Abstract
The temperature-sensitive hemagglutinin Tsh is a member of the autotransporter group of proteins and was first identified in avian-pathogenic Escherichia coli (APEC) strain χ7122. The prevalence of tsh was investigated in 300 E. coli isolates of avian origin and characterized for virulence in a 1-day-old chick lethality test. Results indicate that among the tsh-positive APEC isolates, 90.6% belonged to the highest virulence class. Experimental inoculation of chickens with χ7122 and an isogenic tsh mutant demonstrated that Tsh may contribute to the development of lesions within the air sacs of birds but is not required for subsequent generalized infection manifesting as perihepatitis, pericarditis, and septicemia. Conjugation and hybridization experiments revealed that the tsh gene is located on a ColV-type plasmid in many of the APEC strains studied, including strain χ7122, near the colicin V genes in most of these strains. DNA sequences flanking the tsh gene of strain χ7122 include complete and partial insertion sequences and phage-related DNA sequences, some of which were also found on virulence plasmids and pathogenicity islands present in various E. coli pathotypes and other pathogenic members of the Enterobacteriaceae. These results demonstrate that the tsh gene is frequently located on the ColV virulence plasmid in APEC and suggest a possible role of Tsh in the pathogenicity of E. coli for chickens in the early stages of infection.
Avian-pathogenic Escherichia coli (APEC) comprise a specific subset of pathogenic E. coli that cause extraintestinal diseases of poultry. Of the various forms of E. coli disease in poultry, the most common syndrome starts as a respiratory tract infection in 3- to 12-week-old broiler chickens and turkeys and frequently becomes more generalized. The air sacs are the first organs affected, and systemic spreading may result in pericarditis, perihepatitis, and an often fatal septicemia (15, 29). APEC infections are frequently enhanced or initiated by predisposing factors, which include environmental conditions and viral or Mycoplasma infection (15, 29). O1, O2, and O78 are the most commonly encountered serogroups among APEC (15, 29), and the majority of strains have been shown to belong to a limited number of clonal lineages (69, 70). APEC strains of high virulence are lethal for 1-day-old chicks when administered subcutaneously. Attributes associated with APEC strains include F1 (type 1) and P fimbrial adhesins (16, 21, 53, 66), resistance to serum and phagocytosis (21, 22, 52, 71), the aerobactin siderophore system (21, 41, 65), and colicin V (7, 23, 65, 71) (reviewed in references 15 and 29). Recently the tsh gene, encoding a temperature-sensitive hemagglutinin, first identified by Provence and Curtiss (54), was shown to be associated with APEC but not with E. coli isolated from the feces of healthy chickens (45).
The tsh gene was first identified from APEC O78:K80 strain χ7122 and, when cloned into E. coli K-12, was shown to impart mannose-resistant hemagglutination of chicken erythrocytes if bacteria were grown at 26°C on low-osmolarity solid medium (54). The deduced protein encoded by the tsh gene exhibits 50% similarity to immunoglobulin A (IgA) proteases of Neisseria gonorrhoeae and Haemophilus influenzae (54) and, as demonstrated by Stathopoulos et al. (60), the Tsh protein was the first identified member of an expanding subclass of the IgA protease family of autotransporters present in Shigella spp. and numerous pathotypes of E. coli. Autotransporters are a family of autonomously secreted proteins from gram-negative bacteria that are processed as three functional domains, comprising a sec-dependent amino-terminal leader sequence, an extracellular or surface-secreted mature protein (passenger domain), and an outer membrane-associated carboxy-terminal β-barrel domain that mediates secretion of the passenger domain (32, 44). These proteins exhibit diverse functions involved in virulence and include adhesins, proteases, cytotoxins, and cell invasion proteins (32, 44). Autotransporters recently identified from pathogenic E. coli or Shigella spp. include EspC (61) and AIDA-I (6) from enteropathogenic E. coli; EspP/PssA from enterohemorrhagic/Shiga toxin-producing E. coli (12, 17); Pet and Pic from enteroaggregative E. coli (24, 31); TibA from enterotoxinogenic E. coli (42); Hbp from E. coli associated with a human wound infection (49); and VirG/IcsA (62), SepA (5), and ShMu (56) from Shigella spp. With the exception of EspC, ShMu/Pic, and TibA, the genes encoding these various autotransporter proteins are located on plasmids.
The Tsh autotransporter is processed as a 106-kDa secreted domain, Tshs, exported through a 33-kDa β-barrel domain, Tshβ, and contains a serine protease motif but does not demonstrate detectable proteolysis of human IgA, chicken IgA, or casein (60). Recently a nearly identical Tsh protein, with only two amino acid differences (Q209-K209 and A842-T842), named Hbp, was shown to specifically degrade human hemoglobin and bind heme (49). Although the hemagglutination activity of Tsh occurs predominantly at 26°C, Tsh is also produced at temperatures as high as 42°C and in strain χ7122 is increasingly liberated into the extracellular medium at higher temperatures (60). Currently, it is unknown whether Tsh contributes to the pathogenesis of APEC infection. Furthermore, the location of tsh within the genome of strain χ7122 and other APEC strains has not been precisely determined.
This report determines the prevalence and the location of the tsh gene in APEC strains and investigates the possible role of tsh in the pathogenesis of avian respiratory colibacillosis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The APEC strain χ7122 and derivatives, avian E. coli clinical isolates, E. coli K-12 strains, and plasmids used are presented in Table 1. Clinical isolates included 300 avian E. coli originating from chickens (117 isolates), turkeys (175 isolates), and ducks (8 isolates). They were collected in France (211 isolates) and Canada (89 isolates), mainly from lesions of colisepticemia and in a few cases from the vitellus of day-old turkey poults, over a period of 10 years. Serogroup was determined by slide agglutination with specific antisera raised against O1, O2, and O78 antigens (Biovac, Angers, France, or the Escherichia coli Laboratory, St. Hyacinthe, Québec, Canada). The number of isolates in each serogroup were 28 O1, 49 O2, 84 O78, and 139 other. Lennox (L) broth and L agar (47) were routinely used for growing E. coli strains and clones, and strain DH5α was routinely used for plasmid cloning and recovery. For infection studies, strain χ7122 and derivatives were grown in brain heart infusion broth (BHI; Difco Laboratories, Detroit, Mich.). Ampicillin (100 μg ml−1), kanamycin (25 μg ml−1), chloramphenicol (25 μg ml−1), nalidixic acid (12.5 μg ml−1), and tetracycline (10 μg ml−1) were used as required unless indicated otherwise.
TABLE 1.
Bacterial strains and plasmids
| Bacterial strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| Clinical isolates (n = 300) | Avian E. coli clinical isolates from France and Canada | This study |
| MGN-617 | thi thr leu tonA lacY glnV supE ΔasdA4 recA::RP4 2-Tc::Mu [λpir] Kmr | 38 |
| DH5α | F− λ− Φ80 Δ(lacZYA-argF) endA1 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 | Bethesda Research Laboratories |
| χ7122 | APEC O78:K80:H9, gyrA Nalr | 55 |
| χ7273 | χ7122 tsh::tetAR(B), Nalr Tcr | This study |
| χ7274 | χ7273 cured of pAPEC-1, Nalr | This study |
| χ7275 | χ7122 pAPEC-1 replaced by pColVK30::lacZ, Nalr Tcr | This study |
| χ7276 | E. coli K-12 MG1655 Tn10::kan, Kmr | This study |
| χ7277 | χ7276(pAPEC-1 tsh::tetAR), Tcr Kmr | This study |
| Plasmids | ||
| pSBA383 | 2.2-kb PCR fragment of tetAR(B) cloned into HindIII sites of pUC4-KIXX, Apr | 56 |
| pBSL86 | nptII Kmr cassette retrieval vector, Kmr Apr | 1 |
| pYA3104 | Cosmid clone of χ7122 containing tsh, Apr | 54 |
| pYA3107 | 10-kb EcoRI subclone of pYA3104 region encompassing tsh in pACYC184, Apr | 54 |
| pYA3108 | 7-kb ClaI subclone of tsh region of pYA3107 in pBluescript II SK, Apr | 54 |
| pHK11 | 9.4-kb HindIII-SalI fragment containing colicin V gene cluster from pColV-K30 | 27 |
| pColV-K30 | Prototype colicin V plasmid | 27 |
| pAPEC-1 | Native colicin V plasmid of strain χ7122 | This study |
| pColV-K30:lacZ | pColV-K30::Tn10 iucC::lacZ, Tcr | 4 |
| pMEG-375 | sacRB mobRP4 oriR6K, Cmr Apr | S. Tinge, Megan Health |
| pYA3418 | PCR product of tsh cloned in EcoRI-BamHI sites of pWKS30, Apr | 60 |
| pYA3442 | tetAR(B) fragment of pSBA383 cloned into HindIII sites of pBSL86, Apr | This study |
| pYA3444 | tetAR(B) PstI fragment of pYA3442 cloned into NsiI sites of tsh in pYA3418, Apr Tcr | This study |
| pYA3448 | BssHII fragment from pYA3444 cloned into AscI sites of pMEG375, Cmr Apr Tcr | This study |
Ap, ampicillin; Cm, chloramphenicol; Km, kanamycin; Nal, nalidixic acid; Tc, tetracycline.
DNA and genetic manipulations.
Total bacterial genomic DNA was prepared using a small-scale preparation method (3). Restriction endonucleases and DNA-modifying and ligase enzymes (New England Biolabs and Promega) were used according to the manufacturer's guidelines. Native plasmids from APEC strains were isolated as described by Kado and Liu (37) and separated by electrophoresis. Conjugation and transformation of bacterial cells were performed by standard techniques (47). Counterselection for loss of tetracycline resistance was achieved using a tetracycline-sensitive selective (TSS) agar containing fusaric acid (5 g of tryptone, 5 g of yeast extract, 10 g of NaCl, 15 g of agar, 10 g of NaH2PO4 · H2O, 12 μg of fusaric acid, 50 μg of chlortetracycline HCl, and 13.6 μg of ZnCl2 per liter of medium) (9).
PCR and DNA hybridization.
The primers used for PCR amplification and generation of DNA probes are indicated in Table 2. The locations of primers used for probe generation and PCR analysis are presented in Fig. 1. DNA crude extracts prepared by a rapid boiling method were tested in a 25-μl PCR mixture containing 12.5 pmol (or 6.25 pmol each) of the forward and reverse primers, 5 nmol of each deoxynucleoside triphosphate, and 0.5 U of Taq DNA polymerase in 1× buffer. The PCR conditions were as follows: 94°C for 3 min; annealing as indicated in Table 2 for 1 min, and 72°C for 1 min for 1 cycle; 94°C for 1 min, annealing for 1 min, 72°C for 1 min for 26 cycles; and a final extension at 72°C for 10 min.
TABLE 2.
Primers used for PCR amplifications and DNA probe synthesis
| Primer set | Annealing tempa (°C) | Primerb | Gene specificity | Sequence (5′→3′) | Accession no. and referencec |
|---|---|---|---|---|---|
| Tsh1 | 53 | 1 (>) | tsh | GGTGGTGCACTGGAGTGG | L27423 (54) |
| 2 (<) | tsh | AGTCCAGCGTGATAGTGG | L27423 (54) | ||
| Tsh2 | 51 | 3 (>) | tsh | CCAAATGCAGAGCGTTC | L27423 (54) |
| 4 (<) | tsh | TTTACCGGCGTGATGGC | L27423 (54) | ||
| Tsh-3′ | 60 | 5 (>) | tsh | GGGCTGGAAGTTGAACGCTC | L27423 (54) |
| 6 (<) | ORF4d | CGGGCGGTCAGTCTGGGTC | AF218073 (this study) | ||
| ColV1 | 51 | 7 (>) | cvi | TCTCTGCATTAATGTCTGC | X57525 (27) |
| 8 (<) | cvaB | GATATGGGGCCAATATCCC | X57524 (27) | ||
| Tsh mut. | 52 | 9 (>) | tsh | TCAGTTGAAGGCGGCAGATT | L27423 (54) |
| 10 (<) | tsh | ACGGTGCCGTTGAAGACACTT | L27423 (54) | ||
| 11 (>) | tetR | CCGCGAAATATAATGACCC | V00611 (34) |
Annealing temperature used for PCR.
The locations of primers, except for the Tsh mut. set, are presented in Fig. 1. >, forward-direction primer; <, reverse-direction primer in amplification reaction.
GenBank accession number of sequence from which primers were designed, followed by reference number in parentheses.
ORF4 is 99% identical to the 5′ end of P4-like phage 933L ORF L0015 (50).
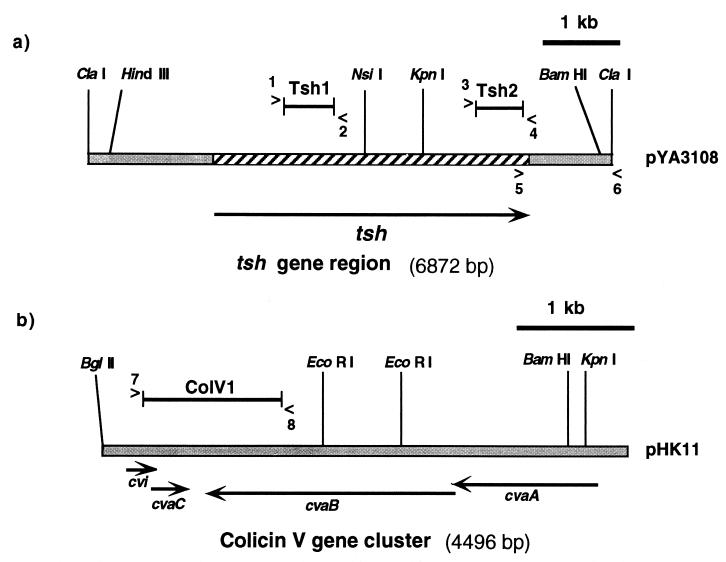
FIG. 1.
Restriction maps and genetic organization of (a) the tsh gene region and (b) the colicin V (ColV) gene cluster. Organization of the ColV gene cluster is derived from Gilson et al. (27). Genes are indicated below the restriction maps with thick black arrows that point in the direction of transcription. Forward (>) and reverse (<) primers for PCR amplifications of DNA from avian E. coli isolates and synthesis of DNA probes are indicated with numbers. Oligonucleotide primers are described in Table 2. DNA probes Tsh1 (620 bp), Tsh2 (616 bp), and ColV1 (1,203 bp) are indicated with solid lines above the restriction maps. The Tsh1 and Tsh2 probes were generated from plasmid pYA3108 (54), and the ColV1 probe was generated from pHK11 (27). Experimental procedures are detailed in the text.
For Southern blots, plasmid extracts or digested genomic DNA was separated through agarose gel electrophoresis and transferred to Magnacharge nylon membranes (MSI, Westborough, Mass.) by capillary transfer. DNA probes Tsh1, specific for the tsh gene, and ColV1, specific for the cvi and cvaCB genes of the ColV gene cluster, were obtained by PCR using the Tsh1 and ColV1 primer sets (Table 2 and Fig. 1) and the conditions described above. In addition, a second DNA probe, Tsh2, specific for the end of the tsh gene was generated by PCR using the Tsh2 primer set (Table 2 and Fig. 1) and the conditions described above. Fragments used to make probes were isolated and purified by gel extraction (Qiaex II gel extraction kit; Qiagen, Santa Clarita, Calif.). Labeling of the DNA probes, hybridization, and detection of the hybridized fragments were performed using the DIG High Prime Kit (Boehringer, Mannheim, Germany). The presence of aerobactin genes in isolates was determined by colony hybridization as previously described (21).
DNA sequencing and analysis.
Nucleotide sequences of the regions flanking the tsh gene from strain χ7122 was determined using pYA3108, pYA3104, and derivatives of pYA3107 containing Tn5seq1 or TnphoA insertions (54) as the templates. Primers used for sequencing included pBluescript SK and KS, SP6, and custom-synthesized oligonucleotide primers. Sequencing was performed using ABI prism fluorescent Big Dye Terminators according to the manufacturer's instructions (PE Biosystems, Norwalk, Conn.) and a 480 thermal cycler (PE Biosystems). Sequencing gels were run at the Protein and Nucleic Acid Chemistry Laboratory of Washington University. Comparison of the DNA sequences and predicted open reading frames (ORFs) with sequences in the GenBank genetic sequence database were performed using the BLASTN, BLASTP, and BLASTX programs (2) accessed from the National Center for Biotechnology Information (NCBI). Putative ORFs within the sequence were selected using ORF Finder at NCBI.
Virulence assay for 1-day-old chicks.
APEC isolates were classified for virulence based on lethality for 1-day-old chicks following subcutaneous inoculation as previously described (14). Lethality classes (LC) were defined as follows: LC1, 50% lethal dose (LD50) < 108 CFU; LC2, LD50 ≥ 108 CFU; LC3, not lethal at ≥108 CFU.
Construction of a tsh::tetAR(B) mutation in strain χ7122 and selection of tetracycline-sensitive derivatives.
In order to construct an isogenic tsh mutant of strain χ7122, we inserted the tetAR(B) cassette derived from Tn10. To construct a tetAR cassette for insertion into the tsh gene, a 2.2-kb HindIII fragment encoding tetAR(B) from pSBA383 (56) was cloned into the HindIII sites of pBSL86 (1), replacing the nptII kanamycin resistance gene in this vector. The resulting plasmid, pYA3442, contained the tetAR(B) cassette flanked by convenient restriction sites. A PstI fragment bearing the tetAR cassette from pYA3442 was cloned into the compatible NsiI site of the tsh gene on plasmid pYA3418 (60), resulting in plasmid pYA3444. A 6.6-kb BssHII fragment containing the tetAR-interrupted tsh gene from pYA3444 was ligated to the AscI sites of suicide vector pMEG-375. The resulting plasmid, pYA3448, was used for allelic replacement of tsh in APEC strain χ7122. The pYA3448 suicide vector containing the tsh::tetAR(B) insert was conjugated from E. coli MGN-617 to χ7122 by overnight plate mating on L agar plus 50 μg of diaminopimelic acid per ml. Transconjugants were selected by growth on L agar plates containing tetracycline without diaminopimelic acid. Selection for double-crossover allele replacement was obtained by sacB counterselection on L agar plates without NaCl and containing 5% sucrose (38). A χ7122 derivative, strain χ7273, was confirmed to contain an insertion of the tsh::tetAR(B) allele resulting from a double crossover, as determined by absence of resistance to ampicillin and chloramphenicol encoded on the suicide vector, PCR amplification using specific oligonucleotide primers 9, 10, and 11 (Table 2), and lack of Tsh protein production as determined by Western blot (data not shown).
The tsh::tetAR mutant strain χ7273 provided a means to test the stability of the tsh-encompassing region by counterselection for loss of tetracycline resistance (9, 56). Comparison of colony counts between χ7273 plated on standard L agar and TSS agar demonstrated a high reversion rate (10−3 to 10−4) to tetracycline sensitivity (Tcs). Tcs derivatives of χ7273, such as strain χ7274, no longer contained tsh or tetAR genes as determined by PCR, suggesting that loss of resistance to tetracycline was due to spontaneous loss of an unstable genetic region such as a plasmid or genomic island containing the tsh gene. Strain χ7274 was conserved for further experiments.
Experimental infection of chickens via the air sacs.
Three groups of 10 3-week-old White Leghorn specific-pathogen-free chickens from the Institut National de la Recherche Agronomique experimental farm were reared in separate cages with food and water available ad libitum. Each chicken was inoculated in the right thoracic air sac with 0.1 ml (107 CFU) of a bacterial inoculum consisting of a diluted 24-h BHI culture of E. coli χ7122, χ7273, or χ7274. Blood samples (50 μl) were collected aseptically from each chicken 6, 24, and 48 h following bacterial inoculation and diluted 1:4 in phosphate-buffered saline (PBS, pH 7.4), and 0.1 ml was plated on Drigalski agar (Diagnostics Pasteur, Marnes la Coquette, France) supplemented with nalidixic acid (40 μg ml−1) or with nalidixic acid and tetracycline in the case of E. coli χ7273. Another 50-μl volume of blood was incubated in 2 ml of BHI for qualitative detection of E. coli. Positive growth of E. coli in BHI was confirmed by plating enriched cultures on Drigalski agar.
All birds were euthanized at 48 h postinfection by inoculation of Nesdonal (Rhône-Mérieux, Lyon, France) and necropsied. Macroscopic fibrinous lesions were observed and scored (air sacs, 0 to 4; heart, 0 to 2; and liver, 0 to 2), and organs were aseptically removed. The left lung, liver, and spleen were weighed, suspended in PBS, and homogenized with an Ultra-Turrax apparatus (19). Dilutions of homogenates were plated onto Drigalski agar with appropriate antibiotics for bacterial quantification, and 1 ml was incubated in BHI for qualitative detection of E. coli. Several randomly selected colonies per organ were verified for serogroup O78, presence or absence of the wild-type tsh gene using the Tsh mut. primer set (Table 2), and antibiotic resistance.
Serum bactericidal assay.
Bacterial survival in chicken serum was determined as previously described (21), with an initial bacterial inoculum of approximately 107 CFU ml−1 incubated in fresh 90% normal chicken serum. The serum-bacterium suspensions were incubated at 37°C for 3 h in a 5% CO2 atmosphere, and counts of viable cells were estimated at the 1- and 3-h time points.
Statistical analyses.
The prevalence of the tsh gene among the different E. coli isolates relative to virulence was analyzed using the chi-square test. In experimental-infection assays, lesion scores and bacterial counts were compared by analysis of variance between groups of chickens; the chi-square test was used to compare the number of contaminated chickens in the case of qualitative detection of E. coli.
Nucleotide sequence accession number.
The tsh DNA region of pAPEC-1 of E. coli strain χ7122 has been entered as GenBank nucleotide accession number AF218073.
RESULTS
Association of tsh with lethality of avian E. coli isolates.
To determine the prevalence of tsh among avian E. coli, 300 clinical isolates from poultry were examined by PCR amplification using the tsh gene-specific primer set Tsh1 (Fig. 1 and Table 2). The presence of the tsh gene was detected in about half of the E. coli isolates tested (49.7%). Its occurrence among isolates from either diseased chickens or turkeys, irrespective of their geographic origin (France or Québec), was significantly associated (P < 0.001) with high lethality for chicks. Among isolates belonging to the high-lethality class (LC1), 61.6% were tsh positive, whereas 30.0 and 9.8% tsh-positive isolates were found in the low lethality class (LC2) and in the nonlethal class (LC3), respectively. When considering tsh-positive isolates, 90.6% belonged to LC1, whereas only 6.0 and 3.4% were LC2 and LC3, respectively.
Among tsh-positive isolates belonging to LC1, there was no significant difference between the frequency of isolates of serogroups O1, O2, and O78 (97.8%) compared to isolates belonging to other serogroups (79.3%) (Table 3). In contrast, among tsh-negative isolates, 88.6% of isolates from serogroups O1, O2, and O78 belonged to LC1, whereas only 27.2% of isolates from other serogroups belonged to LC1. Among the LC3 tsh-negative isolates, 42 of 46 (91.3%) belonged to serogroups other than O1, O2, or O78.
TABLE 3.
Relationship between lethality for 1-day-old chicks and presence of tsh in APEC
Experimental infection of chickens with strain χ7122 and derivatives.
To determine whether tsh contributes to the pathogenesis of respiratory colibacillosis, wild-type APEC strain χ7122 and tsh mutant derivatives χ7273 and χ7274 were compared in a chicken experimental infection model. In contrast to chickens inoculated with the wild-type strain χ7122, chickens inoculated with the tsh::tetAR mutant χ7273 exhibited fewer and less pronounced lesions (P < 0.01) in the air sacs (Table 4). However, compared to strain χ7122, mutant χ7273 caused similar lesions of pericarditis and perihepatitis and persisted in organs and blood to a similar degree (Table 4). Birds infected with mutant χ7273 complemented with tsh on plasmid pYA3108 exhibited air sac lesions similar to those seen in chickens infected with wild-type strain χ7122 (data not shown). As with mutant χ7273, mutant χ7274 caused reduced lesions in the air sacs of birds, but, interestingly, it was much more attenuated. Strain χ7274 caused few lesions of pericarditis and perihepatitis, did not persist in the blood, and poorly colonized the lung, spleen, and liver (Table 4). These results suggest that other genes linked to tsh that contribute to APEC pathogenesis were concurrently lost following loss of the tsh::tetAR allele from strain χ7274 by fusaric acid selection.
TABLE 4.
Comparison of ability of APEC strain χ7122 and derivatives to induce colibacillosis lesions and persist in internal organs and blood of chickens experimentally inoculated via the air sacsa
| E. coli strain | Mean lesion scoreb ± SD
|
Mean no. of bacteriac ± SD
|
Mean no. of bacteria in blood ± SD
|
|||||
|---|---|---|---|---|---|---|---|---|
| Air sacs | Heart and liver | Lung | Liver | Spleen | 6 h | 12 h | 24 h | |
| χ7122 | 3.0 ± 1.3 | 3.1 ± 0.9 | 5.04 ± 1.3 | 3.96 ± 1.1 | 4.54 ± 1.0 | 3.00 ± 0.6 | 2.98 ± 0.5 | 2.41 ± 1.4 |
| χ7273 | 1.5 ± 0.5* | 3.4 ± 0.7 | 4.55 ± 1.3 | 3.08 ± 0.9 | 3.81 ± 0.6 | 3.21 ± 0.3 | 2.65 ± 0.4 | 1.55 ± 0.5 |
| χ7274 | 1.4 ± 1.5* | 0.3 ± 0.5* | 2.00 ± 1.4* | 0.2 ± 0.4* | 0.70 ± 0.1* | 1.94 ± 0.6 | <1.3* | <1.3* |
Asterisks indicate values significantly lower (P < 0.001) than those observed for wild-type strain χ7122.
Lesion scoring values: 0 to 4 for air sacs, 0 to 2 for heart, and 0 to 2 for liver.
Bacterial counts are presented as the mean log10 CFU per gram (per milliliter for blood) ± standard deviation for 10 birds from each infected group. Counts in organs were made 48 h postinfection.
Association of the tsh gene with ColV plasmids in APEC isolates.
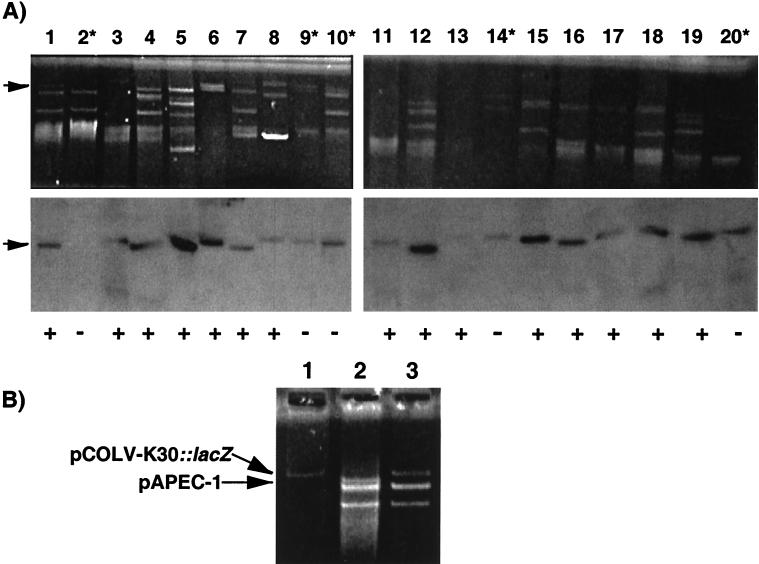
In a recent report by Otto and coworkers (49), a second tsh allele encoding a protein termed Hbp was shown to be located on a ColV-type plasmid of an E. coli O8 strain isolated from a human wound abscess. To determine whether tsh is located on ColV plasmids in APEC isolates, plasmid extracts from 14 highly lethal tsh-positive APEC isolates from Québec, Canada, belonging to different serogroups (eight O78, two O2, and one each O1, O35, O22, and O45) and from strains χ7122 and tsh mutants χ7273 and χ7274 were tested by Southern hybridization using the Tsh1 and ColV1 probes (Fig. 1). Plasmid extracts of the APEC isolates demonstrated one or more plasmids of high molecular weight (Fig. 2A). Wild-type APEC strain χ7122 and tsh insertion mutant χ7273 contained three large plasmids, whereas its fusaric acid-selected Tcs revertant χ7274 had lost the largest of these three plasmids, which we termed pAPEC-1 (Fig. 2A). With the exception of strain χ7274, the Tsh1 probe hybridized to one plasmid of various sizes from each of the APEC isolates and also hybridized to plasmid pColV-K30, which was used as a reference. Furthermore, for all but four of the APEC isolates, the same plasmid that hybridized to the Tsh1 probe also hybridized to the ColV1 probe. For the other four isolates, plasmid extracts did not hybridize to the ColV1 probe. PCR amplification using the ColV1 primer set (Fig. 1) demonstrated the same results as the Southern blots (Fig. 2A). All except two of the APEC isolates analyzed, including strain χ7122, were positive for the aerobactin system (data not shown). Unlike its wild-type parent, strain χ7274 no longer contained tsh-, colicin V-, or aerobactin gene-specific sequences, indicating that these genes are encoded on plasmid pAPEC-1 in strain χ7122.
FIG. 2.
(A) Localization of tsh to ColV-type plasmids in APEC isolates and pColV-K30. Analysis of plasmids of tsh-positive APEC isolates, χ7122, and derivatives by ethidium bromide staining (upper panels) and Southern hybridization of plasmid extracts (lower panels). Asterisks indicate samples from ColV-negative isolates obtained by PCR. Hybridizations depicted are with the Tsh1 probe. Hybridization with the ColV1 probe is marked below the lower panels as positive (+) or negative (−). In positive samples, the ColV1 probe hybridized with the same plasmid as the Tsh1 probe. Arrows indicate the pAPEC-1 plasmid containing tsh in APEC strain χ7122. Lane 1, χ7273; lane 2, χ7274; lanes 3 and 11, pColV-K30; lanes 4 and 12, χ7122; lane 5, TK27; lane 6, CN30; lane 7, TK40; lane 8, TK60; lane 9, CN137; lane 10, CN139; lane 13, CN144; lane 14, CN151; lane 15, CN163; lane 16, CN165; lane 17, CN69; lane 18, TK49; lane 19, CN14; lane 20, CN71. CN (isolated from chicken) and TK (isolated from turkey) samples are clinical isolates from Québec, Canada. (B) Replacement of plasmid pAPEC-1 from strain χ7122 with a Tn10-tagged pColV-K30 plasmid; plasmid profiles of donor, recipient, and transconjugant strains. Lane 1, donor strain MEG-617(pColV-K30::lacZ); lane 2, recipient χ7122 native plasmid profile before conjugation; lane 3, strain χ7275, a transconjugant of χ7122 mated with MEG-617(pColV-K30::lacZ) following selection on medium containing tetracycline and nalidixic acid.
We investigated whether the tsh-containing plasmid pAPEC- 1 of strain χ7122 is in the same incompatibility group as the reference ColV plasmid pColV-K30. Strain χ7122 was mated as the recipient with E. coli strain MGN-617 harboring the Tn10-marked plasmid pColV-K30::lacZ (4). Analysis of Tcr Nalr transconjugants of χ7122 after three serial passages showed that they had lost pAPEC-1, whereas pColV-K30::lacZ had been gained, as represented by strain χ7275 (Fig. 2B). Hence, pAPEC-1 belongs to the same incompatibility group (IncFI) as pColV-K30. Together, the results of the hybridization and conjugation experiments demonstrate that pAPEC-1 of strain χ7122 is a ColV plasmid containing the tsh gene, that tsh is encoded on similar large plasmids in most of the other APEC isolates, and that fusaric acid selection for strain χ7274 resulted in complete loss of the pAPEC-1 plasmid.
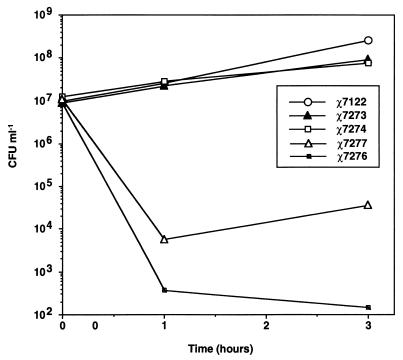
Bactericidal effect of serum.
To determine whether tsh or the pAPEC-1 plasmid contributes to serum resistance, APEC strain χ7122 and derivatives as well as an E. coli K-12 strain, χ7276, and a K-12 transconjugant containing pAPEC-1 (tsh::tetAR), χ7277, were tested for survival in normal chicken serum (Fig. 3). Wild-type APEC strain χ7122 was the most serum-resistant strain and exhibited a 10-fold growth increase after 3 h of incubation (Fig. 3). In addition, the tsh insertion mutant χ7273 and pAPEC-1 (fusaric acid)-cured derivative χ7274 were also serum resistant and exhibited a two- to threefold increase in growth after 3 h of incubation (Fig. 3). In contrast, the K-12 E. coli strain χ7276 was serum sensitive and decreased in viability nearly 105-fold after 3 h of incubation. The same K-12 strain bearing pAPEC-1 (tsh::tetAR), χ7277, was also serum sensitive, but to a lesser extent than strain χ7276, and demonstrated a 103-fold decrease in viability after 3 h of incubation (Fig. 3).
FIG. 3.
Effect of 90% normal chicken serum on survival of APEC strain χ7122 and derivatives and E. coli K-12 with and without the pAPEC-1 plasmid. Strains: χ7122, APEC wild-type strain; χ7273, χ7122 tsh::tetAR; χ7274, χ7273 ΔpAPEC-1; χ7276, E. coli K-12; and χ7277, χ7276(pAPEC-1). Results are from a representative experiment from three independent assays.
Proximity of tsh to the ColV gene cluster in APEC isolates.
Initial Southern hybridizations of HindIII- or EcoRI-digested χ7122 DNA with the Tsh1 or ColV1 DNA probes demonstrated that both probes hybridized to either a 20-kb HindIII fragment or a 15-kb EcoRI fragment (data not shown), suggesting that tsh and the ColV gene cluster are in close proximity on plasmid pAPEC-1. Furthermore, restriction maps of pYA3107 (54) and of the ColV gene cluster of pColV-K30 (26) (Fig. 1) suggested that the ColV gene cluster was situated downstream of tsh, with the cvi gene proximal to tsh. As single KpnI sites are present within the tsh gene and at the end of the ColV gene cluster (Fig. 1), KpnI digests of genomic DNA from eight tsh-positive and ColV-positive APEC isolates (χ7122, TK27, CN30, TK40, TK60, CN144, CN163, and CN165) were hybridized with DNA probes ColV1 and Tsh2 (Fig. 1) to determine if these genes are closely linked in other APEC isolates. DNA digested with KpnI from all the APEC isolates except TK60 exhibited hybridization of Tsh2 and ColV1 probes to a single KpnI fragment that varied in size from 11 to 8.5 kb. Based on the locations of the KpnI sites within tsh and the ColV gene cluster (Fig. 1), in these isolates the suggested distance from the end of the tsh gene to the start of the ColV gene cluster is between 3.2 and 5.7 kb. In contrast, KpnI digests of DNA from strain TK60 exhibited multiple fragments that hybridized with the Tsh2 or ColV1 probe. These results indicate that, with the exception of isolate TK60, the tsh and ColV genes are closely located on the ColV plasmids of the APEC isolates examined.
Sequence analysis of the tsh region of strain χ7122.
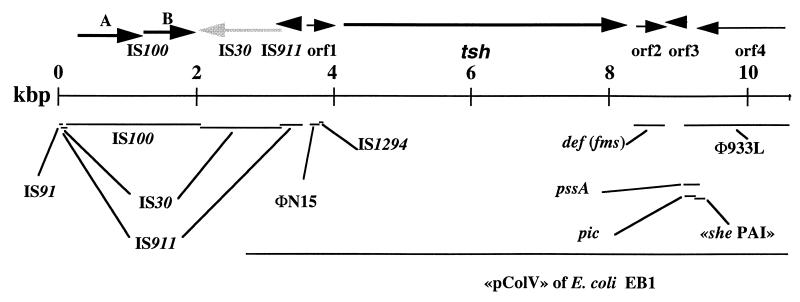
Including the previously sequenced 4,699-bp region containing tsh (54), a total of 10,587 bp of the tsh region of pAPEC-1 was sequenced (Fig. 4). With a G+C content of 50.4%, the region is similar to the E. coli K-12 mean G+C content (50.8%) (8). Regions flanking the tsh gene exhibit identities to insertion sequence (IS) elements IS91, IS911, IS100, IS30, and IS1294, bacteriophage N15, and prophage 933L (50) DNA, and the def (fms) gene of E. coli K-12 (Fig. 4). In addition, the sequenced tsh region of plasmid pAPEC-1 starting at position 2760 and including the tsh gene is 99.8% identical to the sequenced portion of a ColV plasmid that encodes the Hbp autotransporter from E. coli strain EB1 (49) (Fig. 4), which is identical to Tsh of strain χ7122 except for two residue substitutions.
FIG. 4.
Organization and analysis of the tsh region of pAPEC-1 from strain χ7122. The scale is given in kilobase pairs. ORFs are represented by arrows above the scale. Solid arrows represent complete coding (black arrows) or interrupted (grey arrow) sequences showing high identity to known genes or IS elements. Putative ORFs are represented by hatched arrows. Positions and predicted lengths of the ORF products are presented in Table 5. Horizontal lines below the scale represent sequences exhibiting nucleotide identity to the specified region. The percent nucleotide identity and accession numbers of sequences exhibiting nucleotide identity to the represented regions are as follows: IS91, 100% (K04543); IS100, 97% (Z32853); IS30, 100% (X00792); “pColV-K30” of E. coli EB-1, 99.8% (AJ223631); IS911, 100% (X17613); bacteriophage N15, 79% (AF064539); IS1294, 92% (X82430); def (fms), 97% (X63666); prophage 933L, 99% (AF097644); she PAI, 97% (U97493); pic, 100% (AF097644); and pssA, 98% (Y13614).
The sequenced region encompassing tsh contains nine ORFs that include three IS elements (IS100, IS30, and IS911), tsh, and four additional putative ORFs (Fig. 4 and Table 5). The deduced ORF products of the IS elements (IS100, IS30, and IS911) in the tsh region of pAPEC-1 are 99 to 100% identical to IS100 ORFs A and B of Yersinia pestis (51), the first 369 residues of E. coli IS30 (13), and IS911 (49), respectively (Table 5).
TABLE 5.
Summary of ORFs within tsh region of pAPEC-1 and homologies to known sequencesa
| ORF or sequence | Positions (bp)b | Product length (aa) | Homology (% identity/ % similarity) | Region showing identity | Accession no.c (reference) |
|---|---|---|---|---|---|
| IS100 ORFA | 291>1213 | 340 | IS100 ORFA (100) | Full length | Z32853d (51) |
| IS100 ORFB | 1213>1971 | 252 | IS100 ORFB (99) | Full length | Z32853d (51) |
| IS30e | 2001<3161 | 386 | IS30 (100) | 2054<3161 | P37246 (13) |
| IS911 | 3118<3486 | 122 | IS911 (100) | Full length | CAA11505 (49) |
| ORF1 | 3582>4004 | 140 | Orf1 (99) | Full length | CAA11506 (49) |
| gp48 (86/96) | 3589>3795 | AAC19087 | |||
| IS801 Tnp (51/63) | 3793<3960 | P24607 (57) | |||
| tsh | 4127>8260 | 1,377 | Tsh (100) | Full length | L27423 (54) |
| Hbp (99) | Full length | CAA11507 (49) | |||
| ORF2 | 8369>8821 | 150 | PDF (99) | 8369>8761 | P27251 (46) |
| ORF3 | 8808<9065 | 85 | ParA resolvase (35/56) | 8829<9065 | P22996 (25) |
| ORF4 | 9245<10504 | 419 | L0015 (99) | Full length | AAC31494 (50) |
Only the most relevant homologies are presented.
Position on the 10,587-bp tsh region, with coding direction indicated as forward (>) or reverse (<) in relation to tsh.
GenBank accession number.
DNA sequence entry. Protein identity was derived from the predicted ORFs of the DNA sequence.
IS30 ORF interrupted by IS100 sequence.
ORF1 encodes a hypothetical polypeptide of 140 amino acids (aa) that is 99% identical to a hypothetical gene product from the ColV plasmid of E. coli EB1 (49) (Table 5). However, peptide and DNA sequence analyses suggest that ORF1 consists of vestiges of an N15 phage-related gene and IS-related sequences (Table 5 and Fig. 4). ORF2, located downstream of tsh, encodes a hypothetical polypeptide of 150 aa that is 99% identical to the first 131 aa of E. coli polypeptide deformylase (Table 5) encoded by the def (fms) gene (46). ORF3 encodes a hypothetical 85-aa polypeptide that exhibits 35% identity and 56% similarity to the carboxyl end of E. coli RP4 ParA resolvase (25) (Table 5) and similar homology to putative resolvases from Y. pestis (43) and other resolvase-related recombinases from gram-negative and gram-positive bacteria.
The DNA region starting 9 bp from the start of ORF3 until the end of the sequenced tsh region exhibits 99% identity to ORF L0015 from prophage 933L (Φ933L) (Fig. 4), a recently identified P4-like prophage flanking the locus of enterocyte effacement pathogenicity island of enterohemorrhagic E. coli strains (50). Furthermore, DNA sequences that flank the pic, she, and pssA autotransporter encoding genes have 97 to 100% identity to portions of the pAPEC-1 sequenced region that is similar to L0015 of Φ933L (Fig. 4). The Φ933L homologous region encompasses ORF4, whose 419-aa putative product exhibits 99% identity to L0015 of Φ933L (Table 5).
Prophage 933L related DNA was also shown to be present at the 3′ end of the tsh genes in other APEC isolates. PCR amplification was conducted using primers 5 and 6 of the Tsh 3 primer set (Fig. 1 and Table 2), which specifically amplify a 1,148-bp region spanning from the end of the tsh gene to the end of ORF4. Thirteen of 15 tsh-positive isolates tested, including χ7122, produced a 1,150-bp amplification product, indicating the presence of ORF L0015-related sequences located 3′ of tsh in these isolates. The two isolates which did not produce Tsh-3′-specific products were ColV negative.
DISCUSSION
The virulence of APEC is associated with the presence of unique DNA regions in the chromosome that are absent from E. coli K-12 strains (11). The tsh gene, which is absent from E. coli K-12, was identified in APEC strain χ7122 and encodes a protein that shows homology to the IgA proteases of Haemophilus and Neisseria spp. (54) and was more recently shown to be processed and secreted as an autotransporter (60). The autotransporters are a family of secreted proteins from gram-negative bacterial pathogens, and many of these proteins have been implicated as actual or probable virulence factors (32, 44). Maurer et al. (45) noted that tsh was present in APEC strains but not in E. coli isolated from the feces of healthy birds, suggesting that tsh could be associated with the virulence of E. coli in chickens.
Herein, we have confirmed that tsh is associated with the virulence of avian E. coli isolates. Of the 300 avian E. coli isolates examined in the current report, half of the isolates were tsh positive, and tsh was specifically more frequent (P < 0.001) in high-lethality isolates compared to low-lethality isolates. In addition, of the tsh-positive isolates identified, most (90.6%) were from the high-lethality group (Table 3). The presence of tsh was similar among isolates from diseased chickens and turkeys and was not any more associated with serogroups O1, O2, and O78, commonly incriminated in avian colibacillosis, than with isolates from other serogroups (Table 3). In the tsh-positive strains examined, tsh was always plasmid encoded and was linked to colicin V genes, when they were present, on the same plasmid. As we have demonstrated that tsh is encoded on a ColV-type transmissible plasmid related to pColV-K30 in strain χ7122, it is likely that tsh is also encoded on transmissible plasmids in other APEC isolates. As such, the presence of tsh among diverse serogroups of virulent APEC isolates is not surprising. At present it is unknown whether tsh may also occur on the chromosome of certain strains.
The association of tsh with lethal APEC isolates suggested that tsh may be a virulence factor and/or could be physically linked to some independent virulence determinant(s). Experimental infection of chickens with an isogenic tsh knockout derivative of APEC strain χ7122 demonstrated that Tsh contributes to the development of lesions and fibrin deposition in the air sacs. In preliminary studies on the dynamics of air sac infection by E. coli, we have determined that the onset of airsacculitis in chickens is more rapid for APEC strain χ7122 than for the tsh mutant χ7273, suggesting that Tsh increases the rate of colonization at this site and development of airsacculitis. Tsh was first identified as a temperature-sensitive hemagglutinin for chicken erythrocytes (54), suggesting that it may act as an adhesin, particularly in the initial stages of colonization of the avian respiratory tract. The in vivo results from the infection studies further support the likelihood that Tsh plays a role in colonization in the air sacs. Other autotransporters such as Pic, TibA, and Hap also act as adhesins or hemagglutinins (31, 33, 42). In the case of Pic, which is closely related to Tsh, it has recently been suggested that it is involved in the early stages of pathogenesis and most probably promotes intestinal colonization by enteroaggregative E. coli (31).
In addition to its potential role as an adhesin, Tsh may act as a protease on a specific substrate in the air sacs. Tsh belongs to a group of autotransporter proteins that contain serine protease sites (32). A number of these proteins have been shown to exhibit protease activity against substrates such as casein (PssA) (17), pepsin A and coagulation factor V (EspP) (12), and gelatin (Pic) (31). In our hands, Tsh did not cleave human or chicken IgA, casein, or pepsin A (60). However, Otto et al. demonstrated that the gene product of the tsh allele (hbp) from E. coli strain EB1 degrades hemoglobin (49).
The hemoglobin-degrading and heme-binding properties of Tsh do not appear to be required for APEC χ7122 infection of deeper tissues. Although it has not been demonstrated that heme bound to Tsh (Hbp) can be utilized by extraintestinal E. coli strains (49), the presence of the aerobactin siderophore on the pColV plasmid in strain χ7122 (unpublished data), as well as on most other APEC strains (18, 65), likely compensates for inactivation of Tsh or obviates any such role for Tsh in the survival of APEC in blood and systemic tissues. Whether Tsh-mediated proteolysis of a substrate within the air sacs results in lesion formation and fibrin deposition remains to be demonstrated.
DNA sequencing of the region flanking the tsh gene on plasmid pAPEC-1 of strain χ7122 identified IS elements and phage-related DNA (Fig. 4). As has been observed for other plasmids and regions flanking pathogenicity islands, recombination has occurred in the sequenced regions flanking tsh in strain χ7122, as demonstrated by sequential insertion of different IS elements (Fig. 4). However, the region between the tsh gene and ColV gene cluster in the APEC isolates examined appears to be quite conserved, based on the presence and similar distance of phage 933L-related DNA 3′ of tsh and linkage analysis between tsh and ColV sequences. Sequences similar to those flanking tsh have been identified adjacent to pathogenicity islands or on virulence plasmids in other enterobacteria. In particular, IS100 sequences are present on plasmids of Yersinia spp. (35, 43), the EAF plasmid of enteropathogenic E. coli strain B171 (64), adjacent to certain high-pathogenicity islands of Y. pestis and Yersinia pseudotuberculosis (30), and within pathogenicity island 5 (PAI-5) of uropathogenic E. coli J96 (63). P4-like cryptic prophage-related sequences similar to prophage 933L of enterohemorrhagic strain EDL933 (50) are frequently associated with genes that were probably acquired through horizontal transfer, including Shigella flexneri pathogenicity island 2 (48, 67), PAI-6 of uropathogenic E. coli strain CFT073 (20, 39), the 3′ junction of PAI-1 from uropathogenic E. coli 536 (40), and the EAF plasmid of certain enteropathogenic E. coli strains (10) (accession no. AF119170). The presence of P4-related phage genes adjacent to pathogenicity islands and virulence genes suggests that intact phages or portions of these sequences may have mediated horizontal acquisition of these virulence genes through recombination (20). Interestingly, the genes encoding the ShMu, Pic, and PssA autotransporters, which are closely related to Tsh, are flanked by the same phage 933L DNA adjacent to tsh in strain χ7122 (Fig. 4) and most other APEC isolates in this study. Furthermore, as with tsh from strain χ7122, pic is also flanked by IS911-related sequences at its 5′ end (31). The presence of these common flanking sequences suggests that in addition to sharing protein similarities with these autotransporters, tsh, she, pic, and pssA may have been acquired by different E. coli pathotypes through similar prophage- or recombination-mediated mechanisms.
The demonstration that tsh is encoded on large plasmids, usually containing the colicin V gene cluster, in APEC isolates (Fig. 2) and the decreased pathogenicity of strain χ7274, which has lost the ColV-type plasmid bearing tsh (Table 4), strongly suggest that tsh is linked to other genes that contribute to APEC pathogenesis. It is well established that ColV plasmids may enhance the virulence of extraintestinal E. coli (58, 59, 68), and virulent APEC isolates are more often ColV positive than less-virulent clinical isolates or E. coli from healthy birds (7, 18). Ike et al. (36) demonstrated that curing of plasmid pKI100 from an APEC strain of serogroup O2 resulted in loss of lethality for chicks, a decrease in serum resistance, and loss of aerobactin hydroxamate siderophore expression. Recently, a conjugative plasmid encoding a hydroxamate but no colicin activity was shown to contribute to respiratory tract colonization and virulence of an APEC strain following aerosol infection of chickens (28). The traits and virulence determinants associated with ColV plasmids include the aerobactin iron-sequestering siderophore system, resistance to killing by serum complement and phagocytosis, motility, and adherence to intestinal cells (68). In APEC strains, the most clearly established of these traits is the presence of the aerobactin operon, which is often located on ColV plasmids in these strains (18, 65). In addition, in APEC strains, ColV production is associated with serum resistance (71), which may be encoded by genes such as iss or traT (68).
Loss of the ColV-type plasmid pAPEC-1 from strain χ7122 was achieved by fusaric acid counterselection for loss of tetracycline resistance using the tsh::tetAR(B) derivative strain χ7273. Infection experiments with the pAPEC-1-cured strain χ7274 and the tsh insertion mutant χ7273 clearly demonstrated that pAPEC-1 contains genes in addition to tsh that are involved in the pathogenicity of the wild-type strain χ7122 in the lower respiratory tract and extrarespiratory tissues of experimentally infected chickens (Table 4). Although the presence of pAPEC-1 did increase the ability of a K-12 strain to survive in serum by about 100-fold, pAPEC-1 appears to play a limited role in the serum resistance of strain χ7122, as the pAPEC-1-cured derivative χ7274 was also resistant to the bactericidal effects of 90% chicken serum (Fig. 3). However, unlike strain χ7122, χ7274 has lost the aerobactin system encoded on pAPEC-1. Moreover, it is possible that attenuation of strain χ7274 is partly due to a reduced ability to obtain iron from the iron-restrictive environment of the avian host following loss of the aerobactin siderophore system. Construction of aerobactin and aerobactin-Tsh double-knockout mutants would further elucidate the role of iron acquisition by aerobactin and possibly of the Tsh heme-binding hemoglobin protease in the pathogenesis of E. coli infection in poultry.
ACKNOWLEDGMENTS
We are grateful to R. Kolter and K. Rajakumar for supplying plasmids and thank B. Otto for sharing unpublished results. We thank Christian Mouline for skillful technical assistance in experimental infection of chickens and Christos Stathopoulos for Western blotting experiments.
This work was supported by U.S. Department of Agriculture National Research Initiative Competitive Grants Program grant 94-37204-1091 to R.C. III and C.M.D., Formation des Chercheurs et à l'Aide de la Recherche du Québec (FCAR) grant 0214, and Natural Sciences and Engineering Research Council of Canada (NSERC) grant 2294 to J.M.F. C.M.D. is an NSERC fellow.
REFERENCES
- 1.Alexeyev M F. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques. 1995;18:52–56. [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D M, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley-Interscience; 1991. [Google Scholar]
- 4.Bagg A, Neilands J B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985;161:450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjelloun-Touimi Z, Sansonetti P J, Parsot C. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol Microbiol. 1995;17:123–135. doi: 10.1111/j.1365-2958.1995.mmi_17010123.x. [DOI] [PubMed] [Google Scholar]
- 6.Benz I, Schmidt M A. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol Microbiol. 1992;6:1539–1546. doi: 10.1111/j.1365-2958.1992.tb00875.x. [DOI] [PubMed] [Google Scholar]
- 7.Blanco J E, Blanco M, Mora A, Blanco J. Production of toxins (enterotoxins, verotoxins, and necrotoxins) and colicins by Escherichia coli strains isolated from septicemic and healthy chickens: relationship with in vivo pathogenicity. J Clin Microbiol. 1997;35:2953–2957. doi: 10.1128/jcm.35.11.2953-2957.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 9.Bochner B R, Huang H C, Schieven G L, Ames B N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143:926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bortolini M R, Trabulsi L R, Keller R, Frankel G, Sperandio V. Lack of expression of bundle-forming pili in some clinical isolates of enteropathogenic Escherichia coli (EPEC) is due to a conserved large deletion in the bfp operon. FEMS Microbiol Lett. 1999;179:169–174. doi: 10.1111/j.1574-6968.1999.tb08723.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown P K, Curtiss R., III Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci USA. 1996;93:11149–11154. doi: 10.1073/pnas.93.20.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunder W, Schmidt H, Karch H. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol Microbiol. 1997;24:767–778. doi: 10.1046/j.1365-2958.1997.3871751.x. [DOI] [PubMed] [Google Scholar]
- 13.Dalrymple B, Caspers P, Arber W. Nucleotide sequence of the prokaryotic mobile genetic element IS30. EMBO J. 1984;3:2145–2149. doi: 10.1002/j.1460-2075.1984.tb02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dho M, Lafont J P. Adhesive properties and iron uptake ability in Escherichia coli lethal and nonlethal for chicks. Avian Dis. 1984;28:1016–1025. [PubMed] [Google Scholar]
- 15.Dho-Moulin M, Fairbrother J M. Avian pathogenic Escherichia coli (APEC) Vet Res. 1999;30:299–316. [PubMed] [Google Scholar]
- 16.Dho-Moulin M, van den Bosch J F, Girardeau J P, Bree A, Barat T, Lafont J P. Surface antigens from Escherichia coli O2 and O78 strains of avian origin. Infect Immun. 1990;58:740–745. doi: 10.1128/iai.58.3.740-745.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djafari S, Ebel F, Deibel C, Kramer S, Hudel M, Chakraborty T. Characterization of an exported protease from Shiga toxin-producing Escherichia coli. Mol Microbiol. 1997;25:771–784. doi: 10.1046/j.1365-2958.1997.5141874.x. [DOI] [PubMed] [Google Scholar]
- 18.Doetkott D M, Nolan L K, Giddings C W, Berryhill D L. Large plasmids of avian Escherichia coli isolates. Avian Dis. 1996;40:927–930. [PubMed] [Google Scholar]
- 19.Dozois C M, Chanteloup N, Dho-Moulin M, Bree A, Desautels C, Fairbrother J M. Bacterial colonization and in vivo expression of F1 (type 1) fimbrial antigens in chickens experimentally infected with pathogenic Escherichia coli. Avian Dis. 1994;38:231–239. [PubMed] [Google Scholar]
- 20.Dozois C M, Curtiss R., III Pathogenic diversity of Escherichia coli and the emergence of ‘exotic’ islands in the gene stream. Vet Res. 1999;30:157–179. [PubMed] [Google Scholar]
- 21.Dozois C M, Fairbrother J M, Harel J, Bosse M. pap- and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys. Infect Immun. 1992;60:2648–2656. doi: 10.1128/iai.60.7.2648-2656.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis M G, Arp L H, Lamont S J. Serum resistance and virulence of Escherichia coli isolated from turkeys. Am J Vet Res. 1988;49:2034–2037. [PubMed] [Google Scholar]
- 23.Emery D A, Nagaraja K V, Shaw D P, Newman J A, White D G. Virulence factors of Escherichia coli associated with colisepticemia in chickens and turkeys. Avian Dis. 1992;36:504–511. [PubMed] [Google Scholar]
- 24.Eslava C, Navarro-Garcia F, Czeczulin J R, Henderson I R, Cravioto A, Nataro J P. Pet, an autotransporter enterotoxin from enteroaggregative Escherichia coli. Infect Immun. 1998;66:3155–3163. doi: 10.1128/iai.66.7.3155-3163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerlitz M, Hrabak O, Schwab H. Partitioning of broad-host-range plasmid RP4 is a complex system involving site-specific recombination. J Bacteriol. 1990;172:6194–6203. doi: 10.1128/jb.172.11.6194-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilson L, Mahanty H K, Kolter R. Four plasmid genes are required for colicin V synthesis, export, and immunity. J Bacteriol. 1987;169:2466–2470. doi: 10.1128/jb.169.6.2466-2470.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilson L, Mahanty H K, Kolter R. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990;9:3875–3894. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginns C A, Benham M L, Adams L M, Whithear K G, Bettelheim K A, Crabb B S, Browning G F. Colonization of the respiratory tract by a virulent strain of avian Escherichia coli requires carriage of a conjugative plasmid. Infect Immun. 2000;68:1535–1541. doi: 10.1128/iai.68.3.1535-1541.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross W B. Diseases due to Escherichia coli in poultry. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 237–259. [Google Scholar]
- 30.Hare J M, Wagner A K, McDonough K A. Independent acquisition and insertion into different chromosomal locations of the same pathogenicity island in Yersinia pestis and Yersinia pseudotuberculosis. Mol Microbiol. 1999;31:291–303. doi: 10.1046/j.1365-2958.1999.01172.x. [DOI] [PubMed] [Google Scholar]
- 31.Henderson I R, Czeczulin J, Eslava C, Noriega F, Nataro J P. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect Immun. 1999;67:5587–5596. doi: 10.1128/iai.67.11.5587-5596.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson I R, Navarro-Garcia F, Nataro J P. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 33.Hendrixson D R, de la Morena M L, Stathopoulos C, St Geme J W., III Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol Microbiol. 1997;26:505–518. doi: 10.1046/j.1365-2958.1997.5921965.x. [DOI] [PubMed] [Google Scholar]
- 34.Hillen W, Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983;11:525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu P, Elliott J, McCready P, Skowronski E, Garnes J, Kobayashi A, Brubaker R R, Garcia E. Structural organization of virulence-associated plasmids of Yersinia pestis. J Bacteriol. 1998;180:5192–5202. doi: 10.1128/jb.180.19.5192-5202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ike K, Kawahara K, Danbara H, Kume K. Serum resistance and aerobactin iron uptake in avian Escherichia coli mediated by conjugative 100-megadalton plasmid. J Vet Med Sci. 1992;54:1091–1098. doi: 10.1292/jvms.54.1091. [DOI] [PubMed] [Google Scholar]
- 37.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaniga K, Compton M S, Curtiss III R, Sundaram P. Molecular and functional characterization of Salmonella enterica serovar Typhimurium poxA gene: effect on attenuation of virulence and protection. Infect Immun. 1998;66:5599–5606. doi: 10.1128/iai.66.12.5599-5606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao J S, Stucker D M, Warren J W, Mobley H L. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect Immun. 1997;65:2812–2820. doi: 10.1128/iai.65.7.2812-2820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knapp S, Hacker J, Jarchau T, Goebel W. Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol. 1986;168:22–30. doi: 10.1128/jb.168.1.22-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafont J P, Dho M, d'Hauteville H M, Brée A, Sansonetti P J. Presence and expression of aerobactin genes in virulent avian strains of Escherichia coli. Infect Immun. 1987;55:193–197. doi: 10.1128/iai.55.1.193-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindenthal C, Elsinghorst E A. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67:4084–4091. doi: 10.1128/iai.67.8.4084-4091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindler L E, Plano G V, Burland V, Mayhew G F, Blattner F R. Complete DNA sequence and detailed analysis of the Yersinia pestis KIM5 plasmid encoding murine toxin and capsular antigen. Infect Immun. 1998;66:5731–5742. doi: 10.1128/iai.66.12.5731-5742.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loveless B J, Saier M H., Jr A novel family of channel-forming, autotransporting, bacterial virulence factors. Mol Membr Biol. 1997;14:113–123. doi: 10.3109/09687689709048171. [DOI] [PubMed] [Google Scholar]
- 45.Maurer J J, Brown T P, Steffens W L, Thayer S G. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin tsh among avian Escherichia coli. Avian Dis. 1998;42:106–118. [PubMed] [Google Scholar]
- 46.Mazel D, Pochet S, Marliere P. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 1994;13:914–923. doi: 10.1002/j.1460-2075.1994.tb06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller J H. A short course in bacterial genetics: a laboratory manual for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 48.Moss J E, Cardozo T J, Zychlinsky A, Groisman E A. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol Microbiol. 1999;33:74–83. doi: 10.1046/j.1365-2958.1999.01449.x. [DOI] [PubMed] [Google Scholar]
- 49.Otto B R, van Dooren S J, Nuijens J H, Luirink J, Oudega B. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J Exp Med. 1998;188:1091–1103. doi: 10.1084/jem.188.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perna N T, Mayhew G F, Posfai G, Elliott S, Donnenberg M S, Kaper J B, Blattner F R. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1998;66:3810–3817. doi: 10.1128/iai.66.8.3810-3817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Podladchikova O N, Dikhanov G G, Rakin A V, Heesemann J. Nucleotide sequence and structural organization of Yersinia pestis insertion sequence IS100. FEMS Microbiol Lett. 1994;121:269–274. doi: 10.1111/j.1574-6968.1994.tb07111.x. [DOI] [PubMed] [Google Scholar]
- 52.Pourbakhsh S A, Boulianne M, Martineau-Doize B, Fairbrother J M. Virulence mechanisms of avian fimbriated Escherichia coli in experimentally inoculated chickens. Vet Microbiol. 1997;58:195–213. doi: 10.1016/s0378-1135(97)00163-6. [DOI] [PubMed] [Google Scholar]
- 53.Pourbakhsh S A, Dho-Moulin M, Brée A, Desautels C, Martineau-Doize B, Fairbrother J M. Localization of the in vivo expression of P and F1 fimbriae in chickens experimentally inoculated with pathogenic Escherichia coli. Microb Pathog. 1997;22:331–341. doi: 10.1006/mpat.1996.0116. [DOI] [PubMed] [Google Scholar]
- 54.Provence D L, Curtiss R., III Isolation and characterization of a gene involved in hemagglutination by an avian pathogenic Escherichia coli strain. Infect Immun. 1994;62:1369–1380. doi: 10.1128/iai.62.4.1369-1380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Provence D L, Curtiss R., III Role of Crl in avian pathogenic Escherichia coli: a knockout mutation of crl does not affect hemagglutination activity, fibronectin binding, or curli production. Infect Immun. 1992;60:4460–4467. doi: 10.1128/iai.60.11.4460-4467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajakumar K, Sasakawa C, Adler B. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect Immun. 1997;65:4606–4614. doi: 10.1128/iai.65.11.4606-4614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romantschuk M, Richter G Y, Mukhopadhyay P, Mills D. IS801, an insertion sequence element isolated from Pseudomonas syringae pathovar phaseolicola. Mol Microbiol. 1991;5:617–622. doi: 10.1111/j.1365-2958.1991.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 58.Smith H W. A search for transmissible pathogenic characters in invasive strains of Escherichia coli: the discovery of a plasmid-controlled toxin and a plasmid-controlled lethal character closely associated, or identical, with colicine V. J Gen Microbiol. 1974;83:95–111. doi: 10.1099/00221287-83-1-95. [DOI] [PubMed] [Google Scholar]
- 59.Smith H W, Huggins M B. Further observations on the association of the colicine V plasmid of Escherichia coli with pathogenicity and with survival in the alimentary tract. J Gen Microbiol. 1976;92:335–350. doi: 10.1099/00221287-92-2-335. [DOI] [PubMed] [Google Scholar]
- 60.Stathopoulos C, Provence D L, Curtiss R., III Characterization of the avian pathogenic Escherichia coli hemagglutinin Tsh, a member of the immunoglobulin A protease-type family of autotransporters. Infect Immun. 1999;67:772–781. doi: 10.1128/iai.67.2.772-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein M, Kenny B, Stein M A, Finlay B B. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J Bacteriol. 1996;178:6546–6554. doi: 10.1128/jb.178.22.6546-6554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki T, Lett M C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 63.Swenson D L, Bukanov N O, Berg D E, Welch R A. Two pathogenicity islands in uropathogenic Escherichia coli J96: cosmid cloning and sample sequencing. Infect Immun. 1996;64:3736–3743. doi: 10.1128/iai.64.9.3736-3743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tobe T, Hayashi T, Han C G, Schoolnik G K, Ohtsubo E, Sasakawa C. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect Immun. 1999;67:5455–5462. doi: 10.1128/iai.67.10.5455-5462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valvano M A. Diphenylamine increases cloacin DF13 sensitivity in avian septicemic strains of Escherichia coli. Vet Microbiol. 1992;32:149–161. doi: 10.1016/0378-1135(92)90102-y. [DOI] [PubMed] [Google Scholar]
- 66.van den Bosch J F, Hendriks J H, Gladigau I, Willems H M, Storm P K, de Graaf F K. Identification of F11 fimbriae on chicken Escherichia coli strains. Infect Immun. 1993;61:800–806. doi: 10.1128/iai.61.3.800-806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vokes S A, Reeves S A, Torres A G, Payne S M. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol Microbiol. 1999;33:63–73. doi: 10.1046/j.1365-2958.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 68.Waters V L, Crosa J H. Colicin V virulence plasmids. Microbiol Rev. 1991;55:437–450. doi: 10.1128/mr.55.3.437-450.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White D G, Dho-Moulin M, Wilson R A, Whittam T S. Clonal relationships and variation in virulence among Escherichia coli strains of avian origin. Microb Pathog. 1993;14:399–409. doi: 10.1006/mpat.1993.1039. [DOI] [PubMed] [Google Scholar]
- 70.White D G, Wilson R A, Emery D A, Nagaraja K V, Whittam T S. Clonal diversity among strains of Escherichia coli incriminated in turkey colisepticemia. Vet Microbiol. 1993;34:19–34. doi: 10.1016/0378-1135(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 71.Wooley R E, Nolan L K, Brown J, Gibbs P S, Giddings C W, Turner K S. Association of K-1 capsule, smooth lipopolysaccharides, traT gene, and colicin V production with complement resistance and virulence of avian Escherichia coli. Avian Dis. 1993;37:1092–1096. [PubMed] [Google Scholar]