Abstract
Background
Surgery is the cornerstone in curative treatment of colorectal cancer. Unfortunately, surgery itself can adversely affect patient health. 'Enhanced Recovery After Surgery' programmes, which include multimodal interventions, have improved patient outcomes substantially. However, these are mainly applied peri‐ and postoperatively. Multimodal prehabilitation includes multiple preoperative interventions to prepare patients for surgery with the aim of increasing resilience, thereby improving postoperative outcomes.
Objectives
To determine the effects of multimodal prehabilitation programmes on functional capacity, postoperative complications, and quality of life in adult patients undergoing surgery for colorectal cancer.
Search methods
We searched CENTRAL, MEDLINE, Embase and PsycINFO in January 2021. We also searched trial registries up to March 2021.
Selection criteria
We included randomised controlled trials (RCTs) in adult patients with non‐metastatic colorectal cancer, scheduled for surgery, comparing multimodal prehabilitation programmes (defined as comprising at least two preoperative interventions) with no prehabilitation. We focused on the following outcomes: functional capacity (i.e. 6‐minute walk test, VO2peak, handgrip strength), postoperative outcomes (i.e. complications, mortality, length of hospital stay, emergency department visits, re‐admissions), health‐related quality of life, compliance, safety of prehabilitation, and return to normal activities.
Data collection and analysis
Two authors independently selected studies, extracted data, assessed risk of bias and used GRADE to assess the certainty of the evidence. Any disagreements were solved with discussion and consensus. We pooled data to perform meta‐analyses, where possible.
Main results
We included three RCTs that enrolled 250 participants with non‐metastatic colorectal cancer, scheduled for elective (mainly laparoscopic) surgery. Included trials were conducted in tertiary care centres and recruited patients during periods ranging from 17 months to 45 months. A total of 130 participants enrolled in a preoperative four‐week trimodal prehabilitation programme consisting of exercise, nutritional intervention, and anxiety reduction techniques. Outcomes of these participants were compared to those of 120 participants who started an identical but postoperative programme.
Postoperatively, prehabilitation may improve functional capacity, determined with the 6‐minute walk test at four and eight weeks (mean difference (MD) 26.02, 95% confidence interval (CI) ‐13.81 to 65.85; 2 studies; n = 131; and MD 26.58, 95% CI ‐8.88 to 62.04; 2 studies; n = 140); however, the certainty of evidence is low and very low, respectively, due to serious risk of bias, imprecision, and inconsistency. After prehabilitation, the functional capacity before surgery improved, with a clinically relevant mean difference of 24.91 metres (95% CI 11.24 to 38.57; 3 studies; n = 225). The certainty of evidence was moderate due to downgrading for serious risk of bias. The effects of prehabilitation on the number of complications (RR 0.95, 95% CI 0.70 to 1.29; 3 studies; n = 250), emergency department visits (RR 0.72, 95% CI 0.39 to 1.32; 3 studies; n = 250) and re‐admissions (RR 1.20, 95% CI 0.54 to 2.65; 3 studies; n = 250) were small or even trivial. The certainty of evidence was low due to downgrading for serious risk of bias and imprecision. The effects on VO2peak, handgrip strength, length of hospital stay, mortality rate, health‐related quality of life, return to normal activities, safety of the programme, and compliance rate could not be analysed quantitatively due to missing or insufficient data. The included studies did not report a difference between groups for health‐related quality of life and length of hospital stay. Data on remaining outcomes were not reported or were reported inadequately in the included studies.
Authors' conclusions
Prehabilitation may result in an improved functional capacity, determined with the 6‐minute walk test both preoperatively and postoperatively. A solid effect on the number of complications, postoperative emergency department visits and re‐admissions could not be established. The certainty of evidence ranges from moderate to very low, due to downgrading for serious risk of bias, imprecision and inconsistency. In addition, only three heterogeneous studies were included in this review. Therefore, the findings of this review should be interpreted with caution. Numerous relevant RCTs are ongoing and will be included in a future update of this review.
Keywords: Adult, Humans, Colorectal Neoplasms, Colorectal Neoplasms/surgery, Digestive System Surgical Procedures, Postoperative Complications, Postoperative Complications/prevention & control, Preoperative Exercise, Quality of Life
Plain language summary
Preparing a patient with bowel cancer for surgery with multiple interventions
Aim of this review
The aim of this review is to find out whether multiple interventions introduced in the period prior to surgery for bowel cancer could prepare a patient by increasing the patient's overall fitness, and thus improve outcomes after surgery. Cochrane researchers collected and analysed all available randomised controlled trials on this topic.
Key messages
Only three studies met the inclusion criteria for this review, information was not available for all outcomes and the overall certainty of evidence was very low to moderate. More and larger studies are needed to gather evidence on this topic.
What was studied in the review?
Surgery is often given to cure patients diagnosed with early stage bowel cancer. Surgery has a negative impact on the overall fitness of the patient. The energy level decreases, patients are more dependent in their daily living activities, and quality of life decreases. Furthermore, complications may occur after surgery causing a further decrease of fitness. Preoperative interventions, such as exercise programmes, nutritional advice and supplements, as well as mental support, may increase the fitness of the patient, prior to surgery. This concept is called prehabilitation. The impact of surgery is diminished and consequently results in faster and better recovery. Combining such preoperative interventions results in better preparation for surgery because each interventions may help to strengthen the effects of the others. The review authors aimed to study the effect of such multiple‐intervention preparation programmes before surgery for patients with bowel cancer. The review authors focused on these outcomes: physical fitness, number of complications after surgery, death rate, quality of life (assessed with questionnaires), length of stay in the hospital, number of emergency department visits, number of re‐admissions after surgery, safety of the programme and adherence to the programme. They compared groups with prehabilitation programmes to groups not receiving any preparation prior to surgery, other than standard care.
Main results of this review
The review authors found three studies with 250 participants with bowel cancer, without metastases, scheduled for surgery. Studies were conducted in Canada. A total of 130 participants followed four‐week prehabilitation programmes prior to surgery, which included exercises, nutritional advice and supplements, as well as techniques to reduce anxiety about their cancer and its treatment. Another 120 participants followed identical programmes, but only started them after the surgery, when they were discharged from hospital.
Overall, the review authors did not find an improvement in either group of participants. The certainty of evidence was very low to moderate, mainly because of the small numbers of studies and participants included in the review. Physical fitness potentially improves in patients receiving prehabilitation programmes prior to surgery. The effects of such a programme on the number of complications, emergency department visits and re‐admissions are small or even trivial. Because data on death rates, quality of life, length of stay in the hospital, safety of the programme and adherence to the programme was not complete or not reported, the review authors did not analyse these outcomes. Due to the mostly low or very low certainty of the evidence, the findings of this review should be interpreted with caution.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to January 2021 and also looked for unpublished, ongoing studies up to March 2021. In a future update of this review, many ongoing studies will likely have been completed, which can be included to collect more evidence on this subject.
Summary of findings
Summary of findings 1. Prehabilitation compared to no prehabilitation in adult patients undergoing surgery for colorectal cancer.
| Prehabilitation compared to no prehabilitation in adult patients undergoing surgery for colorectal cancer | ||||||
| Patient or population: adult patients undergoing surgery for colorectal cancer Setting: in‐hospital, outpatient or home‐based interventions Intervention: multimodal prehabilitation Comparison: no prehabilitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no prehabilitation | Risk with multimodal prehabilitation | |||||
| Functional capacity 4 weeks postoperatively assessed with: 6MWT in metres | The mean functional capacity four weeks postoperatively ranged from 286.1 to 444 metres | MD 26.02 meters higher (13.81 lower to 65.85 higher) | Not estimable | 131 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Functional capacity 8 weeks postoperatively assessed with: 6MWT in metres | The mean functional capacity eight weeks postoperatively ranged from ‐21.8 to 11 metres | MD 26.58 metres higher (8.88 lower to 62.04 higher) | Not estimable | 140 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | The values reported in the 'Risk with no prehabilitation' column are mean changes from baseline. |
| Complications within 30 days postoperatively | 417 per 1.000 | 396 per 1.000 (292 to 538) | RR 0.95 (0.70 to 1.29) | 250 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | |
| Health‐related quality of life | See comment | See comment | Not estimable | 182 (2 RCTs) | See comment | SF‐36 and HADS results were reported in two studies (Gillis 2014, Carli 2020). We were not able to pool data. Both trials did not report between‐group differences. |
| Functional capacity pre‐surgery assessed with: 6MWT in metres | The mean functional capacity pre‐surgery ranged from ‐16.4 to 315.8 metres | MD 24.91 metres higher (11.24 higher to 38.57 higher) | Not estimable | 225 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Both post‐intervention scores and mean change from baseline are displayed in the "Risk with no prehabilitation" column. |
| Length of hospital stay | See comment | See comment | Not estimable | 250 (3 RCTs) | See comment | Meta‐analysis could not be performed. The three studies (Gillis 2014, Bousquet‐Dion 2018, Carli 2020) found that results were similar between groups. |
| Mortality | See comment | See comment | Not estimable | ‐ | See comment | Not reported in either study |
| Safety of the programme (dropout, SAE) | See comment | See comment | Not estimable | ‐ | See comment | Meta‐analysis could not be performed. Information was insufficient to draw conclusions. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6‐MWT: 6‐minute walk test; CI: Confidence interval; kg: Kilogram; MD: Mean difference; ml: Millilitre; OR: Odds ratio; RCT: randomised controlled trial; RR: Risk ratio; SAE: Serious adverse event; VO2peak: Peak oxygen uptake. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded for risk of bias. Participants and personnel were not blinded (due to the nature of the programme), outcome assessors were blinded.
2 Downgraded for imprecision. Information size was not reached and the confidence intervals encompass both considerable benefit and considerable harm.
3 Downgraded for inconsistency. Results were inconsistent between studies and heterogeneity is substantial with an I2 of 65%.
Background
Description of the condition
Colorectal cancer (CRC) is the second‐most commonly diagnosed type of cancer in women, and the third‐most commonly diagnosed in men. In 2018, over 1.8 million new CRC cases and nearly 862,000 deaths were registered worldwide (Bray 2018). The cornerstone in treatment of CRC remains surgery. Surgery is known to be a major stressor. Subsequently, colorectal surgery is associated with significant postoperative morbidity (Tevis 2016). Complications strongly impact the postoperative and long‐term outcome of the patient as well as the long‐term quality of life (Khuri 2005; Tevis 2016). Furthermore, complications impact healthcare systems due to prolonged length of stay, higher re‐admission rates, and increased costs (West 2017). Key in the development of postoperative comorbidities is the surgical stress response, with subsequent changes in organ functioning (Kehlet 1997). Enhanced Recovery After Surgery (ERAS) programmes consist of multimodal interventions applied peri‐operatively to minimise this stress response. Furthermore, with the aim to maintain physiological function and accelerate recovery after surgery. ERAS after major colorectal surgery has resulted in reduced morbidity rates and reduced length of stay, as well as improved recovery (Gustafsson 2019). However, the majority of the interventions focus on the intra‐and postoperative factors. Since the postoperative period is associated with fatigue, lack of sleep, weakness, anorexia, and mental burdens such as anxiety and depression, it may not seem the most optimal time period to introduce recovery‐enhancing interventions (Baldini 2018; Carli 2018). Furthermore, patients are more psychologically receptive to behavioural interventions in the preoperative period while facing major surgery (Levett 2016; West 2017). Hence, the preoperative period, though limited to several weeks, can be optimally used to introduce prehabilitation.
Description of the intervention
Prehabilitation consists of multidisciplinary preoperative interventions aiming to prevent or attenuate the functional decline and subsequent consequences caused by surgery (Minnella 2018a). It includes assessment of physical, nutritional, and psychological status to determine baseline functional capacity, identify impairments and intervene in order to improve the patients’ preoperative functional reserve prior to treatment (Carli 2017; Silver 2013). The interventions used in prehabilitation address modifiable risk factors (Carli 2017; Minnella 2017). The risk of severe complications is associated with the number of preoperative modifiable risk factors (Van Rooijen 2017). Prehabilitation can alter postoperative outcomes on the short‐term and additionally result in behavioural changes in the long term (Levett 2016; West 2017). The concept has been introduced in recent decades and while awaiting better‐certainty evidence, it is being implemented as part of peri‐operative care. Accordingly, prehabilitation is included in the latest ERAS guideline for colorectal cancer surgery (Gustafsson 2019).
There is a rationale to combine various interventions in a multimodal approach, since the functional impairment in oncology patients is multi‐factorial (Minnella 2018a). Combining interventions induces a synergistic effect (Scheede‐Bergdahl 2019). Apart from the synergistic effect, use of only a single modality, such as an exercise programme, could potentially harm a patient without physiological reserves; while combining exercise with protein supplementation is necessary to make the intervention beneficial (Carli 2017). Apart from the multimodal approach, there is no consensus yet on the design and content of a prehabilitation programme or what group of patients would benefit most. Minnella et al. describe what the screening, assessment, and intervention in prehabilitation should generally contain (Minnella 2018a). Descriptions in recent literature include multiple modalities involving exercise, nutritional and mental support, as well as behaviour modification (Baldini 2018; Carli 2018; Levett 2016; Minnella 2018a; Silver 2013; West 2017). Furthermore, there is a slight preference for supervised training sessions, three times per week of moderate‐ to high‐intensity training, instead of daily moderate‐intensity training (Minnella 2018a). Meaningful changes can be achieved in three to eight weeks preoperatively (Mayo 2011; West 2017). Studies reported thus far vary in methodology used in terms of the type, frequency, duration, and timing of the interventions. Because of the heterogeneity of interventions, published reviews cannot draw firm conclusions (Levett 2016).
As mentioned above, there is no consensus on which patients might benefit most from prehabilitation. However, the peak incidence of colorectal cancer occurs in patients older than age 70 (Papamichael 2015). Higher age is associated with frailty and frailty is associated with limited reserves and an increased risk for poorer functional capacity, complications, and even mortality postoperatively (Bruns 2016; Ommundsen 2017; Papamichael 2015). Treatment in older patients with CRC might be challenging and should take age‐related factors into consideration (Bruns 2016). The updated International Society of Geriatric Oncology recommendations therefore advises to identify patients with CRC who need a formal comprehensive geriatric assessment prior to surgery. Additionally, a prehabilitation programme and postponement of major resection should especially be considered in frail patients with comorbidities (Papamichael 2015).
How the intervention might work
Poor functional capacity preoperatively is associated with postoperative complications and increased mortality (Wilson 2010). Increasing the functional capacity preoperatively results in an improved recovery after surgery (Mayo 2011; Minnella 2019a). Conversely, patients with a decrease in functional capacity have an increased rate of severe complications (Mayo 2011).
Exercise in the context of prehabilitation can be described as regular physical activity incorporated in a structured programme that should be tailored to the patient (Carli 2017). Aerobic and muscular strength training should be incorporated in the exercise programme (West 2017) as well as implementation of balance and flexibility training (Baldini 2018).
Cancer directly affects the nutritional status in patients and nutritional status is further compromised by surgery. The goal of nutritional intervention is to optimise nutrient stores prior to surgery and to compensate for the catabolic response after surgery. Another goal is to stimulate muscle protein synthesis after exercise training (Baldini 2018).
Psychological distress is common in cancer patients. Preoperative psychological interventions appeared to benefit patient‐reported outcome measures in several studies (Tsimopoulou 2015). Furthermore, psychological preparation prior to surgery may result in lower postoperative pain, shorter length of hospital stay, and diminished negative affect (Powell 2016). Active participation of patients in the process to prepare for treatment may contribute to diminishing the emotional distress due to their facing major colorectal surgery (Mayo 2011). Depressive symptoms in patients with colorectal cancer are associated with poorer functional status. Whether the depressive symptoms or the poor functional status comes first is unknown (Barrett‐Bernstein 2019). Furthermore, presence of anxiety is a predictor for poorer recovery (Mayo 2011) and potentially decreases adherence to exercise programmes (Scheede‐Bergdahl 2019). Thus, interventions to improve mental well‐being could improve surgical outcome by itself and improve the adherence to a prehabilitation programme, further improving effectiveness of the programme.
Preoperative interventions focused on smoking and excessive alcohol consumption are generally implemented as part of a prehabilitation programme. The risk of postoperative complications is increased due to smoking (Thomsen 2014). Additionally, current smoking in newly diagnosed colonic cancer patients seems to be related to a decreased 5‐year cancer‐specific survival rate (Sharp 2017). Some advise to use intense counselling and nicotine replacement therapy to cease smoking four weeks prior to surgery in order to reduce postoperative pulmonary and wound healing complications (Gustafsson 2019; Thomsen 2014). Intensive interventions to cease alcohol consumption, initiated four to eight weeks prior to surgery, may also reduce postoperative complication rates (Egholm 2018).
Preoperative anaemia correction is another intervention that could be used in a prehabilitation programme. Anaemia is common in colorectal cancer patients and increases the risk of morbidity and implicates survival (Van Rooijen 2016; Wilson 2010). Furthermore, anaemia can hinder patients in exercise training. The latest ERAS guideline for colorectal surgery include a strong recommendation, based on high‐certainty evidence, to screen and treat anaemia prior to surgery (Gustafsson 2019). Intravenous iron therapy was found in the IVICA trial to be more effective than oral iron therapy in treating preoperative iron deficiency and anaemia (Keeler 2017). This also translated into improved quality of life scores with intravenous iron therapy (Keeler 2019).
Finally, polypharmacy should be addressed prior to surgery. However, this is usually implemented as standard care.
As mentioned before, a multimodal approach is prescribed in many studies, as it produces a synergistic intervention effect. Exercise and dietary protein intake affect anabolism and muscle protein synthesis when used independently as well as when combined (Gillis 2019). Furthermore, a positive mental status will benefit participation in exercise and other lifestyle interventions, while exercise presumably affects cerebral circuits involved in reward and stress resistance (Herrera 2016).
Prehabilitation improves nutritional status (Gillis 2019; Santa Mina 2018), increases functional capacity (Barberan‐Garcia 2018; Gillis 2014; Li 2013; Liu 2019; Minnella 2017; Minnella 2018b), and benefits mental status (Lindbäck 2018; Mayo 2011; Santa Mina 2018). Furthermore, it seems to result in a reduction of complications (Barberan‐Garcia 2018; Hughes 2019), accelerated recovery (Gillis 2014; Li 2013; Minnella 2019b; Van Rooijen 2019a), a diminished length of hospital stay (Gillis 2018; Santa Mina 2014), and improved quality of life (Lindbäck 2018 Dunne 2016). Potentially, a reduction in costs can be achieved by prehabilitation due to shorter length of stay, lower rate of re‐admissions, faster return to work, and a decrease in the use of primary care after discharge (Barberan‐Garcia 2019; Mouch 2019; Nielsen 2008). A recently published pooled analysis of three studies concluded that trimodal prehabilitation was not associated with improved overall survival and disease‐free survival in stage I‐III colorectal cancer (Trépanier 2019). However, subgroup analysis did show an improved 5‐year disease free survival in patients with stage III disease (Trépanier 2019).
Why it is important to do this review
Over the past two decades, an increasing number of studies on prehabilitation have been published. Most studies in colorectal cancer patients were unimodal and consisted of an exercise programme (Heldens 2016; Karlsson 2019; Loughney 2017; Moug 2019; West 2015) or nutritional intervention alone (Gillis 2016). Only a few studies included a multimodal prehabilitation programme (Bousquet‐Dion 2018; Gillis 2014; Li 2013; Van Rooijen 2019a).
Multiple systematic reviews have been conducted on prehabilitation prior to surgery (Bolshinsky 2018; Heger 2019; Hijazi 2017; Hughes 2019; Luther 2018; Piraux 2018). Conclusions were mainly limited due to the heterogeneity of studies. A recently published systematic review on prehabilitation included both cohort and randomised studies investigating unimodal as well as multimodal programmes for major abdominal and cardiothoracic surgery (Kamarajah 2019). The studies showed large variations across type of surgery and prehabilitation regimes. This resulted in heterogeneous study populations, providing limited ability to generalise study results for routine clinical practice (Kamarajah 2019).
Some systematic reviews aimed to study multimodal prehabilitation as a whole. Bolshinsky 2018 performed a systematic review that aimed to determine the effect of multimodal prehabilitation as a bundle of care. The review included 20 studies, with only two studies containing a multimodal prehabilitation programme. Data were insufficient to show any benefit of prehabilitation as a bundle of care in gastro‐intestinal cancer patients (Bolshinsky 2018). Luther 2018 also performed a systematic review to assess the collective impact of "total body prehabilitation" before major abdominal surgery on postoperative outcomes. They included 16 articles assessing prehabilitation on four domains: nutritional and mental optimisation, physical exercise, and negative health behaviours. Luther 2018 identified no studies containing interventions in all four domains. Although data were again insufficient, they concluded that a multimodal programme is likely to have more impact, compared to unimodal programmes (Luther 2018). To our knowledge, none of the reviews has included only studies with multimodal prehabilitation programmes. Considering the consensus that prehabilitation should be multimodal, a Cochrane Review on multimodal prehabilitation in colorectal cancer surgery aims to provide an overview of the current multimodal initiatives as well as the evidence.
Objectives
To determine the effects of a multimodal prehabilitation programme for adult colorectal cancer patients undergoing elective resection on functional capacity, postoperative outcomes, and health‐related quality of life (HRQoL).
Methods
Criteria for considering studies for this review
Types of studies
We conducted this review according to a previously published Cochrane protocol (Van Rooijen 2019a). Randomised controlled trials (RCTs) comparing multimodal prehabilitation to no prehabilitation were eligible for inclusion. Pilot RCTs, multi‐arm RCTs and cluster‐RCTs were also eligible. We included trials irrespective of whether an intention‐to‐treat analysis had been carried out. Blinding was not a prerequisite for inclusion. Studies could contain an ERAS programme as well as standard care. The latter inclusion criterion may have contributed to heterogeneity among the studies. We assessed heterogeneity as described in Assessment of heterogeneity.
Types of participants
Studies with adult participants (age 18 years and older) with non‐metastatic colorectal cancer undergoing elective resection with or without (neo)adjuvant therapy were eligible for inclusion. Studies were excluded if they reported additional intraoperative therapy and/or reported multi‐organ resection.
Types of interventions
As described above, there is no consensus on the design and content of a prehabilitation programme. However, some common denominators are described in the literature. Therefore, any intervention to improve participants' functional capacity, nutritional status, mental status, and/or to decrease the use of substances such as tobacco could qualify for inclusion. Since we were interested in multimodal prehabilitation, eligible studies contained at least two of the following interventions: physical exercise programmes (endurance and/or resistance training, as well as breathing exercises), any nutritional support, any mental support, and/or interventions addressing substance use (e.g. smoking cessation programmes). Studies were excluded when the intervention lasted less than seven days and/or when follow‐up was less than four weeks postoperatively. Control group participants could receive standard care or no prehabilitation.
Types of outcome measures
We were specifically interested in the following outcomes listed below. However, we did not exclude relevant studies that did not report these outcomes.
Primary outcomes
Functional capacity determined with the 6‐minute walk test (6MWT) (maximum number of metres walked in six minutes in a corridor at least 20 metres long) postoperatively
Postoperative complication rate (Clavien‐Dindo scale (CD) or Comprehensive Complication Index (CCI) (Slankamenac 2013)) within 30 days
Patient‐reported HRQoL, measured using the following questionnaires: Short Form‐36 (SF‐36), Hospital Anxiety and Depression Scale (HADS), EuroQol‐5D (EQ‐5D), European Organisation for Research and Treatment of Cancer Quality of Life Questionnaires Core module and ColoRectal cancer module (EORTC QLQ‐C30/‐CR29))
Secondary outcomes
6MWT pre‐surgery after completion of the prehabilitation programme
VO2peak (ml/kg) as determined by the steep ramp test or cardiopulmonary exercise test (CPET)
Handgrip strength (kg)
Length of hospital stay (in days)
Overall mortality at maximal follow‐up period
Compliance rate to the programme
Safety of prehabilitation interventions (dropouts, serious adverse events)
Return to normal activities as measured by PROMIS (Hedrick 2017; Van der Meij 2016)
Emergency department visits within 30 days postoperatively
Readmission rate within 30 days postoperatively
Outcomes should preferably have been available at baseline, pre‐surgery (after prehabilitation programme), and four and/or eight weeks postoperatively.
If data were not fully available, we aimed to retrieve missing data from the study author for further analysis.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases with no language restriction.
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, searched 2021 week 4) (Appendix 1)
MEDLINE (Ovid, 1950 to 2021 week 4) (Appendix 2)
Embase (Ovid, 1974 to 2021 week 4) (Appendix 3)
PsycINFO (EBSCOhost, 1967 to 2021 week 4) (Appendix 4)
We also searched the following registers for ongoing or completed trials (Appendix 5).
US National Library of Medicine clinical trials register (www.clinicaltrials.gov; searched 4 March 2021)
Google Scholar (scholar.google.com; searched 4 March 2021)
Netherlands Trial Register (trialregister.nl; searched 2 March 2021)
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch; searched 4 March 2021)
Searching other resources
We handsearched bibliographies of included studies and any relevant systematic reviews to identify any further eligible studies.
Data collection and analysis
Selection of studies
Two review authors (SR and CM) independently screened the titles and abstracts of all articles identified by the searches.
We screened articles in the following manner. First, we excluded studies that were not RCTs. Subsequently, we assessed eligibility of the papers following the population, intervention, comparison, outcome (PICO) framework. We checked whether the study population (participants) met the inclusion criteria. If not, the article was excluded. If the population met the inclusion criteria, we examined the intervention. In this way, we systematically screened all articles.
We retrieved full‐text articles when a paper was considered eligible based on its title and abstract, or when information was insufficient to determine eligibility. Disagreements regarding eligibility of selected trials were resolved by discussion. In case of doubt or remaining disagreement, a third review author (LJ) assessed the eligibility of the trial, which was then discussed until consensus was reached.
Multiple reports of a given study were collated, and we indicated which report was the study's primary data source. We contacted trial authors in case clarification was necessary and requested additional or missing data.
Data extraction and management
Two review authors (CM and LJ) independently extracted data using a standard data collection form, and entered data into RevMan Web (RevMan Web 2020).
We extracted the following from the included studies:
general information: study title, first author, source, publication date, contact address, language;
study characteristics: study setting (including design and duration), sample size (powered, randomised and analysed), population characteristics (disease, age, gender, comorbidities, treatment modality), description of the prehabilitation programme (number of interventions, duration of the programme), description of the interventions (including frequency and duration per session), implementation of an Enhanced Recovery Programme (ERP) or standard care, and follow‐up;
outcomes: 6MWT preoperatively and postoperatively, postoperative complication rate (CD or CCI), HRQoL (SF‐36, HADS, EQ‐5D, EORTC QLQ‐CR29 or QLQ‐C30), VO2peak, handgrip strength, length of hospital stay, mortality at maximal follow‐up period, compliance to the prehabilitation programme, safety of the programme (including dropouts and adverse events), return to normal activities, emergency department visits postoperatively, and re‐admission rate.
We requested additional or missing data from the study authors when information in articles was insufficient.
We resolved any disagreements by discussion and consensus.
Assessment of risk of bias in included studies
Two review authors (CM and HF) independently assessed risk of bias of the included studies using the revised 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2021). We assessed risk of bias based on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete reporting of outcome data, selectivity of outcome reporting, and other bias. We categorised each domain as being at 'low', 'unclear', or 'high' risk of bias, according to the criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions(Chapter 8, Higgins 2021), and present our assessments both in both 'Risk of bias' tables and graphic summaries. We resolved any disagreements by discussion and consensus.
Measures of treatment effect
For the continuous primary outcome (6MWT), we retrieved mean change from baseline or post‐intervention scores and the standard deviations for each group. We calculated the mean difference (MD) with 95% confidence intervals (CI). For dichotomous outcomes (complications, emergency department visits, and re‐admissions), we calculated the risk ratios (RR) and the 95% CI.
Unit of analysis issues
We did not include any cluster or cross‐over controlled trials. Furthermore, we did not encounter any (other) unit for analysis issues in the included trials. If we had included cluster trials, we would have determined the intra‐cluster correlation coefficient (ICC) or would have used the ICC from another source (Chapter 23, Higgins 2021). We intended to perform sensitivity analysis to study the effect of variability in the ICC. If we had included cross‐over trials, we would have excluded those trials in a sensitivity analysis to assess whether this type of trial could have affected pooled estimates (Chapter 23, Higgins 2021).
Dealing with missing data
We aimed to analyse all data based on the intention‐to‐treat (ITT) principle. We reported the numbers of participants lost to follow‐up and assessed this as a potential source of bias. We performed analyses on the available data in the event missing data were not available.
Assessment of heterogeneity
We assessed heterogeneity according to the Cochrane Handbook (Chapter 10, Higgins 2021). We assessed heterogeneity visually in forest plots and statistically using the Chi2 test (P<0.10). We set the P value to 0.10 to determine statistical significance, because the Chi2 test has low power to assess heterogeneity when studies have small sample sizes or are few in number. We calculated the I2 statistic as a measure of heterogeneity, representing the percentage of variation across studies that can be explained by heterogeneity. To limit the influence of clinical and methodological heterogeneity, we pooled studies with similar study design and with a comparable patient population. We interpreted the I2 statistic value according to the Cochrane Handbook as follows: 0% to 40% might not be important, 30% to 60% moderate heterogeneity, 50% to 90% substantial heterogeneity, and 75% to 100% considerable heterogeneity (Chapter 10, Higgins 2021). Although investigations of heterogeneity might be inaccurate, due to the small number of trials identified, we applied the methods above all outcomes. We did not display pooled data if heterogeneity was clinically or statistically high (i.e. if the I2 statistic value was greater than 75%).
Assessment of reporting biases
To prevent language bias, we did not impose a language restriction. Due to the small number of included studies, we were not able to generate funnel plots to identify publication bias.
Data synthesis
We pooled data and performed meta‐analyses using the aggregated effect parameters and confidence intervals reported by trial investigators.
Where outcomes were dichotomous, we used the Mantel‐Haenszel method to run both the fixed‐effect and random‐effects models. We used the inverse variance method for continuous data. We used the random‐effects model if heterogeneity was high. Otherwise, we used a fixed‐effect model.
Normal distribution of data was assumed, according to the authors' statements in the publication. Since individual data was not available, we could not visually check the distribution using histograms. For studies with non‐parametric results, we intended to calculate mean and standard deviation (SD) by dividing the interquartile range (IQR) by 1.35, according to the Cochrane Handbook (Chapter 6, Higgins 2021). However, this was only applied when the outcome's distribution was similar to a normal distribution.
We summarised the data in forest plots and calculated summary estimates with a 95% CI. We considered using a two‐sided P < 0.05 as statistically significant, except for assessment of heterogeneity, for which the recommended levels are P < 0.10. We performed statistical analyses with RevMan Web (RevMan Web 2020).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were not possible due to the limited number of studies and the limited availability of subgroup data. We intended to examine individual study effects by excluding trials with high heterogeneity. Furthermore, regarding the patient population, we intended to perform subgroup analyses on participants receiving neoadjuvant therapy, open versus laparoscopic surgery, and colonic versus rectal cancer.
Sensitivity analysis
Unfortunately, the number of studies was too small to examine individual study effects on the results.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table with GRADEPro GDT software for the prespecified outcomes.
Functional capacity measured with 6MWT pre‐surgery, four and eight weeks postoperatively
Postoperative complications within 30 days after surgery
Health‐related quality of life
Length of hospital stay
Mortality
Safety of the programme
To assess the certainty of a body of evidence for a given outcome, the following GRADE considerations were used to grade the evidence: study limitations (i.e. risk of bias), inconsistency of results, indirectness of evidence, imprecision, and publication bias. For all five considerations, if there were very serious concerns (for example, if most information came from studies at high risk of bias), we rated down two levels. In the absence of downgrading, had there been a large magnitude of effect, a dose‐response gradient, or if the demonstrated effect could have been reduced by all plausible confounders, the certainty of evidence could have been upgraded. The evidence can be graded as high‐certainty evidence, moderate‐certainty evidence, low‐certainty evidence and very low‐certainty evidence (Chapter 14, Higgins 2021).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search in electronic databases resulted in 8385 records. Additionally, nine studies were identified through searching other resources (Figure 1). After removing duplicates, 8136 records were screened by title and abstract. Twenty‐seven titles were further assessed for eligibility. Two of them were poster presentations, but appeared to be non‐randomised (Astin 2014a; Astin 2014b). Three records were published in abstract form only, containing unimodal programmes (Brown 2018; Cramer 2014; Hernon 2016) and were therefore not eligible for inclusion. One completed RCT has not yet been published (NCT03096951). We retrieved 21 full‐text articles, from which we included three studies and excluded 18 studies.
1.
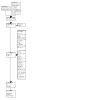
Study flow diagram.
Included studies
We included three trials with a total of 250 participants. Of these, 130 were assigned to prehabilitation groups and 120 were assigned to control groups (Bousquet‐Dion 2018; Carli 2020; Gillis 2014).
Study design
Included trials were parallel‐arm single‐blinded RCTs comparing a multimodal prehabilitation programme to a control group with a similar rehabilitation programme starting postoperatively, after discharge from the hospital. All studies applied an ERAS programme as standard of care.
Participants
The population consisted of adult participants with non‐metastatic colorectal cancer, scheduled for elective resection. Additionally, the subjects in Carli 2020 were frail, as determined by a score of ≥ 2 in the Fried Frailty Index (FFI). Two studies (Bousquet‐Dion 2018; Gillis 2014) had a slightly higher percentage of male participants. Most of the participants in Carli 2020 were ≥75 years of age. In all three trials, surgery was mainly performed laparoscopically, and colonic resections were more common than rectal resections.
Intervention
The studies included a multimodal prehabilitation programme consisting of moderate‐intensity exercise, nutritional, and mental health support. All programmes started approximately four weeks preoperatively. Programmes were resumed for eight weeks after surgery in Gillis 2014 and Bousquet‐Dion 2018, but not in Carli 2020 (Table 2).
1. Multimodal prehabilitation programmes.
| Gillis 2014 | Bousquet‐Dion 2018 | Carli 2020 | |
| Exercise | General:
Aerobic:
Resistance:
|
General:
Aerobic:
Resistance:
|
General:
Aerobic:
Resistance:
|
| Nutrition |
|
|
|
| Mental health |
|
|
|
This programme was being offered to the patients in the prehabilitation group four weeks preoperatively in Bousquet‐Dion 2018, Carli 2020, and Gillis 2014. In Bousquet‐Dion 2018 and Gillis 2014 patients resumed this programme in the prehabilitation group postoperatively until eight weeks after surgery (minus the supervised sessions in Bousquet‐Dion 2018). The programme did not continue postoperatively in Carli 2020.
The rehabilitation or control group did not follow a preoperative programme. Subjects started this identical, postsurgical rehabilitation programme until eight weeks postoperatively inBousquet‐Dion 2018 and Gillis 2014, and until four weeks after surgery in Carli 2020.
Comparison
Control groups did not receive any interventions preoperatively. All groups received a rehabilitation programme containing similar interventions and instructions as the prehabilitation programme, which started once each participant was discharged from hospital.
Outcome
All three trials included assessment of the 6MWT at baseline and pre‐surgery. Bousquet‐Dion 2018 and Carli 2020 reported the 6MWT four weeks after surgery and Gillis 2014 and Bousquet‐Dion 2018 also included an assessment eight weeks postoperatively. Results of the 6MWT were presented as change from baseline or post‐intervention values for the above‐mentioned time points. Where possible, we did not combine these variable scores. However, when necessary, pooling a mixture of scores is allowed when it comes to meta‐analysis of mean differences (Chapter 10, Higgins 2021).
Postoperative complications within 30 days were reported in the included articles. Handgrip strength was reported at baseline only in all three RCTs and could therefore not be analysed as an outcome in this review. HRQoL was reported in Gillis 2014 and Carli 2020 using the SF‐36 and HADS. However, both studies displayed different subscales for the SF‐36. Data could not be pooled for that reason. HADS results were reported as median plus IQR in Carli 2020. Length of hospital stay was also reported as mean plus IQR in the three included studies. Because the range was not displayed for these outcomes, we could not calculate the mean and SD of these variables. Moreover, length of hospital stay is assumed to be highly skewed. For these reasons, we could not include these variables in the quantitative analyses. Compliance rate was not determined in the control group before surgery since they had not started the programme preoperatively. Emergency department visits and re‐admissions were published in the included articles. VO2peak, mortality, safety, and return to normal activities were not reported in either study.
Excluded studies
We excluded 18 studies after assessing the full‐text articles. Two of the excluded articles were non‐randomised trials (Bruns 2019; Lim 2019); three contained an ineligible population, including participants with other diseases than colorectal cancer or starting the intervention postoperatively (Fulop 2021; Klinkhammer‐Schalke 2020; Zhang 2014); four included unimodal prehabilitation (Gillis 2016; Karlsson 2019; Moug 2019; Ommundsen 2017); one was a protocol publication of an RCT with a unimodal programme (Onerup 2017); and one included two prehabilitation groups and no control (Minnella 2020). Seven meta‐analyses were also excluded. Two of these analysed data of two included RCTs in this review and did not publish new data or did not meet the inclusion criteria of this review (Chen 2017; Gillis 2019); four contained non‐randomised data as well, not separately reported (Barrett‐Bernstein 2019; Minnella 2016; Minnella 2017; Trépanier 2019), and one analysed only the prehabilitation groups, with no control group (Awasthi 2019).
Studies awaiting classification
One RCT is registered as completed but not yet published (NCT03096951) and four potentially eligible trials are still ongoing (NCT04595604; NCT04167436; NCT03097224; NL5784).
These studies await classification and will be assessed in the update of this review.
Risk of bias in included studies
Our evaluations of the risk of bias in each study are described in detail in the 'Risk of bias' tables (included in the Characteristics of included studies tables). The overall risk of bias for all three studies in each domain is presented in Figure 2, and the risk of bias for each domain of each trial is presented in Figure 3. Using the revised Cochrane risk of bias tool for randomised trials (Chapter 8, Higgins 2021), we assessed the overall risk of bias in the included studies to be at high risk of bias, because at least one domain in each trial was judged to be at high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
All three studies described the randomisation procedure and used computer‐generated random numbers. The risk of bias is therefore low.
Allocation concealment
Sequentially numbered sealed envelopes were used in the included trials resulting in a low risk of bias assessment.
Blinding
Blinding of personnel and participants
Included studies were assessed as high risk of bias. No blinding of personnel or participants was used. Therefore, the outcome was possibly influenced by the lack of blinding. Bousquet‐Dion 2018 mentioned bias in the form of contamination in the control group, since participants in the control group improved the 6MWT result in the pre‐surgery measurement.
Blinding of outcome assessment
For the 6MWT, the outcome assessor was blinded in Gillis 2014 and Bousquet‐Dion 2018. The risk of bias for judgement of complications, clinical outcome (length of stay, emergency department visits, re‐admissions) is unclear since there is insufficient information to permit judgement. Carli 2020 reported that outcome assessors, surgeons and statisticians were blinded for group allocation. We deem the risk of bias to be low.
Incomplete outcome data
The studies did not include all randomised participants within their final analyses. Therefore, ITT analysis was not performed.
The RCTs excluded participants with loss to follow‐up from analyses. Gillis 2014 did not specify the reason and at what time point these participants were lost to follow‐up. Carli 2020 did specify the reason, however not the time point. Furthermore, the sample size mentioned in the publication of Carli 2020 did not correspond to the sample size included in the attached study protocol. Risk of bias was assessed to be high.
Finally, Bousquet‐Dion 2018 specified the reasons participants were lost to follow‐up. However, four participants were lost to follow‐up due to complications. Since complications are included as an outcome of the trial, it is not clear why these participants were not included in the analyses. Risk of bias was assessed as high for all three trials.
Selective reporting
The study protocol was attached to the publication for Carli 2020. All prespecified outcomes were included in the published study. The other studies did not publish their protocols. However, the prespecified outcomes for Gillis 2014, as mentioned in the trial register, correspond to the outcomes reported in the published paper. This was not the case with the reported outcomes of Bousquet‐Dion 2018. Therefore, the risk of reporting bias is low for Gillis 2014 and Carli 2020, and high for Bousquet‐Dion 2018.
Other potential sources of bias
Information is insufficient to assess whether or not another important risk of bias exists.
Effects of interventions
See: Table 1
See: Table 1.
Primary outcomes
1. Functional capacity determined with the 6MWT postoperatively
The post‐intervention scores of the 6MWT four weeks postoperatively were pooled for Bousquet‐Dion 2018 and Carli 2020. The mean difference was 26.02 metres, in favour of prehabilitation (n = 131, 95% CI ‐13.81 to 65.85; P = 0.20; I2 = 41%; low certainty evidence; Figure 4).
4.

6MWT four weeks postoperatively: post‐intervention scores four weeks postoperatively were used in this analysis.
Data for all patients included in the study of Carli 2020 were available for analysis 1.3, 1.5 and 1.6, while data for a various number of patients are missing in analysis 1.1 and 1.4
Neither study reported significant differences between groups four weeks after surgery. Bousquet‐Dion 2018 found that both groups had a lower score for the 6MWT four weeks postoperatively, compared to baseline. The prehabilitation group in Carli 2020 had a higher mean for the 6MWT four weeks after surgery, while the control group did not recover to their baseline mean results. The percentage of participants who recovered to their baseline level at four weeks postsurgery was 50% in both groups for Bousquet‐Dion 2018. In Carli 2020 the percentage was 68.4% and 53.3% for the prehabilitation and control groups, respectively.
Mean change scores from baseline were analysed for the eight week postoperative assessment of the 6MWT for Gillis 2014 and Bousquet‐Dion 2018. The common effect for change in distance walked during the test was 26.58 metres (n = 140;CI ‐8.88 to 62.04; P = 0.14; I2= 65%; very low‐certainty evidence; Figure 5) in favour of the prehabilitation group.
5.

6MWT eight weeks postoperatively: in contrast with analysis 1.1 mean change from baseline instead of post‐intervention scores eight weeks postoperatively were used for this analysis.
Gillis 2014 described a statistically significant and clinically important increase in the amount of metres in the prehabilitation group (mean change +23.4 metres, SD 54.8) compared to the control group (mean change ‐21.8 metres, SD 80.7). Bousquet‐Dion 2018 did not find any significant differences.
2. Postoperative complication rate (CD or CCI) within 30 days
Included studies reported the number of participants having at least one complication within 30 days, expressed as number and percentage. However, the complications were specified without reporting the way they were treated. Furthermore, the grade of the most severe complications was reported using the CD grade. Information was insufficient to either use the reported CD grades or to calculate CCI for all three trials. Therefore, we have analysed the number of participants having at least one complication within 30 days (n = 250; RR 0.95, 95% CI 0.70 to 1.29; P = 0.75; I2 = 0%; low‐certainty evidence; Figure 6). The RR of 0.95 may favour prehabilitation; however, considering the size of the effect, it is probably not of clinical relevance.
6.

Number of patients with complication
Data for all patients included in the study of Carli 2020 were available for analysis 1.3, 1.5 and 1.6, while data for a various number of patients are missing in analysis 1.1 and 1.4
Included studies described similar complication rates between groups.
3. Patient‐reported HRQoL
The SF‐36 results were reported in Gillis 2014 for all eight subscales, while Carli 2020 displayed the composite total physical and total mental subscale scores. We were thus unable to pool data for the SF‐36.
HADS scores were reported as mean (SD) in Gillis 2014 and as median (IQR) in Carli 2020. We were not able to convert the latter scores into mean (SD); therefore, we could not perform a meta‐analysis for this outcome.
Neither study reported between‐group differences for SF‐36 and HADS.
Secondary outcomes
1. 6MWT pre‐surgery, after completion of the prehabilitation programme
The presurgical results were included as post‐intervention scores for Carli 2020 and as mean changes from baseline for Gillis 2014 and Bousquet‐Dion 2018. The mean difference in 6MWT results was 24.91 metres in favour of prehabilitation (n = 225, 95% CI 11.24, 38.57; P = 0.0004; I2 = 55%; moderate certainty of evidence; Figure 7).
7.

6MWT presurgery: for this analysis both post‐intervention scores (Carli 2020) and mean change from baseline (Bousquet‐Dion 2018 and Gillis 2014) were used.
Data for all patients included in the study of Carli 2020 were available for analysis 1.3, 1.5 and 1.6, while data for a various number of patients are missing in analysis 1.1 and 1.4
Gillis 2014 reported a statistically significant, also clinically relevant increase (at least 20 metres) in the 6MWT in the prehabilitation group compared to a decrease of the 6MWT in the rehabilitation group. Bousquet‐Dion 2018 and Carli 2020 detected no significant differences between groups.
2. VO2peak (ml/kg) as determined by the steep ramp test or cardiopulmonary exercise test
None of the studies reported this outcome.
3. Handgrip strength (kg)
The handgrip strength was only determined at baseline in the included trials. Thus, we could not analyse handgrip strength as an outcome.
4.Length of hospital stay (in days)
Length of stay in the hospital was reported in the included studies. However, it was reported as median and IQR. Since the authors reported most of the results as mean and SD, we concluded that there must have been a skewed distribution of data. Moreover, since we only had the IQR and not the range, we could not calculate the mean and SD. Therefore, a meta‐analysis could not be performed.
All three studies reported that length of stay was similar between groups.
5. Overall mortality at maximal follow‐up period
No study reported on mortality.
6. Compliance rate to the programme
Compliance to the prehabilitation programme was mentioned in included papers. However, since the control groups did not receive a preoperative programme, compliance could not be compared between groups.
Gillis 2014 described an overall compliance of 78% to the programme, where Bousquet‐Dion 2018 found a 98% compliance rate to the exercise programme and 100% compliance to the nutritional intervention. Neither of these studies described how compliance was determined.
Carli 2020 assessed compliance to the in‐hospital programme and self‐reported adherence to the home‐based programme through a study diary. Mean adherence (SD) in the prehabilitation group was 68% (38%) to the in‐hospital programme and 80% (27%) to the home‐based programme.
7. Safety of prehabilitation interventions (dropouts, serious adverse events)
Information was insufficient to determine how many participants dropped out of the prehabilitation programme. Included studies did mention lost to follow‐up of participants. No serious adverse events were reported during the trial of Carli 2020. The other two papers did not mention adverse events.
8. Return to normal activities as measured by PROMIS
No study reported on this outcome.
9. Emergency department visits postoperatively
The number of participants visiting the emergency department within 30 days postoperatively were reported in included studies. The results of the meta‐analysis are in favour of prehabilitation with a RR risk ratio of 0.72 (n = 250; RR of 0.72; CI 0.39 to 1.32; P = 0.28; I2= 0%; low‐certainty evidence; Figure 8).
8.

Emergency department visits
Data for all patients included in the study of Carli 2020 were available for analysis 1.3, 1.5 and 1.6, while data for a various number of patients are missing in analysis 1.1 and 1.4
The studies did not report a between‐group difference regarding the number of emergency department visits.
10. Re‐admission rate
Re‐admission rate within 30 days from surgery was expressed in terms of the number of participants. This outcome favoured control, with a RR ratio of 1.20 (n = 250; RR 1.20, 95% CI 0.54 to 2.65; P = 0.65; I2= 43%; low‐certainty evidence; Figure 9).
9.

Readmissions
Data for all patients included in the study of Carli 2020 were available for analysis 1.3, 1.5 and 1.6, while data for a various number of patients are missing in analysis 1.1 and 1.4
All three studies found no statistical difference between the prehabilitation and control groups.
Discussion
Summary of main results
In the past two decades, the evidence on prehabilitation has grown. There has been a shift from an unimodal towards a multimodal approach. Unfortunately, the evidence on multimodal prehabilitation programmes prior to colorectal cancer surgery is sparse, with only three RCTs meeting the inclusion criteria of the current review. The RCTs analysed a total of 250 participants; 130 in the prehabilitation group and 120 in the control group. The overall risk of bias was assessed to be high because at least one domain in each included study was assessed to be at high risk of bias. Functional capacity, determined with the 6MWT before surgery, and four and eight weeks postoperatively, may improve after prehabilitation. The effects on complication rate, emergency department visits and re‐admission rates were small or even trivial. Altogether, no decisive evidence was found, since the certainty of evidence was rated moderate to very low due to serious risk of bias, imprecision and inconsistency.
Overall completeness and applicability of evidence
To our knowledge, this is the first review assessing multimodal prehabilitation programmes prior to resection for colorectal cancer. Most systematic reviews published on prehabilitation were limited due to studies including both heterogeneous study populations and heterogeneous prehabilitation interventions. By focusing on a specific population, namely non‐metastatic colorectal cancer patients undergoing surgery, we aimed for a homogeneous population. Although, one of the included RCTs focused on a frail population, all RCTs analysed participants with colorectal cancer.
The prehabilitation programmes studied in the included RCTs were rather similar. However, regimens for the control groups differed. We included two studies that offered a similar rehabilitation programme in both groups, and only offered the prehabilitation programme in the intervention group; thereby studying solely the effect of adding prehabilitation on the outcomes. The third study compared a programme preoperatively in the intervention group to a similar programme postoperatively in the control group. Ideally, the perioperative care regimen would have been similar in both groups within included trials.
Overall, included RCTs fairly addressed the aim and review question of the current review. However, due to the limited number of studies included and moderate to very low certainty of evidence of this review, applicability of the evidence is limited.
Quality of the evidence
We used GRADE methods to determine the certainty of the evidence for each outcome across all studies (Chapter 14, Higgins 2021). Because the included studies were RCTs, the certainty of evidence for all outcomes started as 'high'. Due to the high risk of bias, the imprecision and inconsistency of effect estimates, the small number of included studies, and the high levels of statistical heterogeneity, we downgraded the certainty of evidence for all outcomes to 'moderate', 'low' or 'very low'.
Research with prehabilitation does not allow double‐blinding of the participants and personnel. In particular, not blinding participants potentially affects the results, since participants in the control group who heard about a possible effect of prehabilitation may start to exercise themselves. Therefore, by definition, risk of bias is high. This limits the certainty of evidence, although the RCTs assessed were well‐executed. This will not differ in future updates of this review, since it is not possible to blind participants to the intervention. Blinding of all outcome assessments, and not only the primary, as described in some of the included studies, could improve overall risk of bias and certainty of the evidence.
Potential biases in the review process
As we conducted the current review according to Cochrane guidelines, including a thorough and systematic search through electronic databases, reference lists, and other resources, we can conclude that we have a complete overview of the evidence currently available. However, because prehabilitation is a rather new term comprising of various (combinations of) interventions, studies indexed differently or including interventions not prespecified in our search could have been missed. Another limitation is that both the number of studies and total amount of included participants are small. We were therefore not able to perform all prespecified analyses as described in the published protocol of this review and have altered the outcomes and analyses to complete the current review (e.g. analysed number of complications instead of CD or CCI). In future updates, we aim to complete the analyses according to the published Cochrane protocol (Van Rooijen 2019b).
Agreements and disagreements with other studies or reviews
As mentioned earlier, the effects of multimodal prehabilitation have previously been studied in two systematic reviews; however, these did not include only participants with colorectal cancer (Bolshinsky 2018; Luther 2018). Similar to our results, the heterogeneity of included studies precluded the authors from drawing firm conclusions.
In general, the evidence on the beneficial effects of prehabilitation is growing. At first, studies gathered evidence that prehabilitation improved fitness (Li 2013; Mayo 2011). However, evidence that increased fitness translates into reduced perioperative risk and improved postoperative outcome was sparse. Since the postoperative outcomes, e.g. length of stay, improved due to implementation of ERAS, a further reduction could perhaps be difficult to achieve.
To date, several studies on prehabilitation in abdominal surgery have been published. Barberan‐Garcia 2018 conducted an RCT containing a prehabilitation programme consisting of motivational interview, high‐intensity endurance training, and promotion of physical activity in participants undergoing major abdominal surgery. The programme resulted in a 51% reduction in number of participants having postoperative complications and a decrease in the rate of complications per patient in the intervention group (Barberan‐Garcia 2018). Additionally, several systematic reviews, including meta‐analyses mainly focusing on unimodal programmes, found that prehabilitation is associated with significant lower rates of overall postoperative morbidity (Heger 2019; Hughes 2019; Kamarajah 2019; Moran 2016), pulmonary (Heger 2019; Hughes 2019; Kamarajah 2019) and cardiac complications (Kamarajah 2019). However, prehabilitation was not associated with decreased surgical site infections (Kamarajah 2019), major complication rates (CD ⪰ Grade III) (Kamarajah 2019), diminished length of hospital stay (Heger 2019; Hughes 2019; Kamarajah 2019; Lau 2019), or mortality (Kamarajah 2019; Lau 2019).
Prehabilitation has been studied in other cancers as well. In three recently published systematic reviews on preoperative exercise in lung cancer patients, one including a meta‐analysis, a reduction was found in length of hospital stay and a decrease in postoperative complication rates (Cavalheri 2017; Rosero 2019; Steffens 2018). An RCT found that participants with colorectal liver metastases seemed to gain a better physical fitness and improved quality of life due to a four‐week exercise programme (Dunne 2016). Additionally, multimodal programmes have resulted in physical improvement and decreased anxiety symptoms in patients undergoing radical prostatectomy (Santa Mina 2018), and faster postoperative recovery after surgery for bladder, oesophagogastric, and lung cancer (Liu 2019; Minnella 2018b; Minnella 2019b).
Prehabilitation could also be used prior to other treatment modalities besides surgery, such as chemotherapy and/or radiotherapy. Similarly to surgery, these are known stressors. The REx trial studied a preoperative exercise programme prior to and during long‐course neoadjuvant chemoradiotherapy for rectal cancer. The programme was deemed feasible without compromising the planned treatment pathway. Both groups deteriorated in daily walking expressed as steps per day. Though not significantly, the prehabilitation group deteriorated less than the control group (Moug 2019).
Finally, prehabilitation has also been studied in other populations. In the trial conducted by Liang and colleagues, 118 obese participants with a ventral hernia scheduled for surgical repair were randomised to either multimodal prehabilitation or control. Participants receiving prehabilitation had significantly lower recurrence and complication rates compared to control (Liang 2018).
Unfortunately, a common remark of nearly all systematic reviewers is that definitive conclusions cannot be made due to the heterogeneity of the included trials, and the low certainty of the evidence (Bolshinsky 2018; Heger 2019; Hijazi 2017; Hughes 2019; Kamarajah 2019; Luther 2018; Piraux 2018, Rosero 2019).
Future studies still have to decide which patients would benefit most from multimodal prehabilitation. As mentioned previously, patients are generally diagnosed with colorectal cancer at a higher age. Comprehensive geriatric assessment could depict frail patients, and with prehabilitation risk factors associated with frailty, could be attenuated in order to improve the patient's resilience. Recent guidelines have therefore already adapted prehabilitation for this subgroup of patients (Papamichael 2015).
Authors' conclusions
Implications for practice.
Prehabilitation may result in an improved functional capacity determined with the 6‐minute walk test both preoperatively and postoperatively. Solid effects on the number of complications, emergency department visits and re‐admissions could not be established. The certainty of evidence ranges from moderate to very low, due to serious risk of bias, imprecision and inconsistency. Also, only three heterogeneous studies were included in this review. Therefore, the current review was unable to find decisive evidence for the benefits of multimodal prehabilitation in patients with colorectal cancer undergoing surgery.
Implications for research.
Many reviews discussed the heterogeneity of both programmes and outcomes in prehabilitation studies. Future trials could assess the current evidence and use similar prehabilitation interventions and similar outcomes as described in the literature. Furthermore, blinding should be applied for assessment of all outcomes to decrease the risk of bias. To date, several trials are being conducted. An update of this review after completion of those trials will hopefully gather further evidence on multimodal prehabilitation programmes for patients undergoing colorectal cancer surgery.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2023 | Amended | Fixing typographical error in the Authors' conclusions section of review |
History
Protocol first published: Issue 2, 2019 Review first published: Issue 5, 2022
| Date | Event | Description |
|---|---|---|
| 10 May 2023 | Amended | Amended to reflect comments made on Cochrane Library |
| 10 May 2023 | New citation required and conclusions have changed | Amended to reflect comments made on Cochrane Library |
| 30 October 2021 | Feedback has been incorporated | Alterations after associate editor's comments |
| 11 March 2021 | Amended | Search, analyses and text updated. |
| 2 December 2020 | Amended | Track changes accepted after approval from the reviewers |
| 24 November 2020 | Feedback has been incorporated | Changes in response to reviewers' comments |
| 14 February 2020 | Feedback has been incorporated | Feedback first editorial evaluation incorporated |
| 20 September 2019 | New search has been performed | 20th Of September search and draft of the review |
| 8 July 2019 | Amended | Minor corrections to protocol following editorial comments |
Acknowledgements
We thank E. Delvaux, medical librarian at Máxima MC, for her support with the search strategy. We would also like to thank both peer reviewers for their useful feedback: Calvin Heal from the Centre for Biostatistics of the University of Manchester and Cecilia Lund, MD, PhD from the Department of Medicine, Copenhagen University Hospital in Herlev and Gentofte, Denmark. Finally, we thank Hacsi Horváth for editing the initial copy.
Appendices
Appendix 1. CENTRAL search strategy
#1 ("colorectal neoplasm*" or ((neoplasm* or carcinoma* or tumour* or tumor* or cancer or oncol* or malignan* or carcinogen* or oncogen*) and (colorectal or colon* or rectal*))):ti,ab,kw
#2 surger* or "operative surgical procedure*" or surgeon* or "perioperative period" or "perioperative care" or "preoperative care" or surgical* or operation* or operative* or perioperati* or preoperati* or pre operati* or peri operati* or anesthe* or anaethe* or incisi* or excisi* or invasive* or prehab*
#3 ((exercise* and therap*) or "physical education and training" or "exercise movement technique*" or "remedial exercis*" or "rehabilitation exercis*" or exercis* or "physical activit*" or "physical exercis*" or "aerobic exercis*" or "exercise training" or "isometric exercis*"))
#4 ((Psychosocial or psychologic* or "cognitive behavioral therap*" or "cognitive behav*" or "cognitive psychotherap*" or psychoeducation or "psycho education" or (cogniti* and therap*))
#5 "nutrition therap*" or "nutritional status" or "medical nutrition therap*" or "nutrition therap*" or nutrition
#6 "smoking cessation*" or "smoking" or "tobacco use cessation" or "stopping smoking" or "giving up smoking" or "quitting smoking" or smoking or "smoking behav*" or "smoking habit*" or "tobacco cessation"
#7 #1 AND #2
#8 #7 AND (#3 or #4 or #5 or #6)
Of which 334 Cochrane Review Matches
Appendix 2. MEDLINE search strategy
# 1 Search ((("Colorectal Neoplasms"[Mesh] OR ((“Neoplasms”[Mesh] OR carcinoma*[tiab] OR neoplas*[tiab] OR tumour*[tiab] OR tumor*[tiab] OR cancer*[tiab] OR cancer[sb] OR oncolog*[tiab] OR malignan*[tiab] OR carcinogen*[tiab] OR oncogen*[tiab] AND (colorectal*[tiab] OR colon*[tiab] OR rectal*[tiab])))))
# 2 Search "surgery"[Subheading] OR "Surgical Procedures, Operative"[Mesh] OR "Surgeons"[Mesh] OR "Perioperative Period"[Mesh] OR "Perioperative Care"[Mesh] OR "Preoperative Care"[Mesh] OR "Perioperative Care"[Mesh:NoExp] OR surger*[tiab] OR surgical*[tiab] OR surgeon*[tiab] OR operation*[tiab] OR operative*[tiab] OR perioperati*[tiab] OR preoperati*[tiab] OR pre operati*[tiab] OR peri operati*[tiab] OR anesthe*[tiab] OR anaesthe*[tiab] OR incisi*[tiab] OR excisi*[tiab] OR invasive*[tiab] OR Prehab*[tiab]
#3 Search (#1 AND #2)
#4 Search "Exercise Therapy"[Mesh] OR "Exercise"[Mesh] OR "Physical Education and Training"[Mesh] OR "Exercise Movement Techniques"[Mesh] OR remedial exercis*[tiab] OR exercise therap*[tiab] OR rehabilitation exercis*[tiab] OR exercis*[tiab] OR physical activit*[tiab] OR physical exercis*[tiab] OR aerobic exercis*[tiab] OR exercise training*[tiab] OR isometric exercis*[tiab] OR (Physical Education*[tiab] AND training) OR exercise movement Techni*[tiab]
#5 Search (Psychosocial[tiab] OR psychologic*[tiab] OR "Cognitive Behavioral Therapy"[Mesh] OR cognitive behav*[tiab] OR cognitive psychotherap*[tiab] OR (cogniti*[tiab] AND therap*[tiab]) OR psychoeducation[tiab] OR psycho‐education[tiab])
#6 Search "Nutrition Therapy"[Mesh] OR "Nutritional Status"[Mesh] OR medical nutrition therap*[tiab] OR nutrition therap*[tiab] OR nutrition[tiab]
#7 Search "Smoking Cessation"[Mesh] OR “Smoking”[Mesh] OR "Tobacco Use Cessation"[Mesh] OR stopping smoking[tiab] OR smoking cessation*[tiab] OR giving up smoking*[tiab] OR quitting smoking[tiab] smoking[tiab] OR smoking behav*[tiab] OR smoking habit*[tiab] OR tobacco cessation[tiab]
#8 Search #3 AND #4
#9 Search #3 AND #5
#10 Search # 3 AND #6
#11 Search #3 AND #7
Appendix 3. Embase search strategy
#1 exp colorectal tumor/
#2 exp neoplasm/
#3 (carcinoma* or neoplasm* or tumour* or tumor* or cancer* or oncolog* or malignan* or carcinogen* or oncogen*).ab,ti.
#4 2 or 3
#5 (colorectal* or colon* or rectal*).ab,ti.
#6 4 and 5
#7 1 or 6
#8 exp surgery/
#9 exp surgeon/
#10 exp perioperative period/
#11 exp preoperative period/
#12 (surger* or surgical* or surgeon* or operation* or operative* or perioperati* or preoperati* or pre operati* or peri operati* or anesthe* or anaesthe* or incisi* or excisi* or invasive* or Prehab*).ab,ti.
#13 8 or 9 or 10 or 11 or 12
#14 (remedial exercis* or exercise therap* or rehabilitation exercis* or exercis* or physical activit* or physical exercis* or aerobic exercis* or exercise training* or isometric exercis* or exercise movement techni*).ab,ti.
#15 exp kinesiotherapy/
#16 14 or 15
#17 7 and 13 and 16
#18 exp diet therapy/
#19 exp nutritional status/
#20 (medical nutrition therap* or nutrition therap* or nutrition).ab,ti.
#21 18 or 19 or 20
#22 7 and 13 and 21
#23 exp cognitive behavioral therapy/
#24 (Psychosocial or psychologic* or cognitive behav* or cognitive psychotherap* or psychoeducation or psycho‐education).ab,ti.
#25 (cogniti* and therap*).ab,ti.
#26 23 or 24 or 25
#27 7 and 13 and 26
#28 exp smoking cessation/
#29 exp smoking/
#30 (stopping smoking or smoking cessation* or giving up smoking* or quitting smoking or smoking or smoking behav* or smoking habit* or tobacco cessation).ab,ti.
#31 28 or 29 or 30
#32 7 and 13 and 31
Appendix 4. PsycINFO search strategy
S1 DE "Neoplasms" S2 TI colorectal cancer* OR AB colorectal cancer* S3 DE "Surgery" S4 TI surger* OR AB surger* S5 S1 OR S2 S6 S3 OR S4 S7 S5 AND S6 S8 DE "Psychosocial Rehabilitation" S9 TI psychosocial OR TI psychologic* OR TI psychotherap* OR TI cognitive behaviour therap* OR TI psycho‐education* S10 AB psychosocial OR AB psychologic* OR AB psychotherap* OR AB cognitive behaviour therap* OR AB psycho‐education* S11 S8 OR S9 OR S10 S12 S7 AND S11 S13 DE "Exercise" S14 TI ( exercise movement techniques OR exercise* ) OR AB ( exercise movement techniques OR exercise* ) S15 S13 OR S14 S16 S7 AND S15 S17 DE "Nutrition" S18 TI nutrition* OR AB nutrition* S19 S17 OR S18 S20 S7 AND S19 S21 DE "Smoking Cessation" S22 TI ( smok* or tobacco or cigarette* ) OR AB ( smok* or tobacco or cigarette* ) S23 S21 OR S22 S24 S7 AND S23
Appendix 5. Search trial registers
Clinicaltrials.gov
Status: all studies
Study type: interventional (clinical trial)
Condition or disease: colorectal cancer
Other terms: prehabilitation
25 studies found (not including trials not recruiting yet). Trial registration number only displayed when potentially eligible for inclusion in future updates.
Recruiting:
1. NCT04595604
2. NCT04167436
3. NCT03097224
Completed and published:
1. NCT03758209
2. NCT01356264
3. NCT02586701
4. NCT03361150
5. NCT01727570
6. NCT02502760
7. NCT02321813
Completed, not (yet) published:
1. NCT03096951
Status Unknown:
1. NCT03618329
Google Scholar
Search terms:
‐ Prehabilitation
‐ Preoperative optimization
‐ Randomised controlled trial
‐ Colorectal cancer
‐ Colorectal carcinoma
No new studies found (next to literature search and clinicaltrials.gov)
WHO‐ICTRP
Search terms:
‐ Prehabilitation
‐ Colorectal cancer
No new studies found.
Netherlands trial register
Search term:
‐ Prehabilitation
Recruiting:
1. NL5784
Data and analyses
Comparison 1. Prehabilitation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 6MWT four weeks postoperatively | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | 26.02 [‐13.81, 65.85] |
| 1.2 6MWT eight weeks postoperatively | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 26.58 [‐8.88, 62.04] |
| 1.3 Number of patients with complication | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.29] |
| 1.4 6MWT presurgery | 3 | 225 | Mean Difference (IV, Fixed, 95% CI) | 24.91 [11.24, 38.57] |
| 1.5 Emergency department visits | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 1.6 Re‐admissions | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.54, 2.65] |
1.1. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 1: 6MWT four weeks postoperatively
1.2. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 2: 6MWT eight weeks postoperatively
1.3. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 3: Number of patients with complication
1.4. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 4: 6MWT presurgery
1.5. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 5: Emergency department visits
1.6. Analysis.

Comparison 1: Prehabilitation versus control, Outcome 6: Re‐admissions
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bousquet‐Dion 2018.
| Study characteristics | ||
| Methods | Design: parallel‐arm single‐blinded, single centre randomised controlled trial. Setting: single tertiary care centre, Montreal, Quebec, Canada. Recruitment: participants were enrolled from December 2013 to August 2015. Consecutive patients scheduled for surgery were approached during the first consult to the surgeon. After consent, participants started with a baseline assessment approximately four weeks before surgery. They were assessed by a nutritionist, kinesiologist and psychology‐trained research team member. By computer‐generated random numbers in sealed envelopes, patients were randomly assigned on a 1:1 ratio to either PREHAB+ or REHAB. Follow‐up: follow‐up was up to eight weeks postoperatively. Surgical care followed ERAS guidelines. Blinding: outcome assessors of the primary outcome were blinded to group assignment. |
|
| Participants | Screened: 88 patients randomised: 80 patients analysed: 63 patients Inclusion criteria: adult patients with non‐metastatic colorectal cancer resection. Exclusion criteria: patients were ineligible in case of metastases, did not speak French or English, and/or had a contraindication for exercise. Baseline characteristics: median age in PREHAB+ group was 74 and 71 in the REHAB group. The majority was male, had a colonic resection and was operated laparoscopically in both groups. |
|
| Interventions | The content of the multimodal programme was identical in both groups. However, the timing of the start of the programme differed between groups. PREHAB+ (n = 37): the home‐based programme commenced immediately after baseline assessment. In the pre‐surgical period, patients attended an in‐laboratory exercise session supervised by a kinesiologist. Period between baseline assessment and surgery was approximately four weeks. After surgery, patients resumed the programme, only without the supervised sessions for an additional eight weeks. REHAB (n = 26): patients preoperatively received standard of care according to ERAS guidelines. Two days before surgery, an eight weeks home‐based post‐surgical rehabilitation programme was prescribed to the participants. Exercise Home‐based exercise (both groups): whole body exercise prescription, following the guidelines of the American College of Sports Medicine, individualised to participants' fitness level. The intensity of aerobic exercise was based on rate of perceived exertion (using Borg scale) and the 6MWT results at baseline. Aerobic exercise consisted of walking, cycling or jogging and participants were prescribed to perform 30 minutes of moderate intensity exercise (60‐70% of maximum heart rate calculated with Karvonen formula) three to four days per week. Resistance exercises were based on eight repetitions maximum test. Participants were instructed to perform three to four days per week up to two sets of 8‐15 repetitions of resistance exercise, consisting of eight exercises targeting major core, upper and lower limb muscle groups. Patients were provided with an elastic resistance band. Exercise intensity was evaluated and adjusted using the Borg scale. In‐hospital supervised sessions (PREHAB+): supervised by a kinesiologist patients returned to the hospital once a week to train for 30 minutes on a recumbent stepper or a standard treadmill, and to perform resistance exercises for 25 minutes. In‐hospital exercise (both groups): as soon as they were mobilised, patients were instructed to exercise. The REHAB group was able to review the post‐surgical programme. The PREHAB+ group recommenced the programme. Nutritional intervention A registered dietitian provided nutritional counselling based on the nutritional status as determined with the baseline assessment (SGA, NRS2002, 3‐day food diary, assessment of macronutrient intake and food choices). In case the participants did not meet the protein requirement of 1.2 g/kg of body weight per day (ESPEN guidelines, requirement in surgical patients) by diet alone, whey protein supplements were provided. Patients were instructed to ingest proteins within one hour of the exercise training. Mental intervention A psychology trained member of the research team provided personalised techniques, such as relaxation and breathing exercises, to alleviate anxiety in a 60‐minute session. Patients were asked to perform these techniques two to three times per week, using a compact disc with audio guidance. Furthermore, coping strategies were assessed. Booklet All patients received an information booklet, including a diary to record all activities. Follow‐up Patients were contacted on a weekly basis, by telephone. |
|
| Outcomes |
Primary Functional walking capacity as determined by the 6MWT at baseline, before surgery, and at four and eight weeks postoperatively. A change of at least 20 metres was considered to be clinically meaningful. The assessor used a standardized protocol and script and was blinded to group assignment. The results of the 6MWT were given in metres (mean, SD) per time point, per group as well as mean change from baseline per time point, per group. The number of patients (n, %) who improved more or less than 20 metres were reported as well. Secondary Energy expenditure was determined using the Community Healthy Activity Model Programme for Seniors (CHAMPS) questionnaire and were interpreted using the recommendations by the American Cancer Society (ACS) guidelines. CHAMPS was measured at baseline, before surgery, and at four and eight weeks postoperatively. The results were given in kcal/kg/week (median, IQR) per time point in both the PREHAB+ and in the REHAB group. Additionally, the number of patients (n, %) was given who met the ACS recommendations. Body composition determined with anthropometric measurements (bioelectrical impedance analysis and grip strength) are assessed at all four time points, however only the baseline results are presented. Psychological status determined with the Hospital Anxiety and Depression Scale (HADS) was assessed at four time points, however, only the baseline result is displayed. Mean compliance with the programme is presented as % since the previous measurement in both groups and is divided into compliance to the exercise and nutritional intervention. Reported postoperative outcomes 30 days after surgery included length of hospital stay, complications, re‐admissions and emergency department visits. Primary length of stay in the hospital and total hospitalisation were presented as median and IQR for both groups. The number of emergency department visits and re‐admissions are presented for both groups. For these outcomes the intention‐to‐treat analysis is displayed as well. Complications are presented as number and percentage of patients having at least one complication within 30 days, the type of complications are specified and the grade of most severe complication is given using the Clavien‐Dindo classification. |
|
| Notes | Trial registration number: NCT02586701 Funding source: this trial was funded by the Perioperative Programme Charitable Foundation and the Montreal General Hospital Foundation. No conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by computer‐generated random numbers." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed envelopes" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...indicating that bias in the form of contamination is also possible." Comment: No blinding. The authors discuss possible bias in the form of contamination in the control group; the primary outcome improved before the rehabilitation programme started. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome: 6MWT Quote: "The assessor...was blinded to group assignment" Comment: probably done Secondary outcomes Comment: Insufficient information on secondary outcomes to permit judgement of low or high risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: No intention‐to‐treat analysis is performed. Some patients where lost to follow‐up due to complications and are withdrawn from the analysis. Complication rate is one of the outcomes of this article. |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is not available and only the primary outcome is included in the trial registration. |
| Other bias | Unclear risk | Comment: there is insufficient information to assess an other potential bias. |
Carli 2020.
| Study characteristics | ||
| Methods | Design: parallel‐arm single‐blinded, 2‐site randomised controlled trial. Setting: two tertiary centres, Montreal, Quebec, Canada. Recruitment: participants were enrolled from the 7th of September 2015 to the 19th of June 2019. Consecutive patients eligible for participation were screened for frailty. Eligible patients were randomised on a 1:1 ratio to receive either a 4‐week prehabilitation programme (Prehab group) or a similar 4‐week postoperative rehabilitation programme (Rehab group). Both groups were assessed after randomisation by a kinesiologist, nutritionist and psychology‐trained nurse. Follow‐up: follow‐up was up to four weeks postoperatively. Surgical care followed ERAS guidelines. Blinding: outcome assessors, surgeons and statisticians were blinded to group assignment. |
|
| Participants | Screened: 418 patients randomised: 120 patients analysed: 110 patients Inclusion criteria: frail patients older than 65 years of age, scheduled for surgical treatment of non‐metastatic colorectal cancer. Patients were considered frail when scores of the Fried Frailty Index were ≥2. Exclusion criteria: patients were excluded in case of a Fried Frailty Index of 1, did not speak French or English, had metastatic disease and/or had a contraindication for exercise. Baseline characteristics: age in the Rehab group was higher (≥ 75 years of age: Prehab 32 (58.2%), Rehab 42 (76.4%) and patients had higher American Society of Anesthesiologists scores (ASA score of 3: Prehab 33 (60.0%), Rehab 43 (78.2%). The majority had a colonic resection with a minimal invasive surgical approach. |
|
| Interventions | The content of the multimodal programme was identical in both groups. However, the timing of the start of the programme differed between groups. Prehab (n = 55): the personalised, home‐based programme was prescribed by a kinesiologist, nutritionist and psychology‐trained nurse after the baseline visit. The programme continued for four weeks until surgery; no postoperative programme. Rehab (n = 55): baseline assessment was similar. The patients were prescribed an identical, personalised home‐based programme. However, the programme started postoperatively after discharge from the hospital and continued for four weeks. Patients were informed about the programme only a few days before surgery. Exercise Home‐based exercise (both groups): a personalised home‐based programme was prescribed containing aerobic activities (moderate‐intensity, 30‐minute daily walk) and resistance training (three times per week elastic band routine). Guidelines of the American College of Sports Medicine were followed. In‐hospital supervised sessions (both groups): once a week patients performed an in‐hospital training session supervised by a trained kinesiologist. The training sessions consisted of a 30‐minute moderate‐intensity exercise on a recumbent stepper (including a 5‐minute warming up), a 25‐minute resistance exercise using an elastic band, and five minutes of stretching. Details of the programme were similar to Bousquet‐Dion 2018. Nutritional intervention Nutritional status was determined by a registered dietitian using a 3‐day food diary, the SGA, and assessment of macronutrient intake and food choices. Dietary advices were provided together with counselling on caloric balance, bowel movement regularity, and glycaemic control. In case the patient did not meet a daily protein intake of 1.5g/kg of body weight (ESPEN guidelines), whey protein supplementation was prescribed and patients were instructed to ingest the supplementation within one hour of the exercise. Mental intervention The psychological intervention focused on perioperative fatigue, anxiety, and depression. A psychology‐trained nurse provided personalised coping strategies together with a compact disc containing instructions for guidance with the home‐based exercises. counselling regarding smoking and alcohol cessation was included in the consult and when indicated, nicotine replacement therapy was offered. Booklet All patients received an instructional booklet, including a diary to record daily activities. Follow‐up Patients were contacted on a weekly basis, by telephone, to report adherence to the home‐based programme. |
|
| Outcomes |
Primary Postoperative complications within 30 days postoperatively was the primary outcome. Complication rate was expressed as mean and median CCI. Furthermore, the number and percentages of patients having a complication scored with Clavien‐Dindo grade was displayed, together with the number and percentages of severe complications (definition of severe not specified). Secondary Reported postoperative outcomes 30 days after surgery included length of hospital stay, re‐admissions and emergency department visits. Primary length of stay in the hospital and total hospitalisation were presented as median and IQR for both groups. The number of emergency department visits and re‐admissions are presented for both groups as numbers (%). The following outcomes were assessed at baseline, before surgery, and four weeks after surgery: ‐ 6MWT. Results were given in metres (mean, SD) and number of patients (n, %) who improved their scores preoperatively compared to baseline, and number of patients who recovered to their baseline score four weeks after surgery. A change of at least 20 metres was considered to be clinically meaningful. ‐ Energy expenditure was determined using the Community Healthy Activity Model Programme for Seniors (CHAMPS) questionnaire. The results were given in kcal/kg/week (median, IQR) per time point in both groups. The results were dichotomised to light and moderate‐vigorous energy expenditure. ‐ SF‐36‐scores were presented as the total physical and total mental subscales (mean, SD) for all three time points. ‐ Anxiety and depression symptoms were assessed using HADS and were expressed as median with IQR for all three time points. ‐ Mean compliance with the programme is presented as % for both groups. Both compliance to the in‐hospital training sessions and the self‐reported compliance to the home‐based exercises. |
|
| Notes | Trial registration number: NCT02502760 Funding source: this trial was funded by a research grant from the Peri Operative Program charitable foundation and a peer‐reviewed grant from the Rossy Cancer Network. Conflict of interest: Dr. Carli reported the grant from the Rossy Cancer Network. Dr. Liberman reported nonfinancial support from Servier Laboratories and personal fees from Ipsen, Merck & Co, and Pfizer, Inc. The sponsors had no role in the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...achieved via computer‐generated random numbers..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "...placed in sealed, opaque, consecutively numbered envelopes" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...nature of the intervention, it was not possible to blind patients or intervention staff" Comment: No blinding. The authors did try to minimise performance bias; they did not present one of the programmes as potentially superior to the other. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcome assessors, surgeons and statisticians were blinded to group assignment." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: protocol published as supplementary material. Sample size per group differs between protocol and article. Not all randomised patients were included in the intention‐to‐treat analysis (reasons for dropout were mentioned). |
| Selective reporting (reporting bias) | Low risk | Comment: protocol available in supplementary material. All outcomes discussed. |
| Other bias | Unclear risk | Comment: there is insufficient information to assess an other potential bias. |
Gillis 2014.
| Study characteristics | ||
| Methods | Design: parallel‐arm single‐blind single centre superiority randomised controlled trial. Setting: single university‐affiliated tertiary care centre, Montreal, Quebec, Canada. Recruitment: participants were enrolled between November 2011 and March 2013. Consecutive patients scheduled for surgery were approached during the first office visit to the surgeon. After consent, approximately four weeks prior to surgery, participants underwent baseline assessment containing medical examination, baseline questionnaires, biochemical, functional and anthropometric measurements. Baseline assessment was performed by a kinesiologist, dietitian and psychologist. Consequently, patients were randomly assigned by computer‐generated random numbers on a 1:1 ratio, without stratification. Sequentially numbered sealed envelopes concealed group allocation. Follow‐up: follow‐up was up to eight weeks postoperatively. Perioperative care followed ERAS guidelines. Blinding: the team member conducting the measurements was not aware of group allocation. |
|
| Participants | Screened: 106 patients randomised: 89 patients analysed: 77 patients Inclusion criteria: adult patients with non‐metastatic colorectal cancer resection. Exclusion criteria: patients were ineligible if they did not speak French or English, and/or had a contraindication for exercise. Baseline characteristics: mean age in prehabilitation group was 65.7 and 66 in the rehabilitation group. The majority was male, had a colonic resection and was operated laparoscopically in both groups. |
|
| Interventions | The content of the multimodal programme was identical in both groups. However, the timing of the start of the programme differed between groups. Prehabilitation (n = 38): the home‐based programme commenced immediately after baseline assessment. Period between baseline assessment and surgery was approximately four weeks. After surgery, patients resumed the programme for an additional eight weeks. Rehabilitation (n = 39): patients preoperatively received standard of care according to ERAS guidelines. Within one week prior to surgery, the baseline assessment was performed and patients were instructed to start the programme at home after surgery; an eight weeks home‐based post‐surgical rehabilitation programme. Exercise Prescribed by a certified kinesiologist. Patients demonstrated the exercises and the kinesiologist provided feedback as necessary. Home‐based exercise (both groups): total body exercise of 50 minutes three times per week, following the guidelines of the American College of Sports Medicine, alternating between aerobic and resistance training and individualised to participants' fitness level. The intensity of aerobic exercise was based on rate of perceived exertion (using Borg scale) and the 6MWT results at baseline. The Karvonen formula was used to determine the heart rate to achieve the prescribed intensity. Aerobic exercise consisted of 20 minutes walking, jogging, cycling or swimming after a 5‐minute warm‐up. Resistance exercises consisted of 20 minutes of eight exercises targeting major muscle groups at an intensity of 8‐12 repetitions maximum. Exercise intensity was evaluated and adjusted using the Borg scale and the number of completed repetitions. Nutritional intervention A registered dietitian provided nutritional counselling based on the nutritional status as determined with the baseline assessment (3‐day food diary, assessment of macronutrient intake and food choices). Protein requirements were calculated as 1.2 g/kg of body weight per day (ESPEN guidelines, requirement in surgical patients). All patients received whey protein supplements in a quantity that matched the estimated dietary deficit. Patients were instructed to ingest proteins within one hour of the exercise regimen. Mental intervention All participants visited a psychologist for up to 60 minutes. The psychologist provided anxiety reducing techniques, such as relaxation and breathing exercises guided by audio provided on a compact disc. Furthermore, motivation enhancing suggestions were given to comply with the programme. Booklet All patients received an information booklet, including a diary to record all activities. Follow‐up Patients were contacted on a weekly basis, by telephone. |
|
| Outcomes |
Primary Functional walking capacity as determined by the 6MWT eight weeks postoperatively. A change of at least 20 metres was considered to be clinically meaningful. The 6MWT was conducted at baseline, before surgery, and at four and eight weeks postoperatively. The assessor was blinded to group assignment. The results of the 6MWT are given in mean change from baseline in metres (mean, SD) before surgery and eight weeks postoperatively for both groups. The number and percentage of patients who deteriorated, had no change or improved compared to baseline are provided as well. Secondary Self‐reported physical activity was determined using the Community Healthy Activity Model Programme for Seniors (CHAMPS) questionnaire and expressed as energy expenditure (kcal/kg per week). This outcome is presented as mean plus SD per time point for both groups. Health‐related quality of life was determined using the 36‐Item Short Form (SF‐36) Survey and is displayed as mean plus SD per subscale per time point for both groups. Anxiety and depression was assessed using the Hospital Anxiety and Depression Scale (HADS). Mean plus SD is presented for anxiety and depression subscales separately, for both groups. These outcomes were determined at baseline, before surgery, and four and eight weeks after surgery. Mean compliance with the programme is presented as % since the previous measurement in both groups. Complication rates are presented as number and percentages of patients having at least one complication within 30 days, the type of complications are specified and the grade of most severe complication is given using the Clavien‐Dindo classification. Primary length of stay in the hospital and total hospitalisation are presented as median and IQR for both groups. The number of emergency department visits and re‐admissions are presented for both groups. |
|
| Notes | Trial registration number: NCT01356264 Funding source: no information available. No conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by computer‐generated random numbers." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was concealed using sequentially numbered sealed envelopes." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "...including the potential bias that ensues from not being able to blind patients to group assignment." Comment: No blinding. The authors discuss the potential bias arising from not being able to blind the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome: 6MWT Quote: "...was conducted ... by an assessor blinded to group assignment." Comment: probably done Secondary outcomes Comment: Insufficient information on secondary outcomes to permit judgement of low or high risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: reasons for lost to follow were not clarified. Those patients were not included in the analyses and therefore an intention‐to‐treat analysis was not applied. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available, however, all prespecified outcomes included in the trial registration are reported in the publication. |
| Other bias | Unclear risk | Comment: there is insufficient information to assess an other potential bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Awasthi 2019 | Meta‐analysis, including prehabilitation data only (no control) |
| Barrett‐Bernstein 2019 | Meta‐analysis, including non‐randomised cohort data |
| Bruns 2019 | Non‐randomised design |
| Chen 2017 | Meta‐analysis of two studies already included in this review, no new data to assess the outcomes of this review |
| Fulop 2021 | Included colorectal surgery in general. Colorectal cancer patients not described separately in analysis. |
| Gillis 2016 | Unimodal prehabilitation |
| Gillis 2019 | Meta‐analysis of two studies already included in this review, no new data to assess the outcomes of this review |
| Karlsson 2019 | Unimodal prehabilitation |
| Klinkhammer‐Schalke 2020 | Programme initiated after surgery. |
| Lim 2019 | Non‐randomised design |
| Minnella 2016 | Meta‐analysis, including non‐randomised cohort data |
| Minnella 2017 | Meta‐analysis, including non‐randomised cohort data |
| Minnella 2020 | Comparison of two prehabilitation programmes, no control group. |
| Moug 2019 | Unimodal prehabilitation |
| Ommundsen 2017 | No structured intervention programme, multimodal programme not guaranteed |
| Onerup 2017 | Protocol publication of an unimodal programme |
| Trépanier 2019 | Meta‐analysis, including non‐randomised cohort data |
| Zhang 2014 | Postoperative intervention |
Characteristics of studies awaiting classification [ordered by study ID]
NCT03096951.
| Methods | Single‐blinded, parallel‐arm randomised controlled trial |
| Participants | Patients with colon cancer requiring surgery, more than 40 years of age with frailty phenotype criteria <3 (n = 70) |
| Interventions | Pre and postoperative tele‐supervised rehabilitation vs tele‐supervised rehabilitation postoperatively |
| Outcomes | Cardiorespiratory fitness assessed with 6MWT, muscle strength measured with dynamometer, muscle endurance determined with 1‐minute sit‐to‐stand test, QoL assessed with Euroqol‐5D‐3L (EQ‐5D‐3L) questionnaire, fatigue using Functional Assessment of Cancer Therapy: Fatigue questionnaire, physical activity assessed with International Physical Activity Questionnaire Short Form, executive functions using Trail Making test and Fluency test. Weight, lean body mass and fat body mass are measured with bioelectrical impedance analysis (BIA). Energy expenditure using Sensewear armband. A blood test is used to measure fasting glucose, fasting insulin, cholesterol, neutrophil/lymphocyte, C‐reactive protein. Adherence expressed as the number of sessions completed. |
| Notes |
Study completion date October 2019
Characteristics of ongoing studies [ordered by study ID]
NCT03097224.
| Study name | Prehabilitation before surgery in colorectal cancer with improved fast track rehabilitation: part 2 |
| Methods | Single‐blinded, parallel‐arm randomised controlled trial |
| Participants | Patients with colon cancer requiring surgery, more than 65 years of age with Frailty phenotype criteria ⪰3 (n = 40) |
| Interventions | Four weeks of tele‐supervised prehabilitation vs usual care |
| Outcomes | Cardiorespiratory fitness assessed with 6MWT, muscle strength measured with dynamometer, muscle endurance determined with 1‐minute sit‐to‐stand test, QoL assessed with Euroqol‐5D‐3L (EQ‐5D‐3L) questionnaire, fatigue using Functional Assessment of Cancer Therapy: Fatigue questionnaire, physical activity assessed with International Physical Activity Questionnaire Short Form, executive functions using Trail Making test and Fluency test. Weight, lean body mass and fat body mass are measured with bioelectrical impedance analysis (BIA). Energy expenditure using Sensewear armband. A blood test is used to measure fasting glucose, fasting insulin, cholesterol, neutrophil/lymphocyte, C‐reactive protein. Adherence expressed as the number of sessions completed. |
| Starting date | April 2017 |
| Contact information | Gilles Caty, gilles.caty@uclouvain.be & Elise Piraux, elise.piraux@uclouvain.be |
| Notes |
NCT04167436.
| Study name | Fit for Surgery. Multimodal Prehabilitation in Colorectal Cancer Patients |
| Methods | Single‐blinded, parallel‐arm randomised controlled trial |
| Participants | Patients with non‐metastatic colorectal cancer and WHO performance status I and II (n = 48) |
| Interventions | Intervention consists of a multimodal programme: supervised high‐intensity interval and strength training, nutritional counselling, protein and vitamin supplements, relaxation strategies, smoking cessation programme. |
| Outcomes | Quality of recovery 15, change in physical fitness pre‐surgery expressed as VO2 max, complications within 30 days after surgery expressed as the CCI, and change in immunological function (serum and tissue). pre‐surgery, four, eight and 52 weeks after surgery: quality of life (EORTC QLQ‐C30, EORTC QLQ‐C29, SF‐36), depression (PHQ‐9), anxiety (GAD‐7). pre‐surgery and four and eight weeks postoperatively: physical function determined with 6MWT, sit‐to‐stand test, stair climb test, leg extension test and handgrip strength, and nutritional status. Remission and cancer free survival until five years follow‐up. |
| Starting date | May 2019 |
| Contact information | Ramus D Bojesen, rasmus.bojesen@gmail.com |
| Notes |
NCT04595604.
| Study name | Long Term Effect of Trimodal Prehabilitation Compared to ERAS in Colorectal Cancer Surgery |
| Methods | Double‐blinded, parallel‐arm randomised controlled trial (2:1) |
| Participants | Patients with colorectal cancer requiring surgery (n = 500) |
| Interventions | Trimodal prehabilitation and ERAS vs ERAS and nutritional prehabilitation |
| Outcomes | Length of hospital stay, number of days on intensive care unit, morbidity (Clavien‐Dindo) after 7 and 30 days, and 30 and 90‐day mortality. Preoperative functional status determined with the 6MWT and forced vital capacity pre‐surgery, and four and eight weeks postoperatively. Change in diversity of fecal microbiota. |
| Starting date | September 2020 |
| Contact information | Balazs Banky, bankybalazs@tatabanyakorhaz.hu & Andris Fulop, fulop.andras2@gmail.com |
| Notes |
NL5784.
| Study name | Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and lower postoperative complications |
| Methods | Unblinded, parallel‐arm randomised controlled trial |
| Participants | Adult patients with non‐metastatic colorectal cancer undergoing surgery |
| Interventions | Intervention consist of a multimodal programme: supervised high‐intensity interval training and strength training, nutritional counselling, protein and vitamin supplements, relaxation strategies, smoking cessation programme. |
| Outcomes | Postoperative complications within 30 days expressed as CCI, functional capacity measured with 6MWT, stair climb test, sit‐to‐stand test and CPET, strength measured with indirect 1 repetition measures (1‐RM) and hand grip strength, HRQoL measured with EORTC QLQ‐C30 and ‐CR29, SF‐26, depression and anxiety measured with GAD‐7 and PHQ‐9 questionnaires. Physical activity level measured through activity questionnaire, nutritional status determined with PG‐SGA and measurements of skin folds and circumferences, compliance rate, length of hospital stay, and costs. |
| Starting date | October 2016 |
| Contact information | Gerrit Slooter, +31408886230 |
| Notes |
Differences between protocol and review
After reconsideration, we did not contacted experts in the field of prehabilitation as described in the protocol (section: electronic searches) since this is not usually done in a systematic review and would not have provided better certainty of evidence.
After publication of the protocol, during the start of the review, we decided to add two more secondary outcome measures of interest (Secondary outcomes): emergency department visits and re‐admission rate. Both outcomes contribute to assessing the effectiveness of a prehabilitation programme and are often included in published papers.
Regarding the PICO as described in the protocol, we have made several alterations in the final review. First, we have added that blinding was not a prerequisite for inclusion in the current review (Types of studies). The protocol did not include a statement on how to act toward the concept of blinding in our selection process. We believe that how this concept should be handled should optimally be described, since this is also an important criterion in the assessment of risk of bias. After discussion and consensus we decided that blinding was not a prerequisite for inclusion. Secondly, we removed the statement that patients not able to exercise due to an inability or contraindication should be excluded for this review. This is done so because after reconsideration we believe that this exclusion criterion is redundant, since the patient not able to exercise will presumably not be included in the trials of interest. Finally, the description of how we aimed to assess the 6MWT as an outcome has been reframed because we believed that the description in the protocol could be improved to make it more clearer for the reader.
Due to insufficient information, we have analysed the number of patients with a complication instead of including the Clavien‐Dindo scores or the Comprehensive Complication Index to assess the effect of prehabilitation on complication rate.
Many of the intended analyses, such as sensitivity and subgroup analyses, could not be performed due to the limited number of trials included. In future updates of this review, this will again be assessed.
Contributions of authors
Search for trials: Charlotte Molenaar Select which trials to include: Charlotte Molenaar, Stefan van Rooijen, Loes Janssen Extract data from trials: Charlotte Molenaar, Loes Janssen Enter data into RevMan: Charlotte Molenaar, Loes Janssen Carry out the analysis: Charlotte Molenaar, Loes Janssen Interpret the analysis: Charlotte Molenaar, Stefan van Rooijen, Hugo Fokkenrood, Rudi Roumen, Loes Janssen, Gerrit Slooter Draft the final review: Charlotte Molenaar, Stefan van Rooijen, Hugo Fokkenrood, Rudi Roumen, Loes Janssen, Gerrit Slooter Language and stylistics: Charlotte Molenaar, Loes Janssen, Gerrit Slooter
Sources of support
Internal sources
-
Salary , Netherlands
Authors were supported by their institutions in the form of a salary (not specifically provided for the conduct of this review)
External sources
-
No support was provided., Other
No support
Declarations of interest
SvR is author of the commercially available book ‘Fit4Life – het doktersrecept’ on the positive effects of a healthy lifestyle in general (not related to surgery nor to colorectal cancer).
GS is a paid consultant for Johnson & Johnson, a medical device company, since 2005.
SvR and GS are unpaid board members of the not‐for‐profit foundation ‘Fit4Surgery’ that aims to facilitate prehabilitation in Dutch hospitals.
There are no other known conflicts of interests.
Edited (no change to conclusions)
References
References to studies included in this review
Bousquet‐Dion 2018 {published data only}
- Bousquet-Dion G, Awasthi R, Loiselle S, Minnella EM, Agnihotram RV, Bergdahl A, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncologica 2018;57(6):849-59. [DOI: 10.1080/0284186X.2017.1423180] [DOI] [PubMed] [Google Scholar]
Carli 2020 {published data only}
- Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surgery 2020;155(3):233-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 2014 {published data only}
- Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized controlled trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121(5):937-47. [DOI: 10.1097/ALN.0000000000000393] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Awasthi 2019 {published data only}
- Awasthi R, Minnella EM, Ferreira V, Ramanakumar AV, Scheede-Bergdahl C, Carli F. Supervised exercise training with multimodal pre-habilitation leads to earlier functional recovery following colorectal cancer resection. Acta Anaesthesiologica Scandinavica 2019;63(4):461-7. [DOI: 10.1111/aas.13292] [DOI] [PubMed] [Google Scholar]
Barrett‐Bernstein 2019 {published data only}
- Barrett-Bernstein M, Carli F, Gamsa A, Scheede-Bergdahl C, Minnella EM, Ramanakumar AV, et al. Depression and functional status in colorectal cancer patients awaiting surgery: impact of a multimodal prehabilitation program. Health Psychology 2019;38(10):900-9. [DOI: 10.1037/hea0000781] [DOI] [PubMed] [Google Scholar]
Bruns 2019 {published data only}
- Bruns ER, Argillander TE, Schuijt HJ, Duijvendijk P, Zaag ES, Wassenaar EB, et al. Fit4SurgeryTV at-home prehabilitation for frail older patients planned for colorectal cancer surgery: a pilot study.. American Journal of Physical Medicine and Rehabilitation 2019;98(5):399-406. [DOI: 10.1097/PHM.0000000000001108] [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen BP, Awasthi R, Sweet SN, Minnella EM, Bergdahl A, Santa Mina D, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Supportive Care in Cancer 2017;25(1):33-40. [DOI: 10.1007/s00520-016-3379-8] [DOI] [PubMed] [Google Scholar]
Fulop 2021 {published data only}
- Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia 2021;76(1):82-90. [DOI: 10.1111/anae.15215] [DOI] [PubMed] [Google Scholar]
Gillis 2016 {published data only}
- Gillis C, Loiselle SE, Fiore JF Jr, Awasthi R, Wykes L, Liberman AS, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial. Journal of the Academy of Nutrition and Dietetics 2016;116(5):802-12. [DOI: 10.1016/j.jand.2015.06.007] [DOI] [PubMed] [Google Scholar]
Gillis 2019 {published data only}
- Gillis C, Fenton TR, Sajobi TT, Minnella EM, Awasthi R, Loiselle SÈ, et al. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: a pooled analysis of randomized controlled trials. Clinical Nutrition 2019;38(3):1053-60. [DOI: 10.1016/j.clnu.2018.06.982] [DOI] [PubMed] [Google Scholar]
Karlsson 2019 {published data only}
- Karlsson E, Farahnak P, Franzén E, Nygren-Bonnier M, Dronkers J, Meeteren N, et al. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery - a randomized controlled design. PLOS One 2019;14(7):e0219158. [DOI: 10.1371/journal.pone.0219158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinkhammer‐Schalke 2020 {published data only}
- Klinkhammer-Schalke M, Steinger B, Koller M, Zeman F, Fürst A, Gumpp J, et al. Diagnosing deficits in quality of life and providing tailored therapeutic options: results of a randomised trial in 220 patients with colorectal cancer. European Journal of Cancer 2020;130:102-13. [DOI: 10.1016/j.ejca.2020.01.025] [DOI] [PubMed] [Google Scholar]
Lim 2019 {published data only}41915584
- Lim SH, Chan SW, Lai JH, He HG. A qualitative evaluation of the STOMA psychosocial intervention programme for colorectal cancer patients with stoma. Journal of Advanced Nursing 2019;75(1):108-18. [DOI: 10.1111/jan.13821] [DOI] [PubMed] [Google Scholar]
Minnella 2016 {published data only}
- Minnella EM, Awasthi R, Gillis C, Fiore JF Jr, Liberman AS, Charlebois P, et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery 2016;160(4):1070-9. [DOI: 10.1016/j.surg.2016.05.036] [DOI] [PubMed] [Google Scholar]
Minnella 2017 {published data only}
- Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncologica 2017;56(2):295-300. [DOI: 10.1080/0284186X.2016.1268268] [DOI] [PubMed] [Google Scholar]
Minnella 2020 {published data only}
- Minnella EM, Ferreira V, Awasthi R, Charlebois P, Stein B, Liberman AS, et al. Effect of two different pre-operative exercise training regimens before colorectal surgery on functional capacity: a randomised controlled trial. European Journal of Anaesthesiology 2020;37(11):969-78. [DOI: 10.1097/EJA0000000000001215] [DOI] [PubMed] [Google Scholar]
Moug 2019 {published data only}
- Moug SJ, Mutrie N, Barry SJE, Mackay G, Steele RJC, Boachie C, et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the REx trial. Colorectal Disease 2019;21(5):548-62. [DOI: 10.1111/codi.14560] [DOI] [PubMed] [Google Scholar]
Ommundsen 2017 {published data only}
- Ommundsen N, Wyller TB, Nesbakken A, Bakka AO, Jordhøy MS, Skovlund E, et al. Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: a randomized controlled trial. Colorectal Disease 2018;20(1):16-25. [DOI: 10.1111/codi.13785] [DOI] [PubMed] [Google Scholar]
Onerup 2017 {published data only}
- Onerup A, Angenete E, Bock D, Börjesson M, Fagevik Olsén M, Grybäck Gillheimer E, et al. The effect of pre- and post-operative physical activity on recovery after colorectal cancer surgery (PHYSSURG-C): study protocol for a randomised controlled trial. Trials 2017;18(1):212. [DOI: 10.1186/s13063-017-1949-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trépanier 2019 {published data only}
- Trépanier M, Minnella EM, Paradis T, Awasthi R, Kaneva P, Schwartzman K, et al. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Annals of Surgery 2019;270(3):493-501. [DOI: 10.1097/SLA.0000000000003465] [DOI] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang M, Chan SW, You L, Wen Y, Peng L, Liu W, et al. The effectiveness of a self-efficacy-enhancing intervention for chinese patients with colorectal cancer: a randomized controlled trial with 6-month follow up. International Journal of Nursing Studies 2014;51(8):1083-92. [DOI: 10.1016/j.ijnurstu.2013.12.005] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT03096951 {unpublished data only}
- NCT03096951. Prehabilitation in colorectal cancer [Prehabilitation before surgery in colorectal cancer with improved fast track rehabilitation: part 1]. clinicaltrials.gov/ct2/show/NCT03096951 (first received 30 March 2017).
References to ongoing studies
NCT03097224 {unpublished data only}
- NCT03097224. Prehabilitation in frail colon cancer [Prehabilitation before surgery in colorectal cancer with improved fast track rehabilitation: part 2]. clinicaltrials.gov/ct2/show/NCT03097224 (first received 31 March 2017).
NCT04167436 {unpublished data only}
- NCT04167436. Fit for surgery. MultimodalpPrehabilitation in colorectal cancer patients [Fit for surgery. Clinical randomized trial of multimodal prehabilitation strategy in patients with colorectal cancer]. clinicaltrials.gov/ct2/show/NCT04167436 (first received 18 November 2019).
NCT04595604 {unpublished data only}
- NCT04595604. Long term effect of trimodal prehabilitation compared to ERAS in colorectal cancer surgery. (Prehab_2) [Long term effect of trimodal prehabilitation compared to ERAS in colorectal cancer surgery]. clinicaltrials.gov/ct2/show/NCT04595604 (first received 20 October 2020).
NL5784 {unpublished data only}NL5784
- NL5784. Prehabilitation for bowel cancer patients undergoing surgery to improve fitness and reduce complications [Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and lower postoperative complications]. trialregister.nl/trial/5784 (first received 01 August 2016).
Additional references
Astin 2014a
- Astin R, West MA, Loughney L, Grocott M, Jack S, Kemp G. Exercise training following neoadjuvant chemoradiotherapy in rectal cancer restores physical fitness and is associated with improved mitochondrial bioenergetics. American Journal of Respiratory and Critical Care Medicine 2014;189:Meeting abstracts. [ABSTRACT NUMBER: A3523] [Google Scholar]
Astin 2014b
- Astin R, Jack S, Loughney L, Barben C, Grocott M, Brown G, et al. Training response to a structured exercise program is associated with greater tumour regression in rectal cancer following neoadjuvant chemoradiotherapy. American Journal of Respiratory and Critical Care Medicine 2014;189:Meeting abstracts. [ABSTRACT NUMBER: A6663] [Google Scholar]
Baldini 2018
- Baldini G, Ferreira V, Carli F. Preoperative preparations for enhanced recovery after surgery programs: a role for prehabilitation. Surgical Clinics of North America 2018;98(6):1149-69. [DOI: 10.1016/j.suc.2018.07.004] [DOI] [PubMed] [Google Scholar]
Barberan‐Garcia 2018
- Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Annals of Surgery 2018;267(1):50-6. [DOI: 10.1097/SLA.0000000000002293] [DOI] [PubMed] [Google Scholar]
Barberan‐Garcia 2019
- Barberan-Garcia A, Ubre M, Pascual-Argente N, Risco R, Faner J, Balust J, et al. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: secondary results from a randomised controlled trial. British Journal of Anaesthesia 2019;123(4):450-6. [DOI: 10.1016/j.bja.2019.05.032] [DOI] [PubMed] [Google Scholar]
Bolshinsky 2018
- Bolshinsky V, Li MH, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Diseases of the Colon and Rectum 2018;61(1):124-38. [DOI: 10.1097/DCR.0000000000000987] [DOI] [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA - A Cancer Journal for Clinicians 2018;68(6):394-424. [DOI: 10.3322/caac.21492] [DOI] [PubMed] [Google Scholar]
Brown 2018
- Brown VSF, Prabhu P, Jourdan IC, Scott MJ, Rockall TA. Does dietary nitrate supplementation improve performance in cardiopulmonary exercise testing in patients with colorectal cancer? Surgical Endoscopy 2018;32:S430–S482. [DOI: 10.1007/s00464-018-6180-6] [ORAL PRESENTATION NUMBER: O034] [DOI] [Google Scholar]
Bruns 2016
- Bruns ER, den Heuvel B, Buskens CJ, Duijvendijk P, Festen S, Wassenaar EB, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Disease 2016;18(8):O267-77. [DOI: 10.1111/codi.13429] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carli 2017
- Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject matter experts. Physical Medicine and Rehabilitation Clinics of North America 2017;28(1):49-64. [DOI: 10.1016/j.pmr.2016.09.002] [DOI] [PubMed] [Google Scholar]
Carli 2018
- Carli F, Ferreira V. Prehabilitation: a new area of integration between geriatricians, anesthesiologists, and exercise therapists. Aging Clinical and Experimental Research 2018;30(3):241-4. [DOI: 10.1007/s40520-017-0875-8] [DOI] [PubMed] [Google Scholar]
Cavalheri 2017
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD012020. [DOI: 10.1002/14651858.CD012020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapter 10, Higgins 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Chapter 14, Higgins 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Chapter 23, Higgins 2021
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Chapter 6, Higgins 2021
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Chapter 8, Higgins 2021
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Cramer 2014
- Cramer H, Pokhrel B, Gass F, Eisenmann C, Lauche R, Meier B, et al. Hatha yoga for patients with colorectal cancer: a randomized controlled mixed-methods study. The Journal of Alternative and Complementary Medicine 2015;20(5):A52–3. [DOI: ] [Google Scholar]
Dunne 2016
- Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. British Journal of Surgery 2016;103(5):504-12. [DOI: 10.1002/bjs.10096] [DOI] [PubMed] [Google Scholar]
Egholm 2018
- Egholm JW, Pedersen B, Møller AM, Adami J, Juhl CB, Tønnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD008343. [DOI: 10.1002/14651858.CD008343.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 2018
- Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology 2018;155(2):391-410.e4. [DOI: 10.1053/j.gastro.2018.05.012] [DOI] [PubMed] [Google Scholar]
GRADEPro GDT [Computer program]
- GRADEpro GDT. Developed by Evidence Prime, Inc, Version accessed November 2019. Hamilton (ON): McMaster University (developed by Evidence Prime). Available from gradepro.org.
Gustafsson 2019
- Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World Journal of Surgery 2019;43(3):659-95. [DOI: 10.1007/s00268-018-4844-y] [DOI] [PubMed] [Google Scholar]
Hedrick 2017
- Hedrick TL, Harrigan AM, Thiele RH, Friel CM, Kozower BD, Stukenborg GJ. A pilot study of patient-centered outcome assessment using PROMIS for patients undergoing colorectal surgery. Supportive Care in Cancer 2017;25(10):3103-12. [DOI: 10.1007/s00520-017-3718-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heger 2019
- Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). Journal of Gastrointestinal Surgery 2020;24(6):1375-85. [DOI: 10.1007/s11605-019-04287-w] [DOI] [PubMed] [Google Scholar]
Heldens 2016
- Heldens AF, Bongers BC, Vos-Geelen J, Meeteren NL, Lenssen AF. Feasibility and preliminary effectiveness of a physical exercise training program during neoadjuvant chemoradiotherapy in individual patients with rectal cancer prior to major elective surgery. European Journal of Surgical Oncology 2016;42(9):1322-30. [DOI: 10.1016/j.ejso.2016.03.021] [DOI] [PubMed] [Google Scholar]
Hernon 2016
- Hernon J. Supportive exercise programmes for accelerating recovery after major abdominal cancer surgery (PREPARE-ABC). Colorectal Disease 2016;18(Suppl 1):126-8. [ABSTRACT NUMBER: T02] [DOI: 10.1111/codi.13446] [DOI] [PubMed] [Google Scholar]
Herrera 2016
- Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ, Gray N, et al. Neurochemical and behavioural indices of exercise reward are independent of exercise controllability. European Journal of Neuroscience 2016;43(9):1190-202. [DOI: 10.1111/ejn.13193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hijazi 2017
- Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. International Journal of Surgery 2017;39:156-62. [DOI: 10.1016/j.ijsu.2017.01.111] [DOI] [PubMed] [Google Scholar]
Hughes 2019
- Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJ, et al. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World Journal of Surgery 2019;43(7):1661-8. [DOI: 10.1007/s00268-019-04950-y] [DOI] [PubMed] [Google Scholar]
Kamarajah 2019
- Kamarajah SK, Bundred J, Weblin J, Tan BH. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery 2020;167(3):540-9. [DOI: 10.1016/j.surg.2019.07.032] [DOI] [PubMed] [Google Scholar]
Keeler 2017
- Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. British Journal of Surgery 2017;104(3):214-21. [DOI: 10.1002/bjs.10328] [DOI] [PubMed] [Google Scholar]
Keeler 2019
- Keeler BD, Dickson EA, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia 2019;74(6):714-25. [DOI: 10.1111/anae.14659] [DOI] [PubMed] [Google Scholar]
Kehlet 1997
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. British Journal of Anaesthesia 1997;78(5):606-17. [DOI: 10.1093/bja/78.5.606] [DOI] [PubMed] [Google Scholar]
Khuri 2005
- Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ, et al. Determinants of long-term survival after major surgery and the adverse effect on postoperative complications. Annals of Surgery 2005;242(3):326-43. [DOI: 10.1097/01.sla.0000179621.33268.83] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2019
- Lau CS, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. Journal of Gastrointestinal Surgery 2020;24(12):2829-37. [DOI: 10.1007/s11605-019-04436-1] [DOI] [PubMed] [Google Scholar]
Levett 2016
- Levett DZ, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Practice and Research: Clinical Anaesthesiology 2016;30(2):145-57. [DOI: ] [DOI] [PubMed] [Google Scholar]
Li 2013
- Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surgical Endoscopy 2013;27(4):1072-82. [DOI: 10.1007/s00464-012-2560-5] [DOI] [PubMed] [Google Scholar]
Liang 2018
- Liang MK, Bernardi K, Holihan JL, Cherla DV, Escamilla R, Lew DF, et al. Modifying risks in ventral hernia patients with prehabilitation: a randomized controlled trial. Annals of Surgery 2018;268(4):674-80. [DOI: 10.1097/SLA.0000000000002961] [DOI] [PubMed] [Google Scholar]
Lindbäck 2018
- Lindbäck Y, Tropp H, Enthoven P, Abbott A, Öberg B. PREPARE: presurgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial. Spine Journal 2018;18(8):1347-55. [DOI: 10.1016/j.spinee.2017.12.009] [DOI] [PubMed] [Google Scholar]
Liu 2019
- Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesthesia and Analgesia 2020;131(3):840-9. [DOI: 10.1213/ANE.0000000000004342] [DOI] [PubMed] [Google Scholar]
Loughney 2017
- Loughney L, West MA, Dimitrov BD, Kemp GJ, Grocott MP, Jack S. Physical activity levels in locally advanced rectal cancer patients following neoadjuvant chemoradiotherapy and an exercise training programme before surgery: a pilot study. Perioperative Medicine 2017;16(6):3. [DOI: 10.1186/s13741-017-0058-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luther 2018
- Luther A, Gabriel J, Watson RP, Francis NK. The impact of total body prehabilitation on post-operative outcomes after major abdominal surgery: a systematic review. World Journal of Surgery 2018;42(9):2781-91. [DOI: 10.1007/s00268-018-4569-y] [DOI] [PubMed] [Google Scholar]
Mayo 2011
- Mayo N, Feldman L, Carli F. Impact of preoperative change in physical function on postoperative recovery, argument supporting prehabilitation for colorectal surgery. Surgery 2011;150(3):505-14. [DOI: 10.1016/j.surg.2011.07.045] [DOI] [PubMed] [Google Scholar]
Minnella 2018a
- Minnella EM, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. European Journal of Surgical Oncology 2018;44(7):919-26. [DOI: 10.1016/j.ejso.2018.04.016] [DOI] [PubMed] [Google Scholar]
Minnella 2018b
- Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surgery 2018;153(12):1081-9. [DOI: 10.1001/jamasurg.2018.1645] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minnella 2019a
- Minnella EM, Liberman AS, Charlebois P, Stein B, Scheede-Bergdahl C, Awasthi R, et al. The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncologica 2019;58(5):573-8. [DOI: 10.1080/0284186X.2018.1557343] [DOI] [PubMed] [Google Scholar]
Minnella 2019b
- Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C, et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. European Urology Focus 2021;7(1):132-8. [DOI: 10.1016/j.euf.2019.05.016] [DOI] [PubMed] [Google Scholar]
Moran 2016
- Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016;160(5):1189-201. [DOI: 10.1016/j.surg.2016.05.014] [DOI] [PubMed] [Google Scholar]
Mouch 2019
- Mouch CA, Kenney BC, Lorch S, Montgomery JR, Gonzalez-Walker M, Bishop K, et al. Statewide prehabilitation program and episode payment in medicare beneficiaries. Journal of the American College of Surgeons 2020;230(3):306-13.e6. [DOI: 10.1016/j.jamcollsurg.2019.10.014] [DOI] [PubMed] [Google Scholar]
Nielsen 2008
- Nielsen PR, Andreasen J, Asmussen M, Tønnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Services Research 2008;8:209. [DOI: 10.1186/1472-6963-8-209.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papamichael 2015
- Papamichael D, Audisio RA, Glimelius B, Gramont A, Glynne-Jones R, Haller D, et al. Treatment of colorectal cancer in older patients: international society of geriatric oncology (SIOG) consensus recommendations 2013. Annals of Oncology 2015;26(3):463-76. [DOI: 10.1093/annonc/mdu253] [PMID: ] [DOI] [PubMed] [Google Scholar]
Piraux 2018
- Piraux E, Caty G, Reychler G. Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: a systematic review of randomised trials. Surgical Oncology 2018;27(3):584-94. [DOI: 10.1016/j.suronc.2018.07.007] [DOI] [PubMed] [Google Scholar]
Powell 2016
- Powell R, Scott NW, Manyande A, Bruce J, Vögele C, Byrne-Davis LM, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD008646. [DOI: 10.1002/14651858.CD008646.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rosero 2019
- Rosero ID, Ramírez-Vélez R, Lucia A, Martínez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-Analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers 2019;11(7):E944. [DOI: 10.3390/cancers11070944] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santa Mina 2014
- Santa Mina D, Clarke H, Ritvo P, Leung YW, Matthew AG, Katz J, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy 2014;100(3):196-207. [DOI: 10.1016/j.physio.2013.08.008] [DOI] [PubMed] [Google Scholar]
Santa Mina 2018
- Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SM, et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surgical Oncology 2018;27(2):289-98. [DOI: 10.1016/j.suronc.2018.05.010] [DOI] [PubMed] [Google Scholar]
Scheede‐Bergdahl 2019
- Scheede-Bergdahl C, Minnella EM, Carli F. Multi-modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia 2019;74(Suppl 1):20-6. [DOI: 10.1111/anae.14505] [DOI] [PubMed] [Google Scholar]
Sharp 2017
- Sharp L, McDevitt J, Brown C, Comber H. Smoking at diagnosis significantly decreases 5-year cancer-specific survival in a population-based cohort of 18166 colon cancer patients. Alimentary Pharmacology and Therapeutics 2017;45(6):788-800. [DOI: 10.1111/apt.13944] [DOI] [PubMed] [Google Scholar]
Silver 2013
- Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. American Journal of Physical Medicine and Rehabilitation 2013;92(8):715-27. [DOI: 10.1097/PHM.0b013e31829b4afe] [DOI] [PubMed] [Google Scholar]
Slankamenac 2013
- Slankamenac K, Graf R, Barkun J, Puhan M, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Annals of Surgery 2013;258(1):1-7. [DOI: 10.1097/SLA.0b013e318296c732] [DOI] [PubMed] [Google Scholar]
Steffens 2018
- Steffens D, Beckenkamp PR, Hancock M, Solomon M, Young J. Preoperative exercise halves the postoperative complication rate in patients with lung cancer: a systematic review of the effect of exercise on complications, length of stay and quality of life in patients with cancer. British Journal of Sports Medicine 2018;52(5):344. [DOI: 10.1136/bjsports-2017-098032] [DOI] [PubMed] [Google Scholar]
Tevis 2016
- Tevis SE, Kennedy GD. Postoperative complications: looking forward to a safer future. Clinics in Colon and Rectal Surgery 2016;29(3):246-52. [DOI: 10.1055/s-0036-1584501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomsen 2014
- Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD002294. [DOI: 10.1002/14651858.CD002294.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsimopoulou 2015
- Tsimopoulou I, Pasquali S, Howard R, Desai A, Gourevitch D, Tolosa I, et al. Psychological prehabilitation before cancer surgery: a systematic review. Annals of Surgical Oncology 2015;22(13):4117-23. [DOI: 10.1245/s10434-015-4550-z] [DOI] [PubMed] [Google Scholar]
Van der Meij 2016
- Meij E, Huirne JA, Bouwsma EV, Dongen JM, Terwee CB, de Ven PM, et al. Substitution of usual perioperative care by ehealth to enhance postoperative recovery in patients undergoing general surgical or gynecological procedures: study protocol of a randomized controlled trial. JMIR Research Protocols 2016;5(4):e245. [DOI: 10.2196/resprot.6580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rooijen 2016
- Rooijen SJ, Huisman D, Stuijvenberg M, Stens J, Roumen RM, Daams F, et al. Intraoperative modifiable risk factors of colorectal anastomotic leakage: why surgeons and anesthesiologists should act together. International Journal of Surgery 2016;36(Pt A):183-200. [DOI: 10.1016/j.ijsu.2016.09.098] [DOI] [PubMed] [Google Scholar]
Van Rooijen 2017
- Rooijen S, Carli F, Dalton SO, Johansen C, Dieleman J, Roumen R, et al. Preoperative modifiable risk factors in colorectal surgery: an observational cohort study identifying the possible value of prehabilitation. Acta Oncologica 2017;56(2):329-34. [DOI: 10.1080/0284186X.2016.1267872] [DOI] [PubMed] [Google Scholar]
Van Rooijen 2019a
- Rooijen SJ, Molenaar CJ, Schep G, Lieshout RH, Beijer S, Dubbers R, et al. Making patients fit for surgery: introducing a four pillar multimodal prehabilitation program in colorectal cancer. American Journal of Physical Medicine and Rehabilitation 2019;98(10):888-96. [DOI: 10.1097/PHM.0000000000001221] [DOI] [PubMed] [Google Scholar]
West 2015
- West MA, Loughney L, Lythgoe D, Barben CP, Sripadam R, Kemp GJ, et al. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: a blinded interventional pilot study. British Journal of Anaesthesia 2015;114(2):244-51. [DOI: 10.1093/bja/aeu318] [DOI] [PubMed] [Google Scholar]
West 2017
- West MA, Wischmeyer PE, Grocott MP. Prehabilitation and nutritional support to improve perioperative outcomes. Current Anesthesiology Reports 2017;7(4):340-9. [DOI: 10.1007/s40140-017-0245-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2010
- Wilson RJ, Davies S, Yates D, Redman J, Stone M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. British Journal of Anaesthesia 2010;105(3):297-303. [DOI: 10.1093/bja/aeq128] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Rooijen 2019b
- Rooijen SJ, Molenaar CJ, Fokkenrood HJ, Roumen RM, Slooter GD. Prehabilitation versus no prehabilitation to improve functional capacity, reduce postoperative complications and improve quality of life in colorectal cancer surgery. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD013259. [DOI: 10.1002/14651858.CD013259] [DOI] [Google Scholar]


