Abstract
Opioids are commonly prescribed for pain despite growing evidence of their low efficacy in the treatment of chronic inflammatory pain and the high potential for misuse. There is a clear need to investigate non-opioid alternatives for the treatment of pain. In the present study, we tested the hypothesis that acute and repeated dopamine agonist treatment would attenuate mechanical hypersensitivity in male Long-Evans rats experiencing chronic inflammatory pain. We used two clinically available therapeutics, l-DOPA (precursor of dopamine biosynthesis) and pramipexole (dopamine D2/3 receptor agonist), to examine the functional role of dopamine signaling on mechanical hypersensitivity using an animal model of chronic inflammatory pain (complete Freund’s adjuvant, CFA). We found that both acute and repeated pramipexole treatment attenuated hyperalgesia-like behavior in CFA-treated animals but exhibited no analgesic effects in control animals. In contrast, there was no effect of acute or repeated l-DOPA treatment on mechanical hypersensitivity in either CFA- or saline-treated animals. Notably, we discovered some extended effects of l-DOPA and pramipexole on decreasing pain-like behavior at three days and one week post-drug treatment. We also examined the effects of pramipexole treatment on glutamatergic and presynaptic signaling in pain- and reward-related brain regions including the nucleus accumbens (NAc), dorsal striatum (DS), ventral tegmental area (VTA), cingulate cortex (CC), central amygdala (CeA), and periaqueductal gray (PAG). We found that pramipexole treatment decreased AMPA receptor phosphorylation (pGluR1845) in the NAc and DS but increased pGluR1845 in the CC and CeA. A marker of presynaptic vesicle release, pSynapsin, was also increased in the DS, VTA, CC, CeA, and PAG following pramipexole treatment. Interestingly, pramipexole increased pSynapsin in the NAc of saline-treated animals, but not CFA-treated animals, suggesting blunted presynaptic vesicle release in the NAc of CFA-treated animals following pramipexole treatment. To examine the functional implications of impaired presynaptic signaling in the NAc of CFA animals, we used ex vivo electrophysiology to examine the effects of pramipexole treatment on the intrinsic excitability of NAc neurons in CFA- and saline-treated animals. We found that pramipexole treatment reduced NAc intrinsic excitability in saline-treated animals but produced no change in NAc intrinsic excitability in CFA-treated animals. These findings indicate alterations in dopamine D2/3 receptor signaling in the NAc of animals with a history of chronic pain in association with the anti-hyperalgesic effects of pramipexole treatment.
1. Introduction
Chronic pain is a pervasive and physiologically damaging condition that impacts an estimated 20% of the population (Goldberg and McGee, 2011; Yong et al., 2021), which is roughly 50 million adults in the United States (Dahlhamer et al., 2018). It is estimated that 8–10% of the US population experience chronic pain that limits their ability to work and actively engage in everyday life activities (Dahlhamer et al., 2018; Yong et al., 2021). Pain is one of the most frequent causes for individuals to seek medical care and treatment (Mäntyselkä et al., 2001), costing the US healthcare system over $600 billon per year (Institute of Medicine, 2011). In addition to seeking medical care and treatment, chronic pain is often associated with significant emotional distress (Treede et al., 2019). Pain is multidimensional with numerous different types, sources, and symptoms of pain, which include both physical and emotional manifestations. Two broad classifications of chronic pain include chronic inflammatory pain (due to persistent inflammation) and chronic neuropathic pain (due to nerve injury). The majority of chronic pain complaints arise from inflammatory pain conditions including arthritis, which is a leading cause of disability among adults (Hootman et al., 2016).
For successful management of pain, prescription opioids continue to be the most powerful and effective tools used by clinicians to achieve analgesia. Opioids are commonly prescribed for the treatment of chronic pain despite growing evidence of their limited efficacy in the long-term management of pain (Dowell et al., 2016). Use of opioids is a cause for concern because of the development of analgesic tolerance and hyperalgesia (increased pain sensitivity) as well as the heightened misuse liability of opioids (Pahng and Edwards, 2021). Published reports by the Substance Abuse and Health Services Administration and the Centers for Disease Control and Prevention found that 10.1 million people reported opioid misuse and 70,630 people died from an opioid overdose in the 2019 (2019 National Survey on Drug Use and Health, 2020; Hedegaard et al., 2020). The past over-prescribing of opioids followed by more recent prescribing restrictions have led to unintended increases in illegal opioid use (Cicero et al., 2015) and a rise in overdose due to synthetic opioids (2019 National Survey on Drug Use and Health, 2020). With all these factors combined, there is a clear need for the development and/or utilization of non-opioid alternative treatments for individuals living with chronic pain. Currently, there are few FDA-approved, non-opioid pain medications and these are often limited by side effects, low efficacy, and slower drug onset compared to opioids (Volkow et al., 2018), providing additional need for the exploration of non-opioid pharmacotherapies (Kaye et al., 2018; Skolnick and Volkow, 2016).
Both clinical and preclinical research have illustrated the importance of the mesolimbic dopamine system in registering the salience of both reward and pain. Within mesolimbic circuitry, dopamine projections can detect both the onset and offset of pain (Becerra and Borsook, 2008; Navratilova et al., 2012). Activation of ventral tegmental area (VTA) dopamine neurons projecting to the nucleus accumbens (NAc) increase NAc dopamine levels and can produce pain relief, suggesting that relief from pain can be rewarding (Navratilova et al., 2012; Watanabe et al., 2018). Furthermore, neuroadaptations in the mesolimbic dopamine system have been implicated in both addiction and the transition from acute to chronic pain states. While the underlying mechanisms contributing to the chronification of pain are not well understood, there is compelling evidence that chronic pain can produce functional abnormalities in brain regions involved in the salience of pain perception, affective pain processing, and descending pain control (Thompson and Neugebauer, 2019). Some of these cortical and subcortical brain regions include the prefrontal cortex (Ong et al., 2019; Vogt, 2005), NAc (Baliki et al., 2012; Chang et al., 2014), amygdala (Neugebauer, 2015; Simons et al., 2014), and periaqueductal gray (PAG) (Mills et al., 2018; Ong et al., 2019). Two common abnormalities hypothesized in chronic pain states include reward deficiency (reflected by a hypodopaminergic state) and dopamine receptor dysfunction (Borsook et al., 2016; Taylor et al., 2016). Accordingly, the goal of this study was to examine the effects of dopamine agonist medications on chronic inflammatory pain at both a dopamine biosynthesis and dopamine receptor level using clinically available therapeutics.
In the present study, we tested the hypothesis that acute and repeated dopamine agonist treatment would attenuate mechanical hypersensitivity in rats with chronic inflammatory pain. We used the complete Freund’s adjuvant (CFA) model to induce chronic inflammatory pain in male Long-Evans rats. We utilized clinically available therapeutics, l-DOPA (a precursor of dopamine biosynthesis) and pramipexole (a dopamine D2/3 receptor agonist), to test the functional role of dopamine system modulation on mechanical hypersensitivity. We implemented two different dosing regimens for each drug to examine the differences between acute and repeated drug treatment on mechanical hypersensitivity. For the therapeutic treatments, l-DOPA and pramipexole were administered on separate test days, and the order of drug presentation was counterbalanced. To mimic clinical conditions and prevent the peripheral breakdown of l-DOPA, the drug was coadministered with DOPA decarboxylase inhibitor, benserazide. In a separate experiment, we examined the neurobiological consequences of pramipexole treatment on neuronal signaling in pain-related brain regions including the NAc, dorsal striatum (DS), VTA, cingulate cortex (CC), central amygdala (CeA), and PAG. Finally, using ex vivo electrophysiology, we examined the effects of pramipexole treatment on the intrinsic excitability of NAc neurons in rats experiencing chronic inflammatory pain.
2. Materials and methods
2.1. Animals
Ninety-two adult male Long-Evans rats weighing 225–275 grams (approximate age 8–9 weeks) at the time of arrival were purchased from Charles River (Wilmington, MA). The animal numbers for each experiment are as follows: experiment 1 (N = 40), experiment 2 (N = 44), experiment 3 (N = 8). Rats were pair-housed and given ad libitum access to food (Purina Rat Chow, Ralston Purina, St. Louis, MO) and water throughout all experimental procedures. Rats were maintained on a reverse 12-h light/dark cycle (lights off at 8:00 a.m.) and were handled regularly. Rats were given one week to acclimate to the colony room prior to the start of experimental procedures. All animal care, use, and procedures in this study were approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center and were in accordance with the National Institute of Health guidelines.
2.2. Induction of chronic inflammatory pain
Rats were anesthetized with isoflurane (4% for 3 min). After being removed from anesthesia, rats were immediately placed on their backs and the left hindpaw of each rat was wiped with an alcohol prep pad (70% isopropyl). Rats received subcutaneous injections of 150 μL of either 50% CFA or sterile saline (control) into the left hindpaw. Rats were returned to their home-cage as soon as they were able to right themselves and become ambulatory. Rats were observed by the investigators for one week for obvious signs of distress or complications from the procedure. CFA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was diluted to 50% CFA in sterile saline the morning of injections.
2.3. Testing mechanical hypersensitivity
Von Frey tests of mechanical hypersensitivity were conducted as previously described (Pahng et al., 2017). Briefly, rats were habituated to the testing room for at least 30 min prior to testing. Rats were placed in individual compartments (26 x 11 × 20 cm) in elevated cages with stainless-steel mesh floors until grooming and exploratory behaviors ceased (5 min). Measurements for each rat were taken when all four paws were placed on the steel mesh floors. In order to assess the presence of mechanical hypersensitivity, the mid-plantar area of the hindpaw was perpendicularly stimulated with electronic von Frey anesthesiometer (Ugo Basile; Gemonio, Italy), which measures the grams of pressure applied to the paw with the probe. A brisk withdrawal of the paw, which is often followed by a sustained retraction and/or licking, is recorded as a positive response.
2.4. Drug administration
Pramipexole dihydrochloride (PHR1598-500 MG), l-DOPA (L-3,4-dihydroxyphenylalanine methyl ester hydrochloride) (D1507-1G), and benserazide hydrochloride (B7283-1G) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs were prepared fresh the day of testing and protected from light exposure. Pramipexole dihydrochloride was dissolved in sterile saline (1 mg/kg) for subcutaneous (s.c.) administration (2 ml/kg). Benserazide (DOPA decarboxylase inhibitor) was added to l-DOPA to prevent the peripheral breakdown of l-DOPA to dopamine. l-DOPA and Benserazide were dissolved in sterile water together at 50 mg/kg and 10 mg/kg, respectively, for intraperitoneal (i. p.) administration (1 ml/kg). These doses of l-DOPA and benserazide were previously shown to not impact general motor activity (Antinori et al., 2018). Controls animals received either i.p. or s.c. injections of drug vehicle.
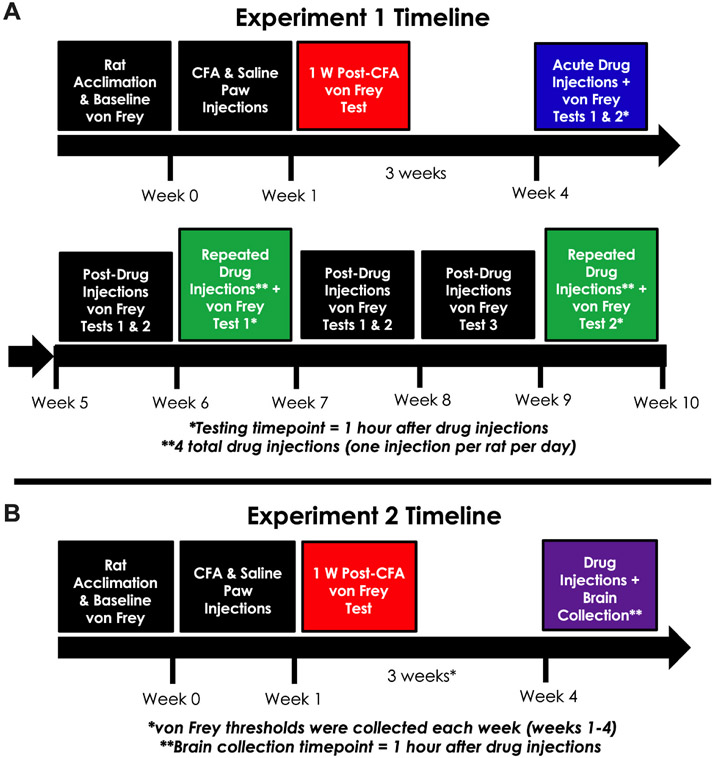
2.5. Experiment 1 design: behavioral effects of acute and repeated dopamine agonism treatment on rats with chronic inflammatory pain
The complete timeline for experiment 1 is shown in Fig. 1A. All rats underwent one week of habituation to the colony room and handling prior to baseline mechanical hypersensitivity testing. Animals were split into two equivalent groups (CFA and saline) based on baseline paw withdrawal thresholds of the left paw. Paw withdrawal thresholds were measured in animals one week after receiving saline or CFA injections to the left paw. CFA and saline animals were split into a total of four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug) based on one-week paw withdrawal thresholds of the left paw. The saline-vehicle and CFA-vehicle groups consisted of saline-injected and CFA-injected animals that received vehicle prior to von Frey testing. The saline-drug and CFA-drug groups consisted of saline-injected animals and CFA-injected animals that received either l-DOPA or pramipexole prior to von Frey testing. All animals in each group received both drugs, but the drugs were administered on separate days. To prevent potential order effects, half of the animals in the drug treatment groups received l-DOPA treatment first and the other half received pramipexole treatment first. The order was flipped during the subsequent test day, so all animals in drug treatment groups received both drugs, but in different test orders.
Fig. 1. Design and timelines for Experiments 1 and 2.
(A) Experiment 1. Rats were given one week for housing room acclimation, handling by the experimenter, and baseline testing of mechanical hypersensitivity. Rats were given either CFA or saline intra-plantar injections based on initial mechanical hypersensitivity testing. One week after CFA and saline injections, paw withdrawal thresholds were measured. Animals were split into four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug) based on one-week paw withdrawal thresholds. For the acute treatment tests during week 4, rats received injections of l-DOPA (50 mg/kg), pramipexole (1 mg/kg), or vehicle 1 h prior to measuring paw withdrawal thresholds. l-DOPA was always co-administered with a DOPA decarboxylase inhibitor, benserazide (10 mg/kg). The two acute treatment tests were separated by more than 48 h, with half of the drug animals receiving l-DOPA first and the other half receiving pramipexole first. Paw withdrawal thresholds were measured in all animals three days and one week after the acute drug treatment testing. For the repeated treatment testing, animals received four daily injections of l-DOPA (50 mg/kg) + benserazide (10 mg/kg), pramipexole (1 mg/kg), or vehicle. The fourth drug injection took place 1 h prior to measuring mechanical hypersensitivity. Repeated drug treatment tests 1 & 2 were separated by two weeks. Paw withdrawal thresholds were measured in all animals four days, one week, and two weeks after the repeated drug treatment test 1. (B) Experiment 2. Rats were given one week for housing room acclimation, handling by the experimenter, and baseline mechanical hypersensitivity testing. Rats were given either CFA or saline paw injections based on initial mechanical hypersensitivity testing. One week after CFA and saline injections, paw withdrawal thresholds were measured. Animals were split into four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug) based on one-week paw withdrawal thresholds. Thresholds were measured in all animals at two weeks, three weeks, and four weeks. All animals were given injections of vehicle 24 h after the last mechanical hypersensitivity test. Rats received injections of either pramipexole (1 mg/kg) or vehicle 1 h prior to brain collection for Western blotting the following day.
For acute drug treatment, rats received either l-DOPA or pramipexole 1 h prior to von Frey testing. Acute treatment test days 1 & 2 were separated by more than 48 h and occurred during week 4 of the experiment. Paw withdrawal thresholds were measured in all four groups four days and one week after the last acute treatment test day. For repeated drug treatment, rats received four days of daily drug or vehicle injections (1 injection per 24 h). The fourth drug or vehicle injection took place 1 h prior to von Frey testing. Due to some evidence of drug carry over effects in our acute treatment results, repeated treatment test days 1 & 2 were separated by two weeks. Paw withdrawal thresholds were measured in all four groups: three days, one week, and two weeks after the first repeated treatment test day. This experimental procedure was completed across two cohorts of animals (Cohort 1: N = 24, n = 6/per group; Cohort 2: N = 16, n = 4/per group) to maximize rigor and reproducibility. There were no differences in left paw withdrawal thresholds between the two cohorts during baseline testing (F (1,36) = 0.0064, p = 0.9362, data not shown) or one week post-CFA/saline injections (F(1,36) = 0.2856, p = 0.5963, data not shown), so the data from the two cohorts were pooled together (N = 40, n = 10/per group) for subsequent analyses.
2.6. Experiment 2 design: neurobiological effects of acute pramipexole treatment on rats with chronic inflammatory pain
The complete timeline for experiment 2 is shown in Fig. 1B. Identical to experiment 1, all rats underwent one week of habituation to the colony room and handling prior to baseline mechanical hypersensitivity testing where they were spilt into two equivalent groups (CFA and saline). Paw withdrawal thresholds were measured in all animals once a week for a total of four weeks. To habituate the animals to drug injections, all animals were given s.c. injections of vehicle 24 h after the last mechanical hypersensitivity test. On the day of brain tissue collection, CFA and saline animals were split into a total of four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug) based on one-week paw withdrawal thresholds (left paw). Rats received injections of either pramipexole or vehicle 1 h prior to euthanization by decapitation under light isoflurane anesthesia. This experimental procedure was completed over two cohorts of animals (Cohort 1: N = 24, n = 6/per group; Cohort 2: N = 20, n = 5/per group) to maximize rigor and reproducibility. There were no differences in left paw withdrawal thresholds between the two cohorts during baseline testing (F(1,40) = 0.4186, p = 0.5213, data not shown) or one week post-CFA/saline injections (F(1,40) = 1.513, p = 0.2258, data not shown). Accordingly, the data were pooled together and brain samples from individual rats were analyzed via Western blotting (N = 44, n = 11/per group).
2.7. Western blot analysis
Western blot analyses for brain regional changes in protein phosphorylation were conducted as previously described (Pahng et al., 2019). After the rats were euthanized, the brains were rapidly removed, snap-frozen in isopentane at −20 °C, and stored at −80 °C until dissection. Brains were placed in a −20 °C freezer the night before dissection. Brains were mounted and sliced using a cryostat at −12 °C during brain region dissection. Regional brain punches (0.5 mm thick) were taken from frozen tissue using 13–16 gauge needles (inner diameter: 1.8–1.19 mm) according to the Rat Brain Atlas (Paxinos and Watson, 1998) Supplementary Fig. 1. Brain punches were homogenized by sonication in a lysis buffer (320 mm sucrose, 5 mm HEPES, 1 mm EGTA, 1 mm EDTA, 1 %SDS, protease inhibitor cocktail (diluted 1:100), and phosphatase inhibitor cocktails II and III (diluted 1:100; Sigma-Aldrich). Tissue homogenates were heated at 90 °C for 5 min. Total protein concentration was measured using a detergent-compatible Lowry method and tissue homogenates were aliquoted based on total protein concentrations (Bio-Rad, Hercules, CA, USA). Aliquoted tissue homogenates were stored at −80 °C until Western blotting. Samples of protein (20 μg) were separated by SDS-polyacrylamide gel electrophoresis on 8% acrylamide gels using a Tris/Glycine/SDS buffer system (Bio-Rad). The gels were electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes overnight (GE Healthcare, Piscataway, NJ, USA). Membranes were blocked for 1 h in 5% non-fat milk at room temperature and incubated overnight in 2.5% non-fat milk with primary antibodies at 4 °C. The primary antibodies used include phospho-AMPA receptor 1 Ser845 (1:500–5000, Cell Signaling Technology; Cat #8084) and phospho-Synapsin Ser9 (1:5000, Cell Signaling Technology; Cat #2311). The following day, membranes were washed and incubated with a species-specific peroxidase-conjugated secondary antibody (1:10, 000; Bio-Rad) for 1 h at room temperature. Membranes were washed and incubated in a chemiluminescent reagent (Immobilon Crescendo Western HRP substrate; Millipore Sigma, Temecula, CA, USA), and exposed to film for varying times depending on the antibody and brain region. Following film development, membranes were stripped for 30 min at room temperature (Restore; Thermo Scientific) and reprobed for total protein and Beta-tubulin levels. The primary antibodies used include total AMPA receptor 1 (1:1000–5000, Cell Signaling Technology; Cat #13185), total Synapsin (1:5000–10000, Cell Signaling Technology; Cat #2312), and Beta-tubulin (1:1000000, Santa Cruz Biotechnology, Cat# sc-53140). The immunoreactivity of the individual bands was detected using densitometry (Image J 1.45S; Bethesda, MD). To normalize the data across the blots, the densitized values were expressed as a percentage of the mean of the saline-vehicle for each gel. The gel order of saline-vehicle, saline-drug, CFA-vehicle, and CFA-drug was repeated for all animals over four gels. Individual protein phosphorylation levels were normalized to either individual total protein levels or individual β-tubulin levels to generate phosphorylation: total or β-tubulin ratio values for statistical comparison. Phosphorylation: β-tubulin ratio values were used in the event of changes to total protein levels or a similar main effect for phosphorylated and total levels of the same protein.
2.8. Experiment 3: Ex vivo electrophysiology
To keep the timeline consistent with experiments 1 & 2, all rats underwent one week of habituation to the colony room and handling prior to receiving intra-plantar CFA or saline injections. Animals (N = 8, n = 4/per group) were examined for a proper inflammatory response one week later and were kept in the colony room for an additional three weeks. Under isoflurane anesthesia, rats were transcardially perfused with room temperature (~25 °C) NMDG artificial cerebrospinal fluid (aCSF) containing the following (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 0.5 CaCl2, 10 MgSO4·7 H2O, 5 Na-ascorbate, 3 Na-pyruvate (Avegno et al., 2019). 300 μm-thick coronal sections containing the NAc were collected using a vibratome (Leica VT1200S, Nussloch, Germany). Sections were incubated in NMDG aCSF at 37 °C for 12 min, then transferred to a room temperature holding aCSF solution containing the following (in mM): 92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 2 CaCl2, 2 MgSO4·7 H2O, 5 Na-ascorbate, 3 Na-pyruvate. Slices were allowed to recover for 1 h prior to recording.
Recordings were performed throughout the NAc (including the medial shell, lateral shell, and core), and positions were noted for each recording Supplementary Fig. 2. Sections were transferred to a recording aCSF solution containing the following (in mM): 130 NaCl, 3.5 KCl, 2 CaCl2, 1.25 NaH2PO4, 1.5 MgSO4·7 H2O, 24 NaHCO3, 10 glucose. Recording aCSF was maintained at 32–34 °C using an in-line heater (Warner Instruments, Hamden, CT, USA). Whole cell recordings were performed using an internal recording solution containing the following (in mM): 140 K-gluconate, 5 KCl, 0.2 EGTA, 10 HEPES, 2 MgCl2·6 H2O, 4 Mg-ATP, 0.3 Na2-GTP, 10 Na2-phosphocreatine (pH 7.2–7.3, 285–295 mOsm). Signals were acquired via a Multiclamp 700B amplifier (Axon Instruments) and were digitized and analyzed via pClamp 10.2 software (Axon Instruments). Liquid junction potentials were not corrected during recordings. Experiments with a series resistance >30 MΩ or a >20% change in series resistance were excluded from analysis. Current clamp recordings were performed to assess the intrinsic excitability of NAc neurons (n = 7–8 cells per group). Following break in and once a stable seal was achieved (usually ~ 3 min), a series of current injections (0–240 pA, 1.5 s duration, 20 pA increments) was delivered to determine rheobase values, defined as the minimum current required to elicit an action potential, as well as the number of action potentials fired in response to varying current injections. Excitability was measured from each cell’s resting membrane potential. Resting membrane potential and input resistance were measured by generating an I–V curve after applying a series of current steps from −20 pA to +20 pA (5 pA increments, 500 msec duration). After recording baseline data, pramipexole (1 μM) was bath-applied to the slice, and excitability measures were recorded following a minimum of 5 min incubation time.
2.9. Statistical analysis
All data were analyzed using Prism 9 (GraphPad Software, Inc; La Jolla, CA, USA). Changes in mechanical hypersensitivity following CFA treatment alone were analyzed using two-way repeated-measures ANOVAs with the between-subjects factor of CFA treatment (CFA vs. saline paw injections) and the within-subjects factor of time (baseline vs. post-treatment). Changes in mechanical hypersensitivity following CFA and drug treatment were analyzed using two-way between-subjects ANOVAs with CFA treatment (CFA vs. saline paw injections) and drug treatment (l-DOPA vs. vehicle or pramipexole vs. vehicle) as factors. Western blotting data was analyzed using two-way between-subjects ANOVAs with CFA treatment (CFA vs. saline paw injections) and pramipexole treatment (pramipexole vs. vehicle) as factors. Rheobase data was analyzed using two-tailed paired and unpaired t-tests. The number of action potentials fired in response to varying current injections was analyzed using two-way and three-way repeated measures ANOVAs with current injections (0–240 pA), pramipexole treatment (pramipexole vs. vehicle), and CFA treatment (CFA vs. saline paw injections) as factors. All post-hoc analyses were completed using Tukey’s and Sidak’s multiple comparisons tests. Potential statistical outliers were examined using the Grubbs outlier test. Significance levels for statistical tests was set at p < 0.05.
3. Results
3.1. Experiment 1. Behavioral effects of acute and repeated dopamine system agonism in rats experiencing chronic inflammatory pain
3.1.1. Experiment 1. Effects of chronic inflammatory pain on mechanical hypersensitivity in rats
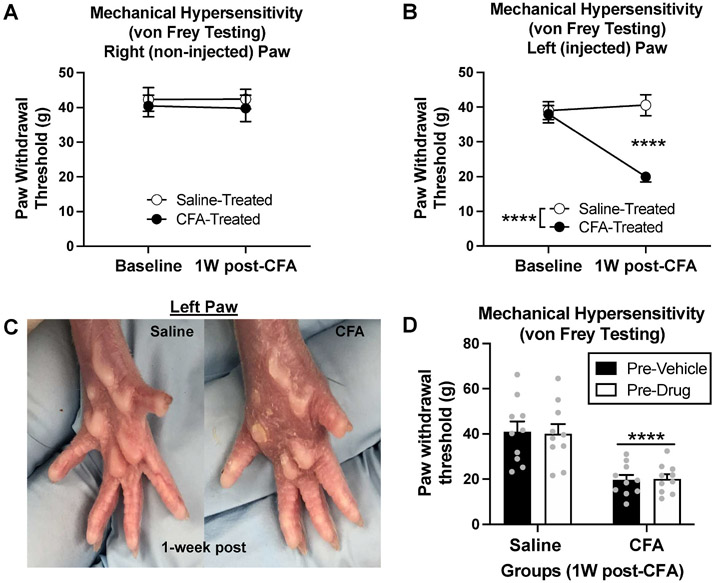
We used intra-plantar CFA injections to model chronic inflammatory pain in rats and tested consequent changes in paw sensitivity using the von Frey test of mechanical hypersensitivity. Rats were split into two equivalent groups based on their baseline paw withdrawal thresholds. Animals received s.c. injections of either saline or CFA into the left hindpaw. One week later, paw withdrawal thresholds were measured to examine the effects of CFA treatment on mechanical hypersensitivity (measure of pain-like behavior). As expected, we did not find a significant effect of CFA treatment (saline vs. CFA) [F(1,38) = 0.4062, p = 0.5277], time (baseline vs. 1-week) [F(1,38) = 0.0091, p = 0.9244], or interaction [F(1,38) = 0.0166, p = 0.8981] for paw withdrawal thresholds in the non-injected right hindpaw Fig. 2A. In the injected left hindpaw, however, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,38) = 19.39, p < 0.0001], time (baseline vs. 1-week) F (1,38) = 11.32, p = 0.0018], and interaction F(1,38) = 16.10, p = 0.0003], for paw withdrawal thresholds Fig. 2B. Post-hoc analysis revealed a significant decrease in paw withdrawal thresholds in CFA-treated animals at one week (p < 0.0001) Fig. 2B. This demonstrates that CFA produces significant mechanical hypersensitivity due to persistent inflammation in the injected hindpaw.
Fig. 2. Experiment 1: Effects of CFA on mechanical hypersensitivity in rats.
(A) There was no effect of CFA treatment or time on paw withdrawal thresholds in the right (non-injected) hindpaw (p > 0.05). (B) At one week, CFA animals demonstrated a decrease in paw withdrawal thresholds from baseline (****p < 0.0001) and compared to saline controls (****p < 0.0001), indicating mechanical hypersensitivity. (C) One-week post-injection, CFA treatment produces visually evident inflammation in the left (injected) hindpaw compared to the left hindpaw of saline-injected controls. (D) Rats were split into four groups based on one week paw withdrawal thresholds (left paw). Prior to l-DOPA & pramipexole treatment, there was an effect of CFA treatment (****p < 0.0001), but no difference in drug treatment groups (pre-vehicle vs. pre-drug) (p > 0.05). Bars represent means and symbols represent individual data points.
The local inflammatory response was visually evident in the CFA-injected hindpaw compared to the saline-injected hindpaw Fig. 2C. Prior to dopamine system treatment, CFA and saline rats were split into four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug) based on one-week paw withdrawal thresholds. Prior to drug treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 36.44, p < 0.0001], but no effect of drug treatment (pre-vehicle vs. pre-drug) [F(1,38) = 0.0036, p = 0.9526] or interaction [F(1,38) = 0.0424, p = 0.8379] Fig. 2D. This demonstrates that there was a significant difference between CFA and saline animals prior to l-DOPA and pramipexole treatment, but no difference between drug treatment groups (saline-vehicle vs. saline-drug, CFA-vehicle vs. CFA-drug) prior to receiving l-DOPA and pramipexole injections.
3.1.2. Experiment 1. Effects of acute dopamine agonism treatment on mechanical hypersensitivity in rats with chronic inflammatory pain
To test the effects of acute l-DOPA and pramipexole treatment on pain-like sensitivity, CFA and saline-injected rats received systemic injections of either l-DOPA (50 mg/kg), pramipexole (1 mg/kg), or vehicle 1 h prior to von Frey testing of mechanical hypersensitivity. l-DOPA was always co-administered with the DOPA decarboxylase inhibitor, benserazide (10 mg/kg), to prevent the peripheral breakdown of l-DOPA. Acute treatment tests occurred during week 4 of the experiment (i.e., three weeks post-CFA treatment). All drug-treated animals received l-DOPA and pramipexole, but on separate test days. To prevent potential order effects, the two acute treatment tests were separated by more than 48 h, with half of the drug treatment animals receiving l-DOPA first and the other half receiving pramipexole first.
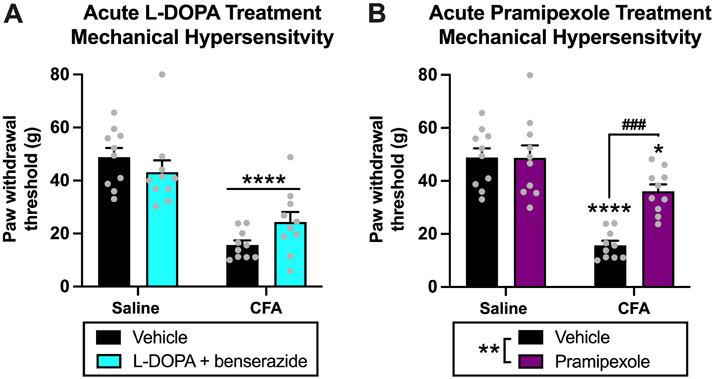
For acute l-DOPA treatment testing, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 54.70, p < 0.0001] and a significant interaction [F(1,36) = 4.125, p = 0.0497], but no effect of l-DOPA treatment (vehicle vs. l-DOPA) [F(1,36) = 0.1782, p = 0.6754] Fig. 3A. Post-hoc analysis with Tukey’s multiple comparisons test revealed the following significant differences in paw withdrawal thresholds: saline-vehicle vs. CFA-vehicle (p < 0.0001), saline-vehicle vs. CFA-l-DOPA (p = 0.0001), saline-l-DOPA vs. CFA-vehicle (p < 0.0001), and saline-l-DOPA vs CFA-l-DOPA (p = 0.0029) Fig. 3A. There was no difference in paw withdrawal thresholds between CFA-vehicle vs. CFA-l-DOPA animals (p = 0.3212), demonstrating that acute administration of l-DOPA did not attenuate mechanical hypersensitivity in CFA animals. Furthermore, there was no difference in thresholds between saline-vehicle vs. saline-l-DOPA animals (p = 0.6690), demonstrating that acute administration of l-DOPA did not produce analgesic effects in saline-treated animals.
Fig. 3. Experiment 1: Effects of acute dopamine system agonism on mechanical hypersensitivity in rats experiencing chronic inflammatory pain.
(A) For acute l-DOPA treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds and an interaction (p < 0.05), but no effect of l-DOPA treatment (p > 0.05). (B) For acute pramipexole treatment, there were effects of CFA treatment (p < 0.0001) and pramipexole treatment (**p < 0.01) on paw withdrawal thresholds, and an interaction (p < 0.001). Post hoc analysis revealed that paw withdrawal thresholds of CFA-vehicle (****p < 0.0001) and CFA-pramipexole (*p < 0.05) animals were decreased compared to saline-vehicle animals. Paw withdrawal thresholds of CFA-pramipexole animals were increased compared to CFA-vehicle animals (###p = 0.001), but there was no difference in paw withdrawal thresholds between saline-vehicle and saline-pramipexole animals (p > 0.05). This demonstrates that acute pramipexole treatment increases paw withdrawal thresholds and reduces mechanical hypersensitivity in CFA animals 1 h after acute administration but produces no analgesic effects in saline animals. Bars represent means and symbols represent individual data points.
For acute pramipexole treatment testing, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 46.81, p < 0.0001], pramipexole treatment (vehicle vs. pramipexole) [F(1,36) = 9.157, p = 0.0046], and an interaction of factors [F(1,36) = 9.449, p = 0.0040] Fig. 3B. Post-hoc analysis revealed the following significant differences in paw withdrawal thresholds: saline-vehicle vs. CFA-vehicle (p < 0.0001), saline-vehicle vs. CFA-pramipexole (p = 0.0494), saline-pramipexole vs. CFA-vehicle (p < 0.0001), and CFA-vehicle vs CFA-pramipexole (p = 0.0007) Fig. 3B. The significant increase in paw withdrawal thresholds of CFA-pramipexole animals compared to CFA-vehicle animals demonstrates that acute administration of pramipexole attenuates mechanical hypersensitivity in CFA animals. There was also a decrease in thresholds of CFA-pramipexole animals compared to saline-vehicle animals (p = 0.0494), which suggests there was not a complete attenuation of pain-like behavior with acute pramipexole treatment. There was no difference in thresholds between saline-vehicle vs. saline-pramipexole animals (p > 0.9999), demonstrating that acute administration of pramipexole did not produce analgesic effects in saline-treated animals.
3.1.3. Experiment 1. Extended effects of acute dopamine system agonist treatment on mechanical hypersensitivity in rats experiencing chronic inflammatory pain
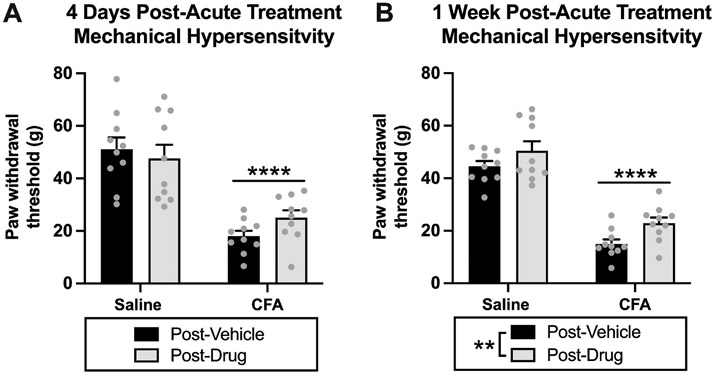
To examine extended effects of acute dopamine system agonism on pain like-behavior, we measured paw withdrawal thresholds in animals four days and one week after the last acute treatment test. We measured the combined post-acute drug treatment effects in four groups (saline-vehicle, saline-drug, CFA-vehicle, CFA-drug). These measurements were taken after the completion of the two acute treatment tests, so we were unable to parse apart pramipexole- and l-DOPA-specific effects. For four-days post-acute drug treatment, we had a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 52.11, p < 0.0001], but no effect of drug treatment (4 days post-vehicle vs. 4 days post-drug) [F(1,36) = 0.2163, p = 0.6446] or interaction [F(1,36) = 1.889, p = 0.1778] Fig. 4A. For one-week post-acute drug treatment, we had a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 133.7, p < 0.0001] and drug treatment (1 week post-vehicle vs. 1 week post-drug) [F(1,36) = 7.843, p = 0.0082], but no interaction [F(1,36) = 0.1619, p = 0.6898] Fig. 4B. These data indicate an increase in paw withdrawal thresholds one week after the last acute treatment test. Because of the potential carry over and/or interactive effects of pramipexole and l-DOPA treatment on pain-like behavior, we adjusted the design of the repeated drug treatment experiment by separating the two repeated drug treatment tests by two weeks. This allowed us to reduce potential drug interactions and to parse apart the post-repeated drug treatment effects for l-DOPA and pramipexole, individually.
Fig. 4. Experiment 1: Extended effects of acute dopamine system agonism on mechanical hypersensitivity in rats with chronic inflammatory pain.
(A) For four-days post-acute drug treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds, but no effect of drug treatment (p > 0.05). (B) For one-week post-acute drug treatment, there was an effect of CFA treatment (****p < 0.0001) and drug treatment (**p = 0.0082) on paw withdrawal thresholds. This suggests that acute drug treatment increases paw withdrawal thresholds one week after acute administration. Bars represent means and symbols represent individual data points.
3.1.4. Experiment 1. Effects of repeated dopamine system agonism on mechanical hypersensitivity in rats experiencing chronic inflammatory pain
To test the effects of repeated l-DOPA and pramipexole treatment on mechanical hypersensitivity, CFA and saline-injected rats received four systemic injections of either l-DOPA (50 mg/kg), pramipexole (1 mg/kg), or vehicle over the course of four days (1 injection every 24 h). All drug-treated animals received four repeated injections of the same drug during repeated testing with half receiving l-DOPA and the other half receiving pramipexole. The drug assignments were switched between repeated tests 1 & 2, so that every drug-treated animal was tested with both drugs. The last injection was given 1 h prior to von Frey testing of mechanical hypersensitivity. Consistent with the acute treatment testing, l-DOPA was co-administered with benserazide (10 mg/kg). Repeated treatment test 1 occurred five weeks post-CFA treatment, while repeated treatment test 2 occurred eight weeks post-CFA treatment. To determine if there were changes in the thresholds of vehicle controls over the course of the 9-week experiment, we measured changes in paw withdrawal thresholds of saline-vehicle and CFA-vehicle animals over time (1 week post, 3 weeks post, 5 weeks post, 6 weeks post, 7 weeks post, and 8 weeks post-CFA). As expected, we found a significant effect of CFA treatment [F(1,18) = 135.5, p < 0.0001], but no effect of time [F(5,90) = 1.409, p = 0.2288] or interaction [F(5,90) = 1.888, p = 0.1042] (graph not shown). This demonstrates that mechanical hypersensitivity remained consistent in vehicle controls over the course of experiment 1.
For repeated l-DOPA treatment testing, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 50.67, p < 0.0001] and interaction [F(1,36) = 5.649, p = 0.0229], but no effect of l-DOPA treatment (vehicle vs. l-DOPA) [F(1,36) = 0.3901, p = 0.5362] Fig. 5A. Post-hoc analysis with Tukey’s multiple comparisons test revealed the following significant differences in paw withdrawal thresholds: saline-vehicle vs. CFA-vehicle (p < 0.0001), saline-vehicle vs. CFA-l-DOPA (p = 0.0003), saline-l-DOPA vs. CFA-vehicle (p < 0.0001), and saline-l-DOPA vs CFA-l-DOPA (p = 0.0098) Fig. 5A. Similar to acute l-DOPA treatment, there was no difference in thresholds between CFA-vehicle vs. CFA-l-DOPA animals (p = 0.1654), demonstrating that even repeated administration of l-DOPA did not significantly attenuate mechanical hypersensitivity in CFA-treated animals. Additionally, there was no difference in thresholds between saline-vehicle vs. saline-l-DOPA animals (p = 0.6067), demonstrating that repeated administration of l-DOPA did not produce analgesic effects in saline-treated animals.
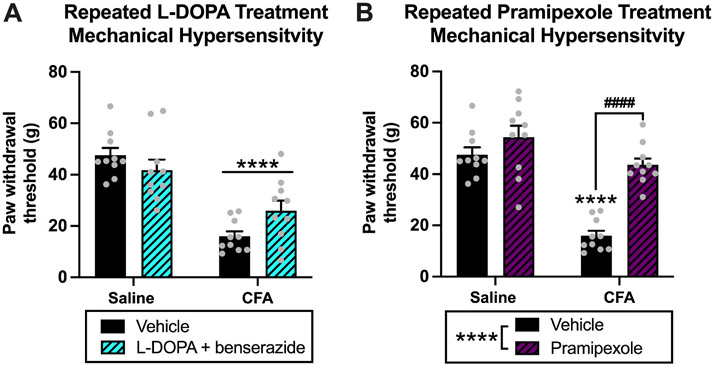
Fig. 5. Experiment 1: Effects of repeated dopamine system agonism on mechanical hypersensitivity in rats with chronic inflammatory pain.
(A) For repeated l-DOPA treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds and an interaction (p < 0.05), but no effect of l-DOPA treatment (p > 0.05). (B) For repeated pramipexole treatment, there were effects of CFA treatment (p < 0.0001) and pramipexole treatment (****p < 0.0001) on paw withdrawal thresholds, and an interaction (p < 0.05). Post hoc analysis revealed that there was a decrease in paw withdrawal thresholds of CFA-vehicle animals compared to saline-vehicle animals (****p < 0.0001) and an increase in paw withdrawal thresholds of CFA-pramipexole animals compared to CFA-vehicle animals (####p < 0.0001). There was no difference in paw withdrawal thresholds between saline-vehicle and saline-pramipexole animals (p > 0.05). This demonstrates that repeated pramipexole treatment increases paw withdrawal and reduces mechanical hypersensitivity in CFA animals 1 h after repeated administration but produces no analgesic effects in saline animals. There was no difference in paw withdrawal thresholds between saline-vehicle animals and CFA-pramipexole animals (p > 0.05), demonstrating a complete attenuation of pain-like behavior in CFA animal following repeated pramipexole treatment. Bars represent means and symbols represent individual data points.
For the repeated pramipexole treatment test, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,36) = 46.81, p < 0.0001], drug treatment (vehicle vs. pramipexole) [F(1,36) = 30.71, p < 0.0001], and an interaction of factors [F(1,36) = 11.37, p = 0.0018] Fig. 5B. Post-hoc analysis revealed the following significant differences in paw withdrawal thresholds: saline-vehicle vs. CFA-vehicle (p < 0.0001), saline-pramipexole vs. CFA-vehicle (p < 0.0001), and CFA-vehicle vs CFA-pramipexole (p < 0.0001) Fig. 5B. Similar to acute pramipexole treatment, the increased paw withdrawal thresholds of CFA-pramipexole animals compared to CFA-vehicle animals demonstrates that repeated administration of pramipexole attenuates mechanical hypersensitivity in CFA animals. In contrast to acute pramipexole treatment, there was no difference in paw withdrawal thresholds of CFA-pramipexole animals compared to saline-vehicle animals (p = 0.7947), which suggests there was a more complete attenuation of pain-like responding with repeated pramipexole treatment. There was no difference in thresholds between saline-vehicle vs. saline-pramipexole animals (p = 0.4285), demonstrating that repeated administration of pramipexole did not produce analgesic effects in saline-treated animals.
3.1.5. Experiment 1. Extended effects of repeated dopamine system agonism on mechanical hypersensitivity in rats experiencing chronic inflammatory pain
To examine the extended effects of repeated dopamine agonism treatment on pain like-behavior, we measured paw withdrawal thresholds in animals three days, one week, and two weeks after the first repeated treatment test. In contrast to the post-acute drug treatment effects, we were able to parse l-DOPA- and pramipexole-specific effects. For three-days post-repeated l-DOPA treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 26.59, p < 0.0001] and an interaction [F(1,26) = 13.37, p = 0.0011], but no effect of drug treatment (vehicle vs. l-DOPA) [F(1,26) = 0.5850, p = 0.4509] Fig. 6A. Post-hoc analysis with Tukey’s multiple comparisons test revealed the following significant differences in von Frey paw withdrawal thresholds: saline-3 days post-vehicle vs. CFA-3 days post-vehicle (p < 0.0001), saline-3 days post-vehicle vs. CFA-3 days post-l-DOPA (p = 0.0222), saline-3 days post-l-DOPA vs. CFA-3 days post-vehicle (p = 0.0015), and CFA-3 days post-vehicle vs. CFA-3 days post-l-DOPA (p = 0.0211) Fig. 6A. The significant increase in thresholds in CFA-l-DOPA animals compared to CFA-vehicle animals three days after repeated l-DOPA treatment was unexpected based on the results for paw withdrawal thresholds 1 h after acute and repeated l-DOPA treatment. However, these results indicate a potential extended beneficial effect of l-DOPA treatment on mechanical hypersensitivity, which was not evident 1 h after treatment, but was evident three days after treatment. For three-days post-repeated pramipexole treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 42.85, p < 0.0001], but no effect of drug treatment (vehicle vs. pramipexole) [F(1,26) = 3.210, p = 0.0848] or an interaction [F(1,26) = 3.427, p = 0.0755] Fig. 6B. There was not a significant difference in thresholds between CFA-pramipexole animals and CFA-vehicle animals three days after repeated pramipexole treatment (p = 0.0714), but there was an indication of a numeric, but not significant increase in thresholds in CFA-pramipexole animals compared CFA-vehicle animals Fig. 6B, which is similar to the three days post-l-DOPA results Fig. 6A.
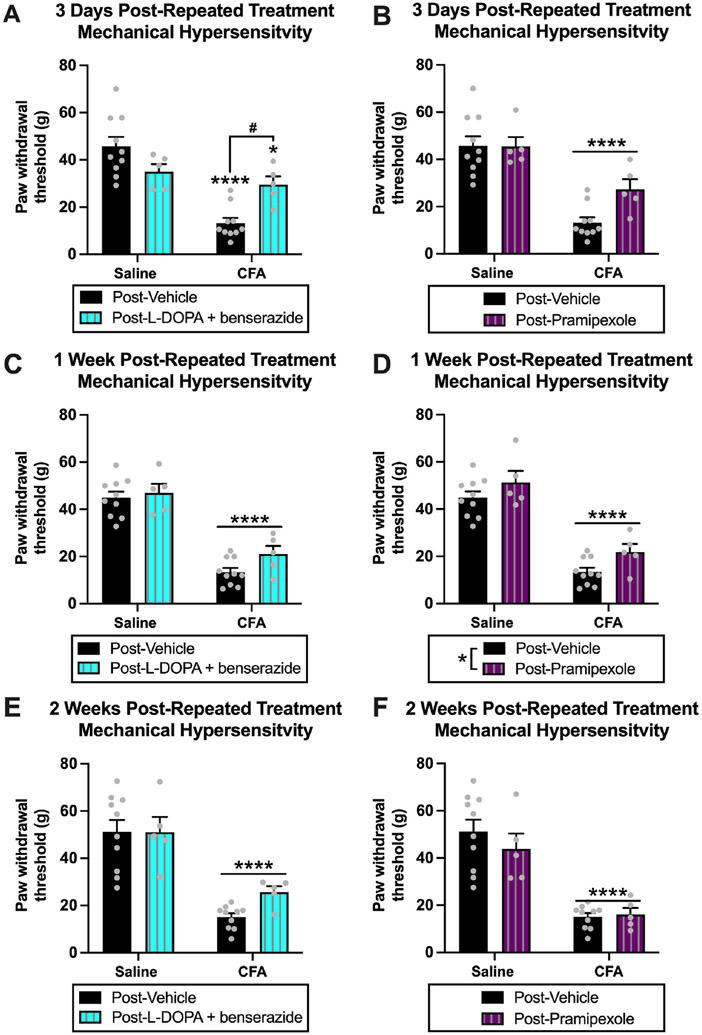
Fig. 6. Experiment 1: Extended effects of repeated dopamine system agonism on mechanical hypersensitivity in rats with chronic inflammatory pain.
(A) At three-days post-repeated l-DOPA treatment, there was an effect of CFA treatment (p < 0.0001) on paw withdrawal thresholds and an interaction (p < 0.01), but no effect of l-DOPA treatment (p > 0.05). Post hoc analysis revealed that paw withdrawal thresholds of CFA-vehicle (****p < 0.0001) and CFA-l-DOPA (*p < 0.05) animals were decreased compared to saline-vehicle animals. There was an increase in paw withdrawal thresholds of CFA-l-DOPA animals compared CFA-vehicle animals (#p < 0.05). This suggests that l-DOPA treatment increases paw withdrawal thresholds and reduces mechanical hypersensitivity in CFA animals three days after repeated administration. (B) At three-days post-repeated pramipexole treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds, but no effect of pramipexole treatment (p > 0.05). (C) At one-week post-repeated l-DOPA treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds, but no effect of l-DOPA treatment (p > 0.05). (D) At one-week post-repeated pramipexole treatment, there was an effect of CFA treatment (****p < 0.0001) and pramipexole treatment (*p < 0.05) on paw withdrawal thresholds. This suggests that pramipexole treatment increases paw withdrawal thresholds one week after repeated administration. (E) At two-weeks post-repeated l-DOPA treatment, there was an effect of CFA treatment (****p < 0.0001) on paw withdrawal thresholds, but no effect of l-DOPA treatment (p > 0.05). (F) At two-weeks post-repeated pramipexole treatment, there was an effect of CFA treatment < 0.0001) on paw withdrawal thresholds, but no effect of pramipexole treatment (p > 0.05). Bars represent means and symbols represent individual data points.
For one-week post-repeated l-DOPA treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 99.67, p < 0.0001], but no effect of drug treatment (vehicle vs. l-DOPA) [F(1,26) = 2.807, p = 0.1058] or an interaction [F(1,26) = 0.9434, p = 0.3403] Fig. 6C. For one-week post-repeated pramipexole treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 100.8, p < 0.0001] and drug treatment (vehicle vs. pramipexole) [F(1,26) = 5.916, p = 0.0222], but no interaction [F(1,26) = 0.1146, p = 0.7377] Fig. 6D, indicating the potential extended beneficial effects of repeated pramipexole treatment on reducing mechanical hypersensitivity. For two-weeks post-repeated l-DOPA treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 46.89, p < 0.0001], but no effect of drug treatment (vehicle vs. l-DOPA) [F(1,26) = 1.343, p = 0.2571] or an interaction [F(1,26) = 1.400, p = 0.2474] Fig. 6E. For two-weeks post-repeated pramipexole treatment, there was a significant effect of CFA treatment (saline vs. CFA) [F(1,26) = 49.80, p < 0.0001], but no effect of drug treatment (vehicle vs. pramipexole) [F(1,26) = 0.4958, p = 0.4876] or interaction [F(1,26) = 0.8526, p = 0.3643] Fig. 6F.
3.1.6. Experiment 2. Verification of CFA-induced mechanical hypersensitivity in rats
Our findings from experiment 1 demonstrate that both acute and repeated treatment with pramipexole attenuates pain-like behavior in rats experiencing chronic inflammatory pain after systemic administration. We conducted a follow-up experiment to examine the neurobiological effects of pramipexole treatment in CFA-treated rats. For experiment 2, we used the same parametric design and a similar timeline Fig. 1B as we did for the previous experiment. We collected brains for Western blot analysis 1 h after acute pramipexole treatment to measure the acute effects of pramipexole on presynaptic and excitatory glutamatergic signaling in pain-related brain regions. Rats were split into two equivalent groups based on baseline mechanical hypersensitivity. Paw withdrawal thresholds were measured weekly until brain collection. In the injected left hindpaw, we found a significant effect of CFA treatment (saline vs. CFA) [F(1,22) = 61.74, p < 0.0001] and interaction [F(4,88) = 9.620, p < 0.0001] for paw withdrawal thresholds, but no effect of time (baseline vs. 1 week vs. 2 weeks vs. 3 weeks vs. 4 weeks) [F (3.545,77.99) = 2.294, p = 0.0742] Supplementary Fig. 3A. Post hoc analysis with Sidak’s multiple comparisons test revealed the following significant differences in paw withdrawal thresholds between saline and CFA animals at one week (p < 0.0001), two weeks (p = 0.0005), three weeks (p < 0.0001), and four weeks (p < 0.0001), but no difference at baseline (p > 0.9999) Supplementary Fig. 3A. Rats were split into four groups (saline-vehicle, saline-pramipexole, CFA-vehicle, CFA-pramipexole) based on paw withdrawal thresholds one week after CFA and saline paw injections. With four groups, there was a significant effect of CFA treatment (saline vs. CFA) [F(3,20) = 19.83, p < 0.0001] and an interaction F(12,80) = 3.744, p = 0.0002] for paw withdrawal thresholds, but no effect of time (baseline vs. 1 week vs. 2 weeks vs. 3 weeks vs. 4 weeks) [F(3.557,71.13) = 2.266, p = 0.0778] Supplementary Fig. 3B. These results show that there was a significant difference between CFA and saline animals prior to pramipexole treatment, but no difference between drug treatment groups (saline-vehicle vs. saline-pramipexole, CFA-vehicle vs. CFA-pramipexole) prior to receiving pramipexole injections.
3.1.7. Experiment 2. Effects of pramipexole treatment on AMPA GluR1 subunit phosphorylation in the NAc, DS, VTA, CC, CeA, and PAG of rats
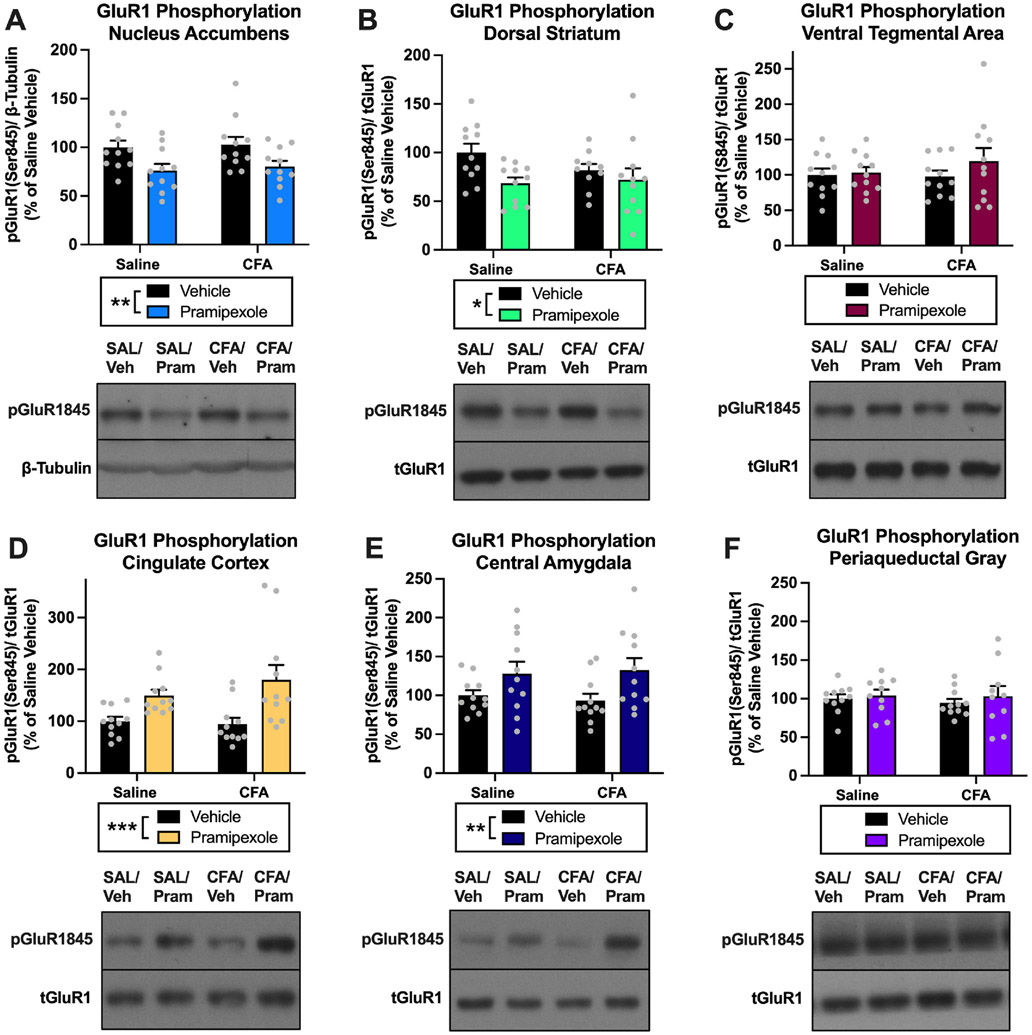
We investigated the effects of pramipexole treatment on postsynaptic glutamatergic signaling in pain-related brain regions by measuring changes in AMPA receptor (AMPAR) GluR1 subunit phosphorylation (Serine 845) in rats experiencing chronic inflammatory pain. For pGluR1845 in the NAc, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,40) = 10.95, p = 0.0020], but no effect of CFA treatment (saline vs. CFA) [F(1,40) = 0.2283, p = 0.6354] or interaction [F(1,40) = 0.0107, p = 0.9182] Fig. 7A. In the DS, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,39) = 5.686, p = 0.0221], but no effect of CFA treatment (saline vs. CFA) [F(1,39) = 0.6771, p = 0.4156] or interaction [F(1,39) = 1.558, p = 0.2194] for pGluR1845 Fig. 7B. Together these data demonstrate that pramipexole treatment decreases pGluR1845 in the NAc and DS of animals. There was no effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,40) = 1.075, p = 0.3059], CFA treatment (saline vs. CFA) [F(1,40) = 0.3694, p = 0.5468] or an interaction [F(1,40) = 0.6172, p = 0.4367] for pGluR1845 in the VTA Fig. 7C. For pGluR1845 in the CC, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,40) = 15.56, p = 0.0003], but no effect of CFA treatment (saline vs. CFA) [F(1,40) = 0.5306, p = 0.4706] or interaction [F(1,40) = 1.100, p = 0.3005] Fig. 7D. In the CeA, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,40) = 7.676, p = 0.0084], but no effect of CFA treatment (saline vs. CFA) [F(1,40) = 0.0078, p = 0.9301] or interaction [F(1,40) = 0.2177, p = 0.6433] for pGluR1845 Fig. 7E. In contrast to the NAc and DS findings, these data demonstrate that pramipexole treatment increases pGluR1845 in the CC and CeA of animals. Finally, for pGluR1845 in the PAG, there was no effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,38) = 0.6030, p = 0.4423], CFA treatment (saline vs. CFA) [F(1,38) = 0.1603, p = 0.6911], or interaction [F(1,38) = 0.0846, p = 0.7728] Fig. 7F.
Fig. 7. Experiment 2: Effects of pramipexole treatment on AMPA receptor phosphorylation in the NAc, DS, VTA, CC, CeA, and PAG of rats.
(A) Pramipexole treatment decreased pGluR1845 in the NAc (**p < 0.01). (B) Pramipexole treatment decreased pGluR1845 in the DS (**p < 0.01). (C) Pramipexole treatment had no effect on pGluR1845 in the VTA (p > 0.05). (D) Pramipexole treatment increased pGluR1845 in the CC (***p < 0.001). (E) Pramipexole treatment increased pGluR1845 in the CeA (**p < 0.01). (F) Pramipexole treatment had no effect on pGluR1845 in the PAG (p > 0.05). Bars represent means and symbols represent individual data points.
3.1.8. Experiment 2. Effects of pramipexole treatment on synapsin phosphorylation in the NAc, DS, VTA, CC, CeA, and PAG of rats
We next investigated the effects of pramipexole treatment on a marker of presynaptic activity in pain- and reward-related brain regions by measuring changes in synapsin phosphorylation (Serine 9) in rats with a history of chronic inflammatory pain. For pSynapsin in the NAc, there was a significant interaction [F(1,39) = 6.399, p = 0.0156], but no effect of pramipexole treatment [F(1,39) = 3.627, p = 0.0642] or CFA treatment (saline vs. CFA) [F(1,39) = 0.6309, p = 0.4318] Fig. 8A. Post-hoc analysis of the interaction effect with Sidak’s multiple comparisons test revealed a significant increase in pSynapsin for saline-vehicle vs. saline-pramipexole animals (p = 0.0059), but no difference in pSynapsin between CFA-vehicle vs. CFA-pramipexole (p = 0.8876) Fig. 8A. In the DS, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,39) = 17.03, p = 0.0002], but no effect of CFA treatment (saline vs. CFA) [F(1,39) = 0.0104, p = 0.9192] or interaction [F(1,39) = 0.0046, p = 0.9464] Fig. 8B for pSynapsin, demonstrating an increase in pSynapsin with pramipexole treatment in the DS. For pSynapsin in the VTA, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,40) = 5.826, p = 0.0205], but no effect of CFA treatment (saline vs. CFA) [F(1,40) = 1.471, p = 0.2324], or an interaction [F(1,40) = 0.0235, p = 0.8789] Fig. 8C, demonstrating increased pSynapsin in the VTA with pramipexole treatment. In the CC, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,37) = 4.984, p = 0.0317], but no effect of CFA treatment (saline vs. CFA) [F(1,37) = 0.0989, p = 0.7549] or interaction [F(1,37) = 0.4307, p = 0.5157] Fig. 8D, demonstrating increased pSynapsin in the CC with pramipexole treatment. There was also a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,39) = 5.611, p = 0.0229], but no effect of CFA treatment (saline vs. CFA) [F (1,39) = 0.8953, p = 0.3499] or interaction [F(1,39) = 0.2887, p = 0.5941] for pSynapsin in the CeA Fig. 8E, demonstrating increased pSynapsin in the CeA with pramipexole treatment. For pSynapsin in the PAG, there was a significant effect of pramipexole treatment (vehicle vs. pramipexole) [F(1,37) = 6.074, p = 0.0185], but no effect of CFA treatment (saline vs. CFA) [F(1,37) = 0.9646, p = 0.3324] or interaction [F(1,37) = 1.159, p = 0.2887] Fig. 8F.
Fig. 8. Experiment 2: Effects of pramipexole treatment on synapsin phosphorylation in the NAc, DS, VTA, CC, CeA, and PAG of rats.
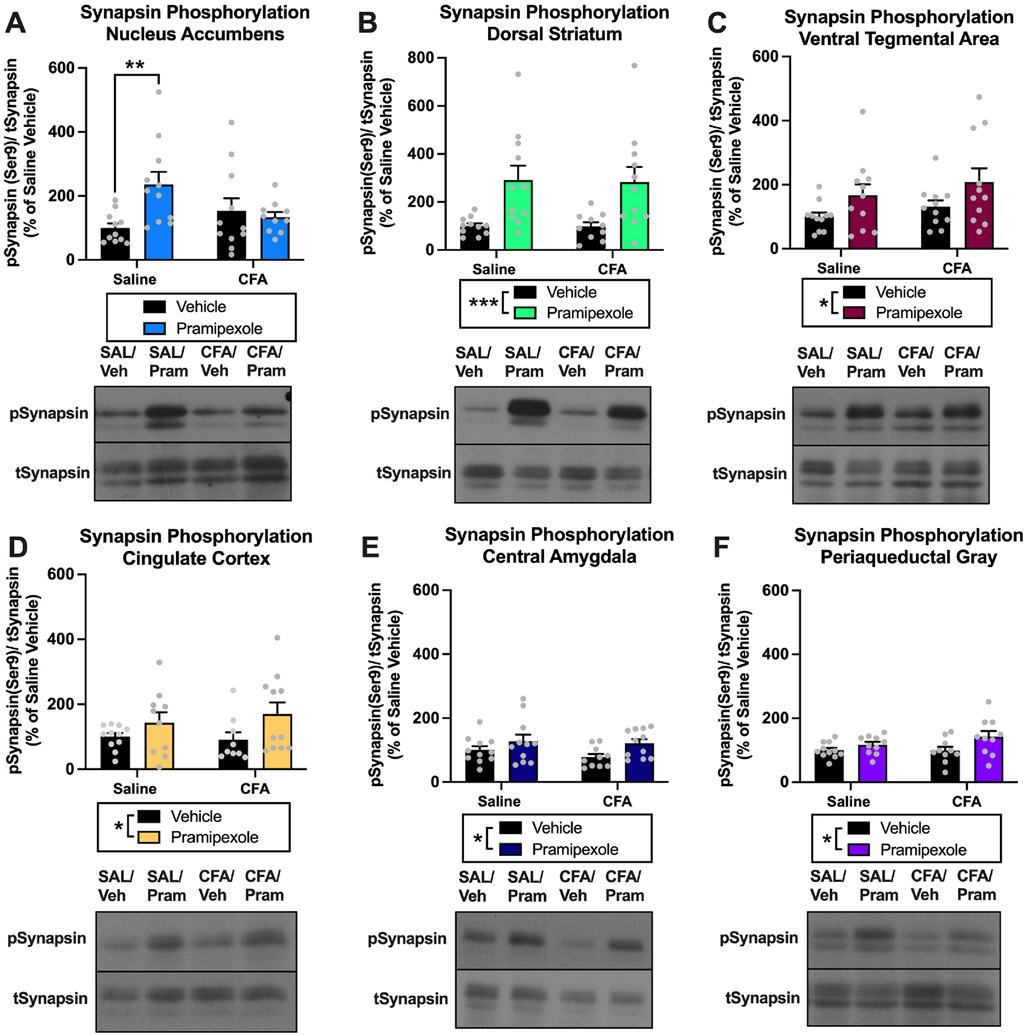
(A) There was a significant interaction between CFA treatment and pramipexole treatment on pSynapsin in the NAc (p < 0.05). Post hoc analysis revealed a significant increase in pSynapsin of saline-pramipexole animals compared to saline-vehicle animals (**p < 0.01), but no difference in pSynapsin between CFA-vehicle and CFA-pramipexole animals (p > 0.05). (B) Pramipexole treatment increased pSynapsin in the DS (***p < 0.001). (C) Pramipexole treatment increased pSynapsin in the VTA (*p < 0.05). (D) Pramipexole treatment increased pSynapsin in the CC (*p < 0.05). (E) Pramipexole treatment increased pSynapsin in the CeA (*p < 0.05). (F) Pramipexole treatment increased pSynapsin in the PAG (*p < 0.05). Bars represent means and symbols represent individual data points.
3.1.9. Experiment 3. Effects of pramipexole treatment on the intrinsic excitability of NAc neurons in rats with a history of chronic inflammatory pain
To follow up on the blunted pSynapsin response in the NAc of CFA animals after pramipexole treatment, we next examined the functional implications of blunted presynaptic activity onto NAc neurons in CFA animals following pramipexole treatment. Specifically, we used ex vivo electrophysiology to examine the effects of pramipexole treatment on the intrinsic excitability of NAc neurons in both saline and CFA animals. In cells from saline-treated animals, we found a significant increase in the rheobase current of NAc neurons following bath application of pramipexole [t(7) = 3.129, p = 0.0166], demonstrating decreased intrinsic excitability Fig. 9A & C. In contrast, there was no change in the rheobase current of NAc neurons following bath application of pramipexole in cells from CFA-treated animals [t(6) = 1.260, p = 0.2545] Fig. 9B & D. There was no difference in the rheobase current of NAc neurons between saline-treated and CFA-treated animals prior to pramipexole treatment [t(13) = 1.278, p = 0.2236]. No significant difference was observed in the resting membrane potential of NAc neurons following pramipexole treatment in saline-treated (no drug: −72.7 ± 4.7 mV, pramipexole: −78.6 ± 3.1 mV; [t(7) = 1.556, p = 0.1637]) or CFA-treated animals (no drug: −73.8 ± 4.8 mV, pramipexole: −75.1 ± 4.1 mV; [t(6) = 0.7182, p = 0.4997]). Additionally, no significant difference was observed in input resistance of NAc neurons following pramipexole treatment in saline-treated (no drug: 124.0 ± 23.0 MΩ, pramipexole: 104.7 ± 16.7 MΩ; [t(7) = 1.129, p = 0.2960]) or CFA-treated animals (no drug: 194.8 ± 65.7 MΩ, pramipexole: 203.4 ± 73.8 MΩ; [t(6) = 0.3757, p = 0.7201]).
Fig. 9. Experiment 3. Effects of pramipexole treatment on the intrinsic excitability of the NAc neurons in saline rats and rats with chronic inflammatory pain.
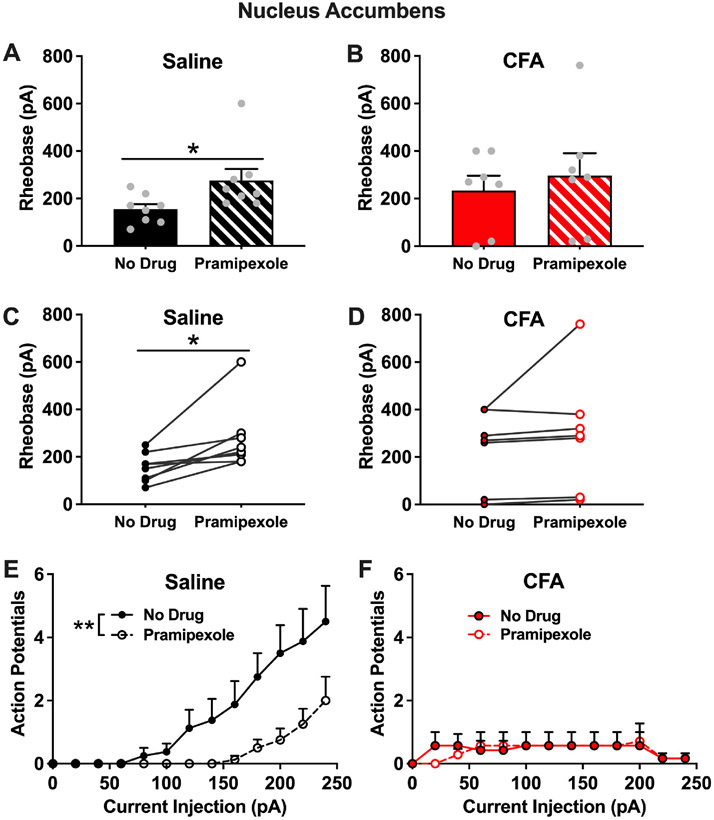
(A). In NAc neurons from saline animals, there was a significant increase in rheobase current after bath application of pramipexole (*p < 0.05). (B). In NAc neurons from CFA animals, there was no change in rheobase current after bath application of pramipexole (p > 0.05). (C) Before and after plot of NAc rheobase current in saline animals following bath application of pramipexole (*p < 0.05). (D) Before and after plot of NAc rheobase current in CFA animals following bath application of pramipexole (p > 0.05). (E) In NAc neurons from saline animals, bath application of pramipexole reduced the number of action potentials generated by varying current injections (**p < 0.05). (F) In NAc neurons from CFA animals, bath application of pramipexole had no effect on the number of action potentials generated by varying current injections (p > 0.05).
We also examined the number of action potentials fired in response to varying current injections before and after bath application of pramipexole. In cells from saline-treated animals, there was a significant effect of pramipexole treatment [F(1,14) = 10.21, p = 0.0065] and current injection [F(2.092,29.29) = 13.15, p < 0.0001] on the number of action potentials as well as an interaction effect [F(12,168) = 3.302, p = 0.0003] Fig. 9E. These findings show that the number of action potentials increase with increasing current injections and that bath application of pramipexole reduces the number of action potentials in saline animals. In cells from CFA animals, there was no effect of pramipexole treatment [F(1,12) = 0.004, p = 0.9515] or current injection [F(1.368,15.97) = 1.864, p = 0.1920] on the number of action potentials and there was no interaction [F(12,140) = 0.6283, p = 0.8154] Fig. 9F. These findings show that the number of action potentials does not increase with increasing current injections and that bath application of pramipexole does not change the number of action potentials in CFA-treated animals. Due to the blunted response of cell firing with increasing current injections in CFA-treated animals compared to saline-treated animals, we completed a follow up three-way repeated measures ANOVA of the action potential data. With this analysis, we found a significant three-way interaction of current x (saline vs. CFA) x (no drug vs. pramipexole) [F(12,308) = 2.866, p = 0.0009] Fig. 9E & F.
4. Discussion
The present study was designed to test the effects of dopaminergic medications on chronic inflammatory pain at both dopamine biosynthesis and dopamine receptor levels using clinically available therapeutics, l-DOPA and pramipexole. For this study, we tested the hypothesis that acute and repeated dopamine agonist treatment would attenuate mechanical hypersensitivity in rats with chronic inflammatory pain as measured by a significant increase in von Frey thresholds, indicating decreased hyperalgesia-like behavior. We also examined the effects of pramipexole treatment on glutamatergic and presynaptic signaling in pain-related brain regions including the NAc, DS, VTA, CC, CeA, and PAG. To examine the functional implications of blunted presynaptic signaling in the NAc of CFA animals, we used ex vivo electrophysiology to examine the effects of pramipexole on the intrinsic excitability of NAc neurons in CFA- and saline-treated animals.
Acute pain activates dopamine neurotransmission in a similar manner to drugs of misuse including opioids (Elman and Borsook, 2016; Scott et al., 2006; Wood et al., 2007b). In contrast, chronic pain can promote a hypodopaminergic and reward deficiency state in the mesolimbic dopamine system comparable to repeated drug use (Borsook et al., 2016; Hipolito et al., 2015; Martikainen et al., 2015; Wood et al., 2007b). l-DOPA is a biosynthetic precursor to dopamine, which is used primarily as a dopamine replacement agent that crosses the blood-brain barrier (BBB) to treat motor symptoms associated with hypodopaminergic states in Parkinson’s Disease. Consistent with our study design, l-DOPA is typically co-administered with a dopamine decarboxylase inhibitor to prevent peripheral breakdown of the drug and to allow more of the drug to cross the BBB. Interestingly, there is evidence of centrally generated increases in pain in Parkinson’s Disease that is attenuated with l-DOPA treatment (Aguirre-Vidal et al., 2020; Blanchet and Brefel-Courbon, 2018; Canavero, 2009; Defazio et al., 2008; Gerdelat-Mas et al., 2007; Schestatsky et al., 2007), which further supports the role of mesolimbic dopamine regulation in pain processing. However, in our model of chronic inflammatory pain, we found that neither acute nor repeated administration of l-DOPA significantly reduced mechanical hypersensitivity in CFA-treated animals immediately (1 h) after drug treatment. These results demonstrate that administration of l-DOPA at 50 mg/kg does not attenuate pain-like behavior in CFA animals or produce analgesia in our control animals. It is a possible that higher doses of l-DOPA may be needed to reduce pain-like behavior in animals with chronic inflammatory pain. At time points consistent with those used in our study, previous investigations found pain-reducing effects of l-DOPA at higher doses (70–200 mg/kg) in animals with neuropathic pain (Cobacho et al., 2010; Park et al., 2013) and visceral pain (Okumura et al., 2016). In contrast, lower doses of l-DOPA (3–25 mg/kg) were sufficient to reduce pain-related symptoms in Parkinsonian animals (Aguirre-Vidal et al., 2020).
One week after the last acute treatment test, we found an unexpected and significant reduction in mechanical hypersensitivity, indicating that either l-DOPA, pramipexole, or an additive combination of the two drugs reduced pain-like behavior at this extended time point. We sur-mised that this extended effect at one week was unlikely to be due to l-DOPA treatment when we were able to parse apart the drug-specific effects following the repeated treatment test. However, at the earlier time point, we found reduced mechanical hypersensitivity in animals with chronic inflammatory pain three days following repeated l-DOPA treatment. This unexpected effect indicates that l-DOPA may have an extended effect that results in a reduction in pain-like behavior a few days after treatment. This extended effect seems to partially disappear by one- and two-weeks post-treatment. These findings suggest that l-DOPA may produce an extended and modest effect on mechanical hypersensitivity in our model of chronic inflammatory pain. Further investigations will be needed to examine the time course of these extended effects and if drug dose or dosing regimen can impact the effects of l-DOPA on pain-like behavior.
As either a cause or consequence of hypodopaminergic states, functional abnormalities in presynaptic dopamine release and dopamine D2/3 receptor (D2/3R) signaling have been reported in chronic pain (Taylor et al., 2016). Pramipexole is a full dopamine receptor agonist with a selective affinity for D2/3Rs. It is a parkinsonian treatment drug that is also widely used for the treatment of restless leg syndrome. Pramipexole has replaced l-DOPA as the primary treatment for restless leg syndrome, because of its good absorption, limited presystemic metabolism, and limited long-term side effects compared to other dopamine agonist medications (Benbir and Guilleminault, 2006). Like Parkinson’s Disease, enhanced pain sensitivity has been reported in restless leg syndrome (Edwards et al., 2011; Hoogwout et al., 2015). There is some clinical evidence that pain reported in restless leg syndrome is attenuated with dopamine agonist treatments including both l-DOPA and pramipexole (Winkelmann et al., 2018). Pramipexole has been used to reduce pain in fibromyalgia patients and has potential efficacy in the treatment of burning mouth syndrome as well as neuropathic pain that is co-morbid spinal cord injuries (Holman and Myers, 2005; Kumru et al., 2016; Stuginski-Barbosa et al., 2008). In the current study, we found that acute and repeated administration of pramipexole reduced mechanical hypersensitivity in animals with chronic inflammatory pain 1 h after drug treatment. Despite a reduction in mechanical hypersensitivity in CFA animals following acute pramipexole treatment, this group was still significantly different from saline animals given vehicle, indicating that there wasn’t a complete attenuation of pain-like responding with acute treatment. In contrast to acute treatment, repeated pramipexole treatment completely attenuated pain-like behavior in CFA animals. With saline animals there was no change in mechanical hypersensitivity with pramipexole treatment, indicating no change in pain-like responding in animals without pain. Together these results indicate that acute and repeated administration of pramipexole produces anti-hyperalgesic effects, attenuates pain-like behavior in CFA animals, but has no analgesic effects in saline animals.
The significant reduction in mechanical hypersensitivity one week after the last acute treatment test was most likely due to pramipexole treatment, because a similar decrease was observed when we were able parse apart the drug-specific effects following repeated treatment testing. This finding suggests potential extended effects of pramipexole treatment on reducing mechanical hypersensitivity, which returns to baseline by two weeks. Our finding that pramipexole attenuates hyperalgesia-like behavior in the context of chronic inflammatory pain contributes to the growing evidence of pramipexole’s efficacy in pain treatment. These data are consistent with other preclinical studies demonstrating pramipexole’s pain reducing/preventing effects in rodent models of fibromyalgia, Parkinson’s Disease, and peripheral nerve injury (Nagakura et al., 2009; Ren et al., 2016; Romero-Sánchez et al., 2020). In addition to highlighting the efficacy of pramipexole in the treatment of chronic inflammatory pain, our findings provide evidence that a repeated dosing regimen may offer superior pain relief compared to acute treatment. Our time course data also provides evidence that pramipexole treatment provides some extended effects on analgesia-like behavior. Further investigations will be needed to examine the short-term and extended behavioral effects of different dosing regimens with pramipexole and other dopamine system modulators.
In a follow-up experiment, we examined the effects of pramipexole treatment on glutamatergic and presynaptic signaling in pain- and reward-related brain areas. These brain regions were chosen based on evidence that the chronification of pain involves a shift from sensory brain structures to limbic/motivational brain circuitry (Apkarian et al., 2013; Thompson and Neugebauer, 2019). These brain regions were also chosen based on our previously published examinations of the neurobiological correlates of pain avoidance-like behavior (Pahng et al., 2017). For glutamatergic signaling, we chose to examine pGluR1S845 in the NAc, DS, VTA, CC, CeA, and PAG. Serine 845 is the PKA site for AMPAR phosphorylation of GluR1, which enhances excitatory currents by increasing channel open time probability, regulating receptor traf-ficking, and promoting insertion of the receptor into the membrane (Blackstone et al., 1994; Oh et al., 2006; Roche et al., 1996). Accordingly, we interept an increase or decrease in pGluR1845 as an increase or decrease in postsynaptic excitatory currents mediated by AMPARs, respectively. In the synapses of NAc neurons, time-dependent increases in pGluR1845 have been reported in neuropathic and inflammatory pain (Goffer et al., 2013; Su et al., 2015). In our study, we did not find a CFA-induced change in pGluR1845 in our whole cell NAc or DS samples. We did, however, discover that pramipexole treatment decreased postsynaptic excitatory currents mediated by AMPARs in the NAc and DS of animals. It is possible that the pramipexole-induced decreases in striatal postsynaptic excitatory currents may contribute to anti-hyperalgesic effects observed in CFA animals. Consistent with this interpretation, authors from a study measuring the anti-hyperalgesic effects of a tricyclic antidepressant medication, desipramine, found that reduced mechanical hypersensitivity in neuropathic pain was associated with decreased pGluR1845 in the NAc (Mitsi et al., 2015). We found no effect of pramipexole treatment on pGluR1845 in the VTA or PAG.
In contrast to our striatal findings, we found that pramipexole treatment increased postsynaptic excitatory currents mediated by AMPARs in the CC and CeA of animals. This data provides a limitation to the interpretation that pramipexole reduces pain-like behavior through widespread reductions in AMPAR activity in pain- and reward-related brain regions. In the literature, there is some evidence that increased AMPAR activity in the CC and CeA is associated with increases in pain sensitivity. In the CeA, modulation of AMPAR activity regulates pain responses during a tail-flick test with AMPAR agonism and antagonism increasing and decreasing pain-like behavior, respectively (Cai et al., 2020). In a study examining opioid-induced hyperalgesia, pGluR1845 was acutely increased in the CC after the development of hyperalgesia, but returned to baseline levels within 24 h (Zeng et al., 2018). It is possible that the increased AMPAR activity in the CC and CeA is a compensatory response to reduced AMPAR activity in striatal brain areas. In future investigations, circuit-based approaches would need to be employed to examine this hypothesis. Together, these findings demonstrate that pramipexole treatment has a bidirectional effect on AMPAR activity in pain- and reward-related brain regions. Future investigations will be needed to examine the potential behavioral implications of this bidirectional effect on both alternative pain measures (e. g., thermal, affective) and non-pain measures (e.g., anxiety, motivation, memory, attention). Additionally, site specific approaches would be needed to examine the brain region specific effects of pramipexole on glutamatergic signaling.
As a general marker of presynaptic activity, we chose to measure synapsin phosphorylation at serine 9, a site regulated by PKA and CaMKII (Czernik et al., 1987; Huttner and Greengard, 1979). Phos-phorylation of serine 9 is associated with a facilitation of presynaptic neurotransmitter release (Chi et al., 2001; Hosaka et al., 1999). Accordingly, we interpret an increase or decrease in pSynapsin as a general increase or decrease in presynaptic neurotransmitter release, respectively. We discovered that pramipexole treatment increased synapsin phosphorylation in the DS, VTA, CC, CeA, and PAG. These data demonstrate that pramipexole treatment increases presynaptic signaling across multiple pain-related brain regions. In future studies, we will examine the potential behavioral implications of increased presynaptic signaling from pramipexole treatment on alternative pain and non-pain behavioral measures. Future investigations will be needed to examine which specific neurotransmitter systems (e.g., dopamine, glutamate, acetylcholine, serotonin etc.) are more impacted by increased presynaptic activity following pramipexole treatment.
In the NAc, we discovered that pramipexole treatment only increased presynaptic neurotransmitter release in saline-treated animals, while this increase was completely blunted in CFA-treated animals. These findings suggest that there is impaired presynaptic function in the NAc of animals with chronic inflammatory pain in response to D2/3R activation with pramipexole. Consistent with our findings, reduced presynaptic release of both dopamine and glutamate in the NAc have been reported with chronic pain conditions (Jääskeläinen et al., 2001; Qi et al., 2018; Taylor et al., 2016; Wood et al., 2007a). Our findings provide novel mechanistic evidence that chronic nociceptive system stim-ulation induces neuroadaptations in the presynaptic vesicle release machinery of NAc neurons. There is normally a complex interplay of dopamine, glutamate, and other neurotransmitter systems involved in regulating presynaptic release within the NAc. In order to examine the functional impact of impaired presynaptic signaling in the NAc with chronic inflammatory pain, we next examined the effects of pramipexole treatment on the intrinsic excitability of NAc neurons in saline- and CFA-treated animals.
Using ex vivo electrophysiology, we discovered that pramipexole treatment increases rheobase current and decreases the number of action potentials generated from current injections in saline-treated animals. In contrast, we found that pramipexole did not change rheobase current or the number of action potentials generated from current injections in animals with a history of chronic inflammatory pain. Additionally, we found that current injection in the NAc of CFA-treated animals did not increase the number of action potentials as expected. Together, our data demonstrates that pramipexole treatment reduces the intrinsic excitability of NAc neurons in saline-treated animals, but not CFA-treated animals as measured by rheobase current and the number of action potentials. Our findings indicate that there exist impaired dopamine D2/3R function and general functional abnormalities in the NAc of animals with chronic inflammatory pain. These findings are consistent with the literature demonstrating functional abnormalities in D2/3R signaling in chronic pain conditions (Taylor et al., 2016). In a PET scan study, non-neuropathic chronic back pain patients had impaired D2/3R binding in the NAc compared to controls (Martikainen et al., 2015). During a pain challenge in this study, there was a lower amount of dopamine release and subsequent D2/3R activation in chronic back pain patients compared to controls. Consistent with this work in human subjects, decreases in NAc D2R levels have been reported following in-duction of chronic pain in rodents (Chang et al., 2014; Sagheddu et al., 2015). In a separate study, ex vivo administration of sulpiride (D2/3R antagonist) in brain slices increased intrinsic NAc excitability in healthy animals but had no effect on excitability in animals with a peripheral nerve injury (Ren et al., 2016). This is consistent with our data showing D2/3R agonism with pramipexole decreases the intrinsic excitability of NAc in saline-treated animals but has no effect on the intrinsic excitability of NAc in the context of chronic inflammatory pain. These combined findings demonstrate that impairment of D2/3R function in the NAc is consistent across different pain modalities (i.e., Inflammatory vs. neuropathic pain) in rodents. Our data provides new evidence of impaired responsiveness of NAc neurons to D2/3R agonism and action potential-generating current in animals with a history of chronic inflammatory pain.
In conclusion, the present study highlights the efficacy of pramipexole in the treatment of mechanical hypersensitivity associated with chronic inflammatory pain. We found that both acute and repeated treatment with pramipexole reduced pain-like behavior in animals but did not produce analgesic effects in pain-free control animals. These results are clinically relevant to investigations into new treatment op-tions for chronic inflammatory pain using medications that are currently approved for use in humans. Our findings also provide critical insight into the neurobiological effects of pramipexole treatment on glutamatergic and presynaptic signaling in animals with and without chronic inflammatory pain. We found that pramipexole treatment reduces GluR1 phosphorylation in the NAc and DS but increases this marker of glutamatergic activity in the CC and CeA. We discovered that pramipexole treatment increases synapsin phosphorylation (marker of presynaptic vesicle release) in the DS, VTA, CC, CeA, and PAG. Pramipexole also increased pSynapsin in the NAc of saline animals but not CFA-treated animals, indicating blunted presynaptic vesicle release in the NAc of CFA-treated animals following pramipexole treatment. We discovered that pramipexole treatment reduced the intrinsic excitability of NAc neurons in saline-treated animals, but not in animals with a history of pain. Consistent with our molecular findings, this demonstrates a blunted response of NAc neurons to pramipexole. Altogether these findings indicate alterations in D2/3R signaling in the NAc of animals with a history of chronic pain in association with the antihyperalgesic effects of pramipexole treatment.
Supplementary Material
Acknowledgements
This work was generously supported by Department of Veterans Affairs Biomedical Laboratory Research and Development Service Grants IK2 BX004334 (ARP) and I01 BX003451 (NWG), and by National Institutes of Health Grants R01 AA025996 (SE), R01 AA023305 (NWG), and U01 AA028709 (NWG).
Footnotes
Author disclosure statement
NWG owns shares in Glauser Life Sciences, Inc, which is a start-up company with interest in the development of therapeutics for treatment of mental illness. The remaining authors declare no competing financial interests or potential conflicts of interest.
CRediT authorship contribution statement
Scott Edwards: Funding acquisition, provided funding, designed the study, and processed brains for western blot experiments. Chelsea N. Callicoatte: performed western blot experiments. Angela E. Barattini: performed western blot experiments. Jessica A. Cucinello-Ragland: Project administration, performed western blot experiments and administered CFA/saline injections for electrophysiology experiments. Alex Melain: aided in injections and tissue collection for western blot experiments. Kimberly N. Edwards: Project administration, administered injections for western blot experiments and processed brains for western blot experiments. Nicholas W. Gilpin: Funding acquisition, provided funding. Elizabeth M. Avegno: designed electrophysiology experiments, performed the electrophysiology experiments, and analyzed electrophysiology data. Amanda R. Pahng: Funding acquisition, Formal analysis, Writing – original draft, designed the study, provided funding, performed CFA/saline injections for behavior and western blotting experiments, performed behavior experiments, collected tissue for western blot experiments, processed brains for western blot experiments, analyzed data, wrote the manuscript, and revised the manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2022.108976.
References
- Aguirre-Vidal Y, Rodríguez-Ramos C, Mendieta L, Romero-Sánchez H, Garza-Mouriño G, Benítez-Díaz Mirón M, Castellanos-Páez M, Pérez-Ramos J, Godínez-Chaparro B, 2020. Synergistic antiallodynic and antihyperalgesic interaction between L-DOPA and celecoxib in parkinsonian rats is mediated by NO-cGMP-ATP-sensitive K + channel. Eur. J. Pharmacol 889 10.1016/j.ejphar.2020.173537. [DOI] [PubMed] [Google Scholar]
- Antinori S, Fattore L, Saba P, Fratta W, Gessa G, Devoto P, 2018. Levodopa prevents the reinstatement of cocaine self-administration in rats via potentiation of dopamine release in the medial prefrontal cortex. Addiction Biol. 23 (2) 10.1111/adb.12509. [DOI] [PubMed] [Google Scholar]
- Apkarian A, Baliki M, Farmer M, 2013. Predicting transition to chronic pain. Curr. Opin. Neurol 26 (4) 10.1097/WCO.0b013e32836336ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avegno E, Middleton J, Gilpin N, 2019. Synaptic GABAergic transmission in the central amygdala (CeA) of rats depends on slice preparation and recording conditions. Physiol. rep 7 (19) 10.14814/phy2.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV, 2012. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci 15 (8), 1117–1119. 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Borsook D, 2008. Signal valence in the nucleus accumbens to pain onset and offset. Eur. J. Pain 12 (7), 866–869. 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbir G, Guilleminault C, 2006. Pramipexole: new use for an old drug - the potential use of pramipexole in the treatment of restless legs syndrome. Neuropsychiatric Dis. Treat 2 (4), 393–405. 10.2147/nedt.2006.2.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C, Murphy T, Moss S, Baraban J, Huganir R, 1994. Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J. Neurosci 14 (12) 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet P, Brefel-Courbon C, 2018. Chronic pain and pain processing in Parkinson’s disease. Prog. neuro-psychopharmacol. biol. psych 87 (Pt B) 10.1016/j.pnpbp.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I, 2016. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev 68, 282–297. 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Cai Y, Hou Y, Pan Z, 2020. GluA1 in central amygdala increases pain but inhibits opioid withdrawal-induced aversion. Mol. Pain 16. 10.1177/1744806920911543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavero S, 2009. Central pain and Parkinson disease. Arch. Neurol 66 (2) 10.1001/archneurol.2008.545. [DOI] [PubMed] [Google Scholar]
- Chang PC, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV, 2014. Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 155 (6), 1128–1139. 10.1016/j.pain.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA, 2001. Synapsin dispersion and reclustering during synaptic activity. Nat. Neurosci 4 (12), 1187–1193. 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- Cicero T, Ellis M, Harney J, 2015. Shifting patterns of prescription opioid and heroin Abuse in the United States. N. Engl. J. Med 373 (18) 10.1056/NEJMc1505541. [DOI] [PubMed] [Google Scholar]
- Cobacho N, De la Calle J, González-Escalada J, Paíno C, 2010. Levodopa analgesia in experimental neuropathic pain. Brain Res. Bull 83 (6) 10.1016/j.brainresbull.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Czernik A, Pang D, Greengard P, 1987. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc. Natl. Acad. Sci. U. S. A 84 (21) 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C, 2018. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb. Mortal. Wkly. Rep 67 (36) 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defazio G, Berardelli A, Fabbrini G, Martino D, Fincati E, Fiaschi A, Moretto G, Abbruzzese G, Marchese R, Bonuccelli U, Del Dotto P, Barone P, De Vivo E, Albanese A, Antonini A, Canesi M, Lopiano L, Zibetti M, Nappi G, Martignoni E, Lamberti P, Tinazzi M, 2008. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch. Neurol 65 (9) 10.1001/archneurol.2008.2. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich T, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA 315 (15). 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Quartana P, Allen R, Greenbaum S, Earley C, Smith M, 2011. Alterations in pain responses in treated and untreated patients with restless legs syndrome: associations with sleep disruption. Sleep Med. 12 (6) 10.1016/j.sleep.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, 2016. Common brain mechanisms of chronic pain and addiction. Neuron 89 (1). 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, Ory-Magne F, Slaoui T, Rascol O, Brefel-Courbon C, 2007. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J. Neurol. Neurosurg. Psychiatry 78 (10). 10.1136/jnnp.2007.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffer Y, Xu D, Eberle S, D’amour J, Lee M, Tukey D, Froemke R, Ziff E, Wang J, 2013. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J. Neurosci 33 (48) 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D, McGee S, 2011. Pain as a global public health priority. BMC Publ. Health 11. 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño A, Warner M, 2020. Drug overdose deaths in the United States, 1999-2019. NCHS data brief(394). https://pubmed-ncbi-nlm-nih-gov.ezproxy.lsuhsc.edu/33395384/. [PubMed] [Google Scholar]
- Hipolito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Moron JA, 2015. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area mu opioid receptors. J. Neurosci 35 (35), 12217–12231. 10.1523/jneurosci.1053-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman A, Myers R, 2005. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 52 (8) 10.1002/art.21191. [DOI] [PubMed] [Google Scholar]
- Hoogwout S, Paananen M, Smith A, Beales D, O’Sullivan P, Straker L, Eastwood P, McArdle N, Champion D, 2015. Musculoskeletal pain is associated with restless legs syndrome in young adults. BMC Muscoskel. Disord 16 10.1186/s12891-015-0765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman J, Helmick C, Barbour K, Theis K, Boring M, 2016. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable Activity limitation among US adults, 2015-2040. Arthritis Rheumatol. 68 (7) 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer R, Südhof T, 1999. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24 (2). 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Huttner W, Greengard P, 1979. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc. Natl. Acad. Sci. U. S. A 76 (10) 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (Us) Committee on Advancing Pain Research, C., and Education, 2011. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. 10.17226/13172. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen S, Rinne J, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J, 2001. Role of the dopaminergic system in chronic pain – a fluorodopa-PET study. Pain 90 (3). 10.1016/S0304-3959(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Kaye A, Cornett E, Hart B, Patil S, Pham A, Spalitta M, KF M, 2018. Novel pharmacological nonopioid therapies in chronic pain. Curr. Pain Headache Rep 22 (4) 10.1007/s11916-018-0674-8. [DOI] [PubMed] [Google Scholar]
- Kumru H, Albu S, Vidal J, Barrio M, Santamaria J, 2016. Dopaminergic Treatment of Restless Legs Syndrome in Spinal Cord Injury Patients with Neuropathic Pain. Spinal Cord Series and Cases, 2. 10.1038/scsandc.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, Halonen P, Takala J, 2001. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 89 (2–3). 10.1016/s0304-3959(00)003614. [DOI] [PubMed] [Google Scholar]
- Martikainen I, Nuechterlein E, Peciña M, Love T, Cummiford C, Green C, Stohler C, Zubieta J, 2015. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J. Neurosci 35 (27) 10.1523/JNEUROSCI.4605-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E, Di Pietro F, Alshelh Z, Peck C, Murray G, Vickers E, Henderson L, 2018. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J. Neurosci 38 (2) 10.1523/JNEUROSCI.1647-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsi V, Terzi D, Purushothaman I, Manouras L, Gaspari S, Neve R, Stratinaki M, Feng J, Shen L, Zachariou V, 2015. RGS9-2–controlled adaptations in the striatum determine the onset of action and efficacy of antidepressants in neuropathic pain states. Proc. Natl. Acad. Sci. U. S. A 112 (36) 10.1073/pnas.1504283112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura Y, Oe T, Aoki T, Matsuoka N, 2009. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain 146 (1–2), 26–33. 10.1016/j.pain.2009.05.024. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health, 2020. Key Subtance Use and Mental Health Indicators in the United States: Results from the 2019 National Survery on Drug Use and Health. [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F, 2012. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. U. S. A 109 (50), 20709–20713. 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, 2015. Amygdala pain mechanisms. Handb. Exp. Pharmacol 227 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M, Derkach V, Guire E, Soderling T, 2006. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem 281 (2) 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Okumura T, Nozu T, Kumei S, Takakusaki K, Miyagishi S, Ohhira M, 2016. Levodopa acts centrally to induce an antinociceptive action against colonic distension through activation of D2 dopamine receptors and the orexinergic system in the brain in conscious rats. J. Pharmacol. Sci 130 (2) 10.1016/j.jphs.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Ong W, Stohler C, Herr D, 2019. Role of the prefrontal cortex in pain processing. Mol. Neurobiol 56 (2) 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng A, Edwards S, 2021. The convergent neuroscience of affective pain and substance use disorder. Alcohol Res. 41 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Edwards KN, Edwards S, 2017. Neurobiological correlates of pain avoidance-like behavior in morphine-dependent and non-dependent rats. Neuroscience 366, 1–14. 10.1016/j.neuroscienee.2017.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Farooq MA, Edwards KN, Edwards S, 2019. Dysregulation of c-jun N-terminal kinase phosphorylation in alcohol dependence. Alcohol 75. 10.1016/j.alcohol.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Joo H, Kim Y, Kwon O, Lee J, Kim E, Moon D, 2013. Anti-allodynic effects of levodopa in neuropathic rats. Yonsei Med. J 54 (2) 10.3349/ymj.2013.54.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The Rat Brain in Stereotaxic Coordinates, fourth ed. Academic Press, San Diego. [Google Scholar]
- Qi C, Guo B, Ren K, Yao H, Wang M, Sun T, Cai G, Liu H, Li R, Luo C, Wang W, Wu S, 2018. Chronic inflammatory pain decreases the glutamate vesicles in presynaptic terminals of the nucleus accumbens. Mol. Pain 14. 10.1177/1744806918781259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ, 2016. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat. Neurosci 19 (2), 220–222. 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche K, O’Brien R, Mammen A, Bernhardt J, Huganir R, 1996. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16 (6). 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Romero-Sánchez H, Mendieta L, Austrich-Olivares A, Garza-Mouriño G, Benitez-Diaz Mirón M, Coen A, Godínez-Chaparro B, 2020. Unilateral lesion of the nigroestriatal pathway with 6-OHDA induced allodynia and hyperalgesia reverted by pramipexol in rats. Eur. J. Pharmacol 869 10.1016/j.ejphar.2019.172814. [DOI] [PubMed] [Google Scholar]
- Sagheddu C, Aroni S, De Felice M, Lecca S, Luchicchi A, M M, Muntoni A, Romano R, Palazzo E, Guida F, Maione S, Pistis M, 2015. Enhanced serotonin and mesolimbic dopamine transmissions in a rat model of neuropathic pain. Neuropharmacology 97. 10.1016/j.neuropharm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Schestatsky P, Kumru H, Valls-Solé J, Valldeoriola F, Marti M, Tolosa E, Chaves M, 2007. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 69 (23). 10.1212/01.wnl.0000295669.12443.d3. [DOI] [PubMed] [Google Scholar]
- Scott D, Heitzeg M, Koeppe R, Stohler C, Zubieta J, 2006. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci 26 (42) 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L, Moulton E, Linnman C, Carpino E, Becerra L, Borsook D, 2014. The human amygdala and pain: evidence from neuroimaging. Hum. Brain Mapp 35 (2) 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Volkow N, 2016. Re-energizing the development of pain therapeutics in light of the opioid epidemic. Neuron 92 (2). 10.1016/j.neuron.2016.09.051. [DOI] [PubMed] [Google Scholar]
- Stuginski-Barbosa J, Rodrigues G, Bigal M, Speciali J, 2008. Burning mouth syndrome responsive to pramipexol. J. Headache Pain 9 (1). 10.1007/s10194-008-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, D’amour J, Lee M, Lin H, Manders T, Xu D, Eberle S, Goffer Y, Zou A, Rahman M, Ziff E, Froemke R, Huang D, Wang J, 2015. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol. Brain 8. 10.1186/s13041-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Becker S, Schweinhardt P, Cahill C, 2016. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157 (6). 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Neugebauer V, 2019. Cortico-limbic pain mechanisms. Neurosci. Lett 702 10.1016/j.neulet.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede R, Rief W, Barke A, Aziz Q, Bennett M, Benoliel R, Cohen M, Evers S, Finnerup N, First M, Giamberardino M, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith B, Svensson P, Vlaeyen J, Wang S, 2019. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 160 (1). 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- Vogt BA, 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci 6 (7), 533–544. 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Benveniste H, McLellan A, 2018. Use and misuse of opioids in chronic pain. Annu. Rev. Med 69 10.1146/annurev-med-011817-044739. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Narita M, Hamada Y, Yamashita A, Tamura H, Ikegami D, Kondo T, Shinzato T, Shimizu T, Fukuchi Y, Muto A, Okano H, Yamanaka A, Tawfik VL, Kuzumaki N, Navratilova E, Porreca F, 2018. Activation of ventral tegmental area dopaminergic neurons reverses pathological allodynia resulting from nerve injury or bone cancer. Mol. Pain 14. 10.1177/1744806918756406, 1744806918756406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J, Allen R, Högl B, Inoue Y, Oertel W, Salminen A, Winkelman J, Trenkwalder C, Sampaio C, 2018. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov. Disord 33 (7) 10.1002/mds.27260. [DOI] [PubMed] [Google Scholar]
- Wood P, Patterson J, Sunderland J, Tainter K, Glabus M, Lilien D, 2007a. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J. Pain 8 (1). 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Wood P, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner E, Bushnell M, Chizh B, 2007b. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci 25 (12) 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- Yong R, Mullins P, Bhattacharyya N, 2021. Prevalence of chronic pain among adults in the United States. Pain. 10.1097/j.pain.0000000000002291. [DOI] [PubMed] [Google Scholar]
- Zeng J, Li S, Zhang C, Huang G, Yu C, 2018. The mechanism of hyperalgesia and anxiety induced by remifentanil: phosphorylation of GluR1 receptors in the anterior cingulate cortex. J. Mol. Neurosci 65 (1) 10.1007/s12031-018-1072-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.