMO predictors of in-mortality for children with respiratory failure.48,49 Data related to procedures such as bronchoscopy, prone positioning, or recruitment maneuvers while on ECMO were not available. Lastly, we acknowledge that much of the data were collected a decade ago. Our results are informative, given the median values of key ventilator parameters such as PIP and breathing frequency, PEEP, dynamic driving pressure, and fall within contemporary guidelines for neonates and pediatric patients with respiratory failure on ECMO, including those with CDH.4,5,34
Conclusions
In a secondary analysis, increased PEEP on ECMO day 1 was independently associated with decreased odds of death in neonates. Other ventilator parameters before ECMO and on ECMO day 1 were not independently associated with mortality in neonatal or pediatric subjects. Although direct recommendations cannot be prescribed to specific PEEP ranges due to inherent limitations of this exploratory analysis, caution should be taken when applying low levels of PEEP for neonates on ECMO, particularly for those with CDH.
Supplementary Material
Footnotes
The data were retrieved and analyzed in the Division of Critical Care Medicine, Salt Lake City, UT.
Dr Miya presented a version of this paper at the Extracorporeal Life Support Organization Annual Conference, held virtually September 25–26, 2020.
Dr Dalton is a consultant to industry of Entegrion, Hemocue, is the medical director of Innovative ECMO Concepts, and is on the advisory board for BREATHE-Oxi 1 (Abiomed), Medtronic. The other authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
REFERENCES
- 1. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369(22):2126–2136. [DOI] [PubMed] [Google Scholar]
- 2. Nieman GF, Satalin J, Andrews P, Habashi NM, Gatto LA. Lung stress, strain, and energy load: engineering concepts to understand the mechanism of ventilator-induced lung injury (VILI). Intensive Care Med Exp 2016;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 1994;149(5):1327–1334. [DOI] [PubMed] [Google Scholar]
- 4. Maratta C, Potera RM, van Leeuwen G, Castillo Moya A, Raman L, Annich GM. Extracorporeal Life Support Organization (ELSO): 2020 Pediatric Respiratory ELSO Guideline. ASAIO J 2020;66(9):975–979. [DOI] [PubMed] [Google Scholar]
- 5. Wild KT, Rintoul N, Kattan J, Gray B. Extracorporeal Life Support Organization (ELSO): guidelines for neonatal respiratory failure. ASAIO J 2020;66(5):463–470. [DOI] [PubMed] [Google Scholar]
- 6. Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc 2014;11(6):956–961. [DOI] [PubMed] [Google Scholar]
- 7. Friedman ML, Abu-Sultaneh S, Slaven JE, Mastropietro CW. Rest ventilator management in children on veno-venous extracorporeal membrane oxygenation. Int J Artif Organs 2022;45(2):174–180. [DOI] [PubMed] [Google Scholar]
- 8. Friedman ML, Barbaro RP, Bembea MM, Bridges BC, Chima RS, Kilbaugh TJ, et al. Mechanical ventilation in children on venovenous ECMO. Respir Care 2020;65(3):271–280. [DOI] [PubMed] [Google Scholar]
- 9. Green TP, Timmons OD, Fackler JC, Moler FW, Thompson AE, Sweeney MF. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Crit Care Med 1996;24(2):323–329. [DOI] [PubMed] [Google Scholar]
- 10. Kattan J, Gonzalez A, Becker P, Faunes M, Estay A, Toso P, et al. Survival of newborn infants with severe respiratory failure before and after establishing an extracorporeal membrane oxygenation program. Pediatr Crit Care Med 2013;14(9):876–883. [DOI] [PubMed] [Google Scholar]
- 11. Keszler M, Ryckman FC, McDonald JV, Jr, Sweet LD, Moront MG, Boegli MJ, et al. A prospective, multicenter, randomized study of high versus low positive end-expiratory pressure during extracorporeal membrane oxygenation. J Pediatr 1992;120(1):107–113. [DOI] [PubMed] [Google Scholar]
- 12. Lin JC. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. Respir Care 2017;62(6):732–750. [DOI] [PubMed] [Google Scholar]
- 13. Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2008(3):CD001340. [DOI] [PubMed] [Google Scholar]
- 14. Dalton HJ, Reeder R, Garcia-Filion P, Holubkov R, Berg RA, Zuppa A, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2017;196(6):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11(2):234–238. [DOI] [PubMed] [Google Scholar]
- 16. Gaies MG, Jeffries HE, Niebler RA, Pasquali SK, Donohue JE, Yu S, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014;15(6):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996;24(5):743–752. [DOI] [PubMed] [Google Scholar]
- 18. van Schelven P, Koopman AA, Burgerhof JGM, Markhorst DG, Blokpoel RGT, Kneyber MCJ. Driving pressure is associated with outcome in pediatric acute respiratory failure. Pediatr Crit Care Med 2022;23(3):e136–e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rauf A, Sachdev A, Venkataraman ST, Dinand V. Dynamic airway driving pressure and outcomes in children with acute hypoxemic respiratory failure. Respir Care 2021;66(3):403–409. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care 2014;18(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med 2015;43(3):654–664. [DOI] [PubMed] [Google Scholar]
- 22. Sharma J, Sherman A, Rimal A, Haney B, Weiner J, Pallotto E. Neonatal respiratory extracorporeal membrane oxygenation and primary diagnosis: trends between two decades. J Perinatol 2020;40(2):269–274. [DOI] [PubMed] [Google Scholar]
- 23. Roy BJ, Rycus P, Conrad SA, Clark RH. The changing demographics of neonatal extracorporeal membrane oxygenation patients reported to the Extracorporeal Life Support Organization (ELSO) Registry. Pediatrics 2000;106(6):1334–1338. [DOI] [PubMed] [Google Scholar]
- 24. Alapati D, Aghai ZH, Hossain MJ, Dirnberger DR, Ogino MT, Shaffer TH; Extracorporeal Life Support Organization Member Centers. Lung rest during extracorporeal membrane oxygenation for neonatal respiratory failure-practice variations and outcomes. Pediatr Crit Care Med 2017;18(7):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. ; ELSO member centers. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grover TR, Murthy K, Brozanski B, Gien J, Rintoul N, Keene S, et al. ; Children's Hospitals Neonatal Consortium. Short-term outcomes and medical and surgical interventions in infants with congenital diaphragmatic hernia. Am J Perinatol 2015;32(11):1038–1044. [DOI] [PubMed] [Google Scholar]
- 27. Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg 2009;44(9):1691–1701. [DOI] [PubMed] [Google Scholar]
- 28. Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 2013;59(3):202–210. [DOI] [PubMed] [Google Scholar]
- 29. Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB; Congenital Diaphragmatic Hernia Study Group. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg 2009;44(7):1315–1321. [DOI] [PubMed] [Google Scholar]
- 30. Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015;132(21):2037–2099. [DOI] [PubMed] [Google Scholar]
- 31. Canadian Congenital Diaphragmatic Hernia Collaborative; Puligandla P, Skarsgard E, Offringa M, Adatia I, Baird R, Bailey M, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ 2018;190(4):E103–E112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, et al. Management of congenital diaphragmatic hernia: a systematic review from the APSA Outcomes and Evidence Based Practice Committee. J Pediatr Surg 2015;50(11):1958–1970. [DOI] [PubMed] [Google Scholar]
- 33. Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. ; CDH EURO Consortium. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology 2010;98(4):354–364. [DOI] [PubMed] [Google Scholar]
- 34. Guner Y, Jancelewicz T, Di Nardo M, Yu P, Brindle M, Vogel AM, et al. ; for the Elso CDH Interest Group. Management of congenital diaphragmatic hernia treated with extracorporeal life support: interim guidelines consensus statement from the Extracorporeal Life Support Organization. ASAIO J 2021;67(2):113–120. [DOI] [PubMed] [Google Scholar]
- 35. Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol 1972;33(6):711–714. [DOI] [PubMed] [Google Scholar]
- 36. Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351(4):327–336. [DOI] [PubMed] [Google Scholar]
- 37. Khemani RG, Parvathaneni K, Yehya N, Bhalla AK, Thomas NJ, Newth CJL. Positive end-expiratory pressure lower than the ARDS Network Protocol is associated with higher pediatric acute respiratory distress syndrome mortality. Am J Respir Crit Care Med 2018;198(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, et al. ; Paediatric Study Group; Australian and New Zealand Intensive Care Society. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med 2007;8(4):317–323. [DOI] [PubMed] [Google Scholar]
- 39. Khemani RG, Conti D, Alonzo TA, Bart RD, III, Newth CJ. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med 2009;35(8):1428–1437. [DOI] [PubMed] [Google Scholar]
- 40. Brogan TV, Zabrocki L, Thiagarajan RR, Rycus PT, Bratton SL. Prolonged extracorporeal membrane oxygenation for children with respiratory failure. Pediatr Crit Care Med 2012;13(4):e249–e254. [DOI] [PubMed] [Google Scholar]
- 41. Minneci PC, Kilbaugh TJ, Chandler HK, Behar BJ, Localio AR, Deans KJ. Factors associated with mortality in pediatric patients requiring extracorporeal life support for severe pneumonia. Pediatr Crit Care Med 2013;14(1):e26–e33. [DOI] [PubMed] [Google Scholar]
- 42. Panico FF, Troster EJ, Oliveira CS, Faria A, Lucena M, Joao PR, et al. Risk factors for mortality and outcomes in pediatric acute lung injury/acute respiratory distress syndrome. Pediatr Crit Care Med 2015;16(7):e194–e200. [DOI] [PubMed] [Google Scholar]
- 43. Yang MJ, Fenton S, Russell K, Yost CC, Yoder BA. Left-sided congenital diaphragmatic hernia: can we improve survival while decreasing ECMO? J Perinatol 2020;40(6):935–942. [DOI] [PubMed] [Google Scholar]
- 44. Chiu L-C, Hu H-C, Hung C-Y, Chang C-H, Tsai F-C, Yang C-T, et al. Dynamic driving pressure associated mortality in acute respiratory distress syndrome with extracorporeal membrane oxygenation. Ann Intensive Care 2017;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta E, Awsare B, Hirose H, Cavarocchi N, Baram M. Don't drive blind: driving pressure to optimize ventilator management in ECMO. Lung 2020;198(5):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serpa Neto A, Schmidt M, Azevedo LCP, Bein T, Brochard L, Beutel G, et al. ; ReVA Research Network and the PROVE Network Investigators. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis: mechanical ventilation during ECMO. Intensive Care Med 2016;42(11):1672–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yehya N, Thomas NJ. Disassociating lung mechanics and oxygenation in pediatric acute respiratory distress syndrome. Crit Care Med 2017;45(7):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barbaro RP, Boonstra PS, Paden ML, Roberts LA, Annich GM, Bartlett RH, et al. Development and validation of the pediatric risk estimate score for children using extracorporeal respiratory support (Ped-RESCUERS). Intensive Care Med 2016;42(5):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bailly DK, Reeder RW, Zabrocki LA, Hubbard AM, Wilkes J, Bratton SL, et al. ; Extracorporeal Life Support Organization Member Centers. Development and validation of a score to predict mortality in children undergoing extracorporeal membrane oxygenation for respiratory failure: pediatric pulmonary rescue with extracorporeal membrane oxygenation prediction score. Crit Care Med 2017;45(1):e58–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.