Abstract
BACKGROUND AND PURPOSE:
The Systolic Blood Pressure Intervention (SPRINT) randomized trial demonstrated that intensive blood pressure management resulted in slower progression of cerebral white matter hyperintensities, compared with standard therapy. We assessed longitudinal changes in brain functional connectivity to determine whether intensive treatment results in less decline in functional connectivity and how changes in brain functional connectivity relate to changes in brain structure.
MATERIALS AND METHODS:
Five hundred forty-eight participants completed longitudinal brain MR imaging, including resting-state fMRI, during a median follow-up of 3.84 years. Functional brain networks were identified using independent component analysis, and a mean connectivity score was calculated for each network. Longitudinal changes in mean connectivity score were compared between treatment groups using a 2-sample t test, followed by a voxelwise t test. In the full cohort, adjusted linear regression analysis was performed between changes in the mean connectivity score and changes in structural MR imaging metrics.
RESULTS:
Four hundred six participants had longitudinal imaging that passed quality control. The auditory-salience-language network demonstrated a significantly larger decline in the mean connectivity score in the standard treatment group relative to the intensive treatment group (P = .014), with regions of significant difference between treatment groups in the cingulate and right temporal/insular regions. There was no treatment group difference in other networks. Longitudinal changes in mean connectivity score of the default mode network but not the auditory-salience-language network demonstrated a significant correlation with longitudinal changes in white matter hyperintensities (P = .013).
CONCLUSIONS:
Intensive treatment was associated with preservation of functional connectivity of the auditory-salience-language network, while mean network connectivity in other networks was not significantly different between intensive and standard therapy. A longitudinal increase in the white matter hyperintensity burden is associated with a decline in mean connectivity of the default mode network.
Hypertension is one of the most prevalent diseases and is associated with significant cardiovascular and cerebrovascular complications, including stroke and dementia.1,2 The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that intensive blood pressure management (systolic blood pressure [SBP] target, <120 mm Hg) improved cardiovascular outcomes compared with standard management (SBP target, <140 mm Hg) during a median follow-up of 3.26 years.3 SPRINT further showed that intensive therapy was associated with a lower incidence of mild cognitive impairment between treatment groups.4 A subset of participants underwent brain MR imaging, with the intensive group showing a slower progression of white matter hyperintensities (WMH) compared with the standard group, though also with a slightly greater decline in brain volume.5 These studies indicate that intensive treatment may provide an overall benefit for cognitive health, though the mechanisms are unclear and remain a key question for further study.6
The SPRINT MR imaging protocol included resting-state fMRI (rs-fMRI), a noninvasive tool measuring blood oxygen level-dependent (BOLD) signal and allowing investigation of changes in brain functional connectivity (FC). In a study of SPRINT MR imaging participants at baseline, the burden of WMH was inversely related to FC of the default mode network (DMN) and of the auditory-salience-language network (ASLN).7 To further determine the impact of hypertension treatment on brain health, we assessed longitudinal changes in brain functional connectivity (ΔFC) to determine whether intensive treatment results in less decline in FC and how ΔFC relates to changes in brain structure.
MATERIALS AND METHODS
Study Cohort
This study is an analysis of SPRINT brain MR imaging data. The trial protocol was approved by the institutional review board at each participating site. All participants provided written informed consent. SPRINT enrolled 9361 adults without diabetes older than 50 years of age with SBP of >130 mm Hg and an elevated cardiovascular risk profile. Exclusion criteria included prior stroke, diabetes, dementia, and a recent cardiovascular event within 3 months. Participants were randomized to intensive or standard SBP management. The main SPRINT outcome publication includes full details on inclusion and exclusion criteria, randomization, locations, and the treatment protocol.3 The intensive treatment group had antihypertensive medications adjusted during the study duration to target an SBP of 120 mm Hg, whereas the standard treatment group had medications adjusted to target an SBP of 135–139 mm Hg, according to the treatment protocol. The SPRINT MR imaging substudy included a subset of participants within 1.5-hour driving distance of an MR imaging center (n = 475) who underwent 2 MR imaging scans: a baseline scan within 3 months of randomization and a follow-up scan approximately 4 years after randomization. Additional participants with chronic kidney disease (n = 73) from the SPRINT ancillary study “Mind the Kidneys” scanned using the same brain MR imaging protocol were also included. Exclusion criteria included claustrophobia and a non-MR imaging–compatible device or foreign object.
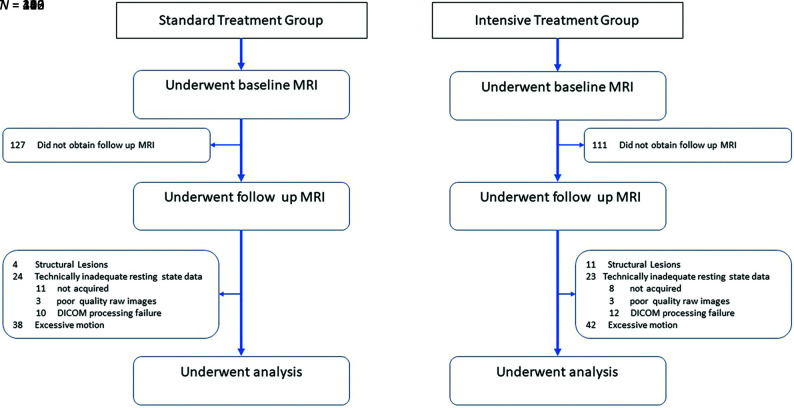
Of the 548 participants who underwent both MR imaging examinations, 15 were excluded due to the presence of structural brain lesions. An additional 47 participants did not have technically adequate resting-state data at both timepoints (19 resting-state data not acquired, 6 poor-quality raw images, 22 failed processing). Excessive motion resulted in exclusion of 80 additional participants as detailed below. Four hundred six participants had adequate longitudinal resting-state data.
MR Imaging Data Acquisition
MR images were obtained on 3T scanners at 11 sites (1 Magnetom Skyra VD11B, 3 Magnetom Trio VB17, 2 Magnetom Verio VB17; Siemens; 4 Achieva 3.2; Philips Healthcare; and 1 Discovery MR750W; GE Healthcare) using a multichannel receiver head coil. The structural MR imaging protocol included sagittal 1-mm isotropic T1-weighted 3D MPRAGE, T2-weighted FLAIR, and T2-weighted fast spin-echo sequences, described previously.5 An rs-fMRI scan was acquired using axial BOLD echo-planar imaging, with TR/TE = 2000/25 ms, isotropic 3.5-mm voxels, and 120 volumes.
Structural Image Processing
Structural imaging was processed in a manner similar to that of prior SPRINT studies.5,8,9 Skull stripping was performed,10 and intracranial tissues were segmented using a multiatlas label fusion method.11 Images were registered into the Montreal Neurological Institute template space. WMH were identified using a deep learning–based segmentation model.5 Classifications of WMH were assessed for quality by a neuroradiologist.
Functional Image Processing
Rs-fMRI data were preprocessed in standard fashion, as reported previously.7 These included removal of the first 6 volumes, section-time correction, motion correction, nuisance variable regression, spatial smoothing (6-mm full width at half maximum), bandpass filtering (0.01–0.1 Hz), and registration via the T1-weighted scan into Montreal Neurological Institute template space, followed by resampling to a 4-mm isotropic resolution. Scans were excluded if any framewise motion exceeded 3.5 mm and if the mean relative displacement was 0.3 mm.12,13 Twenty independent components14 were identified at group-level independent component analysis using Melodic (FSL; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC).15 The 20 identified components can be found in previously published work.7 Canonical networks were identified from these by visual inspection. Subject-level independent components were extracted using group information–guided independent component analysis16,17 and were used to calculate z score maps for each network for each study participant.7
A mean connectivity score (MCS) was calculated for each network in each participant as the participant’s average within-network FC within a 3D mask of the network generated from the group-level independent component analysis.18 This represents a metric of overall network coherence with greater scores reflecting more integrated dynamics within the network18 and in this cohort, it was found to be related to baseline volume of WMH for networks of interest as described below. For each network, the longitudinal change in MCS (ΔMCS) was calculated by subtracting the participant’s baseline MCS from the follow-up MCS, with a positive change with time indicating an overall improvement in within-network connectivity, and a negative change indicating an overall decline. The temporal SD of the resting-state BOLD signal was calculated as the resting-state fluctuation of amplitude to assess neurovascular coupling integrity.19
The DMN and ASLN were considered primary networks of interest based on a baseline assessment of this cohort, which demonstrated that the MCS of these networks correlated with the volume of WMH.7 The MCS of the DMN at baseline was also significantly related to performance on the Montreal Cognitive Assessment, demonstrating a mediation effect. The MCS of the ASLN demonstrated a nonsignificant inverse relationship with performance on the Digit Symbol Coding Test. Additional secondary networks of interest included the left frontoparietal network, dorsal frontal network, basal ganglia network, and a network including the posterior DMN components, which showed a relationship to WMH but not with cognition.7
Group Level Statistical Analyses
Statistical analyses were performed using Matlab (MathWorks).
Treatment Group Comparisons.
For the 2 primary networks of interest, the ΔMCS was compared between the treatment groups using a t test with a Bonferroni correction (P < .025). On the basis of the mean and SD of the MCS in the cohort at baseline, we would expect 80% power to detect ≥8% decline in network MCS for the standard compared with the intensive group. To identify regions of the network with significant differences between treatment groups, FC difference maps were calculated from the spatial network maps of each participant at follow-up and baseline, and a voxelwise t test was performed on the difference maps. The t score map was thresholded at t = 2.588 (P < .01, uncorrected), and Monte Carlo simulation was then performed to determine the cluster size representative of a threshold of P < .0001, family-wise error–corrected. Significant clusters represent the regions within the network where ΔFC is the difference between treatment groups. Treatment groups were also compared using a t test for ΔMCS for secondary networks of interest. Resting-state fluctuation of amplitude was also compared between the groups for both the baseline and follow-up scans using a 2-sample t test to evaluate evidence that group differences may be affected by differences in neurovascular coupling.
To determine whether significant group differences or significant clusters were influenced by differential rates of network-wide or local atrophy, we performed a post hoc analysis in which cortical volumes were calculated for each participant at each time point within the entire ASLN network, within the significant clusters, as well as cortical volumes within atlas ROIs that corresponded to the significant clusters. The longitudinal change in these cortical volumes was calculated for each participant and compared between the 2 treatment groups using 2-sample t tests.
Subgroup Comparisons.
An exploratory subgroup analysis was conducted on the basis of age, baseline WMH burden, change in WMH, and longitudinal change in relative total brain volume (rTBV, defined as the ratio of total brain volume to intracranial volume). Age subgroups were defined as younger than 65 years of age and 65 years or older. Those with high or low baseline burden of WMH were dichotomized by the median value of 1536 mm3 for WMH burden within supratentorial regions of the brain at baseline. Longitudinal change in WMH subgroups were defined as those with minimal or no substantial change (ΔWMH of <250 mm3) or those with some change (ΔWMH of ≥250 mm3). Subgroups for longitudinal change in rTBV were defined on the basis of the median value as those with minimal or no substantial decrease across time (ΔrTBV > −0.019) or those with a larger decrease with time (ΔrTBV ≤ −0.019). The ΔMCS was compared between treatment groups using a 2-sample t test within each subgroup.
Longitudinal Changes in FC across the Entire Longitudinal Cohort.
To determine how longitudinal changes in FC were related to changes in brain structure with time, we created multiple, variable, linear regression models for ΔMCS across the entire SPRINT longitudinal fMRI cohort within primary networks of interest. Models were created using the ΔMCS as the outcome variable, ΔWMH and ΔrTBV as predictors, adjusting for age, sex, and race. Because none of these covariates were significant, these variables were removed from the final models.
RESULTS
Study Cohort
The study cohort included 406 participants: 226 in the intensive arm and 180 in the standard arm (Fig 1). Baseline characteristics are shown in Table 1. Participant demographics, clinical variables, and structural brain volumes were similar between treatment groups at baseline. Hispanic ethnicity and Black race were underrepresented relative to the overall SPRINT cohort. Women made up a slightly larger proportion of the intensive group and a smaller proportion of the standard group relative to the overall SPRINT trial. There was a greater proportion of participants with chronic kidney disease in the current cohort relative to the main SPRINT trial but no difference in the estimated glomerular filtration rate between the treatment groups.
FIG 1.
Flow diagram for SPRINT participants undergoing fMRI.
Table 1:
Characteristics of study cohort at baselinea
| Standard Treatment Group (n = 180) | Intensive Treatment Group (n = 226) | |
|---|---|---|
| Follow-up period (median) (yr) | 3.86 | 3.82 |
| Age (yr) | 67.5 (SD, 8.4) | 68.4 (SD, 8.4) |
| Female sex (No.) (%) | 60 (33.3%) | 89 (39.4%) |
| Race (No.) (%) | ||
| White | 117 (65.0%) | 156 (69.0%) |
| Black | 51 (28.3%) | 59 (26.1%) |
| Hispanic | 9 (5.0%) | 7 (3.1%) |
| Other | 3 (1.7%) | 4 (1.8%) |
| SBP (mm Hg) | 139.6 (SD, 18.4) | 136.2 (SD, 18.2) |
| DBP (mm Hg) | 82.2 (SD, 13.2) | 80.2 (SD, 11.2) |
| eGFR (mL/min/1.73 m2) | 69.1 (SD, 21.3) | 65.2 (SD, 19.0) |
| Serum creatinine level (mg/dL) | 1.1 (SD, 0.4) | 1.1 (SD, 0.4) |
| Chronic kidney disease (eGFR <60 mL/min/1.73 m2) (No.) (%) | 61 (33.9) | 88 (38.9) |
| Framingham 10-year cardiovascular disease risk score (%) | 24.3 (SD, 12.4) | 23.5 (SD, 12.6) |
| TBV (cm3) | 1150.9 (SD, 117.0) | 1139.3 (SD, 117.3) |
| rTBV/ICV) | 0.82 (SD, 0.04) | 0.82 (SD, 0.04) |
| White matter lesion volume (median) (IQR) (cm3) | 1.6 (0.9–3.8) | 1.4 (0.6–3.6) |
Note:—DBP indicates diastolic blood pressure; IQR, interquartile range; eGFR, estimated glomerular rate; ICV, intracranial volume.
All values provided as means unless otherwise specified.
Treatment Group Comparisons
In the ASLN, there was a significant difference between groups in ΔMCS (P = .014), with the intensive treatment group demonstrating a slightly positive change with time (mean, 0.22 [SD, 1.65]) versus a slightly negative change with time in the standard treatment group (mean, −0.19 [SD, 1.64]). In the DMN, there was no significant difference in ΔMCS between groups (P = .24).
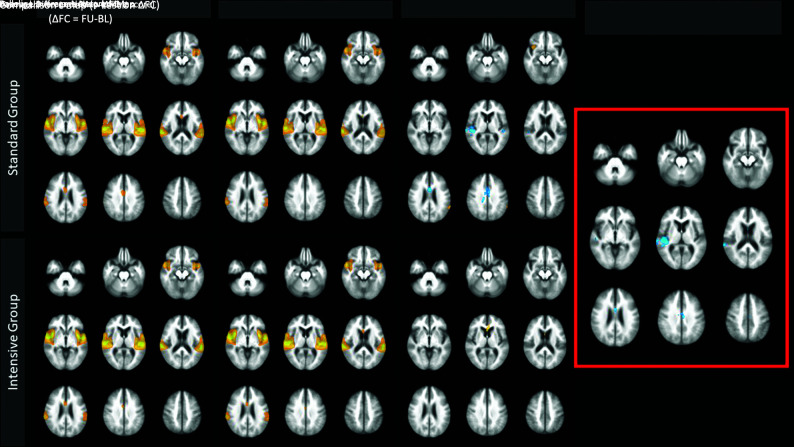
Voxelwise analysis of the longitudinal change in FC (ΔFC) in the ASLN is demonstrated in Fig 2. In the standard treatment group, the ΔFC map shows areas of negative change with time in the bilateral posterior temporal lobes, right insula, and anterior and midcingulate region and some smaller areas of mild positive change across time in the anterior right insula and left parietal lobe. In the intensive treatment group, there is a small region of positive change with time in the anterior cingulate region and no areas of decline with time. The comparison t map demonstrates the results of the voxelwise t test on ΔFC. The standard group shows a significantly larger decrease in FC in the right posterior temporal/insular and cingulate regions, relative to the intensive group. A post hoc comparison of longitudinal change in gray matter volumes in the ASLN network and in cortical regions corresponding to these clusters found no significant difference between the 2 treatment groups (P > .05).
FIG 2.
Voxelwise t test comparing the longitudinal change of the ASLN between treatment groups. Comparison t map on the right (red box) demonstrates regions of significantly more negative change with time in the standard group compared with the intensive group in the cingulate region and right posterior insula and temporal lobe. Clusters are significant to P < .0001 family-wise error–corrected. BL indicates baseline; FU, Follow-up.
Additional secondary networks of interest demonstrated no significant difference in ΔMCS between treatment groups (all, P > .05). There was no difference between treatment groups in resting-state fluctuation of amplitude to suggest systematic differences in neurovascular coupling.
Subgroup Comparisons
Table 2 shows the results of the subgroup analyses. In those with greater longitudinal decline in rTBV, the standard group demonstrated greater decline in the MCS of the DMN relative to the intensive group (P = .024, uncorrected). For the ΔMCS in the ASLN, there were differences between treatment groups in the subgroups with greater baseline WMH volume (P = .044), older age (P = .037), and smaller longitudinal change in rTBV (P = .0058), with the intensive group demonstrating less longitudinal decline.
Table 2:
Subgroup comparisons between treatment groups in longitudinal ΔMCS
| Default Mode Network |
ASLN |
|||||||
|---|---|---|---|---|---|---|---|---|
| Standard Group | Intensive Group | Effect Size (Cohen d) | t | P Value | Effect Size (Cohen d) | t | P Value | |
| Overall | 180 | 226 | –0.12 | –1.17 | .24 | –0.24 | –2.46 | .014a |
| Subgroups | ||||||||
| High baseline WMH | 94 | 108 | –0.14 | –0.97 | .33 | –0.28 | –2.03 | .044b |
| Low baseline WMH | 85 | 118 | –0.09 | –0.66 | .51 | –0.21 | –1.47 | .144 |
| High ΔWMH | 101 | 96 | –0.08 | –0.55 | .59 | –0.24 | –1.67 | .097 |
| Low ΔWMH | 77 | 127 | –0.12 | –0.86 | .39 | –0.27 | –1.86 | .064 |
| Older age | 97 | 131 | –0.12 | –0.90 | .37 | –0.28 | –2.10 | .037b |
| Younger age | 83 | 95 | –0.11 | –0.73 | .47 | –0.19 | –.28 | .20 |
| Larger rTBV decline | 87 | 117 | –0.32 | –2.27 | .024b | –0.09 | –0.64 | .52 |
| Smaller rTBV decline | 93 | 109 | 0.09 | 0.66 | .51 | –0.39 | –2.79 | .0058b |
P <. 05, Bonferroni-corrected.
P <. 05, uncorrected.
Longitudinal Changes in FC across the Entire MR Imaging Cohort
In the multiple variable regression model for ΔMCS of the DMN, ΔWMH demonstrated a negative relationship (P = .013) and ΔrTBV demonstrated a positive relationship (P = .03); however, only ΔWMH was statistically significant after Bonferroni correction for the 2 models. Model parameters are listed in Table 3. Neither ΔWMH nor ΔrTBV were significant predictors in the model for ΔMCS of the ASLN.
Table 3:
Multiple variable linear regression parameters for models of longitudinal ΔMCS
| Predictors | ΔMCS Model Parameters |
|||
|---|---|---|---|---|
| Default Mode Network Model |
ASLN Model |
|||
| β | P Value | β | P Value | |
| ΔWMH | –7.86 ×10−7 | .013 | 7.21 ×10−7 | .067 |
| ΔrTBV | 0.14 | .030 | 0.1106 | .17 |
fMRI findings from the SPRINT randomized clinical trial indicated that intensive blood pressure management results in preservation of FC as measured by MCS in the ASLN, while other networks were not significantly different relative to standard therapy in our sample. Furthermore, we observed that worsening of WMH volume is associated with decreased FC in the DMN.
In the ASLN, FC was preserved in the intensive group compared with the standard group, primarily in the insula and cingulate regions, which correspond primarily to the salience component of the network.20 The salience network has been posited to modulate redirecting attention between internal and external stimuli.20 In the baseline study of this cohort, ASLN FC was also related to WMH burden and demonstrated a nonsignificant inverse relationship with performance on the Digit Symbol Coding Test, which assesses processing speed and working memory.21 However, in the current study, there was no significant relationship between longitudinal change in ASLN FC and change in WMH or rTBV. These results suggest that intensive therapy may impact ASLN FC independent of the effects on global WMH or rTBV. This possibility may perhaps be through more local or regional changes or through other mechanisms related directly to the hypertensive treatments.
The longitudinal changes in ASLN FC were small in magnitude, as were the differences between the treatment groups. This finding is likely related to the short study duration of 4 years relative to the typical duration of hypertension, both study arms being treated with a relatively small difference in achieved blood pressure, and the small impact seen on cognitive function in other SPRINT studies.4,22 The small effect size suggests that there may be a slow impact at the functional network level, which may take years of treatment to manifest as a larger effect or clinically apparent cognitive changes. The treatment group differences in the ASLN FC were more prominent in the older age subgroup and in those with greater WMH burden at baseline. Prior studies have noted that higher blood pressure is associated with poorer processing speed, particularly in older individuals.23 Overall, the neurocognitive significance of our findings in the ASLN is uncertain. Future study on long-term changes in processing speed and working memory would be of interest, particularly in the elderly or in those with greater WMH. However, in a separate SPRINT substudy assessing domain-specific cognitive changes, no significant difference was found in memory scores between groups, and there was a small decline in the processing speed domain score in the intensive treatment group.22
The DMN is disrupted in disease states such as Alzheimer disease,24 including those individuals with hypertension.25 While SPRINT also demonstrated slower progression of WMH with intensive compared with standard therapy,5 no treatment group difference was seen in DMN FC. This result may be because of the opposing effects of the observed changes in WMH and rTBV in SPRINT on DMN FC. We observed an association of higher DMN FC with both lower WMH burden and larger brain volume, and in the prior SPRINT imaging study, intensive therapy resulted in both lower progression of WMH volume and slightly lower brain volumes,5 which would predict opposing effects on DMN connectivity. In fact, in the subgroup of participants who all had a greater decline in rTBV, intensive therapy was associated with less decline in FC in the DMN relative to standard treatment. Changes in FC resulting from structural changes in WMH volume or rTBV may also be less sensitive, either due to resiliency in FC or our ability to quantify small changes. Furthermore, the apparent difference in FC decline between treatment groups was small, and the study may have had insufficient power to detect such a small change. It is possible that studies across a longer time interval or in a larger sample may provide different results. Despite the lack of a beneficial effect of intensive therapy on DMN FC, these results provide some reassurance that intensive lowering of blood pressure does not result in an accelerated decline in connectivity within this network.
Limitations of this study include the longitudinal fMRI cohort being slightly different from the overall SPRINT study, including having less cardiovascular disease but a higher proportion of chronic kidney disease, potentially affecting generalizability. Although hypertension may theoretically impact neurovascular coupling, there was no group difference in resting-state fluctuation of amplitude; thus, group differences in neurovascular coupling are unlikely to have systematically affected the treatment group comparison.
There was a greater rate of loss to follow-up imaging in the standard group and a greater number of exclusions in the intensive group, which could introduce bias. Such bias is difficult to predict; however, if participant losses were due to poorer cognitive function, effects would likely be of small magnitude given the small cognitive differences detected in other SPRINT studies. SPRINT observed little change in domain-specific cognitive score measures22 and adjudicated cases of dementia and mild cognitive impairment in the MR imaging group,4 reducing the power to evaluate associations between cognitive and imaging measures. Finally, because the trial intervention was stopped early due to a significant cardiovascular benefit, this decision likely reduced the magnitude of longitudinal changes in FC, given the shorter time interval, and may have reduced the power to detect group differences.
This last point emphasizes a notable gap in the literature: Despite the well-established role of hypertension as a risk factor for cognitive impairment, cognitive outcomes have primarily been investigated only as a secondary end point in studies of hypertension. Our results are in accord with the results of prior cognitive studies from SPRINT, which found no clinically relevant differences between treatment groups in memory or processing speed,22 no significant difference in incident dementia, and only a small difference in mild cognitive impairment.4 Combined with the results from additional trials, there is mounting evidence that intensive blood pressure management is not harmful and may be beneficial for cognition, even in the elderly.26 However, there remains uncertainty regarding the optimal treatment thresholds with regard to cognitive outcomes, whether those goals may change across the life span, and optimal strategies specific to patients with pre-existing cognitive impairment, who were largely excluded from these trials.27 A recent trial designed with executive function as the primary outcome found that in older adults with mild cognitive impairment, treatment with candesartan was superior to lisinopril.28 There remains a need for additional long-term trials designed and powered to assess the role of blood pressure management in preventing cognitive decline, particularly given the increasing world-wide prevalence of hypertension and the aging population.
CONCLUSIONS
In patients with hypertension, intensive lowering of blood pressure results in preservation of FC in the ASLN, particularly in older subjects and those with greater WMH at baseline. Greater accumulation of WMH is related to a greater decline in the MCS of the DMN; however, intensive blood pressure lowering did not significantly impact longitudinal change in the DMN MCS compared with standard treatment.
Acknowledgments
The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list (https://www.sprinttrial.org/public/dspScience.cfm).
ABBREVIATIONS:
- ASLN
auditory-salience-language network
- BOLD
blood oxygen level–dependent
- DMN
default mode network
- FC
functional connectivity
- MCS
mean connectivity score
- rs-fMRI
resting-state fMRI
- rTBV
relative total brain volume
- SBP
systolic blood pressure
- TBV
total brain volume
- WMH
white matter hyperintensities
Footnotes
The Systolic Blood Pressure Intervention (SPRINT) randomized trial is funded with Federal funds from the National Institutes of Health, including the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; and the National Institute of Neurological Disorders and Stroke, under contract Nos. HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Interagency Agreement No. A-HL-13-002-001. It was also supported, in part, by resources and use of facilities through the Department of Veterans Affairs.
The views expressed in this article are those of the authors and do not represent the official position of the National Institutes of Health, the Department of Veterans Affairs, the US Government, or the SPRINT Research Group. This article was not reviewed or approved by the SPRINT Steering Committee.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: a report from the American Heart Association. Circulation 2016;133:e38–360 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright JT, Williamson JD, Whelton PK; et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson JD, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: a randomized clinical trial. JAMA 2019;321:553–61 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasrallah IM, Pajewski NM, Auchus AP, et al. ; SPRINT MIND Investigators for the SPRINT Research Group, Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019;322:524–34 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iadecola C, Yaffe K, Biller J, et al. ; American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016;68:e67–94 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah C, Srinivasan D, Erus G, et al. Changes in brain functional connectivity and cognition related to white matter lesion burden in hypertensive patients from SPRINT. Neuroradiology 2021;63:913–24 10.1007/s00234-020-02614-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura MK, Pajewski NM, Bryan RN, et al. ; SPRINT Study Research Group. Chronic kidney disease, cerebral blood flow, and white matter volume in hypertensive adults. Neurology 2016;86:1208–16 10.1212/WNL.0000000000002527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Gadegbeku CA, Bryan RN, et al. APOL1 renal-risk variants associate with reduced cerebral white matter lesion volume and increased gray matter volume. Kidney Int 2016;90:440–49 10.1016/j.kint.2016.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doshi J, Erus G, Ou Y, et al. Multi-atlas skull-stripping. Acad Radiol 2013;20:1566–76 10.1016/j.acra.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi J, Erus G, Ou Y, et al. ; Alzheimer's Neuroimaging Initiative. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage 2016;127:186–95 10.1016/j.neuroimage.2015.11.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 2012;60:623–32 10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power JD, Mitra A, Laumann TO, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014;84:320–41 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray KL, McKay DR, Fox PM, et al. ICA model order selection of task co-activation networks. Front Neurosci 2013;7:237 10.3389/fnins.2013.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 2004;23:137–52 10.1109/TMI.2003.822821 [DOI] [PubMed] [Google Scholar]
- 16.Du Y, Fan Y. Group information guided ICA for fMRI data analysis. Neuroimage 2013;69:157–97 10.1016/j.neuroimage.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Allen EA, He H, et al. Artifact removal in the context of group ICA: a comparison of single-subject and group approaches. Hum Brain Mapp 2016;37:1005–25 10.1002/hbm.23086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisner KM, Patzelt EH, Lim KO, et al. An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am J Drug Alcohol Abuse 2013;39:403–13 10.3109/00952990.2013.848211 [DOI] [PubMed] [Google Scholar]
- 19.Kannurpatti SS, Motes MA, Biswal BB, et al. Assessment of unconstrained cerebrovascular reactivity marker for large age-range FMRI studies. PLoS One 2014;9:e88751 10.1371/journal.pone.0088751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon V. Salience Network. In: Toga AW, ed. Brain Mapping: An Encyclopedic Reference. 2nd ed. Academic Press: Elsevier; 2015:597–611 [Google Scholar]
- 21.Jaeger J. Digit symbol substitution test. J Clin Psychopharmacol 2018;38:513–19 10.1097/JCP.0000000000000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapp SR, Gausoin SA, Sachs BC, et al. ; SPRINT Research Group. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol 2020;19:899–907 10.1016/S1474-4422(20)30319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forte G, Pascalis VD, Favieri F, et al. Effects of blood pressure on cognitive performance: a systematic review. J Clin Med 2019;9:34 10.3390/jcm9010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochim Biophys Acta 2012;1822:431–41 10.1016/j.bbadis.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 25.Son SJ, Kim J, Lee E, et al. Effect of hypertension on the resting-state functional connectivity in patients with Alzheimer’s disease (AD). Arch Gerontol Geriatr 2015;60:210–16 10.1016/j.archger.2014.09.012 [DOI] [PubMed] [Google Scholar]
- 26.Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology 2019;92:1017–18 10.1212/WNL.0000000000007543 [DOI] [PubMed] [Google Scholar]
- 27.Gorelick PB, Sorond F. Cognitive function in SPRINT: where do we go next? Lancet Neurol 2020;19:880–81 10.1016/S1474-4422(20)30356-2 [DOI] [PubMed] [Google Scholar]
- 28.Hajjar I, Okafor M, McDaniel D, et al. Effects of Candesartan vs Lisinopril on Neurocognitive Function in Older Adults with Executive Mild Cognitive Impairment: a randomized clinical trial. JAMA Netw Open 2020;3:e2012252 10.1001/jamanetworkopen.2020.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]