Abstract
Brain metastases are the most common brain malignancy. This review discusses the studies presented at the third annual meeting of the Melanoma Research Foundation in the context of other recent reports on the biology and treatment of melanoma brain metastases (MBM). Although symptomatic MBM patients were historically excluded from immunotherapy trials, efforts from clinicians and patient advocates have resulted in more inclusive and even dedicated clinical trials for MBM patients. The results of checkpoint inhibitor trials were discussed in conversation with current standards of care for MBM patients, including steroids, radiotherapy, and targeted therapy. Advances in the basic scientific understanding of MBM, including the role of astrocytes and metabolic adaptations to the brain microenvironment, are exposing new vulnerabilities which could be exploited for therapeutic purposes. Technical advances including single-cell omics and multiplex imaging are expanding our understanding of the MBM ecosystem and its response to therapy. This unprecedented level of spatial and temporal resolution is expected to dramatically advance the field in the coming years and render novel treatment approaches that might improve MBM patient outcomes.
1 ∣. INTRODUCTION
Brain metastases from solid tumors can be a deadly step in cancer progression. Melanoma is the third most common source of brain metastases, exceeded only by lung and breast cancer (Berghoff et al., 2016; Schouten et al., 2002), and is the solid tumor with the highest propensity for homing to the brain (Lamba et al., 2021). The incidence of clinically detected brain metastases in patients with advanced melanoma is approximately 40%, with a higher percentage identified at autopsy (Davies et al., 2011; Amer et al., 1978). Historically, life expectancy for patients with melanoma involving the central nervous system (CNS) is short, with a median survival of less than 6 months (Davies et al., 2011; Zhang et al., 2019). This has changed in the era of checkpoint inhibitors, targeted therapy, and targeted radiation techniques. More recent studies report improved survival up to 21 months in some subsets of patients; however, much work remains to prolong survival, improve quality of life, and meet the challenges of caring for this complex patient population (Knisely et al., 2012; Rauschenberg et al., 2019; Vosoughi et al., 2018; Tio et al., 2018; Bander et al., 2021).
The Melanoma Research Foundation convened the first summit specifically focused on the biology and treatment of melanoma brain metastases (MBM) in 2015. A second meeting was held in 2019. The third meeting was delayed due to the ongoing COVID-19 pandemic but was held in November 2021 in a hybrid fashion. Herein, we outline the current state of research and management for MBM based on discussions at and after that meeting. This includes the status of MBM treatments, clinical and basic research, clinical trial design, and current challenges and future opportunities. A separate publication will focus on these issues as they relate to leptomeningeal disease (LMD) from melanoma, to focus on the unique characteristics and distinct biology of this clinical presentation.
2 ∣. RADIATION THERAPY
In (Chu & Hilaris, 1961) demonstrated the palliative effect of radiation therapy in over 200 patients with brain metastases from multiple solid malignancies. Since that time, national guidelines have been developed and are regularly updated to standardize the use of radiation for brain metastases (Vogelbaum et al., 2022). Dr. Jonathan Knisely from Weill Cornell Medicine reviewed several proposed mechanisms as to how radiation triggers this therapeutic benefit based on its effects on DNA, tissue microvasculature, and immune stimulation. One elucidated mechanism is via the generation of chromosome fragments that escape into the cytoplasm and trigger type-1 interferon secretion via cyclic GMP-AMP synthase (cGAS), which in turn mediates an immune response (Chen et al., 2016; Cai et al., 2014; Fuertes et al., 2011). The presence of DNA fragments can also induce the production of DNA exonuclease Trex1, which degrades the accumulated DNA and abrogates the activation of interferon and the subsequent immune cell repertoire (Vanpouille-Box et al., 2017). Ionizing radiation can also create oxygen free radicals which in turn damage DNA either leading to cell repair or cell death (Ward, 1988; Goldstein & Kastan, 2015).
Dr. Knisely also discussed that radiation therapy can be delivered either in the form of fractionated daily doses, which exploit the differing proliferation and DNA damage repair rates between normal and tumor tissue (Santivasi & Xia, 2014); or as radiosurgery utilizing less frequent but larger daily doses that overwhelm the DNA damage repair machinery and may stimulate the immune system by liberating dsDNA fragments into the tumor microenvironment (Lugade et al., 2005, Lee et al., 2009). Stereotactic radiosurgery (SRS) allows for the sparing of unaffected brain tissue with good local control of metastatic lesions, but still provides only modest improvement in median overall survival (OS) from about 4–7 months (Christ et al., 2015). Whole brain radiation (WBRT) on the other hand is a palliative measure that does not improve OS and is associated with impairment in cognitive performance and function following treatment (McDuff et al., 2013; Brown et al., 2016). In patients with MBM, WBRT over observation reduced the local failure rate but not the distant intracranial failure rate (DICF) or time to DICF, with no improvement in OS (Hong et al., 2019). Based on the limited benefit that WBRT provides patients with MBM and its recognized toxicity profile, its use should be discouraged whenever focal stereotactic radiosurgical treatments can be employed, even when multiple metastases may be present.
It was not until the combination of radiation therapy with active systemic therapy that patients with MBM started to experience more significant improvements in survival (Tazi et al., 2015; Samlowski et al., 2007). Preclinical models have demonstrated that RT combined with immunotherapy can overcome treatment resistance in poorly immunogenic tumor models (Demaria et al., 2005; Pilones et al., 2020). This synergism has been thought to leverage the potential abscopal response in which radiation induces exposure of immunogenic mutations to the immune system. No prospective studies to date have demonstrated synergistic activity, but a large body of retrospective evidence seems consistent with the benefit of combining modalities. In clinical practice, the addition of the immune checkpoint inhibitor ipilimumab to SRS led to an unprecedented median OS of up to 21 months (Knisely et al., 2012; Silk et al., 2013) in patients with MBM. Combining targeted therapy and radiosurgery has also been demonstrated to elicit intracranial disease control and improve survival (Narayana et al., 2013; Wolf et al., 2016; Long et al., 2012; Ahmed et al., 2015). There are challenges to the use of radiation therapy and systemic therapies, such as increased toxicity or confounding effects of one treatment on the other. Retrospective evidence suggests that immunotherapy combined with SRS may increase the likelihood of symptomatic radiation necrosis which can significantly impact the quality of life (Martin et al., 2018). As the use of WBRT in treating MBM has been supplanted by targeted techniques, the panel focused on several remaining open questions, including how to improve the safe delivery of SRS, whether to administer several fractions versus one, whether de-escalating the doses may improve the immune response and decrease inflammation as is currently being investigated by the ABC-X trial (NCT03340129), and what kind of in vivo immune response is truly induced by SRS over and above that of the immunotherapy itself.
3 ∣. SURGICAL THERAPY
Surgical resection has historically been performed in the minority of melanoma patients, typically for solitary brain metastases or with palliative intent of a dominant symptomatic lesion (Wroński & Arbit, 2000). With modern practice improvements, surgical resection of brain metastasis can play an increasingly important role in the integrated management of melanoma patients. Dr. Daniel Cahill from Massachusetts General Hospital and Dana Farber/Harvard Cancer Center presented a treatment algorithm for the surgical management of MBM. Based on studies in melanoma and other tumor histologies, surgery is supported by level I evidence when there is a solitary lesion and the patient has a good functional status, controlled extracranial disease, or if the tumor is large and located in the posterior fossa. If there are multiple lesions, surgery may still be indicated if there is a dominant lesion that is causing symptoms; enlarging hemorrhagic or cystic metastases (less amenable to SRS); or if there is a need for tissue for diagnosis. Surgical intervention can be preferable over the less invasive SRS if there is a need for rapid resolution of mass effect, which allows for the more rapid tapering of high-dose steroids and permits more effective antitumor action of immune checkpoint inhibitors. Indeed, surgery before checkpoint therapy appears to result in longer OS compared to the reverse, possibly by allowing for faster systemic disease control and more expeditious discontinuation of steroids prior to immunotherapy (Alvarez-Breckenridge et al., 2019). On the other hand, SRS has the advantage of being able to treat small, deep lesions with an outpatient procedure that does not require general anesthesia and has a short recovery time that can allow for the rapid initiation of cytotoxic systemic therapies (Suh, 2010). In general, surgical management of MBM requires a multidisciplinary approach that incorporates systemic therapy and/or radiation options.
4 ∣. IMMUNE THERAPY
Since 2011 there have been 12 agents approved for the treatment of stage IV melanoma. Starting with ipilimumab in 2011, the OS of patients with metastatic melanoma has increased from less than 5% at 5 years to 50% with the combination of ipilimumab and nivolumab (Larkin et al., 2019). To date, close to 8000 patients have been included in phase III trials for advanced melanoma, whereas trials that include patients with MBM have amassed only ~800 participants. Historically, patients with MBM have been excluded from clinical trials for multiple reasons, including concerns about the brain penetrance of drugs, and/or the worse prognosis of these patients; belief that the brain is an “immune sanctuary” that would render immune therapies ineffective; and the possibility that surgery and radiation may be better approaches for intracranial diseases over systemic treatments. Evidence has refuted some of these hypotheses; in particular, T cells can infiltrate the brain and improve therapeutic outcomes. Histopathologic analysis of MBM with concurrently performed whole genome expression profiling and immunohistochemistry have demonstrated that MBM are more similar to other extracranial metastases compared to primary melanomas, and that the presence of immune infiltrates is a favorable prognostic factor associated with improved survival (Hamilton et al., 2013). Global interrogation of these tumors by RNA-seq, including MBM patients with paired extracranial metastases, confirmed the prognostic significance of immune infiltrates in MBMs. The study also showed that MBMs harbored significant immunosuppression and enrichment of oxidative phosphorylation compared to the patient-matched non-CNS metastases (Fischer et al., 2019). Whole exome sequencing on different tumor types has demonstrated that brain metastases and primary tumors follow a branched evolution pattern such that each lesion continues to evolve separately and develop its own distinct mutational profile, underscoring the need to study MBM as a distinct disease process in terms of pathophysiology and response to treatment (Brastianos et al., 2015).
Patients with CNS disease often require steroids to control edema and inflammation. This portends a more aggressive disease course and has been used as justification to exclude such patients from clinical trials. In a phase II trial of ipilimumab in patients with MBMs the median OS in asymptomatic patients not on steroids (Cohort A) was 7 months while patients with symptomatic brain metastases and on steroids at study initiation (Cohort B) had a median survival of 3.7 months (Margolin et al., 2012). In a cohort of patients with asymptomatic MBM treated with pembrolizumab, the overall response rate was 22% with a median OS of 17 months (Kluger et al., 2019). A very similar intracranial response rate of 20% was observed for nivolumab in patients with asymptomatic MBMs (Cohort B) in the ABC trial, whereas the response rate was only 6% in patients with neurological symptoms, LMD, or previous CNS-directed treatment (Cohort C) (Long et al., 2018). Dr. Hussein Tawbi from MD Anderson Cancer Center presented the final results of the CheckMate 204 trial which was a phase II study that looked at the long-term outcomes of patients with MBM treated with combination nivolumab plus ipilimumab at standard doses (Tawbi et al., 2021) and included cohorts of both asymptomatic (A) and symptomatic (B) intracranial disease, with patients in cohort B allowed to receive 4 mg or less dexamethasone or an equivalently dosed steroid per day. Both cohorts were treated with up to four cycles of combination checkpoint inhibitor therapy before proceeding to nivolumab maintenance until progression or toxicity. At a longer follow-up of 36 months, the OS in cohort A (101 patients) was 72% and for cohort B (18 patients) was 36%. The partial or complete intracranial clinical benefit rate was 54% in cohort A but only 22% in cohort B. Similar results were obtained for progression-free survival, with rates of response at 36 months for extracranial and global disease (62% and 48% for cohort A, respectively and 36% and 26% for cohort B). The median duration of response had not been reached for either cohort at the time of publication. These results suggest that patients who achieve a response in their MBM tend to have a durable response, and have revolutionized the treatment of patients with MBM to nearly always include this “full dose” ipilimumab (3 m/kg)/nivolumab (1 mg/kg) regimen as a component of therapy.
Although immunotherapy now is recognized to have considerable efficacy, especially in asymptomatic MBM patients, Dr. Tawbi emphasized that there are still significant numbers of patients (those receiving steroids and those with symptomatic MBM) that are not benefiting from our available treatments and research, and who continue to be excluded from trials that are changing the standard of care. There is a need to look further into novel combinations of drugs with CTLA-4/PD-1/PD-L1 inhibitors that may lead to increased efficacy and decreased toxicity and to examine the potentially increased effectiveness of incorporating SRS with active systemic agents as is being done in the ABC-X trial (NCT02374242). This is a phase II, open label, randomized trial of combination ipilimumab/nivolumab with concurrent SRS vs. dual checkpoint inhibitor alone in patients with asymptomatic untreated MBM. Currently, there are clinical trials underway investigating other checkpoint inhibitors such as antagonists to VISTA (NCT02812875) and TIM-3 (NCT04370704) and their efficacy in advanced tumors including melanoma. Recently, blocking LAG-3 has demonstrated efficacy in combination with nivolumab in the treatment of metastatic melanoma patients without CNS disease, and future trials will look to decipher whether there is a role of LAG-3 antagonists in the treatment of brain metastases (Tawbi et al., 2022). Table 1 summarizes the approaches discussed for future clinical trials design, and Table 2 lists the currently recruiting clinical trials enrolling patients with MBM receiving either systemic therapy or in combination with radiation. Combining immunotherapy with VEGF inhibition is being investigated in several studies, as it is a strategy that may improve the intracranial activity of immunotherapy and reduce the need for steroids (NCT02681549, NCT03175432, and NCT04955743) (Banks et al., 2019).
TABLE 1.
Summary of clinical concepts and targets for future research and clinical trial design
| Radiation | Number of fractions: one vs. multiple |
| Dose de-escalation (lower doses to stimulate cGAS-STING) | |
| Sequencing of SRS + systemic therapy vs. concurrent | |
| Synergism between SRS and immunotherapy | |
| IO | Combination of multiple checkpoint inhibitors (CTLA4, PD1/PDL1, LAG3, and TIM3) |
| IO + SRS | |
| Targeted therapies | Brain penetrant formulations |
| Higher doses of BRAF/MEK inhibitors | |
| Combination with inhibitors of metabolic targets such as PHGDH and oxidative phosphorylation | |
| Clinical trial design | Treatment arms to include symptomatic disease and patients receiving steroids |
| Response assessment standardization | |
| Imaging criteria guidelines and standard modalities | |
| Tumor agnostic trial designs | |
| Minimal lesion size (1 cm vs. 5 mm) | |
| CNS-PFS vs. non-CNS-PFS response criteria |
TABLE 2.
Currently enrolling clinical trials of MBM treated with systemic therapy or in combination with radiation
| Trial | Title |
|---|---|
| NCT04129515 | NovoTTF-200A + Pembrolizumab In Melanoma Brain Metastasis |
| NCT03175432 | Bevacizumab and Atezolizumab With or Without Cobimetinib in Treating Patients With Untreated Melanoma Brain Metastases |
| NCT04700072 | Substudy 02D: Safety and Efficacy of Pembrolizumab in Combination With Investigational Agents or Pembrolizumab Alone in Participants With Melanoma Brain Metastasis (MK-3475-02D/KEYMAKER-U02) |
| NCT04899921 | Troriluzole or Placebo Plus Ipi Plus Nivo in Mel Brain Mets |
| NCT03340129 | Anti-PD 1 Brain Collaboration + Radiotherapy Extension (ABC-X Study) (ABC-X) |
| NCT03903640 | Optune Device - TT Field Plus Nivolumab and Ipilimumab for Melanoma With Brain Metastasis |
| NCT04074096 | Binimetinib Encorafenib Pembrolizumab +/− Stereotactic Radiosurgery in BRAFV600 Melanoma With Brain Metastasis (BEPCOME-MB) |
| NCT02681549 | Pembrolizumab Plus Bevacizumab for Treatment of Brain Metastases in Metastatic Melanoma or Non-small Cell Lung Cancer |
| NCT04021420 | Safety and Efficacy of Sonocloud Device Combined With Nivolumab in Brain Metastases From Patients With Melanoma (SONIMEL01) |
| NCT03898908 | Encorafenib and Binimetinib Before Local Treatment in Patients With BRAF Mutant Melanoma Metastatic to the Brain (EBRAIN-MEL) |
| NCT04674683 | Study Comparing Investigational Drug HBI-8000 Combined With Nivolumab vs. Nivolumab in Patients With Advanced Melanoma |
| NCT03332589 | E6201 Plus Dabrafenib for the Treatment of Metastatic Melanoma Central Nervous System Metastases (CNS) |
| NCT04955743 | Pembrolizumab and Lenvatinib in Patients With Brain Metastases From Melanoma or Renal Cell Carcinoma |
| NCT04511013 | A Study to Compare the Administration of Encorafenib + Binimetinib + Nivolumab Versus Ipilimumab + Nivolumab in BRAF-V600 Mutant Melanoma With Brain Metastases |
| NCT03563729 | Melanoma Metastasized to the Brain and Steroids (MEMBRAINS) |
| NCT03873818 | Low Dose Ipilimumab With Pembrolizumab in Treating Patients With Melanoma That Has Spread to the Brain |
| NCT04899908 | Stereotactic Brain-directed Radiation With or Without Aguix Gadolinium-Based Nanoparticles in Brain Metastases |
| NCT04187872 | LITT and Pembrolizumab in Recurrent Brain Metastasis (TORCH) |
| NCT04789668 | Bintrafusp Alfa and Pimasertib for the Treatment of Patients With Brain Metastases |
| NCT04190628 | Safety of ABM-1310 in Patients With Advanced Solid Tumors |
| NCT04543188 | An FIH Study of PF-07284890 in Participants With BRAF V600 Mutant Solid Tumors With and Without Brain Involvement |
| NCT03994796 | Genetic Testing in Guiding Treatment for Patients With Brain Metastases |
5 ∣. TARGETED THERAPY
BRAF mutations are present in a multitude of tumor histologies and can be found in approximately 50% of patients with cutaneous melanoma (Davies et al., 2002). Further genetic classification of non-acral cutaneous melanomas identified NRAS, NF-1, and triple wild-type clusters; a high prevalence of c-KIT mutations in acral and mucosal melanomas; and the high occurrence of GNAQ/GNA11 and BAP1 mutations in uveal melanoma (Cancer Genome Atlas Network 2015; Harbour et al., 2010). Dr. Michael Davies from MD Anderson Cancer Center highlighted opportunities for other molecular targets in MBM. In particular, loss of PTEN, which results in the activation of the PI3K-AKT pathway, has been identified as an important factor associated with the emergence of brain metastases and decreased OS in BRAF mutant melanoma (Bucheit et al., 2014; Cho et al., 2015; Nguyen et al., 2022). Further, increased activation of the PI3K/AKT pathway in MBM (described in more detail below) may serve as a way of overcoming therapy resistance to BRAF inhibitors (Chen et al., 2014; Niessner et al., 2013). The identification of these targets and pathways drives the development of precision drugs and more effective combinations.
Dr. Davies reviewed the results of the COMBI-MB trial which was a phase II trial of dabrafenib and trametinib in patients with BRAF-V600 mutant metastatic melanoma with new or progressive brain metastases (Davies et al., 2017). There were cohorts for untreated and previously treated patients, and steroid use was allowed. The intracranial objective response rate was 44%–58% in the different cohorts, although this was accompanied by a short duration of response of 6.5 months and a median PFS of only 5.6 months. Half of the patients progressed in the brain while their extracranial disease was still controlled. One hypothesis for this observation is that there is less penetrance of small molecule inhibitors into the brain parenchyma (Bollag et al., 2012; Flaherty et al., 2012; Dummer et al., 2018). Results from lung cancer trials have demonstrated that higher doses of osimertinib in patients with EGFR mutant CNS disease can result in meaningful improvements in efficacy even in pretreated patients or those with LMD (Yang et al., 2017, Park et al., 2020). Thus, trials have been designed to explore the activity of higher doses of BRAF/MEK inhibitors (POLARIS trial; NCT03911869). Alternatively, studies in both lung cancer and breast cancer have demonstrated that inhibitors designed to penetrate the blood–brain barrier (BBB) more efficiently may both effectively treat established brain metastases and prevent the development of new brain metastases (Murthy et al., 2020; Lin et al., 2020; Felip et al., 2021; Shaw et al., 2020). This strategy is now being tested in patients with melanoma with new agents with improved penetration of the BBB, such as the ABM-1310 (NCT04190628), PF-07284890 (NCT04543188), or E6201 (NCT03332589).
Another strategy under investigation is to combine clinically available BRAF/MEK inhibitors with checkpoint inhibitors in an attempt to increase the intracranial response (NCT04511013, NCT03625141, and NCT02910700). The TRIDeNT study was presented at ASCO 2021 (NCT02910700) which combined dabrafenib, trametinib, and nivolumab in patients with BRAF mutant metastatic melanoma, including patients previously treated with anti-PD1 and/or with active brain metastases. The objective response rate for the entire population was 92%. For patients with MBMs, the intracranial response rate was 63%, and they had a similar median PFS (8.0 months) compared to patients without CNS involvement (median 8.5 months) (Burton et al., 2021).
Ongoing research in MBM is identifying molecular pathways that could serve as targets for novel inhibitors (see section “Molecular Determinants of Melanoma Brain Metastasis”). As mentioned previously, increased activity of the PI3K-AKT pathway and increased expression of and dependence upon oxidative phosphorylation, have been identified in MBMs and may both be susceptible to targeted inhibition (Chen et al., 2014; Fischer et al., 2019; Fukumura et al., 2021). 3-phosphoglycerate dehydrogenase (PHGDH) is upregulated in aggressive brain metastasis models and clinical samples, including in melanoma and breast cancer. PHGDH is the rate-limiting enzyme of serine synthesis from glucose and PHGDH inhibition results in potent and selective inhibition of breast and melanoma brain metastasis (Ngo et al., 2020). Targeting these novel pathways in combination with other already effective therapies may improve the responses and outcomes of this patient population. Indeed, given the increased effectiveness of PHGDH (and oxidative phosphorylation) inhibition against intracranial tumors compared to extracranial metastases, patients with brain metastases are the ideal patient population in which to study drugs against these pathways. The activity of such inhibitors was demonstrated in melanoma and breast cancer brain metastasis models suggesting that there may be a potential role for future histology agnostic clinical trials targeting common pathways.
6 ∣. TRIAL DESIGN AND RESPONSE CRITERIA
The frequent exclusion of melanoma patients with brain metastasis from the trials that later inform the standard of care leads to results that do not fully represent the needs of patients with this disease. Dr. Patrick Wen from Dana Farber/Harvard Cancer Center reviewed the current state of clinical trial design as it pertains to brain metastases and the consensus guidelines on the response criteria to be used in trials when assessing the activity of regimens in the CNS.
In 2020, the FDA published new guidelines to encourage the inclusion of patients with brain metastases in clinical trials (FDA 2020). Such efforts had already been supported by ASCO and Friends of Cancer Research, who advocated for clinical trials to be less restrictive in the type of patients excluded from enrollment (Gore et al., 2017). Recommendations were made for the inclusion of patients with stable, treated, or active/progressing brain metastases as well as for patients with leptomeningeal involvement. Certain high-risk groups of patients and therapeutic agents continue to be excluded due to safety concerns, including those with risk of hemorrhage or seizures, as well as patients receiving corticosteroids that might affect the efficacy of the investigational treatment. As these are the patients with the worst outcomes and lowest responses to current therapies, they are in greatest need of access to potentially active investigational therapies. The FDA released companion guidance on the evaluation of cancer drugs in patients with CNS metastases in which they recommended that CNS disease should not be evaluated in isolation from the systemic response. Based on this, they will be evaluating the effect of systemic drugs on CNS metastases in the context of the entire burden of metastatic disease regardless of whether the trial was conducted exclusively in patients with CNS disease or if they were only a subset of the studied population. There were also recommendations for imaging assessment and time-to-event endpoints. The overall objective of the efforts is to encourage industry and investigators to limit the exclusion of BM (and MBM) patients from therapeutic trials.
As the inclusion of patients with MBM in trials increases, providers are met with unique challenges in the on-protocol treatment of MBM. Although RECIST criteria exist for assessing responses to extracranial solid tumors until recently there was a lack of consensus criteria for assessing intracranial responses. In addition, there is a need for standardization of the eligibility criteria that are used for enrollment. Both of these issues have begun to be addressed.
In 2015, the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) working group collaborated to propose a standardized response and progression criteria for the assessment of brain metastases in clinical trials (Lin et al., 2015). This guideline proposed definitions for assessing response in CNS disease based on the complete response, partial response, stable disease, and progressive disease. It included clinical status and steroid use within these parameters and proposed that the CNS be considered as a separate compartment when assessing overall response, allowing for more granular reporting of outcomes. Based on this, studies can now report CNS response rate, non-CNS response rate, “bi-compartments PFS,” and separate reporting for CNS-PFS and non-CNS PFS. This can improve the accuracy and applicability of specific trials as those studying local therapies can report on intracranial PFS as a primary endpoint, whereas systemic therapies trials should report on bi-specific PFS since intracranial PFS alone is not an acceptable primary endpoint.
There continues to be debate about the most accurate and appropriate definition of “measurable disease.” Based on RECIST 1.1 criteria, the measurable disease is defined as a contrast-enhancing lesion that can be accurately measured in at least one dimension with a minimum size of 10 mm; however, some trialists have recommended using 5 mm as a size cutoff when the thickness of MRI slices is 2 mm or less (Schwartz, Litière, et al., 2016a; Schwartz, Blacher, et al., 2016b; Qian et al., 2017) given the propensity for brain lesions to recur frequently as multiple lesions less than 10 mm in size. This change would allow for more patients to be included in trials. However, there are concerns about the reproducibility and accuracy of measuring changes in lesions that are <10 mm which will need to be addressed, perhaps using pooled data from ongoing trials.
To maximize reproducibility and consistency across treatment centers, a consensus is needed regarding the standardization of brain tumor imaging protocols, which has also been addressed in new guidelines (Kaufmann et al., 2020). All of these efforts seek to increase inclusion, standardize care delivery and improve the validity of results from trials that treat patients with brain metastases.
7 ∣. MOLECULAR DETERMINANTS OF MELANOMA BRAIN METASTASIS
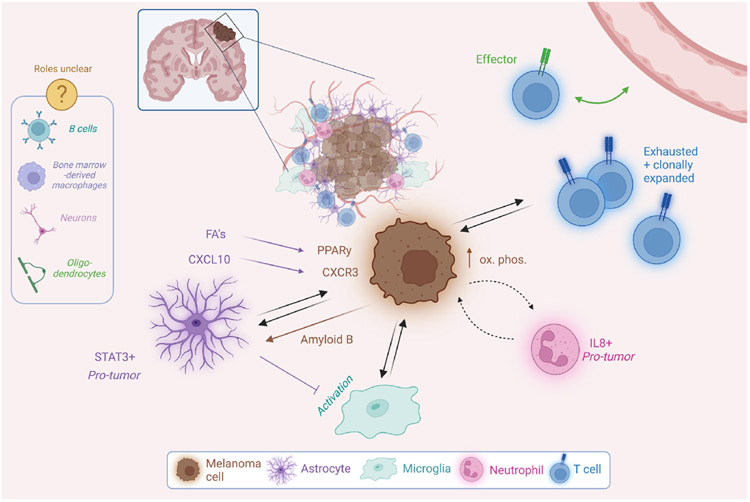
At the second MRF Brain Metastasis Summit in 2019, the study of the tumor microenvironment (TME) was coming into focus as an avenue for the discovery of novel mechanisms and therapeutic targets for MBM. The authors of that summit's report noted Paget's “seed and soil” hypothesis, postulating that both the melanoma cell “seeds” and the brain “soil” contribute to the success of metastasis and cooperate to this end (Eroglu et al., 2019). As predicted, this topic has come to dominate the field (Srinivasan et al., 2021). New insights have increased the granularity in our understanding of the “soil,” as cellular and acellular components of the TME are increasingly recognized for the challenges and support they can present to invading melanoma cells. The BBB is a highly selective filter that isolates the brain from peripheral circulation (Arvanitis et al., 2020). The cerebrospinal fluid, which bathes the brain and spinal cord, is uniquely depleted of growth factors, proteins, and amino acids (Spector et al., 2015; Dolgodilina et al., 2016; Ngo et al. 2020a, 2020b). The cellular components of the brain parenchyma “soil” include neurons and supporting cells, such as astrocytes, microglia, pericytes, and oligodendrocytes. Additionally, immune populations such as T cells, B cells, bone marrow-derived macrophages, and neutrophils are known to patrol the tumor-bearing brain (Klemm et al. 2020; Friebel et al., 2020). Interactions between brain metastases and the microenvironment across cancers are thoroughly explored in Srinivasan et al. 2021; with this review, we will discuss melanoma-specific findings including those presented at the third MRF Brain Metastasis Summit and summarized in Figure 1.
FIGURE 1.
A summary of established molecular players in the interactions between melanoma brain metastasis cells and the cells of the brain microenvironment. Inspired by a similar figure in Srinivasan et al. 2021 and made with Biorender.com
7.1 ∣. Metabolic adaptations
In many ways, melanoma cells navigate the brain TME not just by surviving the challenges it presents, but by manipulating the TME to their advantage. In particular, the metabolic give-and-take between melanoma and its microenvironment has proven critical for MBM progression. For instance, Dr. Michael Pacold of the NYU Grossman School of Medicine presented work showing that cells from multiple cancer types upregulate PHGDH, the rate-limiting enzyme in glucose-derived serine synthesis, to spread in this nutrient-depleted milieu (Ngo et al. 2020). PHGDH was increased in aggressive brain-tropic cell lines as well as patient samples of brain metastasis compared to other sites of metastasis. Inhibition of PHGDH by genetic means impaired brain metastasis development in murine models of triple-negative breast cancer and non-small cell lung cancer. PHGDH-targeting shRNAs also impaired the ability of MBM patient-derived short-term cultures to proliferate in CSF-like nutrient-depleted media. Importantly, prophylactic treatment with a pharmacologic inhibitor of PHGDH decreased brain metastatic burden in a murine model of breast cancer. PHGDH inhibitors may therefore serve as another tool against MBM, perhaps in combination with existing therapies, pending clinical trials.
Other metabolic vulnerabilities of MBM cells are also being explored. For instance, genes involved in oxidative phosphorylation have been found to be upregulated at both the RNA and protein level in MBM patient samples compared to extracranial metastases (Fischer et al. 2019, Fischer et al. 2021, Kleffman et al. 2022). Patients with an increased oxidative phosphorylation signature in their MBMs were found to exhibit decreased survival. In intracranial models, melanoma cells were found to depend upon oxidative phosphorylation for metastatic outgrowth (Fischer et al. 2019). Additionally, oxidative phosphorylation is demonstrated to mediate resistance to MAPK pathway inhibitors in non-CNS melanomas and cell lines, making it an attractive therapeutic target for patients with MBM with mutation status meriting targeted therapy (Gopal et al. 2019). Oxidative phosphorylation has also been implicated in resistance to immune checkpoint inhibitors, both in patients and in preclinical models (Najjar et al. 2019). In both intracranial xenografts into immunocompromised mice and in an autochthonous immune-competent model, treatment with the mitochondrial complex I inhibitor IACS-10759 resulted in decreased MBM burden (Fischer et al. 2019). While clinical development of IACS-10759 has stopped, other inhibitors of oxidative phosphorylation exist that show clinical safety; these agents, including metformin, may represent a promising treatment option for MBM.
7.2 ∣. Astrocyte contributions
Astrocytes are critical players in the brain microenvironment, supporting neuronal activity and coordinating recovery from both acute injury and neurodegeneration (Liddelow et al. 2017). Fatty acid synthesis in astrocytes maintains lipid homeostasis and synapse function in CNS (van Deijk et al. 2017, Garcia Corrales et al. 2021). In the context of inflammation and trauma, astrocytes use fatty acid secretion to respond to neuronal injury (Aizawa 2016, Garcia Corrales et al. 2021). Recent work shows that melanoma cells can co-opt this pathway to further MBM progression: Dr. Qing Chen of the Wistar Institute presented data showing that co-culture with astrocytes enhances melanoma cell proliferation via astrocyte-secreted unsaturated fatty acids triggering the transcription factor-regulating nuclear receptor protein peroxisome proliferator-activated receptor γ (PPARγ) signaling in melanoma cells (Zou et al. 2019). Moreover, a PPARγ antagonist shows promise by inhibiting brain metastasis progression in murine models in which melanoma and breast cancer cells were injected into the left ventricle to generate hematogenously disseminated brain metastases.
Another study illustrates that in both murine models and human patient data, MBM-associated astrocytes upregulate the chemokine ligand CXCL10, and that its receptor CXCR3 is in turn expressed by melanoma cells in the brain (Doron et al. 2019). CXCL10 is a chemokine expressed in response to IFN-γ by various cell types, used by astrocytes in gliosis to coordinate the immune response. One such role for CXCL10 was demonstrated when astrocyte-conditioned media treated with a CXCL10 antibody failed to stimulate T-cell migration in an in vitro invasion assay. Importantly, melanoma cells expressing CXCR3-targeting shRNA's yielded decreased brain metastasis burden in a syngeneic model of MBM.
In these ways, melanoma cells can survive the harsh brain microenvironment, co-opting mechanisms usually involved in injury recovery and immune surveillance to utilize as pro-tumor signals. Melanoma cells can also re-organize their surroundings outright to make them more permissive. MBM cells have been shown to reprogram reactive astrocytes into a tumor-supportive phenotype via various mechanisms (Priego et al. 2018, Klein et al. 2015, Schwartz et al. 2016a, 2016b, Doron et al. 2019). For instance, brain-metastatic cells of several primary tumor origins—including melanoma—have been shown to induce and maintain a subpopulation of STAT3-expressing astrocytes which, in turn, orchestrate a tumor-supportive microenvironment (Priego et al. 2018). This bidirectional communication between astrocytes and cancer cells has clinical importance, as patients with lung adenocarcinoma brain metastases treated with the STAT3 inhibitor silbinin showed increased OS in a small clinical trial.
Despite the evidence for tumor-supportive activity of astrocytes in MBM, antagonistic interactions with the brain microenvironment must also be overcome. One of the first studies to describe a mechanism by which astrocytes block brain metastasis showed that astrocytes can induce apoptosis of brain metastatic cells by secretion of plasminogen activator and Fas ligand, which tumor cells combat by secreting serpins (Valiente et al. 2014). Other recent work shows that melanoma cells secrete amyloidβ (Aβ) to suppress inflammatory activation of astrocytes and protect against phagocytosis by microglia (Kleffman et al. 2022). The authors subjected culture-adapted patient-derived surgical melanoma samples from either MBM or non-brain metastases to mass-spectrometry-based proteomics. These data showed that proteins involved in the processing of amyloid precursor protein (APP) into its product Aβ were more highly expressed in MBM than in extracranial metastasis, suggesting a role for Aβ in MBM. In intracardiac injection models, targeting Aβ by blocking APP expression using shRNAs or CRISP/Cas9 suppressed both the establishment of MBM and the further growth of seeded melanoma cells in the mouse brain without significantly affecting the extracranial metastasis. Multiplex immunofluorescence revealed that astrocytes and microglia surrounding melanoma cells unable to produce Aβ have increased expression of inflammatory markers. Treatment of mice with established brain metastases with a β-secretase inhibitor (which prevents processing of APP into Aβ) resulted in decreased brain metastasis burden.
Work in glial biology sheds light on the apparent contradiction of pro- and anti-metastasis functions of astrocytes: reactive astrocytosis is spatiotemporally heterogeneous, and incompletely described by markers like GFAP (Liddelow et al. 2017). Similarly, the binary model of microglia and macrophage activation is increasingly outmoded and replaced by one of multi-dimensionality and plasticity (Guldner et al. 2020, Xue et al. 2014). These data highlight a continued need for collaboration between the fields of neuroscience and melanoma biology (Monje et al. 2020). In particular, the intriguing finding of a role for Aβ in MBM as a modulator of the brain microenvironment suggests that there may be related mechanisms underlying neurodegenerative pathologies and MBM. Further investigation into the relationship between brain metastasis and neurodegenerative disorders may reveal additional important insights into the complexities of astrocyte and microglia function. A better understanding of glial plasticity and heterogeneity in response to insults can help reveal the mechanisms by which these cell types respond to tumor cell invasion, and how cancer cells can co-opt those responses to survive and adapt to the CNS.
Together, these data point to multiple novel molecular targets with available pharmacologic agents—including but not limited to PHGDH, CXCR3, oxidative phosphorylation, STAT3, PPARγ, PI3K-AKT, and Aβ—as promising tools for the treatment of MBM. Such exciting innovations must be considered against, and perhaps in combination with, the current standards of care including SRS, BRAF and MEK inhibition, and ICB.
Immune infiltrates.
In addition to the role of astrocytes, recent studies have exposed an integral role for myeloid cells, particularly bone marrow-derived macrophages and microglia (collectively known as tumor-associated macrophages, or TAMs) in breast cancer brain metastases. Microglia are the coordinators of the innate immune response in the brain and therefore potentially represent an existential threat to cancer cells taking root in the brain parenchyma. However, recent work highlights that microglia may serve pro-tumor functions as well. An inhibitor of colony-stimulating factor 1 receptor (CSF1R) showed efficacy in murine models of breast-to-brain metastasis by attenuating tumor-associated macrophage activation. Microglia promoted resistance to this treatment by activating CSF2Rb-STAT5 signaling, which was in turn combated with STAT5 inhibition (Klemm et al. 2021). In another study using a syngeneic murine model of breast-to-brain metastasis, myeloid cells were observed to downregulate CX3CR1 in response to brain metastasis. This loss of CX3CR1 was found to trigger a CXCL10-mediated “vicious cycle” resulting in a pro-tumor, immune-suppressed niche (Guldner et al. 2020). In an intracerebral injection model of breast cancer brain metastasis, selective depletion of anti-inflammatory microglia with clodronate resulted in decreased brain metastatic burden (Andreou et al. 2017).
Though a dearth of syngeneic models has somewhat hindered the study of myeloid cells in MBM specifically, the data that have been published points to a pivotal role for microglia in melanoma cells' success in the brain microenvironment, not unlike in breast cancer. For example, prophylactic treatment with a TLR9 agonist has shown efficacy in an intracarotid injection model of MBM by inducing microglial activation (Benbenishty et al. 2019). Other work shows that culture with melanoma cell conditioned media alters the cytokine profile of microglia (Izraely et al. 2019). The roles of this and other cell types in the diverse ecosystem of the brain—including bone marrow-derived macrophages, neutrophils, B and T cells, and neurons—in MBM are less clear at this point as compared to astrocytes. Whereas astrocytes are the most abundant cell type in the brain, others are rarer and therefore represent a technical challenge (Miller 2018).
Moving forward, the study of these constituents of the brain-tumor interface in pairs—for instance, the astrocyte-melanoma relationship, separate from the neuron-melanoma relationship—is important. But cancer and non-cancer constituents of the TME surely communicate among one another contemporaneously and bidirectionally, just as microglia, astrocytes, and immune cells cooperate to address brain injury. High-resolution omics technologies are starting to reveal a dense web of interactions between cell types in MBM at unprecedented spatial and molecular detail (Lawson et al. 2018, Smalley et al. 2021, Gonzalez et al. 2022).
The relative geographic orientation of the cells of the TME has biological import in other malignancies and may therefore represent a dimension of information worth exploring in MBM. For example, glioblastoma cells exhibit distinct phenotypes across the tumor's “heterogeneous landscape of cell populations” (Comba et al. 2021); proximity to synapses allows breast cancer brain metastatic cells to engage with neurons where astrocytes usually do (Zeng et al. 2019); glia and neurons are known to vary their phenotypes across the highly spatially organized brain (Tan et al. 2020, Batiuk et al. 2020). Cutting-edge tools will help the field grapple with phenotypic and spatiotemporal heterogeneity in the dynamic MBM TME. These include scRNA-seq and CITE-seq, spatial transcriptomics, multiplex immunofluorescence imaging technologies (e.g., CODEX and GeoMx), and machine learning-based intercellular communication network inference (Armingol et al. 2021, Goltsev et al. 2018, Khan et al. 2021, Turei et al. 2021). Such studies are already published on brain metastases from other primary tumor origins (Laughney et al. 2020, Guldner et al. 2020). For instance, using CITE-seq to assess tumor-associated macrophage transcriptional heterogeneity, followed by RNA-ISH and IF to interrogate the spatial distribution of those subpopulations, Guldner and colleagues found a subtype of macrophage that exists at the brain-tumor interface specifically (Guldner et al. 2020).
Single-cell RNA-sequencing (scRNA-seq) is also already being applied to MBM samples (Lawson et al. 2018, Smalley et al. 2021, Alvarez-Breckenridge et al. 2022). The untangling of intercellular networks enabled by this technique is especially critical in the context of immunotherapy. Prospective clinical trials of immune checkpoint blockade (ICB) for melanoma brain metastasis are ongoing and promising, but molecular mechanisms of response and resistance are still unclear (see section “Immune Therapy”). Work presented by Dr. Christopher Alvarez-Breckenridge of MD Anderson Cancer Center characterized the response of melanoma in the brain to ICB through scRNA-seq of 27 human MBM samples (Alvarez-Breckenridge et al. 2022). This cohort included 8 patient samples preimmunotherapy and 19 post-immunotherapy, with only some responsive to therapy. This analysis revealed a subpopulation of neutrophils associated with pro-tumoral features. Indeed, tumor-associated neutrophils (TANs) are recognized as critical players in the response to ICB of various cancers, as they can partake in both the anti-tumor immune response and pro-tumor inflammation (Jaillon et al. 2020, Faget et al. 2021). These findings indicate that MBM is no exception, and the role of TANs merits further mechanistic investigation.
In addition to single-cell transcriptomic analyses, this study also included sequencing of T-cell receptors from both patients' blood and MBM tumors. In accordance with previous findings, T cell clonal expansion in the blood was found to correlate with response to checkpoint blockade. Clonally expanded T cells in MBMs, however, were found to adopt a transcriptional signature associated with exhaustion. Further, a significant association was found between a given intracranial T cell having an “effector” transcriptional profile and its clonotype being found in both the MBM and the blood (Alvarez-Breckenridge et al. 2022). Such findings, made possible only by the dual sampling of blood and tumor, underscore the importance of economically designed sample procurement in a field with limited patient numbers, and support peripheral blood sampling for predicting ICB response in MBM patients, as has been proposed with extracranial metastases (Lucca et al. 2021, Pauken et al. 2021).
These single-cell data also provide important context for another study, which described whole-exome sequencing of patient-matched MBMs and extracranial metastases. While T cell clonality—the distribution of receptor sequences across cells—did not differ between pairs, observed richness—the number of unique TCR sequences in a T-cell population—was significantly lower in MBMs (Fischer et al. 2019). Further, the authors observed minimal overlap in T cell repertoires between MBMs and patient-matched extracranial metastases. The scRNA-seq data from Alvarez-Breckenridge et al nonetheless point to concordant phenotypic diversity between MBM and extracranial metastases. Together these data highlight the need for a multi-pronged assessment of the T-cell compartment (e.g., T cell fraction, phenotype, and clonotype) in future studies dissecting molecular mechanisms of intracranial response to ICB in melanoma.
While single-cell data are of a relatively high resolution, its application is still expensive and technically demanding. Dr. Don Nguyen of Yale School of Medicine described a recently developed innovative approach to bulk RNA-sequencing, termed “BMX-Seq,” which deconvolutes tumor and microenvironment gene expression of human models injected into mice more efficiently than existing computational approaches (Wingrove et al. 2019). Importantly, the results are rendered into a user-friendly web interface for exploration by members of the field (http://bmxexplorer.gotdns.org/). As shown by the examples listed in this manuscript, parallel studies on the brain metastasis microenvironment in breast and lung adenocarcinoma often reveal shared mechanisms with MBM. Indeed, BMX-Seq shows 252 genes commonly upregulated when lung, breast, or melanoma were intracranially injected into mouse brains as compared to their expression in 2D culture. Just as important as these similarities, however, are the molecular distinctions between brain metastases of melanoma and other primary tumor origins. For instance, Wingrove et al made the important distinction between forebrain and hindbrain metastases. Unlike breast and lung cancers which show tropism for the cerebellum, melanoma is known to metastasize to the frontal and temporal lobes of the brain more frequently (Cardinal et al. 2021).
8 ∣. BRAIN METASTASIS PRECLINICAL MODELS
Brain metastasis is a complex process, of which only isolated steps can be modeled in vitro. As such, reproducible, clinically relevant in vivo models are paramount for progress in this area. Most studies use cells derived from human patients and employ intracardiac injection into the left ventricle of immunocompromised mice. To facilitate loss-of-function studies, a model must have sufficient penetrance of brain metastasis in this context; such models are rare but increasing in number as the field grows. These intracardiac injection models are highly penetrant but do not reflect early events in the metastatic cascade. While human xenograft models are intrinsically closer to the tumor biology of patients, the use of immunocompromised mice necessitated by injection of human melanoma cells limits insight into the immune microenvironment in MBMs.
Syngeneic murine models—which can be injected into immunocompetent mice to reproducibly yield MBM—have proven especially difficult to generate. This is an urgent problem in the field, as the concept of the brain as an “immune privileged” site quickly wanes and immune checkpoint inhibitors have become the standard of care for patients with metastatic melanoma. As described above (see “Molecular Determinants of Melanoma Brain Metastasis”), molecular associations with response and resistance to ICB are just being uncovered from the analysis of human samples. Functional interrogation of these findings, however, depends upon suitable immunocompetent murine models. Currently, a handful is in use, including B16-F10, YUMM1.7-BrM213, D4M3, and RMS (Nakamura et al. 2002, Zou et al. 2019, Jenkins et al. 2014, Schwartz et al. 2016a 2016b).
Even with the employment of syngeneic cell lines, however, come immune system-related concerns. Recent work shows that the xenobiotic marker GFP (Green Fluorescent Protein) induces T-cell-mediated tumor rejection in a manner proportionate to its expression (Grzelak et al. 2021). GFP and luciferase are widely used tools to facilitate the tracking and measuring of tumors during in vivo experiments. These tools are especially critical in MBM studies because models can be lowly penetrant, and so abandoning them altogether is potentially fraught. This issue is well summarized by Day et al. 2022, which suggest solutions including antigen-specific tolerization techniques like delivery of non-immunogenic mRNA for prolonged expression of luciferase (Krienke et al. 2021). Alternatively, the use of imaging modalities that do not require such markers on the cancer cells (i.e., MRI), may also reduce the impact of such issues. Such strategies may prove invaluable if the field is to model the MBM microenvironment and responses to immunotherapy in mice reproducibly.
In her presentation, Dr. Eva Perez-Guijarro of the National Cancer Institute described a set of four distinct murine models developed in the C57Bl/6J background (M1-M4), each with a unique combination of genetic alterations and carcinogenic insults. This panel promises to provide insights into the biology and ICB responsiveness of various melanoma patient subpopulations (Pérez-Guijarro et al. 2020). Tumors were excised and adapted to culture conditions, after which each “cell line” was injected into the left ventricle to assess its brain metastatic potential. The “M4” line, stemming from tumors with KrasG12D that are sensitive to immune checkpoint blockade, yielded the most brain metastases. This line's brain tropism was increased through several rounds of in vivo selection. The responsiveness of this M4 model to ICB was compared to a triple-negative breast cancer model, 4 T1. Interestingly, the response of mice injected with M4 to ICB mimicked that of human metastatic melanoma patients in two ways: firstly, a combination of anti-CTLA4 and anti-PD-L1 treatment was more effective than either alone, with anti-PD-L1 being superior to anti-CTLA4; secondly, M4 was much more responsive to immunotherapy than the breast model 4 T1. Immunophenotyping analysis of untreated brains showed altered microglia as a prominent component of the altered brain microenvironment in brains bearing M4 melanoma metastases, compared to PBS-injected mice; 4 T1 cells did not induce such a dramatic change in microglia. These findings avail the field of multiple new syngeneic models of MBM, and further underscore the need to dissect the role of microglia in MBM progression.
Activation of the PI3K/Akt pathway has previously been implicated as a driver of melanoma brain metastasis (Cho et al. 2015, Chen et al. 2014a, 2014b, Niessner et al. 2013). Dr. Sheri Holmen of the Huntsman Cancer Institute at the University of Utah elaborated on these findings by describing how activating mutations of AKT promoted spontaneous brain metastasis in an autochthonous murine model of melanoma. BrafV600E, Cdkn2a−/−, Pten−/− mice in which melanocytes were induced to express the mutant Akt1E17K developed significantly more brain metastasis than those with wild-type Akt1 or other Akt1 mutants (Kircher et al. 2019). Further studies revealed that Akt1E17K promotes Focal Adhesion Kinase (FAK) signaling in these tumors, and pharmacological inhibition of FAK significantly reduced brain and lung metastases in the autochthonous BrafV600E, Cdkn2a−/−, Pten−/− model with activated Akt1. Spontaneous models like this, which can be further studied in conjunction with lineage-tracing reporters, represent a considerable advance as they allow tracking all the steps of disease progression since inception in an intact host.
A classic approach to studying BM determinants consists of comparing clones derived from a cell line model that show differing brain metastatic ability upon intracardiac injection into mice (Cruz-Muñoz et al. 2008, Priego et al. 2018, Zou et al. 2019). This method has been used fruitfully in the field for years as a way of identifying molecular features of brain tropism, but the process of single-cell clone isolation and injection, and/or in vivo selection for brain-tropic cells, requires much time and effort. Generation of genetically engineered murine models through complex crossbreeding schemes described above is similarly arduous. Therefore, efforts by members of the field to catalog the behavior and molecular features of the brain metastasis models currently in use represent a major step in the direction of sharing experiences and standardizing methods. The discussion in Cancer Cell by Patton et al. 2021 epitomizes such efforts. The continued investment of all members of the field in the official Brain Metastasis Cell Lines Panel is pivotal to reproducibility and hastened progress (Valiente et al. 2020). This includes not only the addition of new models but also the updating of existing models with observations as they are used in different labs across the world. Day et al., 2022 express a related, powerful sentiment, regarding xenobiotic marker expression in murine models: calling for “a public forum and/or database where researchers can get credit for posting or depositing datasets that reveal untoward and/or confounding effects. This would facilitate more extensive evaluation of the effects of experimental manipulations on outcome because negative results rarely find their way to publication.”
In sharing information on MBM models and in publishing data that make use of them, detailed characterization is critical. For instance, while bioluminescence imaging is an invaluable tool, it lacks anatomic detail. At a minimum, distinguishing between leptomeningeal tumors and those inhabiting the brain parenchyma is crucial because each compartment implies distinct tumor biology. To this end, histopathological characterization is a simple yet pivotal complementary approach.
Time and effort are wasted when a model is inadequately characterized and subsequently disseminated. As such the following are critical data points to be shared from the originating lab as well as labs that make use of the model later:
Culture conditions.
Age, sex, and genetic background of mice.
Cell numbers used in injection and mode of injection.
Time to morbidity and appreciable brain tumor burden.
The distinction between leptomeningeal and parenchymal intracranial metastatic burden, including histopathology.
Enumeration of extracranial sites of metastasis.
9 ∣. CONCLUSIONS AND FUTURE DIRECTIONS
Metastasis is the most common CNS tumor. Molecular markers at the primary tumor level that can predict brain metastatic potential are thus far not clinically applicable for stratifying patients with melanoma; interventional therapies are more germane to the current landscape than preventative ones. Intracardiac models to generate MBM allow testing of interventions on established metastases and have yielded an impressive array of candidate targets in recent years, including PHGDH, STAT3, CXCR3, PPARγ, Aβ, PI3K-AKT, and oxidative phosphorylation. Importantly, some of these targets have readily available pharmacologic agents that are currently undergoing preclinical and clinical testing in the setting of MBM.
To predict and prevent MBM, however, models are required that capture earlier stages of the metastatic cascade, including invasion into the dermis and intravasation into the peripheral circulation. It is estimated that 15-20% of brain metastases present as the isolated first visceral site of disease spread; the associated primary tumors apparently giving rise to these early brain metastases are reportedly thinner and of lower AJCC stage compared to those giving rise to other visceral metastatic sites (Ma et al. 2012, Rabbie et al. 2021). Genomic studies favor a branched evolution model of brain metastasis, wherein clinically actionable mutations are often present in brain metastases that may not be detected in the primary tumor (Brastianos et al. 2015). These data challenge the idea of the brain as the last stop on a long, stepwise progression from the primary site, but mechanisms of early dissemination to the brain are thus far unclear. Lineage tracing experiments and serial sampling of circulating tumor cells may help to answer such questions. Further, there is growing evidence that the unique TME of the CNS significantly impacts the biology and immunology of tumor cells that reach the brain.
The role of the immune microenvironment in MBM is evidently the next frontier in the field. Interestingly, pathways already identified as critical for MBM progression have been implicated in dampening the antitumor immune response and/or promoting the resistance to immunotherapy, including the PI3K-AKT pathway, oxidative phosphorylation, and Aβ. As combination ICB trials proceed, mechanisms of primary resistance and recurrence will need to be more thoroughly investigated in MBM. Such studies hinge upon reproducible, high-penetrance immunocompetent models, such as those characterized in Perez-Guijarro et al. 2020. These and others, along with complementary studies in patient samples, promise to render the brain TME at a higher resolution than ever before and to clarify the roles of various cellular players therein. The severing of ties between melanoma cells and the metastasis-supportive niche they orchestrate in the brain may prove a crucial weapon against these devastating tumors.
The work above has illustrated that the unique molecular features of MBM and the melanoma TME have to be investigated in this specific patient population. In light of this collaborative and innovative work, it becomes paramount that patients with brain metastases are prospectively enrolled in all stages of clinical trial design, and that CNS response rate is evaluated in these patients. The discovery of pathways that may be susceptible to therapeutic interventions across tumor histologies bolsters the idea of implementing clinical trials that are tumor agnostic (i.e., “basket studies” such as Alliance study A071701, NCT03994796), versus histology-specific melanoma studies, to expedite accrual; understanding that sub-analyses of specific histologies may also commence within these studies. The results of the Checkmate-204 trial dispel the notion that the brain is a privileged site and instead demonstrate that patients with MBM can experience durable responses to therapy, changing the treatment landscape and OS of patients with melanoma that is metastatic to the brain. These patients deserve the same rigorous scientific processes that have led to the seismic shift of the therapeutic landscape in patients with extracranial melanoma metastases. We are ushering in a transformation in the biologic, therapeutic, and clinical characteristics of MBM.
FUNDING INFORMATION
Alcida Karz: SPORE P50 CA225450; Maya Dimitrova: None; Kevin Kleffman: SPORE P50 CA225450; Christopher Alvarez-Breckenridge: K12NS080223; Michael B. Atkins: NCI P30 CA051008 to the Georgetown Lombardi Comprehensive Cancer Center; Adrienne Boire: NCI/NIH P30 CA008748, R01 CA245499; Marcus Bosenberg: None; Priscilla Brastianos: National Institutes of Health (5R01CA227156–04; 5R01CA244975–02), Breast Cancer Research Foundation, Demetra fund of the Hellenic Women's Association, and the Terry and Jean de Gunzburg MGH Research Scholar Award; Daniel Cahill: None; Qing Chen: NIH NCI (R01CA241490); Sherise Ferguson; Peter Forsyth: NIH/NCI 1R21CA256289-01A1, R21CA252634-01A1, 1R01CA236034; DOD; Pfizer NCT03719768; Roswell Park; Isabella C. Glitza Oliva: none; Sarah Goldberg: None; Sheri Holmen: NIH/NCI R01CA121118, MRA 347651; Jonathan Knisely: None; Glenn Merlino: NIH Intramural Research Program; Don X. Nguyen: R01CA166376, R01CA191489, and P50CA196530; Michael Pacold: NCI R01 CA211687 (Philips), Damon Runyon-Rachleff Innovation Award DRR 63-20, MRA YIA 688365, Harry J. Lloyd Charitable Trust, ACS Research Scholar Grant RSG-21-115-01-MM, Irma T. Hirschl Career Scientist Award; Eva Perez-Guijarro: NIH Intramural Research Program. FLEX Synergy Award from the NCI Center for Cancer Research; Keiran Smalley: R21CA256289; Hussein Tawbi: None; Patrick Wen: None; Michael A. Davies: MAD is supported by Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the AIM at Melanoma Foundation, the NIH/NCI 1 P50 CA221703-02, the American Cancer Society and the Melanoma Research Alliance, Cancer Fighters of Houston, the Anne and John Mendelsohn Chair for Cancer Research, and philanthropic contributions to the Melanoma Moon Shots Program of MD Anderson; Harriet M. Kluger: None; Janice M. Mehnert: None; Eva Hernando: SPORE P50 CA225450, and a joint Melanoma Research Alliance/American Cancer Society award.
Footnotes
CONFLICT OF INTEREST
Alcida Karz: None; Maya Dimitrova: None; Kevin Kleffman: None; Christopher Alvarez-Breckenridge: None; Michael B. Atkins: Has/had an advisory role for Bristol-Myers Squibb, Merck, Novartis, Eisai, Aveo, Pfizer, Werewolf, Fathom, Pneuma, Leads, Pyxis Oncology, PACT, Elpis, X4Pharma, ValoHealth, ScholarRock, Surface, Takeda, Simcha, Roche, SAB Bio, and GSK and has served as a consultant: Bristol-Myers Squibb, Merck, Novartis, Pfizer, Roche, Exelixis, Iovance, COTA, Idera, Agenus, Apexigen, Asher Bio, Neoleukin, AstraZeneca, Calithera, SeaGen, and Sanofi. He reports research support to his institution from Bristol-Myers Squibb, Merck, and Pfizer. He holds stock/stock options in Pyxis Oncology, Werewolf, and Elpis; Adrienne Boire: Inventor on US Provis. Patent App. No: 62/258,044, 63/052,139. Unpaid member of SAB Evren Scientific; Marcus Bosenberg: AstraZeneca for unrelated activities; Priscilla Brastianos: Consulted for Angiochem, Genentech/Roche, Lilly, Tesaro, ElevateBio, Pfizer (Array), Dantari, SK Life Sciences, Advise Connect Inspire (ACI), Voyager Therapeutics and Sintetica, and has received grant/research support to MGH from Merck, Bristol Myers Squibb, Mirati Therapeutics, and Lilly and honoraria from Merck, Genentech/Roche, Pfizer, and Lilly; Daniel Cahill: Consulted for the Massachusetts Institute of Technology, Advise Connect Inspire, Lilly, GlaxoSmithKline, Boston Pharmaceuticals and serves on the advisory board of Pyramid Biosciences, which includes an equity interest. Honoraria and travel reimbursement from Merck for invited lectures, and from the US NIH and DOD for clinical trial and grant review; Qing Chen: None; Sherise Ferguson; Peter Forsyth: AbbVie; Biocept; Boehringer-Ingelheim; Bristol-Myers Squibb; Novocure; Isabella C. Glitza Oliva: None; Sarah Goldberg: Research funding from AstraZeneca, Boehringer Ingelheim, and Mirati. Consulting/advisory board member for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Amgen, Blueprint Medicine, Sanofi Genzyme, Daiichi-Sankyo, Regeneron, Takeda, and Janssen; Sheri Holmen: None; Jonathan Knisely: None; Glenn Merlino: None; Don X. Nguyen: Has received research funding from AstraZeneca Inc which is unrelated to the work herein; Michael E. Pacold: Holds options and has consulted for Raze Therapeutics. Consultant for aMoon Venture Fund. Consultant and research funds from Novocure. Travel funds from Thermo Fisher Scientific; Eva Perez-Guijarro: None; Keiran Smalley: None; Hussein A. Tawbi: Is a paid consultant for BMS, Merck, Novartis, Genentech, Iovance, Eisai, Pfizer, Karyopharm, Boxer Capital, and receives research funding to institution from BMS, Merck, Novartis, Genentech, GSK, Eisai, Dragongly Therapeutics, RAPT Therapeutics; Patrick Wen: Advisory Board: Agios, Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines; Research Support: Astra Zeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines; Michael A. Davies: Consultant to Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, Iovance, and ABM Therapeutics, and he has been the PI of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, Oncothyreon, and ABM Therapeutics; Harriet M. Kluger: Has received institutional research grants from Merck, Bristol-Myers Squibb, Apexigen. Has received personal fees from Iovance, Immunocore, Celldex, Array Biopharma, Merck, Elevate Bio, Instil Bio, Bristol-Myers Squibb, Clinigen, Shionogi, Chemocentryx, Calithera, Signatero, Giaggen, GI Reviewers; Janice M. Mehnert: Advisory board: BMS, Seagen, Novartis, Eisai, Regeneron; Consulting: Merck; Research Support: BMS, Seagen, Regeneron, Novartis, Incyte, Merck; Eva Hernando: None. The Melanoma Research Foundation thanks BMS, Novartis, Natera, and Istari Oncology for providing financial support for the Brain Metastasis Summit.
REFERENCES
- Ahmed KA, Freilich J, Sloot S, Figura N, Gibney GT, Weber JS, Sarangkasiri S, Chinnaiyan P, Forsyth PA, Etame AB, & Rao NG (2015). Linac-based stereotactic radiosurgery to the brain with concurrent vemurafenib for melanoma metastases. Journal of Neuro-oncology., 122, 121–126. [DOI] [PubMed] [Google Scholar]
- Aizawa F, Nishinaka T, Yamashita T, Nakamoto K, Koyama Y, Kasuya F, & Tokuyama S (2016). Astrocytes release polyunsaturated fatty acids by lipopolysaccharide stimuli. Biological Pharm Bulletin, 39, 1100–1106. 10.1248/bpb.b15-01037 [DOI] [PubMed] [Google Scholar]
- Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM, Bertalan M, Stocking J, Kaplan A, Nayyar N, Lawrence DP, Flaherty KT, Shih HA, Oh K, Batchelor TT, Cahill DP, Sullivan R, & Brastianos PK (2019). Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. The oncologist, 24(5), 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Breckenridge C, Markson SC, Stocking JH, Nayyar N, Lastrapes M, Strickland MR, Kim AE, de Sauvage M, Dahal A , Larson JM, Mora JL, Navia AW, Klein RH, Kuter BM, Gill CM, Bertalan M, Shaw B, Kaplan A, Subramanian M, Jain A, … Carter SL (2022). Microenvironmental landscape of human melanoma brain metastases in response to immune checkpoint inhibition. Cancer Immunology Research, 10(8), 996–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer MH, AI-Sarraf M, Baker LH, & Vaitkevicius VK (1978). Malignant melanoma and central nervous system metastases. Incidence, diagnosis, treatment and survival. Cancer, 42(2), 660–668. [DOI] [PubMed] [Google Scholar]
- Andreou KE, Soto MS, Allen D, Economopoulos V, de Bernardi A, Larkin JR, & Sibson NR (2017). Anti-inflammatory microglia/macrophages as a potential therapeutic target in brain metastasis. Frontiers in Oncology, 7, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armingol E, Officer A, Harismendy O, & Lewis NE (2021). Deciphering cell–cell interactions and communication from gene expression. Nature Review. Genetics, 22, 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitis CD, Ferraro GB, & Jain RK (2020). The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nature Reviews Cancer, 20, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bander ED, Yuan M, Carnevale JA, Reiner AS, Panageas KS, Postow MA, Tabar V, & Moss NS (2021). Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer, 127(12), 2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks PD, Lasocki A, Lau PK, Sandhu S, McArthur G, SShackleton M (2019). Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: A case series. Health Science Reports, 2(3), e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiuk MY, Martirosyan A, Wahis J, de Vin F, Marneffe C, Kusserow C, Koeppen J, Viana JF, Oliveira JF, Voet T, Ponting CP, Belgard TG, & Holt MG (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nature Communications, 11, 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbenishty A, Gadrich M, Cottarelli A, Lubart A, Kain D, Amer M, Shaashua L,Glasner A, Erez N, Agalliu D, Mayo L, Ben-Eliyahu S, & Blinder P (2019). Prophylactic TLR9 stimulation reduces brain metastasis through microglia activation. Plos Biol, 17, e2006859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS, Schur S, Füreder LM, Gatterbauer B, Dieckmann K, Widhalm G, Hainfellner J, Zielinski CC, Birner P, Bartsch R, & Preusser M (2016). Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open, 1(2), e000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, & Hirth P (2012). Vemurafenib: The first drug approved for BRAF-mutant cancer. Nature reviews Drug discovery, 11(11), 873–886. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Allen EMV, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park S-H, McKenna A, … Hahn WC (2015). Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discovery, 5, 1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Ballman KV, Cerhan J, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NN, Ashman JB, Bahary JP, Hadjipanayis CG, Urbanic JJ, Barker FG II, Farace E, Khuntia D, Giannini C, Buckner JC, Galanis E, & Roberge D (2016). N107C/CEC.3: A phase III trial of post-operative stereotactic radiosurgery (SRS) compared with whole brain radiotherapy (WBRT) for resected metastatic brain disease. International Journal of Radiation Oncology, Biology, Physics, 96(5), 937. [Google Scholar]
- Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D , Fox P, Bassett R, Hwu P, Gershenwald JE, Lazar AJ, & Davies MA (2014). Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clinical Cancer Research, 20(21), 5527–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EM, Amaria RN, Glitza IC, Milton DR, Diab A, Patel SP, McQuade JL, Honaker V, Wong MKK, Hwu P, Wargo JA, Davies MA, STawbi HA-H (2021). Phase II study of TRIplet combination nivolumab (N) with dabrafenib (D) and trametinib (T) (TRIDeNT) in patients (pts) with PD-1 naïve or refractory BRAF-mutated metastatic melanoma (MM) with or without active brain metastases. Journal of clinical oncology, 39(15), 9520–9520. 10.1200/JCO.2021.39.15_suppl.9520 [DOI] [Google Scholar]
- Cai X, Chiu YH, & Chen ZJ (2014). The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Molecular cell, 54(2), 289–296. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. (2015). Genomic classification of cutaneous melanoma. Cell, 161(7), 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal T, Pangal D, Strickland BA, Newton P, Mahmoodifar S, Mason J, Craig D, Simon T, Tew BY, Yu M, Yang W, Chang E, Cabeen RP, Ruzevick J, Toga AW, Neman J, Salhia B, & Zada G (2021). Anatomical and topographical variations in the distribution of brain metastases based on primary cancer origin and molecular subtypes: A systematic review. Neuro-oncology Advances., 4(1), Vdab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chakravarti N, Aardalen K, Lazar AJ, Tetzlaff MT, Wubbenhorst B, Kim SB, Kopetz S, Ledoux AA, Gopal YNV , Pereira CG, Deng W, Lee JS, Nathanson KL, Aldape KD , Prieto VG, Stuart D, & Davies MA (2014). Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clinical Cancer Research, 20(21), 5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Sun L, & Chen ZJ (2016). Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nature Immunology, 17, 1142–1149. 10.1038/ni.3558 [DOI] [PubMed] [Google Scholar]
- Cho JH, Robinson JP, Arave RA, Burnett WJ, Kircher DA, Chen G, Davies MA, Grossmann AH, VanBrocklin MW, McMahon M, & Holmen SL (2015). AKT1 activation promotes development of melanoma metastases. Cell reports, 13(5), 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SM, Mahadevan A, Floyd SR, Lam FC, Chen CC, Wong ET, & Kasper EM (2015). Stereotactic radiosurgery for brain metastases from malignant melanoma. Surgical neurology international, 6(Suppl 12), S355–S365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu FC, & Hilaris BB (1961). Value of radiation therapy in the management of intracranial metastases. Cancer, 14(3), 577–581. [DOI] [PubMed] [Google Scholar]
- Comba A, Faisal SM, Varela ML, Hollon T, Al-Holou WN, Umemura Y, Nunez FJ, Motsch S, Castro MG, & Lowenstein PR (2021). Uncovering spatiotemporal heterogeneity of high-grade gliomas: From disease biology to therapeutic implications. Frontiers Oncology, 11, 703–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Munoz W, Man S, Xu P, & Kerbel RS (2008). Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Research, 68, 4500–4505. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, … Futreal PA (2002). Mutations of the BRAF gene in human cancer. Nature, 417(6892), 949–954. [DOI] [PubMed] [Google Scholar]
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, & Bedikian A (2011). Prognostic factors for survival in melanoma patients with brain metastases. Cancer, 117(8), 1687–1696. [DOI] [PubMed] [Google Scholar]
- Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T, Mortier L, Moschos SJ, Hogg D, Márquez-Rodas I, del Vecchio M, Lebbé C, Meyer N, Zhang Y, Huang Y, Mookerjee B, & Long GV (2017). Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. The Lancet Oncology, 18(7), 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C-P, Pérez-Guijarro E, Lopès A, Goldszmid RS, Murgai M, Wakefield L, & Merlino G (2022). Recognition of observer effect is required for rigor and reproducibility of preclinical animal studies. Cancer Cell., 40, 231–232. [DOI] [PubMed] [Google Scholar]
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, & Formenti SC (2005). Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical Cancer Research, 11(2), 728–734. [PubMed] [Google Scholar]
- Dolgodilina E, Imobersteg S, Laczko E, Welt T, Verrey F, & Makrides V (2016). Brain interstitial fluid glutamine homeostasis is controlled by blood-brain barrier SLC7A5/LAT1 amino acid transporter. Journal of Cerebral Blood Flow & Metabolism, 36, 1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doron H, Amer M, Ershaid N, Blazquez R, Shani O, Lahav TG, Cohen N, Adler O, Hakim Z, Pozzi S, Scomparin A, Cohen J, Yassin M, Monteran L, Grossman R, Tsarfaty G, Luxenburg C, Satchi-Fainaro R, Pukrop T, & Erez N (2019). Inflammatory activation of astrocytes facilitates melanoma brain tropism via the CXCL10-CXCR3 signaling Axis. Cell reports, 28, 1785, e6–1798. [DOI] [PubMed] [Google Scholar]
- Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh de Parseval LA, Pickard MD, Sandor V, Robert C, & Flaherty KT (2018). Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. The Lancet Oncology, 19(10), 1315–1327. [DOI] [PubMed] [Google Scholar]
- Eroglu Z, Holmen SL, Chen Q, Khushalani NI, Amaravadi R, Thomas R, Ahmed KA, Tawbi H, Chandra S, Markowitz J, Smalley I, Liu JKC, Chen YA, Najjar YG, Karreth FA, Abate-Daga D, Glitza IC, Sosman JA, Sondak VK, … Smalley KSM (2019). Melanoma central nervous system metastases: An update to approaches, challenges, and opportunities. Pigment Cell & Melanoma Research, 32, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget J, Peters S, Quantin X, Meylan E, & Bonnefoy N (2021). Neutrophils in the era of immune checkpoint blockade. Journal of Immunotherapy of Cancer, 9, e002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felip E, Shaw AT, Bearz A, Camidge DR, Solomon BJ, Bauman JR, Bauer TM, Peters S, Toffalorio F, Abbattista A, Thurm H, Peltz G, Wiltshire R, & Besse B (2021). Intracranial and extracranial efficacy of lorlatinib in patients with ALK-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Annals of Oncology, 32(5), 620–630. [DOI] [PubMed] [Google Scholar]
- Fischer GM, Guerrieri RA, Hu Q, Joon AY, Kumar S, Haydu LE, McQuade JL, Gopal YNV, Knighton B, Deng W, Hudgens CW, Lazar AJ, Tetzlaff MT, & Davies MA (2021). Clinical, molecular, metabolic, and immune features associated with oxidative phosphorylation in melanoma brain metastases. Neuro-oncology Advances, 3(1), vdaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer GM, Jalali A, Kircher DA, Lee WC, McQuade JL, Haydu LE, Joon AY, Reuben A, de Macedo MP, Carapeto FCL, Yang C, Srivastava A, Ambati CR, Sreekumar A, Hudgens CW , Knighton B, Deng W, Ferguson SD, Tawbi HA, … Davies MA (2019). Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer discovery, 9(5), 628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA III, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, … Weber J (2012). Combined BRAF and MEKinhibition in melanoma with BRAF V600 mutations. New England Journal of Medicine, 367(18), 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel E, Kapolou K, Unger S, Núñez NG, Utz S, Rushing EJ, Regli L, Weller M, Greter M, Tugues S, Neidert MC, & Becher B (2020). Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell, 181(7), 1626–1642. [DOI] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, Murphy KM, & Gajewski TF (2011). Host type I IFN signals are required for antitumor CD8 + T cell responses through CD8α + dendritic cells. Journal of Experimental Medicine, 208, 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura K, Malgulwar PB, Fischer GM, Hu X, Mao X, Song X , Hernandez SD, Zhang XH-F, Zhang J, Parra ER, Yu D, Debeb BG, Davies MA, & Huse JT (2021). Multi-omic molecular profiling reveals potentially targetable abnormalities shared across multiple histologies of brain metastasis. Acta Neuropathol, 141, 303–321. 10.1007/s00401-020-02256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Corrales AV, Haidar M, Bogie JFJ, & Hendriks JJA (2021). Fatty acid synthesis in glial cells of the CNS. International Journal of Molecular Sciences, 22, 8159. 10.3390/ijms22158159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M, & Kastan MB (2015). The DNA damage response: Implications for tumor responses to radiation and chemotherapy. Annual review of medicine, 66, 129–143. [DOI] [PubMed] [Google Scholar]
- Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G , Black S, & Nolan GP (2018). Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 174, 968–981.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Mei W, Robles I, Hagerling C, Allen BM, Okholm TLH, Nanjaraj A, Verbeek T, Kalavacherla S, van Gogh M, Georgiou S, Daras M, Phillips JJ, Spitzer MH, Roose JP, & Werb Z (2022). Cellular architecture of human brain metastases. Cell, 185, 729, e20–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal YNV, Gammon S, Prasad R, Knighton B, Pisaneschi F, Roszik J, Feng N, Johnson S, Pramanik S, Sudderth J, Sui D, Hudgens C, Fischer GM, Deng W, Reuben A, Peng W, Wang J, McQuade JL, Tetzlaff MT, … Davies MA (2019). A novel mitochondrial inhibitor blocks MAPK pathway and overcomes MAPK inhibitor resistance in melanoma. Clinical Cancer Research, 25, 6429–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore L, Ivy SP, Balis FM, Rubin E, Thornton K, Donoghue M, Roberts S, Bruinooge S, Ersek J, Goodman N, Schenkel C, & Reaman G (2017). Modernizing clinical trial eligibility: Recommendations of the American Society of Clinical Oncology–friends of cancer research minimum age working group. Journal of Clinical Oncology, 35(33), 3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelak CA, Goddard ET, Lederer EE, Rajaram K, Dai J, Shor RE, Lim AR, Kim J, Beronja S, Funnell APW, & Ghajar CM (2021). Elimination of fluorescent protein immunogenicity permits modeling of metastasis in immune-competent settings. Cancer Cell., 40(1), 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner IH, Wang Q, Yang L, Golomb SM, Zhao Z, Lopez JA, Brunory A, Howe EN, Zhang Y, Palakurthi B, Barron M, Gao H , Xuei X, Liu Y, Li J, Chen DZ, Landreth GE, & Zhang S (2020). CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxci10. Cell, 183, 1234–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R, Krauze M, Romkes M, Omolo B, Konstantinopoulos P, Reinhart T, Harasymczuk M, Wang YY, Lin Y, Ferrone S, Whiteside T, Bortoluzzi S, Werley J, Nukui T, Fallert-Junecko B, Kondziolka D, Ibrahim J, Becker D, Kirkwood J, & Moschos S (2013). Pathologic and gene expression features of metastatic melanomas to the brain. Cancer, 119(15), 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, M Laurin Council, Matatall KA, Helms C, & Bowcock AM (2010). Frequent mutation of BAP1 in metastasizing uveal melanomas. Science, 330(6009), 1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong AM, Fogarty GB, Dolven-Jacobsen K, Burmeister BH, Lo SN, Haydu LE, Vardy JL, Nowak AK, Dhillon HM, Ahmed T, Shivalingam B, Long GV, Menzies AM, Hruby G, Drummond KJ, Mandel C, Middleton MR, Reisse CH, Paton EJ, … Thompson JF (2019). Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: A multicenter, randomized phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 37(33), 3132–3141. [DOI] [PubMed] [Google Scholar]
- Izraely S, Ben-Menachem S, Sagi-Assif O, Telerman A, Zubrilov I , Ashkenazi O, Meshel T, Maman S, Orozco JIJ, Salomon MP, Marzese DM, Pasmanik-Chor M, Pikarski E, Ehrlich M , Hoon DSB, & Witz IP (2019). The metastatic microenvironment: Melanoma–microglia cross-talk promotes the malignant phenotype of melanoma cells. International Joournal of Cancer, 144, 802–817. [DOI] [PubMed] [Google Scholar]
- Jaillon S, Ponzetta A, Mitri DD, Santoni A, Bonecchi R, & Mantovani A (2020). Neutrophil diversity and plasticity in tumour progression and therapy. Nature Reviews Cancer, 20, 485–503. [DOI] [PubMed] [Google Scholar]
- Jenkins MH, Steinberg SM, Alexander MP, Fisher JL, Ernstoff MS, Turk MJ, Mullins DW, & Brinckerhoff CE (2014). Multiple murine BRafV600E melanoma cell lines with sensitivity to PLX4032. Pigment Cell and Melanoma Research, 27, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann TJ, Smits M, Boxerman J, Huang R, Barboriak DP, Weller M, Chung C, Tsien C, Brown PD, Shankar L, Galanis E, Gerstner E, van den Bent MJ, Burns TC, Parney IF, Dunn G, Brastianos PK, Lin NU, Wen PY, & Ellingson BM (2020). Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro-oncology, 22(6), 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Yoo S-J, Clijsters M, Backaert W, Vanstapel A, Speleman K , Lietaer C, Choi S, Hether TD, Marcelis L, Nam A, Pan L , Reeves JW, van Bulck P, Zhou H, Bourgeois M, Debaveye Y, de Munter P, Gunst J, … van Gerven, L. (2021). Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell, 184, 5932, e15–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher DA, Trombetti KA, Silvis MR, Parkman GL, Fischer GM , Angel SN, Stehn CM, Strain SC, Grossmann AH, Duffy KL, Boucher KM, McMahon M, Davies MA, Mendoza MC, VanBrocklin MW, & Holmen SL (2019). AKT1E17K activates focal adhesion kinase and promotes melanoma brain metastasis. Molecular Cancer Research, 17, 1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffman K, Levinson G, Rose IVL, Blumenberg LM, Shadaloey SAA, Dhabaria A, Wong E, Galan-Echevarria E, Karz A, Argibay D, von Itter R, Floristán A, Baptiste G, Eskow NM, Tranos JA, Chen J, Vega EC, de Miera YS, Call M, … Hernando E (2022). Melanoma-secreted amyloid Beta suppresses neuroinflammation and promotes brain MetastasisAmyloid Beta promotes brain metastasis. Cancer Discovery, 12, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Menachem SB, Bengaiev R, Ben-Shmuel A, Nahmias C, & Couraud P (2015). Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. The Journal of Pathology, 236, 116–127. [DOI] [PubMed] [Google Scholar]
- Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, Brouland JP, Iacobuzio-Donahue CA, Brennan C, Tabar V, Gutin PH, Daniel RT, Hegi ME, & Joyce JA (2020). Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell, 181, 1643–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm F, Möckl A, Salamero-Boix A, Alekseeva T, Schäffer A, Schulz M, Niesel K, Maas RR, Groth M, Elie BT, Bowman RL, Hegi ME, Daniel RT, Zeiner PS, Zinke J, Harter PN, Plate KH, Joyce JA, & Sevenich L (2021). Compensatory CSF2-driven macrophage activation promotes adaptive resistance to CSF1R inhibition in breast-to-brain metastasis. Nature Cancer, 2, 1086–1101. [DOI] [PubMed] [Google Scholar]
- Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, Weiss SA, Cohen JV, Yu J, Hegde U, Perrotti E, Anderson G, Ralabate A, Kluger Y, Wei W, Goldberg SB, & Jilaveanu LB (2019). Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. Journal of Clinical Oncology, 37(1), 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely JP, James BY, Flanigan J, Sznol M, Kluger HM, & Chiang VL (2012). Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. Journal of neurosurgery, 117(2), 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, Diken M, Kreiter S, Yogev N, Waisman A, Karikó K, Türeci Ö, & Sahin U (2021). A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science, 371, 145–153. [DOI] [PubMed] [Google Scholar]
- Lamba N, Wen PY, & Aizer AA (2021). Epidemiology of brain metastases and leptomeningeal disease. Neuro-oncology, 23(9), 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, … Wolchok JD (2019). Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine, 381(16), 1535–1546. [DOI] [PubMed] [Google Scholar]
- Laughney AM, Hu J, Campbell NR, Bakhoum SF, Setty M, Lavallèe V-P, Xie Y, Masilionis I, Carr AJ, Kottapalli S, Allaj V , Mattar M, Rekhtman N, Xavier JB, Mazutis L, Poirier JT, Rudin CM, Pe'er D, & Massagué J (2020). Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nature Medicine, 26, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, & Werb Z (2018). Tumour heterogeneity and metastasis at single-cell resolution. Nature Cell Biology, 20, 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, & Fu YX (2009). Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood, The Journal of the American Society of Hematology, 114(3), 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, & Barres BA (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity, 46, 957–967. [DOI] [PubMed] [Google Scholar]
- Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, Hurvitz S, Loi S, Okines A, Abramson V, Bedard PL, Oliveira M, Mueller V, Zelnak A, DiGiovanna MP, Bachelot T, Chien AJ, O'Regan R, Wardley A, … Winer EP (2020). Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. Journal of Clinical Oncology, 38(23), 2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EGE, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, … Response Assessment in Neuro-Oncology (RANO) group. (2015). Response assessment criteria for brain metastases: Proposal from the RANO group. The lancet oncology, 16(6), e270–e278. [DOI] [PubMed] [Google Scholar]
- Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, Menzies AM, & McArthur GA (2018). Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. The Lancet. Oncology, 19(5), 672–681. [DOI] [PubMed] [Google Scholar]
- Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, Puzanov I, Hauschild A, Robert C, Algazi A, Mortier L, Tawbi H, Wilhelm T, Zimmer L, Switzky J, Swann S, Martin AM, Guckert M, Goodman V, … Schadendorf D (2012). Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. The Lancet Oncology., 13, 1087–1095. [DOI] [PubMed] [Google Scholar]
- Lucca LE, Axisa P-P, Lu B, Harnett B, Jessel S, Zhang L, Raddassi K, Zhang L, Olino K, Clune J, Singer M, Kluger HM, & Hafler DA (2021). Circulating clonally expanded T cells reflect functions of tumor-infiltrating T cells. The Journal of Experimental Medicine, 218, e20200921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, & Lord EM (2005). Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. The Journal of Immunology, 174(12), 7516–7523. [DOI] [PubMed] [Google Scholar]
- Ma MW, Qian M, Lackaye DJ, Berman RS, Shapiro RL, Pavlick AC, Golfinos JG, Parker EC, Darvishian F, Hernando E, Shao Y, & Osman I (2012). Challenging the current paradigm of melanoma progression: Brain metastasis as isolated first visceral site. Neuro-Oncology, 14, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF , Richards J, Michener T, Balogh A, Heller KN, & Hodi FS (2012). Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. The lancet oncology, 13(5), 459–465. [DOI] [PubMed] [Google Scholar]
- Martin AM, Cagney DN, Catalano PJ, Alexander BM, Redig AJ, Schoenfeld JD, & Aizer AA (2018). Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA oncology, 4(8), 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuff SG, Taich ZJ, Lawson JD, Sanghvi P, Wong ET, Barker FG, Hochberg FH, Loeffler JS, Warnke PC, Murphy KT, Mundt AJ, Carter BS, McDonald CR, & Chen CC (2013). Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. Journal of Neurology, Neurosurgery & Psychiatry, 84(12), 1384–1391. [DOI] [PubMed] [Google Scholar]
- Miller SJ (2018). Astrocyte heterogeneity in the adult central nervous system. Frontiers in Cellular Neuroscience, 12, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M, Borniger JC, D'Silva NJ, Deneen B, Dirks PB, Fattahi F , Frenette PS, Garzia L, Gutmann DH, Hanahan D, Hervey-Jumper SL, Hondermarck H, Hurov JB, Kepecs A, Knox SM, Lloyd AC, Magnon C, Saloman JL, & Segal RA (2020). Roadmap for the emerging field of cancer neuroscience. Cell, 181, 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL , Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Müller V, Braga S, Duhoux FP, Greil R, … Winer EP (2020). Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. New England Journal of Medicine, 382(7), 597–609. [DOI] [PubMed] [Google Scholar]
- Najjar YG, Menk AV, Sander C, Rao U, Karunamurthy A, Bhatia R , Zhai S, Kirkwood JM, & Delgoffe GM (2019). Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma. JCI Insight, 4(5), el24989. 10.1172/jci.insight.124989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, & Kunitomo M (2002). Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Science, 70(7), 791–798. [DOI] [PubMed] [Google Scholar]
- Narayana A, Mathew M, Tam M, Kannan R, Madden KM, Golfinos JG, Parker EC, Ott PA, & Pavlick AC (2013). Vemurafenib and radiation therapy in melanoma brain metastases. Journal of neuro-oncology, 113(3), 411–416. [DOI] [PubMed] [Google Scholar]
- Ngo B, Kim E, Osorio-Vasquez V, Doll S, Bustraan S, Liang RJ , Luengo A, Davidson SM, Ali A, Ferraro GB, Fischer GM , Eskandari R, Kang DS, Ni J, Plasger A, Rajasekhar VK , Kastenhuber ER, Bacha S, Sriram RK, … Pacold ME (2020). Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer discovery, 10(9), 1352–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen B, Fong C, Luthra A, Smith SA, DiNatale RG, Nandakumar S , Walch H, Chatila WK, Madupuri R, Kundra R, Bielski CM, Mastrogiacomo B, Donoghue MTA, Boire A, Chandarlapaty S, Ganesh K, Harding JJ, Iacobuzio-Donahue CA, Razavi P, … Schultz N (2022). Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell, 185(3), 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, Dummer R, Adam A, Bauer J, Tabatabai G, Flaherty K, Sinnberg T, Beck D, Leiter U, Mauch C, Roesch A, Weide B, Eigentler T, Schadendorf D, … Meier F (2013). Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer medicine, 2(1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee MH, Seong M, Kim ST, Kang JH, Cho BC, Lee KH, Cho EK, Sun J-M, Lee S-H, Ahn JS, Park K, & Ahn MJ (2020). A phase II, multicenter, two cohort study of 160mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Annals of Oncology, 31(10), 1397–1404. [DOI] [PubMed] [Google Scholar]
- Patton EE, Mueller KL, Adams DJ, Anandasabapathy N, Aplin AE, Bertolotto C, Bosenberg M, Ceol CJ, Burd CE, Chi P, Herlyn M, Holmen SL, Karreth FA, Kaufman CK, Khan S, Kobold S, Leucci E, Levy C, Lombard DB, … Merlino G (2021). Melanoma models for the next generation of therapies. Cancer Cell., 39, 610–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Shahid O, Lagattuta KA, Mahuron KM, Luber JM, Lowe MM, Huang L, Delaney C, Long JM, Fung ME, Newcomer K,Tsai KK, Chow M, Guinn S, Kuchroo JR, Burke KP, Schenkel JM, Rosenblum MD, Daud AI, … Singer M (2021). Single-cell analyses identify circulating anti-tumor CD8 T cells and markers for their enrichment. The Journal of Experimental Medicine, 218, e20200920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Guijarro E, Yang HH, Araya RE, Meskini RE, Michael HT, Vodnala SK, Marie KL, Smith C, Chin S, Lam KC, Thorkelsson A, Iacovelli AJ, Kulaga A, Fon A, Michalowski AM, Hugo W, Lo RS, Restifo NP, Sharan SK, … Merlino G (2020). Multimodel preclinical platform predicts clinical response of melanoma to immunotherapy. Nature Medicine, 26, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilones KA, Hensler M, Daviaud C, Kraynak J, Fucikova J, Galluzzi L, Demaria S, & Formenti SC (2020). Converging focal radiation and immunotherapy in a preclinical model of triple negative breast cancer: contribution of VISTA blockade. Oncoimmunology, 9(1), 830–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Cajal SRY, Megías D, Hernández-Encinas E, Blanco-Aparicio C, Martínez L, Zarzuela E, Muñoz J, Fustero-Torre C, Piñeiro-Yáñez E, Hernández-Laín A, … Valiente M (2018). STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nature Medicine, 24, 1024–1035. [DOI] [PubMed] [Google Scholar]
- Qian JM, Mahajan A, Yu JB, Tsiouris AJ, Goldberg SB, Kluger HM, & Chiang VL (2017). Comparing available criteria for measuring brain metastasis response to immunotherapy. Journal of Neuro-Oncology, 132(3), 479–485. [DOI] [PubMed] [Google Scholar]
- Rabbie R, Ferguson P, Wong K, Couturier D-L, Moran U, Turner C, Emanuel P, Haas K, Saunus JM, Davidson MR, Lakhani SR, Shivalingam B, Long GV, Parkinson C, Osman I, Scolyer RA, Corrie P, & Adams DJ (2021). The mutational landscape of melanoma brain metastases presenting as the first visceral site of recurrence. British Journal of Cancer, 124, 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschenberg R, Bruns J, Brütting J, Daubner D, Lohaus F, Zimmer L , Forschner A, Zips D, Hassel JC, Berking C, Kaehler KC, Utikal J, Gutzmer R, Terheyden P, Meiss F, Rafei-Shamsabadi D, Kiecker F, Debus D, Dabrowski E, … Meier F (2019). Impact of radiation, systemic therapy and treatment sequencing on survival of patients with melanoma brain metastases. European Journal of Cancer, 110, 11–20. [DOI] [PubMed] [Google Scholar]
- Samlowski WE, Watson GA, Wang M, Rao G, Klimo P Jr., Boucher K, Shrieve DC, & Jensen RL (2007). Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer, 109(9), 1855–1862. [DOI] [PubMed] [Google Scholar]
- Santivasi WL, & Xia F (2014). Ionizing radiation-induced DNA damage, response, and repair. Antioxidants & redox signaling, 21(2), 251–259. [DOI] [PubMed] [Google Scholar]
- Schouten LJ, Rutten J, Huveneers HA, & Twijnstra A (2002). Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer, 94(10), 2698–2705. [DOI] [PubMed] [Google Scholar]
- Schwartz H, Blacher E, Amer M, Livneh N, Abramovitz L, Klein A , Ben-Shushan D, Softer S, Blazquez R, Barrantes-Freer A, Müller M, Müller-Decker K, Stein R, Tsarfaty G, Satchi-Fainaro R, Umansky V, Pukrop T, & Erez N (2016b). Incipient melanoma brain metastases instigate astrogliosis and neuroinflammation. Cancer Research, 76, 4359–4371. [DOI] [PubMed] [Google Scholar]
- Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, & Seymour L (2016a). RECIST 1.1—Update and clarification: From the RECIST committee. European journal of cancer, 62, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, & Solomon BJ (2020). First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. New England Journal of Medicine, 383(21), 2018–2029. [DOI] [PubMed] [Google Scholar]
- Silk AW, Bassetti MF, West BT, Tsien CI, & Lao CD (2013). Ipilimumab and radiation therapy for melanoma brain metastases. Cancer medicine, 2(6), 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley I, Chen Z, Phadke MS, Li J, Yu X, Wyatt C, Evernden B , Messina JL, Sarnaik A, Sondak VK, Zhang C, Law V, Tran N, Etame A, Macaulay RJB, Eroglu Z, Forsyth PA, Rodriguez PC, Chen YA, & Smalley KSM (2021). Single cell characterization of the immune microenvironment of melanoma brain and leptomeningeal metastases. Clinic cancer research: An official journal of the Americal Association of Cancer Research, 27, 4109–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R, Robert Snodgrass S, & Johanson CE (2015). A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Experiemental Neurology., 273, 57–68. [DOI] [PubMed] [Google Scholar]
- Srinivasan ES, Tan AC, Anders CK, Pendergast AM, Sipkins DA, Ashley DM, Feed PE, & Khasraw M (2021). Salting the soil: Targeting the microenvironment of brain metastases. Molecular Cancer Therapy, 20, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JH (2010). Stereotactic radiosurgery for the management of brain metastases. New England Journal of Medicine, 362(12), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Tan Y-L, Yuan Y, & Tian L (2020). Microglial regional heterogeneity and its role in the brain. Molecular Psychiatry, 25, 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao CD, Moschos SJ, Atkins MB, Lewis K, Postow MA, Thomas RP, Glaspy J, Jang S, Khushalani NI, Pavlick AC, Ernstoff MS, Reardon DA, Kudchadkar R, Tarhini A, … Margolin KA (2021). Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): Final results of an open-label, multicentre, phase 2 study. The Lancet Oncology, 22(12), 1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L , Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, de Menezes JJ, Dalle S, Arance A, Grob J-J, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, … Long GV (2022). Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. New England Journal of Medicine, 386(1), 24–34.RELATIVITY-047 Investigators [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi K, Hathaway A, Chiuzan C, & Shirai K (2015). Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer medicine, 4(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tio M, Wang X, Carlino MS, Shivalingam B, Fogarty GB, Guminski AD, Lo S, Hong AM, Menzies AM, & Long GV (2018). Survival and prognostic factors for patients with melanoma brain metastases in the era of modern systemic therapy. Pigment cell & melanoma research, 31(4), 509–515. [DOI] [PubMed] [Google Scholar]
- Türei D, Valdeolivas A, Gul L, Palacio-Escat N, Klein M, Ivanova O, Ölbei M, Gábor A, Theis F, Módos D, Korcsmáros T, & Saez-Rodriguez J (2021). Integrated intra- and intercellular signaling knowledge for multicellular omics analysis. Molecular Systems Biology, 17, e9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH-F, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, & Massagué J (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Swearingen AEDV, Anders CK, Bairoch A, Boire A, Bos PD, Cittelly DM, Erez N, Ferraro GB, Fukumura D, Gril B, Herlyn M, Holmen SL, Jain RK, Joyce JA, Lorger M, Massague J, Neman J, Sibson NR, … Winkler F (2020). Brain metastasis cell lines panel: A public resource of organotropic cell lines. Cancer Research, 80, 4314–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deijk AF, Camargo N, Timmerman J, Heistek T, Brouwers JF, Mogavero F, Mansvelder HD, Smit AB, & Verheijen MHG (2017). Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia, 65, 670–682. [DOI] [PubMed] [Google Scholar]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, & Demaria S (2017). DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications, 8(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson N, Gondi V, Jordan JT, Lassman AB, Maues J, Mohile N, Redjal N, Stevens G, Sulman E, van den Bent M, Wallace HJ, … Schiff D (2022). Treatment for brain metastases: ASCO-SNO-ASTRO guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 40(5), 492–516. [DOI] [PubMed] [Google Scholar]
- Vosoughi E, Lee JM, Miller JR, Nosrati M, Minor DR, Abendroth R, Lee JW, Andrews BT, Leng LZ, Wu M, Leong SP, Kashani-Sabet M, & Kim KB (2018). Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer, 18, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JF (1988). DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Progress in nucleic acid research and molecular biology, 35, 95–125. [DOI] [PubMed] [Google Scholar]
- Wingrove E, Liu ZZ, Patel KD, Arnal-Estapé A, Cai WL, Melnick M-A, Politi K, Monteiro C, Zhu L, Valiente M, Kluger HM, Chiang VL, & Nguyen DX (2019). Transcriptomic hallmarks of tumor plasticity and stromal interactions in brain metastasis. Cel! reports., 27, 1277, e7–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Zia S, Verma R, Pavlick A, Wilson M, Golfinos JG, Silverman JS, & Kondziolka D (2016). Impact on overall survival of the combination of BRAF inhibitors and stereotactic radiosurgery in patients with melanoma brain metastases. Journal of neuro-oncology, 127(3), 607–615. [DOI] [PubMed] [Google Scholar]
- Wroński M, & Arbit E (2000). Surgical treatment of brain metastases from melanoma: A retrospective study of 91 patients. Journal of neurosurgery, 93(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H , Kraut M, Beyer M, Latz E, Freeman TC, … Schultze JL (2014). Transcriptome-based Network analysis reveals a Spectrum model of human macrophage activation. Immunity, 40, 274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JCH, Cho BC, Kim DW, Kim SW, Lee JS, Su WC, … & Ahn MJ (2017). Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): Updated results from the BLOOM study. [Google Scholar]
- Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galván JA, Robinson HPC, Zlobec I, Ciriello G , & Hanahan D (2019). Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature, 573, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang Z, Shang D, Yu J, & Yuan S (2019). Incidence and prognosis of brain metastases in cutaneous melanoma patients: a population-based study. Melanoma Research, 29(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Zou Y, Watters A, Cheng N, Perry CE, Xu K, Alicea GM, Parris JLD, Baraban E, Ray P, Nayak A, Xu X, Herlyn M, Murphy ME, Weeraratna AT, Schug ZT, & Chen Q (2019). Polyunsaturated fatty acids from astrocytes activate PPARγ signaling in cancer cells to promote brain metastasis. Cancer Discovery, 9, 1720–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]