Abstract
Bioprinting has emerged as one of the most promising strategies for fabrication of functional organs in the lab as an alternative to transplant organs. While progress in the field has mostly been restricted to a few miniaturized tissues with minimal biological functionality until a few years ago, recent progress has advanced the concept of building three-dimensional multicellular organ complexity remarkably. This review discusses a series of milestones that have paved the way for bioprinting of tissue constructs that have advanced levels of biological and architectural functionality. Critical materials, engineering and biological challenges that are key to addressing the desirable function of engineered organs are presented. These are discussed in light of the many difficulties to replicate the heterotypic organization of multicellular solid organs, the nanoscale precision of the extracellular microenvironment in hierarchical tissues, as well as the advantages and limitations of existing bioprinting methods to adequately overcome these barriers. In summary, the advances of the field towards realistic manufacturing of functional organs have never been so extensive, and this manuscript serves as a road map for some of the recent progress and the challenges ahead.
Short description:
Bioprinting has emerged as a promising strategy for fabrication of functional organs in the lab. Until recently, however, this has mostly been restricted to a few miniaturized tissues with rudimentary biological activity. This review discusses a series of milestones that have paved the way for bioprinting of 3D multicellular tissues and organs with advanced levels of biological and architectural mimicry.
1. Introduction
The field of biofabrication has emerged in recent years to encompass a number of advanced engineering strategies, geared towards building biomimetic cellular tissue constructs.[1–5] Bioprinting is one, and arguably the most prominent, of these methods, and has generated enormous attention in the past decade.[6–10] The broad definition of biofabrication converges concepts that were independently developed for fields such as regenerative medicine, developmental biology and process engineering, among other themes.[3] Broadly speaking, each of these areas evolved at their own pace to address different needs in their respective industries. Only relatively recently these areas have converged into what is now a mainstream method in biomedical and regenerative engineering.
From a chronological perspective, it is straightforward to trace back the convergence of the themes that have evolved into what defines contemporary bioprinting. Early in the development of conventional office printers, biomedical researchers were already contemplating the prospects of dispensing cells out of conventional inkjet cartridges (cytoscribing), proposed by Klebe et al back in the late 80s.[11] Obviously, the process at the time did not intend to fabricate functional tissues with viable cells; but arguably that paved the way for far more advanced methods of cell and tissue printing that are utilized nowadays, and which will be discussed more broadly in this review. The emergence of widespread methods to isolate, expand and differentiate cells in the lab,[12] have contributed greatly to the biological developments that enabled the establishment of bioprinting early on.[13] Similarly, the ability to synthesize and manipulate the structural, physical and compositional properties of natural and synthetic biomolecules, have also contributed dramatically to the evolution of the field.[4, 14, 15] Another pillar, obviously, has been the evolution of methods to dispense materials, both synthetic and natural, in three-dimensions (3D), irrespective of the presence of cells.[16] But arguably, it is the intersection of these constituents and processes, rather than an isolated development, that catapulted the field of bioprinting into a mainstream line of science and technology.
For a long time regenerative medicine relied on the use of biocompatible materials that could be implanted in the body as a supporting scaffold for the invasion and repopulation of cells from the host, as originally proposed by Langer and Vacanti in some seminal papers in the 90s.[12, 17] With the popularization of densely cellularized materials in the form of cell-laden hydrogels [14, 18–22] again with substantial contributions from the Langer lab and other active groups in the field, in the early-mid 2000s, the concept of ‘fabricating with biological living matter’ became far more attainable. Similarly, an improved understanding of mechanisms of cell morphogenesis [23, 24] and the development of methods for manipulation of the process of cell and tissue assembly, mostly inspired by embryology and developmental cell biology,[25, 26] led to ability of manipulating high-density cell aggregates as ‘controllable biologic materials’, such as in the form of cell spheroids/aggregates,[27–30] embryoid bodies [31–33] and more recently organoids [34], or even assembloids [35–38]. These latter developments are largely credited to the early work of Forgacs, Mironov and other pioneers in the area of scaffold-free cell printing (Figure 1a). [25, 26] Similarly, this was enabled by a rapid revolution that the field of 3D printing experienced through the first and second decades of the current century, mostly driven by the expiration of protective patents that held the technology to a few pioneering companies profiting from the concept.[39] With the widespread popularization of 3D printing technology for polymer plastics,[40] metals,[41] viscous liquids [42–44] and many other synthetic materials including hydrogels,[45–48] it was only a question of time until the field of biomedical engineering adapted many of the well-developed scaffold materials and cell-manipulating techniques to enable controlled dispensing of these materials in biologically meaningful structures. Remarkable contributions by Anthony Atala’s group, especially in translating some of these early 3D printing technologies from the bench to the clinic, played a major role in facilitating the translation and progress of many of the technologies used today by many in the field, especially (but not only) on extrusion bioprinting of biocompatible materials for patient implantation [9].
Figure 1.
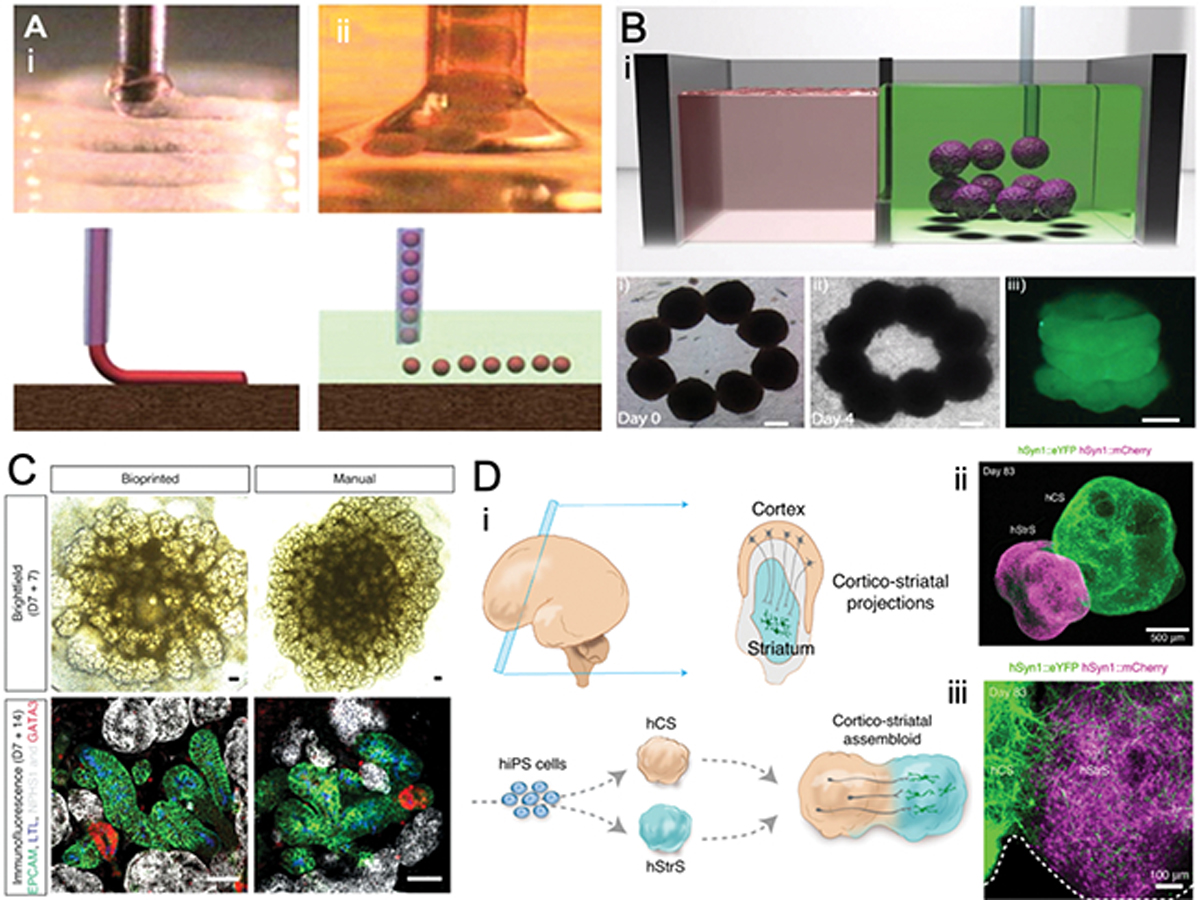
A) General view of high-density cell-laden hydrogel and tissue spheroid printing in air and fluid. Reproduced from [26] with permission. B) Aspiration assisted bioprinting showing the transfer of cell spheroids to fabricate high-density tubular construct in a granular microgel supporting bath. Reproduced from [109] with permission. C) Generation of iPSC-derived kidney organoids by extrusion-based bioprinting. Brightfield (day 7 + 7) and whole-mount immunofluorescence (day 7 + 14) images of manual and bioprinted kidney organoids generated simultaneously from the same batch of iPSC-derived intermediate mesoderm showing patterning and segmented nephrons. Reproduced from [73] with permission. D) (i) Schematic diagram showing cortico-striatal projections in the developing brain, and in-vitro modeling of cortico-striatal projections using human cortical spheroid with human striatal spheroid to form multicellular assembloids derived from hiPS cells. (ii) immunostaining of cortico-striatal at day 83 in low (Scale bar, 500 μm) and high magnification (Scale bar, 100 μm) showing intertwined axonal projections interconnecting the two different regions. Reproduced from [38] with permission.
Currently, there are many modalities of bioprinting that are available, and existing methods have been combined to result in an ever-increasing list of novel bioprinting strategies; we encourage the reader to refer to recent reviews of bioprinting methodologies for further details of such methods [7, 8, 49–51]. In a brief summary, from a fundamental technological perspective, most printers can be linked to one of the four original methods: extrusion, light, inkjet and laser bioprinting. In principle, extrusion relies on dispensing of a fiber in X-Y while the coordinated motion of a build platform or the dispensing head moves in Z [46, 52]. In light-based bioprinting, cells are loaded to a photocrosslinkable biomaterial, and the exposure of the photo-monomers to light, either via rastering or a planar photomask, generates an XY pattern of photopolymerized material, while the build platform moves in Z for a sequenced deposition of layers [53, 54]. On inkjet bioprinting, cells and materials of low viscosity are dispensed using, a jetting system onto a platform that coordinates the XYZ movement of the patterned substrate [55]. Laser bioprinters, on the other hand, use a different approach where cells or materials are loaded to a laser-sensitive tape, and as the rastering of such a laser on the surface of the cell-covered tape occurs, cells are transferred onto an underlying substrate; the coordinated motion of the laser light with the Z movement of the underlying platform ensures the three-dimensionality of the construct [56].
While the field has evolved at an increasingly faster pace, our ability to generate truly meaningful biological substitutes with clinical relevance and size via bioprinting, has remained limited to a few tissues that still rely on the ability of the body to remodel cell-laden tissue constructs.[9] And while it has been suggested that, perhaps, biomimicry may reach a point where increased complexity no longer improves functional outcomes,[9] replicating tissue microstructure and cellular organization remains a critical challenge in the field. Here we discuss the myriad cell, microenvironment, engineering and materials challenges that, we argue, will require some attention before bioprinting can become an efficient tool for fabrication of sizeable complex tissues and organs for both clinical and research use. The specific challenges and possible solutions that will enable the manufacturing of heterotypic tissue constructs with the desired interactions between the multiple populations of cells in an organ are discussed; the engineering challenges and limitations of existing methods in reproducing the microscale complexity of human biology are also presented, focusing mostly on biologic and materials questions, and less so on hardware challenges, which have been covered previously. Additionally, the limited ability of existing methods to control the nanoscale heterogeneous and hierarchical organization of the human native extracellular microenvironment is revisited, together with recent solutions to integrate and feed multiple tissue types via an efficient circulatory system based on bioprinting methods. Lastly, this review covers existing challenges and presents possible solutions that may shine light on potential transformative outcomes for the field of organ and tissue engineering via bioprinting.
One relevant aspect that is worth pointing out is that despite years of discussions in the field, there is still controversy around the definition of bioprinting.[3, 57, 58] On one hand, there is a school of thought that defends the perspective that bioprinting pertains to the dispensing of biological material, be it nonliving biological molecules (proteins, cytokines, etc.) or live components (mammalian, bacterial cells, viruses etc.). On the other hand, there is the competing perspective that bioprinting should only refer to the deposition of biomaterials composed of, at least in part, living biological materials, such as the ones referred above. One agreement that appears to be drawn between these competing definitions is that inert synthetic materials that are printed for applications in medicine or biology, but are not constituted of any form of biological matter, should not fall under the true umbrella of bioprinting. This may be seen as a question of lower relevance, however, one may argue that the complexity of the processes and techniques that are associated with manipulating living biological systems, far exceed those that are associated with printing of non-living matter. Additionally it is worth pointing out that these distinct definitions of bioprinting, considering cell-rich versus cell-free approaches, speak to different objectives of the fabrication process. While cell-free printing requires post-processing of the printed part by cells to result in meaningful biological function, bioprinting with cellularized materials refers to the goal of direct fabrication of living tissues and organs, which is, perhaps, more closely aligned with the overarching goal of controlled organ fabrication. For these reasons, this review will use the definition of bioprinting that considers living cells as a bioink, whereas methods that 3D print with non-living biological matter will be referred to simply as 3D printing (without bio), for clarity.
2. The complexity of bioprinting heterotypic organs with microscale resolution
2.1. The complex cellular architecture of solid organs – the liver as a model
One of the key parameters that define human biology is the complexity of the composition and the biological processes that regulate the human body. Although connective tissues can be perceived as simplified mixtures of proteins and cells that perform relatively simple and repetitive functions of structural support, specialized tissues and organs, especially those that are solid and cellularly dense, are characterized by their increased architectural intricacy.
For instance, one of the tissues that have received extensive attention in the bioprinting community, both in academic[59–63] and commercial settings,[64, 65] is the liver. So we will use this organ as a core example to illustrate the inherent challenges of mimicking the complexity of vital solid organs of comparable difficulty on the microscale and in 3D.
The liver is both subject to irreparable damage due the improper interaction with drugs and exogenous compounds, and is also difficult to transplant and find matched donors. Taking the liver as an example, it is easy to identify many of the biological challenges that may be associated with reproducing the native complexity of human organs. The archetypical building block of the liver is the hepatic lobule.[66] In a single lobular unit, there is a very carefully structured, three-dimensionally organized layer of a few cells, which are closely connected to one another in palisades of hepatocytes. These cells polarize at the single cellular level, and form cell-cell communication to enable the formation of biliary canaliculi between cells, which will drain the liver metabolites into an adjacent biliary duct.[67, 68] This palisade of hepatocytes, which is confined to an area comprising of only a few cells spaced transversally between sinusoids, is bordered by a plasma-rich region, which is known as the space of Disse.[69] This region is populated by a low concentration of stellate and dendritic cells, which participate in responding to liver injury and inducing fibrosis, also playing a critical role in development and regeneration.[69] Adjacent to that, there are endothelial sinusoidal cells, which coat the lumen of the liver circulatory system (sinusoids), and harbor Kupfer cells, connecting the central hepatic vein to the hepatic portal vein, and artery branches.[66] Despite this intricate organization, this actually represents only a simplified view of one single liver lobule at a planar dissecting view, and does not account for the complex three-dimensionality of these same cell-cell and cell-matrix interactions, that repeat themselves over 1 million times (estimated number of individual lobules) in a healthy adult liver. Moreover, this is only restricted to an area of approximately 200 μm in diameter (average size of a single liver lobule). Therefore, from a reverse engineering standpoint, there is hardly anything more complicated in tissue engineering than recreating the architectural organization of a solid organ,[70] like the liver.
In the example above, there are at least 5 different populations of specialized cells that are organized with literally single cell precision in space, and it is well established that their expected function depends almost exclusively on the presence and interaction of the adjacent cell type,[70] as well as the distance from one cell to the next [71]. Therefore, the challenges associated with reproducing this highly intricate set of cell-cell and cell-matrix interactions are many. And while recent organoid 3D printing efforts [72–75] have focused on developing methods to coax stem cells into self-organizing into mature organ-like cell-aggregates, without the need for positioning cells with single-cell precision, the concept of 3D bioprinting is geared towards exactly that – putting the exact cells at their exact place. Therefore, a critical question that comes up is how much bioprinting precision is the minimum necessary to reproduce this set of interactions in a manner that the final outcome is a well-functioning, phenotypically accurate and reliable organ?
2.2. Recent efforts towards bioprinting of complex heterotypic organs
It is critical to acknowledge that the field of bioprinting, and most of the other biofabrication methods available, have enabled the engineering of many complexly structured tissue and organ-like constructs.[7, 9, 76, 77] However, generally speaking, we are only beginning to understand and reproduce the true complexity that is endowed by this set of interactions in engineered tissue constructs. How bioprinting may enable this set of complex interactions remains relatively poorly explored. For instance, very early on, we have enabled the extrusion of hepatocyte-laden methacrylated gelatin (GelMA) hydrogels with minimal phenotypic function, determined by the secretion of trace levels of albumin by bioprinted liver cells.[59] Others have continued in this direction to illustrate the ability of simple bioprinted hepatocyte aggregates embedded in gelatin methacryloyl hydrogels to metabolize different drugs.[78] In both cases no biomimetic structure or architecture were attempted to be reproduced, owning to the relatively low resolution of the extrusion printing system that was utilized. In a far more elegant approach, Ma et al developed a series of light based polymerization bioprinting methods to reproduce the bear-minimal structure of planar lobule-like organization.[79] In more recent efforts, advanced extrusion systems where developed to dispense fiber-like structures with a pre-set liver lobule-like structure.[80, 81] On a separate strategy, others have used a scaffold-free extrusion bioprinting approach to dispense high-density aggregates of liver cells that self-organize to have some liver-like activity.[82] Irrespective of the strategy in question, most, if not all of these methods, do not reproduce the true complexity that is inherent to the actual organ, as explained in the earlier paragraphs. These bioprinted tissues have been typically composed of 2–3 cells types or less, as opposed to five or more, and the extent of structural and biological interactions that are required to ensure adequate organ function remain recognizably difficult to reproduce.[8] This has prevented these bioprinted tissues from achieving any meaningful success in reproducing full organ functionality, despite their early success in detecting drug toxicity in-vitro [78] and replicating some key organ structures. The questions that remain then are, what are the critical biological and engineering challenges that need to be addressed to enable the deposition of structures that can actually recapitulate the level of complexity seen in a native organ?
2.3. Culturing bioprinted multilineage tissue constructs
In the context of building the biological complexity described above, one solution, is the development of methods for rapid deposition of cells in 3D, at single cell resolution, which we discuss further throughout this section and in section 4 below. Associated with this solution, there are challenges related with the basic biology of orchestrating adequate cell-cell communication in heterotypic engineered tissues. In other words, if one is to bioprint tissues as biologically complex as the liver, or any other solid organ with multiple cell types, it is vital that we understand the conditions at which these cells can properly communicate and function in synergy. For instance, it is well known in tissue engineering that co-cultures of tissues with more than 2 cell types can be a challenge if these cells require a different set of nutrients, vitamins and growth factors to perform their desired functions.[83–85] Tissues as diverse as the liver could require as many as 5 different compositions of cell medium, at obviously different ratios, to account for the different concentrations of each cell type. The simple optimization of this medium composition poses a significant challenge, which is not unique to bioprinted constructs, but since printing should facilitate the fabrication of multi-component tissues, this becomes an inherent problem in the field. There has been efforts to develop and test universal media, and some of these alternatives have been developed and tested.[86] But still, this is a critical biological challenge to enable effective and predictable function, as opposed to solely dispensing cells of different lineages next to one another. Similarly, the optimal timing and ratio of these cell interactions remain poorly understood. One approach is to bioprint terminally differentiated cell types at their end stage of maturation, which has been the mainstream strategy in the bioprinting field so far.[7, 8] Another strategy is to bioprint stem cells enriched with the correct cues [45–48, 87, 88] that can then stimulate cells to evolve into different lineages and communicate during such process.[45, 46]
Similarly, with the explosion of organoid technologies in recent years,[89–100] and many successful results obtained with simpler methods for controlled assembly of engineered organoids – commonly referred to as assembloids,[35–38, 101, 102] it is natural to expect that far more complex organoid assembly processes can benefit from existing bioprinting methods, such as those utilized for scaffold free bioprinting in the early days of the technology[5, 26, 103–106] (Figure 1a) and currently marketed by Organovo, as well as more recent spheroid assembly processes.[107–109] Such recent processes have taken advantage of the ability of cell spheroids to be aspirated at the end of a small diameter pipette without entering the pipette channel, such that the spheroids are attached at the end of the pipette like a hanging microtissue that can be transferred from one place to another. This allows tissues to be built up one spheroid at a time to result in heterotypic complexity of the engineered constructs.[108, 109] (Figure 1b,c) Between the referred methods of (a) organoid or undifferentiated stem-cell printing that is induced to self-organize into a functional organ, and (b) printing of terminally differentiated cells that establish cell-cell communication to enable function, it remains to be seen what the better approach may be. Recent efforts on bioprinting of scaffold free kidney organoids point to relevant and successful directions in this area,[72, 75] and in that of bioprinted assembloids (Figure 1d,e). As it has been the case for other successful examples of bioprinted tissues in the field, it may be advantageous to design techniques that combine bioprinting of terminally differentiated cells, such as cells in the vasculature, in connection with organoid-like approaches, where undifferentiated cells can self-organize and differentiate to give rise to more advanced tissue morphologies.
Finally, although many of the efforts to develop improved bioprinting strategies have concentrated on the many engineering questions surrounding the method, it appears that bioprinting creates a set of new biological questions and challenges that are only beginning to be understood. One may argue that the biological challenges may be as consequential as the engineering ones, since reproducing adequate tissue and organ function is invariably dependent on biology, and less so on engineering advances.
2.4. Bioprinting of organ constructs towards advanced functionality
Although bioprinting of complex multi-lineage heterotypic tissue constructs, like the ones exemplified above, remain highly challenging, bioprinting of relatively simpler tissue structures, that also have highly intricate function, have been very successfully reported in the literature in recent years. For instance, a highly successful report by the Atala group showed for the first time the bioprinting of cellularized tissue structures with dimensions and function remarkably similar to that in humans[77] (Figure 2a). The work focused primarily on muskuloskeletal tissues, with advanced examples of cartilage, bone and muscle, and these examples certainly point to the possibilities of bioprinting for more complex organs. Using an extrusion bioprinter equipped with cell-laden hydrogels, sacrificial inks (pluronic) and a supporting ink scaffold composed of PLGA, the authors were able to replicate the architecture of an entire human ear of infant size.[77] Replicating the structure of the ear has been attempted and reported several times before by various groups,[110–118] however, what is remarkable about this achievement was the ability of the bioprinted tissue to develop and mature in-vivo, and virtually match a series of functional parameters from the native counterpart, including microstructure and function. The deposition of collagen, glycosaminoglycans, the histological characteristics, and even many of the mechanical properties at a tissue level, were virtually the same as those from the native tissue.[77] This is an important achievement, especially considering that scalability has been a great challenge in the field. The authors had considerable success by taking advantage of a set of techniques that were developed independently, and then integrated into this method, which points to future directions in the field. For instance, tissue constructs were fabricated by combining a supporting PLGA scaffold of higher rigidity, which was extruded adjacent to cell-laden hydrogels with two cells types, while microchannels that were analogous to a circulatory system were fabricated by printing a sacrificial template material. Each one of these techniques were developed independently in previous works, showing the complexity of creating a successful multicomponent system.
Figure 2.
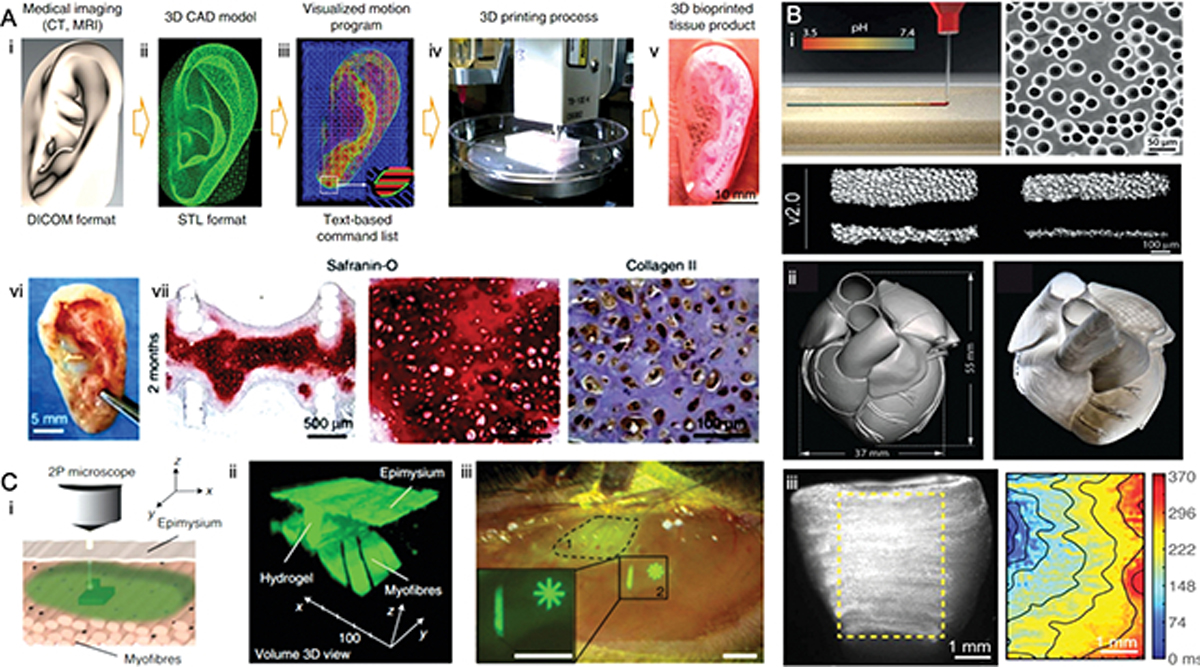
A) An integrated tissue–organ printer (ITOP) for ear cartilage reconstruction. (i) A 3D CAD model of human ear developed from medical image data and a (ii) visualized motion program was used to print 3D architecture of human ear. (iii) Green, blue, and red lines indicate dispensing paths of PCL, Pluronic F-127 and cell-laden hydrogel to achieve layer-by-layer 3D printing of ear cartilage. (vi) Gross appearance, Safranin-O staining and collagen type II immunostaining of the retrieved ear construct after 2 months post-implantation demonstrates the increased (vii) GAG and collagen contents of the bioprinted ear cartilage tissues post-implantation. Reproduced from [77] with permission. B) FRESH 3D-bioprinting of heart tissue using dispensed collagen. Schematic of collagen solution injected into the FRESH support bath (pH 7.4) where collagen undergoes fibrillogenesis (i). The FRESH v2.0 method can print collagen filaments of 20 to 200 μm in diameter. (ii) A 3D model of derived from a magnetic resonance image of an infant human heart (left) and FRESH-printed collagen heart (right). (iii) A dual-material FRESH printing method using a collagen ink and a high-concentration cardiac cell ink was used to build a ventricle model (right) with uniform cell distribution that achieved spontaneous, directional calcium wave propagation with adequate conduction velocity; Reproduced from [134] with permission. C) Intravital 3D bioprinting schematic showing two-photon crosslinking of HCC–hydrogel into dermis across the epidermis and skeletal muscle across epimysium. A 3D-volume reconstruction showing HCC–8-arm PEG structure fabricated between undamaged myofibres and epimysium of skeletal muscle in GFP+ mice. Reproduced from [127] with permission.
The far majority of bioprinting methods depend on the deposition of living biological matter onto a static and stable substrate that prevents cells from being flushed away during the printing process. And without being able to rapidly immerse cells into a medium, the tendency for cells to undergo cell-death is typically high.[59, 119] Not only that, but conventionally, the bigger the cellularized construct, the lower the cell viability will be.[59] This trend is consistent for virtually all bioprinting methods. Many strategies attempted to overcome this limitation, such as the bioprinting of biomaterials connected to syringe pumps that increased the hydration of a cell-laden hydrogels while also promoting biomaterial crosslinking,[47, 120–123] or the use of humidifiers onto the bioprinted structure[124, 125] to ensure immediate hydration and maintenance of temperature and oxygenation, even the integration of an entire incubating system onto commercial bioprinters, equipped with UV light, temperature and humidity control.[126] One very interesting recent solution to this problem, has been the development of methods of intravital bioprinting. To that end, Urciuolo et al. injected cell-laden photosensitive polymer hydrogels that were subsequently cross-linked using a bio-orthogonal two-photon cycloaddition polymerization in live mice inside tissues including skeletal muscle, brain, and skin (Figure 2c).[127] Such an important milestone in the field was mostly enabled by the identification of 7-hydroxycoumarin-3-carboxylic acid as a molecules that, when functionalized into polyethylene glycol or gelatin hydrogels, allowed for deeper penetration of light and cross-linking of the hydrogel pre-polymers at specific wavelengths that enabled both in-vivo hydrogel patterning, as well as well as desirable cell viability. This has been only the first method to 3D bioprinting cells in-vivo using light-based technologies and through the epithelium, however the concept of in-situ bioprinting has been around for nearly a decade,[128–130] and so have been other methods of transdermal hydrogel photopolymerization in-vivo.[131–133]
Despite the emerging methods of intra-vital 3D bioprinting,[127] the seemingly simple problem of printing cells in a liquid bath was only truly overcome with the development of printing methods that enabled the omnidirectional extrusion of viscous fluids or pre-solidified granular materials onto a non-Newtonian liquid bath. [109, 134–141] The development of printing methods that allowed biomaterials and cells to be dispensed within a liquid bath is certainly a stepping-stone in the field towards fabrication of complexly organized tissues and organs. These methods, which were originally developed for fabrication of cell-free biomimetic structures [139, 142] typically work with two components: a bath of granular microgels which are immersed in a precise range of temperature, pH or chemical composition; and an extruder, which dispenses a biomaterial – either with or without cells – which will undergo rapid gelation upon contact with the supporting bath. The granular material allows the print-head to travel freely in X, Y and Z, and to dispense cells in arbitrary locations without the need to fabricate a supporting structure, which has been standard practice in the field. In other words, before these developments, in order to extrusion-print the shape of tissues with complex undercuts and hollowed architectures, one would have to come up with an architectural strategy to prevent the unsupported structures from collapsing. With these recently developed strategies, the granular bath provides the structural support in any direction, while the tissue is printed, and upon hardening of the bioink, the engineered tissue construct can be retrieved by sacrificing the granular supporting material. A number of reports have used these technologies,[109, 134–141] and perhaps the most impacting example of use of this technology was reported on the FRESH-enabled bioprinting of autonomous beating hearts with human-like features (Figure 2c).[134] In that paper, the authors demonstrated the precise imaging, slicing, code-generation and subsequent extrusion printing of a high-density (cell-free) collagen material in the shape of a tricuspid heart valve, and also a full length neonatal heart, with all vessels, chambers and architectural complexity of the native tissue.[134] They then went on to demonstrate that structurally simpler samples filled with high-density cardiac cell inks could actually be manufactured to respond with synchronous beating, with or without stimulation, which is a remarkable advance to the field. In this particular method, the authors took advantage of the controllable dissolution of gelatin microbeads that can be dissolved simply by increasing the liquid temperature. The gelatin beads were immersed in a solution of pH 7.4, which allowed extruded fibers of high-density collagen to gel as the print-head was moved in X-Y-Z. Upon printing, the gelatin was sacrificed and the printed tissues could be retrieved. A similar report was also published around the same time, where a different group used a similar strategy to bioprint a miniaturized beating hearts,[143] but instead of using the FRESH approach, this team developed a simples strategy to dispense an omentum-derived hydrogel on a supporting medium containing sodium alginate, Xanthan gum, and calcium carbonate, without the granular microgels. It is worth noting here that the biological complexity of orchestrating the multi-typic cell-cell interactions that ensure the function of a continuously (long-term) beating heart in these reports was not yet possible;[134, 143] which highlights the immense challenges associated with the biology of printing functional tissues and organs, which are further discussed in section 4.
In summary, while recent examples of bioprinting of cell-laden hydrogels supported by rigid inks and sacrificial biomaterials for fabrication of hollow channels have provided, perhaps, the most advanced examples of replicating structure and function in bioprinted human-sized constructs, it appears as though the bioprinting of materials in granular media is where the state-of-the-art methods of replicating organ complexity will derive from. Advances in light photopolymerization printing also have strong chances of providing substantial developments in the field, and these are discussed more in depth in the following section.
3. The complexity of tissue connectivity – bioprinting the human vasculature
While bioprinting of specific tissues and organs of high cellular heterogeneity and complex structural organization still poses a significant challenge, the intercommunication of such cells in a bioprinted 3D constructs appears to be something that is more advanced in the field. Vascularization is the most basic requirement for tissue survival in the human body. A functional vasculature guarantees adequate tissue oxygenation, nutrient delivery, and removal of waste products.[144] Hence a blood capillary must be ultimately located within at least 200 μm of virtually every cell in the body. Printing of the human vasculature has been a consistent focus of research in biofabrication for a number of years, and to date, there is a variety of methods and printing-based strategies that enable the fabrication of cellularized tissue constructs with a remarkably high degree of success. A couple of review papers and book chapters are noteworthy in this topic. The Miller group has authored a comprehensive review of existing methods for printing of microchannels and vasculature in a recent report in Lab on a Chip, [145] whereas our group discussed a number of biological challenges associated with replicating the true function of vascular capillaries that have been 3D printed on a recent book chapter.[146] Several other reviews have been published on this subject, and the reader is encouraged to refer to these publications for more details on these topics.
One aspect that is noteworthy regarding 3D printing of the human vasculature is that many methods have relied on an indirect printing approach, where the capillary microchannels and vessels are first created and the subsequently seeded with cultures of endothelial cells (ECs) or mixtures of ECs and other supporting perivascular cell types. One of the first methods developed to generate bifurcating 3D capillaries in cell-laden tissue constructs was developed by the Chen group,[147] where the authors used an inexpensive RepRap extrusion 3D printer to dispense a glass carbohydrate type of material that offered sufficient rigidity upon printing. After coating with a protective PLGA layer, the carbohydrate glass could be fully covered with a cell-laden hydrogel pre-polymer, which was then polymerized before sacrificing the 3D printed mold via dissolution. This was a remarkable development in the field of tissue printing at the time, since it enabled the fabrication of much larger vascularized tissues by ensuring that cells across the thickness of larger constructs could receive sufficient oxygen and nutrients to survive, irrespective of the tissue type and dimensions. This work also builds significantly on previous projects that used a similar methodology to microfabricate planar microchannels in cell-laden tissue constructs, which then needed to be stacked to form more complex tissue structures,[119, 148–153] which highlights the importance of adding a third dimension to engineered tissues via printing. A few years later, a similar strategy was published using a pluronic gel as a sacrificial template for the same goals of creating a circulatory system inside of a cell-laden hydrogel,[154, 155] and indeed such methods enabled the creating of pericyte-supported cell-dense vascularized tissues that responded biologically as expected from a fully vascularized human tissue.[154] Contemporary work from our team utilized a similar methodology to 3D print with an agarose ink, which did not require coating or dissolution of the template mold. Instead, the 3D printed gels could be covered with cell-laden biomaterials and subsequently aspirated or pulled out of the construct to avoid any interaction between the template material with the surrounding cells. We were then able to demonstrate that the 3D printed vasculature enhanced the differentiation and viability of osteoprogenitor cells, and that the formed vessels remained patent and stable in solution, even after being dissected out of the surrounding gels.[156] This demonstrated that the printed vessels had the cell-cell communication that is typical of native vessels, enabling their potential manipulation for grafting and suturing, which remains to be tested. Moreover, this same method was used for a dual-ink 3D printing approach where strands of vascular capillaries were printed adjacent to strands of a rigid beta tricalcium phosphate bone scaffold material, paving the way for fabrication of larger pre-vascularized bone grafts [157]. This same method was then used to manipulate fluid flow as a reconfigurable (movable) microfluidic valve in 3D printed hydrogel channels, showing some additional versatility to this approach.[158]
Many other strategies have emerged to address the issue of connectivity between cells in a tissue. One noteworthy development in recent years integrates two of the recent developments cited above to create a technique called SWIFT, short for sacrificial writing into function tissues [141]. In this method cell aggregates and organoids are fabricated in high-throughput and then compacted via centrifugation to result in a granular supporting bath that is comparable to those utilized in the FRESH method, with the caveat that the SWIFT approach uses living cells the non-Newtonian supporting bath, as opposed to granular microgels. At low temperatures of 0–4 °C the high-density cell slurry is soft enough for omnidirectional movement of the print head while it dispenses any material without damaging cells, but viscous enough to hold its shape as this occurs; akin to the FRESH protocol. This allows for a straightforward dispensing of gelatin inks, which harden at low temperature and can be easily sacrificed afterward[141]. Using this method, Skylar-Scott et al were able to 3D printing complex vascular capillaries within constructs made of high-density cell aggregates and enable the adequate interconnection between cells by the presence of a circulatory system fabricated via printing (Figure 3a). Another vasculature 3D printing strategy that has also relied on a sacrificial approach, builds on the concept of sacrificing sugar glass (isomalt) to generate complex vascular capillary structures. A recent paper by the Miller and Stevens’ groups utilized a laser sintering 3D printing approach to fabricate complex patterns of laser sintered isomalt that, like their earlier work on extruded carbohydrate glass, could be sacrificed after covering with a cell-laden hydrogel.[159] Several other methods that rely on extrusion printing of one form or another or sacrificial bioink have been reported, and the reader is encouraged to refer to recent reviews on the topic for a closer look at the specific details of each one of these methods[160–163] (Figure 3b).
Figure 3.

A) 3D printing of alveolar model topologies with entangled vascular networks. i, ii) Images show fabrication of entangled vessel topologies (interpenetrating Hilbert curves and bicontinuous cubic lattice) within the PEGDA hydrogels. iii) Architectural design of an alveolar model topology based on a Weaire-Phelan 3D tessellation with vasculature and shared airway atrium. iv) Photograph of a printed hydrogel shows concave regions of the airway (dashed black circles) squeeze adjacent blood vessels and cause RBC clearance. v) A computational model of airway inflation demonstrates increased displacement at concave regions (dashed yellow circles). Reproduced from [164] with permission. B) Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered (SLS) carbohydrate templates. i) Schematic shows the workflow for the additive fabrication of architectural motifs of the smooth vasculature, hierarchical branching, and unsupported overhangs via SLS. ii) Volumetric reconstruction demonstrates that the metabolically active zone of cells closely follows the perfusable dendritic vascular network. Reproduced from [159] with permission.
Another method that has changed the landscape on the fabrication of complex vascularized tissues uses a completely different approach to that of sacrificial 3D printing. This strategy utilizes the photopolymerization chemistry of light-activated hydrogel pre-polymers, together with the long-standing technology of digital light processing (DLP) 3D printing, to pattern layered structures that will result in the formation of complex channels. Attempts to DLP hydrogel microchannels are not new [165–167], and, one of the greatest challenges has been to control the specificity of light interaction with the existing monomers, such that light does not “bleed” through the regions of interest and prevent the formation of an effective hollow channel. Grigoryan et al addressed this challenge by using molecules that absorb light at specific wave lengths to fine-tune the control of photopolymerization ability using these methods, and with that enabled the printing of very complex microchannel geometries in biocompatible, and even cell-laden, hydrogels.[164] Using this method, the team was able to replicate the complex architecture of the alveoli in the lung, inclusive of the ability to oxygenate incoming blood via induction of breathing-like patters that were activated mechanically on the 3D printed hydrogels (Figure 3a). In summary, 3D biofabrication and printing of complex vascularized constructs has evolved at a rapid pace. Many challenges remain, and again, these seem to pertain the specific biological complexity of what it takes to replicate the biology of a functioning blood vessel,[146] then simply creating conduits of fluids, which appears to be a challenge that has, by and large, been generally overcome.
For instance, while the fabrication of endothelialized 3D printed channels has been accomplished and characterized with extensive success, only a few papers have included the presence of perivascular cells, which are known to regulate important physiologic process of endothelial barrier function, among others [154, 157]. Extensive work by our group has been conducted to characterize the specific microenvironmental and tissue fabrication conditions that enable the consistent and reproducible fabrication of vascular capillaries in vitro [168–172], and many other groups have generated vascularized organoids as well [173]. It has been demonstrated that biological requirements, such as stem cell source [169], mechanical properties [172], hydrogel and medium composition [157], cell ratios [157], and a variety of different conditions, all play a significant role in ensuring adequate endothelial-perivascular cell communication in cell-laden biomaterials. These are aspects that are likely to be needed if one intends to reliably print fully vascularized and functional solid organs. Moreover, the presence of endothelialized channels in bioprinted tissues and organs, does not translate into a functional circulatory system. Vessels have complex blood carrying functions, barrier properties, paracrine signaling, and a myriad of vascular biology specific requirements that tend to be less studied when teams have attempted to bioprint vessels and capillaries [146]. Understandably, the challenges associated with printing blood vessels is substantial, but until these rudimentary vessels demonstrate complex regulatory functions pertaining to vascular biology, they are unlikely to be used as reliable substitutes for native vessels. Moreover, it is important to acknowledge that complex organs are vascularized by complex vascular trees, where larger vessels feed very small capillaries (<20 μm diameter), which we discuss in a recent review [146]. To date, although many examples of 3D printed bifurcating vascular trees exist, the far majority of bioprinting methods have concentrated on larger diameter channels, and even when narrower channels are printed, they typically to not fully replicate the architectural complexity of the native vascular plexus in solid organs. On that note, extrusion methods of sacrificial vascular printing have generally enabled relatively simpler vascular structures, whereas more recent examples of DLP biofabrication have addressed some of the complexity of smaller and more intricate vascular capillaries with higher resolution [164]. It appears as though the existing methods of vasculature printing will facilitate the fabrication of perfusable channels that are sufficient to let endothelial and perivascular cells communicate to generate the very small vascular capillaries via endothelial morphogenesis. Importantly, without the 3D printed channels, these cells themselves cannot get the nutrients required to remodel into capillaries in the core of larger cell-laden biomaterials or cell-aggregated organoids/assembloids. Thus, a likely approach is one where larger vessels are printed to support self-assembled capillaries of small diameter, which collectively can oxygenate an entire 3D solid organ construct.
It must also be highlighted that printing and biofabrication of tissues with terminally differentiated cells, such as endothelial cells, is likely to pose a challenge for translation with respect to their implantation in patients. The current FDA regulation of cell therapies is restricted to a narrow window of isolated stem cells or primary differentiated cells that are not subjected to further processing, except for rare circumstances. This means that the conventional process of cell expansion that is currently needed to obtain sufficient endothelial cell numbers for a graft is not yet regulated. Which brings another important point regarding the need for close communication and evolution of regulatory guidelines that need to be modified in order to allow for adequate translation of bioprinted tissues into the clinic. One consequence of that is that clinical trials with bioprinted pre-vascularized grafts has remained very little active in the recent past.
4. Engineering challenges to replicate microscale organ heterogeneity
There are a growing number of bioprinting methods that have emerged since the implementation of the field, and it is increasingly hard to categorize the specific strategies that are available, owning to their growing intersections and communalities. But generally speaking, the methods have been divided in the form of extrusion, ink-jet, light and laser bioprinting, as described above.[76] There have been several dozens of review papers on the details, advantages and disadvantages of each specific method, [3, 6–8, 57, 58, 174–178] including some of our own.[14, 51, 146] Therefore, we encourage the reader to refer to these earlier publications for details. Here, we will focus on the specific challenges of each bioprinting method in addressing the biological needs of building complex tissues and organs, with a special emphasis on recent developments that have received lesser attention in previous reviews.
In keeping in line with the challenges of replicating the structural and organizational complexity, one of the main questions that remains is what are the engineering challenges that need to be addressed in order to enable meaningful mimicry of such complexity? Previous sections hinted to some of these needs from a biological standpoint, but here, some of the recent engineering challenges are discussed more specifically, without getting so much into the hardware aspects, but rather the engineering challenges that are enablers to complex biology. First and foremost, arguably the primary engineering challenge towards fabrication of physiologically relevant organs, is the ability to recreate tissue heterogeneity with precision. To a great extent, despite the development of many multi-ink bioprinters in recent years,[46, 122, 179] methods that enable a system to replicate the accuracy and precision at which tissue and cell heterogeneity is displayed in the body remain limited. Extrusion methods have been particularly good at attempting to recreate tissue heterogeneity (Figure 4a),[180] owning to the relative ease at which syringe print heads can be positioned on a printing axis and used interchangeably. A critical limitation to these developments, however, is that the true heterogeneity of complex tissues typically happens at a scale that can be orders of magnitude lower than what current extrusion printers can achieve. For instance, even though extrusion bioprinting inks have been developed to be dispensed with the theoretical width of a single cell,[57] these bioinks are generally dispensed as fibers, which makes it extremely difficult to micropattern tissue regions that are discrete with areas that as small as clusters of 2 or 3 cells, surrounded by areas of a different cell type, interconnected by 15–30 μm blood capillaries and innervation. Most systems are just not on par with this level of complexity. Laser and inkjet printers can approximate this precision and resolution to a far greater extent, however, despite growing improvements in printing with multiple inks (cell types) using inkjet and laser printers, it remains remarkably difficult to build tissue layers at a reasonably high speeds in 3D when using these printers.[181–183] Even more important than limitations associated with printing speed, is the fact that laser and inkjet systems have a far narrower window of printability, and thus have far more limited number of compatible bioinks. Moreover, the structural weakness of bioprinted constructs, which is a direct result of the limited ink viscosity needed for these methods, restricts the size of the bioprinted structures to very small dimensions. That is despite the fact that these methods can indeed provide very high accuracy of tissue positioning and resolution.
Figure 4.

A) Design of the digitally tunable continuous multi-material extrusion bioprinter. i) Schematics and photographs showing the seven-channel printhead design connected to reservoirs that are individually actuated by programmable pneumatic valves to print microfibers of seven bioinks. ii) Images show a printed multi-component cell-laden heart-like structure. Reproduced from [180] B) Microfluidics-enabled multi-material maskless stereolithographic bioprinting. iii) The defined CFD model and the velocity profile of PEGDA in the closed microfluidic chamber under sinusoidal fluid flow. iv) The operation of the microfluidic device for consecutive injection of different bioinks. v) Schematics show the skeletal muscle tissue and tendon-to-bone insertion models, the mask for printing, and the fluorescence images of bioprinted GelMA structures containing cells of various types.[184]
On that note, the speed and precision of light polymerization based printers has been a focus of great attention.[53, 185–189] A great deal of effort has been expended towards increasing the speed of light polymerization printers (DLPs, 2PP, Stereolithogrpahy, etc), [53, 185–189] mostly by developing improved chemistries that that are compatible with speed,[190] precision and scalability,[191–195] or by adapting the hardware to improve functionalities and overcome limitations that are built into the process and are known to slow down the fabrication (i.e. calibration, priming, tip cleaning, vat tilting, build platform movement, etc.). Currently, the far majority of light polymerization based bioprinters have the capacity to comfortably pattern regions as little as approximately 40 μm in XYZ. In theory, this would enable micropatterning of very complex regions that are as small as a single cell, potentially enabling very complex tissues to be fabricated. However, this is typically listed as the nominal resolution of the machine, and the reality ends up being far removed from that once the material is encapsulated with cells and other biological molecules. Not only the nominal resolution of the printer does not account for a number of light refraction and blocking issues that arise from the presence of light-scattering components (such as cells); but also, they typically underestimate the complexity of printing with heavily hydrated monomers that are typically composed of a very low concentration of photo-sensitive moieties, which decreases the precision of the monomer-light interactions substantially. In other words, a machine that can print very precise cell-free pure monomers will likely have only a fraction of its accuracy when printing with cell-laden hydrogels at their typical low concentration monomers. Extensive improvements on hydrogel and process conditions have been achieved recently on exactly these fronts, with modification of the biomaterial using light blockers and other strategies,[164, 191–196] but this remains a topic of further development.
Despite the important advances mentioned above, perhaps the major current limitation with light based bioprinters remains that of building heterotypic complexity in tissue constructs. The far majority of light activated bioprinters, both DLP, two-photon polymerization and light/laser rastering lithography, function by using a vat that is filled with a photocrosslinkable biomaterial, and for each added layer, a fresh coat of material needs to be layered onto the printed part. A variety of strategies are used for this. Some rely on the tilting movement of the vat itself, some rely on the coordinated motion of the robotic arm holding the build platform in X-Y, or even up and down. The issue with that strategy is that coverage of the printed biomaterial with a fresh layer of ink is the most straightforward opportunity to provide different composition of the tissue, either via a different biomaterial ink, or a different cell type. The obvious challenge with that method is that the body is not built in flat and even layers, and hence true tissue heterogeneity is difficult to achieve. One method that has been developed to address this goal was to connect hydrogels of different compositions to a microfluidic syringe pump and flow it to a microdevice in a coordinated fashion with the exposure of light, such that different shapes and structures could be built within the device to result in a heterotypic constructs on a chip with a much higher level of microstructural complexity (Figure 4b).[184] This strategy may point to smart alternatives in the future, where tissues can also be built in Z, for increased volumes, as opposed to remaining limited to flat and thin footprints. On that note, another avenue that may be worth pursuing is that of bioprinted microgels that can be fabricated with microscale heterogeneity and then assembled in the form of supramolecular granular hydrogels.[197, 198] Several microgel assembly methods have been developed over the years and this may point to a simpler strategy of tissue biofabrication and assembly.[199–201] For instance, we have recently developed methods for high-throughput bioprinting of injectable pre-vascularized microgels[196] (Figure 5a,b) which can be injected (Figure 5c) into stackable 3D printed microcages with pre-determined functional regions of tissue composition or spatiotemporal release of growth factors (Figure 5e).[202] Interestingly, when these pre-vascularized microgels are implanted and compared to cell-laden hydrogels of the same polymer and cellular composition, the formation of vascularized tissue is significantly improved with the pre-vascularized matrix (data not shown). That is because vessels in the microgels can anastomose with one another (Figure 5d) and with the host from the onset of tissue remodeling, as opposed to being dependent on the assembly of vascular capillaries in the core of conventional constructs, which have poor access to oxygenation.
Figure 5.
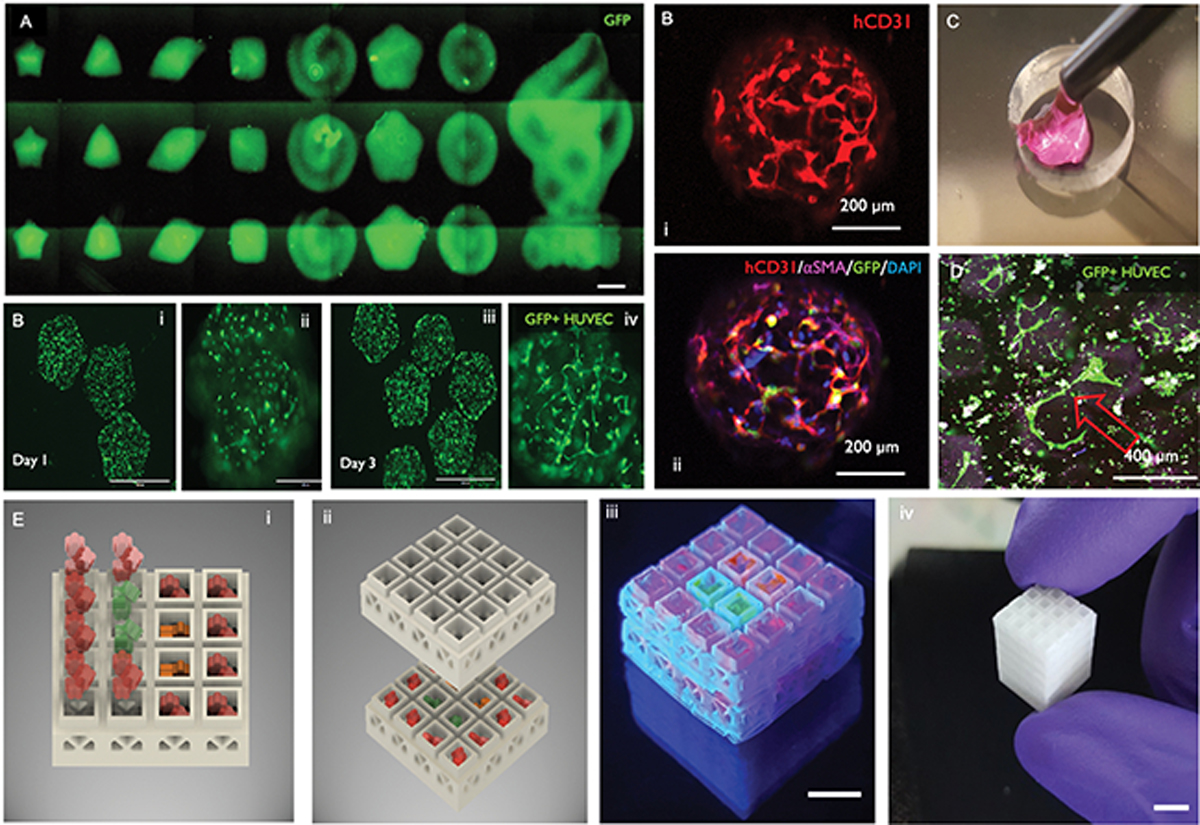
A) DLP bioprinted microarray of cell-laden microgels with controllable geometry and mechanics. Reproduced from [196] with permission. B) Each microgel can be (i) loaded with a co-culture of HUVECs and hMSCs (ii) that self-assemble into (C) pericyte-supported microvascular capillaries in less than 72 h. D) The pre-vascularized microgels can be injected with a built-in vasculature and show ability to anastomose with adjacent capillaries in-vitro. E) These microgels can also be injected into selective regions within 3D printed stackable microcages, to result in complex 3D constructs with predefined tissue regions. Reproduced from [202] with permission.
On the specific advantages of extrusion, versus light and inkjet/laser bioprinting, specifically for the fabrication of complex heterotypic organ constructs, it appears that extrusion methods have an important edge, which is the ease with which newer methods can dispense multiple cell/material types simultaneously. The major disadvantage, remains the relative low resolution of conventionally extruded cell-laden inks, which still revolve around the >100 μm mark, for the most part. On the other hand, the precision and speed of light polymerization bioprinters has shown amazing success in recent publications, with a remarkable ability to build up complex tissue structures in 3D. Nevertheless, the ability to bioprint with multiple cell/material types in 3D has remained limited, and this is a major disadvantage for complex heterotypic organ printing. Laser and inkjet printers, as discussed in other sections of this paper, have lagged behind mostly due to their limited range of viable inks, and undesirable ink properties for manufacturing of large-scale constructs, despite their ability of patterning tissues with single cell resolution.
Another recent topic that has received tremendous attention in the 3D printing community, and is slowly finding its way onto the tissue bioprinting in literature, is that of volumetric/holographic printing, where the idea of additive manufacturing derived from the concept of building layers one on top of the other, is challenged in favor of printing entire 3D constructs from the outside in, so to speak.[203] These technologies are still nascent and pose a set of challenges for biologists and tissue engineers that are only beginning to be explored. But are likely to provide some new impetus in the field.
Similarly, one more other aspect that is noteworthy on the engineering side, as we have hinted to in previous sections, is that pertaining to the interface of microfluidics and bioprinting. Microfluidics has a long history of assisting the development of novel printing methods. Very early approaches developed controllable microfluidic-based systems to dispense highly intricate fiber morphologies with topographical features with extensive level of complexity in their composition.[196] These earlier methods were not attached to a X-Y-Z robot capable of reading G-code or coordinating the motion of a printing robot, but they paved the way for a long range of papers utilizing microfluidics control of ink dispensing capacity to generate tissue complexity. [180] An interesting outcome from these initial methods benefits a lot from materials that have rapid crosslinking in the presence of specific fluids, such as alginate, which has been very extensively characterized for tissue engineering. For instance, using these alginate based systems, Colosi et al reported on the development of what has been known as universal bioinks,[184, 204, 205] where the alginate functions as a carrier material for secondary composition of hydrogel, and once the printing is complete, the alginate is either dissolved, degraded, or maintained in place, enabling straightforward dispensing of multiple types of inks, ranging from dental proteins,[120] to methacrylated hydrogels and others.[184, 204, 205] Another remarkable feature of integrating microfluidics with bioprinting, which has led to the development of some companies altogether, is the possibility of maintaining cells in their designated reservoirs, with their preferred medium, and rapidly switching the dispense of many cell and material types in the middle of a print run (Figure 6a). One commercially available system that has taken significant advantage of this concept is the Biopixlar®, which uses a multi-channel microfluidic device integrated to a four-chamber microdevice, and controller enables the precise opening and closing of microfluidic valves that coordinate the flow of each chamber component down onto an extrusion port (Figure 6b–d). Remarkably, the flow regimes that are created at the end of the print head, result in a dispense-aspirate system where the outflow of cells, even at the single cell level, is controllable to an area of a few micrometers (Figure 6b,d).[206] Once these flow regimes are coordinated with the speed of the dispensing robot, one can deposit even single cells with extensive precision onto a substrate. This is a great example of how microfluidics engineering has been utilized to favor the biology of bioprinting. One key advantage of a system like this, is that it enables rapid switching between various cell types, in the middle of the pointing run, with relative ease, and as many times as needed. Consequently, the idea of building tissue complexity and cell heterogeneity in a construct becomes a lot more attainable (Figure 6c). The main disadvantage of this system, which is not small considering the tissues in the body are three-dimensional and printing leverages precisely that aspect, is that cells can only be deposited in 2D, and building 3D architectures requires stacking of many tissue layers in a labor-intensive sequence of cell deposition, attachment, coverage with a cell-adhesive solution, and follow-on deposition of cells, usually after several hours or overnight (Figure 6d). Nevertheless, there is a list of possibilities to be explored on the interface of microfluidics, printing and biology, which appear to be only beginning to be addressed.
Figure 6.
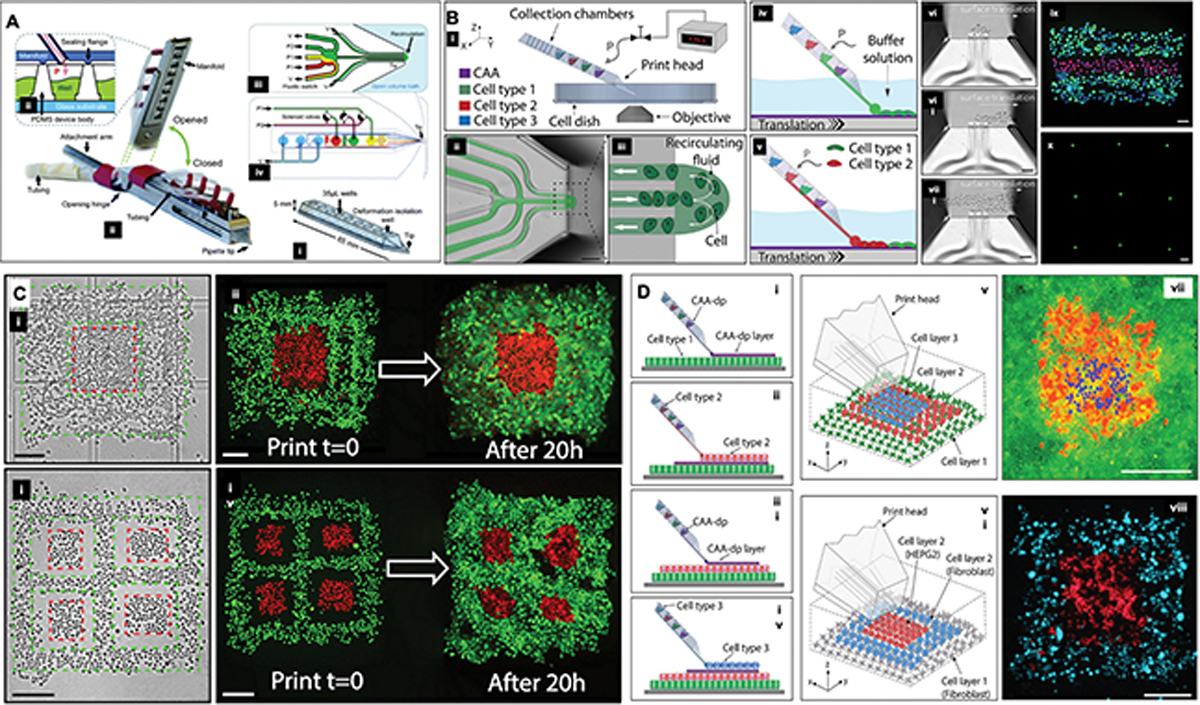
A) Schematic diagram showing a (i) PDMS microfluidic pipette tip, (ii) the holder, the (iii) pneumatic interface between the holder and the tip, wells, and (iii-iv) the microfluidic switching junction near the tip. Reproduced from [206] with permission. B) (i) The microfluidic printhead is connected a controller hardware and software interface, which regulates the deposition of various cell types at a time, by allowing for the formation of a (ii-v) recirculating fluid zone at the end of the printhead within a liquid bath. (vi-viii) The translational movement of the printhead and X and Y, coordinated with the adhesion of the cells to treated substrate, allows for the patterning of cells with (ix) multi- or (x) single cell resolution. C) The bioprinter enables fabrication of multi-cellular two-dimensional (planar) tissues, in this case skin cancer cells (A431, red) surrounded by epithelial cells (HaCaT, green). The scale bars represent 200 μm. D) Three-dimensional tissue constructs where (v,vii) the base cell layer was composed of A431 cells (green), the middle layer being HaCaT cells (red), and the top layer being A431 cells (blue); in (vi,viii), a patch of liver cancer cells (HepG2, in red) surrounded by fibroblasts (3T3-J2, in blue). The scale bars represent 300 μm. Reproduced from [207] with permission.
An additional aspect that has received little attention in comparison to bioink and hardware development for bioprinting is the progress on methods that enable precise and quantitative characterization of bioprinting precision. For instance, if the goal is to position cells with as high precision as possible to match that of a native organ, then there should be methods that allow one to quantify these outcomes in 3D relative to larger organ structures. While confocal microscopy enables imaging and quantification of cellular processes in 3D, quantification of cell positioning in engineering tissues with single cell precision has remained poorly explored. Combinations of clarity microscopy [208] and computational analyses of high-content cell imaging are possible solutions towards that end [209], and should be further explored.
5. Materials challenges to replicate the extracellular matrix and organ complexity
One last relevant aspect pertaining to replicating true tissue and organ complexity relates to the nature of the materials that make up the native extracellular microenvironment in the body. It has become increasingly clear that, while cells are embedded in a protein-rich 3D matrix of relatively consistent composition (mostly collagenous with non-collagenous macromolecules), there is a significant degree of organization to the interaction and composition of these proteins and other biological molecules. This manifests itself in the form of gradients of concentration of soluble molecules, alignment of fibrillar structures, hierarchical organization of assembled proteins, and many more. For the most part, the field has devoted significant efforts to determine the materials characteristics that are required to enable reliable printing of biomaterials via extrusion, light polymerization and several other setting reaction methods. A few reviews have been published on this topic to provide guidance on the material properties leading to improved printability. Nevertheless, the perspective of how these bioprintable materials systems facilitate replicating the heterotypic function and complexity of tissues and organs has been superficially addressed. For instance, while many studies have pointed towards the desirable combination of biomaterials presenting shear-thinning properties and cell-adhesive motifs, these materials of single composition fail to acknowledge that, just like cells in a tissue, the matrix composition can also vary according to the location in a given organ. This points to urgent need to concentrate efforts on the development of printing methods that are amendable to rapid exchange of inks as to allow for the fabrication of more complex heterotypic constructs. Another debate on this question is regarding the emphasis of the biomaterial ink development on strategies that are based on hydrogel patterning versus direct cell patterning. For instance, despite the extensive work of Forgacs, Mirinov et al [23, 25, 26, 103, 105, 106] in implementing bioprinting through the dispensing and pattering of high density cell aggregates, the field has concentrated on cell-laden hydrogels as bionks for many years. However, it should be acknowledged that developmental biology stems from the close interaction of cells in a low concentration of matrix, where cell-laden gels propose the exact opposite; high concentrations of matrix with a low percentage of cells. Recent successful examples of complex organ models, such as the heart [134] and the vasculature [141], have gone back to mimicking this cell-dense approach used by nature, where the material was only utilized as temporary supporting medium for fabrication, or at very low concentration. This brings up the question of where the field should focus more if the intention is to replicate organ function. One perspective that may be relevant to answer this question is, again, the original characteristics of the tissue or organ that is being mimicked. Connective tissues (i.e. cartilage, bone) are matrix-dense by design. Solid vital organs have very little matrix in comparison, and are far more cell-dense. (i.e. liver, heart). This original organization should provide a starting point in the design of bioinks that will facilitate an approximation of the desired organ function.
While the liver is an interesting example to describe the challenges of replicating cellular heterogeneity and architectural complexity for bioprinting, as it was discussed in earlier sections, bone tissue can be used as an interesting example of a seemingly simpler tissue, from a cellular perspective, but much more complex from a matrix biology perspective. For instance, at the microstructural level, compact bone is organized primarily by the lateral positioning of osteon units.[210–212] Each osteon is structured in the form of concentric rings of osteocytes, which result in a unit measuring about 200 μm in diameter. The osteon has a precise organization that is defined by the alignment of groups of collagen fibers, which essentially show a pattern of counter-posed inclination, where groups of fibers in one layer are inclined in the opposite direction of the next. This organization is responsible for a remarkable degree of self-toughening, which makes bone a stiff and tough at the same time.[213–216] One question then is how well can existing bioprinters replicate this level of microstructural detail, for an example? Certainly this question depends on the intentions for the printed tissue. For instance, if the end goal is to simply bioprinting a scaffolds loaded with cells, then we may not require the same level of precision that is intended for a true permanent body replacement part, since the biodegradable scaffold should only stimulate accelerated healing.[12, 17] On the other hand, if the intention is to achieve a material system that replicates the same level of “tissue quality”, so to speak, as the native tissue then that would desirable, especially if it is understood that this is an important component to achieve the desired function of the tissue in question, as it is the case with bone. In other words, from a clinical standpoint, if a patient loses a vital organ, the said patient cannot wait until a scaffold matures into a vital tissue over the course of several months, as it is currently the case. That is the exact purpose of organ transplantation; to provide an immediate remedy for a failed organ structure and immediately recover organ function. Ultimately, if bioprinting is to deliver on the promise of circumventing the problems of organ donation and transplant, the field should strive for these aspirational goals, which poses a significant challenge.
Still using bone as an example, another aspect that deserves recognition with respect to the development of biomaterial inks, is the relative nano and microscale simplicity of existing inks in comparison to the native matrix in the body. For instance, there is an incredibly high number of reports developing bioinks for bone regeneration.[87, 217–226] The far majority of these existing methods use a reductionist process of loading cells in a single component hydrogel that stimulates differentiation of osteoprogenitor cells with some form of osteoinductive ingredient. These hydrogel biomaterials, in their large majority, are composed of either broken down biological molecules that are modified to endow the material with controllable properties, such as photo-crosslinking control, desirable chemistry, or tunable mechanics.[87, 217–226] A number of relevant studies developing tissue specific ECM-derived bionks have followed this trajectory, including some of our own. We have reported dental and bone derived bioinks, where a dentinal tissue was processed to result in an extrusion bioink composed of the extracellular matrix of the native calcified tissues [120], or the bone matrix was functionalized with methacryloil to give rise to a bone-derived phtocrosslinkable bioink for DLP bioprinting, (BoneMA) [196]. Several other examples are available for tissue derived bioinks for a many different organs [227, 228]. Despite these advances, from a matrix biology standpoint, simplified porous hydrogels fail to mimic the nano-structural organization or protein composition of the native extracellular matrix in the body. For instance, while many natural and synthetic hydrogels have been chemically modified with RGD and other cell-adhesive sites [196, 229] to promote integrin binding and cellular traction processes that approximate those in the body, these interactions fail to recapitulate the hierarchical interactions that occur in the extracellular microenvironment in the native tissues. For instance, the structure and mechanics of native fibrillar collagen, which dictate cell response in the ECM in the body, is largely determined by variable modifications of intrafibrillar and extrafibrillar crosslinking,[230] the presence and interaction of inter-fibrillar non-collagenous proteins, the slipping and sliding ability of interconnecting proteoglycans and glycosaminoglycans,[231] as well as the ability of other soluble molecules to interact and bind to these structural proteins.[232] Virtually all of these nuanced aspects of matrix biology are lost in the current reductionist approach to bioink development, where typically proteins are modified to form controllable hydrogels with adequate printability, but overly simplified structure and mechanics. Certainly, this reductionist approach to bioink development is not a subject of concern when engineering regenerative materials,[233, 234] given the temporary nature of the scaffolds. However, these questions may become more significant when organs are to be bioprinted with the desired level of biological precision that will be required to manufacture functional body parts in the lab from the get go.
Again, using bone as an example of such complexity, and highlighting the broad gap between native tissues and engineered inks, it is well known that the bone matrix is composed of a hierarchically organized matrix material, mostly constituted of collagen and hydroxyapatite. The majority of bone bioinks are composed of either simple osteoinductive hydrogels,[235] or basic calcified ceramics and extrudable cements,[51] rather than a matrix that mimics the complexity of bone tissue. At some point, there should be a shift to where designer inks can accommodate the matrix complexity of the native tissues, and enable patterning of cells and biological materials that will result in the manufacturing of fully (or at least more highly) biomimetic tissue constructs right out of the printer, rather than requiring a long process of cell differentiation, remodeling of a scaffold system, and eventual maturation into a functional organ, which is currently the case.[77] Examples of how bioprinting can accommodate for these nuanced questions of materials properties and micro to nanoscale matrix organization already exist. For instance, Martin et al developed a magnetic printing protocol that allow one to replicate not only the structure of microscale osteon-like geometries, but also the specific alignment of mineral particles relative to the Haversian canal in the native bone using magnetic control of the ink in question.[236] The mechanical consequence of a biomimetic “organized” particle distribution in comparison to a random orientation of loaded particles, as expected, was significant; printing to the perspective stated above, that perhaps nanoscale engineering of biological inks may be more important for certain tissues than originally predicted. Of note, the ability of 3D printing the microscale morphology of osteons in bone via DLP printing, while enabling control of the directionality of organic and inorganic components that make up the bone tissue can be highly relevant to mimic the hierarchical (nano to micro) complexity of bone tissue, since not only osteons have a complex architectural organization of interconnected Haversian canals, but also precisely angled collagen fibrils that are reinforced with hydroxyapatite. [236] Our recent work on engineered bone tissue replicating the nanoscale requirements in non-bioprinted constructs, strongly advocates for this perspective, since simply mimicking the nanoscale feature of the native tissue resulted in even better stem cell differentiation and bone-like response than cells treated with gold-standard osteoinductive supplements.[171] Perhaps a combination of a bone derived photo-crosslinkable bioink (i.e. BoneMA [196]), with the nanoscale method of engineering the bone matrix [171], bioprinted with cells, using the 3D magnetic printing method could closely approximate the complexity of human bone. This could form the basis for future studies.
Another aspect that required further consideration is how materials can be developed to ensure that cell growth is guided in a way that is conducive to the desirable structure as the cells expand and process the matrix. Preliminary data in our own lab has shown that hydrogels of low stiffness lose their bioprinted shape far more quickly than hydrogels of higher stiffness, which is expected. However, this loss of shape fidelity over time can also has biological implications, since tissue shape and morphology are well known regulators of development [237]. Further research is also required on this aspect.
6. Conclusion and future outlook
The progress in the file of bioprinting in the past 10–15 years is remarkable. The field has progressed from a few cells dispensed on a plate by a robot, to 3D printed structural alveolar models,[164] beating heart-like tissue constructs,[134] contracting muscles,[77] bendable cartilage,[77] and endothelialized vascular channels.[147, 154, 156] All addressing both engineering developments, as well biological challenges. One may argue that the challenges before us are nothing short of extraordinary. Piecing an entire organ, cell-by-cell, protein-by-protein, using a machine, is not a small feat, and if successful, the outcomes are truly transformative for medicine and engineering. Therefore, all of the challenges stated above need to be read with a clear sense of the true difficulties behind tackling them. In other words, it is not easy to build organs and body parts from scratch, but it has been more than proved by now that this is an achievable goal. Therefore, future work should focus initially on delineating the critical challenges that will truly progress the field forward, rather than laterally. For instance, there has been an explosion of bioprinting hardware development and commercialization in recent years. The cost of bioprinters ranging anywhere from a few thousand to several hundred thousand. Biologists may argue that many of the expensive systems in the market do not necessarily address the most critical problems that are required to actually rebuild the biology of functional tissues. Engineers, on the other hand, may argue that the hardware is highly capable of dispensing biological materials with precision, and it is up to biologists to understand the critical aspects required for ultimate function. Perhaps a more balanced approach would be to develop enabling engineering tools that are rooted on biological problems, rather than heavily focused on speed, precision and cost. For instance, it may be better to identify the critical barriers to bioprinting heterogeneous, complex, vascularized and innervated tissues that function, rather than simply being able to dispense biomaterials with high resolution but limited biological function. Ultimately, it is a well-balanced synergistic effort of engineering and biology, that will show us the viable path to fabrication of ideal organs on the lab bench for implantation in humans, which is the overarching goal of the field of bioprinting.
Another aspect that will shape the future outlook of the field is how these technologies are regulated by regulatory agencies across the globe. While we may be years away from discussing full cellularized bioprinted organs as an alternative to transplantation as a mainstream treatment method, the validation of such engineered systems should be the starting point driving the use of these bioprinted tissues for medical use. This brings up an extremely important point that will advance the field, which is the use of bioprinted tissues as reliable and reproducible subjects of disease models, drug testing and in-vitro models of healthy tissues. While it may take some time until these bioprinted organs are validated clinically, there is an urgent need for better models of disease that can replicate the complexity of drug interactions with multiple cell types, or cell microenvironment, and the progressive nature of disease development. All of these aspects are difficult to study with animal models, which hard to control and visualize in real time, or with conventional 2D models of cell culture. Yet, the overwhelming majority of pre-clinical knowledge available about disease conditions and drug response, comes from these models. Engineering miniaturized and reproducible bioprinted organ constructs that replicate the complexity of human biology on a dish, or on-a-chip [238], is a mutually beneficial step. Not only will this generate valuable knowledge to guide future medical treatments and understanding, but also it can validate much of the function that is expected from bioprinted constructs before they need to implanted as organ substitutes. In other words, bioprinting of diseased tissues and organs should, perhaps, be given as much importance as bioprinting of healthy implantable constructs.
In summary, there are many challenges ahead, but the future of bioprinting is warranted, and the speed of recent developments point to bright outlook where many of these seemingly distant challenges may already be in the works in a lab somewhere.
7. Acknowledgements
The author acknowledges funding from the NIH/National Institute of Dental and Craniofacial Research (R01DE026170 and R01DE029553 to L.E.B.), and the OHSU-Silver Family Innovation Award. The author also thanks Dr. Ramesh Subbiah and Anthony Tahayeri for assistance with preparation of figures of this manuscript.
Biography

Luiz E. Bertassoni is dentist/scientist who received a PhD in Biomaterials from University of Sydney, and completed two postdoctoral fellowships at University of California San Francisco, and the Division of Health Sciences and Technology at Harvard Medical School and MIT. Dr. Bertassoni is a tenured Associate Professor at Oregon Health and Science University (OHSU), where he holds appointments at the Division of Biomaterials and Biomechanics in the School of Dentistry, the Department of Biomedical Engineering in the School of Medicine, and the Cancer Early Detection Advanced Research center (CEDAR) at the Knight Cancer Institute. Dr. Bertassoni’s encompasses various aspects of micro-scale technologies and bioprinting for tissue regeneration; nanoscale structural and mechanical properties of mineralized tissues; and different aspects in the field of calcified ‘organs-on-a-chip’.
8. References
- 1.Moroni L, et al. , Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater, 2018. 3(5): p. 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castilho M, et al. , Multitechnology Biofabrication: A New Approach for the Manufacturing of Functional Tissue Structures? Trends Biotechnol, 2020. 38(12): p. 1316–1328. [DOI] [PubMed] [Google Scholar]
- 3.Groll J, et al. , Biofabrication: reappraising the definition of an evolving field. Biofabrication, 2016. 8(1): p. 013001. [DOI] [PubMed] [Google Scholar]
- 4.Malda J, et al. , 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater, 2013. 25(36): p. 5011–28. [DOI] [PubMed] [Google Scholar]
- 5.Mironov V, et al. , Biofabrication: a 21st century manufacturing paradigm. Biofabrication, 2009. 1(2): p. 022001. [DOI] [PubMed] [Google Scholar]
- 6.Sun W, et al. , The bioprinting roadmap. Biofabrication, 2020. 12(2): p. 022002. [DOI] [PubMed] [Google Scholar]
- 7.Daly AC, et al. , Bioprinting for the Biologist. Cell, 2021. 184(1): p. 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levato R, et al. , From Shape to Function: The Next Step in Bioprinting. Adv Mater, 2020. 32(12): p. e1906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SV, De Coppi P, and Atala A, Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng, 2020. 4(4): p. 370–380. [DOI] [PubMed] [Google Scholar]
- 10.Lenda M, et al. , Ivory crisis: Role of bioprinting technology. Science, 2018. 360(6386): p. 277. [DOI] [PubMed] [Google Scholar]
- 11.Klebe RJ, Cytoscribing: a method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp Cell Res, 1988. 179(2): p. 362–73. [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A and Langer R, A decade of progress in tissue engineering. Nat Protoc, 2016. 11(10): p. 1775–81. [DOI] [PubMed] [Google Scholar]
- 13.Mironov V, Reis N, and Derby B, Review: bioprinting: a beginning. Tissue Eng, 2006. 12(4): p. 631–4. [DOI] [PubMed] [Google Scholar]
- 14.Annabi N, et al. , 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater, 2014. 26(1): p. 85–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele J, et al. , 25th anniversary article: Designer hydrogels for cell cultures: a materials selection guide. Adv Mater, 2014. 26(1): p. 125–47. [DOI] [PubMed] [Google Scholar]
- 16.Lipson H and Kurman M, Fabricated: The New World of 3D Printing. 2013.
- 17.Langer R and Vacanti JP, Tissue engineering. Science, 1993. 260(5110): p. 920–6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YS and Khademhosseini A, Advances in engineering hydrogels. Science, 2017. 356(6337). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan X, et al. , Development of hydrogels for regenerative engineering. Biotechnol J, 2017. 12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauvin R, et al. , Hydrogels and microtechnologies for engineering the cellular microenvironment. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2012. 4(3): p. 235–46. [DOI] [PubMed] [Google Scholar]
- 21.Khademhosseini A and Langer R, Microengineered hydrogels for tissue engineering. Biomaterials, 2007. 28(34): p. 5087–92. [DOI] [PubMed] [Google Scholar]
- 22.Caliari SR and Burdick JA, A practical guide to hydrogels for cell culture. Nat Methods, 2016. 13(5): p. 405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marga F, et al. , Developmental biology and tissue engineering. Birth Defects Res C Embryo Today, 2007. 81(4): p. 320–8. [DOI] [PubMed] [Google Scholar]
- 24.Newman SA, Forgacs G, and Muller GB, Before programs: the physical origination of multicellular forms. Int J Dev Biol, 2006. 50(2–3): p. 289–99. [DOI] [PubMed] [Google Scholar]
- 25.Jakab K, et al. , Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication, 2010. 2(2): p. 022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mironov V, et al. , Organ printing: tissue spheroids as building blocks. Biomaterials, 2009. 30(12): p. 2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bialkowska K, et al. , Spheroids as a Type of Three-Dimensional Cell Cultures-Examples of Methods of Preparation and the Most Important Application. Int J Mol Sci, 2020. 21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrenko Y, Sykova E, and Kubinova S, The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther, 2017. 8(1): p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kijanska M and Kelm J, In vitro 3D Spheroids and Microtissues: ATP-based Cell Viability and Toxicity Assays, in Assay Guidance Manual, Markossian S, et al. , Editors. 2004: Bethesda (MD). [PubMed] [Google Scholar]
- 30.Desoize B, Gimonet D, and Jardiller JC, Cell culture as spheroids: an approach to multicellular resistance. Anticancer Res, 1998. 18(6A): p. 4147–58. [PubMed] [Google Scholar]
- 31.Pettinato G, Wen X, and Zhang N, Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cells Dev, 2015. 24(14): p. 1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daley GQ, From embryos to embryoid bodies: generating blood from embryonic stem cells. Ann N Y Acad Sci, 2003. 996: p. 122–31. [DOI] [PubMed] [Google Scholar]
- 33.Desbaillets I, et al. , Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol, 2000. 85(6): p. 645–51. [PubMed] [Google Scholar]
- 34.Foley KE, Organoids: a better in vitro model. Nat Methods, 2017. 14(6): p. 559–562. [DOI] [PubMed] [Google Scholar]
- 35.Vogt N, Assembloids. Nat Methods, 2021. 18(1): p. 27. [DOI] [PubMed] [Google Scholar]
- 36.Andersen J, et al. , Generation of Functional Human 3D Cortico-Motor Assembloids. Cell, 2020. 183(7): p. 1913–1929 e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim E, et al. , Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature, 2020. 588(7839): p. 664–669. [DOI] [PubMed] [Google Scholar]
- 38.Miura Y, et al. , Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol, 2020. 38(12): p. 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertassoni LE, 3D Printing of Restorative Dental Composites and Ceramics — The Next Frontier in Restorative Dentistry. Californian Dental Association Journal, 2020. 47(10). [Google Scholar]
- 40.Jain A, et al. , Role of Polymers in 3D Printing Technology for Drug Delivery - An Overview. Curr Pharm Des, 2018. 24(42): p. 4979–4990. [DOI] [PubMed] [Google Scholar]
- 41.Ni J, et al. , Three-dimensional printing of metals for biomedical applications. Mater Today Bio, 2019. 3: p. 100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Z, et al. , Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv Sci (Weinh), 2020. 7(17): p. 2001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Placone JK and Engler AJ, Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv Healthc Mater, 2018. 7(8): p. e1701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ning L and Chen X, A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol J, 2017. 12(8). [DOI] [PubMed] [Google Scholar]
- 45.Choi YJ, et al. , 3D Bioprinting of In Vitro Models Using Hydrogel-Based Bioinks. Polymers (Basel), 2021. 13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui X, et al. , Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv Healthc Mater, 2020. 9(15): p. e1901648. [DOI] [PubMed] [Google Scholar]
- 47.Rastogi P and Kandasubramanian B, Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication, 2019. 11(4): p. 042001. [DOI] [PubMed] [Google Scholar]
- 48.Luo Y, Wei X, and Huang P, 3D bioprinting of hydrogel-based biomimetic microenvironments. J Biomed Mater Res B Appl Biomater, 2019. 107(5): p. 1695–1705. [DOI] [PubMed] [Google Scholar]
- 49.Leberfinger AN, et al. , Bioprinting functional tissues. Acta Biomater, 2019. 95: p. 32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Datta P, et al. , Essential steps in bioprinting: From pre- to post-bioprinting. Biotechnol Adv, 2018. 36(5): p. 1481–1504. [DOI] [PubMed] [Google Scholar]
- 51.Obregon F, et al. , Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J Dent Res, 2015. 94(9 Suppl): p. 143S–52S. [DOI] [PubMed] [Google Scholar]
- 52.Panwar A and Tan LP, Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules, 2016. 21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieto D, et al. , Fundamentals of light-cell-polymer interactions in photo-cross-linking based bioprinting. APL Bioeng, 2020. 4(4): p. 041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SH, et al. , Silk Fibroin Bioinks for Digital Light Processing (DLP) 3D Bioprinting. Adv Exp Med Biol, 2020. 1249: p. 53–66. [DOI] [PubMed] [Google Scholar]
- 55.Angelopoulos I, et al. , Engineering inkjet bioprinting processes toward translational therapies. Biotechnol Bioeng, 2020. 117(1): p. 272–284. [DOI] [PubMed] [Google Scholar]
- 56.Devillard R, et al. , Cell patterning by laser-assisted bioprinting. Methods Cell Biol, 2014. 119: p. 159–74. [DOI] [PubMed] [Google Scholar]
- 57.Groll J, et al. , A definition of bioinks and their distinction from biomaterial inks. Biofabrication, 2018. 11(1): p. 013001. [DOI] [PubMed] [Google Scholar]
- 58.Moroni L, et al. , Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol, 2018. 36(4): p. 384–402. [DOI] [PubMed] [Google Scholar]
- 59.Bertassoni LE, et al. , Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication, 2014. 6(2): p. 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gough A, et al. , Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat Rev Gastroenterol Hepatol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, et al. , Current Advances on 3D-Bioprinted Liver Tissue Models. Adv Healthc Mater, 2020. 9(24): p. e2001517. [DOI] [PubMed] [Google Scholar]
- 62.da Silva Morais A, et al. , Advanced Biomaterials and Processing Methods for Liver Regeneration: State-of-the-Art and Future Trends. Adv Healthc Mater, 2020. 9(5): p. e1901435. [DOI] [PubMed] [Google Scholar]
- 63.van Grunsven LA, 3D in vitro models of liver fibrosis. Adv Drug Deliv Rev, 2017. 121: p. 133–146. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen DG and Pentoney SL Jr., Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discov Today Technol, 2017. 23: p. 37–44. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen DG, et al. , Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS One, 2016. 11(7): p. e0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ben-Moshe S and Itzkovitz S, Spatial heterogeneity in the mammalian liver. Nat Rev Gastroenterol Hepatol, 2019. 16(7): p. 395–410. [DOI] [PubMed] [Google Scholar]
- 67.Hundt M, Basit H, and John S, Physiology, Bile Secretion, in StatPearls. 2020: Treasure Island (FL). [PubMed] [Google Scholar]
- 68.Nakanishi Y and Saxena R, Pathophysiology and Diseases of the Proximal Pathways of the Biliary System. Arch Pathol Lab Med, 2015. 139(7): p. 858–66. [DOI] [PubMed] [Google Scholar]
- 69.Haussinger D and Kordes C, Space of Disse: a stem cell niche in the liver. Biol Chem, 2019. 401(1): p. 81–95. [DOI] [PubMed] [Google Scholar]
- 70.Jorgensen AM, Yoo JJ, and Atala A, Solid Organ Bioprinting: Strategies to Achieve Organ Function. Chem Rev, 2020. 120(19): p. 11093–11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui EE and Bhatia SN, Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A, 2007. 104(14): p. 5722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humphreys BD, Bioprinting better kidney organoids. Nat Mater, 2021. 20(2): p. 128–130. [DOI] [PubMed] [Google Scholar]
- 73.Lawlor KT, et al. , Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater, 2021. 20(2): p. 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serex L, et al. , Microfluidic-assisted bioprinting of tissues and organoids at high cell concentrations. Biofabrication, 2020. [DOI] [PubMed] [Google Scholar]
- 75.Brassard JA, et al. , Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat Mater, 2021. 20(1): p. 22–29. [DOI] [PubMed] [Google Scholar]
- 76.Murphy SV and Atala A, 3D bioprinting of tissues and organs. Nat Biotechnol, 2014. 32(8): p. 773–85. [DOI] [PubMed] [Google Scholar]
- 77.Kang HW, et al. , A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol, 2016. 34(3): p. 312–9. [DOI] [PubMed] [Google Scholar]
- 78.Bhise NS, et al. , A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication, 2016. 8(1): p. 014101. [DOI] [PubMed] [Google Scholar]
- 79.Ma X, et al. , Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A, 2016. 113(8): p. 2206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang D, et al. , Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct. Small, 2020. 16(13): p. e1905505. [DOI] [PubMed] [Google Scholar]
- 81.Kang D, et al. , Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication, 2018. 10(3): p. 035008. [DOI] [PubMed] [Google Scholar]
- 82.Norona LM, et al. , Bioprinted liver provides early insight into the role of Kupffer cells in TGF-beta1 and methotrexate-induced fibrogenesis. PLoS One, 2019. 14(1): p. e0208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Battiston KG, et al. , Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials, 2014. 35(15): p. 4465–76. [DOI] [PubMed] [Google Scholar]
- 84.Paschos NK, et al. , Advances in tissue engineering through stem cell-based co-culture. J Tissue Eng Regen Med, 2015. 9(5): p. 488–503. [DOI] [PubMed] [Google Scholar]
- 85.Miki Y, et al. , The advantages of co-culture over mono cell culture in simulating in vivo environment. J Steroid Biochem Mol Biol, 2012. 131(3–5): p. 68–75. [DOI] [PubMed] [Google Scholar]
- 86.van der Sanden B, et al. , Optimizing stem cell culture. J Cell Biochem, 2010. 111(4): p. 801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdollahiyan P, et al. , Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol J, 2020. 15(12): p. e2000095. [DOI] [PubMed] [Google Scholar]
- 88.You F, Eames BF, and Chen X, Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int J Mol Sci, 2017. 18(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garreta E, et al. , Rethinking organoid technology through bioengineering. Nat Mater, 2021. 20(2): p. 145–155. [DOI] [PubMed] [Google Scholar]
- 90.Kang SM, et al. , Engineered Microsystems for Spheroid and Organoid Studies. Adv Healthc Mater, 2021. 10(2): p. e2001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, et al. , Organoid based personalized medicine: from bench to bedside. Cell Regen, 2020. 9(1): p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yuki K, et al. , Organoid Models of Tumor Immunology. Trends Immunol, 2020. 41(8): p. 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Homicsko K, Organoid technology and applications in cancer immunotherapy and precision medicine. Curr Opin Biotechnol, 2020. 65: p. 242–247. [DOI] [PubMed] [Google Scholar]
- 94.Lau HCH, et al. , Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol, 2020. 17(4): p. 203–222. [DOI] [PubMed] [Google Scholar]
- 95.Fan H, Demirci U, and Chen P, Emerging organoid models: leaping forward in cancer research. J Hematol Oncol, 2019. 12(1): p. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montes-Olivas S, Marucci L, and Homer M, Mathematical Models of Organoid Cultures. Front Genet, 2019. 10: p. 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holloway EM, Capeling MM, and Spence JR, Biologically inspired approaches to enhance human organoid complexity. Development, 2019. 146(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grebenyuk S and Ranga A, Engineering Organoid Vascularization. Front Bioeng Biotechnol, 2019. 7: p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blondel D and Lutolf MP, Bioinspired Hydrogels for 3D Organoid Culture. Chimia (Aarau), 2019. 73(1): p. 81–85. [DOI] [PubMed] [Google Scholar]
- 100.Rossi G, Manfrin A, and Lutolf MP, Progress and potential in organoid research. Nat Rev Genet, 2018. 19(11): p. 671–687. [DOI] [PubMed] [Google Scholar]
- 101.Marton RM and Pasca SP, Organoid and Assembloid Technologies for Investigating Cellular Crosstalk in Human Brain Development and Disease. Trends Cell Biol, 2020. 30(2): p. 133–143. [DOI] [PubMed] [Google Scholar]
- 102.Panoutsopoulos AA, Organoids, Assembloids, and Novel Biotechnology: Steps Forward in Developmental and Disease-Related Neuroscience. Neuroscientist, 2020: p. 1073858420960112. [DOI] [PubMed] [Google Scholar]
- 103.Atala A and Forgacs G, Three-Dimensional Bioprinting in Regenerative Medicine: Reality, Hype, and Future. Stem Cells Transl Med, 2019. 8(8): p. 744–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCune M, et al. , Predictive modeling of post bioprinting structure formation. Soft Matter, 2014. 10(11): p. 1790–800. [DOI] [PubMed] [Google Scholar]
- 105.Norotte C, et al. , Scaffold-free vascular tissue engineering using bioprinting. Biomaterials, 2009. 30(30): p. 5910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jakab K, et al. , Three-dimensional tissue constructs built by bioprinting. Biorheology, 2006. 43(3,4): p. 509–13. [PubMed] [Google Scholar]
- 107.Ayan B, et al. , Aspiration-assisted freeform bioprinting of prefabricated tissue spheroids in a yield-stress gel. Commun Phys, 2020. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ayan B, et al. , Aspiration-assisted bioprinting for precise positioning of biologics. Sci Adv, 2020. 6(10): p. eaaw5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daly AC, Davidson MD, and Burdick JA, 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat Commun, 2021. 12(1): p. 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duisit J, et al. , Perfusion-decellularization of human ear grafts enables ECM-based scaffolds for auricular vascularized composite tissue engineering. Acta Biomater, 2018. 73: p. 339–354. [DOI] [PubMed] [Google Scholar]
- 111.Mellott AJ, et al. , Exploiting decellularized cochleae as scaffolds for inner ear tissue engineering. Stem Cell Res Ther, 2017. 8(1): p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xue J, et al. , Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials, 2013. 34(11): p. 2624–31. [DOI] [PubMed] [Google Scholar]
- 113.Zhou L, et al. , Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng Part A, 2011. 17(11–12): p. 1573–81. [DOI] [PubMed] [Google Scholar]
- 114.Haisch A, Ear reconstruction through tissue engineering. Adv Otorhinolaryngol, 2010. 68: p. 108–119. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y, et al. , In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials, 2010. 31(8): p. 2176–83. [DOI] [PubMed] [Google Scholar]
- 116.Jovic TH, et al. , Using 3D Printing Technology to Teach Cartilage Framework Carving for Ear Reconstruction. Front Surg, 2020. 7: p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mussi E, et al. , Ear Reconstruction Simulation: From Handcrafting to 3D Printing. Bioengineering (Basel), 2019. 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee JS, et al. , 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication, 2014. 6(2): p. 024103. [DOI] [PubMed] [Google Scholar]
- 119.Ling Y, et al. , A cell-laden microfluidic hydrogel. Lab Chip, 2007. 7(6): p. 756–62. [DOI] [PubMed] [Google Scholar]
- 120.Athirasala A, et al. , A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication, 2018. 10(2): p. 024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Labowska MB, et al. , A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials (Basel), 2021. 14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piras CC and Smith DK, Multicomponent polysaccharide alginate-based bioinks. J Mater Chem B, 2020. 8(36): p. 8171–8188. [DOI] [PubMed] [Google Scholar]
- 123.Axpe E and Oyen ML, Applications of Alginate-Based Bioinks in 3D Bioprinting. Int J Mol Sci, 2016. 17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahn SH, et al. , A novel cell-printing method and its application to hepatogenic differentiation of human adipose stem cell-embedded mesh structures. Sci Rep, 2015. 5: p. 13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Faramarzi N, et al. , Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds. Adv Healthc Mater, 2018. 7(11): p. e1701347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choudhury D, Anand S, and Naing MW, The arrival of commercial bioprinters - Towards 3D bioprinting revolution! Int J Bioprint, 2018. 4(2): p. 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urciuolo A, et al. , Intravital three-dimensional bioprinting. Nat Biomed Eng, 2020. 4(9): p. 901–915. [DOI] [PubMed] [Google Scholar]
- 128.Ozbolat IT, Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol, 2015. 33(7): p. 395–400. [DOI] [PubMed] [Google Scholar]
- 129.Singh S, et al. , In situ bioprinting - Bioprinting from benchside to bedside? Acta Biomater, 2020. 101: p. 14–25. [DOI] [PubMed] [Google Scholar]
- 130.Hong N, et al. , 3D bioprinting and its in vivo applications. J Biomed Mater Res B Appl Biomater, 2018. 106(1): p. 444–459. [DOI] [PubMed] [Google Scholar]
- 131.Lin RZ, et al. , Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials, 2013. 34(28): p. 6785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elisseeff J, et al. , Transdermal photopolymerization of poly(ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast Reconstr Surg, 1999. 104(4): p. 1014–22. [DOI] [PubMed] [Google Scholar]
- 133.Elisseeff J, et al. , Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A, 1999. 96(6): p. 3104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee A, et al. , 3D bioprinting of collagen to rebuild components of the human heart. Science, 2019. 365(6452): p. 482–487. [DOI] [PubMed] [Google Scholar]
- 135.Highley CB, et al. , Jammed Microgel Inks for 3D Printing Applications. Adv Sci (Weinh), 2019. 6(1): p. 1801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Bryan CS, et al. , Self-assembled micro-organogels for 3D printing silicone structures. Sci Adv, 2017. 3(5): p. e1602800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.LeBlanc KJ, et al. , Stability of High Speed 3D Printing in Liquid-Like Solids. ACS Biomater Sci Eng, 2016. 2(10): p. 1796–1799. [DOI] [PubMed] [Google Scholar]
- 138.Bhattacharjee T, et al. , Liquid-like Solids Support Cells in 3D. ACS Biomater Sci Eng, 2016. 2(10): p. 1787–1795. [DOI] [PubMed] [Google Scholar]
- 139.Bhattacharjee T, et al. , Writing in the granular gel medium. Sci Adv, 2015. 1(8): p. e1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mirdamadi E, et al. , FRESH 3D Bioprinting a Full-Size Model of the Human Heart. ACS Biomater Sci Eng, 2020. 6(11): p. 6453–6459. [DOI] [PubMed] [Google Scholar]
- 141.Skylar-Scott MA, et al. , Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci Adv, 2019. 5(9): p. eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hinton TJ, et al. , Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv, 2015. 1(9): p. e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Noor N, et al. , 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv Sci (Weinh), 2019. 6(11): p. 1900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bae H, et al. , Building vascular networks. Sci Transl Med, 2012. 4(160): p. 160ps23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kinstlinger IS and Miller JS, 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip, 2016. 16(11): p. 2025–43. [DOI] [PubMed] [Google Scholar]
- 146.Parthiban SP, et al. , 3D Bioprinting of Blood Vessels and Vascular Networks: Progress and Challenges Toward Biofabrication of Functional Vascularized Tissues and Organs., in Emerging Technologies for Biofabrication and Biomanufacturing Demirci U, El Assal R, and Cheng P, Editors. 2020, World Scientific Publishing. [Google Scholar]
- 147.Miller JS, et al. , Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater, 2012. 11(9): p. 768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wong KH, et al. , Microfluidic models of vascular functions. Annu Rev Biomed Eng, 2012. 14: p. 205–30. [DOI] [PubMed] [Google Scholar]
- 149.Golden AP and Tien J, Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip, 2007. 7(6): p. 720–5. [DOI] [PubMed] [Google Scholar]
- 150.Tien J, Nelson CM, and Chen CS, Fabrication of aligned microstructures with a single elastomeric stamp. Proc Natl Acad Sci U S A, 2002. 99(4): p. 1758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Song YS, et al. , Engineered 3D tissue models for cell-laden microfluidic channels. Anal Bioanal Chem, 2009. 395(1): p. 185–93. [DOI] [PubMed] [Google Scholar]
- 152.Khademhosseini A, et al. , Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip, 2004. 4(5): p. 425–30. [DOI] [PubMed] [Google Scholar]
- 153.Khademhosseini A, et al. , A soft lithographic approach to fabricate patterned microfluidic channels. Anal Chem, 2004. 76(13): p. 3675–81. [DOI] [PubMed] [Google Scholar]
- 154.Kolesky DB, et al. , Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A, 2016. 113(12): p. 3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kolesky DB, et al. , 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater, 2014. 26(19): p. 3124–30. [DOI] [PubMed] [Google Scholar]
- 156.Bertassoni LE, et al. , Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip, 2014. 14(13): p. 2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Twohig C, et al. , A dual-ink 3D printing strategy to engineer pre-vascularized bone scaffolds in-vitro. Mater Sci Eng C Mater Biol Appl, 2021. 123: p. 111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mansoorifar A, Tahayeri A, and Bertassoni LE, Bioinspired reconfiguration of 3D printed microfluidic hydrogels via automated manipulation of magnetic inks. Lab Chip, 2020. 20(10): p. 1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kinstlinger IS, et al. , Generation of model tissues with dendritic vascular networks via sacrificial laser-sintered carbohydrate templates. Nat Biomed Eng, 2020. 4(9): p. 916–932. [DOI] [PubMed] [Google Scholar]
- 160.Puluca N, et al. , Bioprinting Approaches to Engineering Vascularized 3D Cardiac Tissues. Curr Cardiol Rep, 2019. 21(9): p. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Abdollahi S, Boktor J, and Hibino N, Bioprinting of freestanding vascular grafts and the regulatory considerations for additively manufactured vascular prostheses. Transl Res, 2019. 211: p. 123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morss Clyne A, Swaminathan S, and Diaz Lantada A, Biofabrication strategies for creating microvascular complexity. Biofabrication, 2019. 11(3): p. 032001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sarker MD, et al. , 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J Pharm Anal, 2018. 8(5): p. 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grigoryan B, et al. , Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science, 2019. 364(6439): p. 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Naderi A, Bhattacharjee N, and Folch A, Digital Manufacturing for Microfluidics. Annu Rev Biomed Eng, 2019. 21: p. 325–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bhattacharjee N, et al. , The upcoming 3D-printing revolution in microfluidics. Lab Chip, 2016. 16(10): p. 1720–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Au AK, et al. , 3D-Printed Microfluidics. Angew Chem Int Ed Engl, 2016. 55(12): p. 3862–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Subbiah R, et al. , Prevascularized hydrogels with mature vascular networks promote the regeneration of critical-size calvarial bone defects in vivo. J Tissue Eng Regen Med, 2021. 15(3): p. 219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Parthiban SP, et al. , Engineering pericyte-supported microvascular capillaries in cell-laden hydrogels using stem cells from the bone marrow, dental pulp and dental apical papilla. Sci Rep, 2020. 10(1): p. 21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bertassoni LE, Progress and Challenges in Microengineering the Dental Pulp Vascular Microenvironment. J Endod, 2020. 46(9S): p. S90–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thrivikraman G, et al. , Rapid fabrication of vascularized and innervated cell-laden bone models with biomimetic intrafibrillar collagen mineralization. Nat Commun, 2019. 10(1): p. 3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Monteiro N, et al. , Engineering Microvascular Networks in LED Light-Cured Cell-Laden Hydrogels. ACS Biomater Sci Eng, 2018. 4(7): p. 2563–2570. [DOI] [PubMed] [Google Scholar]
- 173.Zhang S, Wan Z, and Kamm RD, Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip, 2021. 21(3): p. 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zimmerling A and Chen X, Bioprinting for combating infectious diseases. Bioprinting, 2020. 20: p. e00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arslan-Yildiz A, et al. , Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication, 2016. 8(1): p. 014103. [DOI] [PubMed] [Google Scholar]
- 176.Tasoglu S and Demirci U, Bioprinting for stem cell research. Trends Biotechnol, 2013. 31(1): p. 10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang YS, et al. , 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng, 2017. 45(1): p. 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dalton PD, et al. , Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv Sci (Weinh), 2020. 7(11): p. 1902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Twohig C, et al. , A dual-Ink 3D printing strategy to engineer pre-vascularized bone scaffolds in-vitro. Mater Sci Eng C Mater Biol Appl, 2021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu W, et al. , Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater, 2017. 29(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Koch L, et al. , Laser assisted cell printing. Curr Pharm Biotechnol, 2013. 14(1): p. 91–7. [PubMed] [Google Scholar]
- 182.Cui X, et al. , Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul, 2012. 6(2): p. 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Campbell PG and Weiss LE, Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther, 2007. 7(8): p. 1123–7. [DOI] [PubMed] [Google Scholar]
- 184.Miri AK, et al. , Microfluidics-Enabled Multimaterial Maskless Stereolithographic Bioprinting. Adv Mater, 2018. 30(27): p. e1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li W, et al. , Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing. Adv Healthc Mater, 2020. 9(15): p. e2000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ng WL, et al. , Vat polymerization-based bioprinting-process, materials, applications and regulatory challenges. Biofabrication, 2020. 12(2): p. 022001. [DOI] [PubMed] [Google Scholar]
- 187.Van Hoorick J, et al. , (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater, 2019. 97: p. 46–73. [DOI] [PubMed] [Google Scholar]
- 188.Guerra AJ, et al. , Photopolymerizable Resins for 3D-Printing Solid-Cured Tissue Engineered Implants. Curr Drug Targets, 2019. 20(8): p. 823–838. [DOI] [PubMed] [Google Scholar]
- 189.Hribar KC, et al. , Light-assisted direct-write of 3D functional biomaterials. Lab Chip, 2014. 14(2): p. 268–75. [DOI] [PubMed] [Google Scholar]
- 190.Tumbleston JR, et al. , Additive manufacturing. Continuous liquid interface production of 3D objects. Science, 2015. 347(6228): p. 1349–52. [DOI] [PubMed] [Google Scholar]
- 191.Lim KS, et al. , One-Step Photoactivation of a Dual-Functionalized Bioink as Cell Carrier and Cartilage-Binding Glue for Chondral Regeneration. Adv Healthc Mater, 2020. 9(15): p. e1901792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soliman BG, et al. , Stepwise Control of Crosslinking in a One-Pot System for Bioprinting of Low-Density Bioinks. Adv Healthc Mater, 2020. 9(15): p. e1901544. [DOI] [PubMed] [Google Scholar]
- 193.Lim KS, et al. , Fundamentals and Applications of Photo-Cross-Linking in Bioprinting. Chem Rev, 2020. 120(19): p. 10662–10694. [DOI] [PubMed] [Google Scholar]
- 194.Bertlein S, et al. , Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv Mater, 2017. 29(44). [DOI] [PubMed] [Google Scholar]
- 195.Grigoryan B, et al. , Development, characterization, and applications of multi-material stereolithography bioprinting. Sci Rep, 2021. 11(1): p. 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parthiban SP, et al. , BoneMA – synthesis and characterization of methacrylated bone-derived hydrogels for bioprinting of in-vitro vascularized tissue constructs. Biofabrication, 2020. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen MH, et al. , Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol Biosci, 2019. 19(1): p. e1800248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rodell CB, et al. , Evolution of hierarchical porous structures in supramolecular guest-host hydrogels. Soft Matter, 2016. 12(37): p. 7839–7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dong Y, et al. , Multi-stimuli-responsive programmable biomimetic actuator. Nat Commun, 2019. 10(1): p. 4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tasoglu S, et al. , Untethered micro-robotic coding of three-dimensional material composition. Nat Commun, 2014. 5: p. 3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qi H, et al. , DNA-directed self-assembly of shape-controlled hydrogels. Nat Commun, 2013. 4: p. 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Subbiah R, et al. , 3D Printing of Microgel-Loaded Modular Microcages as Instructive Scaffolds for Tissue Engineering. Adv Mater, 2020. 32(36): p. e2001736. [DOI] [PubMed] [Google Scholar]
- 203.Regehly M, et al. , Xolography for linear volumetric 3D printing. Nature, 2020. 588(7839): p. 620–624. [DOI] [PubMed] [Google Scholar]
- 204.Davoodi E, et al. , Extrusion and Microfluidic-based Bioprinting to Fabricate Biomimetic Tissues and Organs. Adv Mater Technol, 2020. 5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colosi C, et al. , Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater, 2016. 28(4): p. 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ainla A, et al. , A multifunctional pipette. Lab Chip, 2012. 12(7): p. 1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jeffries GDM, et al. , 3D micro-organisation printing of mammalian cells to generate biological tissues. Sci Rep, 2020. 10(1): p. 19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chung K, et al. , Structural and molecular interrogation of intact biological systems. Nature, 2013. 497(7449): p. 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Driscoll MK and Zaritsky A, Data science in cell imaging. J Cell Sci, 2021. 134(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weinkamer R, Kollmannsberger P, and Fratzl P, Towards a Connectomic Description of the Osteocyte Lacunocanalicular Network in Bone. Curr Osteoporos Rep, 2019. 17(4): p. 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roschger P, et al. , Bone mineralization density distribution in health and disease. Bone, 2008. 42(3): p. 456–66. [DOI] [PubMed] [Google Scholar]
- 212.Paris O, et al. , Analysis of the hierarchical structure of biological tissues by scanning X-ray scattering using a micro-beam. Cell Mol Biol (Noisy-le-grand), 2000. 46(5): p. 993–1004. [PubMed] [Google Scholar]
- 213.Ascenzi MG and Roe AK, The osteon: the micromechanical unit of compact bone. Front Biosci (Landmark Ed), 2012. 17: p. 1551–81. [DOI] [PubMed] [Google Scholar]
- 214.Avrunin AS, et al. , [Hierarchy of spiral organization of skeletal structures. Interrelationship between structure and functions]. Morfologiia, 2010. 137(6): p. 69–75. [PubMed] [Google Scholar]
- 215.Sahar ND, Hong SI, and Kohn DH, Micro- and nano-structural analyses of damage in bone. Micron, 2005. 36(7–8): p. 617–29. [DOI] [PubMed] [Google Scholar]
- 216.Buckwalter JA and Cooper RR, Bone structure and function. Instr Course Lect, 1987. 36: p. 27–48. [PubMed] [Google Scholar]
- 217.Zhang Y, et al. , Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol, 2020. 8: p. 598607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bahraminasab M, Challenges on optimization of 3D-printed bone scaffolds. Biomed Eng Online, 2020. 19(1): p. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Salah M, et al. , Three-dimensional bio-printing and bone tissue engineering: technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac Plast Reconstr Surg, 2020. 42(1): p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kronemberger GS, et al. , Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J Stem Cells, 2020. 12(2): p. 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sheehy EJ, Kelly DJ, and O’Brien FJ, Biomaterial-based endochondral bone regeneration: a shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater Today Bio, 2019. 3: p. 100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tao O, et al. , The Applications of 3D Printing for Craniofacial Tissue Engineering. Micromachines (Basel), 2019. 10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang L, et al. , Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater, 2019. 84: p. 16–33. [DOI] [PubMed] [Google Scholar]
- 224.Dhawan A, et al. , Three-dimensional Bioprinting for Bone and Cartilage Restoration in Orthopaedic Surgery. J Am Acad Orthop Surg, 2019. 27(5): p. e215–e226. [DOI] [PubMed] [Google Scholar]
- 225.Zhang S and Wang H, Current Progress in 3D Bioprinting of Tissue Analogs. SLAS Technol, 2019. 24(1): p. 70–78. [DOI] [PubMed] [Google Scholar]
- 226.Roseti L, et al. , Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl, 2017. 78: p. 1246–1262. [DOI] [PubMed] [Google Scholar]
- 227.Kabirian F and Mozafari M, Decellularized ECM-derived bioinks: Prospects for the future. Methods, 2020. 171: p. 108–118. [DOI] [PubMed] [Google Scholar]
- 228.Choudhury D, et al. , Organ-Derived Decellularized Extracellular Matrix: A Game Changer for Bioink Manufacturing? Trends Biotechnol, 2018. 36(8): p. 787–805. [DOI] [PubMed] [Google Scholar]
- 229.Cunniffe GM, et al. , (*) Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng Part A, 2017. 23(17–18): p. 891–900. [DOI] [PubMed] [Google Scholar]
- 230.Saito M and Marumo K, Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif Tissue Int, 2015. 97(3): p. 242–61. [DOI] [PubMed] [Google Scholar]
- 231.Bertassoni LE and Swain MV, The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater, 2014. 38: p. 91–104. [DOI] [PubMed] [Google Scholar]
- 232.Jabbari E, Osteogenic peptides in bone regeneration. Curr Pharm Des, 2013. 19(19): p. 3391–402. [DOI] [PubMed] [Google Scholar]
- 233.Messaoudi O, et al. , Stem Cells and Extrusion 3D Printing for Hyaline Cartilage Engineering. Cells, 2020. 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cidonio G, et al. , The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials, 2019. 209: p. 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Askari M, et al. , Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci, 2021. 9(3): p. 535–573. [DOI] [PubMed] [Google Scholar]
- 236.Martin JJ, Fiore BE, and Erb RM, Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat Commun, 2015. 6: p. 8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Blagoev KB, Organ aging and susceptibility to cancer may be related to the geometry of the stem cell niche. Proc Natl Acad Sci U S A, 2011. 108(48): p. 19216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bhatia SN and Ingber DE, Microfluidic organs-on-chips. Nat Biotechnol, 2014. 32(8): p. 760–72. [DOI] [PubMed] [Google Scholar]


